Effect of methionine and lysine supplementation on performance and intestinal morphometrics of finisher broiler chickens fed diets containing processed cassava peel meal
Tolulope O. Adebowale A B * , Muhammed A. Arowolo C , Mukaila Adekola D , Tolulope Adeleye E and Oyegunle E. Oke
A B * , Muhammed A. Arowolo C , Mukaila Adekola D , Tolulope Adeleye E and Oyegunle E. Oke  F
F
A
B
C
D
E
F
Abstract
The current high prices of conventional feedstuffs in developing countries seems to warrant intensified efforts to find sustainable alternatives to the major feed ingredients that would support performance without compromising gut health, reduce cost of production and promote a sustainable environment.
The aim of the study is to evaluate the performance and health-related responses of finisher broiler chickens to the dietary replacement of corn with processed cassava peel meal (high- quality cassava peel meal) at 50% inclusion level, supplemented with methionine and lysine.
Three hundred and twenty Cobb500 broiler chickens were allocated to four dietary treatments on a weight equalization basis. Each treatment was replicated eight times. A control diet based on corn and soybean meal was formulated to meet the recommended digestible methionine and lysine specification of 4.0 and 0.96 g kg−1 respectively (VenCobb 500 Broiler Management Guide, Cobb-Vantress Inc., Siloam Spring, AR) for the finisher phase. The corn in the control diet was replaced with cassava peel meal at 50% replacement level in three other treatments (Diet 2–4). Diets 2–4 were then formulated to contain 100%, 112.5% and 125% of the recommended digestible methionine and 100%, 106.25% and 112.5% of the recommended digestible lysine, yielding 4.0, 4.4, and 4.8 g kg−1 digestible methionine respectively, and 0.96, 1.02, and 1.08 g kg−1 digestible lysine respectively, for the finisher phase.
The highest bodyweight gain, improved feed conversion ratio and increased duodenal and jejunal villus height:crypt depth ratio was found in birds fed Diet 4. The lactobacillus count was highest in birds fed Diet 4 and total bacteria counts was highest in birds fed Diet 2. The feed cost per kilogram weight gain was least for the birds fed diets containing the processed cassava peel meal. However, morphological changes were observed in the liver and intestinal tissues of the birds.
The study showed that the adequate supplementation of methionine and lysine to processed cassava peel meal can improve production performance of finisher broiler chickens but may not prevent morphological changes in the liver and intestine of the birds.
The result implied that processed and fortified cassava peel meal can reduce high dependence on maize in the production of finisher broiler chickens, while mitigating the enviromental challenges associated with the disposal of the agrowaste. The morphological changes in the liver and intestine of the birds require further investigation.
Keywords: blood profile, cassava peel, intestinal health, liver health, lysine, methionine, microbial load, performance.
Introduction
The production of animal feeds that would meet the nutritional requirement of monogastric animals is a great challenge because of the high cost of protein and energy feed ingredients. Corn and wheat are major energy sources in monogastric diets, often making up 50–70% of the feed formulation. This has resulted in increased cost of finished feeds and an eventual hike in the cost of animal production and products. This anomaly has resulted in the search for alternative feed ingredients to reduce production costs without compromising animal performance, and the use of agro-industrial by-products in poultry production has been identified (Gungor and Erener 2020).
Cassava is the third-largest source of carbohydrates for human food in the world (Poku et al. 2018). Cassava peels (5–15% of tuber weight), an important by-product of cassava, when processed could be used to replace corn or wheat in animal production (Oyebimpe et al. 2006). However, its use in monogastric animal diets is limited by the high level of structurally indigestible carbohydrates (cellulose, hemicellulose, pectin, and lignin) and high antinutrients (hydrogen cyanide, tannin and phytate), together with low protein and amino acid (methionine) concentrations. These nutritional limitations of cassava peel result in poor feed intake and impaired digestibility, leading to reduced performance when it is used in the feeding program of broiler chickens. Tewe (1991); Cardoso et al. (2005) observed that cytotoxic hypoxia is expressed as a result of compromised phosphorylation owing to cyanide inhibition of the terminal enzyme of the respiratory chain (cytochrome C oxidase). This accurately leads to compromised nutrient digestibility and absorption by the animals fed cassava peel-based diets (Lukuyu et al. 2014). This mechanism of action associated with the observed metabolic aberration involves the continuous depletion of the sulfur-containing amino acids (methionine) in the circulatory system of the animal consuming diets high in cyanide, as obtained in unprocessed cassava peels. Hence, for increased utilization of cassava peels in monogastric nutrition, it has to be processed to reduce the cyanide content and the amino acid concentration must be balanced.
Methionine, a sulfur-containing amino acid has long been suggested to be involved in cassava detoxification (Ayernor 1985). Si et al. (2004) and Cafe and Waldroup (2006) also noted that as methionine requirement and supplementation increases, the level of dietary lysine intake would require adjustments. The importance of lysine is not often appreciated in cassava or cassava by-product-based diets, mainly because it is the second limiting amino acid, the effect of the second limiting amino acid is not obvious until the requirement for the first limiting amino acid is satisfied (Oke 1978). Similarly, Baker and Han (1994) reported that the concentration of lysine in a diet may affect the response of birds to methionine. Therefore, increasing the methionine content of a cassava peel meal-based diet (naturally low in methionine content) may require additional supplementation of lysine.
Overall, it is supposed that application of improved processing technique to reduce the antinutritional factors in cassava peel and balancing the digestible methionine/lysine content of the diet could enhance the utilization of the cassava peel as a potential and sustainable alternative to the increasingly expensive energy feed stuffs (corn or wheat) in several developing countries. This would reduce the problem of disposal associated with cassava peel, while providing a cheap and sustainable feed resource to animal feed producers. The aim of the study was to utilize processed and supplemented (methionine and lysine) cassava peel meal at high inclusion level (50% dietary replacement of corn) in the feeding regime of finisher broiler chickens. The effects on performance, microbial population, gut morphology, histopathology, hemato-biochemical indices, and economics of production of the birds were evaluated.
Materials and methods
This study was conducted at the Federal University of Agriculture, Abeokuta (FUNAAB) and all procedures followed the Animal Care and Use Committee of the University (FUNAAB). Feed grade DL-methionine (white crystalline powder, 99.8% purity, 215 g kg−1 sulfur content) and lysine (brown crystalline powder, 99.3% purity) were obtained from reputable feed additive store.
