Seroprevalence and potential risk factors of Leptospira interrogans serovar Hardjo infection in dairy cattle of Tanahun district, Nepal
Sabina Lamsal A * , Subir Singh A , Ram Chandra Sapkota B and Surendra Karki C
A * , Subir Singh A , Ram Chandra Sapkota B and Surendra Karki C
A
B
C
Abstract
Leptospirosis is a zoonotic disease, with significant economic and public health implications. Although the government of Nepal has enlisted leptospirosis as a prioritized zoonotic disease, limited studies and control policies have been designed to estimate its prevalence at the human–animal interface. Hence, the burden of Leptospira infection in the cattle population of Nepal is poorly understood.
A cross-sectional study was conducted from August 2022 to December 2022, to estimate the seroprevalence and identify potential risk factors for Leptospira interrogans serovar Hardjo infection in cattle of the Tanahun district, Nepal.
In total, 407 blood samples were collected from 216 dairy cattle herds ranging in size from 1 to 140 animals and covering all 10 municipalities of Tanahun. Extracted serum was examined using an indirect antibody ELISA test. Concurrently, a questionnaire survey was conducted among 216 farmers to understand their husbandry practices, along with their knowledge of zoonoses. Univariable followed by multivariable logistic regression was performed to determine the association of potential risk factors, with the presence of Leptospira infection.
Results showed that the herd-level and animal-level seroprevalence of Leptospira exposure was 6.48% (n = 14/216, 95% confidence interval (CI): 3.59–10.64) and 4.91% (n = 20/407, 95% CI: 3.20–7.47) respectively. In the final multivariable model, four herd-level factors, namely, buying and selling of animals within the district (P = 0.046, OR: 0.14, 95% CI: 0.02–0.97), working nature of farmers, i.e. both in field and in cow shed (P = 0.028, OR: 3.66, 95% CI: 1.15–11.61), presence of rodents (P = 0.037, OR: 26.92, 95% CI: 1.22–593.87), and a herd size of greater than three (P = 0.031, OR: 8.52, 95% CI: 1.21–59.89), were associated with seropositivity to Leptospira organism. Similarly, two animal-level factors, i.e. cattle origin (P = 0.018, OR: 2.76, 95% CI: 1.19–6.38) and tick infestations (P = 0.035, OR: 0.30, 95% CI: 0.10–0.92), were associated with Leptospira exposure. It was found that 58.3% (126/216) of the respondents were aware of zoonosis, whereas only 5.6% (12/216) of the farmers knew about leptospirosis as a zoonotic disease.
On the basis of these research findings, the farmers and general public should be made aware of this zoonosis and the key precautionary measures to be adopted.
A comprehensive study to estimate the national-level prevalence of Leptospira spp. is required for the development of control strategies following one health approach.
Keywords: dairy cattle, ELISA, Leptospira interrogans serovar Hardjo, leptospirosis, livestock, seroprevalence, Tanahun district, zoonosis.
Introduction
The agriculture sector contributes approximately 24% of gross domestic product (GDP) to the national economy of Nepal (AITC 2023), with the livestock sector contributing approximately a quarter of this (Department of Livestock Services 2022). Most Nepalese agriculture households rear at least one type of livestock such as cattle, buffalo, goat, sheep, pigs and poultry. Cattle rearing is a common practice in Nepal, with a total of 7.41 million head in the national herd (Ministry of Agriculture and Livestock Development 2022). Nepalese livestock farming is facing several challenges that include low production and productivity and a high burden of diseases, many of which are zoonotic.
Leptospirosis is one of the zoonotic diseases that are occasionally detected in Nepal in both animal and human populations (Shrestha et al. 2018). It is a globally recognised, yet a neglected, bacterial zoonotic disease (Bharti et al. 2003; Adler and de la Peña Moctezuma 2010). The global burden of leptospirosis is unknown but it is estimated that there are more than 500,000 human cases annually, with a fatality rate of up to 25% in some regions (Zakharova et al. 2021). Among the livestock population, species commonly affected by leptospirosis include cattle, buffalo, goat, sheep, horse and pig, resulting in huge economic losses to the farming communities because of the reproductive problems caused by the disease that include infertility, abortion, stillbirths, weak calves and decreased milk yield (Srivastava 2008; Hensley 2016; Fávero et al. 2017). This disease is caused by an aerobic, Gram-negative, actively motile spirochete of the genus Leptospira spp. The Leptospira interrogans serovar Hardjo is commonly isolated in cattle (Bolin 2000; Leonard et al. 2004; Guerra 2013). Disease transmission, in both humans and animals, can occur through direct contact with urine, tissue or body fluids of carrier animals and indirectly via contaminated soil or water. Human to human transmission has also been recorded through breast-feeding (Bolin and Koellner 1988).
Variable prevalence of Leptospira infection among domestic animals has been reported. Srivastava (2008) showed varying seroprevalence of Leptospira exposure in different species (5.4% in buffaloes, 7.5% in cattle, 12.5% in sheep, 14.6% in horses and 15.9% in dogs) in various states of India. Likewise, a seroprevalence of 40.5%, 31% and 16% in cattle, buffalo and pig respectively, has been reported in West Malaysia (Bahaman et al. 1987). The prevalence of leptospiral serotypes varies with Balamurugan et al. (2013) in Odisha, India, reporting the seroprevalence of L. interrogans serovar Hardjo antibodies to be 23.5%, whereas that for L. australis was as high as 50.9%, with others being lower in abundance. Similarly, in Gujarat, India, L. interrogans serovar Hardjo and L. pomona seroprevalence was found to be 15.56% and 28.89% respectively, in 398 sera screened from cattle (Patel et al. 2014).
Cattle are the major livestock reared in South-east Asian countries including Nepal, with L. interrogans serovar Hardjo being the predominant serovar in cattle. It poses a threat to cattle health in the region, as well as a significant risk to human health in rural and farming communities. Moreover, differences in prevalence among and within countries in the region, owing to variations in geography, climatic conditions, and management practices, together with the resistance exhibited by various breeds, are likely to occur (Hoffmann 2010). The prevalence of this disease is likely to be underestimated in both human and animal populations because of the lack of reliable diagnostic methods, under-reporting and inefficient surveillance facilities across countries in the region (Victoriano et al. 2009; Hartskeerl et al. 2011). The burden of leptospirosis is poorly understood in Nepal because of limited research being conducted, although this disease has been listed in the Nepal Gazette as a top 10 prioritised zoonotic disease in Nepal.
The aim of this study was to gain a greater understanding of the epidemiology of Leptospira infections in Nepal by focusing on serovar Hardjo prevalence in dairy cattle. Tanahun is an emerging district for commercial dairy farming in Nepal. However, only limited studies have been conducted to understand the burden of animal disease, including the zoonotic diseases in this district. To the best of our knowledge, no study has been conducted yet in Tanahun to understand the burden of Leptospira spp. in the cattle population. The objective of this research was to determine seroprevalence of Leptospira exposure in the cattle population of Tanahun, identify the associated risk factors in the herd and in individual animals and to know farmers awareness and level of understanding on zoonoses. The insights provided by this research should help guide disease control strategies for leptospirosis in Nepal.
Materials and methods
Study site and study population
The Tanahun district lies in Gandaki province, Nepal, latitude: 27.91666, longitude: 84.24999, with an area of 1546 km2 (864.25 m2) and a population of 323,288. Of the total area, 88% of it belongs to a tropical climate, with elevation ranging from 300 to 1000 m. Tanahun is an emerging district for commercial livestock farming and is a home to more than 51,000 cattle (Ministry of Agriculture and Livestock Development 2022).
