Reducing in vitro rumen methanogenesis for two contrasting diets using a series of inclusion rates of different additives
M. O’Brien A , A. Navarro-Villa A B , P. J. Purcell A B , T. M. Boland B and P. O’Kiely A CA Animal and Grassland Research and Innovation Centre, Teagasc, Grange, Dunsany, Co. Meath, Ireland.
B School of Agriculture, Food Science and Veterinary Medicine, University College Dublin, Belfield, Dublin 4, Ireland.
C Corresponding author. Email: padraig.okiely@teagasc.ie
Animal Production Science 54(2) 141-157 https://doi.org/10.1071/AN12204
Submitted: 13 April 2012 Accepted: 1 February 2013 Published: 24 April 2013
Abstract
Eleven individual additives were incubated with either perennial ryegrass or with grass silage+barley grain (50 : 50) and the in vitro methane output was assessed using the gas production technique (GPT). Additives were: fatty acids (lauric, oleic, linoleic and linolenic acids), halogenated methane analogues (bromoethanesulfonate and bromochloromethane), pyromellitic diimide, statins (mevastatin and lovastatin), a probiotic (Saccharomyces cerevisiae) and an unsaturated dicarboxylic acid (fumaric acid). Each additive was included at a range of concentrations. Effects on methane output per gram of feed dry matter (DM) incubated (CH4/DMi) and disappeared (CH4/DMd), as well as other fermentation variables, were evaluated after 24 h of incubation. The addition of increased concentrations of individual fatty acids, bromoethanesulfonate and pyromellitic diimide caused a dose-dependent decline in methane output (CH4/DMi, CH4/DMd), when incubated with either perennial ryegrass or grass silage+barley grain. No methane output was detected for either feed with the addition of ≥5 µM bromochloromethane. The statins were ineffective inhibitors of methane output regardless of feed type. For perennial ryegrass, S. cerevisiae caused a dose-dependent decline in CH4/DMd and fumaric acid a dose-dependent decline in CH4/DMi and CH4/DMd. The effectiveness of lauric, oleic, linoleic and linolenic acids and bromoethanesulfonate to reduce methane output was more pronounced when incubated with grass silage+barley grain than with perennial ryegrass, and therefore the type of feed is an important component for any future in vitro and in vivo studies to be undertaken with these additives. Thus, incorporating different feed types in the initial in vitro screening protocols of all new additives is recommended.
Additional keywords: in vitro total gas production technique, rumen fermentation modifiers, ruminant diets.
Introduction
The ruminant diet has a major effect on enteric methane production (Moss et al. 2000). The contribution of enteric methane to greenhouse gas emissions and the substantial loss of ruminant feed energy as methane has prompted research into chemical and biological compounds which can be either an integral part of the animal diet (e.g. dietary inclusion of fatty acids using coconut oil as demonstrated by Lovett et al. (2003)) or added as an additive (e.g. ionophoric antibiotics (monensin) as shown by Goodrich et al. (1984)) to reduce enteric methane losses. The use of ionophores to manipulate rumen fermentation and, thus, reduce methane emissions is being limited because of human health concerns (Nikolich et al. 1994; Moss et al. 2000). Consequently, there has been an increasing interest in fatty acids, halogenated methane analogues (HMA), probiotics (predominantly Saccharomyces cerevisiae and Aspergillus oryzae) and dicarboxylic acids as enteric methane inhibitors. The mode of action of these compounds to reduce ruminant methane emissions are quite variable, with some being directly inhibitory to methanogens (e.g. fatty acids (Zhang et al. 2008), HMA (Prins et al. 1972)) and/or protozoa (e.g. fatty acids (Matsumoto et al. 1991)), while the same or different compounds cause a shift towards the production of propionate in the rumen (e.g. fatty acids (Machmüller et al. 2003), dicarboxylic acid (López et al. 1999)). The responses of rumen fermentation to yeast cultures are highly variable and yeast cultures do not consistently decrease methane production in vivo (Moss et al. 2000). The use of statins (e.g. mevastatin, lovastatin) is a relatively new approach to reduce enteric methane emissions and was demonstrated by Miller and Wolin (2001) who inhibited the growth of pure strains of Methanobrevibacter isolated from the rumen.
Regardless of the compound chosen to reduce enteric methane emissions, the basal diet on which the animal is fed appears to be important for the effectiveness of many of the aforementioned compounds. For example, Machmüller et al. (2003) found that the decrease in methane emissions achieved in response to myristic acid was twice as large in the rumen of sheep consuming a concentrate- rather than a forage-based diet. For other compounds such as HMA, Cole and McCroskey (1975) suggested that the level of concentrate supplementation could affect their efficacy as methane inhibitors. The implications of these differences for practical farming systems could be important in countries such as Ireland where grassland is the dominant (~0.9) crop on agricultural land and provides the lowest-cost feed available for ruminant production systems (O’Riordan and O’Kiely 1996; Finneran et al. 2012). Concentrate feeds (with grass silage) are fed when livestock are accommodated indoors and these feeds can also constitute a major proportion of the lifetime feeding costs of ruminants (O’Riordan and O’Kiely 1996).
Some limitations of previous research in this area include the absence of data across more than one feed type. There is a need to identify the dietary situations in which an additive is more or less effective. Therefore, the objective of the present study was to determine the dose-rate effects of 11 additives, representing currently recognised or potential rumen fermentation modifiers on in vitro rumen methane output for two feed substrates (perennial ryegrass and grass silage+barley grain) that represent the major dietary ingredients fed to ruminants in Ireland and in other countries with similar livestock production systems. The minimum effective concentration of each additive to reduce methane output when co-incubated with either perennial ryegrass or grass silage+barley grain will also be reported, and compared with similar in vitro batch studies in the literature.
Materials and methods
Experimental design
In vitro rumen methanogenesis was assessed within randomised complete block (n = 3) design experiments with treatments in a 2 by 5, 2 by 6 or 2 by 7 factorial arrangement, dependent on the number of concentration levels of each additive. Two feed types (i.e. perennial ryegrass, grass silage+barley grain) were incubated separately with a range of concentrations (see below) of each additive. Except for yeast, replication was in triplicate for all additives. The probiotic yeast additive, Saccharomyces cerevisiae (SC), was incubated in triplicate on two different occasions (n = 6 replicates).
