Response of roe deer (Capreolus capreolus) to seasonal and local changes in dietary energy content and quality
Andreas König A C , Martina Hudler A , Sarah-Alica Dahl
A C , Martina Hudler A , Sarah-Alica Dahl  A , Carmen Bolduan B , Daniel Brugger B and Wilhelm Windisch B
A , Carmen Bolduan B , Daniel Brugger B and Wilhelm Windisch B
A Technical University of Munich, Wildlife Biology and Management Unit, Hans-Carl-von-Carlowitz Platz 2, D-85354 Freising, Germany.
B Technical University of Munich, Chair of Animal Nutrition, Liesel-Beckmann-Strasse 2, D-85354 Freising, Germany.
C Corresponding author. Email: koenig@wzw.tum.de
Animal Production Science 60(10) 1315-1325 https://doi.org/10.1071/AN19375
Submitted: 6 July 2019 Accepted: 26 January 2020 Published: 30 April 2020
Journal Compilation © CSIRO 2020 Open Access CC BY-NC-ND
Abstract
Context: In terms of their nutritional physiology, roe deer have been called ‘concentrate selectors’. This implies that they select proteins in their diet and are not able to digest fibre. It is, thus, suggested that in an agricultural landscape, they are unable to digest the high fibre content of arable crops and, thus, suffer and need to be given supplementary feed.
Aims: Our aim was to determine the nutrient composition and energy content of the roe deer diet in an agricultural habitat compared with that in a natural forest habitat.
Methods: Rumen contents of 245 roe deer were collected to represent each month of the year for 3 years, weighed, and analysed by standard methods for nutrient and fibre content.
Key results: Roe deer in the agricultural habitat had rumen contents with significantly higher metabolisable energy (ME) concentrations, with a median of 6.2 MJ ME/kg DM, than did roe deer in the forest habitat, where the median was 5.4 MJ ME/kg DM. The mass of rumen contents in the forest habitat was, on average, 240 g higher than that in the agricultural habitat. Roe deer in the forest habitat compensate for the lower energy concentration in their natural diet by increasing their food intake. The concentration in the agricultural habitat is a result of the higher proportion of easily digestible carbohydrates in the diet. The concentration of crude protein in the rumen contents did not differ statistically between the two areas. In both habitats, the mean crude fibre concentration varied between 24% DM and 34% DM, and was significantly higher in the forest. The concentration of crude fibre selected by the roe deer is similar to the concentration of crude fibre known to be selected by red deer and fallow deer (intermediate feeders) and mouflon (a grazer) (Hofmann 1989).
Conclusions: The term ‘concentrate selector’ should be replaced by ‘selector’ to avoid misinterpretations. Energy shortfalls were not observed in either of the populations during the study period.
Implications: Supplemental feeding was not necessary to maintain the population.
Additional keywords: cervid, crude fibre, metabolisable energy, nutrition, ruminants, selector.
Introduction
The European roe deer is currently the most common and, of the naturally occurring species, the smallest deer species in central Europe. (Stubbe 1997; Andersen et al. 1998; Geist 1999; Rowell-Schäfer et al. 2001). It currently inhabits the same area as before the last ice age, and occurs from Scandinavia to the Mediterranean, and from the Atlantic coast to well into eastern Europe and western Asia (Sempere et al. 1996; Geist 1999). Here roe deer are to be found between sea level and up to the tree line in the Alps (Linnell et al. 1998). At the end of the 19th century, the roe deer had almost died out in wide swathes of central Europe (Stubbe 1997; Andersen et al. 1998), which is why in some books at the end of the 19th century and beginning of the 20th, roe deer were described only for the sake of completeness (Diezel 1921).
The roe deer is a typical representative of the brushwood-edge dwellers (Cederlund 1983; Wölfel 2005); so, forest clearings and forest-edge areas are its original habitat (Stubbe 1997; Wölfel 2005). Today, roe deer inhabit not only their original forest habitats, but also occur in all agricultural and natural landscape habitats in their range (Sempere et al. 1996; Stubbe 1997). With regard to their nutritional behaviour, roe deer belong to the ‘concentrate selector or browser’ group of wild ruminants (Hofmann 1989; Rowell-Schäfer et al. 2001). These differ from the groups of ‘grass and roughage eaters or grazers’ as well as ‘intermediate, opportunistic mixed feeders’, in that they select diet with a higher nutritional value in terms of a high protein content, and avoid high-fibre diet (Onderscheka and Jordan 1976; Drescher-Kaden and Seifelnasr 1977b; Cederlung and Nyström 1981; Hofmann 1982a; Drescher-Kaden 1984; Hofmann 1989; Duncan et al. 1998). Their rumens are thought to lack fibre-digesting microorganisms such as cellulolytic bacteria (Drescher-Kaden and Seifelnasr 1977a; Duncan et al. 1998). Additionally, because of the high flow rate of digesta, they should not have sufficient time to break down the fibre (Ellenberg 1975; Hofmann 1989). The high flow rate of digesta is partly due to the small rumen volume of 3–6 L, which prevents an increase of the food intake in autumn and winter (Hofmann 1989), although this has not been confirmed by studies in Scandinavia (Holand et al. 1998). Because of their lower metabolism in winter and to save digestive energy, roe deer limit their food intake in winter, as do other ruminants (Bubenik and Lochman 1956; Weiner 1977; Drożdż 1979; Dissen and Hartfiel 1985; Arnold 2013).
However, there are some studies that, on the basis of their data, have refuted the classification and conclusions drawn by Hofmann (1989) on the capabilities of the digestive systems of the individual ruminant species (Holand 1994; Robbins et al. 1995; Behrend et al. 2004; Lechner et al. 2009; Clauss et al. 2010; Marchand et al. 2013; Obidzinski et al. 2017). With regard specifically to the fibre content of the roe deer browsing, even in the results of authors who support the theses of Hofmann, such as Drescher-Kaden and Seifelnasr (1977b), we find crude fibre (CF) content values for the roe deer browsing which, with a CF concentration of 20–24% DM, correspond to those of mouflon at 22–34% DM or those of fallow deer at 24–25%. Other studies have shown CF concentrations in the roe deer food intake of between 13% and 32% DM (Onderscheka and Jordan 1976; Schmidl 1996; Deutsch et al. 1998; Djordjevic et al. 2006). Comparative studies on the food quality in Africa showed no differences in the fibre content of the diet of browsers and grazers (Woodall 1992). The grazer mouflon was also considerably more varied in its choice of food than would be expected in accordance with the findings of Hofmann (Hofmann 1989; Marchand et al. 2013; Obidzinski et al. 2017).
Despite this scientific evidence based on data, and although roe deer occur in high densities in agricultural areas and do exploit agricultural crops (Anke et al. 2005; Abbas et al. 2011), it is often claimed in Germany, because of Hofmann’s classification of the ruminant in particular (Hofmann 1978, 1982a, 1982b, 1989), that roe deer browsing in areas of intensively farmed land struggle because of the high fibre content of the food (Bayern 1997; Bauer 2007, 2009). It is also implied that the quality of the food available for browsing in winter is relatively poor (Onderscheka and Jordan 1976). The consequence is that they cannot build up sufficient fat reserves in autumn, which means they have a shortfall in their energy supply in late winter (Hofmann and Kirsten 1982; Helm 2015), and have to be given supplementary feed from late autumn (Hofmann and Kirsten 1982; Ueckermann 1986; Bauer 2007, 2009, 2014; Helm 2015).
