Feed additives as a strategic approach to reduce enteric methane production in cattle: modes of action, effectiveness and safety
M. Honan A , X. Feng A , J.M. Tricarico B and E. Kebreab
B and E. Kebreab  A C
A C
A Department of Animal Science, University of California, Davis, 2111 Meyer Hall, One Shields Avenue, Davis, CA, 95618, USA.
B Innovation Center for US Dairy, 10255 West Higgins Road, Suite 900, Rosemont, IL 60018, USA.
C Corresponding author. Email: ekebreab@ucdavis.edu
Animal Production Science - https://doi.org/10.1071/AN20295
Submitted: 22 May 2020 Accepted: 23 November 2020 Published online: 2 February 2021
Journal compilation © CSIRO 2021 Open Access CC BY-NC-ND
Abstract
Increasing consumer concern in greenhouse-gas (GHG) contributions from cattle is pushing the livestock industry to continue to improve their sustainability goals. As populations increase, particularly in low-income countries, the demand for animal-sourced foods will place further pressure to reduce emission intensity. Enteric methane (CH4) production contributes to most of the GHG from livestock; therefore, it is key to mitigating such emissions. Feed additives have primarily been used to increase animal productivity, but advances in understanding the rumen has resulted in their development to mitigate CH4 emissions. The present study reviewed some of the main feed additives with a potential to reduce enteric CH4 emissions, focusing on in vivo studies. Feed additives work by either inhibiting methanogenesis or modifying the rumen environment, such that CH4 production (g/day) is reduced. Feed additives that inhibit methanogenesis or compete with substrate for methanogens include 3-nitroxypropanol (3NOP), nitrates, and halogenated compounds containing organisms such as macroalgae. Although 3NOP and macroalgae affect methyl–coenzyme M reductase enzyme that is necessary in CH4 biosynthesis, the former is more specific to methanogens. In contrast, nitrates reduce CH4 emissions by competing with methanogens for hydrogen. However, nitrite could accumulate in blood and be toxic to ruminants. Rumen modifiers do not act directly on methanogens but rather on the conditions that promote methanogenesis. These feed additives include lipids, plant secondary compounds and essential oils. The efficacy of lipids has been studied extensively, and although supplementation with medium-chain and polyunsaturated fatty acids has shown substantial reduction in enteric CH4 production, the results have been variable. Similarly, secondary plant compounds and essential oils have shown inconsistent results, ranging from substantial reduction to modest increase in enteric CH4 emissions. Due to continued interest in this area, research is expected to accelerate in developing feed additives that can provide options in mitigating enteric CH4 emissions.
Keywords: greenhouse gases, methanogens, rumen function, ruminants.
Introduction
The livestock sector is crucial for food and nutrition security globally, with a projected increase of 80% in consumer demand by 2050 for beef (Nadathur et al. 2017). Approximately 83% of global milk is produced from cattle (Visioli and Strata 2014) and, by the end of the decade, milk output is anticipated to have grown by 33% and 9% in developing and developed countries respectively (OECD/FAO 2018). Globally, beef is the third-most consumed meat, contributing 320 million tons of product to world food supply, representing 79% of total sourced meat (Opio et al. 2013; Ritchie and Roser 2019). Nutritional benefits from ruminants are pronounced as they have the ability to convert fibre-dense forages that are indigestible to humans into high-quality bioavailable nutrient sources. In fact, 86% of the feed consumed by livestock worldwide is not considered edible for human consumption (Mottet et al. 2017). At the same time, ruminants occupy more land than do any other livestock species and their enteric methane (CH4) emissions contribute to total anthropogenic greenhouse gases (GHG; Knapp et al. 2014). Enteric CH4 is under increased scrutiny due to its heightened potency compared with carbon dioxide (CO2) in the atmosphere, and the 39% it contributes to the sector’s total emissions (Gerber et al. 2013; IPCC 2013).
Heightened attention on climate change by scientists, governments and consumers is challenging the livestock industry to reduce GHG emissions. Arguments for consumers to shift towards plant-based diets have gained traction; however, constructing diets on the basis of the level of GHG emissions will not necessarily have a positive correlation with nutritional provision (Payne et al. 2016). Dietary manipulation has been studied over the past few decades as a strategy to reduce enteric CH4 emissions and could be assimilated into management practices, notably through feed additives (Cottle et al. 2011). Feed additives are used in livestock diets to improve feed-use efficiency, quality of animal-source foods, and animal performance and health. These additives include vitamins, amino acids, fatty acids, minerals, pharmaceutical compounds, fungal products and steroidal compounds. Recent advances in understanding methanogenesis have led to the development and discovery of feed additives that can reduce CH4 emissions to varying degrees. The present review aims to provide a concise summary of feed additives currently available, or in development, with some potential to reduce CH4 emissions from ruminants. The secondary objective of the review is to summarise information on mode of action, efficacy, safety and readiness for adoption of anti-methanogenic feed additives. Although the focus is on feed additives tested in vivo, some in vitro studies are also discussed if there is paucity of in vivo trials for an additive or to help explain mode of action.
Rumen methanogenesis
Methane production can be substantial in ruminants, representing up to 12% of gross energy intake that could potentially be utilised for physiological processes, but, instead, is released into the atmosphere through eructation (Beauchemin et al. 2009a). However, CH4 synthesis represents a significant metabolic sink for reducing equivalents (hydrogen, H2) that would otherwise accumulate in the rumen and create an unfavourable environment for fermentative digestion processes (Morgavi et al. 2010). Hydrogen itself does not accumulate due to methanogen activity, instead, methanogens participate in interspecies H2 transfer, and dispose of the reducing equivalents from other metabolic processes (Bergman 1990; McAllister et al. 1996). Hydrogen synthesis is a self-limiting process that relies on separate and distinct reducing equivalent consumption pathways so as to continue production. Cellulose-degrading activity in both bacteria and fungi increases in the presence of methanogens, which contributes to the principle of rumen syntrophic relationships (Bauchop and Mountfort 1981; Sasaki et al. 2012).
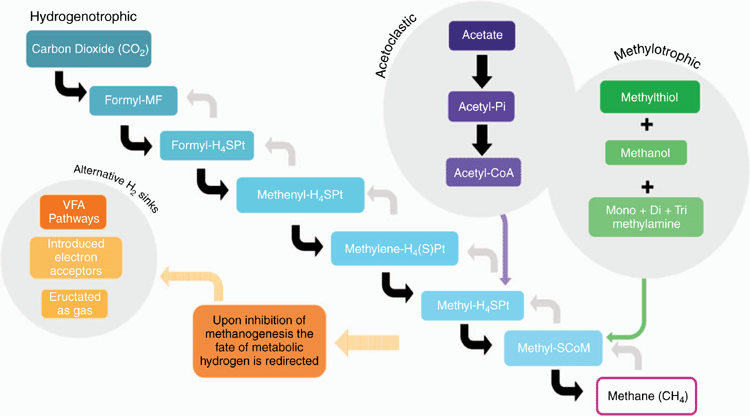
Rumen methanogenesis is performed strictly by archaea (Hook et al. 2010). A methanogenesis pathway is presented in a simplified diagram (Fig. 1), which includes the convergence of pathways known to occur in a Methanosarcina spp. Lambie et al. (2015) categorised methanogens on the basis of their metabolic pathways, as follows: hydrogenotrophic, acetoclastic and methylotrophic that can yield CH4 in the rumen from Methanosarcina spp. Methanogens reduce CO2 with H2 (hydrogenotrophic), source a methyl group from acetate (acetoclastic), or a methyl group from compounds such as methanol, methylthiol, dimethylamine, and mono-, di-, tri- methylamine (methylotroph). Formate contributes to methanogenesis as an electron donor within the hydrogenotrophic pathway, representing ~16–18% of CH4 in batch- and continuous-culture experiments (Seedorf et al. 2014; Ungerfeld 2015; Hungate et al. 1970). Coenzyme M requires a methyl group for the reduction to CH4, which is provided through each of these pathways. Methane mitigation could be achieved by directly targeting methanogens or modifying the rumen environment to shift the metabolic pathways away from methanogenesis or reduce substrates for the archaea.
|
Rumen inhibitors
Feed additives classified as CH4 inhibitors directly act on the methanogenesis pathway (Fig. 1) in a way that can disrupt the process and reduce CH4 production (g/day). Methanogens prevent H2 accumulation in the rumen, which otherwise may lead to adverse effects on fibre degradability and animal performance (Ellis et al. 2008). Given the importance of efficient fibre digestion, the use of CH4 inhibitors must balance between reducing CH4 production and avoiding negative impacts on animal performance and welfare. Inhibition of methanogenesis requires a redirection of reducing equivalents, H2 in this case, to alternative sinks, instead of CO2, unless the inhibitor’s mode of action is a highly competitive electron acceptor. Malik et al. (2015) argued that H2 clearance through pathways such as reductive acetogenesis and propionogenesis also has the advantage of energy conservation into end products such as meat and milk. Several of these alternative sinks will be reviewed herein and may also be implemented as independent feed additives or with an inhibitor. Studies have shown a decrease in CH4 emissions paired with an increase in H2 emissions without the addition of an alternative sink (e.g. Roque et al. 2019b), indicating that elevated H2 concentration in the rumen may not necessarily result in decreased fermentation, and hence, productivity.
3-nitroxypropanol (3NOP, marketed as Bovaer in the European Union)
Methyl–coenzyme M reductase (MCR) is the enzyme that catalyses the final step of the methanogenesis pathway from intermediate methyl–CoM to CH4 as illustrated in Fig. 1. As a nickel enzyme, MCR can catalyse this step only when its Ni ion is in the +1-oxidation state and can be inactivated due to the existing redox potential (Duin et al. 2016). The position of 3NOP binding to an active site of MCR places the reducing nitrate group in close proximity to Ni(I), a distance in which electrons could be transferred. Although 3NOP inhibits methanogenesis and reduces methanogen growth, it does not negatively affect other microbial groups in the rumen (Duin et al. 2016).
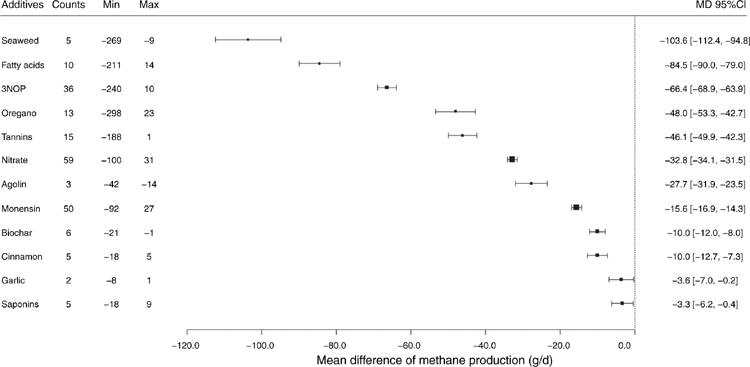
More than 15 studies have been conducted using 3NOP, showing a marked reduction of enteric CH4 emissions with a range of effectiveness. 3NOP added to ruminant diets in small quantities has been shown to persistently reduce enteric CH4 emissions by inhibiting an important step in the methanogenesis metabolic pathway, without apparent negative side effects (Hristov et al. 2015). Figure 2 shows a forest plot illustrating the effect sizes as a mean difference between the control and treatment-group mean CH4 production. For example, Vyas et al. (2016) reported that with 0.2 g 3NOP/kg dry-matter (DM) supplementation, CH4 production in backgrounding and finishing beef cattle reduced 37.6% and 84.3% compared with the control group, whereas Vyas et al. (2018), using the same amount of supplementation in backgrounding phase (0.2 g/kg DM) of beef cattle, found a 54.1% reduction in CH4 production. These authors reduced the level of supplementation of 3NOP to 0.125 g/kg DM during the finishing phase and reported 53.8% reduction in CH4 production. There was also an improvement in gain-to-feed ratio during treatment, with a 7% drop in DM intake (DMI). Similarly, Martinez-Fernandez et al. (2018) reported a decrease in CH4 production of 38% and daily weight gain of 0.571 kg/day compared with the control in steers supplemented with 0.30 g 3NOP/kg DM. Hristov et al. (2015) demonstrated that CH4 production in lactating cows was reduced by 30% by feeding 3NOP at 0.04–0.08 g/kg DM without affecting feed intake and milk production. Lopes et al. (2016) reported a 31% decrease in CH4 production in lactating dairy cattle fed diets supplemented with 0.06 g/kg DM. In a meta-analysis of the anti-methanogenic effects of 3NOP, Dijkstra et al. (2018) reported that enteric CH4 production was reduced 39% in dairy and 22% in beef cattle at a mean dose of 0.123 g/kg DM. Additive dose and the neutral detergent fibre (NDF) content of diet had a significant impact on the effectiveness of 3NOP in reducing enteric CH4 emissions. Furthermore, an increase in 3NOP dose of 0.010 g/kg DM from the mean dose further reduced CH4 production by 2.56 ± 0.55%. Similarly, Jayanegara et al. (2018) reported that the methanogenic archaea population was reduced through 3NOP supplementation and the magnitude of reduction was positively correlated with 3NOP dose in small and large ruminants. Addition of 3NOP is also associated with shifting H2 production in the rumen and results in an increase in molar proportion for propionate and decreases acetate production (Haisan et al. 2014; Kim et al. 2019; Lopes et al. 2016).
There are no known adverse effects of supplementing 3NOP on the animal or the subsequent product. The feed additive 3NOP continues to be studied and, after approval by regulatory bodies, it is expected to be on the market in the near future.