Study design and time frame
A cross-sectional study was conducted from August 2022 to December 2022, covering all 10 municipalities of the Tanahun district. Blood samples were collected from dairy cattle by experienced technicians under the supervision of a veterinarian for the detection of Leptospira spp. A questionnaire survey was conducted concurrently with verbal consent obtained from the farmers before the sample was collected.
Sampling technique
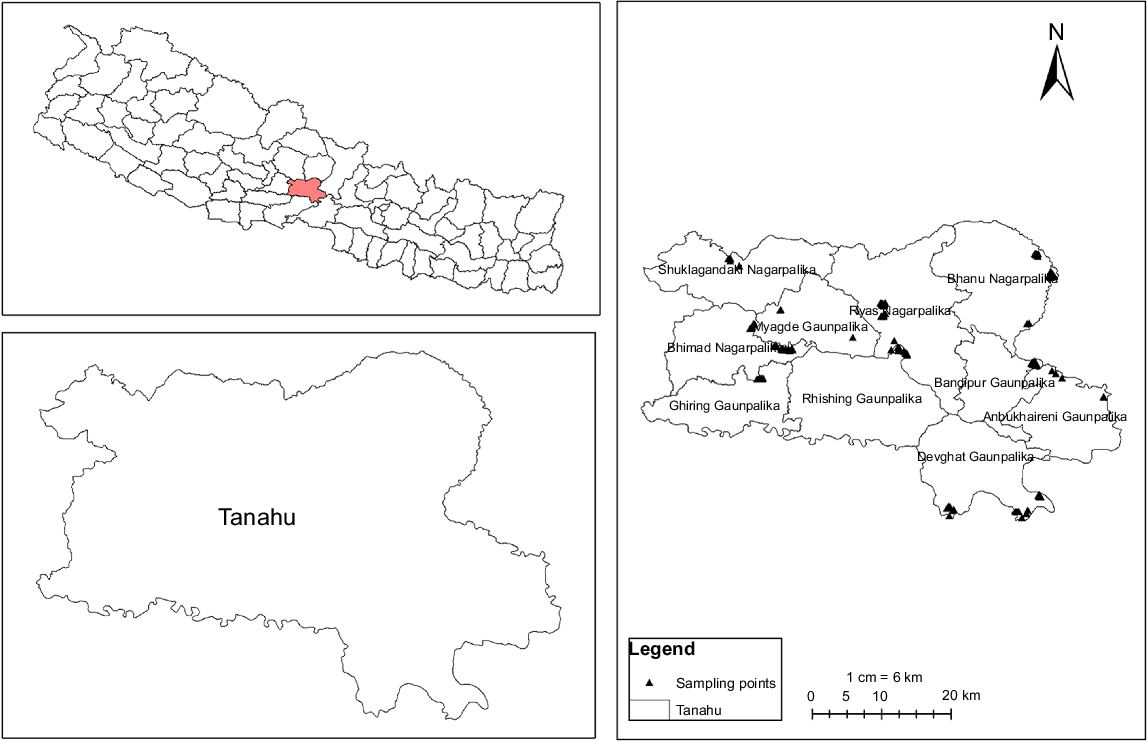
Samples were collected from each of the 10 municipalities located in Tanahun district by using a three-stage random sampling procedure (Fig. 1). The distribution of the cattle population present in each of the municipalities was assessed before finalizing the sampling regimen. At first, 42 villages or wards were selected randomly within the Tanahun district and then 216 farms or herds from the selected areas were randomly chosen for the study on the basis of the farmers willingness to participate and availability. Herd size was found to be ranging from 1 to 140 animals. On chosen farms, cattle were selected randomly and, on average, most farms had three cattle sampled.
Sample size calculation
Sample size was determined using an open-source epidemiological software package ‘Epi InfoTM’ (ver. 7.2.5.0; Epi InfoTM; Centers for Disease Control and Prevention 2022). The expected prevalence was set as 50%, with 5% desired accuracy at 95% confidence level. The total required sample size was estimated to be 381. Altogether, 407 blood samples from 216 herds were collected covering all 10 municipalities (Table 1).
| Serial number | Name of municipality | Cattle population | Number of cattle sampled | Percentage of total sample taken (%) | |
|---|---|---|---|---|---|
| 1. | Vyas (Vy) | 9240 | 72 | 17.69 | |
| 2. | Vimad (Vi) | 6967 | 54 | 13.27 | |
| 3. | Suklagandaki (Suk) | 3532 | 28 | 6.88 | |
| 4. | Bhanu (Bha) | 8473 | 67 | 16.46 | |
| 5. | Myagde (My) | 1247 | 10 | 2.46 | |
| 6. | Bandipur (Ban) | 7464 | 58 | 14.25 | |
| 7. | Aabukhaireni (Aab) | 1265 | 10 | 2.46 | |
| 8. | Devghat (Dev) | 8602 | 69 | 16.95 | |
| 9. | Rising (Ris) | 1750 | 14 | 3.44 | |
| 10. | Ghiring (Ghi) | 3177 | 25 | 6.14 | |
| Total | 51, 717 | 407 | 100 |
Questionnaire survey
A questionnaire survey was conducted in conjunction with the blood sampling by taking face to face interviews with farmers to understand their husbandry practices and awareness of zoonotic diseases. The questions basically included demographic information, farming practices and awareness of zoonotic diseases. Specific questions included age, sex, any physical abnormalities in the past or in present among family members, interaction with animals, urine and water contact, abundance of rodents, types of species/pets reared, daily milk production of cattle, biosecurity practices, food and nutrition, frequency of animal movements, and use of antibiotics. In addition, factors such as frequency of vehicle movements, agricultural land nearby, housing type, bedding type, feed contamination with soil, body condition score (BCS), tick infestations etc. were assessed by surveyor team themselves. The survey questions were pre-tested among 25 farmers residing in the study area and some were adjusted on the basis of the farmers’ responses.
Sample collection, storage and transport
Blood samples (n = 407) were collected from the jugular vein of dairy cattle within a period of 2 weeks. Whole blood (5 mL) was collected from each animal and serum was extracted by centrifugation at 1510g for 5 min at room temperature on the day of collection. Serum samples were stored at −20°C until processed for serology. An enzyme linked immunosorbent assay (ELISA) was then performed in the Central Veterinary Laboratory, Kathmandu.
Test for detection of Leptospira interrogans serovar Hardjo antibody
An indirect ELISA test was performed for in vitro detection of antibodies against Leptospira interrogans serovar Hardjo in cattle serum by using a kit manufactured by Prionics AG Lelystad B.V., Netherlands, i.e. PrioCHECK® L. interrogans serovar Hardjo Ab (ver. 1.0.e).
All reagents in the kit were equilibrated to room temperature (22 ± 3°C) before use. Solutions were prepared, and all ELISA procedures were undertaken as per the manufacturer’s guidelines. Finally, the ELISA plates were read using an ELISA reader (Multiskan™ FC Microplate Photometer from Thermo Fisher Scientific) at an optical density (OD) of 450 nm. To estimate the number of L. interrogans serovar Hardjo sero-positive samples, the readings were computed using the software and protocol included in the kits.
The percentage positivity (PP) cut-off for each serum sample was set as follows:
Statistical analyses
Data were entered using Microsoft Excel 2019®. Descriptive and analytical analyses were conducted using ‘Epi InfoTM’ ver. 7.2.5.0 (Epi InfoTM|Centers for Disease Control and Prevention 2022). The seropositive herds were considered positive if at least one animal tested positive for L. interrogans serovar Hardjo antibodies. Suspected samples obtained from the ELISA test result (n = 17) were excluded for risk factor analysis (N = 390).
