Assessment of atherogenic index, long-chain omega-3 fatty acid and phospholipid content of prime beef: a survey of commercially sourced New Zealand Wagyu and Angus beef cattle
Emma N. Bermingham A B H , Michael Agnew C , Mariza Gomes Reis C , Kevin Taukiri A , Arjan Jonker D , David Cameron-Smith B E F G and Cameron R. Craigie A
A B H , Michael Agnew C , Mariza Gomes Reis C , Kevin Taukiri A , Arjan Jonker D , David Cameron-Smith B E F G and Cameron R. Craigie A
A Meat Quality Team, AgResearch Ltd, Te Ohu Rangahau Kai, Cnr University Ave and Riddet Road, Massey University, Palmerston North, New Zealand.
B High-Value Nutrition National Science Challenge, The University of Auckland, 85 Park Road, Grafton, Private Bag 92019, Auckland 1023, New Zealand.
C Dairy Foods Team AgResearch Ltd, Te Ohu Rangahau Kai, Cnr University Ave and Riddet Road, Massey University, Palmerston North, New Zealand.
D Animal Nutrition & Physiology Team, AgResearch Ltd, Tennent Drive, Private Bag 11008, Palmerston North 4442, New Zealand.
E Liggins Institute, The University of Auckland, 85 Park Road, Grafton, Private Bag 92019, Auckland 1023, New Zealand.
F Riddet Institute, Massey University, Private Bag Palmerston North, New Zealand.
G Singapore Institute for Clinical Sciences, Agency for Science, Technology and Research (A*STAR), Brenner Centre for Molecular Medicine, 30 Medical Drive, 117609, Singapore.
H Corresponding author. Email: emma.bermingham@agresearch.co.nz
Animal Production Science 61(2) 179-190 https://doi.org/10.1071/AN19427
Submitted: 5 August 2019 Accepted: 24 August 2020 Published: 30 September 2020
Journal Compilation © CSIRO 2021 Open Access CC BY-NC-ND
Abstract
Context: There is increasing interest in the composition of lipids in beef from pasture-fed Wagyu-cross cattle and how they compare to beef from traditional beef breeds.
Aim: The present study aimed to investigate the differences in fatty acid and phospholipid content of the ribeye, striploin and tenderloin obtained from commercially sourced beef.
Hypothesis: We hypothesised that long-chain omega-3 fatty acid and phospholipid concentrations would be higher in Wagyu beef sourced from pasture-based production systems than in those sourced from grain-finished Wagyu.
Methods: Beef was either derived from commercial cattle exclusively fed pasture (Angus, Wagyu × Angus and Wagyu × Dairy (Friesian × Jersey); n = 10 per breed) or finished on grain (Angus and Wagyu; n = 10 per breed).
Key results: Phosphatidylcholine was the predominant phospholipid observed in beef surveyed, followed by phosphatidylethanolamine and sphingomyelin. All classes of phospholipid measured were affected by where the commercial beef was obtained, with the concentrations of total phospholipids being highest (P < 0.05) in the ribeye, striploin and tenderloin from pasture-fed beef compared with grain-fed beef. The fatty acid composition also varied among the commercial cuts; typically total saturated fatty acid and monounsaturated fatty acid concentrations were highest in beef finished on grain, whereas the concentrations of long-chain omega-3 fatty acids were two-fold higher (P < 0.05) in striploin and tenderloin derived from pasture-fed Wagyu × Dairy cross cattle than in those from grain-fed cattle. Despite different fatty acid concentrations among the commercial beef breeds surveyed, the calculated atherogenic index was similar for ribeye and striploin. In contrast, the tenderloin obtained from pasture-fed Wagyu × Dairy had the lowest (P = 0.017) atherogenic index.
Conclusions: Depending on the cut of meat studied, pasture-fed Wagyu × Angus and Wagyu × Dairy have different lipid compositions, including lipids that have been associated with both health benefits and risks in human.
Implications: These results show that a serving of meat obtained from non-traditional beef breeds such as pasture-fed Wagyu × Dairy may contribute significantly to the recommended daily intake of long-chain omega-3 fatty acids.
Keywords: docosahexaenoic acid (DHA), docosapentaenoic acid (DPA), eicosapentaenoic acid (EPA), phosphatidylcholine, phosphatidylethanolamine, phosphatidylinositol, phosphatidylserine, sphingomyelin.
Introduction
Meat is a complex food matrix of proteins, lipids, vitamin and minerals. The role of red meat in the human diet is of interest to meat producers, consumers and health agencies, including the World Health Organisation (Bouvard et al. 2015; De Smet and Vossen 2016). Due to the potential role of meat-derived lipids on eating experience (Wood et al. 2004) and consumer health (Smith 2016; Vargas-Bello-Perez and Larrain 2017), the lipid composition of meat is of increasing interest. The research has focussed largely on the major lipid species, including saturated fatty acids (SFA), monounsaturated fatty acids (MUFA) and polyunsaturated fatty acids (PUFA). However, red meat can also contain an appreciable quantity of the long-chain omega-3 fatty acids, eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), and, to a lesser extent, docosahexaenoic acid (DHA). One way to assess the nutritive value of beef is to calculate an atherogenic index (Ulbricht and Southgate 1991; Nantapo et al. 2014). Typically, the lower the atherogenic index, the less atherogenic the food, that is, the healthier the food.
Beyond the fatty acids, there is a complex array of lipid species, including phospholipids. The phospholipids are essential as structural elements of biological membranes and typically comprise a glycerol backbone with a phosphate head group (Cui and Decker 2016). Phospholipids identified in red meat include phosphatidylinositol (PI), phosphatidylethanolamine (PE), phosphatidylserine (PS), phosphatidylcholine (PC) and sphingomyelin (SM). Depending on the study, PC tends to be the dominant phospholipid found in beef, followed by PE, PI, SM and PS (Larick and Turner 1989; Larick et al. 1989; Dannenberger et al. 2007; Bermingham et al. 2018). Phospholipids have both pro- and anti-oxidant roles (Cui and Decker 2016), and may contribute to the flavour of meat (Larick et al. 1989; Huang et al. 2010), and there is increasing interest in their role in human health (Kullenberg et al. 2012).
While finishing system has a large impact on the lipid profiles of beef, with pasture-fed beef having greater concentrations of PUFA, and lower concentrations of MUFA, the genetic propensity to deposit intramuscular fat (i.e. marbling; see Gotoh and Joo 2016) can also influence lipid profiles. Wagyu, or Japanese Black cattle, is a highly marbled breed of beef cattle that is traditionally raised on grain. In recent years, New Zealand farmers have begun using Wagyu genetics in the beef (Angus) or dairy (Friesian × Jersey) herd to produce offspring with increased genetic potential for marbled beef production. Typically, this involves full-blood Wagyu bulls being mated with either Angus or Dairy (Friesian × Jersey) cows. These Wagyu × cross cattle are exclusively pasture raised, and current research is investigating the introgression of Wagyu and dairy genetics for premium beef production (Industries MoP 2016). However, little is known about the fatty acid and lipid composition of pasture-fed Wagyu × Angus and Wagyu × Dairy (Friesian × Jersey) and how they compare to both traditional beef breeds such as pasture-fed Angus, and their grain-fed counterparts. We hypothesised that long-chain omega-3 fatty acids and phospholipid content would be higher in Wagyu beef sourced from pasture-based production systems than from grain-finished Wagyu and that this would be reflected in its atherogenic index. Therefore, we undertook a survey of commercially obtained ribeye, striploin, and tenderloin sourced from pasture-fed (Angus, Wagyu × Angus and Wagyu × Dairy (Friesian × Jersey)) and grain-fed (Wagyu and Angus) beef breeds to understand these differences. This study is part of a longer-term project that aims to look at the health impacts of pasture- and grain-finished beef on human health.
Materials and methods
Sample collection
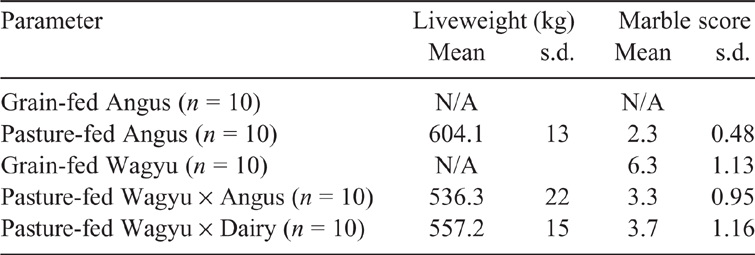
To minimise the impacts of the finishing system on pasture-fed beef, commercial Wagyu × Dairy (Friesian × Jersey) cross (n = 10) Wagyu × Angus (n = 10) and Angus steers (n = 10; Table 1) that were raised as a single group on the same commercial farm and exclusively fed perennial ryegrass-based pastures were sourced. The steers were transported as a single mob and slaughtered at the same abattoir and butchered into tenderloin, striploin and ribeye. Additionally, striploin, ribeye and tenderloin of grain-fed Wagyu (n = 10) and Angus (n = 10) were obtained from commercial abattoirs. The grain-fed Angus cattle were finished for a minimum of 200 days, whereas the grain-fed Wagyu cattle were finished for 300 days.