Halogens
Plant species that accumulate halogenic compounds in their tissues have been investigated for their potential to reduce enteric CH4 emissions. Halogens are elements that hold a large, negative electron affinity and seek to combine with other compounds to reach stability through satisfaction of the valence shell in the rumen environment (Gribble 2004). Bromoform and chloroform are halogens that have been found to interfere directly with the methanogenesis pathway by serving as competitive inhibitors (or analogues) of the MCR, preventing the final catalysis step (Goel et al. 2009). The mode of action is through reacting with reduced vitamin B12 and inhibiting the cobamide-dependent methyl-transferase step of methanogenesis (Wood et al. 1968; Chalupa 1977). The B12-dependent methyl-transferases also play an important role in one carbon metabolism in acetogenic bacteria (Banerjee and Ragsdale 2003), and, therefore, halogenated compounds may have an effect on reductive acetogenesis.
At supplementation level of 1.50–1.59 g/kg DM (2.6 g/100 kg liveweight; mean liveweight = 288 kg) of chloroform– cyclodextrin, steers have demonstrated a 30–35% reduction in enteric CH4 production, with no detectable differences in rumen fermentability (Martinez-Fernandez et al. 2016). Steers dosed daily with 0.267 g/kg DM of chloroform were shown to decrease 94–95% of CH4 production within 4–5 days of treatment. However, CH4 production has been shown to slowly recover to 62% of the pre-treatment levels by Day 42 of treatment (Knight et al. 2011). The macroalgae species Asparagopsis taxiformis and A. armata have been evaluated for their mitigation potential both in vitro and in vivo (Roque et al. 2019a, 2019b). Asparagopsis spp. contain relatively high concentrations of bromoform and other halogenated compounds such as bromochloromethane (Paul et al. 2006; Machado et al. 2016). An in vitro trial analysing effectiveness across seaweed species found A. taxiformis to be the most effective species among 20 freshwater and marine macroalgae in reducing CH4 output (98.9%), but also reduced total gas production (62%), likely indicating inhibition of digestion (Machado et al. 2016). Increasing the dose to 5% in vitro, Roque et al. (2019a) reported a 95% reduction in the level of CH4 production. Three papers have been published so far, reporting the effect of Asparagopsis spp. in sheep, dairy and beef cattle in vivo. Li et al. (2018) supplemented A. taxiformis at 67.5 g/kg DM (30 g/kg of organic matter, OM) in sheep diets and reported a reduction of up to 80% in enteric CH4 production. However, rumen volatile fatty acid (VFA) concentrations in the 0%, 0.5%, 1.0%, 2.0% and 3.0% macroalgae inclusion groups declined from 92.0, to 86.5, 74.9, 69.1 and 65.4 mM respectively. Reductions in VFA concentrations are not desirable as they provide energy to the ruminant. In lactating dairy cattle, Roque et al. (2019b) observed up to 67.2% reduction in CH4 intensity (g/kg milk produced) using A. armata at an inclusion rate of 18.3 g/kg DM (10 g/kg of OM). In Brangus beef cattle, Kinley et al. (2020) reported a reduction of enteric CH4 production of up to 98% by supplementing a feedlot diet with A. taxiformis at 3.26 g/kg DM (2 g/kg of OM). In addition, there was an improvement of 42% in average daily gain with a supplementation level of 1.63 g/kg DM (1.0 g/kg of OM) and it went up to 53% at an inclusion rate of 3.26 g/kg DM (2.0 g/kg of OM). The study by Kinley et al. (2020) reported a greater effectiveness at a lower dose than did that of Roque et al. (2019b), which was likely due to the large differences in the bromoform concentration in A. taxiformis and A. armata, while also acknowledging the inclusion of monensin in the Kinley et al. (2020) experimental diets. The bromoform concentration in Roque et al. (2019b) study was 1.32 mg/g compared with 6.55 mg/g in the Kinley et al. (2020) study.
Sourcing naturally occurring halogens circumvents the need to use synthetic halogens. Historically, these synthetics have had detrimental effects on the environment (Gribble 2004). Kinley et al. (2020) and Roque et al. (2019b) tested for residual bromoform content in meat (or edible offal) and milk respectively. In both cases, concentrations of bromoform were either undetectable or not significantly different from the control, suggesting no safety issues arising from the active ingredient. At present, A. taxiformis is not produced commercially; so, accessibility is an issue. The use of macroalgae also needs to be approved by regulatory agencies before widespread use by producers.
Nitrate
Adding nitrate to ruminant diets can be an effective CH4 mitigation strategy because nitrate competes with methanogens for H2 in the rumen. Nitrate (NO3–) is reduced to nitrite (NO2−; NO3− + H2 → NO2− + H2O) and further to ammonia (NH4+; NO2− + 3H2 + 2H+ → NH4+ + 2H2O) by rumen microbes. However, small quantities of nitrous oxide may also be produced (Latham et al. 2016). This pathway is highly competitive with methanogens for H2 utilisation in the rumen due to greater changes in Gibbs energy than with methanogenesis (CO2 + 4H2 → CH4 + 2H2O) pathway (Villar et al. 2020). The result is a redirection of H+ flow from CO2 to nitrate reduction, thereby reducing the generation of CH4 (Olijhoek et al. 2016).
About 24 in vivo studies showed that the efficacy of nitrate additives varied widely, ranging from +1.25% to −29.8%, and may be affected by several factors. A meta-analysis conducted by Feng et al. (2020) investigated the potential explanatory variables for anti-methanogenic effects of in vivo nitrate supplementation in cattle. These included DMI, roughage proportion, NDF content, crude protein (CP) content, bodyweight, nitrate dose, cattle type, and CH4 measurement methods. The authors reported that nitrate significantly reduced CH4 emissions in a dose–response manner and the mitigating effect increased with the level of nitrate inclusion. Methane production reduced 14.6% in cattle supplemented with nitrate at 17.7 g/kg DM (Feng et al. 2020). Hulshof et al. (2012) reported that nitrate supplementation increased ammonia-nitrogen concentrations in the rumen by 34%, decreased propionate concentrations by 16%, but did not affect the total VFA concentrations. Persistency of nitrate was tested by van Zijderveld et al. (2011a), by including 21 g/kg DM during four successive 24-day periods and a consistent 16% reduction in daily CH4 production (g/day) and yield (g CH4/kg DMI) was demonstrated. An additive effect of nitrate and linseed oil was reported by Guyader et al. (2015a) in multiparous, non-lactating dairy cattle. These authors reported that adding 4% linseed oil to 3% calcium nitrate further reduced CH4 production from 22.8% (nitrate only) to 33.0%.
Concerns about the toxicity of the intermediate product of nitrate, namely nitrite, to ruminants necessitate management, as animal poisoning may occur via methaemoglobinemia (Latham et al. 2016). Nitrite is toxic in blood because it converts haemoglobin to methaemoglobin, which is incapable of carrying oxygen. Blood methaemoglobin concentrations in ruminants increase with a greater nitrate consumption and could cause nitrate poisoning (Lee and Beauchemin 2014). Apparent nitrate-poisoning symptoms such as depressed feed intake, slow or no weight gain, reproduction failure, respiratory distress, coma and death have been reported in previous studies with methaemoglobin concentrations of 30–40% of total haemoglobin (Bruning-Fann and Kaneene 1993). Lee and Beauchemin (2014) discussed several critical factors related to nitrate toxicity, including the dietary nitrate concentrations, nitrate consumption rate, incomplete reduction of nitrate and nitrite to ammonia, and rumen outflow rates. Toxic effects of nitrite on the populations of main cellulolytic bacteria, which may be caused by the negative effects of nitrate/nitrite on cellulolytic and xylanolytic activity, have also been observed (Iwamoto et al. 2002; Asanuma et al. 2015; Granja-Salcedo et al. 2019). However, the risk of nitrate toxicity can be reduced by gradual acclimation of ruminants to dietary nitrate or utilisation of encapsulated nitrate (Lee and Beauchemin 2014). Currently, nitrate inclusion may not be advisable in commercial operations due to its potential toxicity. However, a denitrifying probiotic, Paenibacillus fortis, that can enhance nitrite detoxification in nitrate treated ruminants, has been identified (Latham et al. 2019). If successful, nitrate and the probiotic might be a practical mitigation strategy to reduce CH4 production from ruminants.
Rumen modifiers
The rumen environment can be modified with feed additives to limit the growth of methanogens and to suppress CH4 production, without targeting the specific methanogenesis pathway. The factors influencing CH4 production include those involved in H2 and carbohydrate metabolism (Morgavi et al. 2010). Understanding rumen metabolic processes that affect CH4 formation is still advancing; however, feed additives were used to modify the rumen environment to reduce CH4 production without compromising animal health or productivity. This section discusses feed additives that can potentially reduce CH4 production by modifying the rumen environment.
Dietary lipids
Dietary lipids modify the rumen environment in several ways, including (1) toxic characteristics on methanogens and protozoa, (2) hydrogenation of unsaturated fatty acids (alternative H2 sink) and (3) shifts to propionic production, leading to reduction of enteric CH4 production (Johnson and Johnson 1995; Beauchemin et al. 2008, 2009b). Efficacy of lipids to reduce CH4 emissions are dependent on the form and level of supplementation, as well as the source and fatty acid profile (Beauchemin et al. 2008; Eugène et al. 2008). Several meta-analyses were conducted to estimate the impact of dietary lipids on CH4 production (e.g. Beauchemin et al. 2008; Eugène et al. 2008; Martin et al. 2010). For example, Beauchemin et al. (2008) evaluated 17 studies in sheep, beef and dairy cattle and reported a 5.6% reduction in CH4 production for every 1% additional inclusion of supplemental fat. In dairy cattle, Eugène et al. (2008) reported a decrease of 9% through lipid-supplementation (average 6.4%) compared with control diets (average 2.5%), mostly as a consequence of reduced DMI. Similarly, Patra (2013) reported 3.77% decline in CH4 emissions for each percentage inclusion of lipid in dairy cattle diets. Prediction inconsistencies by the inclusion of supplemental lipid are likely to be due to differences in lipid source and diet composition. In a review, Rasmussen and Harrison (2011) reported that the most effective fatty acid profiles that reduce CH4 production were medium-chain (8–16 carbon chains; MCFA) and polyunsaturated (PUFA) fatty acids. However, reductions in DMI due to high levels of dietary lipids are well characterised and ration formulation programs often are set not to exceed 6–7% of total DMI (NRC 2001).
Medium-chain fatty acids
These include lauric, myristic, capric and caprylic acids (Hollmann et al. 2012). In vitro studies have reported coconut oil, which contains 75% of MCFA, to reduce CH4 production by 43–85% (Dong et al. 1997; Machmüller et al. 1998). Application of coconut oil in in vivo trials also showed similar patterns in CH4 reduction (Hollmann et al. 2012). Ruminants fed diets containing 13, 27 and 33 g coconut oil/kg DM had 3%, 37% and 45% reduction in CH4 output compared with the control respectively. DMI, solids-corrected milk yield, and milk fat yield (no difference between the two greatest levels of inclusion on milk fat yield) decreased linearly with an increase in coconut oil application. Inclusion of myristic acid at a rate of 50.0 g/kg DM in dairy cattle diets reduced CH4 production by 36%, but also reduced milk fat by 2.4%, with a tendency to reduce DMI (Odongo et al. 2007). Lauric acid had no negating effects on methanogenesis in dairy cattle when they received it at 10.0 g/kg DM (Hristov et al. 2009). Within the same trial, the treatment group receiving 21.6 g/kg DM of coconut oil reduced their CH4 production by 61% compared with the control.
Polyunsaturated fatty acids
Polyunsaturated fatty acids have also been shown to reduce CH4 production. For example, Bayat et al. (2015) found that enteric CH4 production reduced by 29.5% with supplementation of 60 g/kg DM of camelina oil, but other parameters such as milk yield and milk components were compromised. In contrast, Duthie et al. (2018) did not find significant differences in enteric CH4 production in steers fed increasing amounts of dietary lipid sourced from maize distillers dark grains, which increased diet ether extract from 24 to 37 g/kg DM for 17 weeks. Supplementation of diets with cottonseed oil has been shown to decrease enteric CH4 production by ~42% (Nogueira et al. 2020). These authors suggested that bio-hydrogenation of lipids served as an alternative H2 sink, and with each percentage point of lipid added to the diet, CH4 production was reduced by 8%. Further characterisation and understanding of the impact and longevity of dietary lipid inclusion on methanogenesis would be valuable in selecting plant sources and estimating their impact. Dietary lipid additives (both MCFA and PUFA) show substantial decreases in CH4 production with a wider range of effectiveness compared with other feed additives (Fig. 2).
Probiotics
Microorganisms included in diets are often referred to as probiotics, cultures, or direct-fed microbials. Introducing microorganisms to a digestive microbiome is practiced on farms to influence the rumen flora for improved digestion. Results of feeding fungi, yeast or bacteria to reduce CH4 production have not been consistent in studies conducted in vitro or in vivo. Application of live yeast cultures (various strains of Saccharomyces cerevisiae) have not been shown to significantly change CH4 production, rumen fermentation or apparent total tract nutrient digestibility in dairy cattle (Bayat et al. 2015). Additionally, inclusion of either a dead or live form of S. cerevisiae has little to no impact on nutrient digestibility or rumen fermentation patterns in beef heifers (Vyas et al. 2014). A meta-analysis by Darabighane et al. (2019) using data from 1990–2016 observed no significant reduction in CH4 production through the use of probiotics.
