Chemical lean determination of boneless beef and lamb using a halogen moisture analyser
Peter Watkins
CSIRO Agriculture and Food, 671 Sneydes Road, Werribee, Vic., 3030, Australia. Email: Peter.Watkins@csiro.au
Animal Production Science 61(7) 715-720 https://doi.org/10.1071/AN20445
Submitted: 31 July 2020 Accepted: 21 January 2021 Published: 2 March 2021
Journal Compilation © CSIRO 2021 Open Access CC BY
Abstract
Context: Chemical lean (CL) is an important metric used by the Australian meat industry to describe fat content of meat product. It is a minimum meat specification included in contracts between Australian vendors of bulk packed boneless manufacturing meat and overseas or domestic buyers and can be regarded as the complement of chemically determined fat in meat (% fat, CL = 100-% fat). The microwave moisture method is commonly used for measuring CL content of meat in Australian abattoirs, relying on a relationship between CL and moisture content of boneless meat. It is regarded as a quick and easy method to use. More recently, newer methods for moisture analysis have become available which may also be suitable for CL determination, including commercial halogen moisture analysers (HMAs). HMAs use a halogen lamp as a source of infrared radiation that is absorbed by a sample, which then results in moisture loss.
Aims: This study aimed to compare the use of HMAs for CL determination of beef and lamb to that obtained from Soxhlet extraction technique, which is the AOAC Final Action Method for determining fat in meat and accepted as the reference method for fat determination. Additionally, the study sought to validate the use of HMAs as a method for CL determination in the Australian meat industry.
Methods: HMAs were used to determine the moisture content of six beef and one lamb samples, which were used to calculate the CL content. The fat content of the samples was also determined using Soxhlet fat extraction. Passing-Bablok regression and Bland-Altman plot analysis were used to identify any differences and bias between the methods, respectively.
Key results: Passing-Bablok regression showed that there was no difference between methods, while Bland-Altman plot analysis indicated no bias was evident between the methods. While some differences were apparent between approaches either due to using moisture as a proxy for fat/CL compared with continual solvent extraction or sample heterogeneity, sufficient agreement existed between results to lie within an acceptance criterion of 1.2 CL units.
Conclusion: The use of HMAs was substantiated as a method for CL determination in the Australian meat industry and has been accredited by AUS-MEAT Ltd as a method for boneless beef and lamb.
Implications: HMAs can be used in the Australian meat industry for CL determination and represent a relatively simpler and easier approach for this important industry metric.
Keywords: chemical lean, chemistry, fat determination, meat quality.
Introduction
Chemical lean (CL) is an important metric used by the Australian meat industry to describe the fat content of a meat product. Formally, it is defined as the amount of lean red meat compared with the amount of fat in a meat product, using an approved method of sampling and testing (Pearce 2016), and can be regarded as the complement of chemically determined fat in meat (% fat); CL = 100 – % fat (Eustace and McPhail 2006). It is a minimum meat specification included in contracts between Australian vendors of bulk packed boneless manufacturing meat and overseas or domestic buyers (Eustace and McPhail 2006). In Australia, there are currently 15 approved methods for chemical lean analysis, ranging from classical wet chemical techniques to moisture determination using microwave ovens as well as specific instrumentally based techniques.
These methods are accredited and audited by an Australian national authority (AUS-MEAT Ltd), to ensure accurate trade descriptions for export meat, including CL content. A recent industry study identified the microwave moisture method as the most used for measuring CL content of meat in the Australian meat industry (Watkins et al. 2021). The microwave moisture method relies on a relationship between moisture and fat in boneless meat, which, as a result, can be used to relate moisture to CL content (Eustace et al. 2006). Meat samples are dried in a microwave oven, and the weight loss resulting from drying can be expressed as percent moisture content and thus used to estimate CL content of meat samples. Anecdotal evidence indicates that the method is simple and requires minimal equipment for its deployment, making it suitable for use within small processors where only one technician is available to do CL determinations, along with other required duties and responsibilities (Watkins et al. 2021).