Using quartiles (median as cut-off point), continuous factors such as age and herd size were converted into binary categorical variables (median as cut-off point). Pearson’s chi-square (χ2) goodness of fit test and Fisher’s exact test were performed to determine the association among the categorical variables, such as breed, herd size, or cattle origin, and the seroprevalence of Leptospira infection. Any variables having a P-value of less than or equal to 0.05 (≤0.05) within a 95% confidence interval were deemed as statistically significant and were considered as potential risk factors.
Univariable followed by multivariable analysis of associated risk factors and leptospirosis seroprevalence were performed. Suspected or inconclusive samples were not used in risk factor analysis either at the herd or animal level. Both the herd characteristics and general husbandry practices including herd size, multispecies farming, presence or absence of agricultural land nearby, presence or absence of calving pen facility, frequency of vehicle movements, housing type (intensive/semi-intensive/extensive), bedding type (mat/cemented/earthen), water sources, follow quarantine practices or not, isolation of sick animals, presence or absence of rodents, use of boots/personal protective equipment (PPE) or not, feeding pattern (stall-feeding/grazing/both), feed contaminated with soil, working nature of farmers (in field only/in cow shed only/both), presence or absence of workers, availability of disposal pits, slaughter slabs/meat slab nearby, buying and selling of animals within or out of district or both, awareness of disease transmission among farmers, status of farm sanitation, and breeding method (artificial insemination/bull mating/both/not any) were variables for univariate logistic regression analysis at a herd level.
Likewise, potential animal-level explanatory variables such as age, parity, sex, body condition score (BCS), breed, presence or absence of a bull, pregnancy status, cattle origin, lactation status, reproductive problems, tick infestations, antibiotic intake and milk yield pattern were variables for univariate logistic regression analysis at an animal level. Altogether univariate regressions of 25 herd-level and 13 animal-level risk factors were performed.
Following the univariate analysis, the variables with P ≤ 0.3 were considered as candidate variables for a multivariable model. A map of the study site was created using ArcGIS Desktop (ver. 10.8.2; ESRI Inc., Redlands, California, USA) (Fig. 1).
Results
Descriptive study
Of the 216 herds visited during the study, 61.57% (133/216) were semi-intensive, having at least a cow, bull or calf with exposure to rodents in and around the farms. In 95.37% (206/216) farms, milking was performed manually by farmers, by using their hands. About 72.22% (156/216) of the farmers were rearing multispecies (buffalo, goat, pig, poultry), whereas 27.78% (60/216) of the farmers reared only cattle.
In 82.41% (178/216) of the farms, owners themselves performed both the farm and agricultural field activities, including cultivation of crops. Approximately 30.09% (65/216) of the farmers usually worked bare footed, 49.54% (107/216) occasionally, and 20.37% (44/216) preferred not to work bare footed in the field. Approximately, 38.43% (83/216) preferred wearing boots while working in the field.
A significant proportion (46.30%;100/216) of farmers bought and sold their cattle within the district, with 29.63% (64/216) bought and sold their cattle out of the district, whereas the remaining 24.07% (52/216) bought and sold their cattle both in and out of the district.
The majority (67.59%; 146/216) of floor surfaces on farm were cemented, 21.76% (47/216) were earthen and 10.65% (23/216) had a mat covering the earthen surfaces. Farm sanitation including regular cleaning and disinfection of floors, gutters, and washing of cattle was maintained regularly in 49.54% (107/216) of the farms. Disposal pits were found in 78.70% (170/216) of farms whereas feed was found to be frequently contaminated with soil in 41.67% (90/216) of farms. About 68.98% (149/216) of the farms practiced artificial insemination (AI) to breed their animals, 6.48% (14/216) relied on natural mating only, whereas the remaining 5.09% (11/216) applied both methods, i.e. in case of AI failure, natural mating was performed.
Of the total 407 cattle sampled, the proportions of home-bred, purchased and stray cattle were 57.74% (235/407), 35.38% (144/407) and 6.88% (28/407) respectively. Only 14.5% (59/407) were male cattle, whereas 85.5% (348/407) were female. Altogether, 79.36% of cattle including male (323/407) were between 2 and 10 years of age, whereas an equal proportion of cattle were <2 years of age and >10 years of age (10.32%; 42/407). Jersey cross cows comprized 44.96% (183/407) of the herd, whereas 33.66% (137/407) were Holstein cross and 19.41% (79/407) were local Pahadi cattle. Only 1.47% (6/407) and 0.49% (2/407) were pure-bred Holstein and Jersey respectively.
The proportion of cows with tick infestation was 41.28% (168/407). Only 22.36% (91/407) of the cattle were found to have a history of reproductive, metabolic and management problems such as dystocia, abortion, repeat breeding, retention of placenta, milk fever, and mastitis in their previous parity.
Among the 10 municipalities of Tanahun, seropositivity to Leptospira interrogans serovar Hardjo in cattle was found to be higher in Suklagandaki (17.86%; 5/28), followed by Myagdey (10%; 1/10) and Vimad (9.26%; 5/54) municipalities. Whereas cattle of Ghiring rural municipality, Vyas municipality, Bhanu municipality and Devghat rural municipality displayed seropositivity of 8% (2/25), 4.167% (3/72), 3.03% (2/66) and 2.86% (2/70) respectively. Moreover, leptospirosis antibodies were not detected in samples taken from Bandipur, Aabukhaireni and Rising rural municipalities of Tanahun.
Seroprevalence study
Of the 216 herds tested, 6.48%, (95% CI: 3.59–10.64) tested positive for Leptospira exposure. The herd size ranged from 1 to 140 cattle, with three being the median value. Likewise, the study demonstrated that out of the total samples examined, only 4.91% (n = 20/407, 95%CI: 3.20–7.47) were positive for L. interrogans serovar Hardjo specific antibodies whereas, 4.18% (n = 17/407, 95%CI: 2.62–6.59) were classified as suspected samples.
From univariate regression, only 13/25 herd and 8/13 individual animal explanatory variables, meeting the cut off criteria of P ≤ 0.3, were selected to run in the final multivariable logistic models as shown in Tables 2 and 3. Multivariable logistic regression analysis was performed independently for these selected herd and individual animal explanatory variables.
| Variable | Level | Total number (N = 390) | Number of seropositive (n) | Adjusted OR | 95% CI | P-value | |
|---|---|---|---|---|---|---|---|
| Breed | Holstein cross | 136 | 5 | 1.467 | 0.595–3.620 | 0.406 | |
| Jersey cross | 184 | 7 | 4.06 | 0.20–27.51 | 0.216 | ||
| Local Pahadi and stray cattle | 70 | 8 | Ref | ||||
| Bull | Yes | 85 | 10 | 0.619 | 0.120–3.191 | 0.567 | |
| No | 305 | 10 | Ref | ||||
| Cattle origin | Purchased | 134 | 7 | 2.756 | 1.191–6.381 | 0.018* | |
| Stray/gifts | 26 | 7 | 2.043 | 0.664–6.482 | 0.208 | ||
| Home bred | 230 | 6 | Ref | ||||
| Lactation status | Lactating | 203 | 6 | 1.516 | 0.816–2.815 | 0.188 | |
| Non-lactating | 187 | 14 | Ref | ||||
| Milk yield pattern | Reduced | 132 | 6 | 0.621 | 0.246–1.569 | 0.314 | |
| Normal | 258 | 14 | Ref | ||||
| Pregnancy status | Pregnant | 131 | 6 | 0.765 | 0.421–1.393 | 0.381 | |
| Non-pregnant | 259 | 14 | Ref | ||||
| Reproductive problems | Yes | 86 | 7 | 1.599 | 0.464–5.510 | 0.457 | |
| No | 304 | 13 | Ref | ||||
| Tick infestation | Yes | 158 | 15 | 0.301 | 0.099–0.919 | 0.035* | |
| No | 232 | 5 | Ref |
CI, confidence interval; OR, odds ratio; *P < 0.05, a significant variable; N, total number of sample taken; n, total number of seropositive cattle; Ref, reference category/level.