Introducing propionate-producing bacteria has been evaluated as a possible solution because propionate production consumes H2 as a reducing equivalent and, thereby, competes with methanogenesis (Ungerfeld 2013). This has not been effective with all strains of bacteria but Propionibacterium thoenii T159 reduced CH4 production by 20% and increased VFA production by 21% in a study that screened 31 different strains within in vitro models (Chen et al. 2020). However, in lactating primiparous cows, P. freudenreichii 53-W was shown to increase CH4 production by 27% (Jeyanathan et al. 2019). The mechanisms of reduction in CH4 production (if any) are still unknown and could be either directly by microbes or indirectly through metabolites that affect the rumen microbiome (Doyle et al. 2019). Jeyanathan et al. (2019) found no effect on CH4 output when feeding Lactobacillus pentosus D31, and L. bulgaricus D1 in vivo. Currently, there is no concrete evidence that probiotics are an effective method of CH4 mitigation.
Acetogenesis, or reductive acetogenesis, is another H2-utilising metabolic pathway in which acetogens utilise CO2 and H2 as substrates to produce acetate. While more prevalent in other mammalian guts, acetogens cohabit with methanogens in the rumen, but are either lacking a substantial population density, preferred environment conditions, or the competitiveness to be the favourable pathway of H2 ‘disposal’ (Joblin 1999). Redirection of H2 into the acetogenesis pathway to yield acetate would allow the recapture of energy compared with the loss due to methanogenesis. Enhancing this pathway in the rumen may be approached by sourcing acetogens from other ecosystems and transplanting them into the rumen (Gagen et al. 2014) or uncovering a method to enhance the existing rumen acetogen population if they can outcompete native methanogens.
Biochar
Organic matter that has undergone pyrolysis, commonly known as biochar, has a wide range of impacts on livestock systems due to its unique characteristics. Biochar has been utilised for generations as a remedy for digestive disorders and is sourced by the livestock industry to address issues surrounding animal husbandry, metabolism and waste management (Kalus et al. 2019; Schmidt et al. 2019). Abatement of CH4 production through the application of biochar has been shown in soil (Yu et al. 2013) and compost (Sonoki et al. 2013). Considering that there is already an existing market for biochar as a beneficial feed additive, in vivo evidence for GHG mitigation will be significant (Schmidt et al. 2019). Possible mechanisms have been elucidated through a study that observed that application of biochar to paddy soils stimulated methanotrophic proteobacteria and reduced CH4, despite methanogens also being stimulated (Feng et al. 2012). Additionally, biochar may provide a habitat for methanogens or possibly absorb gases when consumed due to its porous nature, but the mechanisms of action for CH4 mitigation in cattle are not well understood (Terry et al. 2019; Man et al. 2020).
Rice husks sourced for biochar and fed at an inclusion rate of 6 g/kg DM reduced CH4 production by 22%, increased liveweight gain by 25%, and had no impact on DMI over a 98-day period (Leng et al. 2012). Biochar supplemented at 8 g/kg DM reduced CH4 production by 9.5% in growing steers and 18.4% in finishing steers (Winders et al. 2019). Contrary to these findings, inclusion levels of ‘pine-enhanced biochar’ at 5, 10 and 20 g/kg DM in the diets of Angus × Hereford heifers did not reduce CH4 emissions (Terry et al. 2019). However, it altered the microbiota, notably selecting against Fibrobacter species, which is one of the dominating phyla of the rumen responsible for cellulose degradation (Béra-Maillet et al. 2004). The wide variation in effectiveness precludes biochar as proven feed additive to reduce CH4 production at present. More research, particularly in vivo, is required to understand the conditions under which biochar can mitigate CH4 production.
Ionophores
Ionophores, such as monensin, alter rumen microbial populations to improve digestive efficiency by depriving methanogens of substrates that are typically provided by Gram-positive bacterial and ciliate protozoal populations (Russell and Strobel 1989; Hook et al. 2010). This fermentation shift favours the production of propionate over acetate, which reduces the amount of H2 available for methanogens.
A meta-analysis by Appuhamy et al. (2013) quantitatively determined the impact of monensin in cattle. In beef cattle supplemented with monensin at an average monensin dose of 0.032 g/kg DM, CH4 production was reduced by 19 g/day, which was further reduced as the NDF content of the diet increased. In dairy cattle, CH4 production was reduced by 6 g/day at the same average dose and was further reduced as the dietary lipid content increased. Appuhamy et al. (2013) concluded that although there were reductions in CH4 production through supplementation with monensin, the effect was transient, lasting ~6 weeks. In contrast, Benchaar (2020) reported no suppression effect of monensin on CH4 output when it was administered to dairy cattle (0.024 g/kg DM), but there was an increase in the proportion of a biohydrogenation intermediate, thus altering rumen metabolism patterns.
The antimicrobial nature of ionophores has caused a concern to human health (Guan et al. 2006; Hook et al. 2010). Long-term use of ionophores is limited due to a low efficacy, transient nature and safety concerns.
Plant secondary compounds
Plant secondary compounds are primarily synthesised in response to their environmental conditions and not for specific physiological function (Morrissey 2009). Some plant secondary compounds that may possess antimethanogenic properties are variable in composition due to environmental condition in which they are grown. Seasonal variation, pollution, diseases, pests, storage, injuries and pollination activity influence secondary-compound production and composition (Figueiredo et al. 2008). These compounds are not commonly extracted or isolated before feeding to ruminants because of time and cost considerations, which may contribute to their concentrations being inconsistent. These obstacles present a challenge in determining or predicting efficacy.
Tannins
Tannins are soluble, phenolic compounds that accumulate within plant tissues likely due to ongoing metabolic processes and contribute to the plant defence system (Swanson 2003). The CH4 mitigation mechanisms of tannins are not well understood but may be due to a combination of factors, including a reduction in fibre digestibility (decrease in H2 production) or a direct inhibition of methanogens (Tavendale et al. 2005).
Jayanegara et al. (2012) conducted a meta-analysis describing the relationship between rumen CH4 formation and the level of dietary tannin (hydrolysed or condensed) inclusion between in vivo and in vitro models. These authors reported that low levels of inclusions of tannins in animal experiments often yielded inconsistent results on CH4 production, but that variability seemed to diminish at higher doses, leading to setting the threshold for detecting treatment differences in animals to be >20 g/kg DM of tanniferous inhibitors. Furthermore, reduction in CH4 production was often followed by a suppression in OM and fibre digestibility. Methane measurements from goats fed Kobe lespedeza, a forage containing condensed tannins at 151, 101 and 49.9 g/kg of DM led to a 54%, 52% and 32% reduction compared with the control group respectively (Animut et al. 2008). Supplementing beef cattle diets with tannic acid at a 26 g/kg DM inclusion rate, CH4 production decreased 33.6%, but the digestibility of DM and CP, and the concentration of VFA were negatively affected (Yang et al. 2017). Investigating different tannin-containing hays, Stewart et al. (2019) found small burnet (Sanguisorba minor) fed to Angus cows and heifers to reduce CH4 production in comparison to a diet containing alfalfa hay (209 vs 289 g CH4/day respectively). However, CP and DM digestibility was affected negatively.
Grape marc or pomace contains high concentrations of condensed tannins and it is a readily available biowaste from the viticulture industry. Moate et al. (2014) fed dried pelleted (274 g/kg DM) or ensiled grape marc (269 g/kg DM) to dairy cattle and found that the dried form was the most effective in reducing CH4. The authors reported that the CH4 production in dairy cattle fed the control, dried and ensiled grape marc was 470, 375 and 389 g CH4/day. More recently, Caetano et al. (2019) fed ensiled grape marc at a rate of 31.2 g/kg DM, which equates to ~3–4 kg/day of ensiled grape marc (estimated on the basis of reported DMI). Treatment inclusion in the study of Caetano et al. (2019) study led to a 14% reduction in CH4 production; however, it ultimately decreased the energy availability of the diet due to the greater contents of lignin and acid detergent fibre in the treatment diet. Cattle have exhibited intoxication sensitivities to tannins, particularly if diets do not meet nutrient requirements for growth or milk production. However, such issues can be avoided through appropriate dosages and adaptation periods paired with properly formulated diets (Doce et al. 2013).
Flavonoids
Flavonoids are not known to have extensive CH4 reduction potential, but anti-microbial properties of the compounds have been reviewed (Patra and Saxena 2010). Several in vitro trials (Oskoueian et al. 2013; Kim et al. 2015) have been conducted to gain a better understanding of antimicrobial characteristics and its relation to methanogenesis, but studies utilising in vivo models are scarce. Kim et al. (2015) studied the mitigation potential of four plants containing flavonoids in vitro, by using rumen fluid sourced from a single cow. In all treatments, CH4 production was reduced by 39–48%; however, results such as this have not yet been translated into animal models. Flavonoids derived from mulberry leaves (~1.3 g/kg DM) did not influence methanogenesis to a detectable level in sheep, but they increased digestibility (Chen et al. 2015). Rutin trihydrate, a flavonoid, was given to dairy cattle at a dose of 100 mg/kg bodyweight, which led to an elevated plasma glucose, β-hydroxybutyrate and albumin, but did not suppress CH4 production (Stoldt et al. 2016).
Saponins
Saponins have been studied for their capacity to alter rumen fermentation by reducing protozoal communities, thus lowering H2 availability and the production of CH4 (Hess et al. 2003). Saponins are commonly found in low quantities in legume plants such as kidney beans, soya beans, chickpeas and green peas (Shi et al. 2004). Holtshausen et al. (2009) conducted a two-part study on saponins derived from Yucca schidigera and Quillaja saponaria and their effect on CH4 production in vitro and in vivo. Inclusions of 15, 30, or 45 g/kg DM of Y. schidigera and Q. saponaria decreased CH4 production ranging from 6 to 26% in vitro. However, in vivo study in dairy cattle using whole-plant Y. schidigera and Q. saponaria powders at 10 g/kg of DM did not show an impact on rumen fermentation. Cross-bred cattle supplemented with soapnut, a saponin-containing plant, did not have significant reductions in CH4 production (Poornachandra et al. 2019). Tea saponins offered to ewes led to a decrease in CH4 production if scaled to metabolic weight; otherwise, no differences were observed in absolute values (Liu et al. 2019). The same supplement was offered to steers at 2.44 and 3.85 g/kg DM, but no impact on gas output was observed (Ramírez-Restrepo et al. 2016). Lack of results in reducing CH4 production may be linked to low concentrations of saponins within additives. However, in some circumstances, high concentrations of saponins have been linked to bloat through foaming properties, but no strong conclusions have been drawn (Lindahl et al. 1954; Sen et al. 1998). Low-level inclusions may have antiprotozoal and mild antibacterial characteristics and can be incorporated into livestock diets through a variety of plant options.
Essential oils
Essential oils (EO) are naturally occurring chemical compounds extracted from plants and used in fragrances and cosmetics and, to a lesser extent, pharmaceutical products for humans and animals. Volatile in nature, the EO contribute to the phenotypic expression of the plant including colour and scent (Edris 2007; Benchaar et al. 2008). Consumption of EO has been observed to affect rumen microbial communities and fermentation patterns in a varying manner, depending on the EO source (Benchaar and Greathead 2011). Many EO hold a high affinity for lipid and bacterial membranes, leading to disruption, but the broad antimicrobial effect is likely to be due to a combination of mechanisms (Helander et al. 1998). EO are non-specific in nature; therefore, there is a concern for their inclusion in diets because they may affect favourable microbe populations, leading to a decrease in feed efficiency. Numerous plants such as cinnamon, lemongrass, ginger, garlic, juniper berries, eucalyptus, thyme, citrus, oregano, mint, rosemary and coriander have been screened in vitro (Becnhaar et al. 2008; Nanon et al. 2015). However, only few have been studied in vivo. Some studies include the whole plant (Olijhoek et al. 2019) into a diet, while other extract the EO before inclusion in a more concentrated treatment (Lejonklev et al. 2016), which introduces another level of variability.
Oregano contains EO compounds carvacrol and thymol that may stimulate general antimicrobial properties in the rumen (Kolling et al. 2018). Only two in vivo studies (Tekippe et al. 2011; Hristov et al. 2013) have shown reduction of CH4 production of up to 40% in dairy cattle. Hristov et al. (2013) did not observe any adverse effects of supplementation (8.7, 18.9 and 28.2 g Origanum vulgare leaves/kg DM) on feed efficiency, rumen pH or VFA concentrations. In contrast, several other studies have shown no significant impact of supplementing oregano on CH4 production. For example, lactating dairy cattle supplemented with oregano oil and carvacrol at 0.05 g/kg DM did not express any anti-methanogenic properties (Benchaar 2020). Kolling et al. (2018) reported a reduction in CH4 yield (in g/kg digestible DMI), but no reductions surrounding other CH4 emission parameters such as protozoal count, by using 0.56 g oregano extract/kg DM in lactating dairy cattle. Olijhoek et al. (2019) reported no significant reduction in dairy cows supplemented with either 18–53 g oregano plant/kg DM from Origanum vulgare ssp. vulgare containing 0.12% EO of oregano DM, or 7–21 g oregano DM/kg of DM from Origanum vulgare ssp. hirtum containing 4.21% EO of oregano DM. The authors speculated that the differences in reported effectiveness could be related to the duration of measurement (1–8 h post intake in those that reported reductions vs >24 h in studies with no effect). The observation by Hristov et al. (2013) who reported a linear decline in effectiveness after feed intake lends support to measurement duration contributing to differences in reported effectiveness.