To determine any shortfall in energy for roe deer caused by the fibre-rich diet in a particular area, it is first necessary to establish the daily energy requirement of a roe deer. A series of studies on this have been conducted with roe deer living in enclosures or kept in respirometric chambers (Weiner 1977). The maintenance requirement (fasting metabolic rate, FMR) for roe deer is between ~3.3 MJ/roe deer.day and 5.1 MJ/roe deer.day, depending on the season (Drożdż and Osiecki 1973; Weiner 1977; Perzanowski 1978; Hartfiel et al. 1985; Oslage and Strothmann 1988; Mauget et al. 1997). This requirement increases with movement and stress by as much as 200–300% (Weiner 1977). In pregnant does, the resting metabolic rate (RMR), which is ~20% above the FMR, rises in the last 2 months of the pregnancy by 15%, and after parturition by 27% due to lactation (Mauget et al. 1997). Roe deer kept in enclosures had an annual median intake of ~8 MJ ME/roe deer.day, with the highest value in June of 10 MJ ME/roe deer.day (Oslage and Strothmann 1988). These values can be seen as the lower-limit value for energy intake, as animals kept in enclosures have less need for movement. In their case, activities such as the search for mates and migration between seasonal habitats or in search of a food supply with a higher energy content are unnecessary (Aulak and Babinska-Werka 1990; Bideau et al. 1993; Mancinelli et al. 2015). The maximum energy requirement of 17 MJ ME/day assumed by Onderscheka (1999) or Bauer (2007) seems realistic, given the results based on animals kept in enclosures (Oslage and Strothmann 1988).
We aimed to help objectify the discussion in German-speaking countries about the natural diet of roe deer and the necessity of feeding them in times of need, with a special focus on important habitats in Bavaria, southern Germany. We, therefore, compared the energy content and quality of the diet of roe deer in an agricultural habitat (Ag) with those of the diet of roe deer in a near-natural forest habitat (Fo). As well as the crude protein (CP) and nitrogen-free extract (NFE) contents, we were particularly interested in the CF concentration in the two habitats, so as to find out whether this is higher in the agricultural landscape than in the natural landscape. We used CF and NFE for a better comparison with older studies. As neutral detergent fibre (NDF) and water-soluble carbohydrates (WSC) are considered state of the art in nutritional analyses, we added these too (for more details about results of our nutritional analysis, see Dahl et al. 2020). As energy values in particular are related to DM, and the seasonal rhythm of the dietary intake must also be taken into consideration, the rumen volume (L) and rumen content (g) were also to be recorded. The study areas had to be comparable in terms of their climatic conditions. Roe deer were not to be given supplementary feed in either the Ag or the Fo. As roe deer are assumed most likely to have a shortfall in energy between the end of February and the beginning of April, when their fat reserves have been depleted, their metabolism rises in response to daylength, and the vegetation does not yet supply sufficient energy (Hofmann and Kirsten 1982), the sampling process should include this period. For this, the close season for roe deer normally imposed by Bavarian hunting law had to be suspended for the purposes of the research between 15 January and 1 May in the study period.
Generally, the following questions were considered:
-
How does the crude nutrient composition of the diet of roe deer in Ag compare with that of roe deer in Fo?
-
How high is the energy concentration in the diet in cultivated area in comparison to that in a near-natural Fo?
-
What does this energy concentration depend on?
-
How do roe deer respond to a different energy concentration?
-
How much energy is available to the roe deer or how high is the energy concentration in their food intake in the respective habitats?
-
Is there a point at which there is an energy shortfall and, at which, it is necessary to feed roe deer in the cultivated area?
Materials and methods
Site selection
From 2011 to 2014, the rumens of 245 roe deer (Table 1) were collected in both a Fo and in an Ag. The forest area (Fo) is ~20 km south of the city of Munich, southern Germany, in the ‘Jungmoräne’ growth district, at an altitude of ~650 m above sea level, with a mean annual temperature of 8°C and annual precipitation of ~950 mm. It is a state-owned forest covering an area of 2673 ha, of which 90% is forest. Hunting is conducted on the area by the state, in this case, the Bayerische Staatsforsten AöR (Bavarian State Forest) of Munich. The current growing stock consists of 70% Norway spruce (Picea abies), 20% European beech (Fagus silvatica), 2% silver fir (Abies alba) and other hardwoods.
|
The Ag was made up of three hunting districts near the small town of Eggenfelden, ~100 km east of the city of Munich at an altitude of 490 m above sea level in the Tertiary Hill Country. The area covers a total of 2240 ha and is privately owned. The hunting on the area is privately organised. The mean annual temperature here is ~8°C, and precipitation reaches ~840 mm. The proportion of the hunting areas that is forested varies between 27% and 34%. It consists of 75% Norway spruce (Picea abies), 10% Scots pine (Pinus silvestris), 5% silver fir (Abies alba) and 2% European beech (Fagus silvatica). It is typical at the forest edges of these small farm-owned forests to find large numbers of pedunculate oak (Quercus robur), something which is not reflected by the inventory data. In the 3 years of the study period, the agricultural areas consisted of 33–40% grassland; 16–21% were planted with winter wheat, 11–27% with silage maize, 3–10% with grain maize, 5–8% with barley and 3–10% with clover.
For both study areas, the Forest Survey by the Forest Administration on the Situation of Forest Regeneration of 2015 attests a tolerable level of damage to the forest regeneration through browsing (Forstverwaltung 2015). From this, it can be concluded that the food-resource base is sufficient, independently of the forest regeneration, as the damage to forest vegetation from browsing would otherwise be higher and untenable. The quality assessments on ground-level vegetation undertaken as part of the study also showed good-quality vegetation in winter and spring (König et al. 2016).
Sample selection
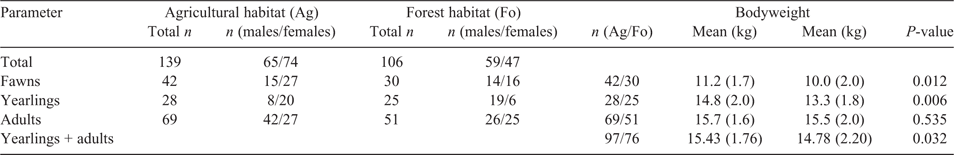
The roe deer were hunted from hunting hides, and in autumn also in driven hunts. So as to observe seasonal influences such as an energy shortfall in October and March (Hofmann and Kirsten 1982), we gathered samples throughout the 12 months of each year. We, thus, acquired a permit from the local hunting authorities (District Council of Rottal am Inn, Az: 31-7512-01/13 and Az: 31-7512-02/13; District Council of Starnberg Az: 7512/311.2W; District Council of Munich Az: 5.3-750/Hei) to take roe deer outside regular hunting seasons. Once killed, the animals were field-dressed immediately, and the digestive tracts were removed and frozen locally at –20°C. The roe deer were also weighed locally and grouped according to sex and age in the categories fawn, yearling und adult (Table 1). The sex ratio of taken deer was 1 male : 0.98 female.
After field-dressing, the weights of the adult animals at 15.7 kg (Ag) and 15.5 kg (Fo) did not differ significantly (P = 0.535) between the habitats. In the Ag, fawns were, on average, 1.2 kg (P = 0.012) and yearlings 1.5 kg (P = 0.006) heavier than they were in the Fo (Table 1). If subadult and adult animals are considered together, the weights of the subadult and adult roe deer in the Ag with an average weight of 15.4 kg differed significantly from those of roe deer in the Fo, with an average of 14.8 kg (t = –2.165; d.f. 171; P = 0.032).
Sample preparation
So as to determine the rumen volume and the entire ingesta, the reticulum, omasum and abomasum (stomach) were separated from the rumen at the pila ruminoreticuloris. The ingesta in the rumen were subsequently removed and weighed to determine rumen content (g). The empty rumen was sewn up again and filled with water. Its volume (L) was determined on the basis of the water displacement.
The ingesta were first homogenised, and then, for further analyses, ~150 g was removed, freeze-dried and ground to 1 mm. After drying, the sample was weighed again to determine the proportion of dry mass (DM) to the fresh mass (FM).
Nutritional analysis
To analyse the composition of the diet, crude nutrients and fibre fractions (NDF) were analysed according to standard procedures (AOAC 2019). For this, freeze-dried samples from each rumen were used.