| Variable | Level | Total number (N = 216) | Number of sero-positive (n) | Adjusted OR | 95% CI | P-value | |
|---|---|---|---|---|---|---|---|
| Herd size (median = 3) | >3 | 106 | 9 | 8.516 | 1.210–59.893 | 0.031* | |
| <=3 | 110 | 5 | Ref | ||||
| Multispecies farming | Yes | 156 | 12 | 0.105 | 0.010–1.057 | 0.056 | |
| No | 60 | 2 | Ref | ||||
| Frequency of vehicle movements | Frequently | 80 | 6 | 0.861 | 0.380–1.951 | 0.720 | |
| Less frequently | 136 | 8 | Ref | ||||
| Housing type | Intensive | 65 | 2 | 5.025 | 0.788–32.062 | 0.088 | |
| Semi-intensive | 133 | 9 | 3.291 | 0.842–16.025 | 0.128 | ||
| Extensive | 18 | 3 | Ref | ||||
| Bedding type | Mat | 23 | 1 | 2.321 | 0.200–27.530 | 0.504 | |
| Cemented | 146 | 7 | 1.813 | 0.089–16.174 | 0.625 | ||
| Earthen | 47 | 6 | Ref | ||||
| Isolation of sick animals | Yes | 94 | 4 | 1.050 | 0.156–7.030 | 0.962 | |
| No | 122 | 10 | Ref | ||||
| Rodents | Yes | 209 | 12 | 26.922 | 1.220–593.867 | 0.037* | |
| No | 7 | 2 | Ref | ||||
| Breeding method | Both | 11 | 0 | 1.851 | 0.434–7.901 | 0.410 | |
| Artificial insemination | 149 | 6 | 1.704 | 0.091–10.257 | 0.651 | ||
| Bull mating | 14 | 4 | 0.921 | 0.164–5.291 | 0.935 | ||
| Not any | 42 | 4 | Ref | ||||
| Feed contamination with soil | Yes | 153 | 11 | 0.490 | 0.160–1.47 | 0.201 | |
| No | 63 | 3 | Ref | ||||
| Working nature of farmers | Both | 178 | 9 | 3.657 | 1.152–11.605 | 0.028* | |
| In cow shed or field only | 38 | 5 | Ref | ||||
| Disposal pits | Yes | 170 | 9 | 0.228 | 0.025–2.059 | 0.188 | |
| No | 46 | 5 | |||||
| Animal Import | Yes | 117 | 11 | 0.240 | 0.035–1.600 | 0.139 | |
| No | 99 | 3 | Ref | ||||
| Buy/sell | Within a district | 152 | 11 | 0.140 | 0.019–0.970 | 0.046* | |
| Out of district | 64 | 3 | Ref |
CI, confidence intervals; OR, odd ratios; *P < 0.05, a significant variable; N, total number of sample taken; n, total number of seropositive cattle; Ref, reference category/level.
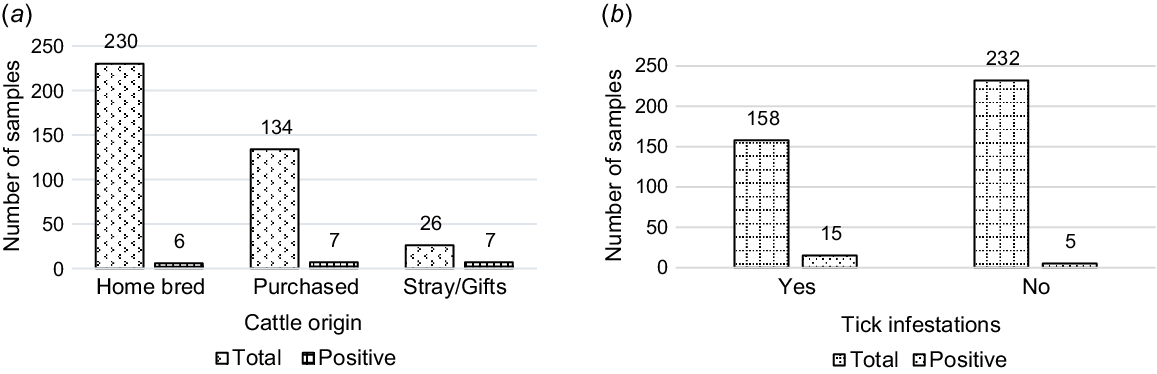
In the final animal-level multivariable regression, only two animal-level risk factors, cattle origin and tick infestation, were significantly associated with seropositivity to Leptospira organism (Table 2). Purchased cattle herds had a significant 2.75-fold increase in the odds of being exposed to L. interrogans serovar Hardjo (P = 0.018, OR: 2.756, 95% CI: 1.191–6.381) relative to stray and home-bred cattle. Herds with tick infestations had a 0.3-fold decrease in the odds of being seropositive (P = 0.035, OR: 0.301, 95% CI: 0.10–0.92), as shown in Fig. 2.
Leptospira exposure in total sampled cattle population. (a) Cattle-origin, P ≤ 0.05. (b) Tick-infestations, P ≤ 0.05.
In the final herd-level multivariable model, the four herd-level risk factors, i.e. buying and selling of animals within and out of district, working nature of farmers, presence or absence of rodents, and herd size, were significantly associated with seropositivity to Leptospira organism (Table 3).
Considering sex-wise prevalence, it was evident that females tended to be less affected 4.75% (16/337) than were males 7.55% (4/53), although this was not significant (P ≥ 0.05). The median age of the cattle in the herd was 5 years, ranging from 2.5 months to 23 years. So, age is categorized as ‘≤5’ or ‘>5’. The prevalence tended to be higher (P ≥ 0.05) with the younger age group, with 5.29% (12/227) being affected relative to the older group (4.91%; 8/163).
With respect to herd size, herds with more than three cattle were found to be significantly more affected 8.49% (9/106) than were herds with three or fewer cattle 4.54% (5/110). Herds with more than three cattle were 8.5 times more likely to be seropositive to Leptospira organism (P = 0.031, OR: 8.516, 95% CI: 1.210–59.893) than were those with three or fewer cattle (Fig. 3).
Leptospira exposure in total sampled cattle population (a) Herd size, P ≤ 0.05; (b) Presence or absence of Rodents P ≤ 0.05.