Experimental birds and management
In total, 350 1-day-old Cobb500 male broiler chickens were purchased from a commercial hatchery (Zartech, Ibadan). The broiler chickens were reared together for 21 days of pre-experimental period and fed commercial diets. The birds were housed in cemented floor pens (1.45 m × 1.54 m) covered with wood shavings from 1 day old till the end of the experiment. The wood shavings were changed once in 7–10 days. At Day 21, 320 broiler chickens were selected and allotted to dietary treatments on weight equalization basis. Each experimental treatment was replicated eight times, with 10 broiler chickens in a pen. Each replicate of a treatment was taken as the experimental unit. Feed and water were provided ad libitum and clean water was given on a daily basis. The experiment lasted for 3 weeks. The commercial feed fed to the chickens at the pre-experiment stage (starter phase) contained metabolizable energy (ME) = 2900 kcal kg−1, crude protein (CP) = 220 g kg−1, which met the Cobb 500 requirements (VenCobb 500 Broiler Management Guide, Cobb-Vantress Inc., Siloam Spring, AR) of the chicks. A control diet based on corn and soybean meal was formulated to meet the recommended digestible methionine and lysine specification of 4.0 and 0.96 g kg−1 respectively (VenCobb 500 Broiler Management Guide, Cobb-Vantress Inc., Siloam Spring, AR) for the finisher phase. Three other diets (2–4) were formulated to have 100%, 112.5% and 125% of the recommended digestible methionine and 100%, 106.25% and 112.5% of the recommended digestible lysine, while 50% of the corn in the control diet was replaced with cassava peel meal in these diets, yielding 4.0, 4.4, and 4.8 g kg−1 digestible methionine respectively, and 0.96, 1.02, and 1.08 g kg−1 digestible lysine respectively (VenCobb 500 Broiler Management Guide, Cobb-Vantress Inc., Siloam Spring, AR) for the finisher phase (Table 1). Diet 1 = 100% maize (4.0 g kg−1 digestible methionine (DM) and 0.96 g kg−1 digestible lysine (DL)). Diet 2 = 50% maize + 50% processed cassava peel (HQCP) (4.0 g kg−1 DM and 0.96 g kg−1 DL). Diet 3 = 50% maize + 50% processed cassava peel (HQCP) (4.4 g kg−1 DM and 1.02 g kg−1 DL). Diet 4 = 50% maize + 50% processed cassava peel (HQCP) (4.8 g kg−1 DM and 1.08 g kg−1 DL).
| Item | Diet 1 | Diet 2 | Diet 3 | Diet 4 | |
|---|---|---|---|---|---|
| Ingredient (kg) | |||||
| Maize | 60.00 | 30.00 | 30.00 | 30.00 | |
| CPM | – | 30.00 | 30.00 | 30.00 | |
| Soyabean meal | 31.00 | 34.00 | 34.00 | 34.00 | |
| Palm oil | 3.00 | 3.20 | 3.20 | 3.20 | |
| Bone meal | 4.50 | 1.30 | 1.30 | 1.30 | |
| Limestone | 0.60 | 0.60 | 0.60 | 0.60 | |
| Methionine | 0.20 | 0.20 | 0.25 | 0.28 | |
| Lysine | 0.10 | 0.10 | 0.12 | 0.14 | |
| Broiler premix A | 0.25 | 0.25 | 0.25 | 0.25 | |
| Salt | 0.35 | 0.35 | 0.35 | 0.35 | |
| Chemical composition (%) | |||||
| ME (kcal/kg) | 3100.00 | 3080.02 | 3090.00 | 3090.00 | |
| Crude protein | 19.55 | 19.54 | 19.56 | 19.58 | |
| Crude fibre | 4.01 | 5.29 | 5.28 | 5.30 | |
| Ether extract | 5.61 | 4.92 | 4.91 | 4.92 | |
| Phosphorus | 0.45 | 0.83 | 0.83 | 0.83 | |
| Calcium | 2.17 | 4.11 | 4.13 | 4.11 | |
| Methionine | 0.402 | 0.401 | 0.440 | 0.480 | |
| Lysine | 0.960 | 0.961 | 1.021 | 1.080 | |
| Energy protein ratio | 158.56 | 157.78 | 157.97 | 157.81 | |
| Methionine + cysteine | 0.771 | 0.780 | 0.810 | 0.830 | |
High-quality cassava peel meal (processed cassava peel meal)
The processed cassava peel meal termed high-quality cassava peel meal (HQCP) was obtained from International Livestock Research Institute (ILRI) in Ibadan. The detailed methods for the production has been documented by Amole et al. (2019). The freshly harvested cassava (TMS 419) was peeled and grated. The grated peels were then dewatered for 24 h (placed in woven bags) using a hydraulic press. Then, pulverized and sieved to pass through 3 mm mesh (separation of coarse fraction), the resultant material was dried locally in the sun and stored in plastic woven bags.
Chemical analysis
The methionine, cysteine and lysine content of the HQCP meal (Table 2) was determined using the methods described by Spackman et al. (1958). Triplicate samples of the processed cassava peel were dried to constant weight, defatted, hydrolyzed, evaporated in a rotary evaporator and loaded into the Technicon Sequential Multi-sample (TSM) Amino Acid Analyzer. Proximate composition of feed and HQCP were analyzed using standard methods (AOAC 2000).
Growth performance
Feed intake and gain in weights were measured at weekly intervals by using a digital electronic weighing scale (Nihon Kohden, A-12). Feed intake was computed as the difference between the feed offered and leftovers. Feed:gain ratio was calculated as the ratio of feed consumed to bodyweight (BW) gain.
Hematological indices
Blood samples were obtained from five birds per replicate for the examination of the hematogical and serum indices. Hemoglobin concentration (Hb) was estimated using the cyanmethemoglobin method (Cannan 1958). Packed cell volume (PCV), red blood cell (RBC), and white blood cell (WBC) counts were determined with a Wintrobe hematocrit tube. From these parameters, the mean corpuscular hemoglobin (MCH), mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC) were calculated following the method of Schalm et al. (1975). Differential leucocyte counts (lymphocytes, basophils, eosinophils, monocytes) were conducted on blood smears stained with May–Grunwald–Giemsa stain and further calculated.
Serum chemistry
Total serum protein and uric acid concentrations were measured according to previous procedures (Wootton and Powell 1964; Varley et al. 1980). The method outlined by Tietz and Andresen (1986) was used to measure the serum creatinine concentration. Serum enzymes such as aspartate aminotransferase (AST) and alanine aminotransferase (ALT) and the concentrations of the total cholesterol and triglyceride were determined with the aid of commercial kits (Roche COBAS testing kits, Roche, Basel, Switzerland). Serum glucose profiles were determined by the glucose oxidase/peroxidase method.