We determined the following crude nutrients:
-
Determination of the DM through heat-drying at 103°C
-
lipid (% DM)
-
CF (% DM)
-
CP (% DM)
-
ash (% DM)
-
NFE (% DM)
-
NDF (% DM)
-
WSC (% DM)
Metabolisable energy estimation
Metabolisable energy (ME) was determined using the in vitro gas-production method according to (Menke and Steingass 1988). For this, ground-up ingesta samples for each dead roe deer were mixed with rumen fluid from a ram, and the gas production after 8 and 24 h was recorded. The analyses for each rumen sample were performed four times.
The metabolisable energy (ME) was calculated using the following formula (Menke and Steingass 1988):
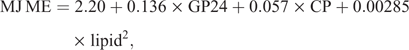
where GP = gas production (mL/200 mg DM).
The results of the estimation of ME made after the analysis of stomach juices from ram and roe deer rumens correlate strongly and significantly at r = 0.6 and P < 0.001 (König et al. 2016).
Total ME estimation (MJ ME/roe deer.day)
As the digesta are retained in the rumen for a short time only, it follows that the roe deer has two rumen fillings per day, with 8–12 browsing periods (Bubenik and Lochman 1956; Hofmann 1982a; Stubbe 1997). At the moment, when the roe deer was taken, we got one rumen filling containing ingesta from four to six browsing periods. As the sampling process with regard to browsing periods for each killed deer was incidental, the energy values of the food found in the rumen represent only part of the roe deer’s daily energy intake. So as to estimate the daily energy intake, we assumed that, per rumen, one-third to one-fourth of the content was undigested material containing the full energy content. To estimate the energy intake over the whole day, the energy concentrations determined per rumen sample (DM) were converted to FM values and extrapolated for the weight of the ingesta found in the rumen. To convert the fresh weight to dry weight, we determined the proportion of DM in the FM for each sample. This value was used for the correction and was, on average, ~0.223 kg DM per kg FM (±0.036, s.d.). This corresponds approximately to the value of 0.2 kg dry weight per 1 kg fresh weight in the literature (Stubbe 1997). This value per rumen was then corrected by one-third or one-fourth per rumen content (g) and multiplied by two, to get the daily energy estimation for the two rumen fillings per day.
ME (MJ/day) was calculated according to the following formula:

Statistics
For identifying normal distribution, Kolmogorov–Smirnov and Shapiro–Wilk test was used. Student’s t-tests for equal and unequal variances were used to back up the statistical differences between means. The decision on whether to use a Student’s t-test for equal or unequal variances was taken using Levene’s test for equality of variances. For non-normal distributions of data, the Mann–Whitney U-test was used to back up the statistical differences between medians (Lozan 1992; Jannsen and Laatz 2017). The calculations were performed using SPSS 24.0 (IBM SPSS Statistics for Windows, Version 24.0; IBM Corp., Armonk NY, USA).
Results
Rumen volume
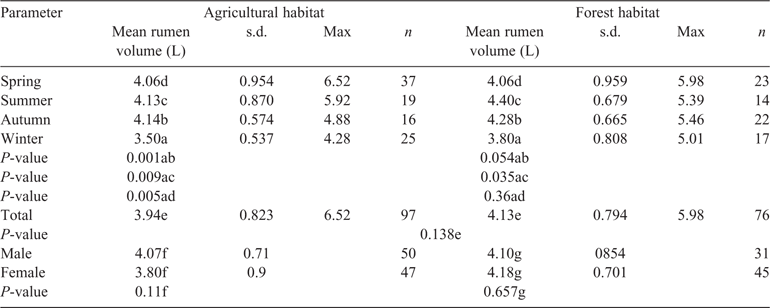
The rumen volume of the roe deer older than 1-year old was, on average, between 3.55 L and 4.54 L (Table 2). The rumen capacities of the agricultural roe deer (Ag roe) were ~4.8% lower than the values for roe deer in forest areas (Fo roe). However, the largest rumen volumes were not found in Fo roe, but in Ag roe, at up to 6.52 L.
The differences in rumen volume found in animals between the two habitats were not significant (t = 1.490; d.f. = 167; P = 0.138). The rumen volumes of the roe deer in the Fo were largest, on average, in summer, whereas those of the roe deer in the Ag were at their largest in summer and autumn. In both habitats, the rumens shrank to a minimum volume in winter. In the Ag, the rumen volume differed significantly between the winter and the other seasons (winter vs autumn, P = 0.005; winter vs summer, P = 0.009; winter vs spring, P = 0.001). In the Fo, there was a significant difference only between the rumen volume in winter and that in summer (P = 0.035).
Rumen content
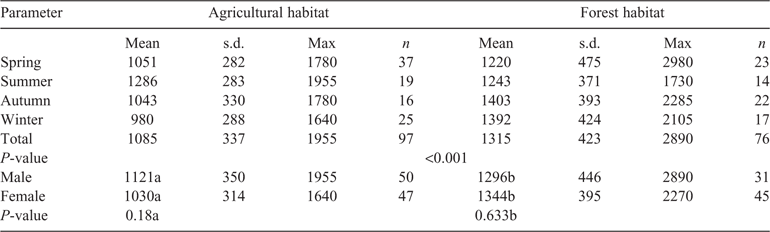
In contrast to the findings on rumen capacity, the differences in rumen content between the two habitats were significant (t = 4.134; d.f. = 171; P < 0.001). The average annual rumen content of Ag roe was ~230 g lower than that of Fo roe (Table 3). Whereas the rumen content of roe deer in agricultural areas decreased in accordance with the rumen volume (L) towards autumn and winter, the rumen content of Fo roe increased from 1220 g in spring and 1243 g in summer to 1403 g in autumn and 1392 g in winter.
|
The differences between the two habitats were particularly great when it came to the quantities of rumen content in autumn and winter, when Fo roe took in ~380 g more food per filling of the rumen, which, assuming two rumen fillings per day (Stubbe 1997), amounted to an additional 770 g per day. The level of filling of the rumen ((rumen content / volume) / 100 (Holand et al. 1998)) differed significantly between autumn and winter and between spring and summer in the case of Fo roe (t = –2.165; d.f. = 96; P = 0.033).
Quality of the diet
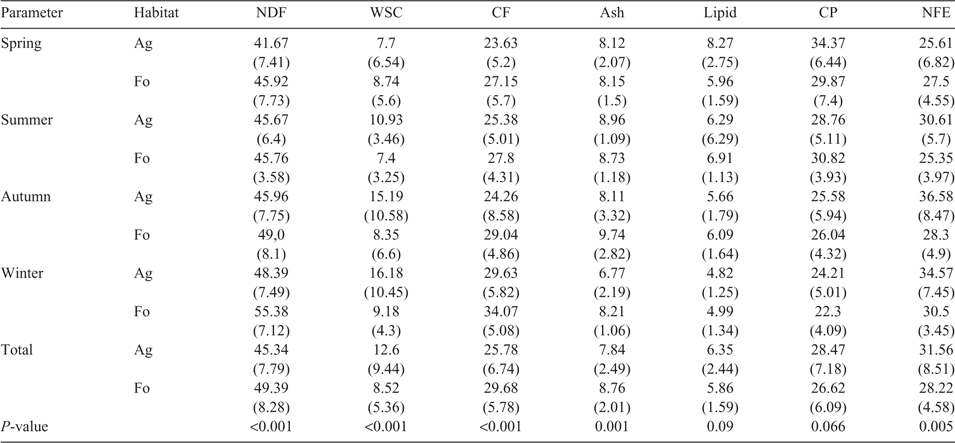
The quality of the diet intake, expressed in terms of the composition of raw nutrients, showed in part clear differences between the two habitats. In both habitats, roe deer consumed significant amounts of CF, with an annual average of 25.8% and 29.7% of CF in rumen content DM of animals from Ag and Fo respectively (CF: t = 4.468; d.f. = 218; P < 0.001; Table 4). The seasonal averages of CF were between 23.6% DM and 29.6% DM in the Ag, and between 27.2% DM and 34.1% DM in the Fo. Although the average CF values were lower in the Ag than for roe deer in the Fo, the gap between the extreme values in the Ag was considerably wider (CF min. 12.7% DM; max. 47.7% DM) than in the Fo (CF min. 16.8% DM; max. 44.3% DM). Other big differences between the two areas in terms of the quality of the diet could be seen in the percentage concentrations of ash and NFE. At 31.56% DM, the average annual concentration of NFE was significantly higher in the Ag than in the Fo at 28.22% DM (NFE: Levene’s F = 26.120; P < 0.001; t = –2.825; d.f. = 229.939; P = 0.005). The top value recorded in the Ag was ~55.4% DM. Only in spring did the concentration of NFE in the Fo exceed the values in the Ag with a slight significance (t = 2.009; df = 69; P = 0.048).