Sample preparation
Pasture-fed beef carcasses were suspended by the Achilles tendon and chilled for 24 h (internal temperature of <4°C). Marbling score was established by chiller assessment at 12/13th rib interface on the left side of the carcass by a trained grader using the Aus–Meat scale, with 9 being the maximum value (Aus-Meat-Limited 2010). The left side of each carcass was quartered at the 12/13th rib, and sent chilled to AgResearch Ruakura (Hamilton, New Zealand). After chill-aging for between 7 and 9 days postmortem (–1.5°C), each carcass was processed into ribeye, striploin and tenderloin by a master butcher, according to the New Zealand Meat Specifications Guide (Beef+Lamb – NewZealand 2010).
So as to determine fatty acid and phospholipid concentrations, the meat samples were prepared according to the methods described by (Bermingham et al. 2018). Briefly, whole cuts were frozen on the day of butchering. Each cut was weighed, labelled and chilled at –5°C before freeze-drying and grinding. Ground samples were stored at –20°C, before being subsampled for phospholipid and fatty acid analysis.
Phospholipid concentrations
Lipids were extracted using pressurised liquid extraction (Dionex ASE 350, Thermo Scientific, Auckland, NZ) as described previously (Zhou et al. 2010), with some modifications (Bermingham et al. 2018). Briefly, 250 mg of freeze-dried meat was ground with sand (2 g) using a mortar and pestle. The mixture was transferred to a 20-mL stainless-steel extraction cell, any void volume in the vial was filled with sand. Lipids were extracted with CHCl3–CH3OH (2 : 1 v/v) at 60°C. The extraction time was set to 10 min per cycle, and two extraction cycles were performed. A volume of 5 mL of 0.9% NaCl was added to the lipid extract, and solvent partitioning was achieved by gentle inversion. After phase separation, the lower organic phase was recovered and concentrated under nitrogen gas. Phospholipids were purified as described previously (Narvaez-Rivas et al. 2011). Phospholipid concentrations were measured by high-performance liquid chromatography coupled with evaporative light-scattering detector (HPLC–ELSD) as described previously (Reis et al. 2013), by using an HPLC–ELSD (Shimadzu, Kyoto, Japan) instrument equipped with two LC-10 Advp pumps (SCL 10 Advp gradient system), a DGU-14 advp module degasser, and a Shimadzu ELSD–LT II ELSD.
Fatty acid concentrations
Fatty acid concentrations were measured using the method described previously (Craigie et al. 2017; Bermingham et al. 2018). Briefly, 300 mg of freeze-dried meat, 4 mL of toluene, 0.3 mL of internal standard (C11 triacylglyceride in toluene), and 4 mL of 5% methanolic sulfuric acid were mixed thoroughly and incubated at 70°C for 2 h, with mixing every 30 min during the incubation time. After 25 min of equilibration at room temperature, 5 mL of saturated NaCl was added, mixed and then centrifuged at room temperature at 1100g for 2 min, to separate solvent layers. The top layer containing the fatty acid methyl esters was transferred into 1.5-mL gas chromatography (GC) autosampler vials (Hewlett Packard, Model 6890). The GC was a GC-2010 plus (Shimadzu) with a flame ionisation detector. The column was a Restek RTX 2330 column of 105-m length, 0.25-mm i.d., and 0.20-μm film thickness (Restek Corporation, Bellefonte, PA, USA). The thermal program had an initial temperature of 175°C for 17 min, which was increased