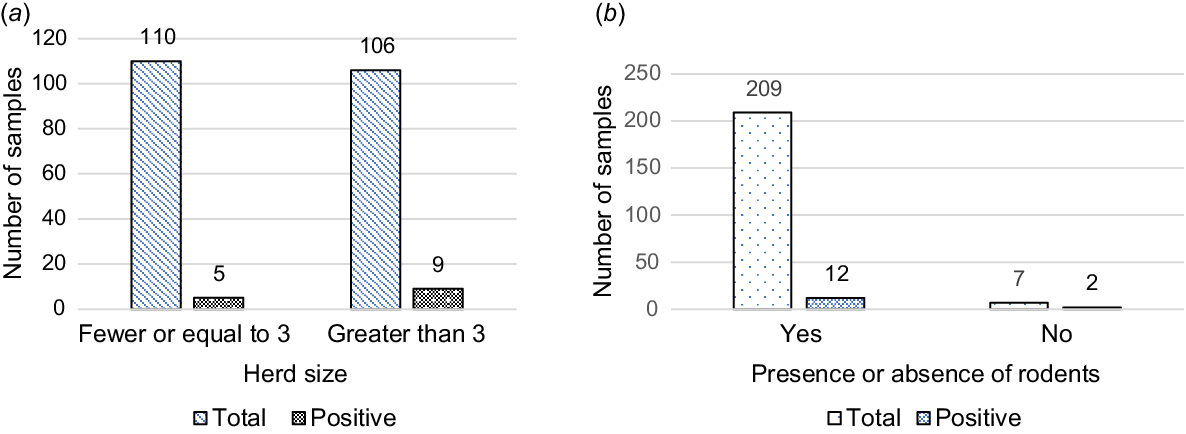
Similarly, infestations of rodents were apparent in the majority (96.76%; 209/216) of herds. Farms where rodents were found had a 26.9-fold increase in the odds of being exposed to L. interrogans serovar Hardjo (P = 0.037, OR: 26.922, 95% CI: 1.220–593.867) than those farms with fewer or no rodents (Fig. 3).
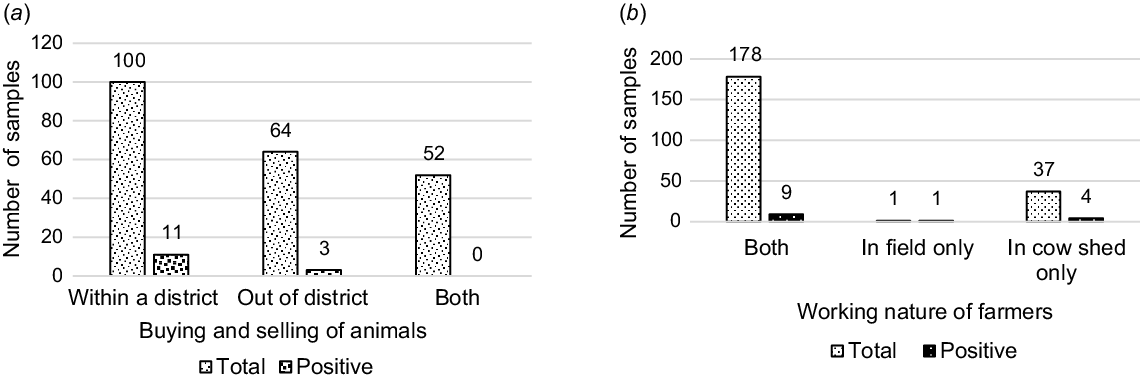
The cattle herds bought and sold within the district were less likely to be affected by leptospirosis (P = 0.046, OR: 0.135, 95% CI: 0.019–0.965) than were those moved among districts or across borders.
In 82.41% (178/216) of the farms, owners themselves performed both the cow shed and field activities. It was found that the cattle herds reared by the farmers working in both the cow shed and field had a significantly increased risk, 3.65 times greater, of developing leptospirosis seropositivity (P = 0.028, OR: 3.657, 95% CI: 1.152–11.605) than those herds reared by farmers working only in the cow sheds (Fig. 4).
Leptospira exposure in total sampled cattle population. (a) Buying and selling of animals, P ≤ 0.05. (b) Working nature of farmers, P ≤ 0.05.
When comparing breeds, local Pahadi and stray cattle tended to be more affected (11.43%; 8/70) than were Jersey cross (3.80%; 7/184) and Holstein cross (3.68%; 5/136) animals. Although the result was insignificant in multivariate analysis, it was significant in univariate analysis.
More than half of the respondents (58.33%; 126/216) were aware of zoonoses. Approximately, 55.56% (120/216) of the respondents knew that disease can be transmitted through contaminated milk, uncooked or raw meat, blood, contaminated soil, contaminated water, body discharges and genital secretions. Of 216 farmers surveyed, 15.28% (33/216) had heard of leptospirosis as an animal disease, whereas only 5.56% (12/216) of the farmers knew that leptospirosis was zoonotic.
Discussion
The present study assessed the serological evidence of L. interrogans serovar Hardjo infections in Tanahun district and identified potential risk variables at the animal and herd levels. The findings showed that the seroprevalence of L. interrogans serovar Hardjo among dairy cattle herds of the Tanahun district of Nepal is very similar to that reported by Khanal et al. (2018). In their study, 6% (10/160) and 4.51% (7/155) of dairy cattle were seropositive to Leptospira interrogans serovar Hardjo infection in the pre-monsoon and the post-monsoon seasons respectively. Another study by Rawal and Shrestha (2019) showed that dairy cattle in the Bhaktapur district of Nepal had a sero-detection rate of 5.11% (9/176), which is consistent with our findings. Similarly, a study conducted by Vongxay et al. (2012) in Lao PDR found 6% antibodies against L. interrogans serovar Hardjo in serum samples of cattle assessed by ELISA. The similarity in Leptospira interrogans serovar Hardjo seroprevalence between our study in Tanahun district and other districts within Nepal can largely be attributed to consistent climatic conditions, and livestock rearing practices in terms of animal husbandry, grazing, and sheltering. These practices contribute to comparable levels of exposure to environmental sources of Leptospira, such as contaminated water and soil, resulting in similar seroprevalence findings. Additionally, the availability and use of ELISA, having similar sensitivity and specificity, as a major serological diagnostic test minimizes variations in the detection and reporting of seroprevalence rates of L. interrogans serovar Hardjo.
However, Joshi and Shrestha (2011) detected a higher seroprevalence of Leptospira exposure in dairy cows (11.0%; 12/114) reared in mid-hills regions of Nepal. Mandal et al. (2008) reported 16.84%, and Savalia and Mahendra (2008) reported 14.77% seroprevalence of Leptospira exposure in dairy cattle of West Bengal and Gujarat state of India respectively, both of which compare well with our present findings. In contrast, higher seroprevalence at the rate of 42.5% (Balamurugan et al. 2013) was recorded in dairy cattle from Orissa and 22% from Uttaranchal, Tamil Nadu, Uttar Pradesh of India (Mariya et al. 2007). Similarly, a study conducted in Lao PDR by Olmo et al. (2019) detected 12.8% antibodies against L. interrogans serovar Hardjo in serum samples of cattle. Moreover, a study by Gamage et al. (2011) reported 20.3% of bovine leptospirosis in Sri Lanka. Prevalence of bovine leptospirosis in dairy herds was reported as 31.3% and 47.3% in research conducted by Tabatabaeizadeh et al. (2011) and Parvez and Faruque (2015) from Iran and Bangladesh respectively. In contrast to our findings, higher seroprevalence observed in studies conducted in neighbouring countries such as India, Bangladesh, Sri Lanka, and in other regions, may reflect the endemic nature of diseases, differences in test methods, livestock rearing practices, and environmental conditions. Because the microscopic agglutination test (MAT) is a serovar-serogroup specific test, having better sensitivity and specificity, it is the preferred choice for sero-epidemiologic studies (WHO 2007). Use of gold standard serological tests, such as MAT that is highly sensitive to detect specific Leptospira antibodies, including L. interrogans serovar Hardjo over ELISA, thus results in higher seroprevalence. Variations in climatic conditions, especially rainfall and humidity, could facilitate the survival and spread of Leptospira in the environment, contributing to higher seroprevalence in those areas. Also, difference in livestock rearing practices among regions can significantly affect exposure rates.