Garlic (Allium sativum) contains organosulfur compounds, specifically diallyl disulfide, as its main EO component. Organosulfur compounds are suspected of having a toxic effect on the enzyme system of the methanogenic archaea, inhibiting their activity, while also suppressing protozoal populations (Busquet et al. 2005a; Soliva et al. 2011). Soliva et al. (2011) reported a 91% reduction in CH4 with 300 mg/L garlic oil in vitro, associated with an increase in bacterial counts and reduction in protozoa. Similarly, Busquet et al. (2005a) observed a 73.6% reduction in CH4 production in vitro by using similar concentrations of garlic oil. However, most in vivo cattle studies have not found an impact of garlic oil on CH4 production. For example, van Zijderveld et al. (2011b) used diallyl disulfide at 0.056 g/kg DM in dairy cattle and observed no reduction in CH4 production. Staerfl et al. (2012) using dried garlic bulbs (treatment standardised for 15 g allicin/kg DM) in feedlot cattle reported no significant effect on CH4 production measured at 5, 9 and 11 months of age. Similarly, Meale et al. (2014) reported no detectable differences in enteric CH4 or CO2 production in animals supplemented with garlic oil (15 g allicin/kg DM). Sheep models have reported similar results of no detectable difference in enteric CH4 production (Patra et al. 2011; Klevenhusen et al. 2011); however, goat models supplemented with L propyl–propane–thiosulfinate, another organosulfur compound found in garlic, suppressed CH4 production by roughly 33% (Martinez-Fernandez et al. 2013). Nevertheless, in their subsequent experiment, Martinez-Fernandez et al. (2014), using the same compound in goats in vivo, did not find a significant reduction in enteric CH4 production.
Lemongrass (Cymbopogon spp.) has been assessed in vitro for potential antimicrobial effects due to citral, an aldehyde sourced from the EO fraction contributing to aromatic characteristic of the plant (Pawar et al. 2014; Joch et al. 2016; Singh et al. 2018). While CH4 was not measured, Wanapat et al. (2008) detected an improvement in microbial protein supply, DM digestibility and microbial populations when Brahman-native beef cattle consumed 18.5 g lemongrass powder/kg DM. In lactating Barki goats, 4 g/kg DM elicited a slight increase in protozoal counts and CH4 production (Khattab et al. 2017).
Supplementing cinnamaldehyde and cinnamon oil (containing 78% cinnamaldehyde) to dairy cattle diet at inclusion rates ranging from 0.003 to 0.16 g/kg did not reduce CH4 production (Benchaar 2016). Methanogen numbers decreased in a study adding 0.5 g/kg DM of cinnamon oil, but the study did not measure gases directly, so any CH4 reduction was speculative (Khorrami et al. 2015). Eugenol, an active EO component of cinnamon, was added to diets at 0.025, 0.050 or 0.075 g/kg DM, but no treatment group demonstrated a difference in enteric CH4 compared with the control (Benchaar et al. 2015). Shifts away from acetate production and towards propionogenesis have been observed in artificial conditions when cinnamon-sourced additives were introduced (Busquet et al. 2005b). Inclusion of EO in livestock diets has not rendered any safety concerns for animal husbandry or consumption of subsequent products.
Essential oil blends
Taking advantage of the unique composition among plants, some studies have used an EO ‘blend’ or ‘complex’ containing extracts from multiple plants. The antimicrobial nature of a variety of the EO may imply a capacity to modify rumen fermentation. EO blends have demonstrated a greater feed efficiency and a higher production of energy-corrected milk in dairy cattle through modification of rumen fermentation (Elcoso et al. 2019; Silva et al. 2020). Blends have become commercially available, typically containing at least two different EO. For example, Agolin Ruminant (Agolin, Bière, Switzerland; AR) contains a blend of eugenol, geranyl acetate and coriander EO. Agolin Ruminant is an antimicrobial EO product and has shown 20% reduction in CH4 intensity in dairy cattle (Hart et al. 2019). Klop et al. (2017) alternated AR (0.17 g/kg DM) with lauric acid (0.65 g/kg DM) for 2-week periods over 10 weeks, but CH4 production was not altered. Elcoso et al. (2019) estimated 15% lower CH4 production in lactating dairy cattle consuming AR. Castro-Montoya et al. (2015) fed 0.0128 and 0.0240 g AR/kg DM to dairy and beef cattle respectively, but detected only tendencies towards CH4 reduction in both groups, with no significant differences occurring.
Mootral© is synthesised from natural products including garlic- and flavonoid-containing citrus extract and has demonstrated anti-methanogenic properties (Eger et al. 2018; Roque et al. 2019c; Vrancken et al. 2019). The garlic component in Mootral© targets methanogenic archaea populations and protozoal communities in the rumen and has led to nearly complete inhibition of CH4 production in vitro at a dosage of 2 g experimental mixture/day, without compromising bacterial population (Eger et al. 2018). The experimental mix contained 1.5% (w/w) allicin and 45% (w/w) polyphenolics (Eger et al. 2018). A 23.2% decrease in CH4 yield (26.8% expressed in CH4 production) was observed in Angus × Hereford crosses after 12 weeks of treatment by supplementing Mootral© at 1.58 g/kg DM (Roque et al. 2019c). Adverse effects on DMI, ADG and feed efficiency were not detected over the 12-week trial. Lactating cattle offered Mootral incorporated in pellets at a rate of 0.640 g/kg DM for Holstein-Friesian and 1.21 g/kg DM for Jersey herd experienced suppression of CH4 of 20.7% and 38.3% respectively (Vrancken et al. 2019). Additionally, 3–5% increase in milk yield across breeds was observed with increased feed efficiency in the Jersey cattle. Further research is required to determine the effective dose and magnitude of reduction from ruminants supplemented with Mootral©.
Conclusions
Several feed additives provide a promising option that could increase the sustainability of animal-sourced foods by substantially reducing enteric CH4 emissions. Rumen inhibitors have shown potential of up to 98% reduction in enteric CH4 production, although they differ in accessibility and risk to animal welfare. Although none of the inhibitors are currently on the market, on the basis of the volume of available literature, 3NOP may be offered to producers in the near future, with nitrate and microalgae to follow after further research. Rumen modifiers including EO, tannins, saponins, biochar and lipids can be sourced globally but vary in composition and are not always effective. Consistency is a factor to consider with plant-based feed additives, but it can be addressed, as demonstrated, in commercial applications such as Mootral© and Agolin Ruminant. Direct-fed microbials or probiotics have not demonstrated strong evidence to be considered a rumen modifier to suppress CH4 production. Due to increased interest in this area, research is expected to accelerate in production of feed additives that reduce enteric CH4 production.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
We are grateful to the California Air Resources Board for supporting the study under Project #17RD018.
References
Animut G, Puchala R, Goetsch AL, Patra AK, Sahlu T, Varel VH, Wells J (2008) Methane emission by goats consuming diets with different levels of condensed tannins from lespedeza. Animal Feed Science and Technology 144, 212–227.| Methane emission by goats consuming diets with different levels of condensed tannins from lespedeza.Crossref | GoogleScholarGoogle Scholar |
Appuhamy JRN, Strathe AB, Jayasundara S, Wagner-Riddle C, Dijkstra J, France J, Kebreab E (2013) Anti-methanogenic effects of monensin in dairy and beef cattle: a meta-analysis. Journal of Dairy Science 96, 5161–5173.
| Anti-methanogenic effects of monensin in dairy and beef cattle: a meta-analysis.Crossref | GoogleScholarGoogle Scholar |
Asanuma N, Yokoyama S, Hino T (2015) Effects of nitrate addition to a diet on fermentation and microbial populations in the rumen of goats, with special reference to Selenomonas ruminantium having the ability to reduce nitrate and nitrite. Animal Science Journal 86, 378–384.
| Effects of nitrate addition to a diet on fermentation and microbial populations in the rumen of goats, with special reference to Selenomonas ruminantium having the ability to reduce nitrate and nitrite.Crossref | GoogleScholarGoogle Scholar | 25439583PubMed |
Banerjee R, Ragsdale SW (2003) The many faces of vitamin B12: catalysis by cobalamin-dependent enzymes. Annual Review of Biochemistry 72, 209–247.
| The many faces of vitamin B12: catalysis by cobalamin-dependent enzymes.Crossref | GoogleScholarGoogle Scholar | 14527323PubMed |
Bauchop T, Mountfort DO (1981) Cellulose fermentation by a rumen anaerobic fungus in both the absence and the presence of rumen methanogens. Applied and Environmental Microbiology 42, 1103–1110.
| Cellulose fermentation by a rumen anaerobic fungus in both the absence and the presence of rumen methanogens.Crossref | GoogleScholarGoogle Scholar | 16345902PubMed |
Bayat AR, Kairenius P, Stefański T, Leskinen H, Comtet-Marre S, Forano E, Chaucheyras-Durand F, Shingfield KJ (2015) Effect of camelina oil or live yeasts (Saccharomyces cerevisiae) on ruminal methane production, rumen fermentation, and milk fatty acid composition in lactating cows fed grass silage diets. Journal of Dairy Science 98, 3166–3181.
| Effect of camelina oil or live yeasts (Saccharomyces cerevisiae) on ruminal methane production, rumen fermentation, and milk fatty acid composition in lactating cows fed grass silage diets.Crossref | GoogleScholarGoogle Scholar | 25726099PubMed |
Beauchemin KA, Kreuzer M, O’mara F, McAllister TA (2008) Nutritional management for enteric methane abatement: a review Australian Journal of Experimental Agriculture 48, 21–27.
| Nutritional management for enteric methane abatement: a reviewCrossref | GoogleScholarGoogle Scholar |
Beauchemin KA, McAllister TA, McGinn SM (2009a) Dietary mitigation of enteric methane from cattle. Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources 4, 1–18.
| Dietary mitigation of enteric methane from cattle.Crossref | GoogleScholarGoogle Scholar |
Beauchemin KA, McGinn SM, Benchaar C, Holtshausen L (2009b) Crushed sunflower, flax, or canola seeds in lactating dairy cow diets: effects on methane production, rumen fermentation, and milk production. Journal of Dairy Science 92, 2118–2127.
| Crushed sunflower, flax, or canola seeds in lactating dairy cow diets: effects on methane production, rumen fermentation, and milk production.Crossref | GoogleScholarGoogle Scholar | 19389969PubMed |
Benchaar C (2016) Diet supplementation with cinnamon oil, cinnamaldehyde, or monensin does not reduce enteric methane production of dairy cows. Animal 10, 418–425.
| Diet supplementation with cinnamon oil, cinnamaldehyde, or monensin does not reduce enteric methane production of dairy cows.Crossref | GoogleScholarGoogle Scholar | 26888487PubMed |
Benchaar C (2020) Feeding oregano oil and its main component carvacrol does not affect ruminal fermentation, nutrient utilization, methane emissions, milk production, or milk fatty acid composition of dairy cows. Journal of Dairy Science 103, 1516–1527.
| Feeding oregano oil and its main component carvacrol does not affect ruminal fermentation, nutrient utilization, methane emissions, milk production, or milk fatty acid composition of dairy cows.Crossref | GoogleScholarGoogle Scholar | 31759586PubMed |
Benchaar C, Greathead H (2011) Essential oils and opportunities to mitigate enteric methane emissions from ruminants. Animal Feed Science and Technology 166, 338–355.
| Essential oils and opportunities to mitigate enteric methane emissions from ruminants.Crossref | GoogleScholarGoogle Scholar |
Benchaar C, Calsamiglia S, Chaves AV, Fraser GR, Colombatto D, McAllister TA, Beauchemin KA (2008) A review of plant-derived essential oils in ruminant nutrition and production. Animal Feed Science and Technology 145, 209–228.
Benchaar C, Hassanat F, Petit HV (2015) Dose–response to eugenol supplementation to dairy cow diets: methane production, N excretion, ruminal fermentation, nutrient digestibility, milk production, and milk fatty acid profile. Animal Feed Science and Technology 209, 51–59.
| Dose–response to eugenol supplementation to dairy cow diets: methane production, N excretion, ruminal fermentation, nutrient digestibility, milk production, and milk fatty acid profile.Crossref | GoogleScholarGoogle Scholar |
Béra-Maillet C, Ribot Y, Forano E (2004) Fiber-degrading systems of different strains of the genus Fibrobacter. Applied and Environmental Microbiology 70, 2172–2179.
| Fiber-degrading systems of different strains of the genus Fibrobacter.Crossref | GoogleScholarGoogle Scholar | 15066810PubMed |
Bergman EN (1990) Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiological Reviews 70, 567–590.
| Energy contributions of volatile fatty acids from the gastrointestinal tract in various species.Crossref | GoogleScholarGoogle Scholar | 2181501PubMed |
Bruning-Fann CS, Kaneene JB (1993) The effects of nitrate, nitrite and N-nitroso compounds on human health: a review. Veterinary and Human Toxicology 35, 521–538.