to 220°C at a rate of 6°C per minute and held for 10 min. The carrier gas was hydrogen with a linear velocity of 50 cm/s (3.05 mL/min). The injection volume was 1 µL, with a split ratio of 80 : 1. The injector temperature was 260°C and the detector temperature was 300°C. The peaks were identified and quantified using an internal standard (C11:0) and theoretical flame ionisation detector response factors. The equations for generating the response and conversion factors to quantify individual fatty acids from fatty acid methyl esters were obtained from American Oil Chemists’ Society (AOCS Ce1f-96, Ce 1h-05 and Ce 1i-07).
Statistical analyses
Data were analysed for each table cut separately by using a linear mixed model, using cattle (pasture-fed Angus, pasture-fed Wagyu × Angus, pasture-fed Wagyu × Dairy, grain-fed Angus, grain-fed Wagyu) as the fixed effect in Genstat (2015, Version 18.1.0.17005, VSN International Ltd, UK). Residual plots were generated in Genstat for all phospholipids and fatty acid data. Normality was assessed by the Shapiro–Wilk test. Both these tests indicated that the data were not normally distributed, and, therefore, all data were transformed using natural log before statistical analysis. Differences between multiple means were determined by the Bonferroni least significant difference test at P < 0.05 in Genstat (2015; Version 18.1.0.17005). All averages were back-calculated from log, and are presented on a fresh-weight basis.
Results
Phospholipids
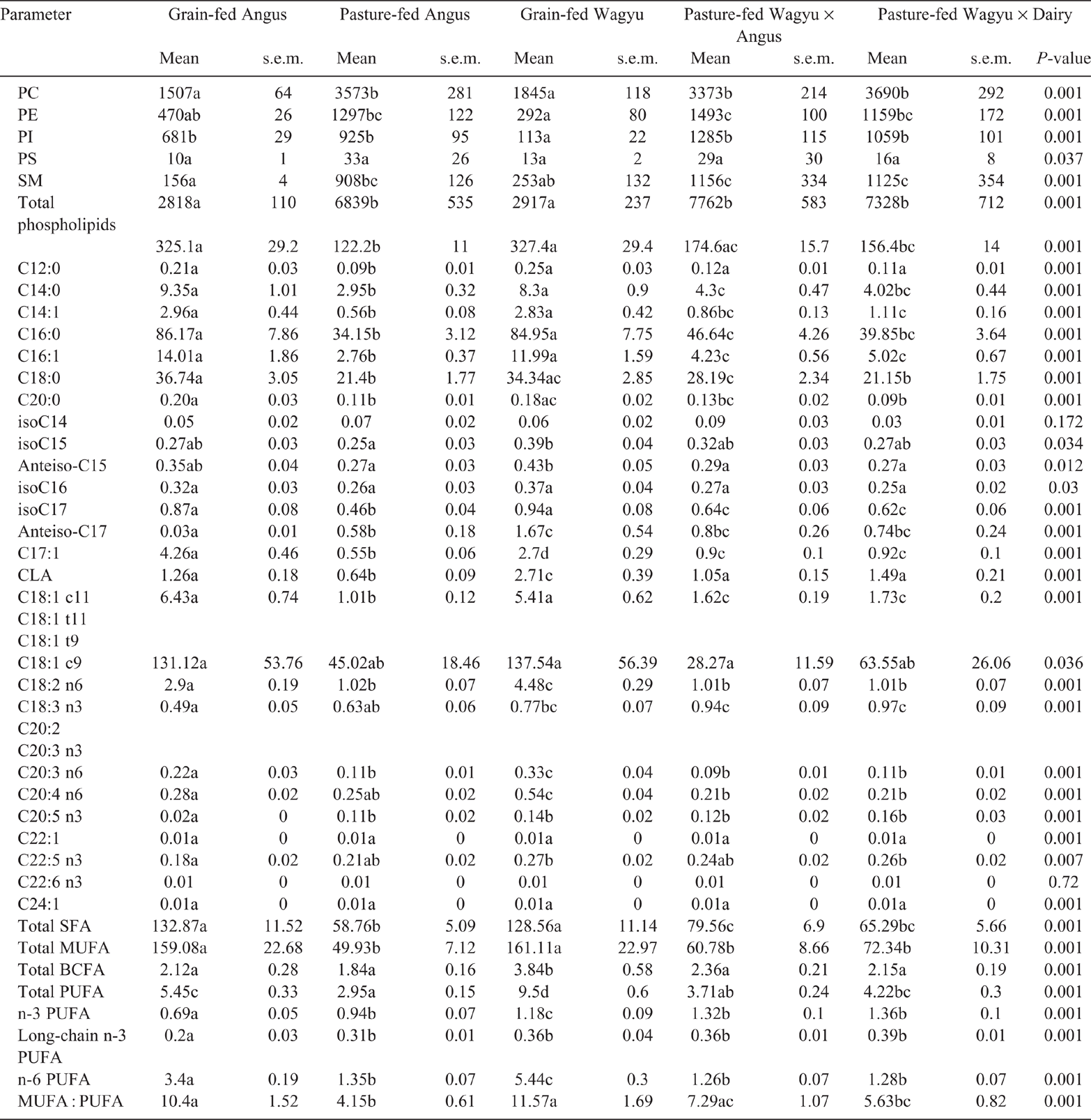
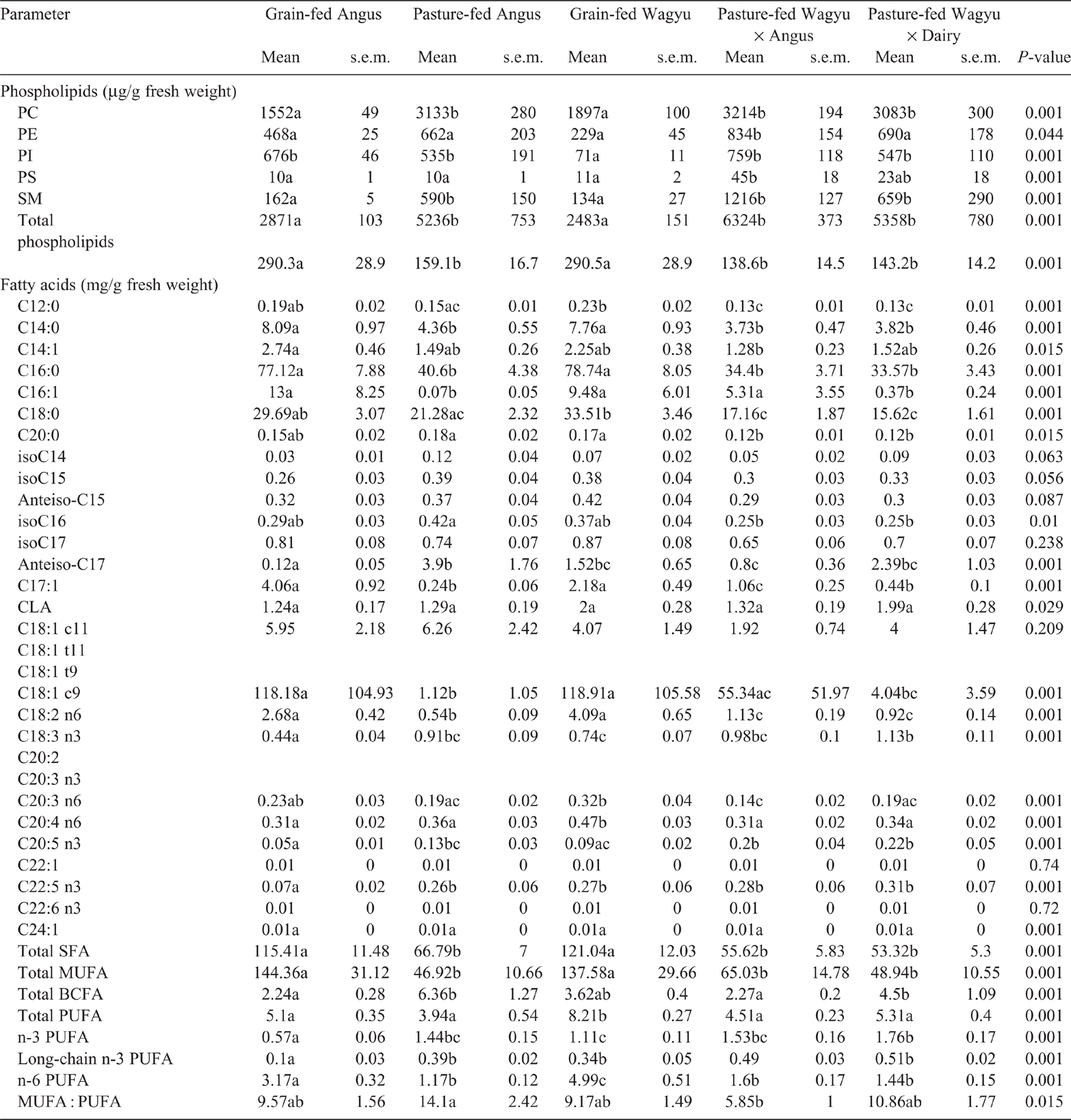
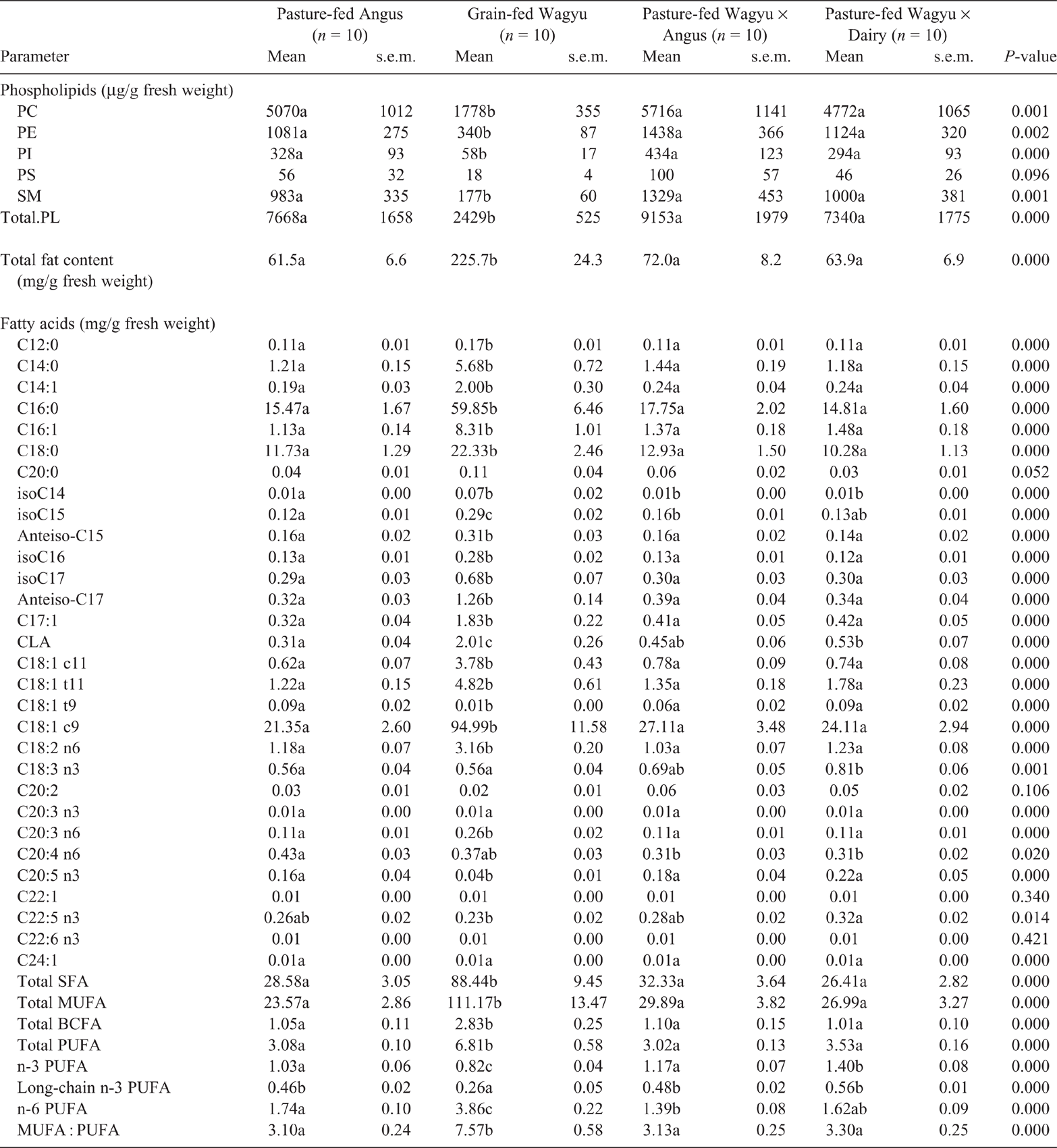
The ribeye (Table 2) and striploin (Table 3) obtained from pasture-fed Angus, Wagyu × Angus and Wagyu × Dairy had greater (P < 0.05) concentrations of PC than did those from grain-fed Angus or Wagyu. The PC concentration of tenderloin obtained from grain-fed Wagyu were lower (P < 0.05) than those obtained from all pasture-fed beef breeds (Table 4).
In the ribeye, PE was least abundant in grain-fed Wagyu, and most abundant in the pasture-fed Wagyu × Angus (P < 0.05; Table 2). Similarly, the PE concentration in striploin (Table 3) and was lowest in the grain-fed Wagyu and highest in pasture-fed Wagyu-Angus (P < 0.05). In the tenderloin (Table 4), the PE concentration was lowest (P < 0.05) in the grain-fed Wagyu compared with the pasture-fed beef.
The concentrations of PI were lowest in the ribeye obtained from grain-fed Wagyu (P < 0.05), but similar across other beef breeds surveyed (Table 2). In striploin, PE was highest in pasture-fed Wagyu × Angus (P < 0.05), whereas PI was lowest in grain-fed Wagyu (P < 0.05; Table 2). In the tenderloin, both PE and PI were lowest (<0.05) in the grain-fed Wagyu compared with the pasture-fed cattle breeds (Table 3).
In the ribeye, PS concentrations were highest (P = 0.037) in pasture-fed Angus, Wagyu × Angus and Wagyu × Dairy (Table 2) beef. In the striploin, PS concentration was highest (P = 0.001) in Wagyu × Angus and Wagyu × Dairy (Table 3) beef. The PS concentration of tenderloin (Table 4) was similar among the different breeds of commercial beef cuts surveyed.
In the ribeye, SM concentrations were greater (P < 0.05) in both pasture-fed Wagyu × Angus and Wagyu × Dairy (Table 1). In the striploin, SM concentrations were greatest (P < 0.05) in the pasture-feed cattle (Angus, Wagyu × Angus and Wagyu × Dairy), compared with the grain-fed cattle (Table 2).