Nevertheless, because of variations in epidemiological study design, study objectives, sample size and geography, leptospirosis prevalence may vary over time. Therefore, even though our findings align closely with other districts within Nepal, the differences observed with respect to other countries underscore the importance of local context in understanding disease prevalence.
In our study, the higher seroprevalence of leptospirosis in purchased and stray cattle herds than in home-bred cattle are symptomatic of transmission between herds. Because Tanahun district connects with the Kaski, Gorkha, Lamjung and Chitwan districts, the frequency of movements of human, vehicles and animal herds is high year-round. Thus, the increased frequency of contact between herds significantly elevates the risk of exposure to Leptospira interrogans serovar Hardjo, because it enhances the opportunities for the organism to spread through direct contact or via contaminating shared water sources, soil, or feed (Hashimoto et al. 2012). Purchased cattle, especially those from different regions or with unknown health histories, may carry and shed the Leptospira without showing symptoms, serving as a hidden reservoir that perpetuates the cycle of Leptospira transmission within and among herds. Within herd settings, close proximity of animals facilitates the transmission of Leptospira through urine, which is the primary mode of shedding for this pathogen. Similarly, free-roaming stray cattle, often of unknown health status, are likely to expose healthy herds to Leptospira infection, especially in environments where they intermingle with domesticated cattle. The increased contact and subsequent exposure not only amplify the risk for the cattle but also pose a greater risk of zoonotic transmission to humans, particularly those working closely with livestock (Balamurugan et al. 2013). Thus, herd management is crucial during transportation of cattle and other susceptible animals to control the spread of the Leptospira organism. Herd management should include implementing quarantine measures for newly purchased cattle before allowing them to join the main herd, and regular health screenings should be standard practice. For stray cattle, relocating them to designated shelters where they can be monitored and treated, along with preventing their access to common grazing areas and water sources, are interventions that further reduce the risk of Leptospira transmission to healthy herds.
Seropositivity to L. interrogans serovar Hardjo was lower in Bandipur, Devghat rural municipality, followed by Bhanu and Vyas municipalities, and higher in Suklagandaki, followed by Myagdey, Vimad and Ghiring municipalities. Lower seroprevalence in these municipalities might be due to closer management of dairy herds where culling of unproductive and unhealthy animals is closely monitored, whereas farm sanitation and knowledge of the disease transmission cycle among farmers helped lower Leptospira exposure in cattle herds. Knowledge of the transmission cycle empowers farmers to implement targeted interventions such as quarantine measures, isolation of suspected animal, routine physical examination, and farm sanitation (Ryan et al. 2012). As such, timely culling helps remove potential carriers from the herd, decreasing the chance of transmission. Farm sanitation, including proper management of waste and water sources, reduces environmental contamination by Leptospira organisms (Sharma et al. 2003; Zakharova et al. 2021). These practices, when implemented effectively, can significantly reduce the risk of Leptospira transmission in cattle populations.
In contrast to this, samples collected from Suklagandaki included more stray and physically unhealthy cattle, which increases the risk of exposure to Leptospira infection. Whereas samples from Vimad municipality were from cattle herds with a history of reproductive problems, including repeat breeding, abortion, metritis and retention of placenta, which indicates an increased exposure to Leptospira infection. Also, in the Myagdey municipality, most of the cattle farms from where samples were taken were located near to the highway. However, lower frequency of animal movement and relatively small number of samples taken from Aabukhaireni and Rising rural municipalities might explain the lower seroprevalence rates. Studies have shown that farms with inadequate biosecurity measures, including uncontrolled animal movement, introduce new sources of infection and spread the pathogen within and among herds, thus increasing the exposure risk (Balamurugan et al. 2013; Picardeau 2013). Additionally, ensuring an adequate sample size in seroprevalence studies is crucial to accurately assess the risk factors and prevalence, because smaller samples may not capture the full extent of population exposure (Skinner 2016).
In relation to herd size, several studies have demonstrated that a larger herd size provides a higher risk for Leptospira exposure (Ryan et al. 2012; Miyama et al. 2018). This is consistent with our results. Larger herd size increases the likelihood of Leptospira transmission because of closer contact among animals, which facilitates the spread of the organism through urine, a primary mode of transmission. Additionally, larger herds often share water and grazing resources, creating more opportunities for environmental contamination and subsequent exposure. Although smaller herds may reduce the risk of Leptospira transmission because of lower animal density and contact, this might not be economically feasible for all types of farmers. Because vaccination against leptospirosis has not been introduced in Nepal, it is recommended that farmers, especially with a larger herd size, implement preventive measures such as improved biosecurity practices to mitigate the risk of Leptospira infection.
Our study showed that rodents were frequently present in herds and could be an important risk factor as rodents play a vital role as a carrier for leptospirosis (Adler and de la Peña Moctezuma 2010). In Tanahun, the timing of our study from August to December included the months of October to November when rice paddy harvesting was conducted and rodents were sighted more frequently. Rats had unrestricted access to paddy hay, the principal dietary component of dairy cattle. Interestingly, study conducted by Olmo et al. (2019) in farms of Lao PDR showed a 9.3% prevalence of rodents and this was not considered to be the major risk factor for leptospirosis infection in cattle. Leptospirosis outbreaks typically correspond to the rainy and paddy harvesting season, with a rise in cases from August and a decrease in cases in November (Zavitsanou and Babatsikou 2008; Victoriano et al. 2009). Poverty and poor education were implicated as conditions leading to rodent-borne transmissions (LaRocque et al. 2005). Rodents should be controlled on the farm because they act as carriers of zoonotic pathogens such as Leptospira and can transmit diseases between animals thus, increasing the risk of outbreaks. However, the risk of leptospirosis transmission can be successfully reduced through the use of rodenticides, animal trapping, and improved sanitation (Mohan Rao 2006).
Furthermore, our findings showed a significant relationship between the nature of farm work and seropositivity to the Leptospira organism (Table 3). Farmers who work both in farms and fields are at a higher risk of contracting leptospirosis because of an increased exposure to environments contaminated with Leptospira organism. Working in fields, especially during the rainy season, can expose farmers to contaminated water and soil, whereas farm work increases direct contact with potentially infected livestock. This dual exposure raises the likelihood of infection. This finding is consistent with previous reports from south-western Iran (Alavi et al. 2013) and Malaysia (Sejvar et al. 2003). To reduce the risk of Leptospira exposure, farmers should practice good hygiene, such as wearing protective clothing and boots, and washing hands and feet after working in potentially contaminated areas. Additionally, implementing measures such as regular health checks of cattle and proper waste management on farms could further reduce the chances of transmission.
Our study also showed that the practice of buying and selling animals within and outside the local district, especially from live animal markets, have a significant effect on increasing the risk of Leptospira infection. The movement of animals through buying and selling, especially across districts, might introduce new sources of Leptospira organisms into existing herds that may have previously been unexposed, thus increasing the overall risk of the disease. Therefore, strict biosecurity measures should be followed when buying or selling animals, including quarantine periods and health screenings, to prevent the introduction of infected animals into healthy herds. In present study, seroprevalence of L. interrogans serovar Hardjo in cattle was found to be insignificant with respect to their age, sex and breed.