There was another statistically confirmed difference in the diets of the roe deer between the two habitats in the average annual percentage concentrations of ash, which were ~1% higher in the Fo at 8.94% DM than in the Ag (ash: Levene’s F = 10.893; P = 0.001; t = 3.217; d.f. = 241.327; P = 0.001).
There were no significant differences between the two habitats with regard to the percentage concentrations of CP and lipid in the food contents of the roe deer (lipid: Levene’s F = 13.828; P < 0.001; t = –1.703; d.f. = 237.559; P = 0.09; and CP: t = –1.845; d.f. = 242; P = 0.066; Table 4).
However, if we distinguished between the CP values according to season, they were significantly higher in spring in the Ag at 34.37% DM than they were in the Fo at 29.87% DM (t = –2.713; d.f. 69; P = 0.008).
Similarly to the differences in CF and NFE, differences between the two habitats with regard to the percentage concentrations of NDF and WSC were significant too (Table 4).
Energy concentration
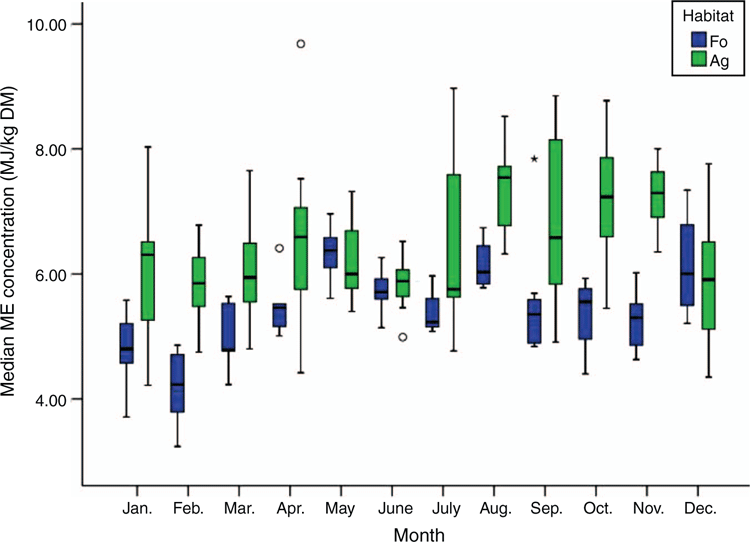
Given the crude nutrients found, the annual median energy concentration of the food in the Ag was significantly higher, at 6.2 MJ ME/day (DM; Fig. 1), than that of the Fo at 5.4 MJ ME/day (DM; Mann–Whitney U = 3631.500; Wilcoxon W = 9302.500; Z = –6.739; P < 0.001).
With the exception of the month of May, the energy concentration in the Ag was always higher than that of the Fo. The maximum monthly median is 6.5 MJ ME/day (DM) in the Fo and 7.5 MJ ME/day (DM) in the Ag.
Daily total estimated energy intake
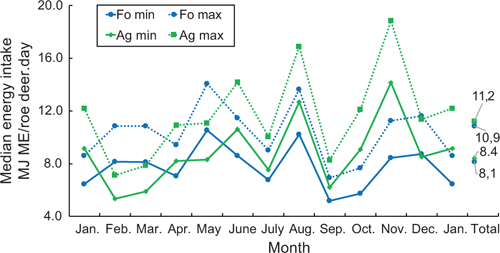
So as to estimate the energy intake of the roe deer, both the rumen content and the energy concentration were taken into account below. In the estimation, account was taken of the fact that a large part of the rumen material examined was always digested or broken down by the microbiota, as roe deer have between four and six browsing intervals per rumen filling, and fill their rumen twice per day. Calculations were made for two scenarios, using one-third (minimum) and one-fourth (maximum) of the energy contained in the rumen content, and multiplied by two fillings to get total energy intake per roe deer per day. The annual medians per day and roe deer were a minimum of 8.4 MJ ME/roe deer.day for the Ag, and 8.1 MJ ME/roe deer.day for the Fo (Fig. 2). An annual median of 11.2 MJ ME/roe deer.day was estimated as a maximum energy intake for the Ag, and 10.9 MJ ME/roe deer.day for the Fo. The differences between the two habitats in terms of minimum and maximum energy per day were minimal and not significant. As an annual daily average, roe deer of both habitats took in approximately the same amount of energy.
|
Whereas the average monthly estimated energy intake of roe deer in the Fo vacillates only slightly around the annual median, the estimated energy intake of the roe deer in the Ag varies considerably throughout the year. Least food was taken in during February and March, while the energy estimation was highest during the grain harvest in August, and during the maize harvest and mast of beech and oak in November, when beechnuts and acorns were ripe.
Discussion
In the present study, the agricultural vegetation provided the roe deer with an annual median energy concentration that was significantly higher by 1 MJ ME/day than that in the Fo. This higher energy concentration resulted from a significantly higher concentration of easily digestible carbohydrates (NFE or WSC), which were apparent even in winter in high concentrations. This confirmed the results of Serrano Ferron et al. (2012), which they found in south-western France. Our roe deer compensated for the lower energy concentration of the diet in the Fo with a significantly higher food intake, which ensured they always had sufficient energy available. On an annual average, the roe deer in both habitats took in approximately the same amount of energy per day.
The actual estimated energy intake per day can only be deduced from the actual ME found in the rumen if we make certain assumptions, as the content of the roe deer’s rumen always consists of a mixture of fresh, pre-digested and digested food because of the 8–12 browsing cycles (Bubenik and Lochman 1956; Klötzli 1965; Hofmann 1989; Stubbe 1997). The pre-digested vegetable material has already released energy; its energy values are lower than those of freshly browsed plant material. Furthermore, low-solubility, indigestible fibre, especially lignin and cellulose, accumulates in the rumen (Esser 1957; Klötzli 1965), producing low levels of gas during the in vitro gas production (Menke and Steingass 1988) and, thus, lower energy values (Giger-Reverdin et al. 1998; Hummel et al. 2006).