More recently, newer methods for moisture analysis have become available, which may also be suitable for CL determination, including halogen moisture analysers (HMAs). HMAs use thermogravimetry in their operation where the sample is heated by absorbing infrared radiation from a halogen lamp, resulting in moisture loss (Anonymous 2015). As the sample is heated, the HMA continually monitors the weight until it reaches a steady-state, at which point it is assumed to have dried and the instrument shows the moisture content (as %; Anonymous 2015). Using the HMA is straightforward; a sample is placed on an aluminium tray, which is then transferred to the HMA and, after closing the lid, it is then ready for analysis. By comparison, the microwave moisture method requires extra equipment and additional steps for its deployment, and so, in terms of efficiency, the HMA represents an alternative and simpler approach for determining moisture content and, by extension, CL content. CSIRO was approached by a small Australian meat processing company (based in Adelaide, SA, Australia) using this approach, and wished to validate it for determining CL content in boneless meat (beef and lamb), as well as accredit it as an AUS-MEAT approved method. This short communication presents the comparison of using HMAs for determining CL content with Soxhlet fat extraction used as the reference method, with the validation of an HMA for determining CL content.
Methods
Meat samples
Six beef samples spanning CL65 to CL95 (or 35 to 5% fat) and a single lamb sample were used for this study. These samples were prepared for an industry supported survey and their preparation is detailed elsewhere (Watkins et al. 2021). Briefly, meat was acquired from two Australian meat processing companies with 2 × 27.2 kg cartons acquired for each sample. After storage at 4°C, the meat was homogenised by combining the two cartons, and was then passed through a Thompson 42 mincer with a 6 mm plate (Thompson Meat Machinery, Crestmead, Qld, Australia). The aggregate was collected and re-passed through the mincer, and mixed using a commercial food/meat mixer (RC-100; Mainca USA, St. Louis, MO, USA). The homogenate was separated and weighed into separate 1 kg packages, which were then vacuum sealed (Cryovac® Barrier Shrink; Sealed Air, St. Neots, UK). These were stored at –20°C until required for analysis. A set of six beef and one lamb 1 kg packages was distributed to the processing facility in Adelaide, whereas at Werribee (Vic, Australia), the samples were removed from frozen storage and stored at 4°C overnight before analysis.
CL determination
HMA moisture analysis
Two HMA models (HE53 and HE73; Mettler Toledo, Melbourne, Vic., Australia) were used in this study. One HE53 was located at the Adelaide meat processing facility (A53), whereas another, along with a HE73, was located at a laboratory in Werribee (W53 and W73). For the purposes of the study, the HE53 and HE73 were, and can be, regarded as functionally equivalent in performance. At Werribee, the six beef and lamb samples were measured using an HE53 and HE73, with another set measured at Adelaide with an HE53.
For analysis, the package was opened, and a portion (10 g) was removed from its centre and placed into a disposable aluminium pan, which was then transferred into the HMA. The analysers were operated at 160°C using the ‘AUTO’ mode using a pre-set proprietary criterion to end the analysis. The pre-set criterion relies on the change in weight to be <1 mg for a predetermined time period (<l min, K. Fordham, Mettler, private communication). The HMA was left unattended until the measurement had finished, after which the moisture content (MC%) was displayed and recorded. The pan and its contents were discarded after each measurement. This series of steps was repeated three times (i.e. 3 × 10 g replicates were measured).
CL calculation
The CL content (CL%) was calculated from the moisture content (MC%) using different formulae, which are species specific, and have been previously reported (Eustace et al. 2006). The formulae are as follows:
Beef
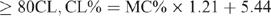
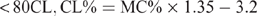
Mutton
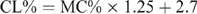
Soxhlet fat extraction
A set of samples was forwarded to three different commercial providers, and the fat content was measured by Soxhlet extraction in duplicate by each provider. The fat content (% fat) was reported and the corresponding CL content was calculated using CL = 100 – % fat. For the purposes of this study, the results were considered as an aggregate, and regarded as the reference values. This method is the Association of Official Analytical Chemists Final Action method for the determination of fat in meat.
Statistical analyses
Analysis of variance (ANOVA) was used to identify differences between techniques on a sample by sample basis. Tukey’s range test was used to determine the associated P-values, and an in-house R function (R Core Team 2018) was used to identify any significant differences between means using a letter summary (see Table 1).