The survey taken in the present study was conducted within a population where approximately 30% were literate, with only approximately 8% having advanced to a higher level of education. Our findings reflect this level of education and assist with understanding the level of awareness on zoonosis in the study population. Various studies have shown that the adoption of intensive awareness campaigns, in combination with vaccination of cattle, is successful in reducing the incidence of leptospirosis (Crump et al. 2001; Thornley et al. 2002; Leptospirosis in Japan 2008). Although implementing awareness campaigns and vaccination program for controlling leptospirosis in Nepal is feasible, it may face challenges because of limited government resources and competing public health priorities. Furthermore, studies similar to the current study should be replicated in other parts of Nepal to determine the overall prevalence of leptospirosis in the animal population, so that targeted awareness and vaccination programs in high-risk areas can be implemented. This is particularly important in areas with significant livestock activity, to prevent disease outbreaks and protect vulnerable populations.
Conclusions and recommendations
Herd and animal levels of seroprevalence of Leptospira exposure were found to be 6.48% (14/216) and 4.91% (20/407) respectively, among the dairy cattle herds in the Tanahun district of Nepal. In the final multivariable model, four potential herd-level risk factors, i.e. buying and selling of animals within and out of district, the nature of farm work performed by farmers, presence of rodents and a herd size greater than three animals were significantly associated with seropositivity to Leptospira organism. Similarly, two animal-level risk factors, i.e. cattle origin and tick infestations, were significant factors with respect to Leptospira exposure. Interestingly, 58.33% (126/216) of the respondents were aware of the concept of zoonosis, but only 5.56% (12/216) of the farmers knew leptospirosis as a zoonotic disease.
Based on these research findings, the farmers and general public, especially in high-risk areas, of Tanahun should be made aware of this zoonosis. The key precautionary measures should be consistently applied to rule out associated risk factors of leptospirosis, including regular maintenance of farm hygiene, use of protective clothing, such as boots and gloves while handling animals or working in fields, especially during the rainy season, and ensuring supply of safe and clean water sources for both animals and humans. Moreover, these findings would be helpful in developing targeted control and contingency plans for the management of this disease. By identifying the seroprevalence and risk factors, the study has provided a foundation for implementing effective interventions, such as improved biosecurity measures. For future studies, it is recommended to conduct longitudinal research to track infection trends over time, explore the genetic diversity of Leptospira strains circulating in livestock, and assess the impact of environmental factors on disease transmission. Investigating the link between seroprevalence and clinical disease in both animals and humans would also provide valuable insights for comprehensive leptospirosis management strategies.
Testing for markers for the molecular characterization of pathogenic isolates, including the introduction of a microscopic agglutination test, needs to be performed to identify different serovars of Leptospira spp., along with L. interrogans serovar Hardjo, to obtain a more detailed picture of their comparative prevalence nationwide. A national action plan for the control of prioritized zoonotic diseases such as leptospirosis should be designed and implemented. This needs to include effective cooperation across the public health sectors in developing an effective one health approach to disease control.
Declaration of funding
The authors are thankful to FAO-ECTAD, Nepal, for partially funding the research field work. Sabina Lamsal was funded by ACIAR (Australia) to present at the 9th SAADC conference 2023, Vientiane, Lao PDR.
Author contributions
Conceptualization and data curation: Sabina Lamsal; Methodology: Sabina Lamsal, Ram Chandra Sapkota; Formal Analysis: Sabina Lamsal, Surendra Karki; Supervision: Subir Singh, Ram Chandra Sapkota, Surendra Karki; Writing – original draft: Sabina Lamsal; Writing – review and editing: Subir Singh, Surendra Karki, Sabina Lamsal.
Acknowledgements
The authors are indebted to the Department of Livestock Services (DLS) for permitting to use laboratory facilities of Central Veterinary Laboratory (CVL) for sample testing. The authors offer thanks to all the associated veterinarians, veterinary technicians, working staffs and all other contributors from CVL, Veterinary Hospital and Livestock Service Expert Center (VHLSEC), Tanahun, for their encouragement, assistance, guidance and kind cooperation during the study period.
References
Adler B, de la Peña Moctezuma A (2010) Leptospira and leptospirosis. Veterinary Microbiology 140, 287-296.
| Crossref | Google Scholar | PubMed |
AITC (2023) Agriculture and livestock diary. Available at https://aitc.gov.np/en/resources/t02u8l1dz23abqozzz3vlvntvbgta1reos0xbzy9 [posted 18 April 2023]
Alavi L, Alavi S, Khoshkho M (2013) Risk factors of leptospirosis in Khuzestan, South West of Iran, 2012. International Journal of Enteric Pathogens 1, 68-71.
| Crossref | Google Scholar |
Bahaman AR, Ibrahim AL, Adam H (1987) Serological prevalence of leptospiral infection in domestic animals in West Malaysia. Epidemiology and Infection 99(2), 379-392.
| Crossref | Google Scholar | PubMed |
Balamurugan V, Thirumalesh SRA, Sridevi R, Mohandoss N, Govindaraj G, Hemadri D, Gajendragad MR, Rahman H (2013) Seroprevalence of bovine leptospirosis in Odisha, India. World Journal of Veterinary Science 1(1), 1-7.
| Crossref | Google Scholar |
Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, Lovett MA, Levett PN, Gilman RH, Willig MR, Gotuzzo E, Vinetz JM (2003) Leptospirosis: a zoonotic disease of global importance. The Lancet Infectious Diseases 3(12), 757-771.
| Crossref | Google Scholar | PubMed |
Bolin CA, Koellner P (1988) Human-to-human transmission of Leptospira interrogans by milk. The Journal of Infectious Diseases 158, 246-247.
| Crossref | Google Scholar | PubMed |
Centers for Disease Control and Prevention (2022) Epi InfoTM. Available at https://www.cdc.gov/epiinfo/index.html [retrieved 23 February 2022]
Crump JA, Murdoch DR, Baker MG (2001) Emerging infectious diseases in an island ecosystem: the New Zealand perspective. Emerging Infectious Diseases 7, 767-772.
| Crossref | Google Scholar | PubMed |
Fávero JF, de Araújo HL, Lilenbaum W, Machado G, Tonin AA, Baldissera MD, Stefani LM, Da Silva AS (2017) Bovine leptospirosis: prevalence, associated risk factors for infection and their cause-effect relation. Microbial Pathogenesis 107, 149-154.
| Crossref | Google Scholar |
Gamage CD, Koizumi N, Muto M, Nwafor-Okoli C, Kurukurusuriya S, Rajapakse JRPV, Kularatne SAM, Kanda K, Lee RB, Obayashi Y, Watanabe H, Tamashiro H (2011) Prevalence and carrier status of leptospirosis in smallholder dairy cattle and peridomestic rodents in Kandy, Sri Lanka. Vector-Borne and Zoonotic Diseases 11(8), 1041-1047.
| Crossref | Google Scholar | PubMed |
Guerra MA (2013) Leptospirosis: public health perspectives. Biologicals 41(5), 295-297.
| Crossref | Google Scholar | PubMed |
Hartskeerl RA, Collares-Pereira M, Ellis WA (2011) Emergence, control and re-emerging leptospirosis: dynamics of infection in the changing world. Clinical Microbiology and Infection 17(4), 494-501.
| Crossref | Google Scholar |
Hashimoto VY, Dias JA, Spohr KAH, Silva MCP, Andrade MGB, Müller EE, et al. (2012) Prevalence and risk factors for Leptospira spp. in cattle herds in the south central region of Paraná state. Pesquisa Veterinária Brasileira 32, 99-105.
| Crossref | Google Scholar |
Hensley T (2016) Leptospirosis in cattle. Texas Veterinary Medical Association (TVMA). Available at TexVetPets.org
Hoffmann I (2010) Climate change and the characterization, breeding and conservation of animal genetic resources. Animal Genetics 41(s1), 32-46.