Nevertheless, so as to estimate the approximate daily intake of ME, we assumed that, on the basis of the 8–12 browsing cycles that make up two rumen fillings (Bubenik and Lochman 1956; Klötzli 1965; Hofmann 1989; Stubbe 1997), between one-third and one-fourth of the content of the rumen is non-digested food, and, thus, still has its full energy content. Calculated on this basis, we estimate the annual median ME intake of roe deer in both habitats to be between ~8 MJ ME/roe deer.day and 11 MJ ME/roe deer.day. The slight differences in the estimation of ME in the two habitats are not significant. If we compare our estimates of the energy estimation with values from animals kept in enclosures or cages, they seem plausible. In most experiments, the FMR or the RMR for roe deer are determined. These are between 3.3 MJ/roe deer.day in winter and 5.7 MJ/roe deer.day in summer (Drożdż and Osiecki 1973; Ellenberg 1975; Weiner 1977; Perzanowski 1978; Hartfiel et al. 1985). Oslage and Strothmann (1988) found an annual average energy requirement of ~8 MJ ME/roe deer.day for roe deer in enclosures and peak values in June of 10 MJ ME/roe deer.day, which is in line with our estimate of the lowest ME concentration (1/3 non-digested material). As roe deer in enclosures take part only in normal activities such as running away, defending their territories, looking for mates or accumulating fat reserves to a very limited extent, the ME estimation for free and wild roe deer must be higher than these values. The RMR increases with normal movement by 45%. During escape behaviour, for example, RMR can increase by up to 210–300% (Weiner 1977; Ellenberg 1978; Bubenik 1984), without the roe deer having to draw on their reserves. For lactating does, a daily energy requirement of between 8 and 14 MJ/day is assumed (Eisfeld 1984; Mauget et al. 1997; Kamphues et al. 2009), which is consistent with the upper estimates (1/4 non-digested material) for May and June (birth of the kids) in our calculation. Onderscheka (1999) gave a maximum estimated energy requirement, derived from studies with animals in enclosures, of 17 MJ ME/roe deer per day. Our maximum values calculated on the basis of the recorded grazing for the Ag reached 18.9 MJ ME/roe deer per day in November, which seems realistic in relation to the energy values calculated in this way for May and June. In this period, the roe deer in the Ag also build up large fat reserves (König et al. 2016), which they live on in winter. There is, thus, strong seasonal variation in the estimated energy intake of roe deer in the Ag, whereas the average estimated energy intake of the roe deer in the forest area varies only very slightly around the median. This seasonal variation in the estimated energy intake of the roe deer in the Ag is very similar to the seasonal cycle in the measured energy intake of roe deer kept in enclosures (Ellenberg 1978; Hofmann 1978; Drożdż 1979; Oslage and Strothmann 1988). The roe deer in enclosures are provided with an ad libitum supply of food and energy. The roe deer reduce their food intake in late winter and increase it to a maximum in the autumn. In contrast, the roe deer in the Fo do not have any such strong variations in the energy concentration of their natural diet. The energy concentration in the Fo reaches the annual median of the Ag only in May. Roe deer in the Fo, thus, take in a relatively constant level of energy over the year. Pivotal to this daily energy supply of the roe deer in the Fo is the quantity of food intake. Although the rumen volume decreases in roe deer in both habitats in winter, the roe deer in the Fo graze more in autumn–winter to compensate for the lower energy concentration in their browsing. With two rumen fillings a day (Stubbe 1997), roe deer in the Fo take in ~770 g more food than do roe deer in the Ag, despite the fact that, when subadult and adult roe deer are considered together, they have lower bodyweights on average in the Fo than those in the Ag. Similar results were recorded for roe deer in Scandinavia, which also had a higher percentage filling of the rumen in winter. The rumen volume diminished from summer to autumn and winter (means: 4.3 L in summer, 4.0 L in autumn, 3.7 L in winter), while the average browsing intake rose from 1.0 kg to 1.4 kg in autumn and 1.9 kg in winter (Holand et al. 1998). The fact that Hofmann (1978) disputed this ability of the roe deer may be due to the test material used. The higher food intake recorded in Scandinavia (Holand et al. 1998) is either due to the lower energy concentration of the natural vegetation browsed and/or caused by a higher energy consumption due to the harsher winter. The three winters in our study period were not unusually cold, so that we can assume that temperatures barely fell or fell only for a short time below the thermoneutral temperature of –10°C (Bubenik 1984). The energy absorbed by the roe deer, thus, predominantly did not have to be used to regulate their temperature.
The higher energy concentration of the natural roe deer diet in the Ag in comparison with that in the Fo is due to the lower concentration of CF and a higher concentration of NFE. The differences in the concentrations of these two crude nutrients in the two habitats are significant. The mean levels of CF recorded in the two habitats are between 23.63% DM and 34.07% DM. These high CF values do not match the myth that roe deer as ‘concentrate selecters’ cannot digest CF (Eisfeld 1974; Ellenberg 1975; Drescher-Kaden 1976; Onderscheka and Jordan 1976; Drescher-Kaden and Seifelnasr 1977b; Cederlung and Nyström 1981; Hofmann 1982a, 1989; Duncan et al. 1998; Onderscheka 1999). However, high CF concentrations similar to ours are to be found in studies on crude nutrients in roe deer browsing. All of these studies were conducted using the same methods of analysis as we used. The CF means varied between 12.6% DM and 32% DM (Onderscheka and Jordan 1976; Drescher-Kaden and Seifelnasr 1977b; Schmidl 1996; Djordjevic et al. 2006). However, a CF concentration of 12% DM was found in one of our roe deer in the Ag. The mean average did not fall below 18% DM. One explanation for this could be the fact that our data were collected over 12 months. Drescher-Kaden and Seifelnasr (1977b), thus, examined 13 and 21 roe deer, which had been killed only in autumn in two areas respectively.
Even today, it is still usual to feed roe deer from November/December across wide swathes of central Europe. Recommended feeds have a CF content of less than 10% DM (Onderscheka 1999), which actually lowers the concentration of CF in the rumen of roe deer in former studies. By contrast, our study areas were chosen to ensure that the roe deer had no access to feeding. If we compare our CF means in roe deer with the CF means in red deer (Cervus elaphus; intermediate feeders) and mouflon (Ovis ammon musimon; grazers; Hofmann 1989), there is a high degree of similarity. For mouflon, a mean CF concentration of 22–34% DM (Drescher-Kaden and Seifelnasr 1977b) was found, and for red deer it ranged from 25% DM to 38% DM (Briedermann et al. 1988). The values in our study on roe deer did not differ from these values for red deer and mouflon. As we took our roe deer samples from freely roaming wild animals, these roe deer voluntarily took in and utilised high concentrations of fibre in their diet. Other studies have also shown adaptation towards better utilisation of the CF, especially in winter (Cederlung and Nyström 1981; Rehbinder and Ciszuk 1985), and this has also been shown for red deer (Arnold et al. 2015). Although CF provides less ME, relatively speaking, than do CP or NFE extracts, it still contributes to heat generation through fermentation in the rumen (Silver et al. 1971), lowers the thermoneutral range (Bubenik 1984), and, thus, saves ME otherwise needed to maintain body temperature.
Besides showing differences in the concentration of CF, the roe deer diet differed between the two habitats in that there was a significantly higher concentration of NFE as well as the WSCs in the Ag, which, thus, provides more easily digestible carbohydrates. Differentiated according to season, there were, however, slightly significantly higher concentrations of NFE in the Fo only in spring, while, in the remaining seasons, the NFE values for the Ag far exceeded those for the Fo. Viewing both habitats together, the mean NFE concentrations varied between 25.4% DM and 36.6% DM. All in all, these results are in line with data from previous literature, although they are among the higher values. In two separate studies in Serbia, NFE concentrations of between 18% DM and 28% DM (n = 20; Djordjevic et al. 2006) were recorded, and between 15% DM and 25% DM (n = 43; Popovic et al. 2009). Slightly higher NFE values, on average, were found in Austria in a forest district, with NFE concentrations ranging between 33.8% DM and 44.7% DM (Schmidl 1996), although, in this case, no samples were taken between February and April. In two forest areas in Bavaria, one 30 km east of Munich (n = 21) and one 100 km north of Munich (n = 13), NFE concentrations of 29% and 28% DM respectively (Drescher-Kaden and Seifelnasr 1977b), were found, which correlates well with the NFE concentrations of 28% DM in our Fo in autumn.
Generally, the CP concentrations in our study did not provide any indication of the different energy concentrations in the two habitats. The annual mean CP concentrations were not significant, although, in spring, the concentration in the Ag was significantly higher than that in the Fo. These higher values in spring can be explained by the winter wheat grown here.
The highest CP concentrations were found as early as in spring in the Ag, with a concentration of 34.4% DM, and not until the summer in the Fo, with a concentration of 30.8%. This result can be explained by the vegetation growth, as the CP content of the plants rises parallel to the temporal development of the young plants (Buchgraber 2005). The CP concentrations found in our two study areas are more or less comparable with those found in other studies (Drescher-Kaden and Seifelnasr 1977b; Schmidl 1996; Djordjevic et al. 2006; Popovic et al. 2009).