Fatty acids
In the ribeye (Table 1), striploin (Table 2) and tenderloin (Table 3), the total SFA (C12, C14, C16, C18 and C20) were highest (P < 0.05) in the grain-fed cattle.
The concentrations of total monounsaturated fatty acids (C14:1, C16:1, C17:1, C18:1c9, C18:1c11, C22:1 and C24:1) in grain-fed beef (Angus and Wagyu) were higher (P < 0.05) than those in grass-fed beef for ribeye, striploin and tenderloin.
The concentrations of total poly-unsaturated fatty acids (PUFA; conjugated linoleic acid isomers, C18:2n6, C18:3n3, C20:3n3, C:20:3n6, C20:4n3, C20:4n6, C20:5n3, C22:5n3 C22:6n3) were affected (P < 0.001) by cattle breed in the ribeye (Table 1), striploin (Table 2) and tenderloin (Table 3). Grain-fed Angus and Wagyu had the highest total PUFA in ribeye (Table 1), striploin (Table 2) and tenderloin (Table 3), compared with the pasture-fed cattle; this was largely driven by increased concentrations (P < 0.05) of omega-6 fatty acids (C18:2n6, C20:3n6, and C20:4n6). The concentrations of total omega-3 fatty acids (C18:3n3, C20:3n3, C20:4n3, C20:5n3, C22:5n3 and C22:6n3) were significantly higher in the pasture-fed cattle, especially the in Wagyu-cross breeds.
Discussion
This survey aimed to understand the differences in fatty acid and phospholipid profiles in commercially sourced prime beef. As expected, the pasture-fed beef cattle had higher concentrations of both long-chain omega-3 fatty acids and phospholipids than did the grain-finished beef cattle. As a consequence of these changes, the calculated atherogenic index of the beef differed between the breeds and finishing systems surveyed, with lower scores being observed in grain-fed beef than in pasture-fed beef cuts. It is important to note that these results are reflective of beef available to the consumer and are based on commercially obtained beef cuts. Therefore, genetics, rearing location and management and finishing duration were not consistent between the cattle sampled, and any differences observed can only be attributed in a general manner.
The dominance of PC in the current study is consistent with previous work in pasture-fed Wagyu × cross cattle (Bermingham et al. 2018). The PC supplied by a 150-g serving of red meat studied herein would range from 0.23 g (ribeye and striploin) to 0.86 g (tenderloin from pasture-fed Wagyu × Angus). While 3 g/day of a PC-rich (~35% of total phospholipids) milk-based supplement improved markers associated with cardiovascular disease in humans (Weiland et al. 2016), it has been hypothesised that high PC intake could lead to increased risk of cardiovascular disease (Tang et al. 2013; Heianza et al. 2017; Zeisel and Warrier 2017), via conversion of PC to trimethylamine-N-oxide (TMAO) by the intestinal microbiota. To our knowledge, only one study has linked beef consumption with increased TMAO; however, in this case, the rodents were fed diets containing 65% of red meat every day for 14 days (Van Hecke et al. 2016), rather than the World Health Organisations’ recommendation of no more than 500 g cooked red meat per week (~750 g raw) in three or four servings over a week. The relationship between meat intake and TMAO is not clear cut as many other foods, including fatty fish (Landfald et al. 2017), fruit and vegetables (Zhang et al. 1999) and dairy foods (Rohrmann et al. 2016) also increase TMAO. For example, urinary TMAO concentrations arising from beef consumption (227 g) were 76.5 μmol/8 h whereas the consumption of 227 g of fish increased TMAO to 301.8–5135.3 μmol/8 h depending on the fish species consumed (Zhang et al. 1999). While the relationship between PC intake and cardiovascular disease risk is plausible on the basis of rodent studies, this has not been confirmed in humans. It is, therefore, important to investigate this relationship between beef consumption and disease risk more fully.
Phosphatidylethanolamine plays a role in neurological diseases such as Parkinson’s disease (Patel and Witt 2017) and may play a role in cardiovascular disease through lowering plasma cholesterol. In rats, diets containing 2% PE for 2 weeks reduced plasma cholesterol concentrations (Imaizumi et al. 1991). PI plays a role in metabolic disease (Holub 1982; Dinicola et al. 2017) and supplementation of 2.8–5.6 g PI per day increases high-density lipoprotein (HDL) cholesterol in humans (Burgess et al. 2005); this level is much higher than the 0.1–0.2 g present in a serve of 150 g of red meat observed in the current study. PE is the major phospholipid found in the brain; supplementation with 300 mg of PS (derived from soybeans) improved cognitive function in elderly humans (Kato-Kataoka et al. 2010; Richter et al. 2013). Unsurprisingly, PS was the least abundant phospholipid measured in the cuts in the current study, comprising <2% of total phospholipid and was not different among beef sources studied. This is consistent with the results of previous work on pasture-fed New Zealand Wagyu-cross cattle (Bermingham et al. 2018). Furthermore, Dannenberger et al. (2007) were unable to detect PS in the longissimus muscle of Simmental or Holstein cattle breeds, Larick and Turner (1989) observed PS at low concentrations (~2.5% total phospholipids) in the pectoralis muscle of Angus and Hereford cattle breeds and Larick et al. (1989) observed PS at ~5% of total phospholipids in L. dorsi from Bison, Hereford and Brahman steers.
Sphingomyelin (SM) was less abundant in ribeye, striploin and tenderloin (ranging 6–20% of total phospholipids). Dietary SM has been associated with improved health outcomes (Vesper et al. 1999; Bartke and Hannun 2009). For example, supplementation with 1 g/day of dietary SM in humans increased HDL cholesterol (Ramprasath et al. 2013); however, there is no current nutritional recommendation for this phospholipid (Vesper et al. 1999). The SM content of a 150-g serve of pasture-fed beef from the cattle examined in the current study would range from 0.15 to 0.20 g, suggesting that a 150-g serve of red meat could significantly contribute to positive health outcomes associated with SM intake.
Total phospholipid concentrations were similar among pasture-fed Angus, Wagyu × Angus and Wagyu × Dairy cattle, and these breeds generally had greater (P < 0.05) concentrations of total phospholipids than did grain-fed cattle. To our knowledge, only two studies have been conducted to investigate the effects of beef source, of different breeds fed concentrate or pasture only, on the phospholipid concentration of beef. While Larick and Turner (1989) did not see any differences in phospholipid concentrations in beef from Angus and Hereford × Angus cattle fed grain or grass+grain, Dannenberger et al. (2007) observed greater concentrations of SM and lysophosphatidylcholine in the longissimus muscle from pasture-fed Simmental cattle than from concentrate-fed Simmental; however, there was no effect of finishing system on the phospholipid concentration of beef from Holstein cattle.
Overall, the phospholipid concentration of the pasture-fed Wagyu × Dairy and Wagyu × Angus were higher than previously reported in work on pasture-fed Wagyu × Dairy cattle (Bermingham et al. 2018). While the pasture-fed Wagyu in the current study had an average carcass weight (~292 kg) similar to that reported previously (291 kg), the marbling score averaged 3.5/9, compared with 7.5/9 in the previous study. While the relationship between marbling score and phospholipid composition has not been studied, to our knowledge, the intramuscular fat content and fatty acid composition are affected by marble score in Wagyuy cattle (Kazala et al. 1999).
Fatty acids
Generally, the fatty acid concentrations reported herein are in agreement with those reported previously in literature, indicating a dominance of 16:0, C18:0 and 18:1c9 fatty acids in the muscle of both Wagyu (Sturdivant et al. 1992; Xie et al. 1996; Kazala et al. 1999; Mir et al. 2000) and other (Knight et al. 2003; Purchas et al. 2005; Coleman et al. 2016) beef breeds. As expected, fatty acid composition differed among table cuts, in agreement with previous research (Sturdivant et al. 1992; Kazala et al. 1999; Bermingham et al. 2018).
Typically, grain-feeding is believed to increase the concentration of SFA present in beef muscle; however, several studies have shown that this is not the case (Daley et al. 2010; Turner et al. 2015; Hwang and Joo 2017). Saturated fats are typically associated with an increased risk of cardiovascular disease in humans (de Souza et al. 2015; Ooi et al. 2015), and even the lower concentrations of SFA observed in pasture-fed beef are considered in excess of what is allowable by Australian and New Zealand Food Standards (28% SFA; FSANZ 2016). However, as discussed in Bermingham et al. (2018) and by others (Bier 2016), the SFA story is not clear-cut, with SFA in natural whole foods (e.g. meat, milk) being less atherogenic than that in manufactured foods (Thorning et al. 2015). The increase in SFA associated with grain feeding in the current study was associated with higher C16:0 (palmitic acid) concentrations, which were about two-fold higher in grain-fed than in the grass-fed cattle. Palmitic acid (i.e. C16:0) has been linked to poor cardiovascular health through its links with elevating cholesterol (Daley et al. 2010; Ebbesson et al. 2015; Agostoni et al. 2016). In a recent systematic review, increased palmitic acid intake, rather than SFA intake, was linked to an increased risk of myocardial infarction; however, the review acknowledged that there were many confounding factors that may weaken these observations (Ismail et al. 2018). Similarly, the concentrations of C14:0 (myristic acid), which is another fatty acid linked to elevated cholesterol concentrations (Daley et al. 2010; Ebbesson et al. 2015), were also higher in grain-fed beef than in the pasture-fed beef in the current study. Stearic acid (C18:0), a cholesterol-neutral fatty acid (Grundy 1994), was also lower in the pasture-fed beef in the current study.