Busquet M, Calsamiglia S, Ferret A, Carro MD, Kamel C (2005a) Effect of garlic oil and four of its compounds on rumen microbial fermentation. Journal of Dairy Science 88, 4393–4404.
| Effect of garlic oil and four of its compounds on rumen microbial fermentation.Crossref | GoogleScholarGoogle Scholar | 16291631PubMed |
Busquet M, Calsamiglia S, Ferret A, Cardozo PW, Kamel C (2005b) Effects of cinnamaldehyde and garlic oil on rumen microbial fermentation in a dual flow continuous culture. Journal of Dairy Science 88, 2508–2516.
| Effects of cinnamaldehyde and garlic oil on rumen microbial fermentation in a dual flow continuous culture.Crossref | GoogleScholarGoogle Scholar | 15956313PubMed |
Caetano M, Wilkes MJ, Pitchford WS, Lee SJ, Hynd PI (2019) Effect of ensiled crimped grape marc on energy intake, performance and gas emissions of beef cattle. Animal Feed Science and Technology 247, 166–172.
| Effect of ensiled crimped grape marc on energy intake, performance and gas emissions of beef cattle.Crossref | GoogleScholarGoogle Scholar |
Castro-Montoya J, Peiren N, Cone JW, Zweifel B, Fievez V, De Campeneere S (2015) In vivo and in vitro effects of a blend of essential oils on rumen methane mitigation. Livestock Science 180, 134–142.
| In vivo and in vitro effects of a blend of essential oils on rumen methane mitigation.Crossref | GoogleScholarGoogle Scholar |
Chalupa W (1977) Manipulating rumen fermentation. Journal of Animal Science 45, 585–599.
| Manipulating rumen fermentation.Crossref | GoogleScholarGoogle Scholar |
Chen D, Chen X, Tu Y, Wang B, Lou C, Ma T, Diao Q (2015) Effects of mulberry leaf flavonoid and resveratrol on methane emission and nutrient digestion in sheep. Animal Nutrition 1, 362–367.
| Effects of mulberry leaf flavonoid and resveratrol on methane emission and nutrient digestion in sheep.Crossref | GoogleScholarGoogle Scholar | 29767046PubMed |
Chen J, Harstad OM, McAllister T, Dörsch P, Holo H (2020) Propionic acid bacteria enhance ruminal feed degradation and reduce methane production in vitro. Acta Agriculturæ Scandinavica. Section A, Animal Science 69, 169–175.
| Propionic acid bacteria enhance ruminal feed degradation and reduce methane production in vitro.Crossref | GoogleScholarGoogle Scholar |
Cottle DJ, Nolan JV, Wiedemann SG (2011) Ruminant enteric methane mitigation: a review. Animal Production Science 51, 491–514.
| Ruminant enteric methane mitigation: a review.Crossref | GoogleScholarGoogle Scholar |
Darabighane B, Salem AZM, Aghjehgheshlagh FM, Mahdavi A, Zarei A, Elghandour MMMY, López S (2019) Environmental efficiency of Saccharomyces cerevisiae on methane production in dairy and beef cattle via a meta-analysis. Environmental Science and Pollution Research International 26, 3651–3658.
| Environmental efficiency of Saccharomyces cerevisiae on methane production in dairy and beef cattle via a meta-analysis.Crossref | GoogleScholarGoogle Scholar | 30535735PubMed |
Dijkstra J, Bannink A, France J, Kebreab E, van Gastelen S (2018) Antimethanogenic effects of 3-nitrooxypropanol depend on supplementation dose, dietary fiber content, and cattle type. Journal of Dairy Science 101, 9041–9047.
| Antimethanogenic effects of 3-nitrooxypropanol depend on supplementation dose, dietary fiber content, and cattle type.Crossref | GoogleScholarGoogle Scholar | 30055923PubMed |
Doce RR, Belenguer A, Toral PG, Hervás G, Frutos P (2013) Effect of the administration of young leaves of Quercus pyrenaica on rumen fermentation in relation to oak tannin toxicosis in cattle. Journal of Animal Physiology and Animal Nutrition 97, 48–57.
| Effect of the administration of young leaves of Quercus pyrenaica on rumen fermentation in relation to oak tannin toxicosis in cattle.Crossref | GoogleScholarGoogle Scholar | 21992033PubMed |
Dong Y, Bae HD, McAllister TA, Mathison GW, Cheng KJ (1997) Lipid-induced depression of methane production and digestibility in the artificial rumen system (RUSITEC). Canadian Journal of Animal Science 77, 269–278.
| Lipid-induced depression of methane production and digestibility in the artificial rumen system (RUSITEC).Crossref | GoogleScholarGoogle Scholar |
Doyle N, Mbandlwa P, Kelly WJ, Attwood G, Li Y, Ross RP, Stanton C, Leahy S (2019) Use of lactic acid bacteria to reduce methane production in ruminants, a critical review. Frontiers in Microbiology 10, 2207
| Use of lactic acid bacteria to reduce methane production in ruminants, a critical review.Crossref | GoogleScholarGoogle Scholar | 31632365PubMed |
Duin EC, Wagner T, Shima S, Prakash D, Cronin B, Yáñez-Ruiz DR, Duval S, Rümbeli R, Stemmler RT, Thauer RK, Kindermann M (2016) Mode of action uncovered for the specific reduction of methane emissions from ruminants by the small molecule 3-nitrooxypropanol. Proceedings of the National Academy of Sciences of the United States of America 113, 6172–6177.
| Mode of action uncovered for the specific reduction of methane emissions from ruminants by the small molecule 3-nitrooxypropanol.Crossref | GoogleScholarGoogle Scholar | 27140643PubMed |
Duthie CA, Troy SM, Hyslop JJ, Ross DW, Roehe R, Rooke JA (2018) The effect of dietary addition of nitrate or increase in lipid concentrations, alone or in combination, on performance and methane emissions of beef cattle. Animal 12, 280–287.
| The effect of dietary addition of nitrate or increase in lipid concentrations, alone or in combination, on performance and methane emissions of beef cattle.Crossref | GoogleScholarGoogle Scholar | 28701247PubMed |
Edris AE (2007) Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: a review. Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives 21, 308–323.
| Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: a review.Crossref | GoogleScholarGoogle Scholar |
Eger M, Graz M, Riede S, Breves G (2018) Application of MootralTM reduces methane production by altering the archaea community in the rumen simulation technique. Frontiers in Microbiology 9, 2094
| Application of MootralTM reduces methane production by altering the archaea community in the rumen simulation technique.Crossref | GoogleScholarGoogle Scholar | 30233557PubMed |
Elcoso G, Zweifel B, Bach A (2019) Effects of a blend of essential oils on milk yield and feed efficiency of lactating dairy cows. Applied Animal Science 35, 304–311.
| Effects of a blend of essential oils on milk yield and feed efficiency of lactating dairy cows.Crossref | GoogleScholarGoogle Scholar |
Ellis JL, Dijkstra J, Kebreab E, Bannink A, Odongo NE, McBride BW, France J (2008) Aspects of rumen microbiology central to mechanistic modelling of methane production in cattle. The Journal of Agricultural Science 146, 213–233.
| Aspects of rumen microbiology central to mechanistic modelling of methane production in cattle.Crossref | GoogleScholarGoogle Scholar |
Eugène M, Massé D, Chiquette J, Benchaar C (2008) Meta-analysis on the effects of lipid supplementation on methane production in lactating dairy cows. Canadian Journal of Animal Science 88, 331–337.
| Meta-analysis on the effects of lipid supplementation on methane production in lactating dairy cows.Crossref | GoogleScholarGoogle Scholar |
Feng Y, Xu Y, Yu Y, Xie Z, Lin X (2012) Mechanisms of biochar decreasing methane emission from Chinese paddy soils. Soil Biology & Biochemistry 46, 80–88.
| Mechanisms of biochar decreasing methane emission from Chinese paddy soils.Crossref | GoogleScholarGoogle Scholar |
Feng XY, Dijkstra J, Bannink A, van Gastelen S, France J, Kebreab E (2020) Anti-methanogenic effects of nitrate supplementation in cattle: a meta-analysis. Journal of Dairy Science 103, 11375–11385.
| Anti-methanogenic effects of nitrate supplementation in cattle: a meta-analysis.Crossref | GoogleScholarGoogle Scholar | 32981733PubMed |
Figueiredo AC, Barroso JG, Pedro LG, Scheffer JJ (2008) Factors affecting secondary metabolite production in plants: volatile components and essential oils. Flavour and Fragrance Journal 23, 213–226.
| Factors affecting secondary metabolite production in plants: volatile components and essential oils.Crossref | GoogleScholarGoogle Scholar |
Gagen EJ, Wang J, Padmanabha J, Liu J, de Carvalho IPC, Liu J, Webb RI, Al Jassim R, Morrison M, Denman SE, McSweeney CS (2014) Investigation of a new acetogen isolated from an enrichment of the tammar wallaby forestomach. BMC Microbiology 14, 314
| Investigation of a new acetogen isolated from an enrichment of the tammar wallaby forestomach.Crossref | GoogleScholarGoogle Scholar | 25495654PubMed |
Gerber PJ, Steinfeld H, Henderson B, Mottet A, Opio C, Dijkman J, Falcucci A, Tempio G (2013) ‘Tackling climate change through livestock: a global assessment of emissions and mitigation opportunities.’ (Food and Agriculture Organization of the United Nations (FAO): Rome, Italy)
Goel G, Makkar HP, Becker K (2009) Inhibition of methanogens by bromochloromethane: effects on microbial communities and rumen fermentation using batch and continuous fermentations. British Journal of Nutrition 101, 1484–1492.
| Inhibition of methanogens by bromochloromethane: effects on microbial communities and rumen fermentation using batch and continuous fermentations.Crossref | GoogleScholarGoogle Scholar |
Granja-Salcedo YT, Fernandes RM, Araujo RCD, Kishi LT, Berchielli TT, Resende FDD, Berndt A, Siqueira GR (2019) Long-term encapsulated nitrate supplementation modulates rumen microbial diversity and rumen fermentation to reduce methane emission in grazing steers. Frontiers in Microbiology 10, 614
| Long-term encapsulated nitrate supplementation modulates rumen microbial diversity and rumen fermentation to reduce methane emission in grazing steers.Crossref | GoogleScholarGoogle Scholar | 30984141PubMed |
Gribble GW (2004) Amazing organohalogens: although best known as synthetic toxicants, thousands of halogen compounds are, in fact, part of our natural environment. American Scientist 92, 342–349.
| Amazing organohalogens: although best known as synthetic toxicants, thousands of halogen compounds are, in fact, part of our natural environment.Crossref | GoogleScholarGoogle Scholar |
Guan H, Wittenberg KM, Ominski KH, Krause DO (2006) Efficacy of ionophores in cattle diets for mitigation of enteric methane. Journal of Animal Science 84, 1896–1906.
| Efficacy of ionophores in cattle diets for mitigation of enteric methane.Crossref | GoogleScholarGoogle Scholar | 16775074PubMed |
Guyader J, Eugène M, Meunier B, Doreau M, Morgavi DP, Silberberg M, Rochette Y, Gerard C, Loncke C, Martin C (2015a) Additive methane-mitigating effect between linseed oil and nitrate fed to cattle. Journal of Animal Science 93, 3564–3577.
| Additive methane-mitigating effect between linseed oil and nitrate fed to cattle.Crossref | GoogleScholarGoogle Scholar | 26440025PubMed |
Haisan J, Sun Y, Guan LL, Beauchemin KA, Iwaasa A, Duval S, Barreda DR, Oba M (2014) The effects of feeding 3-nitrooxypropanol on methane emissions and productivity of Holstein cows in mid lactation. Journal of Dairy Science 97, 3110–3119.
| The effects of feeding 3-nitrooxypropanol on methane emissions and productivity of Holstein cows in mid lactation.Crossref | GoogleScholarGoogle Scholar | 24630651PubMed |
Hart KJ, Jones HG, Waddams KE, Worgan HJ, Zweifel B, Newbold CJ (2019) An essential oil blend decreases methane emissions and increases milk yield in dairy cows. Open Journal of Animal Sciences 9, 259
| An essential oil blend decreases methane emissions and increases milk yield in dairy cows.Crossref | GoogleScholarGoogle Scholar |
Helander IM, Alakomi HL, Latva-Kala K, Mattila-Sandholm T, Pol I, Smid EJ, Gorris LG, von Wright A (1998) Characterization of the action of selected essential oil components on Gram-negative bacteria. Journal of Agricultural and Food Chemistry 46, 3590–3595.
| Characterization of the action of selected essential oil components on Gram-negative bacteria.Crossref | GoogleScholarGoogle Scholar |
Hess HD, Monsalve LM, Lascano CE, Carulla JE, Diaz TE, Kreuzer M (2003) Supplementation of a tropical grass diet with forage legumes and Sapindus saponaria fruits: effects on in vitro ruminal nitrogen turnover and methanogenesis. Australian Journal of Agricultural Research 54, 703–713.
| Supplementation of a tropical grass diet with forage legumes and Sapindus saponaria fruits: effects on in vitro ruminal nitrogen turnover and methanogenesis.Crossref | GoogleScholarGoogle Scholar |
Hollmann M, Powers WJ, Fogiel AC, Liesman JS, Bello NM, Beede DK (2012) Enteric methane emissions and lactational performance of Holstein cows fed different concentrations of coconut oil. Journal of Dairy Science 95, 2602–2615.
| Enteric methane emissions and lactational performance of Holstein cows fed different concentrations of coconut oil.Crossref | GoogleScholarGoogle Scholar | 22541489PubMed |
Holtshausen L, Chaves AV, Beauchemin KA, McGinn SM, McAllister TA, Odongo NE, Cheeke PR, Benchaar C (2009) Feeding saponin-containing Yucca schidigera and Quillaja saponaria to decrease enteric methane production in dairy cows. Journal of Dairy Science 92, 2809–2821.
| Feeding saponin-containing Yucca schidigera and Quillaja saponaria to decrease enteric methane production in dairy cows.Crossref | GoogleScholarGoogle Scholar | 19448015PubMed |
Hook SE, Wright ADG, McBride BW (2010) Methanogens: methane producers of the rumen and mitigation strategies. Archaea 2010, 945785
| Methanogens: methane producers of the rumen and mitigation strategies.Crossref | GoogleScholarGoogle Scholar | 21253540PubMed |
Hristov AN, Vander Pol M, Agle M, Zaman S, Schneider C, Ndegwa P, Vaddella VK, Johnson K, Shingfield KJ, Karnati SKR (2009) Effect of lauric acid and coconut oil on ruminal fermentation, digestion, ammonia losses from manure, and milk fatty acid composition in lactating cows. Journal of Dairy Science 92, 5561–5582.
| Effect of lauric acid and coconut oil on ruminal fermentation, digestion, ammonia losses from manure, and milk fatty acid composition in lactating cows.Crossref | GoogleScholarGoogle Scholar | 19841218PubMed |
Hristov AN, Lee C, Cassidy TT, Heyler K, Tekippe JA, Varga GA, Corl B, Brandt RC (2013) Effect of Origanum vulgare L. leaves on rumen fermentation, production, and milk fatty acid composition in lactating dairy cows. Journal of Dairy Science 96, 1189–1202.