The winter is seen by many as a critical period for the diet of wild ruminants because of the poor quality and low energy content of the vegetation (Onderscheka and Jordan 1976; Sommer 2004; Storms et al. 2008; Arnold 2013). A shortfall in the diet of the roe deer could not be confirmed in either the Ag or the Fo in the present study. The level of damage caused in both habitats by browsing to natural regeneration in the forest was described by the forest authorities as being tolerable (Forstverwaltung 2015). From this, it can be concluded that on the whole, there was sufficient availability of vegetation for the roe deer in winter and spring, or there would have been more browsing damage to the tree vegetation. Furthermore, even after the grain harvest in summer and autumn in the Ag, good-quality vegetation was available to roe deer on 79% of the habitat area, so that the roe deer were able to build up good reserves here (König et al. 2016). We cannot, on the basis of our results, confirm the need to feed roe deer, particularly in the Ag, so as to prevent them from starving in winter, as has been suggested by some (Hofmann and Kirsten 1982; Ueckermann 1986; Bauer 2014; Helm 2015; Bauer and Schwarz 2017).
Even if the classification and attribution of ruminants on the basis of their digestive system in accordance with Hofmann (1989) is problematic and has been refuted many times because of the pronounced adaptability of ruminants to different habitats (Dissen and Hartfiel 1985; Woodall 1992; Deutsch et al. 1998; Sommer et al. 2005; Clauss et al. 2010; Obidzinski et al. 2017), a characterisation of the browsing behaviour of roe deer seems sensible. On the basis of their browsing behaviour, this can be considered to be selective behaviour, independent of the plant species consumed, crude nutrients or habitat. Because of this behaviour, it makes sense to describe roe deer as selectors.
Conclusions
-
Roe deer can convert agricultural crops with great efficiency.
-
The mean CF content of the rumen is never below 18% DM.
-
Carbohydrates are an important energy resource in Ag.
-
A lower energy concentration in diet is compensated for by more grazing.
-
There were no deficits because of a lack of energy.
-
There is no need for supplemental feeding.
-
Roe deer are selectors or browsers, but not ‘concentrate selectors’, as they take in and exploit CF to a similar extent as do ruminants classed as grazers or intermediate feeders.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
We gratefully acknowledge the financial support given by the Bavarian Ministry of Agriculture, Forestry and Nutrition. We also thank the colleagues of the Bavarian State Forest as well as Klaus Urban and all students involved for their practical support.
References
Abbas F, Morellet N, Hewison AJM, Merlet J, Cargnelutti B, Lourtet B, Angibault J-M, Daufresne T, Aulagnier S, Verheyden H (2011) Landscape fragmentation generates spatial variation of diet composition and quality in a generalist herbivore. Oecologia 167, 401–411.| Landscape fragmentation generates spatial variation of diet composition and quality in a generalist herbivore.Crossref | GoogleScholarGoogle Scholar | 21519885PubMed |
Andersen R, Duncan P, Linnell JDC (Eds) (1998) ‘The European roe deer: the biology of success.’ (Scandinavian University Press: Oslo, Norway)
Anke M, Dittrich G, Groppel B, Schäfer U, Müller R, Hoppe C (2005) Zusammensetzung und Aufnahme von Winteräsung durch Reh-, Muffel-, Dam- und Rotwild. In ‘Beiträge zur Jagd- und Wildforschng. Vol. 32’. (Ed. C Stubbe) Jana Vertriebs GmbH, Melsungen pp. 379–398.
AOAC (Ed.) (2019) ‘Official methods of analysis of AOAC International.’ (AOAC International: Gaithersburg, MD)
Arnold W (2013) Jahreszeitliche Anpassung bei Wildwiederkäuern: wo steht das Rehwild? Hege und Bejagung des Rehwildes. Feldkirchen. 20, 13–21.
Arnold W, Beiglbock C, Burmester M, Guschlbauer M, Lengauer A, Schroder B, Wilkens M, Breves G (2015) Contrary seasonal changes of rates of nutrient uptake, organ mass, and voluntary food intake in red deer (Cervus elaphus). American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 309, R277–R285.
| Contrary seasonal changes of rates of nutrient uptake, organ mass, and voluntary food intake in red deer (Cervus elaphus).Crossref | GoogleScholarGoogle Scholar | 26017492PubMed |
Aulak W, Babinska-Werka J (1990) Preference of different habitats and age classes of forest by roe deer. Acta Theriologica 35, 289–298.
| Preference of different habitats and age classes of forest by roe deer.Crossref | GoogleScholarGoogle Scholar |
Bauer J (2007) Rehwildfütterung und Waldschutz. Tierschutz in der Jagd. Bayerischer Landesjagdverband 16, 67–72.
Bauer J (2009) Wann Fütterung geboten ist. Jagd in Bayern 20–21.
Bauer J (2014) Füttern ohne Idiologie. Revierkurier 4–5.
Bauer J, Schwarz H (2017) Notzeit Es liegt in unseren Händen. Jagd in Bayern 16–17.
Bayern Av (1997) ‘Das jagdliche Vermächtnis von Herzog Albrecht von Bayern.’ (Paul Parey Zeitschriftenverlag GmbH & Co Kg: Singhofen, Germany)
Behrend A, Lechner-Doll M, Streich WJ, Clauss M (2004) Seasonal faecal excretion, gut fill, liquid and particle marker retention in mouflon Ovis ammon musimon, and a comparison with roe deer. Acta Theriologica 49, 503–515.
| Seasonal faecal excretion, gut fill, liquid and particle marker retention in mouflon Ovis ammon musimon, and a comparison with roe deer.Crossref | GoogleScholarGoogle Scholar |
Bideau E, Gerard JF, Vincent JP, Maublanc ML (1993) Effects of age and sex on space occupation by European roe deer. Journal of Mammalogy 74, 745–751.
| Effects of age and sex on space occupation by European roe deer.Crossref | GoogleScholarGoogle Scholar |
Briedermann L, Dittrich G, Lockow K-W (1988) Rotwild Cervus elaphus L. In ‘Buch der Hege Band 1 Haarwild’. (Ed. H Stubbe) pp. 2–57. (Verlag Harri Deutsch: Thun, Franfurt am Main, Germany)
Bubenik A, Lochman J (1956) Futterverbrauch und Tagesrhythmus der Futteraufnahme bei Reh- und Rotwild. Zeitschrift fur Jagdwissenschaft 2, 112–118.
Bubenik AB (1984) ‘Ernährung, Verhalten und Umwelt des Schalenwildes.’ (BLV Verlagsgesellschaft: München, Germany)
Buchgraber K (2005) Nahrungsangebot für Rehwild auf Grünland- und Ackerflächen. In ‘11. Östereichische Jägertagung. Raumberg– Gumpenstein’, 15 and 16 February 2005. (Ed. HB-uFf Landwirtschaft) pp. 27–32. (Irdning, Austria)
Cederlund G (1983) Home range dynamics and habitat selection by roe deer in a boreal area in central Sweden. Acta Theriologica 28, 443–460.
| Home range dynamics and habitat selection by roe deer in a boreal area in central Sweden.Crossref | GoogleScholarGoogle Scholar |
Cederlung G, Nyström A (1981) Seasonal differences between moose and roe deer in ability to digest browse. Holarctic Ecology 4, 59–65.
Clauss M, Hume ID, Hummel J (2010) Evolutionary adaptions of ruminants and their potential relevance for modern production systems. Animal Behaviour 4, 979–992.
Dahl S-A, Hudler M, Windisch W, Bolduan C, Brugger D, König A (2020) High fibre selection by roe deer (Capreolus capreolus): evidence of ruminal microbiome adaptation to seasonal and geographical differences in the nutrient composition. Animal Production Science.
| High fibre selection by roe deer (Capreolus capreolus): evidence of ruminal microbiome adaptation to seasonal and geographical differences in the nutrient composition.Crossref | GoogleScholarGoogle Scholar |
Deutsch A, Lechner-Doll M, Wolf G (1998) Activity of cellulolytic enzymes in the contents of reticulorumen and caecolon of roe deer (Capreolus capreolus). Comperative Biochemistry and Physiology. Part A 119, 925–930.
| Activity of cellulolytic enzymes in the contents of reticulorumen and caecolon of roe deer (Capreolus capreolus).Crossref | GoogleScholarGoogle Scholar |
Diezel CE (1921) ‘Erfahrungen aus dem Gebiete der Niederjagd.’ (Kosmoas: Stuttgart, Germany)
Dissen J, Hartfiel W (1985) Beobachtungen zum Äsungsverhalten sowie Untersuchungen zur Nährstoffverdaulichkeit von Rehwild. Zeitschrift fur Jagdwissenschaft 31, 83–91.