Feed preparation
Three feeds (perennial ryegrass, grass silage and barley grain) were oven-dried at 40°C for 48 h and milled through a 1-mm screen. The dried milled grass silage and barley grain were combined at a 50 : 50 ratio on a gravimetric DM basis. The two substrates for fermentation therefore were ryegrass and grass silage+barley grain (GS+B) and both were stored under dry, cool, dark conditions in sealed containers before use. Although the oven-drying and milling preparation of the grass silage was to facilitate using a physically homogeneous sample in the in vitro gas production technique (GPT), some loss of silage volatiles is likely to have occurred (Porter and Murray 2001). However, as the same silage sample was used for all batches of incubations, the loss of some volatiles would therefore affect all additive concentrations similarly. In the present study, we were interested in the relative effects across concentrations of an additive, rather than quantifying the absolute outputs of methane.
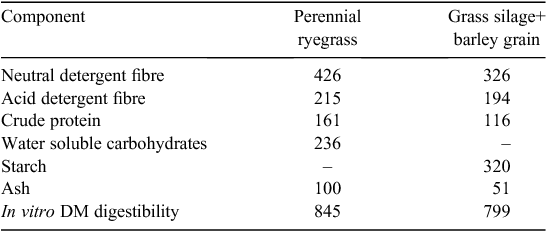
Duplicate samples of each feed mix were assayed for ash, crude protein (CP), acid detergent fibre (ADF), neutral detergent fibre (NDF), in vitro DM digestibility (DMD) and, in the case of GS+B, for its starch concentration. Concentration of water-soluble carbohydrate was determined for perennial ryegrass by using the anthrone method (Thomas 1977). The DMD procedure used was that described by Tilley and Terry (1963), with the modification that the final residue was isolated by filtration through a 1.6-μm Whatman GF/A filter (Whatman, Maidstone, England), rather than by centrifugation. Ash concentration was determined following complete combustion of samples in a muffle furnace at 550°C for 5 h. CP concentration (N × 6.25) was determined using a LECO FP-528 N analyser (Leco Corporation, St Joseph, MI, USA), on the basis of the method #990-03 of the AOAC (1990). The ADF (expressed exclusive of residual ash) and NDF (assayed with heat-stable amylase and sodium sulfite, and expressed exclusive of residual ash) concentrations were determined using an ANKOM fibre analyser (ANKOM Technology, Fairport, NY, USA) according to Van Soest et al. (1991). Starch concentration was determined using the method of McCleary et al. (1997) in a segmented flow analyser (Bran+Luebbe, Norderstedt, Germany). The chemical composition of the feeds is summarised in Table 1. At the time of the in vitro GPT incubations, subsamples (2 g) of each feed were oven-dried at 98°C for 48 h for residual moisture determination.
|
Additives
Additives co-incubated with each feed were selected on the basis of their actual or potential ability to inhibit methanogenesis using different modes of action and their commercial availability. The additives were as follows: the fatty acids oleic, linoleic, and linolenic (purity of 99%) at 0, 1.25, 2.5, 5 and 10 mL/L, lauric acid (purity of 99%) at 0, 1.25, 2.5, 5 and 10 g/L; HMA 2-bromoethanesulfonate (BES, Na salt) and bromochloromethane (BCM, in a α-cyclodextrin matrix) at 0, 1, 5, 10, 20 and 40 µM; pyromellitic diimide (PMDI, purity of 97%) at 0, 1, 5, 10 and 20 mg/L; the statins mevastatin (purity of ≥95%) at 0, 0.25, 0.5, 1, 2, and 4 µM and lovastatin (purity of ≥98%) at 0, 0.25, 0.5, 1, 2, 4 and 8 µM; probiotic yeast (SC, Yea-sacc1026) at 0, 0.1, 0.2, 0.5, 1 and 2 g/L; and the unsaturated dicarboxylic acid fumaric (purity of 99%) at 0, 1, 5, 10 and 20 mM. Concentrations of each additive was based on the in vitro studies of Hristov et al. (2004) (fatty acids), Choi et al. (2004) (BES, PMDI), Miller and Wolin (2001) (mevastatin, lovastatin), Lila et al. (2004) (SC) and López et al. (1999) (fumaric acid), with some modifications to favour the likelihood that methane output would be suppressed within the range of concentrations. The concentrations of BCM added were based on those for BES. Additives were purchased from Sigma-Aldrich (St Louis, MO, USA), with the exception of Yea-sacc1026 which was supplied by Alltech (Sarney, Dunboyne, Co. Meath, Ireland) and BCM which was supplied by Nigel Tomkins (CSIRO, Townsville, Australia). Stock solutions of HMA and PMDI were prepared in distilled water. Statins were dissolved in 70% ethanol as per Miller and Wolin (2001), with ethanol making up no more than 0.28% v/v of the fermentation medium. The equivalent amount of either ethanol or water was added to the respective blank and control-treatment fermentation bottles. Stock solutions were stored at 4°C. The concentration given for each additive was the final concentration in the fermentation medium.
In vitro fermentation
Effects of each supplement on rumen fermentation were tested in vitro by using the GPT of Theodorou et al. (1994), with some modifications made according to Mauricio et al. (1999). These modifications were made because the total volume of gas produced and its methane concentration were of interest, rather than a profile of the kinetics of their production. Each dried milled feed (0.5 g) was weighed into individual 160-mL fermentation bottles and incubated with the range of concentrations of each additive. Replication was provided by the use of three different rumen-fluid inoculum sources, obtained from three fistulated adult steers fed a grass silage and concentrate diet (60 : 40 on a gravimetric DM basis). Therefore, additives at each concentration were incubated separately with the rumen fluid of each steer. Samples of rumen digesta were obtained from the rumen before morning feeding and strained separately through four layers of cheesecloth. Artificial saliva was prepared according to McDougall (1948). Each rumen-fluid source was mixed with the artificial saliva at a ratio of 1 : 4. A sample (0.8 mL) of each rumen fluid–artificial saliva mixture was stored at −18°C in eppendorf tubes containing 20 μL of 9 M H2SO4 for subsequent ruminal volatile fatty acid (VFA) analysis. The pH of each mixture was determined before incubation and subsequently dispensed (50 mL) with a peristaltic pump into fermentation bottles under CO2 flushing. Bottles were sealed with butyl rubber stoppers and aluminium crimp caps and placed in an incubator at 39°C for 24 h. Fermentation bottles were manually shaken at the start of incubation (0 h) and at 2 and 8 h during incubation. It was necessary to perform six batches of incubations to test all additives (one batch assessed per week), with two additives incubated per batch. The yeast additive was incubated in triplicate in two separate batches.