Intestinal morphology
Tissue samples (3 cm) were obtained from the middle part of the duodenum and jejunum washed with 10% formalin, and fixed in phosphate-buffered saline (PBS) (40 g of NaH2PO4, 65 g of Na2HPO4, and 1000 mL of 10% formalin) for 24–48 h (Gunal et al. 2006). Tissues were dehydrated with an automatic tissue processor. The process consisted of dehydrating the tissues in a series of increasing concentrations of alcohol, clearing the tissue in xylene, and embedding it in paraffin. A microtome was used to make three cuts of 5-μm width. The cuts were stained with hematoxylin and eosin. For each treatment, 16 glass slides were prepared and the mean of 10 villi per slide was used as the average value for further analysis. Villus height (VH), villus width (VW), crypt depth (CD), and the ratios of VH:VW and VH:CD were determined at a magnification of ×10 by using a light microscope.
Microbial enumeration
Enumeration of the microbial population in the ileal contents was performed at 42 days of age in eight birds per treatment. Various dehydrated media were prepared and sterilized according to the manufacturer’s instructions before being poured into sterile Petri dishes. The media consisted of nutrient agar for total bacteria culture, De Man–Rogosa–Sharpe agar for Lactobacillus culture, and eosin methylene blue agar for Escherichia coli culture. A 10-fold serial dilution method using sterilized water was adopted to determine colony-forming units in each gram of digesta harvested from the ileum by means of the pour plate method (Jang et al. 2007; Shams Shargh et al. 2012).
Statistical analyses
Data were subjected to ANOVA by using Statistical Analysis System (SAS) Institute (2002) and where significant (P < 0.05), treatment means were compared using Duncan’s multiple range test.
Results
Feed intake and productivity
The results are presented in Table 3. No mortality occurred during the study. The highest final bodyweight and body-weight gain (P < 0.05) were recorded in birds fed with Diet 4 and the smallest bodyweight gain was observed in birds fed with Diet 2. The lowest feed intake was recorded in birds fed the control diet (Diet 1) and Diet 4 (P > 0.05). The lowest feed conversion ratio (FCR) and best feed cost per kilogram of gain was found in birds fed Diet 4 (P < 0.05), whereas birds fed Diet 2 showed the highest FCR (P < 0.05).
| Parameter | Diet 1 | Diet 2 | Diet 3 | Diet 4 | s.e.m. | P = value | |
|---|---|---|---|---|---|---|---|
| Initial bodyweight (g) | 941.00 | 942.00 | 943.00 | 942.00 | n.s. | n.s. | |
| Final bodyweight per bird (kg) | 2.61b | 2.58c | 2.61b | 2.68a | 0.05 | 0.04 | |
| Body weight gain per bird (kg) | 1.68b | 1.64c | 1.66c | 1.74a | 0.08 | 0.03 | |
| Feed intake per bird | 3.59 | 3.61 | 3.60 | 3.58 | 0.06 | 0.07 | |
| Feed conversion ratio | 2.14b | 2.20a | 2.17b | 2.06c | 0.04 | 0.02 | |
| Cost of feed per kg gain | 0.90a | 0.81b | 0.81c | 0.77d | 0.15 | 0.02 |
Diet 1 = control diet (corn–soyabean meal-based diet) containing 4.0 g kg−1 digestible methionine, 0.96 g kg−1 digestible lysine; Diet 2 = a diet containing 50% replacement of maize in Diet 1 with high-quality cassava peel meal and containing 4.0 g kg−1 digestible methionine, 0.96 g kg−1 digestible lysine; Diet 3 = a diet containing 50% replacement of maize in Diet 1 with high-quality cassava peel meal and containing 4.4 g kg−1 digestible methionine, 1.02 g kg−1 digestible lysine’ Diet 4 = a diet containing 50% replacement of maize in Diet 1 with high-quality cassava peel meal and containing 4.8 g kg−1 digestible methionine, 1.08 g kg−1 digestible lysine. s.e.m., standard error of the mean, n.s., not significant.
Within a row, values followed by different letters are significantly different (P < 0.05).
Intestinal morphology
Table 4 shows that in the duodenum of the chickens, villi width, crypt depth and intestinal muscle thickness were not significantly influenced by the dietary treatments. The villi height and villi height:crypt depth ratio were highest in birds fed Diet 4. In the jejunum, villi height, width, depth and villi height:crypt depth ratio were significantly (P < 0.05) affected by the dietary treatment. The highest villi height and villi height:crypt depth ratio were recorded in birds fed Diet 4 (P < 0.05), whereas the highest crypt depth was found in birds fed Diet 2 (P < 0.05) (Figs 1, 2).
| Gut section | Diet 1 | Diet 2 | Diet 3 | Diet 4 | s.e.m. | P = value | |
|---|---|---|---|---|---|---|---|
| Duodenum | |||||||
| Villi height | 1243.18b | 1246.25b | 1025.34c | 2246.68a | 300.87 | 0.02 | |
| Villi width | 201.23 | 282.42 | 229.11 | 303.08 | 55.00 | 0.05 | |
| Crypt depth | 475.44c | 563.13b | 377.56d | 681.88a | 102.45 | 0.03 | |
| Crypt width | 274.19 | 262.17 | 225.43 | 329.36 | 50.09 | 0.05 | |
| Muscle thickness | 290.50 | 284.04 | 251.12 | 291.17 | 65.67 | 0.07 | |
| VH:CD | 2.61c | 2.21d | 2.72b | 3.29a | 0.07 | 0.04 | |
| Jejunum | |||||||
| Villi height | 1251.18b | 2287.47a | 1106.32c | 2201.84a | 302.98 | 0.04 | |
| Villi width | 196.13b | 306.42a | 194.34b | 323.46a | 75.87 | 0.03 | |
| Crypt depth | 455.63b | 747.12a | 343.18c | 546.49b | 200.01 | 0.02 | |
| Crypt width | 294.29 | 330.78 | 197.25 | 328.58 | 50.98 | 0.09 | |
| Muscle thickness | 300.80 | 269.87 | 328.40 | 289.11 | 65.05 | 0.05 | |
| VH:CD | 2.75d | 3.06c | 3.22b | 4.03a | 0.09 | 0.03 | |
Diet 1 = control diet (corn–soyabean meal-based diet) containing 4.0 g kg−1 digestible methionine, 0.96 g kg−1 digestible lysine; Diet 2 = a diet containing 50% replacement of maize in Diet 1 with high-quality cassava peel meal and containing 4.0 g kg−1 digestible methionine, 0.96 g kg−1 digestible lysine; Diet 3 = a diet containing 50% replacement of maize in Diet 1 with high-quality cassava peel meal and containing 4.4 g kg−1 digestible methionine, 1.02 g kg−1 digestible lysine; Diet 4 = a diet containing 50% replacement of maize in Diet 1 with high-quality cassava peel meal and containing 4.8 g kg−1 digestible methionine, 1.08 g kg−1 digestible lysine. s.e.m., standard error of the mean; VH:CD, villus height:crypt depth.