Djordjevic N, Popovic Z, Grubic G (2006) Chemical composition of the rumen contents in roe deer (Capreolus capreolus) as potetial quality indicator of their feeding. Journal of Agricultural Sciences 51, 133–140.
| Chemical composition of the rumen contents in roe deer (Capreolus capreolus) as potetial quality indicator of their feeding.Crossref | GoogleScholarGoogle Scholar |
Drescher-Kaden U (1976) Untersuchungen am Verdauungstrakt von Reh, Damhirsch und Mufflon Mitteilungen 1: Gewichtserhebungen und Kapazitätsmessungen am Verdauungstrakt, insbesondere am Pansen-Haubenraum von Reh, Damhirsch und Mufflon. Zeitschrift fur Jagdwissenschaft 22, 184–190.
Drescher-Kaden U (1984) Stellung des Rehs im System der Wiederkäuer. In ‘Rehwild Biologie, Hege. Nationalpark Bayerischer Wald’. (Ed. BSf ELF) (Bay STMELF: München, Germany)
Drescher-Kaden U, Seifelnasr EA (1977a) Untersuchungen am Verdauungstrakt von Reh, Damhirsch und Mufflon. Mitteilungen 3: Mikroorganismen im Pansen von Reh, Damhirsch und Mufflon. Zeitschrift fur Jagdwissenschaft 23, 64–69.
Drescher-Kaden U, Seifelnasr EA (1977b) Untersuchungen zum Verdauungstrakt von Rehwild, Damwild und Mufflon. Mitteilung 2: Rohnährstoffe im Panseninhalt von Reh, Damhirsch und Mufflon. Zeitschrift fur Jagdwissenschaft 23, 6–11.
Drożdż A (1979) Seasonal intake and digestbility of natural foods by roe-deer. Acta Theriologica 24, 137–170.
| Seasonal intake and digestbility of natural foods by roe-deer.Crossref | GoogleScholarGoogle Scholar |
Drożdż A, Osiecki A (1973) Intake and digestibility of natural feeds by roe-deer. Acta Theriologica 18, 81–91.
| Intake and digestibility of natural feeds by roe-deer.Crossref | GoogleScholarGoogle Scholar |
Duncan P, Tixier H, Hofmann RR, Lechner-Doll M (1998) Feeding strategies and the physiology of digestion in roe deer. In ‘The European roe deer: the biology of success’. (Eds R Andersen, P Duncan, JDC Linnell) pp. 91–116. (Scandinavian University Press: Oslo, Norway)
Eisfeld D (1974) Der Proteinbedarf dew Rehes (Capreolus capreolus L.) zur Erhaltung. Zeitschrift fur Jagdwissenschaft 20, 43–48.
Eisfeld D (1984) Nahrungsbedarf des Rehwildes. In ‘Rehwild Biologie, Hege. Nationalpark Bayerischer Wald’. (Ed. BSf ELF) pp. 36–40. (Bay STMELF: München, Germany)
Ellenberg H (1975) Wilddichte, Ernährung und Vermehrung beim Reh. Verhandlungen der Gesellschaft für Ökologie 1974, 59–76.
Ellenberg H (1978) Zur Populationsökologie des Rehes in Mitteleuropa. Spixiana.
Esser W (1957) Beitrag zur Untersuchung der Äsung des Rehwildes. Zeitschrift fur Jagdwissenschaft 3, 1–40.
Forstverwaltung B (2015) ‘Forstliches Gutachten zur Situation der Waldverjüngung 2015. Ergebnis Hegegemeinschaft.’ (Bayerisches Staatsministerium für Ernährung, Landwirtschaft und Forsten, München, Germany)
Geist V (1999) ‘Deer of the world. Their evolution, behaviour, and ecology.’ (Swan Hill Press: Shrewsbury, UK)
Giger-Reverdin S, Maaroufi C, Bontems V, Jousse C (1998) Interest and limits of the HFT method to evaluate the energy value of compound feeds for small ruminants. Cahiers Options Méditerranéennes 52, 39–42.
Hartfiel W, Pheiffer J, Dissen J (1985) Energetische Untersuchungen an Reh und Schaf mit Hilfe der quantitativen Thermographie zur Beurteilung des Energiebedarfs im Winter. Zeitschrift fur Jagdwissenschaft 31, 34–41.
Helm G (2015) Ernteschock und Verbiss. Volle Silos, leere Mägen. Jagd in Bayern 16–17.
Hofmann RR (1978) Die Ernährung des Rehwildes im Jahresablauf nach dem Modell Weichselboden. In ‘Wildbiologische Informationen für den Jäger Jagd + Hege Ausbildungsbuch II’. (Ed. RR Hofmann) pp. 121–136. (Ferdinand Enke Verlag: Stuttgart, Germany)
Hofmann RR (1982a) Die Stellung der europäischen Wildwiederkäuer im System der Äsungstypen. In ‘Wildbiologische Informationen für den Jäger. Jagd + Hege - Ausbildungsbuch I’. (Ed. RR Hofmann) pp. 9–18. (Ferdinand Enke Verlag: Stuttgart, Germany)
Hofmann RR (1982b) Die Verdauungsorgane des Rehes und ihre Anpassung an die besondere Ernährungsweise. In ‘Wildbiologische Informationen für den Jäger. Vol. I’. (Ed. RR Hofmann) pp. 103–126. (Ferdinand Enke Verlag: Stuttgart, Germany)
Hofmann RR (1989) Evolutionary steps of ecophysiological adaption and diversification of ruminants: a comperative view if their digestives system. Oecologia 78, 443–457.
| Evolutionary steps of ecophysiological adaption and diversification of ruminants: a comperative view if their digestives system.Crossref | GoogleScholarGoogle Scholar | 28312172PubMed |
Hofmann RR, Kirsten N (1982) ‘Die Herbstmast: Simulation.’ (Stuttgart, Germany)
Holand O (1994) Seasonal dynamics of digestion in relation to diet quality and intake in European roe deer (Capreolus capreolus). Oecologia 98, 274–279.
| Seasonal dynamics of digestion in relation to diet quality and intake in European roe deer (Capreolus capreolus).Crossref | GoogleScholarGoogle Scholar | 28313903PubMed |
Holand O, Mysterud A, Wannang A, Linnell JDC (1998) Roe deer in northern environments: physiology and behaviour. In ‘The European roe deer: the biology of success’. (Eds R Andersen, P Duncan, JDC Linnell) pp. 117–138. (Scandinavian University Press: Oslo, Norway)
Hummel J, Südekum KH, Streich WJ, Clauss M (2006) Forage fermentation patterns and their implications for herbivore ingesta retention times. Functional ecology 20, 989–1002.
| Forage fermentation patterns and their implications for herbivore ingesta retention times.Crossref | GoogleScholarGoogle Scholar |
Jannsen J, Laatz W (2017) ‘Statistische Datenanalyse mit SPSS.’ (Springer Gabler: Berlin, Germany)
Kamphues J, Coenen M, Iben C, Kienzle E, Pallauf J, Simon O, Wanner M, Zentek J (Eds) (2009) ‘Supplement zur Vorlesung und Übung in der Tierernährung.’ (M. & H. Schaper: Hannover, Germany)
Klötzli F (1965) ‘Qualität und Quantität der Rehäsung.’ (ETH Zürich: Zurich, Switzerland)
König A, Scheingraber M, Mitschke J (2016) ‘Energiegehalt und Qualität der Nahrung von Rehen (Capreolus capreolus) im Jahresverlauf in zwei unterschiedlich geprägten Habitaten.’ (Zentrum Wald Forst Holz: Freising, Germany)
Lechner I, Barboza P, Collins W, Günther D, Hattendorf B, Hummel J, Clauss M (2009) No ‚bypass’ in adult ruminants: passage of fluid ingested vs. fluid inserted into the rumen in fistulates muskoxen (Ovisbos moschatus), reeindeer (Rangifer tarandus) and moose (Alces alces). Comperative Biochemistry and Physiology. Part A 154, 151–156.
