Genetic parameters and trends for lamb survival following long-term divergent selection for number of lambs weaned in the Elsenburg Merino flock
C. L. Nel A B D , A. A. Swan
A B D , A. A. Swan  C , K. Dzama A , A. J. Scholtz B and S. W. P. Cloete
C , K. Dzama A , A. J. Scholtz B and S. W. P. Cloete  A B
A B
A Department of Animal Sciences, Stellenbosch University, Merriman Avenue, Stellenbosch, 7602 South Africa.
B Directorate: Animal Sciences, Western Cape Department of Agriculture, Muldersvlei Road, Elsenburg, 7607 South Africa.
C Animal Genetics and Breeding Unit, University of New England, Ring Road, Armidale, NSW 2351, Australia.
D Corresponding author. Email: NeliusN@elsenburg.com
Animal Production Science - https://doi.org/10.1071/AN21198
Submitted: 9 April 2021 Accepted: 11 August 2021 Published online: 5 October 2021
Journal compilation © CSIRO 2021 Open Access CC BY-NC-ND
Abstract
Context: Mortality of new-born lambs is well known to have important implications for both animal production and welfare. Improving survival rates by genetic selection is very desirable, but the low heritability of survival traits challenges the prospect of useful genetic gain.
Aim: This study aimed to derive genetic and environmental parameters for lamb survival in the Elsenburg Merino resource flock. It also investigated correlations to possible indicator traits birthweight and birth coat score and reported genetic progress in breeding values for lamb survival following 33 years of divergent selection.
Methods: The flock was divergently selected for number of lambs weaned and was separated by the High (positive) and Low (negative) selection groups. The pedigree file identified 8138 lambs as the progeny of 273 sires and 2062 dams. The study considered total survival from birth to weaning (Tsv) that was also partitioned into perinatal survival to 3 days of age, and the remaining period. Variance components were derived by linear mixed models by using the ASREML® program. Genetic trends were derived by predicting mean breeding values for selection groups within each year and evaluated by fitting linear and broken-stick regression models.
Results: Predicted Tsv of H-line lambs (0.81 ± 0.01) was higher (P < 0.01) than that of L-line lambs (0.68 ± 0.01). Heritability was significant but low for survival traits (0.03–0.07), moderate for birthweight (0.16) and high for birth coat score (0.54). Genetic trends of the H-line trended divergently (P < 0.01) to the L-line for survival traits, but a changepoint (P < 0.01) in trend suggested that the H-line reached a selection plateau following 19–22 years of selection. Preceding this period, the rate of genetic change equalled ~1% of the mean for Tsv.
Conclusions: Despite the low heritability of survival, the genetic trends reported in this study contradicted the premise that genetic selection is not a worthwhile method to reduce incidences of lamb mortality.
Implications: It is recommended that lamb survival phenotypes should be recorded and incorporated into indices individually where possible, but composite traits are a viable alternative.
Keywords: sheep, (co)variance components, mortality, breeding values, heritability.
Introduction
Mortality of new-born lambs is a well known constraint to small stock production systems (Alexander 1984). A high margin of loss is common, with average mortality ranging between 20% and 25% across different breeds (Hinch and Brien 2014). Merinos have been estimated to deliver only two-thirds of potential at weaning (Kilgour 1992), or to lose as many as 59 lambs per 100 ewes mated (Kleemann and Walker 2005). Besides the issue of economic wastage, lamb mortality is also a concern from an emotional and welfare perspective, which provides further reason that the improvement of lamb mortality should not be neglected.
Survival depends on an interaction between the environment, physiology as well as the behaviour of both the ewe and the lamb. Ewes need to facilitate optimal in utero development, give birth without difficulty and provide adequate colostrum along with tactile stimulation and mothering. In turn, the lamb must adapt to the extra-uterine environment, thermoregulate and display favourable vigour in its ability to stand and suckle (see reviews by Dwyer 2008b; Brien et al. 2014; Hinch and Brien 2014).
The environment plays an important role in lamb survival (Hinch and Brien 2014). Among other factors, nutrition of the ewe is critical, and malnourishment adversely affects both physical and behavioural performance of the ewe (Dwyer 2008a), which leads to higher lamb mortality rates (Kleemann et al. 1993). Intervention at the environmental level can alleviate stress factors that increase risk. Housing and shepherding have been associated with a lower incidence of stillbirths (Binns et al. 2002) and early mortality has been reduced by the provision of shelter (Alexander et al. 1980; Lynch and Alexander 1980). However, such strategies are expensive, time consuming, and have other associated risks such as increased infection rates (Binns et al. 2002). Also, mortality rates of 19% can occur even under favourable circumstances (Behrendt et al. 2011), suggesting room for improvement on a genetic level. Genetic selection for higher survival rates is highly desirable but would depend on practical selection criteria and useful genetic components contributing to the survival outcome.
Survival contributes to composite phenotypes of the ewe such as the total weight weaned (TWW) or total number of lambs weaned per ewe mated (NLW). In addition to survival, these phenotypes are underpinned by other measurable components such as fertility, growth and litter size. Selection for TWW or NLW could provide a balanced approach to simultaneously improving underlying components (see review by Snowder and Fogarty 2009). A concern is that records such as NLW and TWW do not necessarily account for incidences of mortality unless survival of the lamb or rearing ability of the ewe is expressed explicitly. Multiples are well known to be more susceptible than singles (Slee et al. 1991; Kleemann and Walker 2005; Snowder and Fogarty 2009; Bunter et al. 2018), and a higher number of lambs born could be accompanied by mortalities not necessarily reflected in a trait such as NLW. Accordingly, studies and reviews have recommended that the genetic improvement of lamb survival should be included in index selection individually (Swan 2009; Brien et al. 2011, 2014; Hinch and Brien 2014; Bunter and Brown 2015; Bunter et al. 2018). In New Zealand, Sheep Improvement Limited (SIL; http://www.sil.co.nz) has provided breeding values for lamb survival since 1999 (Newman et al. 2000), but other examples of selection for survival as a trait of the lamb are scarce in both the research or industry sectors.
Useful genetic components to survival are supported by differences in lamb mortality across breeds (Alexander 1984; Kleemann et al. 2000; Fogarty et al. 2005), Merino bloodlines (Mortimer and Atkins 1997) and Romney sire lines (Gudex et al. 2005). However, genetic gain by selection within breeds is considered as challenging. A generally low or very low heritability (h2; 0.01–0.05) has been reported in Merinos (Brien et al. 2009; Hatcher et al. 2010) and other breeds (e.g. Lopez-Villalobos and Garrick 1999; Morris et al. 2000; Riggio et al. 2008; Vanderick et al. 2015). Studies have reported maternal genetic effects (m2) as influential, but also low, and often associated with negative and varying estimates of the direct-maternal covariance (ram; Brien et al. 2014). Moreover, survival is a threshold character (Falconer 1989) that may not be amenable to analysis by linear models. Subsequently, studies analysing survival data have varied in their approach, causing some complexity in the comparison and interpretation of estimates.
Using threshold analysis, Cloete et al. (2009) reported promising genetic trends in lamb survival derived from the Elsenburg Merino flock, a selection experiment subject to divergent selection for NLW since 1986. Genetic gain achieved in the Elsenburg flock proved contradicting to the notion that genetic selection would not be worthwhile in improving mortality rates. However, the study by Cloete et al. (2009) reported a large negative ram estimate, an unfavourable result that could be symptomatic of analysis rather than a true biological underpinning (Robinson 1996). Also, evaluating survival data on the underlying scale can cause difficulty in deriving accurate and comparable threshold-linear covariance components between survival and continuous traits (Cloete et al. 2009). Given the low h2 of survival, there is interest in identifying favourably correlated traits that could improve realised gain (Brien et al. 2010). However, such an objective would hinge on accurate genetic and environmental correlations. Birthweight has long been known as an important predictor of lamb survival (Smith 1977; Hinch et al. 1983; Gama et al. 1991), but is complicated by a non-linear relationship to mortality (Dwyer 2008b; Hinch and Brien 2014). A birth coat score that grades the hairyness or woollyness of coats could play a part in lamb viability in the early neonatal period (Alexander 1961; Slee 1978; Slee et al. 1991). However, this has been lowly correlated with survival in Merinos in a previous study (Brien et al. 2010). The objectives of the current study are thus (1) to revisit genetic parameters for direct and maternal components for lamb survival in the Elsenburg flock with alternative methods to those of Cloete et al. (2009), (2) to investigate the relationships of survival with the possible indicator traits birthweight and birth coat score and (3) to report genetic progress (or lack thereof) in lamb survival following divergent selection that currently extends to 33 years since the onset of the experiment.
Materials and methods
The Elsenburg Merino flock
The Elsenburg Merino flock is a resource flock managed as a selection experiment for divergent selection based on NLW since 1986 (see review by Schoeman et al. 2010). The flock is separated by the High (H-line; positive selection) and Low (L-line; negative selection) lines that originated from the same base population. At the start of the experiment, ewes were allocated at random within age groups to each of the two lines. Subsequent selection proceeded by screening ewes according to their ranking for NLW. Besides instances of death or severe health-related problems, replacement ewes remained in the flock for at least five joinings. At the beginning of the experiment, roughly 120 ewes were assigned to each selection line. The H-line has since grown to 130–200 ewes, while numbers in the L-line dwindled to ~40–80 breeding ewes. Selection of rams was predominantly based on the progeny of dams based on at least three joinings. Following the results reported by Cloete et al. (2004), selection from 2003 was also guided by the use of best linear unbiased prediction (BLUP) breeding values derived from a single-trait repeatability model fitted to annual NLW records of ewes. During this period, the original ranking values were still considered in tandem to BLUP-derived breeding values, but BLUP values have been the only selection criterion since 2010. In the latter years of the selection experiment, breeding management required the use of external sires to manage levels of inbreeding. From 2008 to 2019, 349 H-line lambs and 151 L-line lambs were born as the progeny of external sires. These animals were also considered for selection and 740 H-line and 118 L-line lambs were subsequently born as grand-progeny of external sires between 2010 and 2019.
Details pertaining to management and nutrition are available elsewhere (Cloete et al. 2004, 2009) and only a short summary will be repeated here. During this experiment, the flock was maintained at the Elsenburg Research Farm near the town of Stellenbosch (33°51′S, 18°50′E) in the Western Cape Province, South Africa. Besides during joining in single-sire groups within lines, the two lines were maintained as a single flock for most of the year. The flock grazed on irrigated kikuyu grass (Pennisetum clandestinum) during the summer (January–February) and during the lambing period in winter (June–July). In the remaining periods, the flock grazed on dryland as well as irrigated lucerne (Medicago sativa) and dryland medics (M. truncatula). Oats (Avena sativa) fodder crops were also available during winter to supplement the slow-growing legume pastures. Ewes were shorn within 3–4 weeks before lambing. Lambing took place in 10–20 kikuyu lambing paddocks of ~0.3–0.4 ha each, where ewes were set-stocked in groups ranging from 15 to 25, depending on the research needs. After a variable period of 3 to ~14 days, lambed ewes and their lambs were randomly drifted to somewhat larger (1.0–1.5 ha) irrigated lucerne paddocks in groups of 20–40. As the irrigated paddocks became depleted, these groups were joined in larger groups on dryland lucerne, medic and oat paddocks.