Hristov AN, Oh J, Giallongo F, Frederick TW, Harper MT, Weeks HL, Branco AF, Moate PJ, Deighton MH, Williams SRO, Kindermann M (2015) An inhibitor persistently decreased enteric methane emission from dairy cows with no negative effect on milk production. Proceedings of the National Academy of Sciences of the United States of America 112, 10663–10668.
| An inhibitor persistently decreased enteric methane emission from dairy cows with no negative effect on milk production.Crossref | GoogleScholarGoogle Scholar | 26229078PubMed |
Hulshof RBA, Berndt A, Gerrits WJJ, Dijkstra J, Van Zijderveld SM, Newbold JR, Perdok HB (2012) Dietary nitrate supplementation reduces methane emission in beef cattle fed sugarcane-based diets. Journal of Animal Science 90, 2317–2323.
| Dietary nitrate supplementation reduces methane emission in beef cattle fed sugarcane-based diets.Crossref | GoogleScholarGoogle Scholar |
Hungate RE, Smith W, Bauchop T, Yu I, Rabinowitz JC (1970) Formate as an intermediate in the bovine rumen fermentation. Journal of Bacteriology 102, 389–397.
| Formate as an intermediate in the bovine rumen fermentation.Crossref | GoogleScholarGoogle Scholar | 5419259PubMed |
IPCC (2013) ‘Climate change: the physical science basis.’ Working Group I contribution to the IPCC fifth assessment report. (IPCC: Cambridge, UK)
Iwamoto M, Asanuma N, Hino T (2002) Ability of Selenomonas ruminantium, Veillonella parvula, and Wolinella succinogenes to reduce nitrate and nitrite with special reference to the suppression of ruminal methanogenesis. Anaerobe 8, 209–215.
| Ability of Selenomonas ruminantium, Veillonella parvula, and Wolinella succinogenes to reduce nitrate and nitrite with special reference to the suppression of ruminal methanogenesis.Crossref | GoogleScholarGoogle Scholar |
Jayanegara A, Leiber F, Kreuzer M (2012) Meta‐analysis of the relationship between dietary tannin level and methane formation in ruminants from in vivo and in vitro experiments. Journal of Animal Physiology and Animal Nutrition 96, 365–375.
| Meta‐analysis of the relationship between dietary tannin level and methane formation in ruminants from in vivo and in vitro experiments.Crossref | GoogleScholarGoogle Scholar | 21635574PubMed |
Jayanegara A, Sarwono KA, Kondo M, Matsui H, Ridla M, Laconi EB, Nahrowi (2018) Use of 3-nitrooxypropanol as feed additive for mitigating enteric methane emissions from ruminants: a meta-analysis. Italian Journal of Animal Science 17, 650–656.
| Use of 3-nitrooxypropanol as feed additive for mitigating enteric methane emissions from ruminants: a meta-analysis.Crossref | GoogleScholarGoogle Scholar |
Jeyanathan J, Martin C, Eugène M, Ferlay A, Popova M, Morgavi DP (2019) Bacterial direct-fed microbials fail to reduce methane emissions in primiparous lactating dairy cows. Journal of Animal Science and Biotechnology 10, 41
| Bacterial direct-fed microbials fail to reduce methane emissions in primiparous lactating dairy cows.Crossref | GoogleScholarGoogle Scholar | 31069075PubMed |
Joblin KN (1999) Ruminal acetogens and their potential to lower ruminant methane emissions. Australian Journal of Agricultural Research 50, 1307–1314.
| Ruminal acetogens and their potential to lower ruminant methane emissions.Crossref | GoogleScholarGoogle Scholar |
Joch M, Cermak L, Hakl J, Hucko B, Duskova D, Marounek M (2016) In vitro screening of essential oil active compounds for manipulation of rumen fermentation and methane mitigation. Asian–Australasian Journal of Animal Sciences 29, 952
| In vitro screening of essential oil active compounds for manipulation of rumen fermentation and methane mitigation.Crossref | GoogleScholarGoogle Scholar | 26954157PubMed |
Johnson KA, Johnson DE (1995) Methane emissions from cattle. Journal of Animal Science 73, 2483–2492.
| Methane emissions from cattle.Crossref | GoogleScholarGoogle Scholar | 8567486PubMed |
Kalus K, Koziel JA, Opaliński S (2019) A review of biochar properties and their utilization in crop agriculture and livestock production. Applied Sciences 9, 3494
| A review of biochar properties and their utilization in crop agriculture and livestock production.Crossref | GoogleScholarGoogle Scholar |
Khattab MSA, El-Zaiat HM, El Tawab AA, Matloup OH, Morsy AS, Abdou MM, Ebeid HM, Attia MFA, Sallam SMA (2017) Impact of lemongrass and galangal as feed additives on performance of lactating Barki goats. International Journal of Dairy Science 12, 184–189.
| Impact of lemongrass and galangal as feed additives on performance of lactating Barki goats.Crossref | GoogleScholarGoogle Scholar |
Khorrami B, Vakili AR, Mesgaran MD, Klevenhusen F (2015) Thyme and cinnamon essential oils: potential alternatives for monensin as a rumen modifier in beef production systems. Animal Feed Science and Technology 200, 8–16.
| Thyme and cinnamon essential oils: potential alternatives for monensin as a rumen modifier in beef production systems.Crossref | GoogleScholarGoogle Scholar |
Kim ET, Le Luo Guan SJL, Lee SM, Lee SS, Lee ID, Lee SK, Lee SS (2015) Effects of flavonoid-rich plant extracts on in vitro ruminal methanogenesis, microbial populations and fermentation characteristics. Asian-Australasian Journal of Animal Sciences 28, 530
| Effects of flavonoid-rich plant extracts on in vitro ruminal methanogenesis, microbial populations and fermentation characteristics.Crossref | GoogleScholarGoogle Scholar | 25656200PubMed |
Kim SH, Lee C, Pechtl HA, Hettick JM, Campler MR, Pairis-Garcia MD, Beauchemin KA, Celi P, Duval SM (2019) Effects of 3-nitrooxypropanol on enteric methane production, rumen fermentation, and feeding behavior in beef cattle fed a high-forage or high-grain diet. Journal of Animal Science 97, 2687–2699.
| Effects of 3-nitrooxypropanol on enteric methane production, rumen fermentation, and feeding behavior in beef cattle fed a high-forage or high-grain diet.Crossref | GoogleScholarGoogle Scholar | 31115441PubMed |
Kinley RD, Martinez-Fernandez G, Matthews MK, de Nys R, Magnusson M, Tomkins NW (2020) Mitigating the carbon footprint and improving productivity of ruminant livestock agriculture using a red seaweed. Journal of Cleaner Production 259, 120836
| Mitigating the carbon footprint and improving productivity of ruminant livestock agriculture using a red seaweed.Crossref | GoogleScholarGoogle Scholar |
Klevenhusen F, Zeitz JO, Duval S, Kreuzer M, Soliva CR (2011) Garlic oil and its principal component diallyl disulfide fail to mitigate methane, but improve digestibility in sheep. Animal Feed Science and Technology 166, 356–363.
| Garlic oil and its principal component diallyl disulfide fail to mitigate methane, but improve digestibility in sheep.Crossref | GoogleScholarGoogle Scholar |
Klop G, Dijkstra J, Dieho K, Hendriks WH, Bannink A (2017) Enteric methane production in lactating dairy cows with continuous feeding of essential oils or rotational feeding of essential oils and lauric acid. Journal of Dairy Science 100, 3563–3575.
| Enteric methane production in lactating dairy cows with continuous feeding of essential oils or rotational feeding of essential oils and lauric acid.Crossref | GoogleScholarGoogle Scholar | 28237592PubMed |
Knapp JR, Laur GL, Vadas PA, Weiss WP, Tricarico JM (2014) Invited review: enteric methane in dairy cattle production: quantifying the opportunities and impact of reducing emissions. Journal of Dairy Science 97, 3231–3261.
| Invited review: enteric methane in dairy cattle production: quantifying the opportunities and impact of reducing emissions.Crossref | GoogleScholarGoogle Scholar | 24746124PubMed |
Knight T, Ronimus RS, Dey D, Tootill C, Naylor G, Evans P, Molano G, Smith A, Tavendale M, Pinares-Patino CS, Clark H (2011) Chloroform decreases rumen methanogenesis and methanogen populations without altering rumen function in cattle. Animal Feed Science and Technology 166, 101–112.
| Chloroform decreases rumen methanogenesis and methanogen populations without altering rumen function in cattle.Crossref | GoogleScholarGoogle Scholar |
Kolling GJ, Stivanin SCB, Gabbi AM, Machado FS, Ferreira AL, Campos MM, Tomich TR, Cunha CS, Dill SW, Pereira LGR, Fischer V (2018) Performance and methane emissions in dairy cows fed oregano and green tea extracts as feed additives. Journal of Dairy Science 101, 4221–4234.
| Performance and methane emissions in dairy cows fed oregano and green tea extracts as feed additives.Crossref | GoogleScholarGoogle Scholar | 29477520PubMed |
Lambie SC, Kelly WJ, Leahy SC, Li D, Reilly K, McAllister TA, Valle ER, Attwood GT, Altermann E (2015) The complete genome sequence of the rumen methanogen Methanosarcina barkeri CM1. Standards in Genomic Sciences 10, 57
| The complete genome sequence of the rumen methanogen Methanosarcina barkeri CM1.Crossref | GoogleScholarGoogle Scholar | 26413197PubMed |
Latham EA, Anderson RC, Pinchak WE, Nisbet DJ (2016) Insights on alterations to the rumen ecosystem by nitrate and nitrocompounds. Frontiers in Microbiology 7, 228
| Insights on alterations to the rumen ecosystem by nitrate and nitrocompounds.Crossref | GoogleScholarGoogle Scholar | 26973609PubMed |
Latham EA, Pinchak WE, Trachsel J, Allen HK, Callaway TR, Nisbet DJ, Anderson RC (2019) Paenibacillus 79R4, a potential rumen probiotic to enhance nitrite detoxification and methane mitigation in nitrate-treated ruminants. The Science of the Total Environment 671, 324–328.
| Paenibacillus 79R4, a potential rumen probiotic to enhance nitrite detoxification and methane mitigation in nitrate-treated ruminants.Crossref | GoogleScholarGoogle Scholar | 30933788PubMed |
Lee C, Beauchemin KA (2014) A review of feeding supplementary nitrate to ruminant animals: nitrate toxicity, methane emissions, and production performance. Canadian Journal of Animal Science 94, 557–570.
| A review of feeding supplementary nitrate to ruminant animals: nitrate toxicity, methane emissions, and production performance.Crossref | GoogleScholarGoogle Scholar |
Lejonklev J, Kidmose U, Jensen S, Petersen MA, Helwing ALF, Mortensen G, Weisbjerg MR, Larsen MK (2016) Effect of oregano and caraway essential oils on the production and flavor of cow milk. Journal of Dairy Science 99, 7898–7903.
| Effect of oregano and caraway essential oils on the production and flavor of cow milk.Crossref | GoogleScholarGoogle Scholar | 27522414PubMed |
Leng RA, Preston TR, Inthapanya S (2012)
Li X, Norman HC, Kinley RD, Laurence M, Wilmot M, Bender H, de Nys R, Tomkins N (2018) Asparagopsis taxiformis decreases enteric methane production from sheep. Animal Production Science 58, 681–688.
| Asparagopsis taxiformis decreases enteric methane production from sheep.Crossref | GoogleScholarGoogle Scholar |
Lindahl IL, Cook AC, Davis RE, Maclay WD (1954) Preliminary investigations on the role of alfalfa saponin in ruminant bloat. Science 119, 157–158.
| Preliminary investigations on the role of alfalfa saponin in ruminant bloat.Crossref | GoogleScholarGoogle Scholar | 13122048PubMed |
Liu Y, Ma T, Chen D, Zhang N, Si B, Deng K, Tu Y, Diao Q (2019) Effects of tea saponin supplementation on nutrient digestibility, methanogenesis, and ruminal microbial flora in Dorper crossbred ewe. Animals (Basel) 9, 29
| Effects of tea saponin supplementation on nutrient digestibility, methanogenesis, and ruminal microbial flora in Dorper crossbred ewe.Crossref | GoogleScholarGoogle Scholar |
Lopes JC, de Matos LF, Harper MT, Giallongo F, Oh J, Gruen D, Ono S, Kindermann M, Duval S, Hristov AN (2016) Effect of 3-nitrooxypropanol on methane and hydrogen emissions, methane isotopic signature, and ruminal fermentation in dairy cows. Journal of Dairy Science 99, 5335–5344.