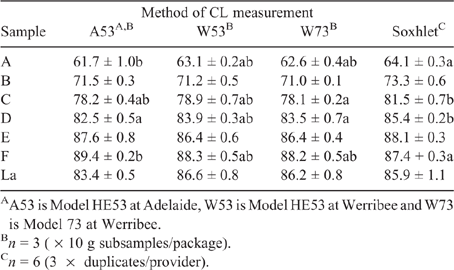
|
Method comparison
Passing–Bablok regression
Passing–Bablok (PB) regression was used to the compare each set of measurements on a sample by sample basis (Passing and Bablok 1983). The PB approach is robust, non-parametric, assumes that measurements are continuously distributed (covering a broad concentration range) and particularly suited for method comparison studies (Bilić-Zulle 2011). This is in contrast to the use of least-squares linear regression, which is a parametric approach and not recommended for use in method comparison studies (Payne 1997). The methods are also assumed to be linearly related (Bilić-Zulle 2011). The regression calculates the coefficients for the linear equation (y = b0 + b1.x), as well as the associated 95% confidence intervals (95% CI) for each coefficient, b0 and b1, which (if the linear relation was valid) would mean that b0 = 0 and b1 = 1. Thus, if the span of CI intervals (i.e. from –CI to CI) for b0 and b1 = 1 embrace 0 and 1 respectively, then it is presumed that no difference exists between the methods (Bilić-Zulle 2011), and they are regarded as equivalent. The analysis was performed using the ‘PBreg’ command available in the ‘MethComp’ package (Carstensen et al. 2020) in R (R Core Team 2018).
Bland–Altman analysis
Bland–Altman plot analysis was used to assess the presence of any bias present on a method by method comparative basis. In this case, a plot of the mean difference (or bias) between measurements from two methods is made against the mean of the measurements, along with the limits of the agreements associated with the differences (Kopp-Schneider and Hielscher 2019). The lines of agreement are calculated as ±1.96 × s, where 1.96 is the coverage factor for 95% statistical confidence, and s is the standard deviation associated with the differences. Plots were created using the ‘BA.plot’ command, whereas estimates of the difference and associated limits of agreements were undertaken using ‘BA.est’, both available in the MethComp package (Carstensen et al. 2020) in R (R Core Team 2018).
Results and discussion
Table 1 shows the CL content of the six beef (A to F) and lamb (La) samples, determined using Soxhlet fat extraction and moisture analysis with the HMAs. In the case of two samples (beef B and lamb), no differences between the results from the HMA and Soxhlet methods were apparent. For beef samples A, C, D and E, the Soxhlet values were higher than the HMA results, which could be attributable to the differences between methods. On the one hand, Soxhlet fat extraction meat is a continuous (almost exhaustive) process performed over several hours, whereas, on the other hand, the HMA dries the sample over a shorter time (typically <1 h), which could impact on the measurements. Sample F did not follow this trend, though, with the Soxhlet results lower than those from the HMAs. It is not clear why this was the case. This may be an effect related to the fat content, as this sample had the lowest of all meats. Another reason may be due to sample heterogeneity. Subsequent unpublished work showed that differences did exist in the CL content of different packages for the samples. When the packages were produced, it had been assumed that the meat had been extensively homogenised. However, if this was not the case, then there would be discrepancies observed in the analysis. The sample size is also a consideration (Watkins et al. 2021). Only 10 g is used for each measurement, so it is feasible that some micro-heterogeneity could be present in the subsamples compared with the package (1 kg). Other AUS-MEAT accredited methods use larger sample sizes (e.g. 300 g for near-infrared transmission/reflectance and 22.0 kg for X-ray analysis; Watkins et al. 2021) and thus would less likely be impacted by any (micro)-differences in terms of CL distribution present within the meat. That considered, it is also known, though, that variation of CL content will exist across samples (Vander Heyden and Smeyers-Verbeke 2007).