Increased concentration of MUFA in grain-fed Angus and Wagyu is consistent with the results of other studies (Turner et al. 2015), and was driven largely by an increased (P < 0.05) concentration of oleic acid (C18:1c9) in these cattle. Oleic acid, as recently reviewed (Lopez-Huertas 2010; Sales-Campos et al. 2013), is associated with improved human health; however, other studies have indicated that excessive intakes may decrease life expectancies in humans (Delgado et al. 2017). While plant-derived oleic acid (e.g. olive oils) is typically associated with positive health outcomes, high concentrations of oleic acid in beef burgers have been observed to increase HDL cholesterol and reduce low-density lipoprotein (LDL) cholesterol in humans (Gilmore et al. 2011a).
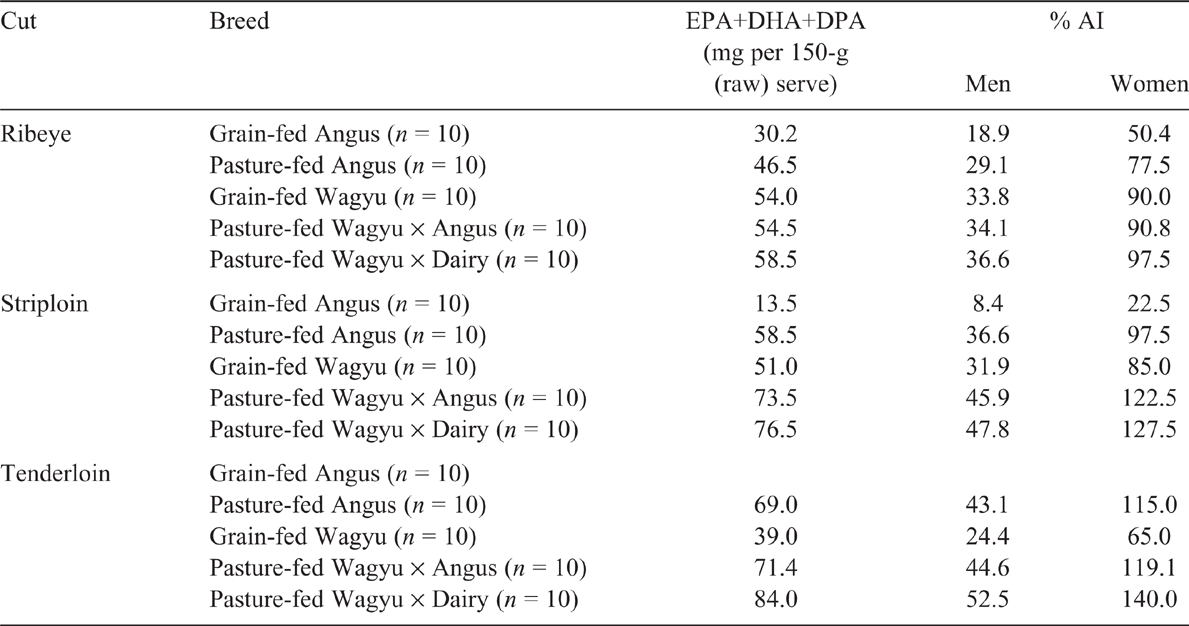
The health benefits of long-chain omega-3 fatty acids are well established. EPA and DHA, the long-chain omega-3 found in oily fish, are frequently associated with health benefits (Ian Givens and Gibbs 2008), but there also is increasing interest around the benefits of DPA for human health (Kaur et al. 2011). DPA is a direct elongation product of EPA (Byelashov et al. 2015), and may play a vital role in brain health and immunity (reviewed by Kaur et al. 2016). In New Zealand and Australia (Byelashov et al. 2015), the recommended minimum intake (adequate intake) of long-chain omega-3 fatty acids (EPA+DHA+DPA) in adult men is 160 mg of (EPA+DHA+DPA)/day and 90 mg of (EPA+DHA+DPA)/day for adult women (National Health and Medical Research Council (Aus) and Ministry of Health (NZ)). The anticipated long-chain omega-3 present in a 150-g (wet weight) serving of the various table cuts are shown in Table 5. In all cuts examined, pasture-fed Wagyu × Angus and Wagyu × Dairy cattle contributed more of the recommend intakes of long-chain omega-3, compared with pasture-fed Angus. Similarly, grain-fed Wagyu provided more of the recommended intake than did grain-fed Angus. Typically, pasture-fed Wagyu × Dairy provided the largest quantity of long-chain omega-3 per 150-g serve, providing 30 and 90% (ribeye), 45 and 120% (striploin) and 45 and 140% (tenderloin) of the recommended adequate intake for men and women respectively. This suggests that pasture-fed Wagyu × Dairy could be a good source of dietary long-chain omega-3 fatty acids.
|
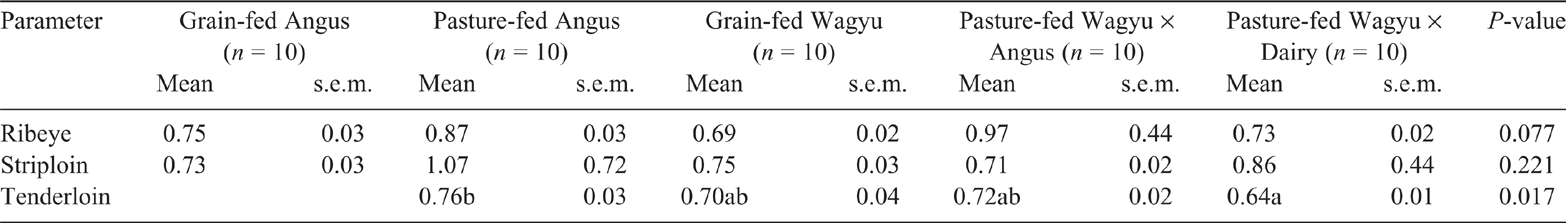
The atherogenic index is often used to assess the nutritional value of foods (Ulbricht and Southgate 1991; Nantapo et al. 2014). It is calculated using a ratio between SFA (C12:0, C14:0 and C16:0) and the sum of MUFA and PUFA. In the tenderloin (Table 6), the Wagyu × Dairy had the lowest atherogenic index (P < 0.05), compared with the tenderloin of the other beef breeds surveyed, whereas the atherogenic indexes calculated for ribeye (P = 0.077) and striploin (P = 0.221) were similar among the beef breeds surveyed. Previous studies have investigated differences in the calculated atherogenic index between pasture- and grain-finished cattle. For example, Hwang and Joo (2017) compared the atherogenic index of striploin between grain- and pasture-finished marbled (Hanwoo) and traditional beef breeds. They observed that grain-fed marbled beef had the lowest atherogenic index, whereas grass-fed traditional beef had the highest atherogenic index. This was most likely due to the increased MUFA concentration of grain-fed beef in Hwang and Joo (2017).
To our knowledge, only three studies have addressed the impacts of beef obtained from feeding grain and concentrate or pasture on human health, focussing on the impacts of oleic acid, total MUFA concentration or n-3 PUFA. Diets high in oleic acid (using fat and lean trim from Angus beef finished on grain; Gilmore et al. 2011b) or MUFA (from traditional beef and Wagyu; Adams et al. 2010) increased HDL cholesterol concentrations compared with a low oleic acid- or high-SFA burger (using fat and lean trims obtained from beef finished on pasture) respectively. In contrast, while beef diets high in long-chain omega-3 increased plasma concentrations of long-chain omega-3 fatty acids, there was no effect on plasma cholesterol concentrations (McAfee et al. 2011). These studies suggest that beef obtained finished on different diets and/or from different breeds have the potential to influence human health outcomes.
Conclusions
Overall, our results suggest that, depending on the cut of meat surveyed, pasture-fed Wagyu × Angus and Wagyu × Dairy have a different lipid composition, including for those lipids that have been associated with both health benefits and risks in humans. The concentrations of phospholipids were higher in the ribeye, striploin and tenderloin obtained from pasture-fed beef than in those obtained from grain-fed beef. Furthermore, the concentrations of long-chain omega-3 fatty acids in striploin and tenderloin were two-fold higher in cuts derived from pasture-fed Wagyu × Dairy-cross cattle than in those derived from grain-fed cattle. However, while the calculated atherogenic index was similar among the beef cattle breeds surveyed, clinical studies are needed to test whether altered lipid composition due to feed and breed could have an effect on markers of disease risk.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This study was funded by a grant from the High-Value Nutrition, New Zealand National Science Challenge (HVN3710040) and First Light Foods (Hastings, New Zealand). The funders did not contribute to the analysis or the interpretation of the results. The technical support of Frederik Knol (AgResearch, New Zealand) is greatly appreciated. Statistical analysis was conducted by Ms Catherine McKenzie and Mr Shen Hea (AgResearch). Additionally, the contributions of Mr Matt Crowther and Gareth Stillman (First Light Foods) are acknowledged.