| Effect of 3-nitrooxypropanol on methane and hydrogen emissions, methane isotopic signature, and ruminal fermentation in dairy cows.Crossref | GoogleScholarGoogle Scholar | 27085412PubMed |
Machado L, Magnusson M, Paul NA, Kinley R, de Nys R, Tomkins N (2016) Identification of bioactives from the red seaweed Asparagopsis taxiformis that promote antimethanogenic activity in vitro. Journal of Applied Phycology 28, 3117–3126.
| Identification of bioactives from the red seaweed Asparagopsis taxiformis that promote antimethanogenic activity in vitro.Crossref | GoogleScholarGoogle Scholar |
Machmüller A, Ossowski DA, Wanner M, Kreuzer M (1998) Potential of various fatty feeds to reduce methane release from rumen fermentation in vitro (Rusitec). Animal Feed Science and Technology 71, 117–130.
| Potential of various fatty feeds to reduce methane release from rumen fermentation in vitro (Rusitec).Crossref | GoogleScholarGoogle Scholar |
Malik PK, Bhatta R, Gagen EJ, Sejian V, Soren NM, Prasad CS (2015) Alternate H2 sinks for reducing rumen methanogenesis. In ‘Climate change impact on livestock: adaptation and mitigation’. (Eds V Sejian, J Gaughan, L Baumgard, C Prasad) pp. 303–320. (Springer: New Delhi, India)
Man KY, Chow KL, Man YB, Mo WY, Wong MH (2020) Use of biochar as feed supplements for animal farming. Critical Reviews in Environmental Science and Technology 1–31.
| Use of biochar as feed supplements for animal farming.Crossref | GoogleScholarGoogle Scholar |
Martin C, Morgavi DP, Doreau M (2010) Methane mitigation in ruminants: from microbe to the farm scale. Animal 4, 351–365.
| Methane mitigation in ruminants: from microbe to the farm scale.Crossref | GoogleScholarGoogle Scholar | 22443940PubMed |
Martinez-Fernandez G, Abecia L, Martín García AI, Ramos Morales E, Hervás G, Molina Alcaide E, Yáñez Ruiz DR (2013) In vitro–in vivo study on the effects of plant compounds on rumen fermentation, microbial abundances and methane emissions in goats. Animal 7, 1925–1934.
| In vitro–in vivo study on the effects of plant compounds on rumen fermentation, microbial abundances and methane emissions in goats.Crossref | GoogleScholarGoogle Scholar | 24237672PubMed |
Martinez-Fernandez G, Abecia L, Ramos-Morales E, Martin-García AI, Molina-Alcaide E, Yáñez-Ruiz DR (2014) Effects of propyl propane thiosulfinate on nutrient utilization, ruminal fermentation, microbial population and methane emissions in goats. Animal Feed Science and Technology 191, 16–25.
| Effects of propyl propane thiosulfinate on nutrient utilization, ruminal fermentation, microbial population and methane emissions in goats.Crossref | GoogleScholarGoogle Scholar |
Martinez-Fernandez G, Denman SE, Yang C, Cheung J, Mitsumori M, McSweeney CS (2016) Methane inhibition alters the microbial community, hydrogen flow, and fermentation response in the rumen of cattle. Frontiers in Microbiology 7, 1122
| Methane inhibition alters the microbial community, hydrogen flow, and fermentation response in the rumen of cattle.Crossref | GoogleScholarGoogle Scholar | 27486452PubMed |
Martinez-Fernandez G, Duval S, Kindermann M, Schirra HJ, Denman SE, McSweeney CS (2018) 3-NOP vs. halogenated compound: methane production, ruminal fermentation and microbial community response in forage fed cattle. Frontiers in Microbiology 9, 1582
| 3-NOP vs. halogenated compound: methane production, ruminal fermentation and microbial community response in forage fed cattle.Crossref | GoogleScholarGoogle Scholar | 30131771PubMed |
McAllister TA, Cheng KJ, Okine EK, Mathison GW (1996) Dietary, environmental and microbiological aspects of methane production in ruminants. Canadian Journal of Animal Science 76, 231–243.
| Dietary, environmental and microbiological aspects of methane production in ruminants.Crossref | GoogleScholarGoogle Scholar |
Meale SJ, Chaves AV, McAllister TA, Iwaasa AD, Yang WZ, Benchaar C (2014) Including essential oils in lactating dairy cow diets: effects on methane emissions1. Animal Production Science 54, 1215–1218.
| Including essential oils in lactating dairy cow diets: effects on methane emissions1.Crossref | GoogleScholarGoogle Scholar |
Moate PJ, Williams SRO, Torok VA, Hannah MC, Ribaux BE, Tavendale MH, Eckard RJ, Jacobs JL, Auldist MJ, Wales WJ (2014) Grape marc reduces methane emissions when fed to dairy cows. Journal of Dairy Science 97, 5073–5087.
| Grape marc reduces methane emissions when fed to dairy cows.Crossref | GoogleScholarGoogle Scholar | 24952778PubMed |
Morgavi DP, Forano E, Martin C, Newbold CJ (2010) Microbial ecosystem and methanogenesis in ruminants. Animal 4, 1024–1036.
| Microbial ecosystem and methanogenesis in ruminants.Crossref | GoogleScholarGoogle Scholar | 22444607PubMed |
Morrissey JP (2009) Biological activity of defence-related plant secondary metabolites. In ‘Plant-derived natural products’. (Eds Osbourne AE, Lanzotti V) pp. 283–299. (Springer: New York, NY, USA)
Mottet A, de Haan C, Falcucci A, Tempio G, Opio C, Gerber P (2017) Livestock: on our plates or eating at our table? A new analysis of the feed/food debate. Global Food Security 14, 1–8.
| Livestock: on our plates or eating at our table? A new analysis of the feed/food debate.Crossref | GoogleScholarGoogle Scholar |
Nadathur SR, Wanasundara JPD, Scanlin L (2017) Proteins in the diet: challenges in feeding the global population. In ‘Sustainable protein sources’. (Eds Nadathur SR, Wanasundara JPD, Scanlin L) pp. 1–19. (Academic Press: San Diego, CA, USA)
Nanon A, Suksombat W, Wen ZY (2015) Use of essential oils for manipulation of rumen microbial fermentation using batch culture. Wetchasan Sattawaphaet 45, 167–180.
National Research Council (2001) ‘Nutrient requirements of dairy cattle.’ 7th revised addition 2001. (National Research Council: Washington, DC)
Nogueira RGS, Perna F, Pereira ASC, Cassiano ECO, Carvalho RF, Rodrigues PHM (2020) Methane mitigation and ruminal fermentation changes in cows fed cottonseed and vitamin E. Scientia Agrícola 77, e20180247
| Methane mitigation and ruminal fermentation changes in cows fed cottonseed and vitamin E.Crossref | GoogleScholarGoogle Scholar |
Odongo NE, Or-Rashid MM, Kebreab E, France J, McBride BW (2007) Effect of supplementing myristic acid in dairy cow rations on ruminal methanogenesis and fatty acid profile in milk. Journal of Dairy Science 90, 1851–1858.
| Effect of supplementing myristic acid in dairy cow rations on ruminal methanogenesis and fatty acid profile in milk.Crossref | GoogleScholarGoogle Scholar | 17369226PubMed |
OECD/FAO (2018) ‘OECD–FAO agricultural outlook 2018–2027.’ (Organization for Economic Cooperation and Development Paris, Food and Agriculture Organization: Rome, Italy)
Olijhoek DW, Hellwing ALF, Brask M, Weisbjerg MR, Højberg O, Larsen MK, Dijkstra J, Erlandsen EJ, Lund P (2016) Effect of dietary nitrate level on enteric methane production, hydrogen emission, rumen fermentation, and nutrient digestibility in dairy cows. Journal of Dairy Science 99, 6191–6205.
| Effect of dietary nitrate level on enteric methane production, hydrogen emission, rumen fermentation, and nutrient digestibility in dairy cows.Crossref | GoogleScholarGoogle Scholar | 27236758PubMed |
Olijhoek DW, Hellwing ALF, Grevsen K, Haveman LS, Chowdhury MR, Løvendahl P, Weisbjerg MR, Noel SJ, Højberg O, Wiking L, Lund P (2019) Effect of dried oregano (Origanum vulgare L.) plant material in feed on methane production, rumen fermentation, nutrient digestibility, and milk fatty acid composition in dairy cows. Journal of Dairy Science 102, 9902–9918.
| Effect of dried oregano (Origanum vulgare L.) plant material in feed on methane production, rumen fermentation, nutrient digestibility, and milk fatty acid composition in dairy cows.Crossref | GoogleScholarGoogle Scholar | 31495619PubMed |
Opio C, Gerber P, Mottet A, Falcucci A, Tempio G, MacLeod M, Vellinga T, Henderson B, Steinfeld H (2013) ‘Greenhouse gas emissions from ruminant supply chains: a global life cycle assessment.’ (Food and Agriculture Organization of the United Nations: Rome, Italy)
Oskoueian E, Abdullah N, Oskoueian A (2013) Effects of flavonoids on rumen fermentation activity, methane production, and microbial population. BioMed Research International 2013, 349129
| Effects of flavonoids on rumen fermentation activity, methane production, and microbial population.Crossref | GoogleScholarGoogle Scholar | 24175289PubMed |
Patra AK (2013) The effect of dietary fats on methane emissions, and its other effects on digestibility, rumen fermentation and lactation performance in cattle: a meta-analysis. Livestock Science 155, 244–254.
| The effect of dietary fats on methane emissions, and its other effects on digestibility, rumen fermentation and lactation performance in cattle: a meta-analysis.Crossref | GoogleScholarGoogle Scholar |
Patra AK, Saxena J (2010) A new perspective on the use of plant secondary metabolites to inhibit methanogenesis in the rumen. Phytochemistry 71, 1198–1222.
| A new perspective on the use of plant secondary metabolites to inhibit methanogenesis in the rumen.Crossref | GoogleScholarGoogle Scholar | 20570294PubMed |
Patra AK, Kamra DN, Bhar R, Kumar R, Agarwal N (2011) Effect of Terminalia chebula and Allium sativum on in vivo methane emission by sheep. Journal of Animal Physiology and Animal Nutrition 95, 187–191.
| Effect of Terminalia chebula and Allium sativum on in vivo methane emission by sheep.Crossref | GoogleScholarGoogle Scholar | 20666858PubMed |
Paul NA, de Nys R, Steinberg PD (2006) Chemical defence against bacteria in the red alga Asparagopsis armata: linking structure with function. Marine Ecology Progress Series 306, 87–101.
| Chemical defence against bacteria in the red alga Asparagopsis armata: linking structure with function.Crossref | GoogleScholarGoogle Scholar |
Pawar MM, Kamra DN, Agarwal N, Chaudhary LC (2014) Effects of essential oils on in vitro methanogenesis and feed fermentation with buffalo rumen liquor. Agricultural Research 3, 67–74.
| Effects of essential oils on in vitro methanogenesis and feed fermentation with buffalo rumen liquor.Crossref | GoogleScholarGoogle Scholar |
Payne CL, Scarborough P, Cobiac L (2016) Do low-carbon-emission diets lead to higher nutritional quality and positive health outcomes? A systematic review of the literature. Public Health Nutrition 19, 2654–2661.
| Do low-carbon-emission diets lead to higher nutritional quality and positive health outcomes? A systematic review of the literature.Crossref | GoogleScholarGoogle Scholar | 26975578PubMed |
Poornachandra KT, Malik PK, Dhali A, Kolte AP, Bhatta R (2019) Effect of combined supplementation of tamarind seed husk and soapnut on enteric methane emission in crossbred cattle. Carbon Management 10, 465–475.
| Effect of combined supplementation of tamarind seed husk and soapnut on enteric methane emission in crossbred cattle.Crossref | GoogleScholarGoogle Scholar |
Ramírez-Restrepo CA, Tan C, López-Villalobos N, Padmanabha J, Wang J, McSweeney CS (2016) Methane production, fermentation characteristics, and microbial profiles in the rumen of tropical cattle fed tea seed saponin supplementation. Animal Feed Science and Technology 216, 58–67.
| Methane production, fermentation characteristics, and microbial profiles in the rumen of tropical cattle fed tea seed saponin supplementation.Crossref | GoogleScholarGoogle Scholar |
Rasmussen J, Harrison A (2011) The benefits of supplementary fat in feed rations for ruminants with particular focus on reducing levels of methane production. ISRN Veterinary Science 2011, 613172
| The benefits of supplementary fat in feed rations for ruminants with particular focus on reducing levels of methane production.Crossref | GoogleScholarGoogle Scholar | 23738103PubMed |
Ritchie H, Roser M (2019) Meat and dairy production. Our World in Data. Available at https://ourworldindata.org/meat-production [Verified 22 December 2020]
Roque BM, Brooke CG, Ladau J, Polley T, Marsh LJ, Najafi N, Pandey P, Singh L, Kinley R, Salwen JK, Eloe-Fadrosh E (2019a) Effect of the macroalgae Asparagopsis taxiformis on methane production and rumen microbiome assemblage. Animal Microbiome 1, 3
| Effect of the macroalgae Asparagopsis taxiformis on methane production and rumen microbiome assemblage.Crossref | GoogleScholarGoogle Scholar | 33499933PubMed |
Roque BM, Salwen JK, Kinley R, Kebreab E (2019b) Inclusion of Asparagopsis armata in lactating dairy cows’ diet reduces enteric methane emission by over 50 percent. Journal of Cleaner Production 234, 132–138.