Within a row, values followed by different letters are significantly different (P < 0.05).
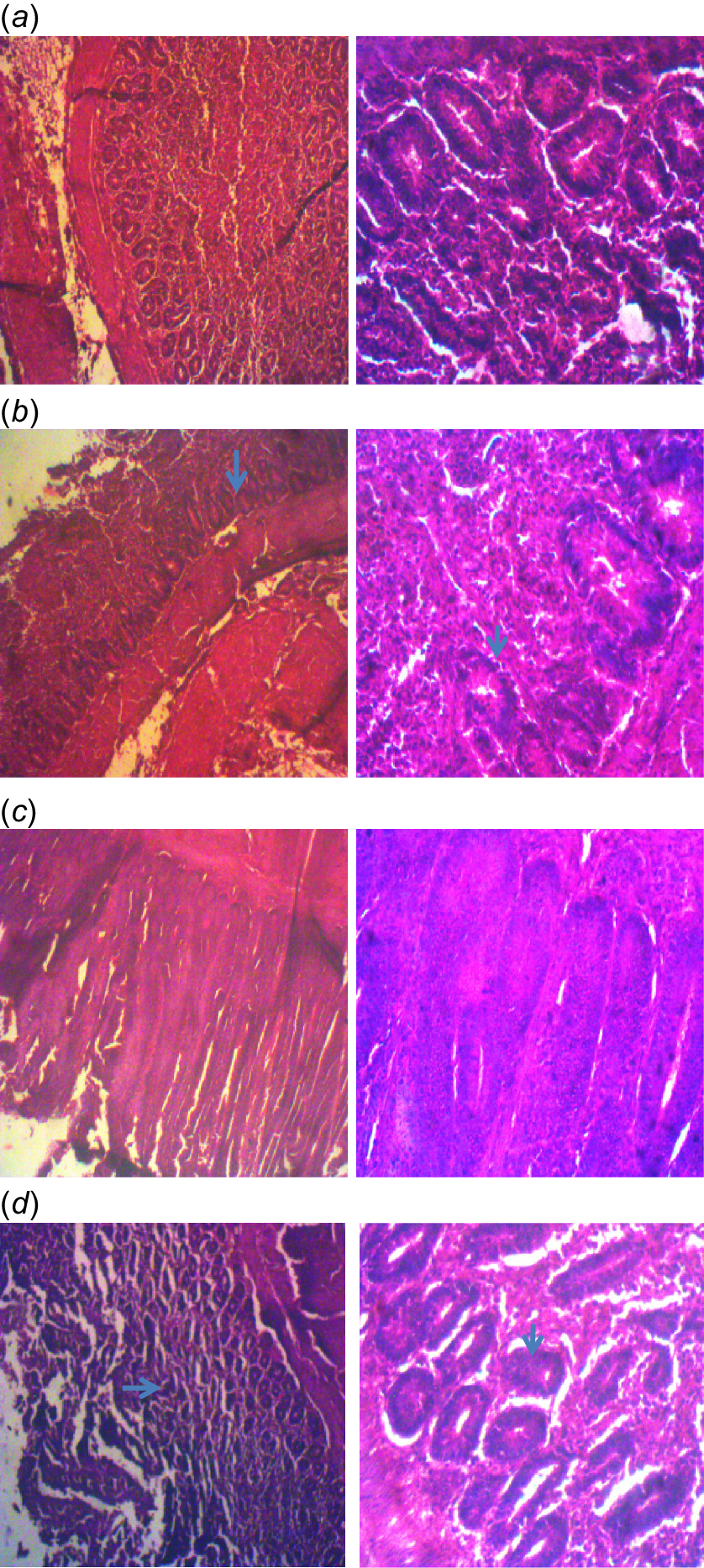
The histopathology slide of intestinal tissue (jejunal) of finisher broiler chickens fed diets containing high-quality cassava peel meal. (a) There is no observable lesion in the jejunal tissue of the birds fed Diet 1. HE ×100, ×400. (b) There is atrophy of villi and hyperplasia of the crypts in the jejunal tissue of the birds fed Diet 2. HE ×100, ×400. (c) There is no observable lesion in the jejunal tissue of the birds fed Diet 3. HE ×100, ×400. (d) There is atrophy of villi and hyperplasia of the crypts in the jejunal tissue of birds fed Diet 4. Diet 1 = control diet (corn-soyabean meal based diet) containing 4.0 g kg−1 digestible MET, 0.96 g kg−1 digestible lysine; Diet 2 = a diet containing 50% replacement of maize in Diet 1 with high-quality cassava peel meal and containing 4.0 g kg−1 digestible MET, 0.96 g kg−1 digestible lysine; Diet 3 = a diet containing 50% replacement of maize in Diet 1 with high-quality cassava peel meal and containing 4.4 g kg−1 digestible MET, 1.02 g kg−1 digestible lysine; Diet 4 = a diet containing 50% replacement of maize in Diet 1 with high-quality cassava peel meal and containing 4.8 g kg−1 digestible MET, 1.08 g kg−1 digestible lysine.