Gas measurements and chemical analyses
After 24-h incubation, gas accumulation in the headspace of each fermentation bottle was measured using a pressure transducer (Gems Sensors and Controls, Basingstoke, UK) following the procedure described by Mauricio et al. (1999). The total amount of gas produced in each bottle was estimated using the following equation of Mauricio et al. (1999):
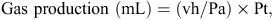
where vh equals head space volume (mL), Pa equals atmospheric pressure (N/m2) and Pt equals the pressure transducer reading (N/m2). A sample of gas (0.8 mL) collected in a graduated syringe was transferred to a 2-mL evacuated tight vial (National Scientific Co., Rochwood, TN, USA) sealed with a silicon–teflon septa (Sun Sri, Rochwood, TN, USA) before the determination of methane. Measurement of the methane concentration in a gas sample accumulated during 24 h of incubation was the same procedure as reported by Bodas et al. (2008) and Navarro-Villa et al. (2011). Although Theodorou et al. (1994) recommended that pressures of no more than 483 hPa should be allowed to accumulate in fermentation bottle head space, López et al. (2007) found an r2 of 0.993 between gas volume and head-space pressure within the range 0–1082 hPa in fermentation bottles. Since head-space pressure in the present experiment ranged from 10 to 1212 hPa (mean = 638 hPa), the pressure inside the fermentation bottles was unlikely to affect fermentation in a manner that would influence the relative methane output of the various treatments being evaluated. In addition, as a result of the extensive flushing of both the rumen fluid–artificial saliva and the fermentation bottle headspace with CO2 until the bottles were sealed, and taking account of the low solubility of methane in the incubation medium (Windholz et al. 1976), it was considered that the gas sample collected from the headspace at the end of the 24-h incubation period was representative of the methane produced during the fermentation.
The gas inside each of the fermentation bottles was released and the bottles were cooled to 4°C to terminate fermentation. The bottles were then opened to measure pH and to take a sample of fermentation medium (0.8 mL) for VFA analysis. Finally, all contents remaining in the bottles were filtered through sintered Pyrex glass crucibles (pore Number 1, 100–160 µm, Corning Inc., Tewksbury, MA, USA) and the residue recovered was oven-dried (98°C for 48 h). Apparent DM disappearance (aDMd) was estimated as the difference in the weight of DM incubated and in the weight of DM in the residue expressed as a proportion of the weight of DM incubated. For the HMA, PMDI and statins, both gas production and aDMd for each feed were corrected for endogenous substrates by the inclusion of blank fermentation bottles containing rumen fluid and artificial saliva only. Preliminary studies in our laboratory showed some additives to be fermentable by the rumen microbiota and/or cause total gas production (TGP) to increase in bottles containing rumen fluid–artificial saliva and additive only; therefore, alternative blanks containing rumen fluid–artificial saliva mixture, plus the corresponding additive, were included for each concentration of lauric acid, oleic acid, linoleic acid, linolenic acid, SC and fumaric acid, to correct TGP and aDMd values for each feed from the endogenous substrates and additive.
Methane concentration in the fermentation gas was determined by gas chromatography (GC) using a Shimadzu GC2014 with AOC 20i autosampler (Shimadzu Corporation, Kyoto, Japan) equipped with a 2.1 m by 5 mm (outer diameter) by 3.2 mm (inner diameter) glass column packed with molecular sieve 5A, 60/80 mesh and a flame ionisation detector. Temperatures were 120°C in the column, 170°C in the injector and 150°C in the detector. The carrier gas nitrogen (N2) flow rate was 40 mL/min. Hydrogen was simultaneously determined using a thermal conductivity detector at 170°C and a current of 75 mA. Each gas sample (8 µL) was injected into the column and the methane and hydrogen concentrations were calculated by external calibration, using a certified gas mixture (Scott-Marrin Inc., Riverside, CA, USA). Chromatograms were integrated using software GC Solutions version 2.30.00 SU6 (Shimadzu, Kyoto, Japan).
A sample of the rumen fluid–artificial saliva mixture (from each animal) and of the post-incubation medium were assayed for VFA (acetic, propionic, iso-butyric + n-butyric (butyric), iso-valeric + n-valeric (valeric)) by GC using a Shimadzu GC17A with AOC 20i autosampler (Shimadzu Corporation, Kyoto, Japan) equipped with a Chrompack column [2.4 m by 5 mm by 3.4 mm glass packed with 9% carbowax 20M + 1% H3PO4 on c-WHP 80/100 mesh (Phenomenex, Cheshire, UK)] and a flame ionisation detector. Temperatures were 150°C in the column and injector and 180°C in the detector. The carrier gas N2 flow rate was 290 mL/min. Iso-caproic acid (0.04 M) was used as an internal standard, as described by Ranfft (1973). Chromatograms were integrated using software EZstart version 7.2.1 (Shimadzu, Kyoto, Japan).
Calculations and statistical analyses
Calculations were performed as per Navarro-Villa et al. (2011). Briefly, the change in total VFA concentration in the medium (ΔtVFA) during incubation was calculated by subtracting the total VFA concentration of the initial inoculum (i.e. rumen fluid–artificial saliva mixture) from the total VFA concentration in the medium after 24 h of incubation. Apparent net production of VFA per g DM incubated (tVFA/DMi) was calculated by multiplying ΔtVFA by the volume of medium (50 mL) and dividing by the weight of feed incubated. Individual VFAs were expressed as molar proportions relative to total VFA (mmol/mol).