Data recording
Data were recorded over a 33-year period from 1986 to 2019. The pedigree file identified the line of origin for most of the 8138 lambs as the progeny of 273 sires and 2062 dams. Recording of all traits commenced during daily lambing rounds at 0800 hours. that identified all lambs born within the previous 24-h period with their dams, thus enabling linkage back to the selection line and sire (Cloete et al. 2004, 2009). Traits recorded were survival, birthweight (BW) and birth coat score (BCS) for both dead and alive lambs. In rare cases, birthweight was unavailable in lambs mutilated by damage-causing animals, mostly crows (Corvus spp.). Survival traits were regarded as total survival from birth to weaning (Tsv), survival to 3 days of age, including stillbirths and ante parturient deaths, as perinatal survival (PNsv), and the remaining period post 3-days to weaning (P3sv). BCS was recorded only since 1994 and included records on 5883 lambs over 25 years. BCS was subjectively recorded according to a linear 5-point scale graded from the most hairy (1) to most woolly (5; Cloete et al. 2003), with half marks allowed where considered appropriate.
Statistical analyses
Variance components
The discrete expression of the survival phenotype as either dead (0) or alive (1) is described as a threshold character (Falconer 1989) that follows a hypothetical normal distribution on the underlying liability scale. Threshold characters violate the assumption of normality on the observed scale, and linear models are theoretically not appropriate for the analysis of binary phenotypes. However, previous examples from the literature analysed survival data on both underlying and observed scales that allowed for the comparison of these methods. Everett-Hincks et al. (2014) and Vanderick et al. (2015) used cross-validation of randomly assigned ‘missing’ phenotypes and reported that linear models were marginally more accurate at predicting missing phenotypes than were logit-transformed alternatives. Also, Matos et al. (2000) reported linear and threshold models to be similar according to predictive and goodness-of-fit parameters. Consequently, in contrast to previous results of survival data from the same flock (Cloete et al. 2009), current data were evaluated by linear analysis for benefit of convenient interpretation on the observed scale.
Estimation of fixed effects and subsequent derivation of variance components commenced by using the ASREML® program (Gilmour et al. 2015). The environmental factors that were considered as fixed effects included year of birth (1986–2019), selection group (H-line or L-line), sex (male or female), age of dam (2–6+ years) and birth type (singles vs pooled multiples). The non-linear phenotypic relationship between survival and BW was investigated by fitting BW as a linear (BW) and quadratic (BW2) covariate in a separate analysis of survival traits. These results were reported separately and were identified by TsvBW, PNsvBW and P3svBW. Otherwise, variance components and genetic trends of survival are reported excluding BW as a covariate.
Random effects considered in the model were direct and maternal genetic effects, the ram and maternal permanent environment. Analysis commenced by fitting various combinations of fixed effects and possible two-way interactions so as to obtain an operational model. In the model used to derive parameter estimates for random components, selection group was included as a fixed effect to avoid possible inflation of genetic variance that could result from the selection program separating the H- and L-lines. Fixed effects and interactions were tested for significance according to Wald statistics derived from conditional least-square methods. Those observed as significant (P < 0.05) were retained throughout subsequent analysis. Random terms were then added to the operational model on a stepwise basis, which resulted in either of the following genetic models used for analysis (in matrix notation):
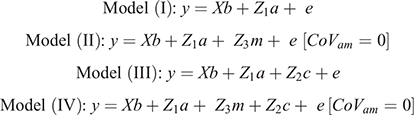
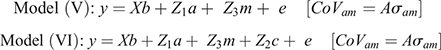
In the preceding equations, y represents the vector of observations of the respective traits, b the vector of fixed effects, a represents the vector of direct genetic variances, m the vector of maternal genetic variances, c the vector of maternal permanent environmental variances and e the vector of residuals. The corresponding incidence matrices of each effect are represented by X, Z1, Z2 and Z3 respectively. A represents the numerator relationship matrix, and σam the covariance between direct and maternal genetic effects.
It was assumed that
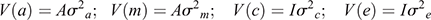
with A representing the numerator relationship matrix, I representing identity matrices and σ2a, σ2m, σ2mc and σ2e the direct genetic variance, maternal genetic variance, maternal permanent environmental variance and environmental (residual) variance respectively. These analyses yielded estimates of genetic and permanent environmental variances, which were used to compute (co)variance ratios for direct additive genetic, maternal genetic, the direct-maternal genetic correlation (where applicable) as well as maternal permanent environmental variances as a proportion of the total observed phenotypic variance. A log-likelihood ratio test (LRT) was used to test for significant differences in model likelihoods, as follows:
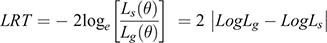
Where Lg(θ) and Ls(θ) represent the likelihood of the general (full) and the simpler (nested) model respectively. The LRT is a chi-square distributed random variable, with degrees of freedom equal to the difference in the number of random effects. When one random effect was added at a time (e.g. (II) vs (I) or (III) vs (II)), a difference of 1.92 was regarded as a significant (P < 0.05) improvement in the log-likelihood compared with the reduced model tested against. If a model with an additional term did not provide a significant (P < 0.05) improvement, the simpler (nested) model was chosen as the operational model for the trait. The terms fitted in the operational model were subsequently used for multi-trait models. The multi-trait analyses were limited to two-trait models that allowed for the estimation of direct genetic (rg), maternal permanent environmental (rmc), environmental (re) and phenotypic (rp) correlations between the respective traits.
Genetic trends
To assess genetic divergence between selection lines, individual breeding values were obtained from a separate analysis that excluded selection group (or any interactions with selection group) from the model. This was considered appropriate for trends because the retention of selection group as a fixed effect would deflate possible genetic differences that could have resulted from selection. These breeding values were used to derive means for each selection group (H-line vs L-line) for each year (1986–2019) as indicative of genetic trends. So as to assess possible change in genetic trends, two approaches were followed. First, standard linear regression lines were fitted for both trends representing the H- and L-lines and tested for significance (P < 0.05) of their regression coefficients and divergence between the lines using individual standard errors (s.e.) and the pooled s.e. respectively. The intercept of linear regressions on genetic trends was fixed at zero (Year 0) because the H- and L-lines originated from the same founding population. Second, trends were investigated for a possible selection plateaux, or a distinct change in trend, by fitting ‘broken stick’ regression models that delivered an estimate of a possible changepoint in genetic trends. This was performed with the segmented package (Muggeo 2008) in the R environment (R Core Team 2020). A piecewise or segmented relationship between the mean response and explanatory variable is modelled by adding an additional term to the linear predictor, as follows:
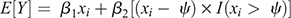
where β1 is the left (or initial) slope, β2 is the difference in slopes, ψ is the breakpoint and I(.) is an indicator function equal to one when the statement is true (χi > ψ) and zero when the statement is false (χi < ψ). The segmented package evaluates a possible difference in slopes by making use of the Davies (1987) test. This is not the most appropriate test for determining the number of breakpoints (Muggeo 2008), but a maximum of one was assumed for the current analysis. In cases where Davies’ test yielded significant (P < 0.01) results, the derived changepoint (CP) and differential slopes/coefficients were reported in addition to standard regression lines. Breeding values, genetic trends and fitted regression lines were plotted with ggplot2 (Wickham 2016) in R.
Results
Fixed effects
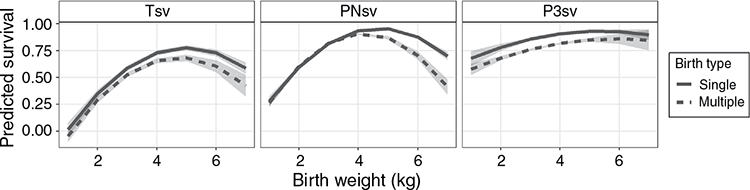
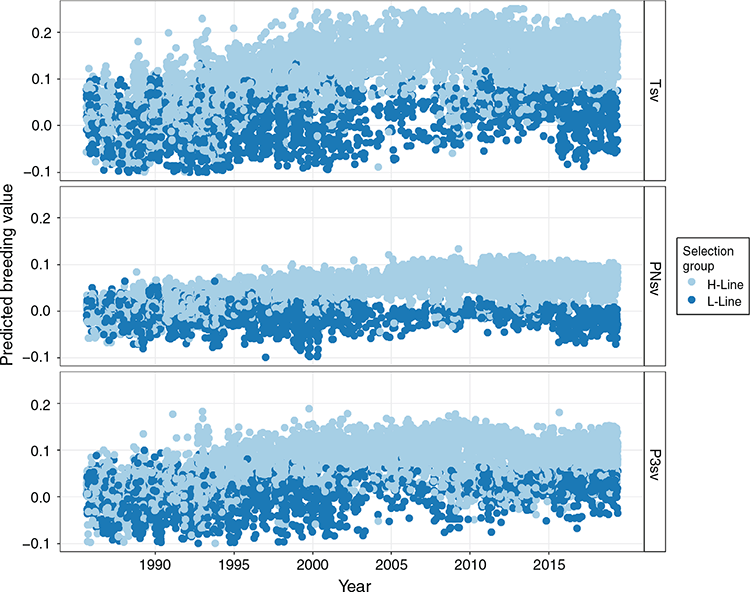
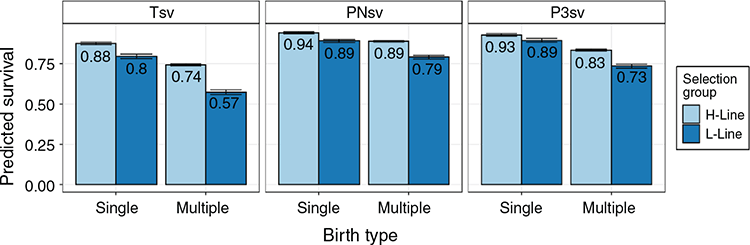
Trait summaries and least-square means of fixed effects can be seen in Table 1. Over the period of the study, ~10% of animals were lost within the first 3 days after birth, which was a large proportion of the ~23% total mortality rate to weaning. The mean BW was 3.85 kg, and mean BCS was 3.2. The effect of year was in a significant (P < 0.05) interaction with selection group for Tsv, PNsv, BW and BCS, and is not tabulated. As the two lines shared the same environment, differences between the H- and L-lines across succeeding years are considered a response to selection and are discussed under genetic trends below. Selection group also interacted (P < 0.05) with birth type in all three survival traits. The advantage of the H-line was more pronounced in multiples than in singles and predicted that Tsv for L-line multiples was especially poor (0.57; Fig. 1). The predicted least square means for fixed effects can be seen in Table 1. Generally, lambs born in the H-line had higher survival rates (P < 0.01) than did L-line lambs in PNsv, P3sv and Tsv. H-line lambs were also slightly heavier (P < 0.05) but had a lower BCS (P < 0.01), indicating that they tended to be hairier and L-line lambs were woollier. Female lambs had a higher (P < 0.01) survival rate than did male lambs in Tsv and P3sv, but sex was not influential in PNsv (P > 0.05). Male lambs were heavier than females (P < 0.01) and had a higher (P < 0.01) BCS. Birth type was influential in all traits except BCS, with singles having higher (P < 0.01) survival rates for all periods, and the significant (P < 0.01) difference in BW amounted to nearly 1 kg. The age of the dam also proved influential (P < 0.01) in all traits. A non-linear relationship best described this influence as ewes of intermediate age tended to have lambs with better survival rates, a higher lamb birthweight, but lower BCS compared with primiparous or mature ewes.
|
When BW was included as linear and quadratic terms in the fixed-effects model of TsvBW, PNsvBW and P3svBW, a non-linear phenotypic relationship of survival and BW was evident (Fig. 2), but more pronounced in PNsv than in P3sv. Lambs were predicted to have the best chances of survival according to an intermediate optimum that ranged between 3 and 5 kg. The effect of selection group was tested for a possible interaction with BW and BW2, which was not significant (P > 0.05). This suggested that advantages of the H-line lambs were generally independent of BW, making inferences in this respect quite robust. However, BW and BW2 were in a significant (P < 0.05) interaction with birth type in the case of PNsvBW, but not for TsvBW or P3svBW. According to Fig. 2, the difference between singles and multiples was largest in cases of high BW, but PNsv was independent of birth type for lambs lighter than 4 kg at birth.