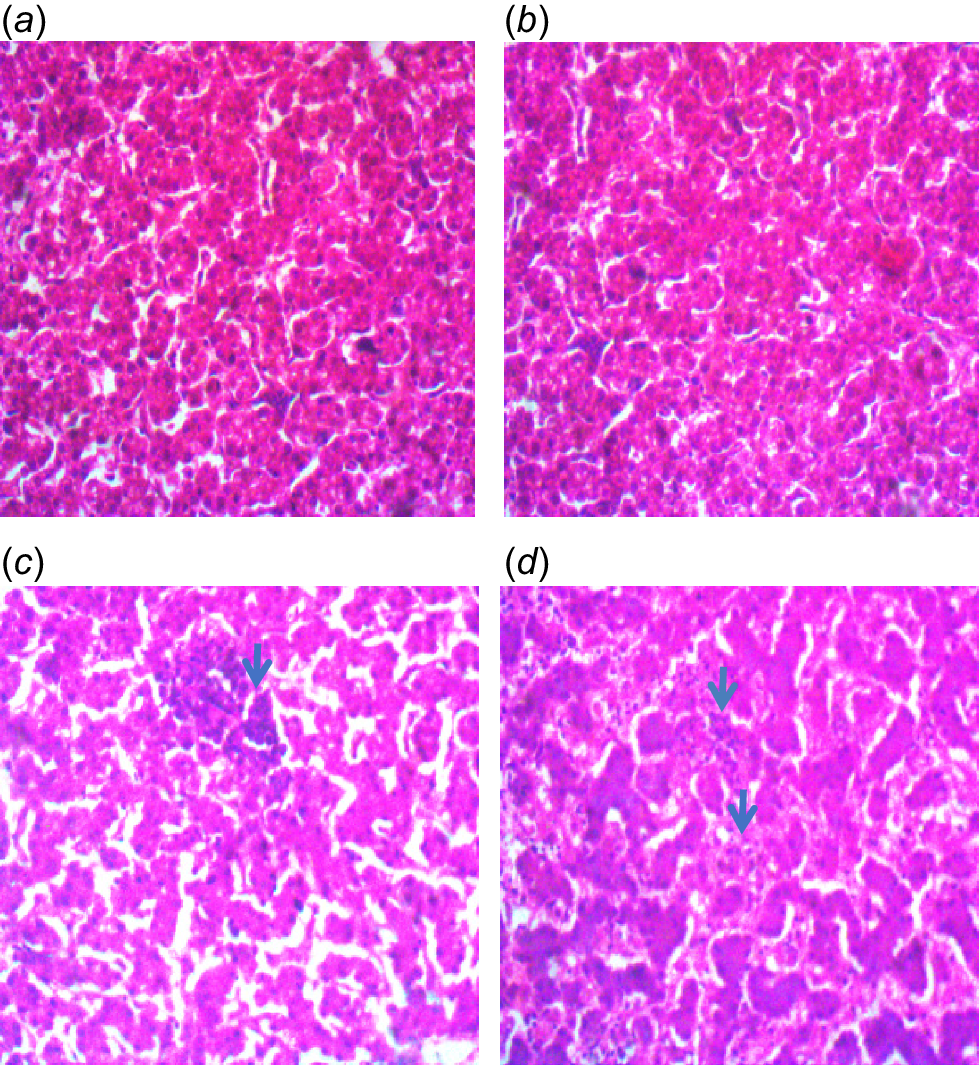
The histopathology slide of liver tissue of finisher broiler chickens fed diets containing high-quality cassava peel meal. (a) There is no observable lesion in the liver tissue of the birds fed Diet 1. HE ×400. (b) There is no observable lesion in the liver tissue of the birds fed Diet 2. HE ×400. (c) There is plate atrophy and foci of necrotizing inflammation (arrow) in the liver of the birds fed Diet 3. HE ×400. (d) There is plate atrophy and severe of necrotizing inflammation (arrows) in the liver of the birds fed Diet 4. HE ×400. Diet 1 = control diet (corn-soyabean meal based diet) containing 4.0 g kg−1 digestible MET, 0.96 g kg−1 digestible lysine; Diet 2 = a diet containing 50% replacement of maize in Diet 1 with high-quality cassava peel meal and containing 4.0 g kg−1 digestible MET, 0.96 g kg−1 digestible lysine; Diet 3 = a diet containing 50% replacement of maize in Diet 1 with high-quality cassava peel meal and containing 4.4 g kg−1 digestible MET, 1.02 g kg−1 digestible lysine; Diet 4 = a diet containing 50% replacement of maize in Diet 1 with high-quality cassava peel meal and containing 4.8 g kg−1 digestible MET, 1.08 g kg−1 digestible lysine.
Microbial count
All birds fed diets containing HQCP meal and supplemented with amino acid had higher intestinal Lactobaccillus spp. count, with the highest (P < 0.05) count being in birds fed Diet 4. The Escherichia coli count was not significantly (P > 0.05) different across treatments. Finisher broiler chickens fed Diet 4 also showed the highest (P < 0.05) total bacteria count (Table 5).
| Item (log10 cfu g−1) | Diet 1 | Diet 2 | Diet 3 | Diet 4 | s.e.m. | P = value | |
|---|---|---|---|---|---|---|---|
| Lactobacillus spp. | 4.54c | 6.71b | 6.83b | 8.16a | 0.22 | 0.03 | |
| Escherichia coli | 6.35 | 6.15 | 6.45 | 6.35 | 0.35 | 0.07 | |
| Total bacteria count | 7.93c | 9.73a | 8.03b | 8.74a | 0.15 | 0.02 |
Diet 1 = control diet (corn–soyabean meal-based diet) containing 4.0 g kg−1 digestible methionine, 0.96 g kg−1 digestible lysine; Diet 2 = a diet containing 50% replacement of maize in Diet 1 with high-quality cassava peel meal and containing 4.0 g kg−1 digestible methionine, 0.96 g kg−1 digestible lysine; Diet 3 = a diet containing 50% replacement of maize in Diet 1 with high-quality cassava peel meal and containing 4.4 g kg−1 digestible methionine, 1.02 g kg−1 digestible lysine; Diet 4 = a diet containing 50% replacement of maize in Diet 1 with high-quality cassava peel meal and containing 4.8 g kg−1 digestible methionine, 1.08 g kg−1 digestible lysine. s.e.m., standard error of the mean.
Within a row, values followed by different letters are significantly different (P < 0.05).
Blood serum and hematology
Table 6 shows that birds fed Diet 1 had the highest packed cell volume (PCV), hemoglobin, and red blood cell (P < 0.05) and white blood cell (P = 0.05) counts. However, the mean corpuscular hemoglobin (MCH), mean corpuscular volume (MCV), and mean corpuscular hemoglobin concentration (MCHC) were not significantly affected by the dietary treatment. The effects of dietary supplementation of methionine and lysine to cassava-meal based diets on serum biochemistry of finisher broiler chickens are provided in Table 7. There were no significant (P > 0.05) effects of the dietary treatments on serum biochemistry of the finisher broiler chickens.
| Parameter | Diet 1 | Diet 2 | Diet 3 | Diet 4 | s.e.m. | P = value | |
|---|---|---|---|---|---|---|---|
| PCV (%) | 34.33a | 31.66b | 32.00b | 25.33c | 1.56 | 0.04 | |
| Hemoglobin (g dL−1) | 11.56a | 10.70b | 10.73b | 8.63c | 1.23 | 0.02 | |
| Red blood cell (×1012 L−1) | 3.13a | 2.63c | 2.80b | 2.30d | 0.37 | 0.02 | |
| White blood cells (×1012 L−1) | 15.27 | 14.13 | 14.33 | 11.63 | 3.56 | 0.05 | |
| Neutrophils (%) | 27.67 | 28.33 | 31.33 | 33.00 | 5.78 | 0.07 | |
| Lymphocytes (%) | 71.00b | 69.66b | 68.00b | 110.00a | 14.56 | 0.001 | |
| Eosinophyl (%) | 0.33a | 0.33a | 0.00 | 0.33b | 0.21 | 0.02 | |
| Monocytes (%) | 0.66b | 1.00a | 0.33c | 0.66b | 0.34 | 0.01 | |
| MCV (fl) | 110.06 | 120.48 | 114.82 | 110.59 | 14.34 | 0.55 | |
| MCH (pg) | 36.99 | 40.70 | 38.48 | 37.58 | 4.89 | 0.45 | |
| MCHC (g dL−1) | 33.66 | 33.88 | 33.58 | 34.26 | 3.34 | 0.93 |
Diet 1 = control diet (corn–soyabean meal-based diet) containing 4.0 g kg−1 digestible methionine, 0.96 g kg−1 digestible lysine; Diet 2 = a diet containing 50% replacement of maize in Diet 1 with high-quality cassava peel meal and containing 4.0 g kg−1 digestible methionine, 0.96 g kg−1 digestible lysine; Diet 3 = a diet containing 50% replacement of maize in Diet 1 with high-quality cassava peel meal and containing 4.4 g kg−1 digestible methionine, 1.02 g kg−1 digestible lysine; Diet 4 = a diet containing 50% replacement of maize in Diet 1 with high-quality cassava peel meal and containing 4.8 g kg−1 digestible methionine, 1.08 g kg−1 digestible lysine. s.e.m., standard error of the mean; PCV, packed cell volume; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration.