Statistical analysis was carried out using the SPSS Version 16 for MS-Windows (SPSS Inc., Chicago, IL, USA). The effects included in the general linear model for each variable were block (rumen fluid source), feed type, additive concentration, feed type × additive concentration interaction and error. Dunnett’s test was used to compare the methane output between the control and additive treatments. Polynomial contrasts were used to test for linear and quadratic effects of concentration of each additive when co-incubated with either ryegrass or GS+B. Effects were considered significant when P < 0.05 and trends at P < 0.1
Results
Fatty acids
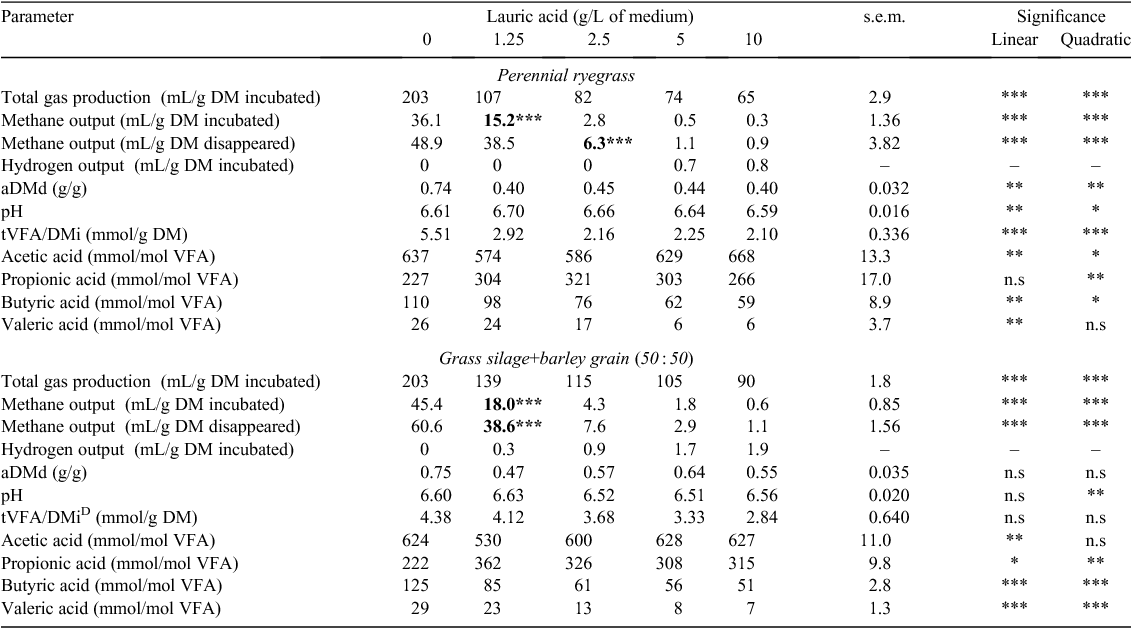
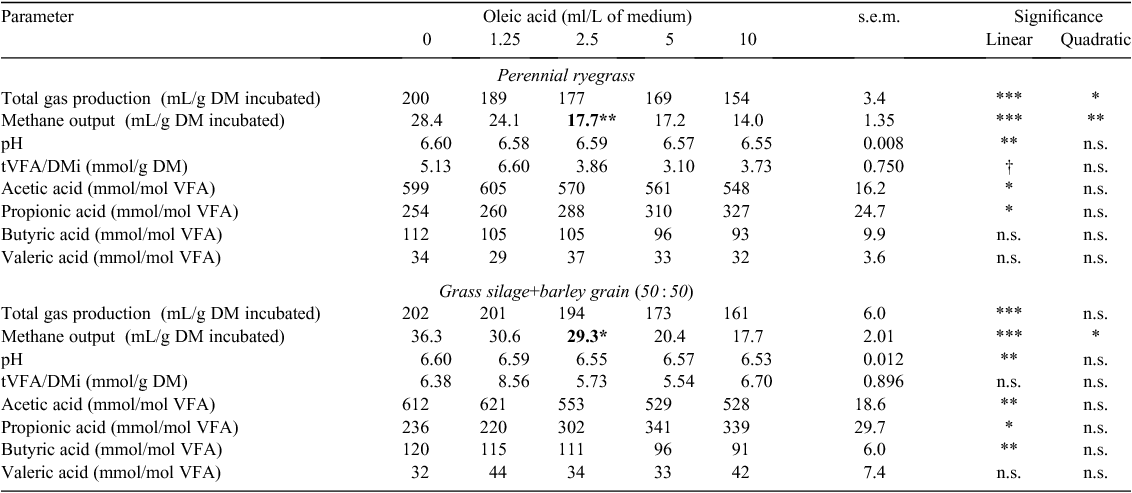
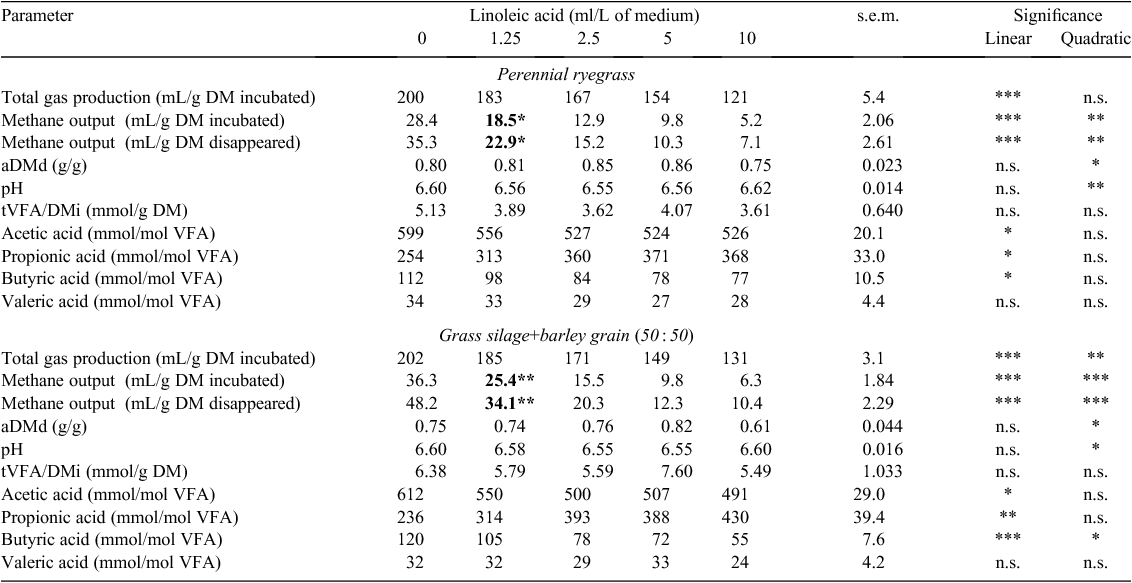
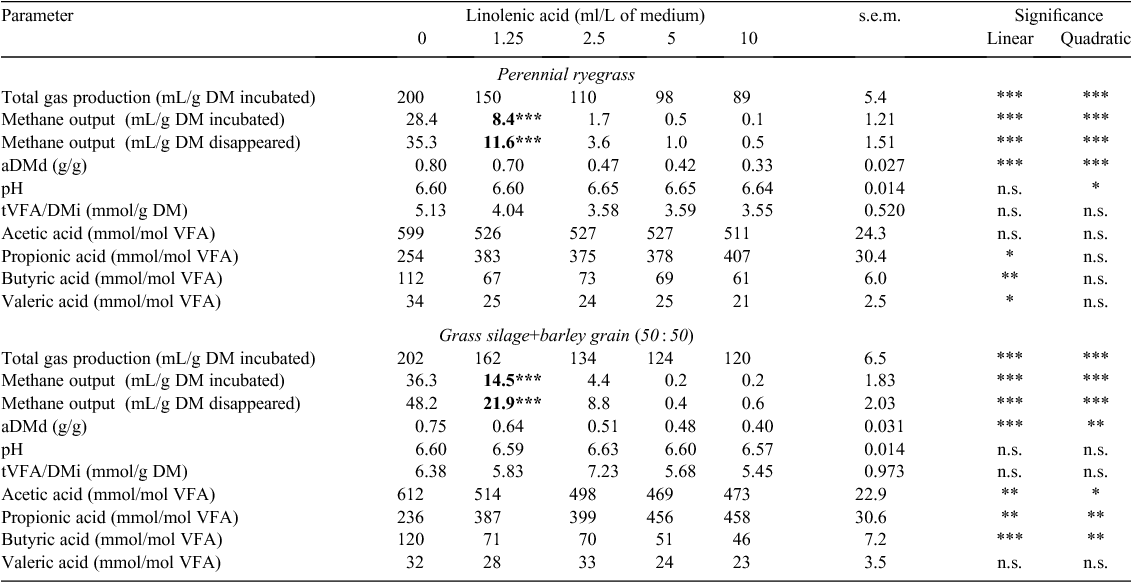
Total gas production (TGP) decreased linearly for ryegrass and GS+B with an increasing concentration of lauric, oleic, linoleic and linolenic acids when expressed in g DM incubated (TGP/DMi) (P < 0.001; Tables 2–5). With an increasing concentration of lauric, linoleic and linolenic acids, there was a linear decrease in methane output per g DM incubated (CH4/DMi) and disappeared (CH4/DMd) when incubated with either feed (P < 0.001; Tables 2, 4, 5). With an increasing concentration of oleic acid, CH4/DMi decreased linearly (P < 0.001) for both feeds (Table 3). Hydrogen output per g DM incubated (H2/DMi) was detected with the addition of ≥5 g/L lauric acid with ryegrass and ≥1.25 g/L with GS+B (Table 2). No hydrogen output was detected with the other fatty acids examined in the present study. Apparent DM disappearance (aDMd) decreased linearly (P < 0.01) with an increasing concentration of lauric acid for ryegrass (Table 2). A quadratic response in aDMd was found for both feeds with an increasing concentration of linoleic acid (P < 0.05; Table 4), and aDMd decreased linearly with an increasing concentration of linolenic acid for both feeds (P < 0.001; Table 5). For ryegrass, the pH of the fermentation medium decreased linearly