References
Adams TH, Walzem RL, Smith DR, Tseng S, Smith SB (2010) Hamburger high in total, saturated and trans-fatty acids decreases HDL cholesterol and LDL particle diameter, and increases TAG, in mildly hypercholesterolaemic men. British Journal of Nutrition 103, 91–98.| Hamburger high in total, saturated and trans-fatty acids decreases HDL cholesterol and LDL particle diameter, and increases TAG, in mildly hypercholesterolaemic men.Crossref | GoogleScholarGoogle Scholar | 19674491PubMed |
Agostoni C, Moreno L, Shamir R (2016) Palmitic acid and health: introduction. Critical Reviews in Food Science and Nutrition 56, 1941–1942.
| Palmitic acid and health: introduction.Crossref | GoogleScholarGoogle Scholar | 25764181PubMed |
Aus-Meat Limited (2010) ‘Australian beef carcase evaluation beef & veal chiller assessment language.’ Available at https://www.ausmeat.com.au/WebDocuments/Producer_HAP_Beef_Small.pdf [Verified 10 September 2020]
Bartke N, Hannun YA (2009) Bioactive sphingolipids: metabolism and function Journal of Lipid Research 50, S91–6.
| Bioactive sphingolipids: metabolism and functionCrossref | GoogleScholarGoogle Scholar | 19017611PubMed |
Beef + Lamb NewZealand (2010) ‘Beef + lamb New Zealand reference guide.’ 3rd edn. (Beef + Lamb New Zealand: Takapuna, Auckland’ Available at http://www.beeflambnz.co.nz/retailer-resources/Reference_Guide.pdf [Verified 10 September 2020]
Bermingham EN, Reis MG, Subbaraj AK, Cameron-Smith D, Fraser K, Jonker A, Craigie CR (2018) Distribution of fatty acids and phospholipids in different table cuts and co-products from New Zealand pasture-fed Wagyu–dairy cross beef cattle. Meat Science 140, 26–37.
| Distribution of fatty acids and phospholipids in different table cuts and co-products from New Zealand pasture-fed Wagyu–dairy cross beef cattle.Crossref | GoogleScholarGoogle Scholar | 29501930PubMed |
Bier DM (2016) Saturated fats and cardiovascular disease: interpretations not as simple as they once were. Critical Reviews in Food Science and Nutrition 56, 1943–1946.
| Saturated fats and cardiovascular disease: interpretations not as simple as they once were.Crossref | GoogleScholarGoogle Scholar | 25774535PubMed |
Bouvard V, Loomis D, Guyton KZ, Grosse Y, Ghissassi FE, Benbrahim-Tallaa L, Guha N, Mattock H, Straif K (2015) Carcinogenicity of consumption of red and processed meat. The Lancet. Oncology 16, 1599–1600.
| Carcinogenicity of consumption of red and processed meat.Crossref | GoogleScholarGoogle Scholar | 26514947PubMed |
Burgess JW, Neville TA-M, Rouillard P, Harder Z, Beanlands DS, Sparks DL (2005) Phosphatidylinositol increases HDL-C levels in humans. Journal of Lipid Research 46, 350–355.
| Phosphatidylinositol increases HDL-C levels in humans.Crossref | GoogleScholarGoogle Scholar | 15576836PubMed |
Byelashov OA, Sinclair AJ, Kaur G (2015) Dietary sources, current intakes, and nutritional role of omega-3 docosapentaenoic acid. Lipid Technology 27, 79–82.
| Dietary sources, current intakes, and nutritional role of omega-3 docosapentaenoic acid.Crossref | GoogleScholarGoogle Scholar | 26097290PubMed |
Coleman LW, Hickson RE, Schreurs NM, Martin NP, Kenyon PR, Lopez-Villalobos N, Morris ST (2016) Carcass characteristics and meat quality of Hereford sired steers born to beef-cross-dairy and Angus breeding cows. Meat Science 121, 403–408.
| Carcass characteristics and meat quality of Hereford sired steers born to beef-cross-dairy and Angus breeding cows.Crossref | GoogleScholarGoogle Scholar | 27448194PubMed |
Craigie CR, Johnson PL, Shorten PR, Charteris A, Maclennan G, Tate ML, Agnew MP, Taukiri KR, Stuart AD, Reis MM (2017) Application of hyperspectral imaging to predict the pH, intramuscular fatty acid content and composition of lamb M. longissimus lumborum at 24h post mortem. Meat Science 132, 19–28.
| Application of hyperspectral imaging to predict the pH, intramuscular fatty acid content and composition of lamb M. longissimus lumborum at 24h post mortem.Crossref | GoogleScholarGoogle Scholar | 28551294PubMed |
Cui L, Decker EA (2016) Phospholipids in foods: prooxidants or antioxidants? Journal of the Science of Food and Agriculture 96, 18–31.
| Phospholipids in foods: prooxidants or antioxidants?Crossref | GoogleScholarGoogle Scholar | 26108454PubMed |
Daley C, Abbott A, Doyle P, Nader G, Larson S (2010) A review of fatty acid profiles and antioxidant content in grass-fed and grain-fed beef. Nutrition Journal 9, 10
| A review of fatty acid profiles and antioxidant content in grass-fed and grain-fed beef.Crossref | GoogleScholarGoogle Scholar | 20219103PubMed |
Dannenberger D, Nuernberg G, Scollan N, Ender K, Nuernberg K (2007) Diet alters the fatty acid composition of individual phospholipid classes in beef muscle. Journal of Agricultural and Food Chemistry 55, 452–460.
| Diet alters the fatty acid composition of individual phospholipid classes in beef muscle.Crossref | GoogleScholarGoogle Scholar | 17227079PubMed |
De Smet S, Vossen E (2016) Meat: the balance between nutrition and health. A review. Meat Science 120, 145–156.
| Meat: the balance between nutrition and health. A review.Crossref | GoogleScholarGoogle Scholar | 27107745PubMed |
de Souza RJ, Mente A, Maroleanu A, Cozma AI, Ha V, Kishibe T, Uleryk E, Budylowski P, Schunemann H, Beyene J, Anand SS (2015) Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. British Medical Journal 351, h3978
| Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies.Crossref | GoogleScholarGoogle Scholar | 26268692PubMed |
Delgado GE, Kramer BK, Lorkowski S, Marz W, von Schacky C, Kleber ME (2017) Individual omega-9 monounsaturated fatty acids and mortality: the Ludwigshafen risk and cardiovascular health study. Journal of Clin Lipidology 11, 126–135.e5.
| Individual omega-9 monounsaturated fatty acids and mortality: the Ludwigshafen risk and cardiovascular health study.Crossref | GoogleScholarGoogle Scholar |
Dinicola S, Minini M, Unfer V, Verna R, Cucina A, Bizzarri M (2017) Nutritional and acquired deficiencies in inositol bioavailability. Correlations with metabolic disorders International Journal of Molecular Sciences 18, 2187
Ebbesson SO, Voruganti VS, Higgins PB, Fabsitz RR, Ebbesson LO, Laston S, Harris WS, Kennish J, Umans BD, Wang H, Devereux RB, Okin PM, Weissman NJ, MacCluer JW, Umans JG, Howard BV (2015) Fatty acids linked to cardiovascular mortality are associated with risk factors. International Journal of Circumpolar Health 74, 28055
| Fatty acids linked to cardiovascular mortality are associated with risk factors.Crossref | GoogleScholarGoogle Scholar | 26274054PubMed |
FSANZ (2016) ‘Food standards code from 1 March 2016: standard 1.2.7 – nutrition, health and related claims food standards Australia New Zealand.’ Available at https://www.foodstandards.govt.nz/code/Pages/default.aspx. [Verified 10 September 2020]
Gilmore LA, Walzem RL, Crouse SF, Smith DR, Adams TH, Vaidyanathan V, Cao X, Smith SB (2011a) Consumption of high-oleic acid ground beef increases HDL-cholesterol concentration but both high- and low-oleic acid ground beef decrease HDL particle diameter in normocholesterolemic men. The Journal of Nutrition 141, 1188–1194.
| Consumption of high-oleic acid ground beef increases HDL-cholesterol concentration but both high- and low-oleic acid ground beef decrease HDL particle diameter in normocholesterolemic men.Crossref | GoogleScholarGoogle Scholar | 21525253PubMed |
Gilmore LA, Walzem RL, Crouse SF, Smith DR, Adams TH, Vaidyanathan V, Cao X, Smith SB (2011b) Consumption of high-oleic acid ground beef increases HDL-cholesterol concentration but both high- and low-oleic acid ground beef decrease HDL particle diameter in normocholesterolemic men. The Journal of Nutrition 141, 1188–1194.
| Consumption of high-oleic acid ground beef increases HDL-cholesterol concentration but both high- and low-oleic acid ground beef decrease HDL particle diameter in normocholesterolemic men.Crossref | GoogleScholarGoogle Scholar | 21525253PubMed |
Gotoh T, Joo ST (2016) Characteristics and health benefit of highly marbled Wagyu and Hanwoo beef. Han-gug Chugsan Sigpum Hag-hoeji 36, 709–718.