| No ‚bypass’ in adult ruminants: passage of fluid ingested vs. fluid inserted into the rumen in fistulates muskoxen (Ovisbos moschatus), reeindeer (Rangifer tarandus) and moose (Alces alces).Crossref | GoogleScholarGoogle Scholar |
Linnell JDC, Duncan P, Andersen R (1998) The European roe deer: a porttait of a successful species. In ‘The European roe deer: the biology of success’. (Eds R Andersen, P Duncan, JDC Linnell) pp. 11–22. (Scandinavian University Press: Oslo, Norway)
Lozan JL (1992) ‘Angewandte Statistik für Naturwissenschaftler.’ (Verlag Paul Parey: Berlin, Germany)
Mancinelli S, Peters W, Boitani L, Hebblewhite M, Cagnacci F (2015) Roe deer summer habitat selection at multiple spatio-temporal scales in an Alpine environment. Hystrix 92,
| Roe deer summer habitat selection at multiple spatio-temporal scales in an Alpine environment.Crossref | GoogleScholarGoogle Scholar |
Marchand P, Redjadj C, Garel M, Cugnasse J-M, Maillard D, Loison A (2013) Are mouflon (Ovis gmelini musimon) really grazers? A review of variation in diet composition. Mammal Review 43, 275–291.
| Are mouflon (Ovis gmelini musimon) really grazers? A review of variation in diet composition.Crossref | GoogleScholarGoogle Scholar |
Mauget C, Mauget R, Sempéré A (1997) Metabolic rate in female European roe deer. Canadian Journal of Zoology 75, 731–739.
| Metabolic rate in female European roe deer.Crossref | GoogleScholarGoogle Scholar |
Menke KH, Steingass H (1988) Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Animal Research and Development 28, 7–55.
Obidzinski A, Miltko R, Bolibok L, Wajdzik M, Skubis J, Nasiadka P (2017) Variation of natural diet of free ranging mouflon affects their ruminal protozoa composition. Smaöö Ruminant Research 157, 57–64.
| Variation of natural diet of free ranging mouflon affects their ruminal protozoa composition.Crossref | GoogleScholarGoogle Scholar |
Onderscheka K (1999) Das Rehwild: seine Ernährung und Fütterung. In ‘Rehwild in der Kulturlandschaft. Nürnberg. Vol. 7’, 19 and 20 March 1990. (Ed. J Reddemann) pp. 37–60. (Schriftenreihe des Landesjagdverbandes Bayern e.V.: München, Germany)
Onderscheka K, Jordan HR (1976) Einfluß der Jahreszeit, des Biotops und der Äsungskonkurrenz auf die botanische Zusammensetzung des Panseninhaltes beim Gams-, Reh-, Muffel- und Rotwild. Die Bodenkultur 27, 202–217.
Oslage HJ, Strothmann A (1988) Zum Energie- und Proteinbedarf von Rehwild. Zeitschrift fur Jagdwissenschaft 34, 164–181.
Perzanowski K (1978) The effect of winter food composition on roe-deer energy budget. Acta Theriologica 23, 451–467.
| The effect of winter food composition on roe-deer energy budget.Crossref | GoogleScholarGoogle Scholar |
Popovic Z, Djordjevic N, Djordjevic M, Grubic G, Stojanovic B (2009) Estimation of the quality of the nutrition of roe deer based on chemical composition of the rumen content. Acta Veterinaria 59, 653–663.
| Estimation of the quality of the nutrition of roe deer based on chemical composition of the rumen content.Crossref | GoogleScholarGoogle Scholar |
Rehbinder C, Ciszuk P (1985) Supplementary feeding of roe deer (Capreolus capreolus L.) with late harvested hay. A pilot study. Rangifer 5, 6–14.
| Supplementary feeding of roe deer (Capreolus capreolus L.) with late harvested hay. A pilot study.Crossref | GoogleScholarGoogle Scholar |
Robbins CT, Spalinger DE, Hoven Wv (1995) Adaptation of ruminants to browse and grass diets: are anatomical based browser–grazer interpretations valid? Oecologia 103, 208–213.
| Adaptation of ruminants to browse and grass diets: are anatomical based browser–grazer interpretations valid?Crossref | GoogleScholarGoogle Scholar | 28306775PubMed |
Rowell-Schäfer A, Lechner-Doll M, Hofmann RR, Streich WJ, Güvern B, Meyer HHD (2001) Metabolic evidence of a rumen bypass or a ruminal escape of nutrients in roe deer (Capreolus capreolus). Comperative Biochemistry and Physiology. Part A 128, 289–298.
| Metabolic evidence of a rumen bypass or a ruminal escape of nutrients in roe deer (Capreolus capreolus).Crossref | GoogleScholarGoogle Scholar |
Schmidl C (1996) Botanische und chemische Panseninhaltsanalysen, chemische Blutuntersuchungen und Untersuchungen der Schwermetallbelastung beim Rehwild. Veterinärmedizinische Universität Wien, Austria.
Sempere AJ, Sokolov VE, Danilkin AA (1996) Capreolus capreolus. Mammalian Species 538, 1–9.
| Capreolus capreolus.Crossref | GoogleScholarGoogle Scholar |
Serrano Ferron E, Verheyden H, Hummel J, Cargnelutti B, Lourtet B, Merlet J, Gonzales-Candela M, Angibault JM, Hewison AJM, Clauss M (2012) Digestive plasticity as a response to woodland fragmentation in roe deer. Ecological Research 27, 77–82.
Silver H, Holter JB, Colovos NF, Hayes HH (1971) Effect of falling temperature on heat production in fasting white-tailed deer. The Journal of Wildlife Management 35, 37–46.
| Effect of falling temperature on heat production in fasting white-tailed deer.Crossref | GoogleScholarGoogle Scholar |
Sommer A (2004) Stoffwechsel der Wildwiederkäuer. In ‘Tagung für die Jägerschaft. Raumberg–Gumpenstein’. (Ed. BfaL Gumpenstein) pp. 3–6. (Irdning, Austria)
Sommer A, Vodnansky M, Petrikovic P, Pozgaj R (2005) Influence of lucerne and meadow hay quality on the digestibility of nutrients in the roe deer. Czech Journal of Animal Science 2, 74–80.
Storms D, Aubry P, Hamann J-L, Saïd S, Fritz H, Saint-Andrieux C, Klein F (2008) Seasonal variation in diet composition and similarity of sympatric red deer Cervus elaphus and roe deer Capreolus capreolus. Wildlife Biology 14, 237–250.
| Seasonal variation in diet composition and similarity of sympatric red deer Cervus elaphus and roe deer Capreolus capreolus.Crossref | GoogleScholarGoogle Scholar |
Stubbe C (1997) ‘Das Rehwild [the roe deer].’ (Parey Buchverlag: Berlin, Germany)
Ueckermann E (1986) ‘Die Fütterung des Schalenwildes.’ (Verlag Paul Parey: Hamburg, Germany)
Weiner J (1977) Energy metabolism of the roe deer. Acta Theriologica 22, 3–24.
| Energy metabolism of the roe deer.Crossref | GoogleScholarGoogle Scholar |
Wölfel H (2005) Biologie des Rehwildes und Konsequenzen für die jagdliche Praxis. oder: Das Reh ist kein Ungeziefer und der Jäger kein Schädlingsbekämpfer. In ‘11. Österreichische Jäger Tagung. Gumpenstein. Vol. 11’. (Ed. HB-uFf Landwirtschaft) pp. 5–8. (BAL: Irdning, Austria)
Woodall PF (1992) An evolution of a rapid method for estimating digestibility. African Journal of Ecology 30, 181–185.
| An evolution of a rapid method for estimating digestibility.Crossref | GoogleScholarGoogle Scholar |