Random variance components
According to the LRT, the addition of a direct additive genetic effect (σ2a) significantly (P < 0.05) improved the log-likelihood of all traits (Model I; Table 2). In the analysis of Tsv and PNsv, the maternal permanent environmental (σ2mc) and the maternal genetic (σ2m) components appeared to partially compete for the same variance. In preliminary analysis, fitting σ2m improved (P < 0.05) log-likelihood (Model II vs Model I), but the further addition of σ2mc (Model IV) deflated σ2m as most maternal variance partitioned to σ2mc. Between the Model II and Model III, the best log-likelihood was found in Model III, which considered only σ2mc in addition to σ2a. Model III also provided the best fit for P3sv, although the LRT statistic (Model III – Model I = 1.93) exceeded the critical chi-square statistic of 1.92 by a very narrow margin. In BW, both σ2m and σ2mc were retained as components of variance in Model IV. However, including a component accounting for the direct-maternal genetic covariance (σ2am, Model VI) did not deliver a better log-likelihood than did the nested model (IV). For BCS, including σ2mc proved influential as Model III had the best log-likelihood of all models tested. Because σ2am was not influential or not applicable in any of the traits, the LRT results of Models V and VI and the correlation (ram) are not shown.
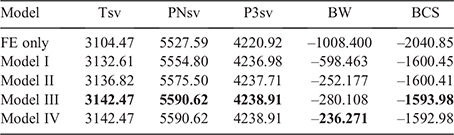
|
All traits were heritable, but the genetic components of survival traits were generally low to very low (Table 3). For Tsv, the direct genetic effect (h2; 0.07) was slightly larger than the permanent maternal environmental component (mc2; 0.04), but h2 and mc2 traded ranking across the partitioned periods of survival. In the PNsv, mc2 was low (0.10), but considerably higher than the very low h2 (0.03). But for P3sv, the h2 was comparatively large (0.07) while mc2 was very low (0.02), indicating that the dam’s influence peaked early on in the life of the lamb. BW was moderately heritable at 0.16, but was more affected by the maternal genetic effect (m2; 0.2). The mc2 component was also influential, but lower than the genetic effects (0.12). The h2 of BCS was high (0.54) and the mc2 ratio was significant, but very low (0.03).
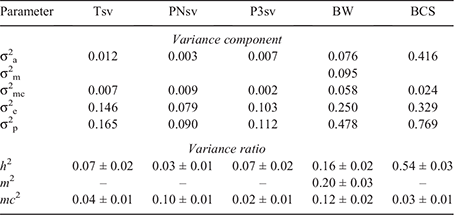
|
Genetic, environmental and phenotypic correlations
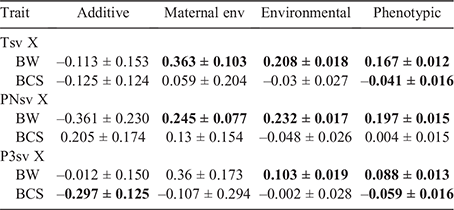
The correlations derived from respective two-trait analyses with BW and BCS did not suggest strong linear relationships with survival (Table 4). The genetic correlation (rg) of the survival traits with BW were all negative in sign, but not significant (P > 0.05). Despite the poor precision of the estimate, the magnitude of the negative genetic relationship between PNsv and BW (rg = –0.36) could be a noteworthy suggestion, favouring low BW lambs in the perinatal period. In contrast, all non-genetic relationships between BW and survival were positive, moderately in favour of lambs of higher BW. The strongest example was the relationship between Tsv and BW on the maternal permanent environmental level by a moderate magnitude (rmc = 0.36), but the benefit of higher BW was lowly reflected on the phenotypic level (rP = 0.17). A similar tendency of environmental and phenotypic relationships was observed between BW and PNsv that also marginally favoured higher BW lambs. For P3sv, the relationship with BW was either insignificant (P > 0.05) or low, suggesting that the benefit of a higher BW was almost negligible once the lamb has survived to 3 days of age.
|
The genetic correlations of BCS with PNsv and P3sv were opposing in sign, but significant (P < 0.05) only in the case of P3sv. Lambs with a genetic tendency for hairy (in contrast to woolly) coats were thus more inclined to survive the post 3-day period to weaning. Negative relationships between BCS and survival were reflected on the phenotypic level for Tsv and P3sv but were negligibly low in magnitude.
Genetic trends
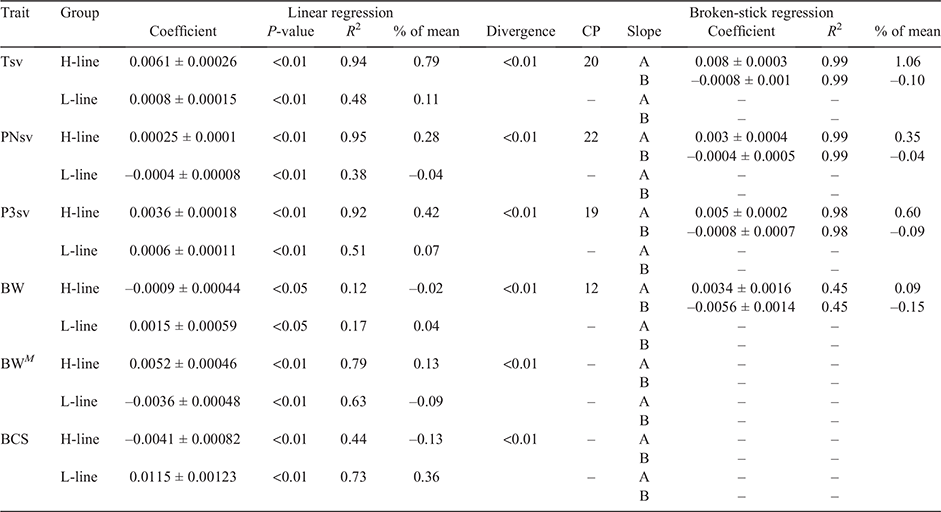
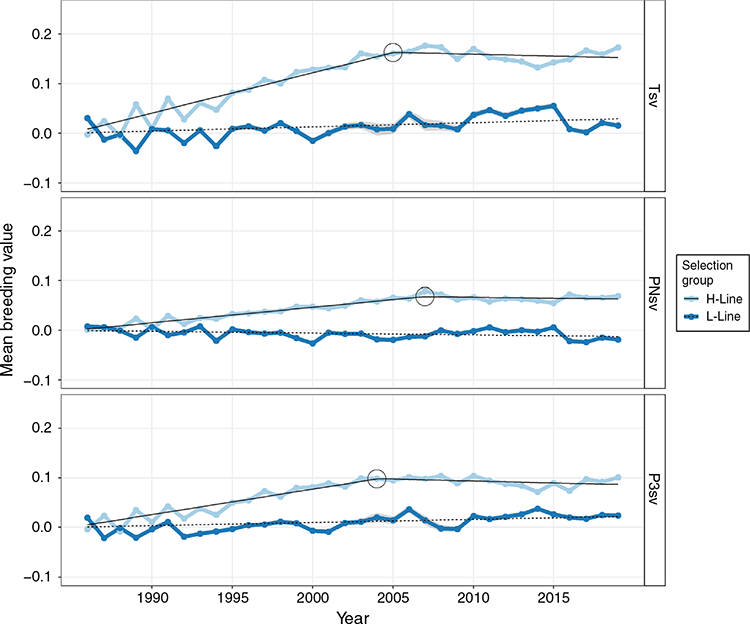
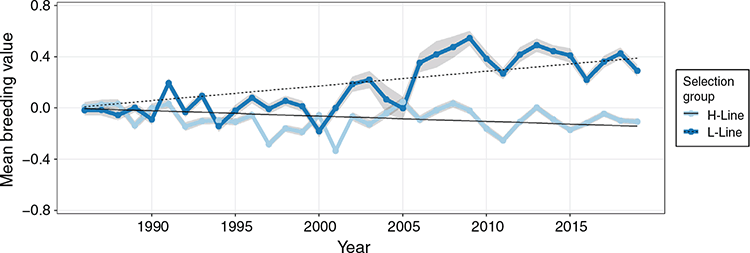
Linear regressions of genetic trends were all significant (P < 0.05; Table 5), indicating that all traits responded to selection to some extent. Respective coefficients were not necessarily opposite in sign for H- and L-line trends but were observed as divergent (P < 0.01) in all traits. However, a linear regression proved a poor fit of the genetic trends in the L-line, with R2-values ranging between 0.38 and 0.51 for survival traits. Mean breeding values of the H-line trended favourably and substantially higher than those of the L-line group for all three survival traits (Fig 3). The genetic difference between selection groups was also clearly reflected in the individual breeding values, which showed favourable ranking for the majority of H-line lambs for the last 20 years of the experiment, whereas a much more mixed result was observed for the period preceding 1995 (Fig. 4). The poor fit of linear regressions of L-line trends is considered to be due to high levels of year-to-year variation with little consistency in a directional response to selection (Figs 3, 5), with the possible exception of BCS (Fig. 6). If coefficients of linear regressions are expressed as % of the overall mean per annum (p.a.), the H-line favourably trended upward at a rate of nearly 0.8% p.a. for Tsv (Table 5). Observed change in the H-line for PNsv (0.28% p.a.) and P3sv (0.42% p.a.) were of a more moderate magnitude, but this can be expected given less variation in partitioned periods. Survival in the L-line also trended upward for Tsv and P3sv, but downward for PNsv. Albeit statistically significant (P < 0.01), the magnitude of the regression coefficients in the L-line were small (–0.04% to 0.11%), indicating only marginal net change in L-line breeding values over the total selection period.
|
In later years of the selection experiment, the individual values appeared to have a threshold bounding breeding values at a value slightly larger than 0.2 for Tsv and 0.1 for PNsv (Fig. 4), and trends showed little further gain in mean breeding values (Fig. 3). This apparent selection plateau was evaluated for a possible CP in trend by fitting broken-stick regression models as an alternative to the average linear trend over 33 years. Davies’ test for a difference in slopes proved significant (P < 0.01) in H-line trends for Tsv, PNsv, P3sv and BW (Table 5). For survival traits, a CP in genetic trends suggested that the selection response of the H-line reached a plateau following 19–22 years of selection (Table 5), with no further increase in mean breeding values after roughly 2005 (Fig. 3). Broken-stick regression lines also showed that the genetic gain made by the H-line appeared to have been manifested in the first 20 years of selection. During this period, the initial (A) coefficient suggested a high rate of genetic change equal to ~1% of the mean for Tsv. The subsequent (B) coefficient indicated a declining trend but was of a small magnitude with a large s.e., indicating little to no genetic gain from 2005 to 2019.