Within a row, values followed by different letters are significantly different (P < 0.05).
| Parameter | Diet 1 | Diet 2 | Diet 3 | Diet 4 | s.e.m. | P = value | |
|---|---|---|---|---|---|---|---|
| Total protein (g L−1) | 6.1 | 5.7 | 6.5 | 5.9 | 0.78 | 0.98 | |
| Albumin (g L−1) | 4.13 | 4.37 | 4.23 | 4.70 | 0.89 | 0.93 | |
| Globulin (g L−1) | 1.93 | 1.30 | 2.27 | 1.13 | 1.98 | 0.68 | |
| Cholesterol (mg dL−1) | 131.10 | 118.63 | 118.63 | 134.30 | 15.78 | 0.84 | |
| Triglyceride (mg dL−1) | 95.90 | 92.50 | 94.60 | 96.73 | 3.89 | 0.90 | |
| Glucose (g L−1) | 118.63 | 120.93 | 125.03 | 128.97 | 3.67 | 1.00 | |
| Uric acid (mg dL−1) | 10.53 | 8.70 | 8.87 | 8.70 | 2.88 | 0.07 | |
| AST (U L−1) | 105.67 | 104.67 | 103.00 | 98.67 | 2.55 | 0.34 | |
| ALT (U L−1) | 60.50 | 56.00 | 57.50 | 60.00 | 4.78 | 0.74 | |
| Creatinine (mg dL−1) | 1.27 | 1.54 | 1.64 | 0.59 | 0.98 | 0.64 | |
| ALP (U L−1) | 31.33 | 28.67 | 31.33 | 31.33 | 4.56 | 0.77 |
Diet 1 = control diet (corn–soyabean meal based diet) containing 4.0 g kg−1 digestible methionine, 0.96 g kg−1 digestible lysine; Diet 2 = a diet containing 50% replacement of maize in Diet 1 with high-quality cassava peel meal and containing 4.0 g kg−1 digestible methionine, 0.96 g kg−1 digestible lysine; Diet 3 = a diet containing 50% replacement of maize in Diet 1 with high-quality cassava peel meal and containing 4.4 g kg−1 digestible methionine, 1.02 g kg−1 digestible lysine; Diet 4 = a diet containing 50% replacement of maize in Diet 1 with high-quality cassava peel meal and containing 4.8 g kg−1 digestible methionine, 1.08 g kg−1 digestible lysine. s.e.m., standard error of the mean.
Within a row, values followed by different letters are significantly different (P < 0.05).
Discussion
This study has demonstrated that the supplementation of methionine and lysine to diets containing HQCP meal at 50% replacement of maize can benefit growth performance of finisher broiler chickens by improving bodyweight gain, feed conversion ratio, and cost of feed per kilogram of gain. Various studies have identified methionine as the most limiting amino acid in cassava roots and peels for the growth of poultry, with lysine being the second-most limiting (Nassar and De Sousa 2007; Ande et al. 2021). Our results align with the previous studies by demonstrating that adequate supplementation of methionine to cassava peel-based diets can improve growth performance of broiler chickens. Hence, the addition of methionine and lysine at adequate or higher concentrations to cassava peel meal-based diets may increase the availability of the amino acids to the birds for growth performance. In addition, methionine is essential not only for protein deposition but also for detoxifying hydrogen cyanide (Tewe et al. 1977), especially in diets that contain high amounts of cassava peels. Thus, the improved growth performance of the birds may be attributed to the reduced metabolic disturbances caused by the continuous depletion of sulfur-containing amino acids (methionine) from the bloodstream, which can occur when animals consume diets high in cyanide, such as cassava peel meal (CPM), and improved tissue accretion. Lysine plays an important role in tissue accretion (Wang et al. 2022). These findings agree with that of Oloruntola (2020), who showed that addition of high concentrations of methionine and multi-enzyme to the diets containing 35% inclusion of sun-dried CPM did not negatively affect the growth performance of rabbits. Our previous findings showed that carcass yield of broiler chickens fed HQCP-based diets supplemented with methionine and lysine were not compromised (Adebowale et al. 2024). It seems that CPM-based diets compromise the growth performance of broiler chickens when CPM is used to replace more than 20% of maize (Aderemi et al. 2012; Olowoyeye et al. 2019; Chang’a et al. 2020), without adequate supplementation of methionine and lysine to the diet. It is possible that the reduced antinutritional factors, including the fiber content of the HQCP affects the utilization of the cassava by-product for growth performance by the birds. Amole et al. (2022) reported that the crude protein, crude fiber and hydrogen cyanide concentrations of CPM were improved during the transformation process to HQCP.
It was observed that dietary supplementation of methionine and lysine to HQCP-based diets did not compromise villus height to crypt depth ratio in the duodenum and jejunum, indicating higher absorptive function of the intestine. Itzá-Ortiz et al. (2019) reported that increased villus height (VH) and crypt depth (CD) of piglets is an indication of higher nutrient absorptive capacity of the intestine, but which may not correlate with bodyweight gain. However, this is contrary to the observation in this study. The higher VH:CD ratio of the birds fed diets containing HQCP fortified with methionine and lysine lead to improved bodyweight gain. This is in agreement with the report of Jia et al. (2010) that suggested that the villus height, crypt depth, and villus height:crypt depth ratio are directly related to the absorptive capacity of the intestinal mucous membrane. Surprisingly, there was an atrophy of the villi and hyperplasia of the crypts in the birds fed diets containing HQCP. The study of Evans and Ozung (2020) showed an alteration in the intestine of rabbits fed diets containing high concentrations of cassava peel as a replacement for maize. Ojediran et al. (2024) found that the histology of the liver and intestine of piglets fed with diets containing high inclusion level of HQCP meal had varying degrees of inflammation. This also supports the findings in this study. Cereda (2023) suggested that cyanogenic glucoside in cassava peels could pose risks to the intestine, if not properly processed (Aminlari and Shahbazi 1994; Ejiro 2015). Interestingly, it was observed that the HCN concentration in the HQCP were within the reported tolerable level for birds (Okoli et al. 2012). However, it is possible that the accumulation of cyanide, even at lower concentrations, may have a gradual impact on growing birds.