| Characteristics and health benefit of highly marbled Wagyu and Hanwoo beef.Crossref | GoogleScholarGoogle Scholar |
Grundy SM (1994) Influence of stearic acid on cholesterol metabolism relative to other long-chain fatty acids. The American Journal of Clinical Nutrition 60, 986S–990S.
| Influence of stearic acid on cholesterol metabolism relative to other long-chain fatty acids.Crossref | GoogleScholarGoogle Scholar | 7977157PubMed |
Heianza Y, Ma W, Manson JE, Rexrode KM, Qi L (2017) Gut microbiota metabolites and risk of major adverse cardiovascular disease events and death: a systematic review and meta‐analysis of prospective studies. Journal of the American Heart Association 6, e004947
| Gut microbiota metabolites and risk of major adverse cardiovascular disease events and death: a systematic review and meta‐analysis of prospective studies.Crossref | GoogleScholarGoogle Scholar | 28663251PubMed |
Holub BJ (1982) The nutritional significance, metabolism, and function of myo-inositol and phosphatidylinositol in health and disease. Advances in Nutritional Research 4, 107–141.
| The nutritional significance, metabolism, and function of myo-inositol and phosphatidylinositol in health and disease.Crossref | GoogleScholarGoogle Scholar | 6278902PubMed |
National Health and Medical Research Council (Aus) and Ministry of Health (NZ). (2017) Nutrient Reference Values for Australia and New Zealand. Fats: total fat & fatty acids. Available at https://www.nrv.gov.au/nutrients/fats-total-fat-fatty-acids [Verified 10 September 2020]
Huang Y-C, Li H-J, He Z-F, Wang T, Qin G (2010) Study on the flavor contribution of phospholipids and triglycerides to pork. Food Science and Biotechnology 19, 1267–1276.
| Study on the flavor contribution of phospholipids and triglycerides to pork.Crossref | GoogleScholarGoogle Scholar |
Hwang YH, Joo ST (2017) Fatty acid profiles, meat quality, and sensory palatability of grain-fed and grass-fed beef from Hanwoo, American, and Australian crossbred cattle. Han-gug Chugsan Sigpum Hag-hoeji 37, 153–161.
| Fatty acid profiles, meat quality, and sensory palatability of grain-fed and grass-fed beef from Hanwoo, American, and Australian crossbred cattle.Crossref | GoogleScholarGoogle Scholar |
Ian Givens D, Gibbs RA (2008) Current intakes of EPA and DHA in European populations and the potential of animal-derived foods to increase them. The Proceedings of the Nutrition Society 67, 273–280.
| Current intakes of EPA and DHA in European populations and the potential of animal-derived foods to increase them.Crossref | GoogleScholarGoogle Scholar | 18498671PubMed |
Imaizumi K, Sekihara K, Sugano M (1991) Hypocholesterolemic action of dietary phosphatidylethanolamine in rats sensitive to exogenous cholesterol. The Journal of Nutritional Biochemistry 2, 251–254.
| Hypocholesterolemic action of dietary phosphatidylethanolamine in rats sensitive to exogenous cholesterol.Crossref | GoogleScholarGoogle Scholar |
Ministry of Primary Industries (2016) Primary growth partnership annual report, 1 July 2015 to 30 June 2016. New Zealand Government, Wellington, New Zealand.
Ismail SR, Maarof SK, Siedar Ali S, Ali A (2018) Systematic review of palm oil consumption and the risk of cardiovascular disease. PLoS One 13, e0193533
| Systematic review of palm oil consumption and the risk of cardiovascular disease.Crossref | GoogleScholarGoogle Scholar | 29489910PubMed |
Kato-Kataoka A, Sakai M, Ebina R, Nonaka C, Asano T, Miyamori T (2010) Soybean-derived phosphatidylserine improves memory function of the elderly Japanese subjects with memory complaints. Journal of Clinical Biochemistry and Nutrition 47, 246–255.
| Soybean-derived phosphatidylserine improves memory function of the elderly Japanese subjects with memory complaints.Crossref | GoogleScholarGoogle Scholar | 21103034PubMed |
Kaur G, Cameron-Smith D, Garg M, Sinclair AJ (2011) Docosapentaenoic acid (22:5n-3): a review of its biological effects. Progress in Lipid Research 50, 28–34.
| Docosapentaenoic acid (22:5n-3): a review of its biological effects.Crossref | GoogleScholarGoogle Scholar | 20655949PubMed |
Kaur G, Guo XF, Sinclair AJ (2016) Short update on docosapentaenoic acid: a bioactive long-chain n-3 fatty acid. Current Opinion in Clinical Nutrition and Metabolic Care 19, 88–91.
| Short update on docosapentaenoic acid: a bioactive long-chain n-3 fatty acid.Crossref | GoogleScholarGoogle Scholar | 26808265PubMed |
Kazala EC, Lozeman FJ, Mir PS, Laroche A, Bailey DR, Weselake RJ (1999) Relationship of fatty acid composition to intramuscular fat content in beef from crossbred Wagyu cattle. Journal of Animal Science 77, 1717–1725.
| Relationship of fatty acid composition to intramuscular fat content in beef from crossbred Wagyu cattle.Crossref | GoogleScholarGoogle Scholar | 10438017PubMed |
Knight TW, Knowles S, Death AF, West J, Agnew M, Morris CA, Purchas RW (2003) Factors affecting the variation in fatty acid concentrations in lean beef from grass‐fed cattle in New Zealand and the implications for human health. New Zealand Journal of Agricultural Research 46, 83–95.
| Factors affecting the variation in fatty acid concentrations in lean beef from grass‐fed cattle in New Zealand and the implications for human health.Crossref | GoogleScholarGoogle Scholar |
Kullenberg D, Taylor LA, Schneider M, Massing U (2012) Health effects of dietary phospholipids. Lipids in Health and Disease 11, 3
| Health effects of dietary phospholipids.Crossref | GoogleScholarGoogle Scholar | 22221489PubMed |
Landfald B, Valeur J, Berstad A, Raa J (2017) Microbial trimethylamine-N-oxide as a disease marker: something fishy? Microbial Ecology in Health and Disease 28, 1327309
| Microbial trimethylamine-N-oxide as a disease marker: something fishy?Crossref | GoogleScholarGoogle Scholar | 28588431PubMed |
Larick DK, Turner BE (1989) Influence of finishing diet on the phospholipid composition and fatty acid profile of individual phospholipids in lean muscle of beef cattle. Journal of Animal Science 67, 2282–2293.
| Influence of finishing diet on the phospholipid composition and fatty acid profile of individual phospholipids in lean muscle of beef cattle.Crossref | GoogleScholarGoogle Scholar |
Larick DK, Turner BE, Koch RM, Crouse JD (1989) Influence of phospholipid content and fatty acid composition of individual phospholipids in muscle from Bison, Hereford and Brahman steers on flavour. Journal of Food Science 54, 521–526.
| Influence of phospholipid content and fatty acid composition of individual phospholipids in muscle from Bison, Hereford and Brahman steers on flavour.Crossref | GoogleScholarGoogle Scholar |
Lopez-Huertas E (2010) Health effects of oleic acid and long chain omega-3 fatty acids (EPA and DHA) enriched milks. A review of intervention studies. Pharmacological Research 61, 200–207.
| Health effects of oleic acid and long chain omega-3 fatty acids (EPA and DHA) enriched milks. A review of intervention studies.Crossref | GoogleScholarGoogle Scholar | 19897038PubMed |
McAfee AJ, McSorley EM, Cuskelly GJ, Fearon AM, Moss BW, Beattie JA, Wallace JM, Bonham MP, Strain JJ (2011) Red meat from animals offered a grass diet increases plasma and platelet n-3 PUFA in healthy consumers. British Journal of Nutrition 105, 80–89.
| Red meat from animals offered a grass diet increases plasma and platelet n-3 PUFA in healthy consumers.Crossref | GoogleScholarGoogle Scholar | 20807460PubMed |
Mir Z, Paterson LJ, Mir PS (2000) Fatty acid composition and conjugated linoleic acid content of intramuscular fat in crossbred cattle with and without Wagyu genetics fed a barley-based diet. Canadian Journal of Animal Science 80, 195–197.
| Fatty acid composition and conjugated linoleic acid content of intramuscular fat in crossbred cattle with and without Wagyu genetics fed a barley-based diet.Crossref | GoogleScholarGoogle Scholar |
Nantapo CT, Muchenje V, Hugo A (2014) Atherogenicity index and health-related fatty acids in different stages of lactation from Friesian, Jersey and Friesian × Jersey cross cow milk under a pasture-based dairy system. Food Chemistry 146, 127–133.
| Atherogenicity index and health-related fatty acids in different stages of lactation from Friesian, Jersey and Friesian × Jersey cross cow milk under a pasture-based dairy system.Crossref | GoogleScholarGoogle Scholar | 24176323PubMed |
Narvaez-Rivas M, Gallardo E, Rios JJ, Leon-Camacho M (2011) A new high-performance liquid chromatographic method with evaporative light scattering detector for the analysis of phospholipids. Application to Iberian pig subcutaneous fat. Journal of Chromatography. A 1218, 3453–3458.