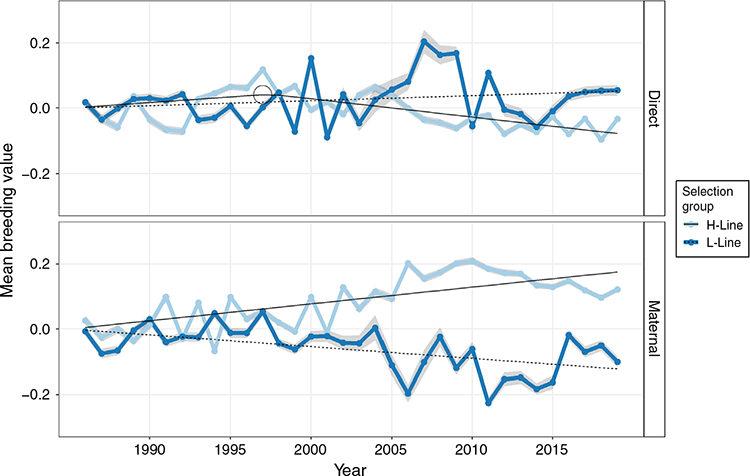
Response p.a. in both H- and L-line breeding values for BW were of a low or negligible magnitude, but it is interesting to note that the regression coefficients of direct and maternal genetic trends were opposite in sign (Table 5). In the H-Line, BW divergently (P < 0.01) trended downward in the direct genetic response, but upward in the maternal genetic response, while the opposite was observed in the L-line (Fig. 5). In trends, the H- and L-lines traded rankings for higher mean breeding values for BW in numerous years, with very weak evidence of a divergent response reported in Table 5. In the maternal genetic trends, the selection groups were more distinguishable than in the direct genetic trends and appeared divergent following the 1995–2000 period. As deduced from regression coefficients, the responses of direct and maternal trends were in opposite directions. According to the broken-stick regression, the H-line trend changed sign to lower BW following 12 years of selection (Year 1998; Table 5). However, both initial (A) and subsequent (B) coefficients were of a low magnitude, and both A and B coefficients had a poor fit (R2 < 0.45) compared with those of survival traits. Also, in both the direct and maternal genetic trends, breeding values over the last ~5 years of the experiment trended in a relatively narrow margin compared with the zero-line used to define the originating population, resembling only marginal net change in BW associated with selection for NLW.
Responses observed for BCS were also low, but the slight upward trend of 0.36% p.a. in the L-line indicated that L-line animals slowly tended to higher BCS values with time, describing a tendency towards woollier coats. According to trends, divergence between the lines became more pronounced following the Year 2000, and the L-line persistently had higher scores from 2005 onward, but Davies’ test for a difference in slopes did not support this observation (P > 0.01).
Discussion
Descriptive statistics and fixed effects
Mean survival to weaning was consistent with what could be expected according to the literature (Hinch and Brien 2014). In Merinos, survival to 7 days has been reported at 85% and to weaning at ~72% (Hatcher et al. 2010). Similarly, Brien et al. (2010) reported an 85% survival rate to 3 days of age, and 80% survival to weaning from a predominantly Merino population. It is evident that the period around birth and the first days following birth are critical for lambs, as a proportionally high mortality rate coincided with this short period. Female, H-line and single lambs were less likely to succumb than were male, L-line and multiple lambs (Table 1). Males accordingly suffered higher mortality rates in Scottish Blackface (Sawalha et al. 2007; Riggio et al. 2008), Texel, Shropshire and Oxford Down (Maxa et al. 2009), Texel and Texel-cross (Morris et al. 2000), Coopworth (Everett-Hincks et al. 2005) and Merino (Brien et al. 2009) lambs. The marked difference in survival between the H- and L-lines is supported by previous experiments that successfully manifested within breed differences by selection on fitness traits (Atkins 1980; Haughey 1983; Knight et al. 1988). The interaction between birth type and selection group (Fig. 1) supported the earlier findings of Cloete and Scholtz (1998), who suggested that advantage of H-line lambs was predominantly derived from better survival of multiples. However, the advantage of the ‘pro-fertility’ Merino selection flock reported by Atkins (1980) was consistent across singles and multiples. Also, Romney ewes selected for survival and rearing ability had a 8.5% higher survival rate than did the control strain ewes, but the effect of selection line did not interact with birth rank (Knight et al. 1988) as in the current study. Generally, an increased litter size is associated with a decreased lamb survival, since the survival of twins is lower than that of singles (Slee et al. 1991; Kleemann and Walker 2005; Table 1), especially in unfavourable circumstances (Hinch et al. 1983). Multiples in Merinos maintained a survival rate of 72–73% compared with 85–89% for singles (Brien et al. 2009). The differences in birth type are not entirely explained by birthweight, since heavy twins have also been shown as susceptible (Morris et al. 2000). The current results implied that birth type affects survival both during the perinatal and subsequent phases of life (Table 1), but some studies have reported other findings. Scottish Blackface lambs born as multiples were more susceptible than were singles only during early neonatal periods (up to ~14 days), although multiples had a greater chance of surviving birth (Sawalha et al. 2007). Unexpectedly, Maxa et al. (2009) reported survival to 24 h as greater in Texel lambs born as twins than in triplets and singles, but Riggio et al. (2008) found triplets more susceptible than singles or twins across all periods considered. In the present study, experienced ewes were found to be better mothers than primiparous ewes (Table 1). Lower offspring mortality rates in older dams are common in the literature (Owens et al. 1985; Morris et al. 2000; Southey et al. 2001; Everett-Hincks et al. 2005; Riggio et al. 2008). However, the best lamb-rearing performance is likely to be found in ewes of intermediate age, since lambs borne by older ewes also have a lower survival rate (Sawalha et al. 2007; Brien et al. 2009; Maxa et al. 2009). Phenotypic results of TsvBW, PNsvBW and P3svBW (Fig. 2) derived from including BW as a covariate to survival traits aligned with the consensus of a curvilinear relationship on the phenotypic level (Hall et al. 1995; Sawalha et al. 2007; Hatcher et al. 2009; Maxa et al. 2009; Everett-Hincks et al. 2014). Both heavy and light lambs are prone to higher mortality rates, but the symptoms surrounding the cause of death tend to be different between very light or heavy lambs (Refshauge et al. 2016). Morris et al. (2000) reasoned that heavier lambs have a good chance of survival once they get through the birth process but could be more susceptible to complications close to birth. Current results suggested that both very light and very heavy lambs are more likely to succumb within the early perinatal phase, with BW being less important to survival at later stages to weaning.
Variance ratios
Random variance components of lamb survival
Lamb survival has often been associated with low to very low estimates of h2, as was also reported in Table 3. These results are suggestive of poor prospects of genetic improvement (Brien et al. 2014). The present study found Tsv to be lowly heritable at 0.07, but with a h2 of more than two-fold the mean h2 estimate of 0.03 derived from earlier literature by Safari et al. (2005), as well as more recent results also derived by linear models for Merinos (0.03; Brien et al. 2009; Hatcher et al. 2010). The method used to derive genetic parameters could be influential in varying estimates across studies. Previous results from the same flock were derived from threshold analysis and yielded estimates that differed by a large margin (Cloete et al. 2009). From that study, a high h2 of 0.28 was reported for Tsv, with a significant maternal genetic effect of 0.07 as well as a negative direct-maternal genetic correlation of –0.61. Comparing these ratios with the current results suggests that differences in parameter estimates could be related to analysing survival data on either the observed or the underlying scale. Similarly, Matos et al. (2000) reported that the h2 of survival from birth to weaning from a threshold model amounted to about three-fold the magnitude of the value derived by linear models in the same study. However, a moderate h2 estimate of 0.11 for Tsv has also been derived from threshold models (Welsh et al. 2006). Compared with the present results, high h2 estimates of 0.18–0.33 have also been reported for postnatal survivability derived from sire models (Sawalha et al. 2007).
However, in numerous instances, estimates of h2 have been consistently low across different methods. Using both logit-transformed and linear analyses, Morris et al. (2000) and Lopez-Villalobos and Garrick (1999) reported h2 estimates not different from zero or 0.01 respectively. All models, including a logit-transformation, estimated h2 values of <0.01 for survival to weaning for a large study of a range of New Zealand composite breeds (Everett-Hincks et al. 2014). For survival to 12 weeks, Riggio et al. (2008) reported relatively larger h2 estimates of 0.05 ± 0.03 and 0.08 ± 0.06 from linear and probit-transformed analyses respectively. While the current h2 estimate is also low, it coincides with the upper end of these estimates, and is the highest thus far reported from linear analysis.
The present study excluded maternal genetic effect from the final model for all survival traits (Tables 2, 3). Maternal effects are generally expected to have both genetic and environmental components (Bradford 1972). Similarly, Muller et al. (2020) found no evidence of a significant m2 component in Tsv of Dormer lambs, but this contradicted a substantial body of evidence that has found m2 to be influential to survival, albeit generally low, with a range between 0.02 and 0.08 (Lopez-Villalobos and Garrick 1999; Morris et al. 2000; Welsh et al. 2006; Brien et al. 2009; Hatcher et al. 2010; Everett-Hincks et al. 2014; Vanderick et al. 2015) and a mean of 0.05 ± 0.01 (Safari et al. 2005). Also, m2 is equivalent to rearing ability when survival is evaluated as a trait of the ewe, and several studies have also reported the genetic component of ewe rearing ability as low, but significant with h2 between 0.01 and 0.08 (Safari et al. 2005; Afolayan et al. 2008; Hebart et al. 2010; Bunter et al. 2016), which provides further evidence to support maternal genetic effects. The exclusion of m2 from the current operational model is therefore unexpected and contradicts the majority of the literature. It is likely that the model choice is an artefact of conflicted partitioning of variance between the m2 and mc2 components. In preliminary analysis, the m2 derived from Model II were significant and consistent with the literature cited above, but the maternal genetic variance was deflated to negligibly low values when both mc2 and m2 were included in Model IV. The support for Model IV was guided by the LRT, but was also preferred by criteria based on the Bayesian information criterion (data not shown). Management of the Elsenburg flock maintains breeding ewes for at least four to five seasons, which resulted in an average >4.3 survival records per dam. This can be considered as a strong structure for partitioning maternal effects in sheep across dam generations, which further makes the contradicting result unexpected. Given the discussion above, the obtained results should be accepted until they are possibly refuted in future studies involving more data. The Elsenburg flock currently lacks estimates of ewe rearing ability, but this could deliver an opportunity to revisit the topic in the near future.