Methionine has been reported to sustain the functional integrity of the intestinal barrier (Gong et al. 2023) and promote regeneration of intestinal epithelium (Ramalingam et al. 2010). This study observed that birds fed diets without additional supplementation of methionine and lysine showed a reduced muscular thickness, together with necrotic lesions on the intestinal tissues. It is suspected that the increased intestinal muscular thickness in birds fed diets supplemented with methionine and lysine influenced the growth performance of the birds by increasing the surface area for nutrient absorption.
Fermented feed products have potential to increase the concentration of health-promoting bacteria (Lactobacillus) in the gut (De Filippis et al. 2020). It was observed that the ileal population of lactic acid bacteria (Lactobacillus) and total bacterial count were higher in treatments containing HQCP with or without additional supplementation of methionine and lysine. The dominant microbiota present in the ileum of chickens are the facultative and microaerophilic bacteria, lactobacilli (Yin et al. 2010). The population of these microbiota plays an important role in nutrient utilization and overall wellbeing of the birds.
In all treatment groups, values recorded for PCV, WBC, and RBC were within the reported normal range for poultry (Adegoke et al. 2018). This observation agrees with previous reports. Ajuonuma and Uchendu (2013) recorded that the replacement of dietary maize with cassava peel did not compromise hematological parameters of growing pullets. Similarly, Olafadehan (2011) demonstrated that incorporating variously HQCP into rabbit diets did not adversely affect their blood constituents.
In conclusion, diets containing HQCP supplemented with methionine and lysine did not compromise growth performance, histomorphometric values (villus height to crypt depth ratio, crypt depth, and villus height) and did not adversely affect the hemato-biochemical indices of finisher broiler chickens. However, morphological changes were observed in the liver and intestinal tissues of the birds fed fed HQCP-based diets. It is possible that the morphological changes can be mitigated by further fortifying the diet with specific health-promoting additives. This study demonstrated that enhanced processing techniques, along with proper fortification with methionine and lysine, can significantly increase the utilization of cassava peels in broiler chicken diets. This approach offers a viable alternative to traditional feed ingredients, promoting sustainability and cost-efficiency in poultry production. Future research should explore long-term effects and mechanisms underlying the observed benefits on growth performance, as well as the morphological alterations in the liver and intestinal tissues of the birds.
Data availability
The data that support this study are available in Mendeley data (10.17632/kzpj9gkkyj.1).
Declaration of funding
This research was supported by the International Foundation for Science, Stockholm, Sweden, through a grant to the first author (Tolulope Adebowale). Tetfund Institutional Based Research Fund is well acknowledged for the basic research grant towards this study. The support of TWAS-UNESCO for a research visit to Izmir Institute of Technology, Türkiye, is crucial for the completion of this study.
References
Adebowale TO, Oso AO, Bamgbose AM (2024) Carcass trait, meat lipid profile and meat quality of broiler chickens fed diets containing high inclusion level of high quality cassava (Manihot esculenta) peel meal. Journal of Agriculture and Rural Development in the Tropics and Subtropics (JARTS) 125(2), 149-157.
| Crossref | Google Scholar |
Adegoke AV, Abimbola MA, Sanwo KA, Egbeyale LT, Abiona JA, Oso AO, Iposu SO (2018) Performance and blood biochemistry profile of broiler chickens fed dietary turmeric (Curcuma longa) powder and cayenne pepper (Capsicum frutescens) powders as antioxidants. Veterinary and Animal Science 6, 95-102.
| Crossref | Google Scholar | PubMed |
Aderemi FA, Adenowo TK, Oguntunji AO (2012) Effect of whole cassava meal on performance and egg quality characteristics of layers. Journal of Agricultural Science 4(2), 195-200.
| Crossref | Google Scholar |
Ajuonuma CO, Uchendu CI (2013) Effect of processed cassava peel meal on the haematology of pullets. IOSR Journal of Agriculture and Veterinary Science 6(3), 27-29.
| Crossref | Google Scholar |
Aminlari M, Shahbazi M (1994) Rhodanese (thiosulfate:cyanide sulfurtransferase) distribution in the digestive tract of chicken. Poultry Science 73(9), 1465-1469.
| Crossref | Google Scholar | PubMed |
Ande KO, Oso AO, Oluwatosin OO, Sanni LO, Adebayo K (2021) Effect of white and yellow cassava root meal diets supplemented with different additives on performance of layers and the quality of eggs laid. Tropical Animal Health and Production 53, 235.
| Crossref | Google Scholar | PubMed |
Ayernor GS (1985) Effects of the retting of cassava on product yield and cyanide detoxication. International Journal of Food Science and Technology 20(1), 89-96.
| Crossref | Google Scholar |
Baker DH, Han Y (1994) Ideal amino acid profile for chicks during the first three weeks posthatching. Poultry Science 73(9), 1441-1447.
| Crossref | Google Scholar | PubMed |
Cafe MB, Waldroup PW (2006) Interactions between levels of methionine and lysine in broiler diets changed at typical industry intervals. International Journal of Poultry Science 5, 1008-1015.
| Crossref | Google Scholar |
Cannan RK (1958) Proposal for a certified standard for use in hemoglobinometry; second and final report. Journal of Laboratory and Clinical Medicine 52(3), 471-476.
| Google Scholar | PubMed |
Cardoso AP, Mirione E, Ernesto M, Massaza F, Cliff J, Haque MR, Bradbury JH (2005) Processing of cassava roots to remove cyanogens. Journal of Food Composition and Analysis 18, 451-460.
| Google Scholar |
Chang’a EP, Abdallh ME, Ahiwe EU, Mbaga S, Zhu ZY, Fru-Nji F, Iji PA (2020) Replacement value of cassava for maize in broiler chicken diets supplemented with enzymes. Asian-Australasian Journal of Animal Sciences 33(7), 1126-1137.
| Crossref | Google Scholar |
De Filippis F, Pasolli E, Ercolini D (2020) The food-gut axis: lactic acid bacteria and their link to food, the gut microbiome and human health. FEMS Microbiology Reviews 44(4), 454-489 https://doi.org/10.1093/femsre/fuaa015.