with increasing concentrations of lauric and oleic acids (P < 0.01; Tables 2, 3) and a quadratic response was evident for linoleic (P < 0.01; Table 4) and linolenic (P < 0.05; Table 5) acids. In the case of GS+B, there was a quadratic response in pH with increasing concentrations of lauric (P < 0.01; Table 2) and linoleic (P < 0.05; Table 4) acids and there was a linear increase (P < 0.01) in pH with increasing concentration of oleic acid (Table 3). Total VFA output per g DM incubated (tVFA/DMi) decreased linearly or tended to decrease linearly with an increasing concentration of lauric (P < 0.001) and oleic (P = 0.07) acids, respectively, for ryegrass (Tables 2, 3). Increasing the concentration of lauric acid incubated with ryegrass and GS+B linearly increased the proportion of acetic acid (P < 0.01), caused a quadratic response in the proportion of propionic acid (P < 0.01), and linearly decreased the proportion of butyric and valeric acids (P < 0.01; Table 2). With increasing concentrations of oleic, linoleic and linolenic acids, the proportion of acetic and butyric acids decreased linearly for both feeds, with the exception of oleic and linolenic acid when added to ryegrass (P < 0.05; Tables 3–5). The proportion of propionic acid increased linearly with increasing concentrations of oleic, linoleic and linolenic acids incubated with both feeds (P < 0.05; Tables 3–5). For ryegrass, valeric acid decreased linearly with increasing concentration of linolenic acid (P < 0.05; Table 5).
The minimum measured concentration of the fatty acids lauric, linoleic and linolenic required to reduce (P < 0.05) methane output in ryegrass and GS+B compared with the control treatment was 1.25 g or ml/L (Tables 2, 4, 5). A measured oleic acid concentration of 2.5 ml/L was required to reduce (P < 0.05) methane output in both feeds (Table 3).
Halogenated methane analogues (HMA) and pyromellitic diimide (PMDI)
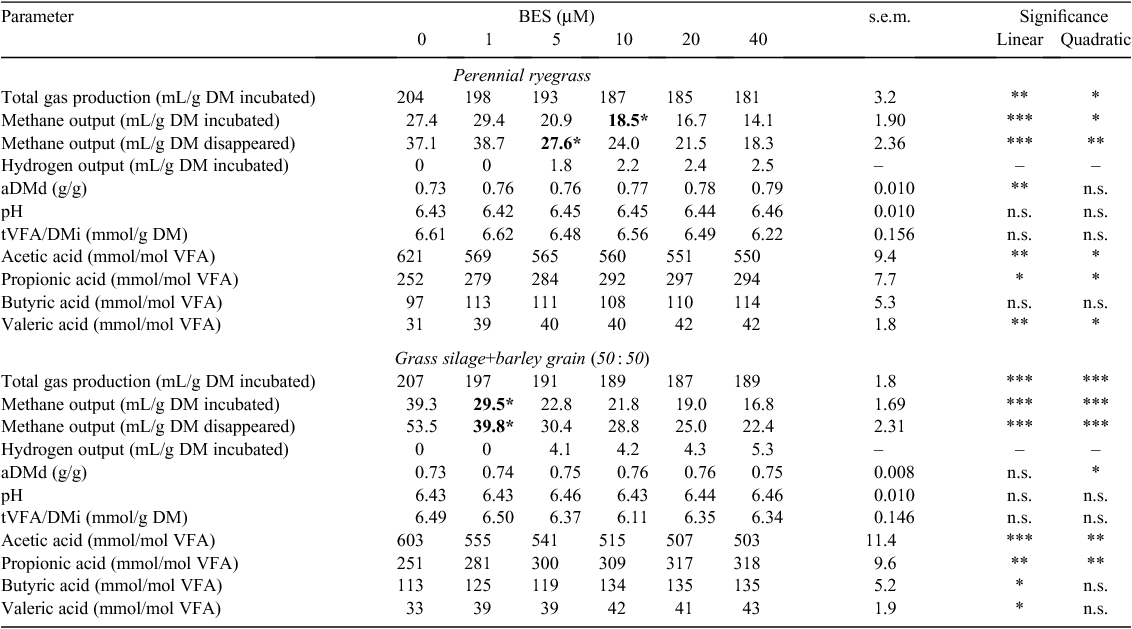
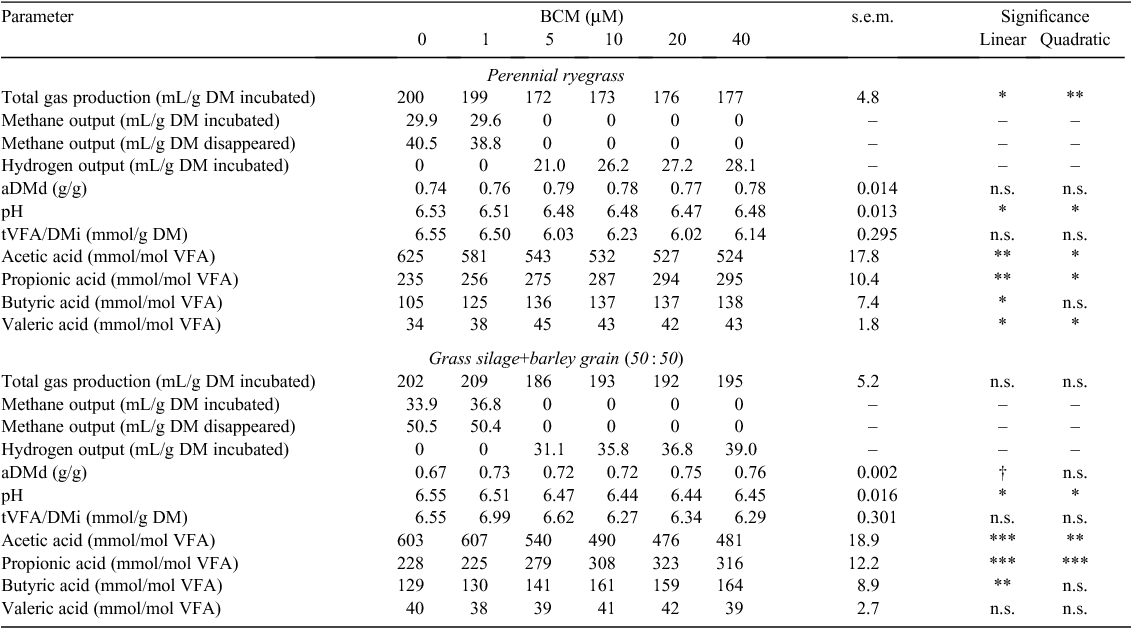
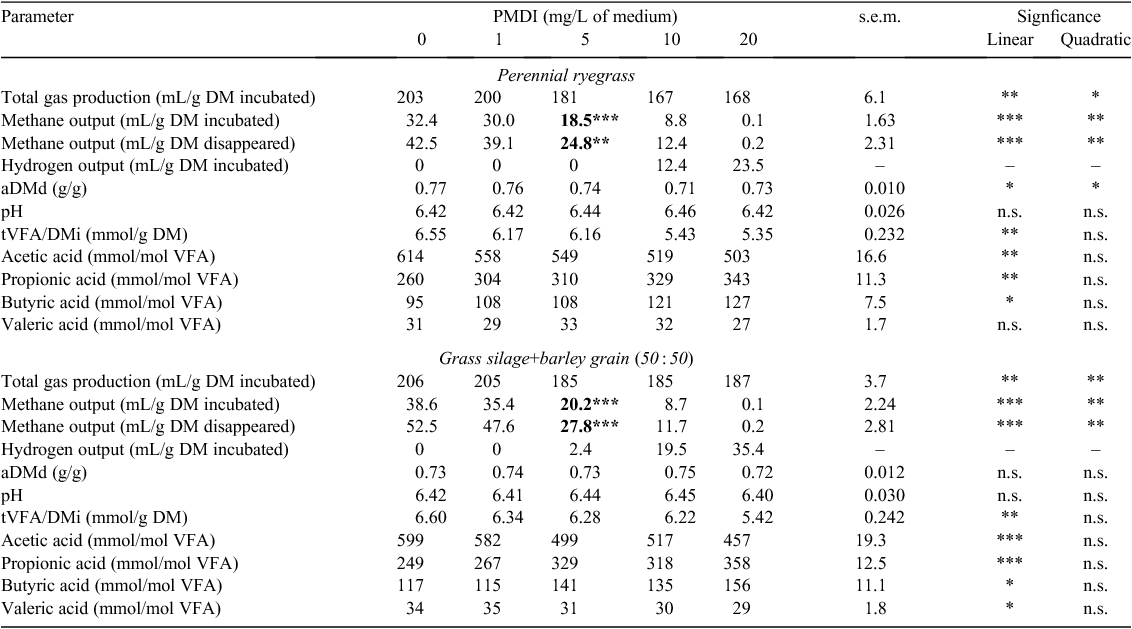