Treating early survival as a partitioned period showed different variance ratios for survival between pre- and post-perinatal (3-days of age) phases, at 0.03 and 0.07 respectively (Table 3). Genetic variation in lamb survival between birth and weaning is influenced by the age of the lamb (Southey et al. 2001; Sawalha et al. 2007; Riggio et al. 2008). Current findings suggest that success of the new-born lamb was predominantly dependent on the environment, with a small direct genetic component. Following the initial 3 days after birth, the influence of the direct genetic effect of the lamb became proportionally more important to survival as h2 increased in magnitude. Similarly, Hatcher et al. (2010) reported a direct h2 of only 0.02 for survival to 7 days of age, but a larger value of 0.05 for the period 7–100 days. In turn, Brien et al. (2009) reported declining h2 estimates associated with an increasing lamb age, being highest at birth (0.07). For survival at 24 h, Riggio et al. (2008) reported a high h2 estimate of 0.33 ± 0.11 from probit-transformed data and an estimate of 0.09 ± 0.03 from a linear analysis. However, generally, a range between 0.01 and 0.07 (Morris et al. 2000; Maxa et al. 2009; Everett-Hincks et al. 2014) suggested that the h2 of perinatal survival was low, but m2 was similar or larger than for direct genetic effects, with a range between 0.02 and 0.10 (Morris et al. 2000; Maxa et al. 2009; Everett-Hincks et al. 2014). However, a recent study compared neonatal mortality and rectal temperature of the H- and L-line lambs across a cold-stress gradient, and reported the differences between lines to be dependent on the severity of cold stress during the neonatal period (Nel et al. 2021). These results were strongly suggestive that a genotype by environment interaction could be important when analysing neonatal mortality/survival, which would affect estimates of h2 by the animal model that assumes genotypes are evaluated under equal opportunity.
An influential mc2 component is well supported in the literature, but it should be considered that the current estimates of mc2 would likely also reflect the influence of maternal genetic effects, as detailed in the discussion above. The study by Muller et al. (2020) similarly reported a mc2 of 0.03, but also included litter variance ratio of 0.12 for Tsv in Dormer lambs. Other studies have been able to separate maternal genetic effects. For survival to weaning, Barwick et al. (1990) and Lopez-Villalobos and Garrick (1999) reported a mc2 estimate of 0.09, larger than the observed h2 and m2 effects combined (0.04; both studies). When considering survival to an early timepoint, Morris et al. (2000) also reported a mc2 component of 0.10 from a linear model for survival up to 24 h. Comparing the magnitude of estimates between PNsv and P3sv suggested that the effect of mc2 declined with age of the lamb (Table 3). Similarly, Hatcher et al. (2010) reported ‘dam repeatability’ as 0.12 between 1 and 7 days, but reported about half this magnitude (0.06) during the period between 7 and 100 days. Observing a relatively high maternal influence in the perinatal period is not surprising, given the high dependence of lambs during the first days of age (Dwyer 2008a), but would further be expected when perinatal survival is defined to include mortalities at birth, which is currently the case.
Since maternal genetic effects were not fitted, the current results also excluded the direct-maternal genetic correlation (ram) as a component of variance affecting lamb survival. This is in contradiction to the negative and large ram estimate of –0.60 previously reported by Cloete et al. (2009), an estimate that was similar to the ram of –0.75 also derived from threshold analysis by Welsh et al. (2006). The issue surrounding ram is pertinent if found to have a biological significance, since it counter-intuitively implies that ewes with a high genetic tendency for survival would tend to be poor mothers. Also, negative ram components inflate estimates of h2, m2 or mc2 (Fozi et al. 2005), causing discrepancies across studies. Hatcher et al. (2010) included σ2am, but found ram to be not different from zero. In another study (Maxa et al. 2009), estimates varied widely across different breeds (–0.79 for Shropshire; –0.52 for Texel; and –0.05 for Oxford Down) in analyses based on the logit-link function. Other logit-transformed models estimated positive ram values at values above the theoretical threshold of 1, while results derived from linear analysis in the same study found ram to be negative, but of a more feasible margin (Morris et al. 2000). Using linear models, Vanderick et al. (2015) reported a low to moderately negative range of ram values between –0.14 and –0.44, while results from logit-transformed phenotypes were positive, but very low at 0.02–0.03. Other negative estimates varied from –0.23 (Lopez-Villalobos and Garrick 1999) to –0.74 (Everett-Hincks et al. 2005), to exceeding –1 (Burfening 1993), while ram was also reported to be positive between 0.01 and 0.62 (Barwick et al. 1990; Matos et al. 2000; Sawalha et al. 2007). It is evident from the literature cited that it is difficult to pinpoint a reasonable expected range for ram, since the reported range of estimates in the literature shows little to no consistency across studies.
Birthweight
The h2 of BW was moderate, and m2 was the largest component affecting the trait (Table 3). The respective values of 0.16 and 0.20 were quite similar to previous estimates derived from the Elsenburg flock using fewer data (Cloete et al. 2003, 2009). The current estimate of h2 (0.16) agrees well with values of 0.15 (Riggio et al. 2008) and 0.18 (Safari et al. 2007), but a slightly higher range between 0.20 and 0.24 has also been reported (Snyman et al. 1995; Al-Shorepy and Notter 1998; Safari et al. 2005; Hatcher et al. 2010). The moderate m2 estimate of 0.20 coincides well with previous estimates of 0.18–0.19 (Safari et al. 2007; Riggio et al. 2008) or the mean of 0.21 ± 0.03 across studies (Safari et al. 2005). However, some previous estimates for m2 are considerably lower at 0.06–0.09 (Snyman et al. 1995; Hatcher et al. 2010), while a uniquely high value of 0.38 has also been reported by Al-Shorepy and Notter (1998). The observed mc2 agreed well with a previous estimate of 0.12 (Snyman et al. 1995). Safari et al. (2007) reported a small mc2 at 0.07, but the study also included a litter effect of 0.33 that was not considered in the current analysis. Permanent environmental effects of a similar magnitude (0.10–0.16) have also been reported in the literature (Safari et al. 2005; Riggio et al. 2008).
The additional ram component accounting for direct-maternal genetic covariance did not provide a better log-likelihood than did the nested model, suggesting no antagonism between high BW ewes and the weight of their new-born lambs. Similarly, Snyman et al. (1995) did not find this effect influential, and Hatcher et al. (2010) reported that ram did not differ from zero. Other studies found the effect significant and negative at between –0.15 and –0.31 (Safari et al. 2005, 2007; Cloete et al. 2009). However, if there is a true antagonism underlying these observations, it would not be considered problematic, since both high and low BW are not desirable on the phenotypic level (Fig. 2). If an ewe is genetically prone to a very high (or low) BW, it could be favourable that the maternal influence on the lamb does not lead to an extreme BW in the same direction or of the same magnitude.
Birth coat score
Very few estimates of h2 of BCS derived from animal models were found in the literature. The current estimate of 0.54 is within the range between very high estimates of 0.65 (Kemper et al. 2003) and 0.70 (Cloete et al. 2003, from the same flock) and a considerably lower estimate of 0.31 (Brien et al. 2010). Despite a considerable difference between these estimates, it can be assumed that fast genetic change is possible for BCS. However, the effect of mc2 was very small at 0.03. Other than a similar estimate of mc2 reported by Cloete et al. (2003) on the same flock but using fewer records, no other estimates were found in the literature.
Correlations
Despite the convenience of two-trait analysis with both traits on the observed scale, derived genetic correlation coefficients were often compromised by a low accuracy as reflected by large standard errors. Birthweight and mortality have long been linked (Smith 1977), but the curvilinear relationship in Fig. 3 complicates the use of BW as a correlated trait to genetically improve lamb survival. In correspondence with results in Table 4, previous results in Merinos have also reported that the genetic relationship between BW and Tsv is not different from zero (Cloete et al. 2009; Brien et al. 2010; Hatcher et al. 2010), but another study found positive genetic correlations of BW with perinatal survival (0.24) and survival to 4 weeks (0.45; Riggio et al. 2008). The current rg for BW and PNsv, albeit non-significant, was unexpected in its moderate magnitude and the fact that it was opposite in direction to the relationships on all non-genetic levels. This contrast was not consistent with other results, since the opposite was reported for Scottish Blackface lambs where neonatal mortality and BW were positively correlated at 0.21, but with a negative phenotypic relationship of –0.25 (Sawalha et al. 2007). Generally, the low-positive relationship of BW with survival on the maternal permanent environmental, environmental and phenotypic levels could be due to the very high susceptibility of extremely small lambs (Fig. 2) that skews the intermediate optimum slightly higher than the mean of 3.85.
The use of BCS as a correlated trait to improve lamb survival is also limited. A hairy birth coat has been lowly correlated with lamb survival in Merinos (Brien et al. 2010), but the current findings found a significant genetic relationship with BCS only for P3sv (Table 4). This moderate negative genetic correlation was in favour of hairier lambs. Earlier studies from climate chambers (Alexander 1961) or water bath tests (Slee 1978; Slee et al. 1991) suggested that longer or hairier coats could alleviate the rate of heat loss in lambs. This, in turn, was associated with the perinatal period and a reduced mortality stemming from exposure (Geenty et al. 2014). In this regard, the genetic correlation with P3sv, and not PNsv, was unexpected, since thermoregulation is considered most critical in the neonatal period (Plush et al. 2016). However, the precision of the genetic correlation in the perinatal period was poor and the 95% confidence interval included 0. It is possible that the benefits of a hairier coat (or low BCS) are restricted to lambs born in severe environmental circumstances (Mullaney 1966), causing difficulty in deriving robust estimates of the genetic (co)variance since the bulk of lambs were born in relatively benign conditions.
Genetic trends
The genetic trends reported in the present study (Table 5) contradicted the premises that the h2 of lamb survival was too low to support genetic gains in the desired direction (Fig. 3). They provided an opposing view to the suggestion that genetic improvement of lamb survival is unlikely to be worthwhile (Everett-Hincks et al. 2005; Everett-Hincks and Cullen 2009). Substantial genetic gains were evident in the genetic trends of the H-line, and selection group differences were also clearly reflected on the phenotypic level (Table 1). Assuming an h2 of 0.03, genetic responses in lamb survival were predicted to amount to only ~14% (Brien et al. 2009) and 26% (Fogarty et al. 2006) of the rate possible for genetic gain in clean fleece weight (CFW, assumed to have a h2 of 0.42 in both studies). As a point of reference in South African Merinos, a resource flock subject to positive selection for CFW reported gains of 1.2% p.a. (Cloete et al. 2007). The current gain in lamb survival of nearly 0.80% per annum is ~66% of the rate achieved for CFW, which far exceeds these previous predictions, especially since gain in Tsv hinged on a correlated response to selection for NLW. However, a more conservative rate of gain of 0.68% p.a. could be considered a more appropriate selection response by adjusting for the slight upward gain observed in the L-line. However, the genetic trend for Tsv appeared to show that most of the genetic gain was made in the initial years of selection. The initial (A) coefficient derived from broken-stick regression suggested genetic change to be as much as 1% of the mean between 1986 and 2006 (Table 5). Accordingly, the H- and L-lines were phenotypically separable by survival performance within 10 years after the onset of the selection experiment (Cloete and Scholtz 1998). The selection plateau indicated by the CP of the H-line genetic trend could, in part, be due to the influence of external genetics introduced to the H-line about the year 2008. Animals born as progeny (50% pure) or subsequent grand-progeny (25% pure) of external sires are likely to have partially counteracted the divergent genetics manifested between the pure H- and L-lines. However, observed breeding values were also likely to be approaching the boundaries of what is achievable under the current selection regime. The upper threshold of ~0.2 observed in breeding values (Fig. 4) could be expected, since mean Tsv was 0.77 and survival is bounded at below unity (Alexander 1984). In a scenario where the best-ranked animals reach this level, further selection for lamb survival would likely be met with diminishing gains, especially since positive results from first-cross animals suggested influential non-additive genetic components to lamb survival (Fogarty 1972; McGuirk et al. 1978).