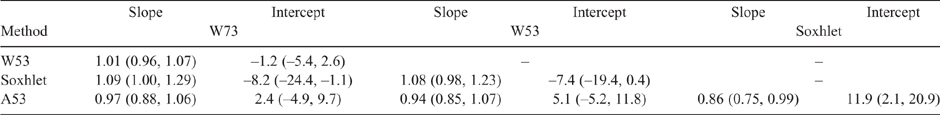
Table 2 shows the regression coefficients obtained from PB regression analysis of the CL results based on a method versus method basis, along with the associated 95% CIs. The column sample identification denotes the dependent variable, whereas the row samples identification denotes the independent variable for the non-parametric regression. As noted above, if the span of CI intervals for the intercept and the slope contains 0 and 1 respectively, it is presumed that no difference exists between the methods (Bilić-Zulle 2011). As can be seen, most of the intercept (b0) and slope (b1) CI intervals for the HMA comparisons between the HMAs contain 0 and 1 respectively, indicating that there was no difference between the CL results using this approach (Bilić-Zulle 2011). There were some exceptions though, notably between A53 and W73, and the Soxhlet results. Presumably, this is related to the differences between techniques, with Soxhlet being more exhaustive in extracting fat from the meat and the HMA approach depending on the heating and drying of the sample. Sample heterogeneity, as previously noted, could also contribute.
Fig. 1 shows the Bland–Altman plots for the CL results for the beef and lamb samples using the HMAs and Soxhlet fat extraction. The bold central line on the ordinate axis in Fig. 1 represents the mean difference between measurements, whereas the upper and lower lines represent the limits of agreement (LoAs) of the comparison, where the LoAs are ±1.96 × s, where 1.96 is the coverage factor for 95% statistical confidence, and s is the standard deviation associated with the differences. Where no bias is present between two methods, the points in a plot (such as Fig. 1) would be expected to be randomly distributed (scattered) above and below zero in the ordinate axis (Kalra 2017). With the alternative, where some bias is present between methods, some structure (such as linearity in the plot) would be pronounced and evident. Inspection of Fig. 1 does not reveal any strong evidence of structure within the plot, with most points randomly scattered along the ordinate axes. Thus, it was concluded that there was no inherent bias between the methods used in this study.
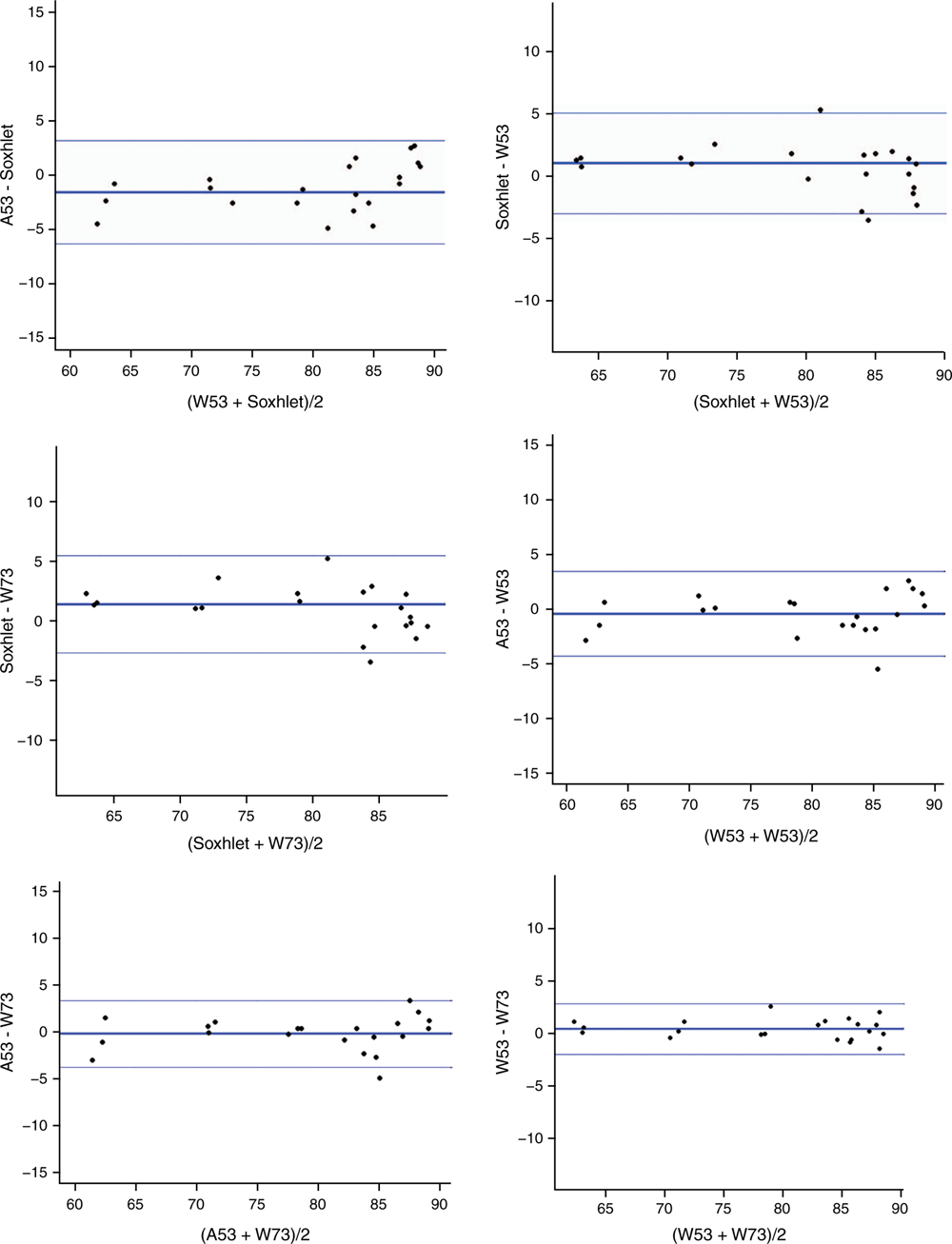
|