Total gas production (TGP/DMi) decreased linearly (P < 0.01) with an increasing concentration of BES and PMDI for both feeds (Tables 6, 8), but with an increasing concentration of BCM, the response in TGP/DMi was quadratic (P < 0.01) for ryegrass (Table 7). Methane output (CH4/DMi, CH4/DMd) decreased linearly with increasing concentration of both BES and PMDI incubated with either feed (P < 0.001; Tables 6, 8). Hydrogen was detected with the addition of ≥5 µM BES and BCM for both feeds (Tables 6, 7), and ≥10 mg/L PMDI for ryegrass and ≥5 mg/L PMDI for GS+B (Table 8). The aDMd increased linearly (P < 0.01) with an increasing concentration of BES for ryegrass and a quadratic response (P < 0.01) was evident for GS+B (Table 6). It increased linearly with BCM for GS+B only (P = 0.05; Table 7) and a linear and quadratic response was evident with PMDI for ryegrass only (P < 0.05; Table 8). There was a linear decrease in pH with an increasing concentration of BCM for ryegrass (P < 0.05) and the response was quadratic (P < 0.05) for GS+B (Table 7). The tVFA/DMi decreased linearly with an increasing concentration of PMDI for both feeds (P < 0.01; Table 8), but this variable was unaffected (P > 0.05) by the addition of either BES or BCM (Tables 6, 7). The proportion of acetic acid decreased linearly (P < 0.01) and the proportion of propionic acid increased linearly (P < 0.01) with an increasing concentration of both HMA and PMDI incubated with ryegrass or GS+B (Tables 6–8). With the exception of BES incubated with ryegrass, the proportion of butyric acid increased linearly with increasing concentrations of HMA and PMDI for both feeds (P < 0.05; Tables 6–8). The proportion of valeric acid increased linearly with an increasing concentration of BES incubated with both feeds (P < 0.05; Table 6), and there was a quadratic response with increasing BCM concentration for ryegrass only (P < 0.05; Table 7). In contrast, increasing concentration of PMDI incubated with GS+B linearly decreased the proportion of valeric acid (P < 0.05; Table 8).