In turn, the L-line technically also responded against the direction of selection for Tsv, but a lower-level plateau is also expected since selection for lower levels of fitness directly opposes the influence of natural selection. Also, while the dams of dead lambs could remain in the flock to produce siblings that survive, selection intensity for reducing NLW is limited by the need to perpetuate the numbers of the L-line. For the partitioned periods of survival, diverging trends were most pronounced in P3sv (Fig. 3). The perinatal period was partially masked by the environment provided by the ewe, but a higher h2 was observed for P3sv, which explains direct genetic gains of a higher magnitude in the H-line for this trait than for PNsv. However, the amount of variation observed for PNsv was high, considering it included mortalities only up to 3 days of age (including stillbirths), and the gains during this period were also favourable and worthwhile despite the low h2.
The low levels of consistency in genetic trends for BW and BCS (Figs 5, 6) compared to the relative consistency of trends of survival traits (Fig. 3) suggested weaker underlying genetic relationships with the NLW phenotype compared to survival traits. The downward direct genetic trend of the H-line for BW was of a very low magnitude in both linear and broken-stick regression lines (Table 5, Fig. 5) and is better considered as a stabilising response that trended within the optimal range for BW. In turn, it was interesting to see conflicting directions in maternal genetic trends as H-line dams tended to support slightly heavier BWs. These opposing responses are suggestive that a negative direct-maternal covariance could exist for BW, but its magnitude was below the threshold for denoting significance in the present analysis. Also, neither direct nor the maternal genetic change p.a. were of a large magnitude. Given the intermediate optimum of BW and the strong phenotypic relationship with survival, it is likely that natural selection also provided a stabilising effect on genetic trends for BW in the L-line. This argument is supported by the fact that the phenotypic relationships of lamb survival with BW were robust across selection lines (Fig. 2).
Regarding BCS, the persistently higher trend of the L-Line (since 2005) suggested that a genetic tendency towards woollier coats were associated with the selection response of the L-line to negative selection for NLW (Fig. 6) and BCS differences between selection groups were also reflected on the phenotypic level (Table 1). However, a genetic relationship of woollier coats with lower survival was only observed in P3sv (Table 4). Most correlation coefficients involving BCS were estimated with poor precision, and further investigation is needed to elucidate a possible effect of BCS on survival.
Lamb survival in selection indices
Snowder and Fogarty (2009) promoted the use of composite trait selection to increase overall ewe productivity, reasoning that the composite traits could act as an appropriate biological index to balance relationships between underlying component traits. The present study has confirmed the general findings of Cloete et al. (2009), namely, that favourable and worthwhile responses in a correlated component trait such as lamb survival are possible by selection for NLW. This reasoning is supported by positive genetic correlations between lamb survival and NLW in Merinos (Swan et al. 2001; Afolayan et al. 2008; Hebart et al. 2010). However, determining an appropriate selection criterion requires multiple factors to be considered. First, composite trait selection is also associated with risks. Selection for NLW also depends on the number of lambs born and would favour ewes bearing multiples. An increase in multiples is important, given substantial and negative genetic and phenotypic correlations between survival and litter size (Swan et al. 2001; Hebart et al. 2010; Bunter et al. 2018). Also, litter size, as a trait of the ewe, has a higher h2 than does lamb survival (Afolayan et al. 2008; Hebart et al. 2010), which could upset the balance assumed by composite trait selection. Combined with the higher fertility rates of the H-line (Cloete et al. 2004), the present study has shown that survival can be improved, along with an increased lambing rate. Phenotypic predictions (Fig. 1) suggested that most of the gains in the H-line were made by better survival rates of multiples. However, the greater likelihood of multiples to succumb, including in the H-line, remained evident. Accordingly, the simulation study by Amer et al. (1999) showed diminishing economic returns from genetic gain in reproductive rate if lamb survival is not simultaneously improved. Furthermore, there is evidence that survival as multiples and singles are different genetic traits (Bunter et al. 2018) and there could be benefit in targeting survival of multiples specifically. Also, while the response observed in the Elsenburg Merino flock was favourable, NLW would not be treated with the same emphasis in traditional indices, and the underlying response in component traits is unlikely to be of the same magnitude. In fact, Brien et al. (2010) predicted a genetic decline in survival if represented only as a component of NLW in a selection index. An alternative to composite trait selection could also be to focus on ewe rearing ability, a trait that is currently being incorporated into Australian Sheep Breeding Values (Bunter et al. 2019, 2021). However, greater clarity on maternal genetic components is needed, given the unexpected lack of a significant m2 for lamb survival in the current study (Table 3).
For these reasons, it is recommended that lamb survival phenotypes should be recorded and incorporated into indices individually. Direct selection will require large, comprehensive datasets (Brien et al. 2010, 2014), which requires the inclusion of dead lambs into pedigrees, which is currently not routine in South African Merinos. However, adequate record keeping of survival is feasible and delivers an opportunity to optimise both breeding and welfare objectives. For example, mortality can be reduced by assistance at birth, but recording of such incidences can contribute to the breeding value of relevant animals. Conversely, animals lost to misadventure or predation can be recorded as alive to avoid random, non-genetic events from interfering with breeding values related to fitness. A good example can be seen in the phenotyping protocol outlined by Vanderick et al. (2015), a system that should not be excessively difficult or expensive to accommodate since most breeding flocks require early identification and tagging of lambs as part of general management and pedigree recording.
Conclusions
Lamb survival in the H-line has been markedly higher than that in the L-line for the past two decades. This result confirmed previous studies on the same resource flock, namely that selection for NLW benefited lamb survival as a correlated response. The present study also confirmed that the direct heritability of survival was low or very low, but maintained that worthwhile gain in survival traits is achievable by genetic selection. Although survival in the H-line reached a plateau from ~2005, the favourable status of the selection line could be maintained despite the introduction of external genetics. The potential benefit of using either BW or BCS as indirect selection criteria was limited, but the significant genetic correlation of BCS with P3sv should be studied further. Wherever possible, survival traits should be recorded and evaluated individually in national ovine evaluation schemes. Although the infrastructure to record Tsv is present in South Africa, it has so far been poorly supported by the industry.
Data availability statement
The data that support this study will be shared on reasonable request to the corresponding author.
Conflicts of interest
The authors declare that there are no competing conflicts of interest to be reported.
Declaration of funding
The study hinged on external funding supplied by the South African Wool Industry (Initially the South African Wool Board, and subsequently, Cape Wools SA), the Western Cape Agricultural Research Trust and the Technology and Human Resources for Industry Program of the South African National Research Foundation. None of the authors stands to benefit in a financial or any other material sense from the results reported in this paper.
Acknowledgements
The authors gratefully acknowledge the technical support of Miss E. du Toit, Mr J. E. Fourie and Mrs A. C. M. Kruger as well their teams for the management and husbandry of the study population as well as the recording of data.
References
Afolayan RA, Fogarty NM, Gilmour AR, Ingham VM, Gaunt GM, Cummins LJ (2008) Reproductive performance and genetic parameters in first cross ewes from different maternal genotypes. Journal of Animal Science 86, 804–814.| Reproductive performance and genetic parameters in first cross ewes from different maternal genotypes.Crossref | GoogleScholarGoogle Scholar | 18156352PubMed |
Al-Shorepy SA, Notter DR (1998) Genetic parameters for lamb birth weight in spring and autumn lambing. Animal Science 67, 327–332.
| Genetic parameters for lamb birth weight in spring and autumn lambing.Crossref | GoogleScholarGoogle Scholar |
Alexander G (1961) Temperature regulation in the new-born lamb. iii. Effect of environmental temperature on metabolic rate, body temperatures, and respiratory quotient. Australian Journal of Agricultural Research 12, 1152–1174.
| Temperature regulation in the new-born lamb. iii. Effect of environmental temperature on metabolic rate, body temperatures, and respiratory quotient.Crossref | GoogleScholarGoogle Scholar |
Alexander G (1984) Constraints to lamb survival. In ‘Reproduction of sheep’. (Eds D Lindsay, D Pearce) pp. 199–209. (Australian Academy of Science and the Australian Wool Corporation: Melbourne, Vic., Australia)
Alexander G, Lynch JJ, Mottershead BE, Donnelly JB (1980) Reduction in lamb mortality by means of grass wind-breaks: results of a five-year study. Proceedings of the Australian Society of Animal Production 13, 329–331.
Amer PR, McEwan JC, Dodds KG, Davis GH (1999) Economic values for ewe prolificacy and lamb survival in New Zealand sheep. Livestock Production Science 58, 75–90.
| Economic values for ewe prolificacy and lamb survival in New Zealand sheep.Crossref | GoogleScholarGoogle Scholar |
Atkins KD (1980) Selection for skin folds and fertility. Proceedings of the Australian Society of Animal Production 13, 174–176.
Barwick SA, Leymaster KA, Keele JW, Harvey WR (1990) Estimates of genetic parameters for lamb survival to weaning and birth weight in the US Suffolk. Proceedings of the Association for the Advancement of Animal Breeding and Genetics 8, 331–334.
Behrendt R, Van Burgel AJ, Bailey A, Barber P, Curnow M, Gordon DJ, Edwards JEH, Oldham CM, Thompson AN (2011) On-farm paddock-scale comparisons across southern Australia confirm that increasing the nutrition of Merino ewes improves their production and the lifetime performance of their progeny. Animal Production Science 51, 805–812.
| On-farm paddock-scale comparisons across southern Australia confirm that increasing the nutrition of Merino ewes improves their production and the lifetime performance of their progeny.Crossref | GoogleScholarGoogle Scholar |
Binns SH, Cox IJ, Rizvi S, Green LE (2002) Risk factors for lamb mortality on UK sheep farms. Preventive Veterinary Medicine 52, 287–303.
| Risk factors for lamb mortality on UK sheep farms.Crossref | GoogleScholarGoogle Scholar | 11849723PubMed |
Bradford GE (1972) The role of maternal effects in animal breeding: VII. Maternal effects in sheep. Journal of Animal Science 35, 1324–1334.
| The role of maternal effects in animal breeding: VII. Maternal effects in sheep.Crossref | GoogleScholarGoogle Scholar | 4570696PubMed |
Brien F, Hebart ML, Hocking Edwards JE, Greeff JC, Hart KW, Refshauge G, Gaunt G, Behrendt R, Thomson K, Hinch G, Geenty KG, Van Der Werf JHJ (2009) Genetics of lamb survival: preliminary studies of the information nucleus flock. Proceedings of the Association for the Advancement in Animal Breeding and Genetics 18, 108–111.
Brien FD, Hebart ML, Smith DH, Edwards JEH, Greeff JC, Hart KW, Refshauge G, Bird-Gardiner TL, Gaunt G, Behrendt R, Robertson MW, Hinch GN, Geenty KG, Van Der Werf JHJ (2010) Opportunities for genetic improvement of lamb survival. Animal Production Science 50, 1017–1025.
| Opportunities for genetic improvement of lamb survival.Crossref | GoogleScholarGoogle Scholar |
Brien FD, Hinch GN, van der Werf JHJ, Brown DJ, Swan AA (2011) Selection strategies for the genetic improvement of reproductive performance in sheep. Proceedings of the Association for the Advancement in Animal Breeding and Genetics 19, 151–158.