Table 3 shows the differences, upper and lower LoAs, and the interval between the upper and lower LoAs. Given that the microwave method remains in use in Australian meat processing facilities, a difference that is ≤1.2 CL units can be taken as a suitable acceptance criterion for comparing methods (Eustace et al. 2006). It should be noted that this criterion of 1.2 CL units is quite conservative, and that the precision found in this study is lower than this (Table 2). The differences between the HMAs were also lower than this value, whereas the magnitude between the Soxhlet results and those for A53 and W73 were of a similar magnitude (1.38 and 1.6 respectively).
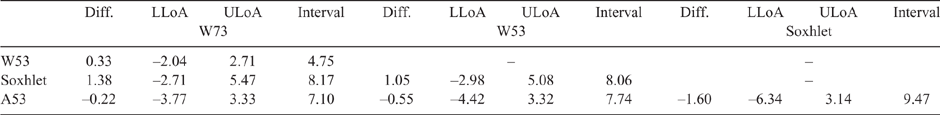
|
The interval between the LoAs represents a 95% confidence level for the mean differences (Doğan 2018), ranging from 4.7 (W53 vs W73) to 9.47 (A53 vs Soxhlet). A smaller interval range would be preferred, as it represents a narrower confidence level, also indicating closer agreement between methods. The other comparisons ranged from 7 to 8, suggesting that there was consistency between the methods. Usually, increasing the number of replicate measurements results in narrower CIs for the mean difference and the agreement limits (Giavarina 2015), which would have been most likely for this study as well.
A small processing facility using an HMA for CL determination would find this approach to be convenient and time efficient. The HMA is simple to use and requires minimal preparation; transfer the sample to a pan, place into the HMA, close the lid and press ‘start’. The apparatus can be left unattended until the drying reaches a steady-state and the moisture content is displaced. Operators within the facility can attend to other duties without the need to attend to the unit or be involved with multi-step procedures. The only requirement for using the HMA is that the sample is prepared in such a way that it is representative and suitable for analysis.
In summary, a comparison has been made on the use of an HMA for CL determination compared with Soxhlet fat extraction. This was undertaken using PB regression and Bland–Altman plot analysis. The former showed no difference between methods, whereas the latter indicated no bias was evident between the methods. Some differences were apparent, though, and this is likely to be either due to the differences between the methods (i.e. moisture as a proxy for fat/CL in meat compared with continual solvent extraction of fat from meat), or sample heterogeneity. That said, there was enough agreement for the HMA results to lie within an acceptance criterion of 1.2 CL units. This approach has been accredited by AUS-MEAT Ltd as a method for the determination CL content in boneless beef and lamb.
Conflict of interests
The author declares no conflict of interest.