Compared with the control treatment, a measured BES concentration of 5–10 µM and 1 µM reduced (P < 0.05) methane output in ryegrass and GS+B, respectively (Table 6). Methane output could not be detected with a BCM concentration ≥5 µM for either feed (Table 7). A similar measured concentration of PMDI (5 mg/L) was required to reduce (P < 0.01) methane output in ryegrass and GS+B (Table 8).
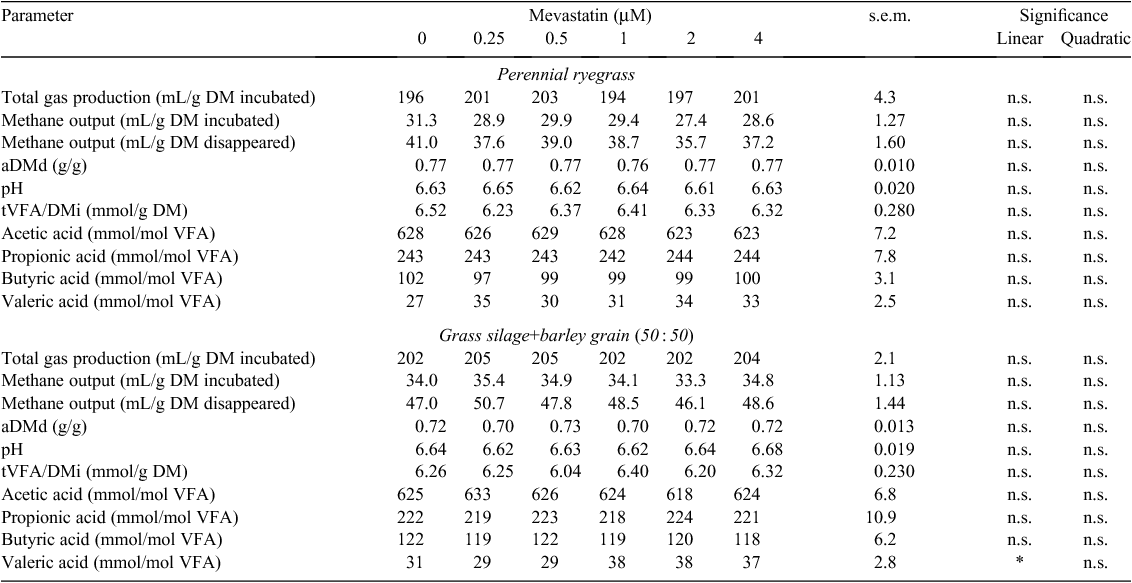
Statins
Neither mevastatin (0.25–4 uM) nor lovastatin (0.25–8 uM) reduced methane output from either ryegrass or GS+B. (P > 0.05; Tables 9, 10). The proportion of valeric acid increased linearly with increasing concentration of mevastatin when incubated with GS+B (P < 0.05; Table 9). No other variables in Tables 9 and 10 were significant (P > 0.05) with the addition of mevastatin or lovastatin, respectively, for either feed type.
Saccharomyces cerevisiae (SC)
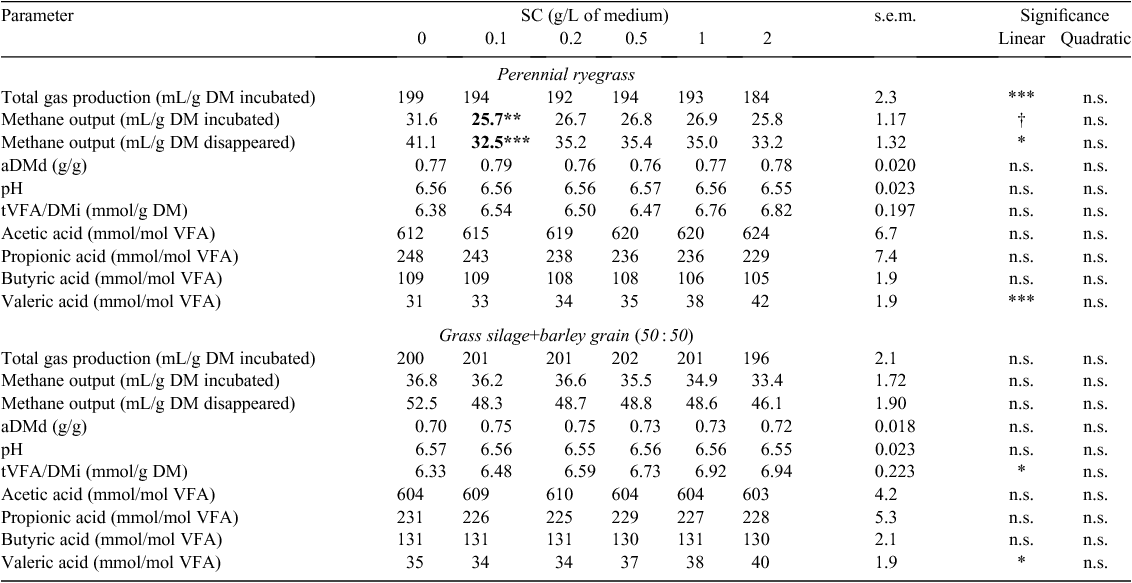
Total gas production (TGP/DMi) and CH4/DMd decreased linearly (P < 0.05) with an increasing concentration of SC incubated with ryegrass, and CH4/DMi showed a tendency (P = 0.06) to decrease (Table 11). Total VFA output (tVFA/DMi) increased linearly with an increasing concentration of SC for GS+B (P < 0.05; Table 11). The proportion of valeric acid increased linearly with an increasing concentration of SC for both feeds (P < 0.05; Table 11). Compared with the control treatment, the addition of SC reduced methane output only in ryegrass and not in GS+B. The minimum measured concentration of SC required to reduce (P < 0.01) methane output in ryegrass was 0.1 g/L (Table 11).