Brien FD, Cloete SWP, Fogarty NM, Greeff JC, Hebart ML, Hiendleder S, Edwards JEH, Kelly JM, Kind KL, Kleemann DO, Plush KL, Miller DR (2014) A review of the genetic and epigenetic factors affecting lamb survival. Animal Production Science 54, 667–693.
| A review of the genetic and epigenetic factors affecting lamb survival.Crossref | GoogleScholarGoogle Scholar |
Bunter KL, Brown DJ (2015) Revisiting total weaning weight as a selection criterion. Proceedings of the Association for the Advancement of Animal Breeding and Genetics 21, 201–204.
Bunter KL, Swan AA, Purvis IW, Brown D (2016) Pregnancy scanning can be used as a source of data for genetic evaluation of reproductive traits of ewes. Animal Production Science 56, 679–689.
| Pregnancy scanning can be used as a source of data for genetic evaluation of reproductive traits of ewes.Crossref | GoogleScholarGoogle Scholar |
Bunter KL, Swan AA, Brown DJ, Brien FD, Smith J (2018) Litter size at lambing influences genetic evaluation of maternal rearing ability. Animal Production Science 58, 791–800.
| Litter size at lambing influences genetic evaluation of maternal rearing ability.Crossref | GoogleScholarGoogle Scholar |
Bunter KL, Swan AA, Gurman PM, Boerner V, McMillan AJ, Brown DJ (2019) Evolution of genetic evaluation for ewe reproductive performance. Proceedings of the Association for the Advancement of Animal Breeding and Genetics 23, 560–563.
Bunter KL, Swan AA, Gurman PM, Brown DJ (2021) New genomically enhanced reproduction breeding values for Merino sheep allow targeted selection for conception rate, litter size and ewe rearing ability. Animal Production Science 61, 333–336.
| New genomically enhanced reproduction breeding values for Merino sheep allow targeted selection for conception rate, litter size and ewe rearing ability.Crossref | GoogleScholarGoogle Scholar |
Burfening PJ (1993) Direct and maternal genetic effects on lamb survival. Small Ruminant Research 11, 267–274.
| Direct and maternal genetic effects on lamb survival.Crossref | GoogleScholarGoogle Scholar |
Cloete SWP, Scholtz AJ (1998) Lamb survival in relation to lambing and neonatal behaviour in medium wool Merino lines divergently selected for multiple rearing ability. Australian Journal of Experimental Agriculture 38, 801–811.
| Lamb survival in relation to lambing and neonatal behaviour in medium wool Merino lines divergently selected for multiple rearing ability.Crossref | GoogleScholarGoogle Scholar |
Cloete SWP, Olivier JJ, Van Wyk JB, Erasmus GJ, Schoeman SJ (2003) Genetic parameters and trends for birth weight, birth coat score and weaning weight in Merino lines divergently selected for ewe multiple rearing ability. South African Journal of Animal Science 33, 248–256.
| Genetic parameters and trends for birth weight, birth coat score and weaning weight in Merino lines divergently selected for ewe multiple rearing ability.Crossref | GoogleScholarGoogle Scholar |
Cloete SWP, Gilmour AR, Olivier JJ, Van Wyk JB (2004) Genetic and phenotypic trends and parameters in reproduction, greasy fleece weight and liveweight in Merino lines divergently selected for multiple rearing ability. Australian Journal of Experimental Agriculture 44, 745–754.
| Genetic and phenotypic trends and parameters in reproduction, greasy fleece weight and liveweight in Merino lines divergently selected for multiple rearing ability.Crossref | GoogleScholarGoogle Scholar |
Cloete SWP, Olivier JJ, Olivier WJ (2007) Genetic change in South African Merino resource flocks. Proceedings of the Association for the Advancement of Animal Breeding and Genetics 17, 320–323.
Cloete SWP, Misztal I, Olivier JJ (2009) Genetic parameters and trends for lamb survival and birth weight in a Merino flock divergently selected for multiple rearing ability. Journal of Animal Science 87, 2196–2208.
| Genetic parameters and trends for lamb survival and birth weight in a Merino flock divergently selected for multiple rearing ability.Crossref | GoogleScholarGoogle Scholar |
Davies RB (1987) Hypothesis testing when a nuisance parameter is present only under the alternative. Biometrika 74, 33–43.
| Hypothesis testing when a nuisance parameter is present only under the alternative.Crossref | GoogleScholarGoogle Scholar |
Dwyer CM (2008a) Genetic and physiological determinants of maternal behavior and lamb survival: implications for low-input sheep management. Journal of Animal Science 86, E246–E258.
| Genetic and physiological determinants of maternal behavior and lamb survival: implications for low-input sheep management.Crossref | GoogleScholarGoogle Scholar | 17709772PubMed |
Dwyer CM (2008b) The welfare of the neonatal lamb. Small Ruminant Research 76, 31–41.
| The welfare of the neonatal lamb.Crossref | GoogleScholarGoogle Scholar |
Everett-Hincks JM, Cullen NG (2009) Genetic parameters for ewe rearing performance. Journal of Animal Science 87, 2753–2758.
| Genetic parameters for ewe rearing performance.Crossref | GoogleScholarGoogle Scholar | 19502504PubMed |
Everett-Hincks JM, Lopez-Villalobos N, Blair HT, Stafford KJ (2005) The effect of ewe maternal behaviour score on lamb and litter survival. Livestock Production Science 93, 51–61.
| The effect of ewe maternal behaviour score on lamb and litter survival.Crossref | GoogleScholarGoogle Scholar |
Everett-Hincks JM, Mathias-Davis HC, Greer GJ, Auvray BA, Dodds KG (2014) Genetic parameters for lamb birth weight, survival and death risk traits. Journal of Animal Science 92, 2885–2895.
| Genetic parameters for lamb birth weight, survival and death risk traits.Crossref | GoogleScholarGoogle Scholar | 24802039PubMed |
Falconer DS (1989) ‘Introduction to Quantitative Genetics.’ (Longmans Green/John Wiley & Sons: Harlow, Essex, UK)
Fogarty NM (1972) Crossbreeding for lamb production. 1. Survival and growth of first cross lambs. Australian Journal of Experimental Agriculture 12, 234–239.
| Crossbreeding for lamb production. 1. Survival and growth of first cross lambs.Crossref | GoogleScholarGoogle Scholar |
Fogarty NM, Ingham VM, Gilmour AR, Cummins LJ, Gaunt GM, Stafford J, Edwards JEH, Banks RG (2005) Genetic evaluation of crossbred lamb production. 1. Breed and fixed effects for birth and weaning weight of first-cross lambs, gestation length, and reproduction of base ewes. Australian Journal of Agricultural Research 56, 443–453.
| Genetic evaluation of crossbred lamb production. 1. Breed and fixed effects for birth and weaning weight of first-cross lambs, gestation length, and reproduction of base ewes.Crossref | GoogleScholarGoogle Scholar |
Fogarty NM, Safari E, Gilmour AR, Ingham VM, Atkins KD, Mortimer SI, Swan AA, Brien FD, Greeff JC, van der Werf JHJ (2006) Wool and meat genetics: the joint possibilities. International Journal of Sheep and Wool Science 54, 22–27.
Fozi MA, Van Der Werf JHJ, Swan AA (2005) The importance of accounting for maternal genetic effects in Australian fine-wool Merino breeding. Australian Journal of Agricultural Research 56, 789–796.
| The importance of accounting for maternal genetic effects in Australian fine-wool Merino breeding.Crossref | GoogleScholarGoogle Scholar |
Gama LT, Dickerson GE, Young LD, Leymaster KA (1991) Effects of breed, heterosis, age of dam, litter size, and birth weight on lamb mortality. Journal of Animal Science 69, 2727–2743.
| Effects of breed, heterosis, age of dam, litter size, and birth weight on lamb mortality.Crossref | GoogleScholarGoogle Scholar | 1885385PubMed |
Geenty KG, Brien FD, Hinch GN, Dobos RC, McCaskill M, Ball AJ, Behrendt R, Gore KP, Harden S, Hocking-Edwards JE, Hart K, van der Werf JHJ (2014) Reproductive performance in the Sheep CRC Information Nucleus across different sheep production environments in southern Australia. Animal Production Science 54, 715–726.
| Reproductive performance in the Sheep CRC Information Nucleus across different sheep production environments in southern Australia.Crossref | GoogleScholarGoogle Scholar |
Gilmour AR, Gogel BJ, Welham SJ (2015) ‘ASReml User Guide Release 4.1. Structural Specification.’ Available at www.vsni.co.uk
Gudex BW, Hickford JGH, Frampton CM (2005) Sire line differences in lamb losses from starvation-exposure. Proceedings of the New Zealand Society of Animal Production 65, 186–190.
Hall DG, Fogarty NM, Gilmour AR (1995) Performance of crossbred progeny of trangie fertility merino and booroola merino rams and poll dorset ewes 1. Lamb birth weight, survival and growth. Australian Journal of Experimental Agriculture 35, 1069–1074.
| Performance of crossbred progeny of trangie fertility merino and booroola merino rams and poll dorset ewes 1. Lamb birth weight, survival and growth.Crossref | GoogleScholarGoogle Scholar |
Hatcher S, Atkins KD, Safari E (2009) Phenotypic aspects of lamb survival in Australian Merino sheep. Journal of Animal Science 87, 2781–2790.
| Phenotypic aspects of lamb survival in Australian Merino sheep.Crossref | GoogleScholarGoogle Scholar | 19502501PubMed |
Hatcher S, Atkins KD, Safari E (2010) Lamb survival in Australian Merino Sheep: a genetic analysis. Journal of Animal Science 88, 3198–3205.
| Lamb survival in Australian Merino Sheep: a genetic analysis.Crossref | GoogleScholarGoogle Scholar | 20562357PubMed |
Haughey KG (1983) Selective breeding for rearing ability as an aid to improving lamb survival. Australian Veterinary Journal 60, 361–363.
| Selective breeding for rearing ability as an aid to improving lamb survival.Crossref | GoogleScholarGoogle Scholar | 6667215PubMed |
Hebart ML, Brien FD, Jaensch KS, Smith DH, Walkom SF, Grimson RJ (2010) Genetics of reproductive efficiency: a study of Merino resource flocks in South Australia. Communication 0685. In ‘Proceedings of the 9th World Congress on Genetics Applied to Livestock Production’, Leipzig, Germany.
Hinch GN, Brien F (2014) Lamb survival in Australian flocks: a review. Animal Production Science 54, 656–666.
| Lamb survival in Australian flocks: a review.Crossref | GoogleScholarGoogle Scholar |
Hinch GN, Kelly RW, Owens JL, Crosbie SF (1983) Patterns of lamb survival in high fecundity Booroola flocks. Proceedings of the New Zealand Society of Animal Production 43, 29–32.
Kemper KE, Smith JL, Purvis IW (2003) The value of birthcoat score as an early age selection criterion for superfine Merino sheep. Proceedings of the Association for the Advancement of Animal Breeding and Genetics 15, 139–142.