| Google Scholar | PubMed |
Ejiro KH (2015) A comparative study on the distribution of cyanide in sections of the digestive tracts and other organs in the domestic chicken (Gallus domesticus L.) exposed to varying levels of cyanide. Annals of Biological Research 6(3), 1-5.
| Google Scholar |
Evans EI, Ozung PO (2020) Histopathological changes of some internal organs and brain regions of rabbits fed dietary cassava peel meal as replacement for Maize. Asian Journal of Animal Sciences 14, 93-102.
| Crossref | Google Scholar |
Gong L, Mahmood T, Mercier Y, Xu H, Zhang X, Zhao Y, Luo Y, Guo Y (2023) Dietary methionine sources and levels modulate the intestinal health status of broiler chickens. Animal Nutrition 15, 242-255.
| Crossref | Google Scholar | PubMed |
Gunal M, Yayli G, Kaya O, Karahan N, Sulak O (2006) The effects of antibiotic growth promoter, probiotic or organic acid supplementation on performance, intestinal microflora and tissue of broilers. International Journal of Poultry Science 5(2), 149-155.
| Crossref | Google Scholar |
Gungor E, Erener G (2020) Effect of dietary raw and fermented sour cherry kernel (Prunus cerasus L.) on digestibility, intestinal morphology and caecal microflora in broiler chickens. Poultry Science 99(1), 471-478.
| Crossref | Google Scholar | PubMed |
Itzá-Ortiz M, Segura-Correa J, Para-Suescún J, Aguilar-Urquizo E, Escobar-Gordillo N (2019) Correlation between body weight and intestinal villi morphology in finishing pigs. Acta Universitaria 29, 1-4.
| Google Scholar |
Jang IS, Ko YH, Kang SY, Lee CY (2007) Effect of a commercial essential oil on growth performance, digestive enzyme activity and intestinal microflora population in broiler chickens. Animal Feed Science and Technology 134(3–4), 304-315.
| Crossref | Google Scholar |
Jia G, Yan J-Y, Cai J-Y, Wang K-N (2010) Effects of encapsulated and non-encapsulated compound acidifiers on gastrointestinal pH and intestinal morphology and function in weaning piglets. Journal of Animal and Feed Sciences 19(1), 81-92.
| Crossref | Google Scholar |
Nassar NMA, De Sousa MV (2007) Amino acid profile in cassava and its interspecific hybrid. Genetics and Molecular Research 6(2), 292-297.
| Google Scholar | PubMed |
Ojediran TK, Olayeni TB, Azeez SA, Amolegbe FD, Emiola IA (2024) High-quality cassava peel meal for growing pigs: implications on carcass, meat quality, organ weights, hepatic and jejunum histology. Journal of microbiology, biotechnology and food sciences 4(1), e10285.
| Crossref | Google Scholar |
Oke OL (1978) Problems in the use of cassava as animal feed. Animal Feed Science and Technology 3(4), 345-380.
| Crossref | Google Scholar |
Okoli IC, Okparaocha CO, Chinweze CE, Udedibie ABI (2012) Physicochemical and hydrogen cyanide content of three processed cassava products used for feeding poultry in Nigeria. Asian Journal of Animal and Veterinary Advances 7(4), 334-340.
| Crossref | Google Scholar |
Olafadehan OA (2011) Haematological parameters, serum constituents and organ development of growing rabbits as affected by feeding of processed cassava peels. Animal Nutrition and Feed Technology 11(1), 41-51.
| Google Scholar |
Oloruntola OD (2020) Effect of dietary cassava peel meal supplemented with methionine and multienzyme on hemo-biochemical indices, digestibility, and antioxidants in rabbits. The Journal of Basic and Applied Zoology 81, 33.
| Crossref | Google Scholar |
Olowoyeye JC, Agbede JO, Igbasan FA, Oloruntola OD, Ayeni AO (2019) Effect of replacing maize with cassava peel-leaf mixture on growth performance of broiler chickens. Livestock Research for Rural Development 31(10), 518-524.
| Google Scholar |
Oyebimpe K, Fanimo AO, Odugwa OO, Biobaku WO (2006) Response of broiler chickens to cassava peel and maize offal in cashewnut meal-based diets. Archivos De Zootecnia 55, 301-304.
| Google Scholar |
Poku A-G, Birner R, Gupta S (2018) Is Africa ready to develop a competitive bioeconomy? The case of the cassava value web in Ghana. Journal of Cleaner Production 200, 134-147.
| Crossref | Google Scholar |
Ramalingam A, Wang X, Gabello M, Valenzano MC, Soler AP, Ko A, Morin PJ, Mullin JM (2010) Dietary methionine restriction improves colon tight junction barrier function and alters claudin expression pattern. American Journal of Physiology-Cell Physiology 299(5), C1028-C1035.
| Crossref | Google Scholar | PubMed |
Shams Shargh M, Dastar B, Zerehdaran S, Khomeiri M, Moradi A (2012) Effects of using plant extracts and a probiotic on performance, intestinal morphology, and microflora population in broilers. Journal of Applied Poultry Research 21(2), 201-208.
| Crossref | Google Scholar |
Si J, Kersey JH, Fritts CA, Waldroup PW (2004) An evaluation of the interaction of lysine and methionine in diets for growing broilers. International Journal of Poultry Science 3, 51-60.
| Google Scholar |
Spackman DH, Stein WH, Moore S (1958) Automatic recording apparatus for use in the chromatography of amino acids. Analytical Chemistry 30(7), 1190-1206.
| Crossref | Google Scholar |
Tewe OO, Maner JH, Gomez G (1977) Influence of cassava diets on placental thiocyanate transfer, tissue rhodanese activity and performance of rats during gestation. Journal of the Science of Food and Agriculture 28(8), 750-756.
| Crossref | Google Scholar |
Varley JM, Macgregor HC, Erba HP (1980) Satellite DNA is transcribed on lampbrush chromosomes. Nature 283, 686-688.
| Crossref | Google Scholar | PubMed |
Wang Z, Shao D, Kang K, Wu S, Zhong G, Song Z, Shi S (2022) Low protein with high amino acid diets improves the growth performance of yellow feather broilers by improving intestinal health under cyclic heat stress. Journal of Thermal Biology 105, 103219.
| Crossref | Google Scholar | PubMed |
Wootton DM, Powell EC (1964) Parahalipegus (gen. n.) for Halipegus aspina Ingles, 1936 (Hemiuridae:Trematoda). The Journal of Parasitology 50(5), 662-663.
| Crossref | Google Scholar | PubMed |
Yin Y, Lei F, Zhu L, Li S, Wu Z, Zhang R, Gao GF, Zhu B, Wang X (2010) Exposure of different bacterial inocula to newborn chicken affects gut microbiota development and ileum gene expression. The ISME Journal 4(3), 367-376.
| Crossref | Google Scholar |