| A new high-performance liquid chromatographic method with evaporative light scattering detector for the analysis of phospholipids. Application to Iberian pig subcutaneous fat.Crossref | GoogleScholarGoogle Scholar | 21507406PubMed |
Ooi EM, Watts GF, Ng TW, Barrett PH (2015) Effect of dietary fatty acids on human lipoprotein metabolism: a comprehensive update. Nutrients 7, 4416–4425.
| Effect of dietary fatty acids on human lipoprotein metabolism: a comprehensive update.Crossref | GoogleScholarGoogle Scholar | 26043038PubMed |
Patel D, Witt SN (2017) Ethanolamine and phosphatidylethanolamine: partners in health and disease. Oxidative Medicine and Cellular Longevity 2017, 18
| Ethanolamine and phosphatidylethanolamine: partners in health and disease.Crossref | GoogleScholarGoogle Scholar |
Purchas RW, Knight TW, Busboom JR (2005) The effect of production system and age on concentrations of fatty acids in intramuscular fat of the longissimus and triceps brachii muscles of Angus-cross heifers. Meat Science 70, 597–603.
| The effect of production system and age on concentrations of fatty acids in intramuscular fat of the longissimus and triceps brachii muscles of Angus-cross heifers.Crossref | GoogleScholarGoogle Scholar | 22063885PubMed |
Ramprasath VR, Jones PJ, Buckley DD, Woollett LA, Heubi JE (2013) Effect of dietary sphingomyelin on absorption and fractional synthetic rate of cholesterol and serum lipid profile in humans. Lipids in Health and Disease 12, 125
| Effect of dietary sphingomyelin on absorption and fractional synthetic rate of cholesterol and serum lipid profile in humans.Crossref | GoogleScholarGoogle Scholar | 23958473PubMed |
Reis MG, Roy NC, Bermingham EN, Ryan L, Bibiloni R, Young W, Krause L, Berger B, North M, Stelwagen K, Reis MM (2013) Impact of dietary dairy polar lipids on lipid metabolism of mice fed a high-fat diet. Journal of Agricultural and Food Chemistry 61, 2729–2738.
| Impact of dietary dairy polar lipids on lipid metabolism of mice fed a high-fat diet.Crossref | GoogleScholarGoogle Scholar | 23394615PubMed |
Richter Y, Herzog Y, Lifshitz Y, Hayun R, Zchut S (2013) The effect of soybean-derived phosphatidylserine on cognitive performance in elderly with subjective memory complaints: a pilot study. Clinical Interventions in Aging 8, 557–563.
Rohrmann S, Linseisen J, Allenspach M, von Eckardstein A, Muller D (2016) Plasma concentrations of trimethylamine-N-oxide are directly associated with dairy food consumption and low-grade inflammation in a German adult population. The Journal of Nutrition 146, 283–289.
| Plasma concentrations of trimethylamine-N-oxide are directly associated with dairy food consumption and low-grade inflammation in a German adult population.Crossref | GoogleScholarGoogle Scholar | 26674761PubMed |
Sales-Campos H, Souza PR, Peghini BC, da Silva JS, Cardoso CR (2013) An overview of the modulatory effects of oleic acid in health and disease. Mini-Reviews in Medicinal Chemistry 13, 201–210.
Smith SB (2016) Marbling and its nutritional impact on risk factors for cardiovascular disease. Han-gug Chugsan Sigpum Hag-hoeji 36, 435–444.
| Marbling and its nutritional impact on risk factors for cardiovascular disease.Crossref | GoogleScholarGoogle Scholar |
Sturdivant CA, Lunt DK, Smith GC, Smith SB (1992) Fatty acid composition of subcutaneous and intramuscular adipose tissues and M. longissimus dorsi of Wagyu cattle. Meat Science 32, 449–458.
| Fatty acid composition of subcutaneous and intramuscular adipose tissues and M. longissimus dorsi of Wagyu cattle.Crossref | GoogleScholarGoogle Scholar | 22059895PubMed |
Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL (2013) Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. The New England Journal of Medicine 368, 1575–1584.
| Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk.Crossref | GoogleScholarGoogle Scholar | 23614584PubMed |
Thorning TK, Raziani F, Bendsen NT, Astrup A, Tholstrup T, Raben A (2015) Diets with high-fat cheese, high-fat meat, or carbohydrate on cardiovascular risk markers in overweight postmenopausal women: a randomized crossover trial. The American Journal of Clinical Nutrition 102, 573–581.
| Diets with high-fat cheese, high-fat meat, or carbohydrate on cardiovascular risk markers in overweight postmenopausal women: a randomized crossover trial.Crossref | GoogleScholarGoogle Scholar | 26178720PubMed |
Turner TD, Jensen J, Pilfold JL, Prema D, Donkor KK, Cinel B, Thompson DJ, Dugan MER, Church JS (2015) Comparison of fatty acids in beef tissues from conventional, organic and natural feeding systems in western Canada. Canadian Journal of Animal Science 95, 49–58.
| Comparison of fatty acids in beef tissues from conventional, organic and natural feeding systems in western Canada.Crossref | GoogleScholarGoogle Scholar |
Ulbricht TL, Southgate DA (1991) Coronary heart disease: seven dietary factors. Lancet 338, 985–992.
| Coronary heart disease: seven dietary factors.Crossref | GoogleScholarGoogle Scholar | 1681350PubMed |
Van Hecke T, Jakobsen LM, Vossen E, Gueraud F, De Vos F, Pierre F, Bertram HC, De Smet S (2016) Short-term beef consumption promotes systemic oxidative stress, TMAO formation and inflammation in rats, and dietary fat content modulates these effects. Food & Function 7, 3760–3771.
| Short-term beef consumption promotes systemic oxidative stress, TMAO formation and inflammation in rats, and dietary fat content modulates these effects.Crossref | GoogleScholarGoogle Scholar |
Vargas-Bello-Perez E, Larrain RE (2017) Impacts of fat from ruminants’ meat on cardiovascular health and possible strategies to alter its lipid composition. Journal of the Science of Food and Agriculture 97, 1969–1978.
| Impacts of fat from ruminants’ meat on cardiovascular health and possible strategies to alter its lipid composition.Crossref | GoogleScholarGoogle Scholar | 27925211PubMed |
Vesper H, Schmelz E-M, Nikolova-Karakashian MN, Dillehay DL, Lynch DV, Merrill AH (1999) Sphingolipids in food and the emerging importance of sphingolipids to nutrition. The Journal of Nutrition 129, 1239–1250.
| Sphingolipids in food and the emerging importance of sphingolipids to nutrition.Crossref | GoogleScholarGoogle Scholar | 10395583PubMed |
Weiland A, Bub A, Barth SW, Schrezenmeir J, Pfeuffer M (2016) Effects of dietary milk- and soya-phospholipids on lipid-parameters and other risk indicators for cardiovascular diseases in overweight or obese men: two double-blind, randomised, controlled, clinical trials. Journal of Nutritional Science 5, e21
| Effects of dietary milk- and soya-phospholipids on lipid-parameters and other risk indicators for cardiovascular diseases in overweight or obese men: two double-blind, randomised, controlled, clinical trials.Crossref | GoogleScholarGoogle Scholar | 27293558PubMed |
Wood JD, Richardson RI, Nute GR, Fisher AV, Campo MM, Kasapidou E, Sheard PR, Enser M (2004) Effects of fatty acids on meat quality: a review. Meat Science 66, 21–32.
| Effects of fatty acids on meat quality: a review.Crossref | GoogleScholarGoogle Scholar | 22063928PubMed |
Xie YR, Busboom JR, Cornforth DP, Shenton HT, Gaskins CT, Johnson KA, Reeves JJ, Wright RW, Cronrath JD (1996) Effects of time on feed and post-mortem aging on palatability and lipid composition of crossbred Wagyu beef. Meat Science 43, 157–166.
| Effects of time on feed and post-mortem aging on palatability and lipid composition of crossbred Wagyu beef.Crossref | GoogleScholarGoogle Scholar | 22060570PubMed |
Zeisel SH, Warrier M (2017) Trimethylamine N-oxide (TMAO), the microbiome, and heart and kidney disease. Annual Review of Nutrition 37, 157-181
Zhang AQ, Mitchell SC, Smith RL (1999) Dietary precursors of trimethylamine in man: a pilot study. Food and Chemical Toxicology 37, 515–520.
| Dietary precursors of trimethylamine in man: a pilot study.Crossref | GoogleScholarGoogle Scholar | 10456680PubMed |
Zhou L, Le Grandois J, Marchioni E, Zhao M, Ennahar S, Bindler F (2010) Improvement of total lipid and glycerophospholipid recoveries from various food matrices using pressurized liquid extraction. Journal of Agricultural and Food Chemistry 58, 9912–9917.
| Improvement of total lipid and glycerophospholipid recoveries from various food matrices using pressurized liquid extraction.Crossref | GoogleScholarGoogle Scholar | 20738132PubMed |


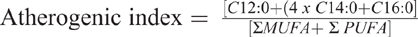 (Ulbricht and Southgate 1991; Nantapo et al. 2014). Means followed by different lowercase letters in the same row are significantly different (at P = 0.05)
(Ulbricht and Southgate 1991; Nantapo et al. 2014). Means followed by different lowercase letters in the same row are significantly different (at P = 0.05)