Acknowledgements
The author thanks Mettler Toledo for access to, and use of, Model HE53 and HE73 HMAs. The author also acknowledges financial support of this study from Subway Meats Ltd, Adelaide, and the Enterprise Solutions Centre of Food Innovation Australia Limited (FIAL).
References
Anonymous (2015) Drying oven vs. halogen moisture analyzer: a practical guide to compare methods. (Mettler Toledo AG: Greifensee, Switzerland)Bilić-Zulle L (2011) Comparison of methods: passing and Bablok regression. Biochemia Medica 21, 49–52.
| Comparison of methods: passing and Bablok regression.Crossref | GoogleScholarGoogle Scholar | 22141206PubMed |
Carstensen B, Gurrin L, Ekstrøm CT, Figurski M (2020) ‘MethComp: analysis of agreement in method comparison studies.’ Available at https://CRAN.R-project.org/package=MethComp.
Doğan NÖ (2018) Bland-Altman analysis: a paradigm to understand correlation and agreement. Turkish Journal of Emergency Medicine 18, 139–141.
| Bland-Altman analysis: a paradigm to understand correlation and agreement.Crossref | GoogleScholarGoogle Scholar | 30533555PubMed |
Eustace IJ, McPhail NG (2006) Meat technology information sheet: a guide to calibration and verification of accuracy for instruments for estimation of chemical lean content for manufacturing. Available at http://meatupdate.csiro.au/chemical-lean.pdf.
Eustace IJ, McPhail NG, Small A (2006) Meat technology information sheet: microwave method for chemical lean determination. Available at http://meatupdate.csiro.au/infosheets/Microwave%20Method%20for%20chemical%20Lean%20Determination%20-%201997.pdf.
Giavarina D (2015) Understanding Bland Altman analysis. Biochemia Medica 25, 141–151.
| Understanding Bland Altman analysis.Crossref | GoogleScholarGoogle Scholar | 26110027PubMed |
Kalra A (2017) Decoding the Bland–Altman plot: basic review. Journal of the Practice of Cardiovascular Sciences 3, 36–38.
| Decoding the Bland–Altman plot: basic review.Crossref | GoogleScholarGoogle Scholar |
Kopp-Schneider A, Hielscher T (2019) How to evaluate agreement between quantitative measurements. Radiotherapy and Oncology: Journal of the European Society for Therapeutic Radiology and Oncology 141, 321–326.
| How to evaluate agreement between quantitative measurements.Crossref | GoogleScholarGoogle Scholar |
Passing H, Bablok W (1983) A new biometrical procedure for testing the equality of measurements from two different analytical methods. Application of linear regression procedures for method comparison studies in clinical chemistry, part I. Clinical Chemistry and Laboratory Medicine 21, 709–720.
| A new biometrical procedure for testing the equality of measurements from two different analytical methods. Application of linear regression procedures for method comparison studies in clinical chemistry, part I.Crossref | GoogleScholarGoogle Scholar |
Payne RB (1997) Method comparison: evaluation of least squares, deming and Passing/Bablok regression procedures using computer simulation. Annals of Clinical Biochemistry 34, 319–320.
| Method comparison: evaluation of least squares, deming and Passing/Bablok regression procedures using computer simulation.Crossref | GoogleScholarGoogle Scholar | 9158833PubMed |
Pearce KL (2016) Improving lamb lean meat yield. Available at https://www.sheepcrc.org.au/files/pages/publications/publications/improving-lamb-lean-meat-yield/Lean_Meat_Yield_Manual_-_Web_Feb16.pdf.
R Core Team (2018) ‘R: a language and environment for statistical computing.’ (R Foundation for Statistical Computing: Vienna, Austria)
Vander Heyden Y, Smeyers-Verbeke J (2007) Set-up and evaluation of interlaboratory studies. Journal of Chromatography A 1158, 158–167.
| Set-up and evaluation of interlaboratory studies.Crossref | GoogleScholarGoogle Scholar | 17339041PubMed |
Watkins P, Stockham K, Stewart S, Gardner G (2021) Contemporary chemical lean determination used in the Australian meat processing industry: a method comparison. Meat Science 171, 108289
| Contemporary chemical lean determination used in the Australian meat processing industry: a method comparison.Crossref | GoogleScholarGoogle Scholar | 32889306PubMed |