| Crossref | Google Scholar |
Joshi BR, Shrestha BS (2011) Studies on normal fertility indices and factors associated with bovine infertility in the hills of Nepal. Nepalese Veterinary Journal 30, 86-102.
| Google Scholar |
Khanal DR, Paudyal N, Khanal S, Prajapati M, Shrestha P, Bowen R, Acharya MP, Shrestha SP, Singh UM, Thakur RP, Naletoski I, Joshi BR (2018) Detection of antibodies against Leptospira hardjo in large ruminants of Nepal. ACTA Scientific Agriculture 2(12), 131-133 https://www.researchgate.net/publication/344272060.
| Google Scholar |
LaRocque RC, Breiman RF, Ari MD, Morey RE, Janan FA, Hayes JM, Hossain MA, Brooks WA, Levett PN (2005) Leptospirosis during dengue outbreak, Bangladesh. Emerging Infectious Diseases 11(5), 766-769.
| Crossref | Google Scholar | PubMed |
Leonard N, Mee JF, Snijders S, Mackie D (2004) Prevalence of antibodies to Leptospira interrogans serovar Hardjo in bulk tank milk from unvaccinated Irish dairy herds. Irish Veterinary Journal 57(4), 226.
| Crossref | Google Scholar |
Leptospirosis in Japan (2008) Infectious Agents Surveillance Report (ISAR). Available at http://idsc.nih.go.jp/iasr/29/335/tpc335.html
Mandal S, Joardar SN, Chakraborty D, Sardar N (2008) Seroepidemiological study of bovine leptospirosis in West Bengal. Indian Journal of Comparative Microbiology, Immunology and Infectious Diseases 29(1&2), 42-44.
| Google Scholar |
Mariya R, Srivastava SK, Thangapandian E (2007) Seroprevalence of leptospiral antibodies in bovine. Indian Veterinary Journal 84, 547-548.
| Google Scholar |
Miyama T, Watanabe E, Ogata Y, Urushiyama Y, Kawahara N, Makita K (2018) Herd-level risk factors associated with Leptospira hardjo infection in dairy herds in the southern Tohoku, Japan. Preventive Veterinary Medicine 149, 15-20.
| Crossref | Google Scholar | PubMed |
Mohan Rao AMK (2006) Preventive measures for leptospirosis: rodent control. Indian Journal of Medical Microbiology 24, 325-328.
| Crossref | Google Scholar | PubMed |
Olmo L, Reichel MP, Nampanya S, Khounsy S, Wahl LC, Clark BA, Thomson PC, Windsor PA, Bush RD (2019) Risk factors for Neospora caninum, bovine viral diarrhoea virus, and Leptospira interrogans serovar Hardjo infection in smallholder cattle and buffalo in Lao PDR. PLoS ONE 14(8), e0220335.
| Crossref | Google Scholar |
Parvez MA, Faruque, R (2015) Seroprevalence and Associated Risk Factors of Leptospira interrogans Serovar Hardjo in Dairy Cattle of Chittagong, Bangladesh. View project Broiler View project. Available at https://www.researchgate.net/publication/282382390
Patel JM, Vihol PD, Prasad MC, Kalyani IH, Raval JK, Patel KM, Thirumalesh SRA, Balamurugan V (2014) Seroepidemiological pattern of leptospirosis in bovine of South Gujarat, India. Veterinary World 7(11), 999-1003.
| Crossref | Google Scholar |
Picardeau M (2013) Diagnosis and epidemiology of leptospirosis. Médecine et Maladies Infectieuses 43, 1-9.
| Crossref | Google Scholar |
Rawal G, Shrestha D (2019) Sero-detection of Leptospira hardjo in cattle of bhaktapur district of Nepal. International Journal of Applied Sciences and Biotechnology 7(3), 378-381.
| Crossref | Google Scholar |
Ryan EG, Leonard N, O’Grady L, Doherty ML, More SJ (2012) Herd-level risk factors associated with Leptospira hardjo seroprevalence in beef/suckler herds in the Republic of Ireland. Irish Veterinary Journal 65(1), 6.
| Crossref | Google Scholar |
Savalia CV, Mahendra P (2008) Studies on the reservoir status of leptospirosis in Gujarat. Indian Journal of Field Veterinary 4(1), 7-9.
| Google Scholar |
Sejvar J, Bancroft E, Winthrop K, Bettinger J, Bajani M, Bragg S, et al. (2003) Leptospirosis in ‘eco-challenge’ athletes, Malaysian Borneo, 2000. Emerging Infectious Diseases 9(6), 702-707.
| Crossref | Google Scholar | PubMed |
Sharma S, Vijayachari P, Sugunan AP, Sehgal SC (2003) Leptospiral carrier state and seroprevalence among animal population – a cross-sectional sample survey in Andaman and Nicobar Islands. Epidemiology and Infection 131(2), 985-989.
| Crossref | Google Scholar |
Shrestha R, McKenzie JS, Gautam M, Adhikary R, Pandey K, Koirala P, BC Gyan Bahadur, Miller LC, Collins-Emerson J, Craig SB, Shrestha S, Heuer C (2018) Determinants of clinical leptospirosis in Nepal. Zoonoses and Public Health 65(8), 972-983.
| Crossref | Google Scholar | PubMed |
Skinner CJ (2016) Probability Proportional to Size (PPS) sampling. In ‘Wiley StatsRef: Statistics Reference Online’. (Eds N Balakrishnan, T Colton, B Everitt, W Piegorsch, F Ruggeri, JL Teugels) pp. 1–5. (John Wiley & Sons) 10.1002/9781118445112.STAT03346.PUB2
Srivastava SK (2008) Current status of leptospirosis in India in animals and humans. Indian Journal of Veterinary Pathology 32(2), 179-186.
| Google Scholar |
Tabatabaeizadeh E, Hashemi Tabar G, Farzaneh N, Seifi HA (2011) Prevalence of Leptospira hardjo antibody in bulk tank milk in some dairy herds in Mashhad suburb. African Journal of Microbiology Research 5(14), 1768-1772.
| Crossref | Google Scholar |
Thornley CN, Baker MG, Weinstein P, Maas EW (2002) Changing epidemiology of human leptospirosis in New Zealand. Epidemiology and Infection 128(1), 29-36.
| Crossref | Google Scholar | PubMed |
Victoriano AFB, Smythe LD, Gloriani-Barzaga N, Cavinta LL, Kasai T, Limpakarnjanarat K, Ong BL, Gongal G, Hall J, Coulombe CA, Yanagihara Y, Yoshida S-I, Adler B (2009) Leptospirosis in the Asia Pacific region. BMC Infectious Diseases 9, 147.
| Crossref | Google Scholar |
Vongxay K, Conlan JV, Khounsy S, Dorny P, Fenwick S, Thompson RCA, Blacksell SD (2012) Seroprevalence of major bovine-associated zoonotic infectious diseases in the Lao people’s democratic republic. Vector-Borne and Zoonotic Diseases 12(10), 861-866.
| Crossref | Google Scholar | PubMed |
Zakharova OI, Korennoy FI, Iashin IV, Toropova NN, Gogin AE, Kolbasov DV, Surkova GV, Malkhazova SM, Blokhin AA (2021) Ecological and socio-economic determinants of livestock animal leptospirosis in the Russian Arctic. Frontiers in Veterinary Science 8, 658675.
| Crossref | Google Scholar |
Zavitsanou A, Babatsikou F (2008) Leptospirosis: epidemiology and preventive measures. Health Science Journal 2(2), 75-82.
| Google Scholar |