| Inclusion of Asparagopsis armata in lactating dairy cows’ diet reduces enteric methane emission by over 50 percent.Crossref | GoogleScholarGoogle Scholar |
Roque BM, Van Lingen HJ, Vrancken H, Kebreab E (2019c) Effect of Mootral: a garlic- and citrus-extract-based feed additive: on enteric methane emissions in feedlot cattle. Translational Animal Science 3, 1383–1388.
| Effect of Mootral: a garlic- and citrus-extract-based feed additive: on enteric methane emissions in feedlot cattle.Crossref | GoogleScholarGoogle Scholar | 32704901PubMed |
Russell JB, Strobel HJ (1989) Effect of ionophores on ruminal fermentation. Applied and Environmental Microbiology 55, 1
| Effect of ionophores on ruminal fermentation.Crossref | GoogleScholarGoogle Scholar | 2650616PubMed |
Sasaki D, Morita M, Sasaki K, Watanabe A, Ohmura N (2012) Acceleration of cellulose degradation and shift of product via methanogenic co-culture of a cellulolytic bacterium with a hydrogenotrophic methanogen. Journal of Bioscience and Bioengineering 114, 435–439.
| Acceleration of cellulose degradation and shift of product via methanogenic co-culture of a cellulolytic bacterium with a hydrogenotrophic methanogen.Crossref | GoogleScholarGoogle Scholar | 22652087PubMed |
Schmidt HP, Hagemann N, Draper K, Kammann C (2019) The use of biochar in animal feeding. PeerJ 7, e7373
| The use of biochar in animal feeding.Crossref | GoogleScholarGoogle Scholar | 31396445PubMed |
Seedorf H, Kittelmann S, Henderson G, Janssen PH (2014) RIM-DB: a taxonomic framework for community structure analysis of methanogenic archaea from the rumen and other intestinal environments. PeerJ 2, e494
| RIM-DB: a taxonomic framework for community structure analysis of methanogenic archaea from the rumen and other intestinal environments.Crossref | GoogleScholarGoogle Scholar | 25165621PubMed |
Sen S, Makkar HP, Becker K (1998) Alfalfa saponins and their implication in animal nutrition. Journal of Agricultural and Food Chemistry 46, 131–140.
| Alfalfa saponins and their implication in animal nutrition.Crossref | GoogleScholarGoogle Scholar | 10554208PubMed |
Shi J, Arunasalam K, Yeung D, Kakuda Y, Mittal G, Jiang Y (2004) Saponins from edible legumes: chemistry, processing, and health benefits. Journal of Medicinal Food 7, 67–78.
| Saponins from edible legumes: chemistry, processing, and health benefits.Crossref | GoogleScholarGoogle Scholar | 15117556PubMed |
Silva RBD, Pereira MN, Araujo RCD, Silva WDR, Pereira RAN (2020) A blend of essential oils improved feed efficiency and affected ruminal and systemic variables of dairy cows. Translational Animal Science 4, 182–193.
| A blend of essential oils improved feed efficiency and affected ruminal and systemic variables of dairy cows.Crossref | GoogleScholarGoogle Scholar |
Singh RK, Dey A, Paul SS, Singh M, Punia S (2018) Responses of lemongrass (Cymbopogon citratus) essential oils supplementation on in vitro rumen fermentation parameters in buffalo. Indian Journal of Animal Nutrition 35, 174–179.
| Responses of lemongrass (Cymbopogon citratus) essential oils supplementation on in vitro rumen fermentation parameters in buffalo.Crossref | GoogleScholarGoogle Scholar |
Soliva CR, Amelchanka SL, Duval SM, Kreuzer M (2011) Ruminal methane inhibition potential of various pure compounds in comparison with garlic oil as determined with a rumen simulation technique (Rusitec). British Journal of Nutrition 106, 114–122.
| Ruminal methane inhibition potential of various pure compounds in comparison with garlic oil as determined with a rumen simulation technique (Rusitec).Crossref | GoogleScholarGoogle Scholar |
Sonoki T, Furukawa T, Jindo K, Suto K, Aoyama M, Sánchez‐Monedero MÁ (2013) Influence of biochar addition on methane metabolism during thermophilic phase of composting. Journal of Basic Microbiology 53, 617–621.
| Influence of biochar addition on methane metabolism during thermophilic phase of composting.Crossref | GoogleScholarGoogle Scholar | 22915326PubMed |
Staerfl SM, Zeitz JO, Kreuzer M, Soliva CR (2012) Methane conversion rate of bulls fattened on grass or maize silage as compared with the IPCC default values, and the long-term methane mitigation efficiency of adding acacia tannin, garlic, maca and lupine. Agriculture, Ecosystems & Environment 148, 111–120.
| Methane conversion rate of bulls fattened on grass or maize silage as compared with the IPCC default values, and the long-term methane mitigation efficiency of adding acacia tannin, garlic, maca and lupine.Crossref | GoogleScholarGoogle Scholar |
Stewart EK, Beauchemin KA, Dai X, MacAdam JW, Christensen RG, Villalba JJ (2019) Effect of tannin-containing hays on enteric methane emissions and nitrogen partitioning in beef cattle. Journal of Animal Science 97, 3286–3299.
| Effect of tannin-containing hays on enteric methane emissions and nitrogen partitioning in beef cattle.Crossref | GoogleScholarGoogle Scholar | 31242504PubMed |
Stoldt AK, Derno M, Das G, Weitzel JM, Wolffram S, Metges CC (2016) Effects of rutin and buckwheat seeds on energy metabolism and methane production in dairy cows. Journal of Dairy Science 99, 2161–2168.
| Effects of rutin and buckwheat seeds on energy metabolism and methane production in dairy cows.Crossref | GoogleScholarGoogle Scholar | 26805964PubMed |
Swanson BG (2003) Tannins and polyphenols. In ‘Encyclopaedia of food sciences and nutrition’. (Eds B Caballero, LC Trugo, PM Finglas) pp. 5729–5733. (Academic Press: London, UK)
Tavendale MH, Meagher LP, Pacheco D, Walker N, Attwood GT, Sivakumaran S (2005) Methane production from in vitro rumen incubations with Lotus pedunculatus and Medicago sativa, and effects of extractable condensed tannin fractions on methanogenesis. Animal Feed Science and Technology 123, 403–419.
| Methane production from in vitro rumen incubations with Lotus pedunculatus and Medicago sativa, and effects of extractable condensed tannin fractions on methanogenesis.Crossref | GoogleScholarGoogle Scholar |
Tekippe JA, Hristov AN, Heyler KS, Cassidy TW, Zheljazkov VD, Ferreira JFS, Karnati SK, Varga GA (2011) Rumen fermentation and production effects of Origanum vulgare L. leaves in lactating dairy cows. Journal of Dairy Science 94, 5065–5079.
| Rumen fermentation and production effects of Origanum vulgare L. leaves in lactating dairy cows.Crossref | GoogleScholarGoogle Scholar | 21943758PubMed |
Terry SA, Ribeiro GO, Gruninger RJ, Vieira Chaves A, Beauchemin KA, Okine E, McAllister TA (2019) A pine enhanced biochar does not decrease enteric CH4 emissions, but alters the rumen microbiota. Frontiers in Veterinary Science 6, 308
| A pine enhanced biochar does not decrease enteric CH4 emissions, but alters the rumen microbiota.Crossref | GoogleScholarGoogle Scholar | 31608292PubMed |
Ungerfeld EM (2013) A theoretical comparison between two ruminal electron sinks. Frontiers in Microbiology 4, 319
| A theoretical comparison between two ruminal electron sinks.Crossref | GoogleScholarGoogle Scholar | 24198813PubMed |
Ungerfeld EM (2015) Shifts in metabolic hydrogen sinks in the methanogenesis-inhibited ruminal fermentation: a meta-analysis. Frontiers in Microbiology 6, 37
| Shifts in metabolic hydrogen sinks in the methanogenesis-inhibited ruminal fermentation: a meta-analysis.Crossref | GoogleScholarGoogle Scholar | 25699029PubMed |
van Zijderveld SM, Gerrits WJJ, Dijkstra J, Newbold JR, Hulshof RBA, Perdok HB (2011a) Persistency of methane mitigation by dietary nitrate supplementation in dairy cows. Journal of Dairy Science 94, 4028–4038.
| Persistency of methane mitigation by dietary nitrate supplementation in dairy cows.Crossref | GoogleScholarGoogle Scholar | 21787938PubMed |
van Zijderveld SM, Dijkstra J, Perdok HB, Newbold JR, Gerrits WJJ (2011b) Dietary inclusion of diallyl disulfide, yucca powder, calcium fumarate, an extruded linseed product, or medium-chain fatty acids does not affect methane production in lactating dairy cows. Journal of Dairy Science 94, 3094–3104.
| Dietary inclusion of diallyl disulfide, yucca powder, calcium fumarate, an extruded linseed product, or medium-chain fatty acids does not affect methane production in lactating dairy cows.Crossref | GoogleScholarGoogle Scholar | 21605778PubMed |
Villar ML, Hegarty RS, Nolan JV, Godwin IR, McPhee M (2020) The effect of dietary nitrate and canola oil alone or in combination on fermentation, digesta kinetics and methane emissions from cattle. Animal Feed Science and Technology 259, 114294
| The effect of dietary nitrate and canola oil alone or in combination on fermentation, digesta kinetics and methane emissions from cattle.Crossref | GoogleScholarGoogle Scholar |
Visioli F, Strata A (2014) Milk, dairy products, and their functional effects in humans: a narrative review of recent evidence. Advances in Nutrition 5, 131–143.
| Milk, dairy products, and their functional effects in humans: a narrative review of recent evidence.Crossref | GoogleScholarGoogle Scholar | 24618755PubMed |
Vrancken H, Suenkel M, Hargreaves PR, Chew L, Towers E (2019) Reduction of enteric methane emission in a commercial dairy farm by a novel feed supplement. Open Journal of Animal Sciences 9, 286–296.
| Reduction of enteric methane emission in a commercial dairy farm by a novel feed supplement.Crossref | GoogleScholarGoogle Scholar |
Vyas D, Uwizeye A, Mohammed R, Yang WZ, Walker ND, Beauchemin KA (2014) The effects of active dried and killed dried yeast on subacute ruminal acidosis, ruminal fermentation, and nutrient digestibility in beef heifers. Journal of Animal Science 92, 724–732.
| The effects of active dried and killed dried yeast on subacute ruminal acidosis, ruminal fermentation, and nutrient digestibility in beef heifers.Crossref | GoogleScholarGoogle Scholar | 24398831PubMed |
Vyas D, McGinn SM, Duval SM, Kindermann M, Beauchemin KA (2016) Effects of sustained reduction of enteric methane emissions with dietary supplementation of 3-nitrooxypropanol on growth performance of growing and finishing beef cattle. Journal of Animal Science 94, 2024–2034.
| Effects of sustained reduction of enteric methane emissions with dietary supplementation of 3-nitrooxypropanol on growth performance of growing and finishing beef cattle.Crossref | GoogleScholarGoogle Scholar | 27285700PubMed |
Vyas D, Alemu AW, McGinn SM, Duval SM, Kindermann M, Beauchemin KA (2018) The combined effects of supplementing monensin and 3-nitrooxypropanol on methane emissions, growth rate, and feed conversion efficiency in beef cattle fed high-forage and high-grain diets. Journal of Animal Science 96, 2923–2938.
| The combined effects of supplementing monensin and 3-nitrooxypropanol on methane emissions, growth rate, and feed conversion efficiency in beef cattle fed high-forage and high-grain diets.Crossref | GoogleScholarGoogle Scholar | 29741701PubMed |
Wanapat M, Cherdthong A, Pakdee P, Wanapat S (2008) Manipulation of rumen ecology by dietary lemongrass (Cymbopogon citratus Stapf.) powder supplementation. Journal of Animal Science 86, 3497–3503.
| Manipulation of rumen ecology by dietary lemongrass (Cymbopogon citratus Stapf.) powder supplementation.Crossref | GoogleScholarGoogle Scholar | 18708607PubMed |
Winders TM, Jolly-Breithaupt ML, Wilson HC, MacDonald JC, Erickson GE, Watson AK (2019) Evaluation of the effects of biochar on diet digestibility and methane production from growing and finishing steers. Translational Animal Science 3, 775–783.
| Evaluation of the effects of biochar on diet digestibility and methane production from growing and finishing steers.Crossref | GoogleScholarGoogle Scholar | 32704845PubMed |
Wood JM, Kennedy FS, Wolfe RS (1968) The reaction of multi-halogenated hydrocarbons with free and bound reduced vitamin B12. Biochemistry 7, 1707–1713.
| The reaction of multi-halogenated hydrocarbons with free and bound reduced vitamin B12.Crossref | GoogleScholarGoogle Scholar | 4870333PubMed |
Yang K, Wei C, Zhao GY, Xu ZW, Lin SX (2017) Effects of dietary supplementing tannic acid in the ration of beef cattle on rumen fermentation, methane emission, microbial flora and nutrient digestibility. Journal of Animal Physiology and Animal Nutrition 101, 302–310.
| Effects of dietary supplementing tannic acid in the ration of beef cattle on rumen fermentation, methane emission, microbial flora and nutrient digestibility.Crossref | GoogleScholarGoogle Scholar | 27272696PubMed |
Yu L, Tang J, Zhang R, Wu Q, Gong M (2013) Effects of biochar application on soil methane emission at different soil moisture levels. Biology and Fertility of Soils 49, 119–128.
| Effects of biochar application on soil methane emission at different soil moisture levels.Crossref | GoogleScholarGoogle Scholar |