Kilgour RJ (1992) Lambing potential and mortality in Merino sheep as ascertained by ultrasonography. Australian Journal of Experimental Agriculture 32, 311–313.
| Lambing potential and mortality in Merino sheep as ascertained by ultrasonography.Crossref | GoogleScholarGoogle Scholar |
Kleemann DO, Walker SK (2005) Fertility in South Australian commercial Merino flocks: sources of reproductive wastage. Theriogenology 63, 2075–2088.
| Fertility in South Australian commercial Merino flocks: sources of reproductive wastage.Crossref | GoogleScholarGoogle Scholar | 15826674PubMed |
Kleemann DO, Walker SK, Walkley JRW, Ponzoni RW, Smith DH, Grimson RJ, Seamark RF (1993) Effect of nutrition during pregnancy on birth weight and lamb survival in FecB Booroola × South Australian Merino ewes. Animal Reproduction Science 31, 213–224.
| Effect of nutrition during pregnancy on birth weight and lamb survival in FecB Booroola × South Australian Merino ewes.Crossref | GoogleScholarGoogle Scholar |
Kleemann DO, Quigley SP, Bright R, Scott Q (2000) Survival and growth of Damara, Dorper, Dorset, Rambouillet, South African Meat Merino first cross lambs in semi arid Queensland. Asian–Australasian Journal of Animal Sciences 13, 173
Knight TW, Lynch PR, Hall DRH, Hockey HUP (1988) Identification of factors contributing to the improved lamb survival in Marshall Romney sheep. New Zealand Journal of Agricultural Research 31, 259–271.
| Identification of factors contributing to the improved lamb survival in Marshall Romney sheep.Crossref | GoogleScholarGoogle Scholar |
Lopez-Villalobos N, Garrick DJ (1999) Genetic parameter estimates for lamb survival in Romney sheep. Proceedings of the New Zealand Society of Animal Production 59, 121–124.
Lynch JJ, Alexander G (1980) The effect of time since shearing on sheltering behaviour by Merino sheep. Proceedings of the Australian Society of Animal Production 13, 325–328.
Matos CAP, Thomas DL, Young LD, Gianola D (2000) Genetic analyses of lamb survival in Rambouillet and Finnsheep flocks by linear and threshold models. Animal Science 71, 227–234.
| Genetic analyses of lamb survival in Rambouillet and Finnsheep flocks by linear and threshold models.Crossref | GoogleScholarGoogle Scholar |
Maxa J, Sharifi AR, Pedersen J, Gauly M, Simianer H, Norberg E (2009) Genetic parameters and factors influencing survival to twenty-four hours after birth in Danish meat sheep breeds. Journal of Animal Science 87, 1888–1895.
| Genetic parameters and factors influencing survival to twenty-four hours after birth in Danish meat sheep breeds.Crossref | GoogleScholarGoogle Scholar | 19251927PubMed |
McGuirk BJ, Bourke ME, Manwaring JM (1978) Hybrid vigour and lamb production. 2. Effects on survival and growth of first-cross lambs, and on wool and body measurements of hogget ewes. Australian Journal of Experimental Agriculture 18, 753–763.
| Hybrid vigour and lamb production. 2. Effects on survival and growth of first-cross lambs, and on wool and body measurements of hogget ewes.Crossref | GoogleScholarGoogle Scholar |
Morris CA, Hickey SM, Clarke JN (2000) Genetic and environmental factors affecting lamb survival at birth and through to weaning. New Zealand Journal of Agricultural Research 43, 515–524.
| Genetic and environmental factors affecting lamb survival at birth and through to weaning.Crossref | GoogleScholarGoogle Scholar |
Mortimer SI, Atkins KD (1997) Improvement of Merino reproductive performance through bloodline substitution and crossing. Proceedings of the Association for the Advancement of Animal Breeding and Genetics 12, 404–407.
Muggeo VMR (2008) Segmented: an R package to fit regression models with broken-line relationships. R News 8, 20–25.
Mullaney PD (1966) The relation of birth coat and lamb survival. Australian Journal of Experimental Agriculture 6, 84–87.
| The relation of birth coat and lamb survival.Crossref | GoogleScholarGoogle Scholar |
Muller A, Brand TS, Scholtz AJ, Kruger ACM, Cloete SWP (2020) Genetic and environmental parameters and trends for early growth and yearling traits in the Elsenburg Dormer resource flock. Small Ruminant Research 191, 106181
| Genetic and environmental parameters and trends for early growth and yearling traits in the Elsenburg Dormer resource flock.Crossref | GoogleScholarGoogle Scholar |
Nel CL, Cloete SWP, Kruger ACM, Dzama K (2021) Long term genetic selection for reproductive success affects neonatal lamb vitality across cold stress conditions. Journal of Thermal Biology 98, 102908
| Long term genetic selection for reproductive success affects neonatal lamb vitality across cold stress conditions.Crossref | GoogleScholarGoogle Scholar | 34016335PubMed |
Newman SA, Dodds KG, Clarke JN, Garrick DJ, McEwan JC (2000) The Sheep Improvement Limited (SIL) genetic engine. Proceedings of the New Zealand Society of Animal Production 60, 195–197.
Owens JL, Bindon BM, Edey TN, Piper LR (1985) Behaviour at parturition and lamb survival of Booroola Merino sheep. Livestock Production Science 13, 359–372.
| Behaviour at parturition and lamb survival of Booroola Merino sheep.Crossref | GoogleScholarGoogle Scholar |
Plush KJ, Brien FD, Hebart ML, Hynd PI (2016) Thermogenesis and physiological maturity in neonatal lambs: a unifying concept in lamb survival. Animal Production Science 56, 736–745.
| Thermogenesis and physiological maturity in neonatal lambs: a unifying concept in lamb survival.Crossref | GoogleScholarGoogle Scholar |
R Core Team (2020) R: a Language and Environment for Statistical Computing. Available at https://www.r-project.org/
Refshauge G, Brien FD, Hinch GN, Van De Ven R (2016) Neonatal lamb mortality: factors associated with the death of Australian lambs. Animal Production Science 56, 726–735.
| Neonatal lamb mortality: factors associated with the death of Australian lambs.Crossref | GoogleScholarGoogle Scholar |
Riggio V, Finocchiaro R, Bishop SC (2008) Genetic parameters for early lamb survival and growth in Scottish Blackface sheep. Journal of Animal Science 86, 1758–1764.
| Genetic parameters for early lamb survival and growth in Scottish Blackface sheep.Crossref | GoogleScholarGoogle Scholar | 18407992PubMed |
Robinson DL (1996) Models which might explain negative correlations between direct and maternal genetic effects. Livestock Production Science 45, 111–122.
| Models which might explain negative correlations between direct and maternal genetic effects.Crossref | GoogleScholarGoogle Scholar |
Safari E, Fogarty NM, Gilmour AR (2005) A review of genetic parameter estimates for wool, growth, meat and reproduction traits in sheep. Livestock Production Science 92, 271–289.
| A review of genetic parameter estimates for wool, growth, meat and reproduction traits in sheep.Crossref | GoogleScholarGoogle Scholar |
Safari E, Fogarty NM, Gilmour AR, Atkins KD, Mortimer SI, Swan AA, Brien FD, Greeff JC, Van Der Werf JHJ (2007) Across population genetic parameters for wool, growth, and reproduction traits in Australian Merino sheep. 1. Data structure and non-genetic effects. Australian Journal of Agricultural Research 58, 169–175.
| Across population genetic parameters for wool, growth, and reproduction traits in Australian Merino sheep. 1. Data structure and non-genetic effects.Crossref | GoogleScholarGoogle Scholar |
Sawalha RM, Conington J, Brotherstone S, Villanueva B (2007) Analyses of lamb survival of Scottish Blackface sheep. Animal 1, 151–157.
| Analyses of lamb survival of Scottish Blackface sheep.Crossref | GoogleScholarGoogle Scholar | 22444218PubMed |
Schoeman SJ, Cloete SWP, Olivier JJ (2010) Returns on investment in sheep and goat breeding in South Africa. Livestock Science 130, 70–82.
| Returns on investment in sheep and goat breeding in South Africa.Crossref | GoogleScholarGoogle Scholar |
Slee J (1978) The effects of breed, birthcoat and body weight on the cold resistance of newborn lambs. Animal Production 27, 43–49.
| The effects of breed, birthcoat and body weight on the cold resistance of newborn lambs.Crossref | GoogleScholarGoogle Scholar |
Slee J, Alexander G, Bradley LR, Jackson N, Stevens D (1991) Genetic aspects of cold resistance and related characters in newborn Merino lambs. Australian Journal of Experimental Agriculture 31, 175–182.
| Genetic aspects of cold resistance and related characters in newborn Merino lambs.Crossref | GoogleScholarGoogle Scholar |
Smith GM (1977) Factors affecting birth weight, dystocia and preweaning survival in sheep. Journal of Animal Science 44, 745–753.
| Factors affecting birth weight, dystocia and preweaning survival in sheep.Crossref | GoogleScholarGoogle Scholar | 558970PubMed |
Snowder GD, Fogarty NM (2009) Composite trait selection to improve reproduction and ewe productivity: a review. Animal Production Science 49, 9–16.
| Composite trait selection to improve reproduction and ewe productivity: a review.Crossref | GoogleScholarGoogle Scholar |
Snyman MA, Erasmus GJ, van Wyk JB, Olivier JJ (1995) Direct and maternal (co) variance components and heritability estimates for body weight at different ages and fleece traits in Afrino sheep. Livestock Production Science 44, 229–235.
| Direct and maternal (co) variance components and heritability estimates for body weight at different ages and fleece traits in Afrino sheep.Crossref | GoogleScholarGoogle Scholar |
Southey BR, Rodriguez-Zas SL, Leymaster KA (2001) Survival analysis of lamb mortality in a terminal sire composite population. Journal of Animal Science 79, 2298–2306.
| Survival analysis of lamb mortality in a terminal sire composite population.Crossref | GoogleScholarGoogle Scholar | 11583416PubMed |
Swan AA (2009) The economics of litter size in meat sheep. In ‘Use FecB (Booroola) gene in sheep breeding programs: proceedings of the Helen Newton Turner Memorial International Workshop’, Pune, India, 10–12 November 2008, pp. 173–176.
Swan AA, Piper LR, Brewer HG, Purvis IW (2001) Genetic variation in reproductive performance of fine wool Merinos. Proceedings of the Association for the Advancement of Animal Breeding and Genetics 14, 417–420.
Vanderick S, Auvray B, Newman SA, Dodds KG, Gengler N, Everett-Hincks JM (2015) Derivation of a new lamb survival trait for the New Zealand sheep industry. Journal of Animal Science 93, 3765–3772.
| Derivation of a new lamb survival trait for the New Zealand sheep industry.Crossref | GoogleScholarGoogle Scholar | 26440155PubMed |
Welsh CS, Garrick DJ, Enns RM, Nicoll GB (2006) Threshold model analysis of lamb survivability in Romney sheep. New Zealand Journal of Agricultural Research 49, 411–418.
| Threshold model analysis of lamb survivability in Romney sheep.Crossref | GoogleScholarGoogle Scholar |
Wickham H (2016) ‘ggplot2: Elegant Graphics for Data Analysis.’ (Springer-Verlag: New York, NY, USA) Available at https://ggplot2.tidyverse.org