Fumaric acid
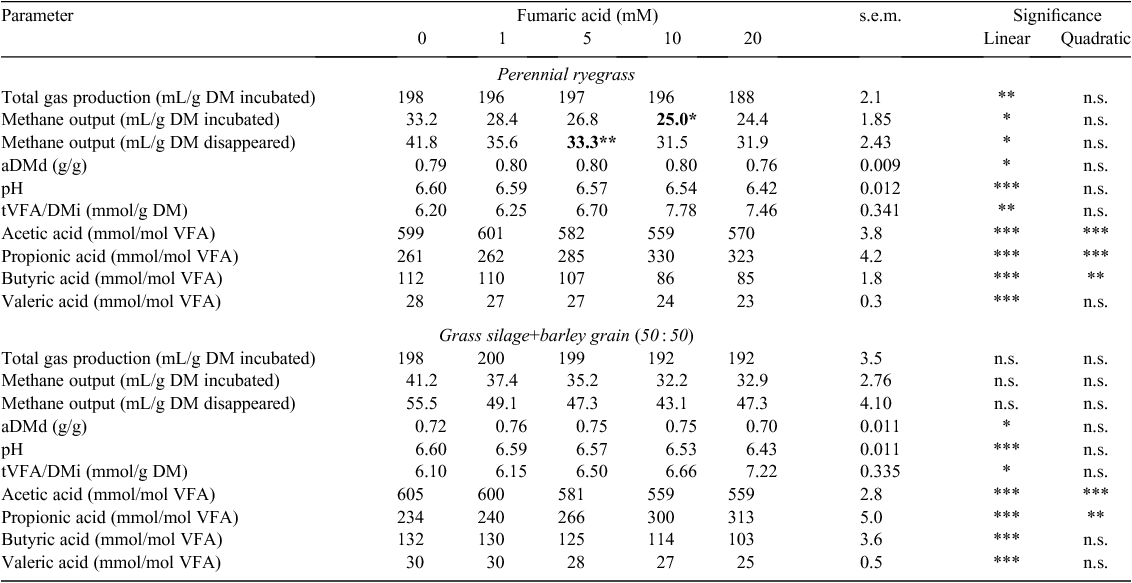
When ryegrass was incubated with an increasing concentration of fumaric acid, TGP/DMi and methane output (CH4/DMi, CH4/DMd) decreased linearly (P < 0.05; Table 12). For both feeds, an increasing fumaric acid concentration caused a linear decrease (P < 0.05) in aDMd and pH and a linear increase (P < 0.05) in tVFA/DMi (Table 12). With an increasing concentration of fumaric acid, there was a linear decrease in the proportions of acetic, butyric and valeric acids and a linear increase in the proportion of propionic acid for both feed types (P < 0.001; Table 12). The minimum measured concentration of fumaric acid required to reduce (P < 0.05) methane output in ryegrass ranged from 5 to 10 mM. Fumaric acid did not reduce (P > 0.05) methane output in GS+B compared with the control treatment (Table 12).
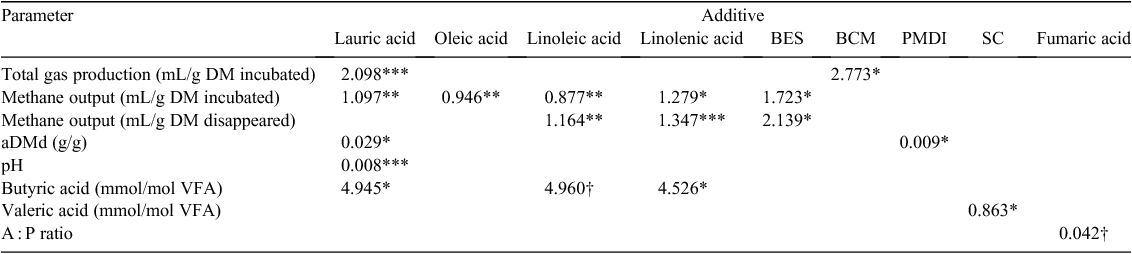
For the relevant additives, the standard error of the mean and significance of two-way interactions are shown in Table 13 for variables that had significant feed by additive concentration interactions.
Discussion
Perspective
The effects of 11 additives on in vitro rumen methane output, VFA profiles and other fermentation characteristics were assessed with perennial ryegrass and GS+B which differed in fibre and CP concentration, but had a high in vitro DMD (Table 1). The individual additives representing actual or potential rumen fermentation modifiers were chosen on the basis of their reported ability to suppress rumen methanogenesis, as well as their commercial availability. The efficacy of medium-chain fatty acids to suppress methane output in vivo is known to be feed-type dependent (Machmüller et al. 2003), and therefore, the present study can assess the sensitivity of the in vitro GPT to register differences between the two contrasting feeds supplemented with different additives. The yeast, S. cerevisiae (SC), was the organism chosen to represent probiotics because of its use as a feed additive on commercial farms. The use of statins could be an alternative way to reduce methane production, but they have not, to our knowledge, been screened with contrasting feeds. To strengthen the representativeness of the comparison of ryegrass and GS+B with different additives, a range of concentrations of each were used. The minimum measured effective concentration of each additive to reduce methane output in the present study was compared with that in similar in vitro 24-h batch-digestion studies in the literature.
Differences in methane output between feeds
Under the assay conditions of the in vitro GPT employed in the present study, a numerically greater methane output was found for GS+B than ryegrass in the control fermentation bottles, which agrees with the results of other similar in vitro studies (Klevenhusen et al. 2008; Mc Geough et al. 2011; Navarro-Villa et al. 2011) but not with some in vivo studies (Beauchemin and McGinn 2005; Mc Geough et al. 2010). These contradictory results have been discussed in greater detail by Mc Geough et al. (2011).
Fatty acids
Fatty acids inhibit methane production through the provision of an alternate means of electron disposal, as well as by direct toxic effects on ruminal microoganisms and protozoa (McAllister et al. 1996; Zhang et al. 2008). With 9–25% of methane production in the rumen attributed to protozoa-associated methanogens (Newbold et al. 1995), the elimination of protozoa will simultaneously reduce the associated methanogens (Jouany 1994). Propionate-producing gram-negative bacteria are not significantly inhibited by fatty acids (Van Nevel and Demeyer 1988); thus, the reduction in methane production shifts fermentation towards the production of propionic acid (McAllister et al. 1996).
Agreeing with the findings of an in vivo study of Machmüller et al. (2003) for myristic acid, in the present study, the highest methane-suppressing effect of lauric acid (on a DM incubated basis) was achieved when it was added to GS+B, a feed with a relatively lower content of structural carbohydrate than in ryegrass. This result confirmed the sensitivity of the in vitro GPT to assess an inhibition effect on rumen methane output across two contrasting feeds. However, using the rumen simulation technique (Rusitec), Machmüller et al. (2001) showed that methane output with lauric acid supplementation was independent of diet type (high vs low structural-carbohydrate diet), but feed-type dependent when supplemented with coconut oil, which is known for its high content of lauric acid (Beare-Rogers et al. 2001).
Methane output declined with the addition of lauric acid, with a corresponding numerical increase in hydrogen output. Depressing the hydrogen-consuming process of methanogenesis by medium chain fatty acids can enhance the in vitro release of gaseous hydrogen (Machmüller et al. 2001). The reduced TGP and methane output with increasing lauric acid supplementation, as well as alterations to the VFA profile, would suggest that lauric acid restricted the activity of the bacterial and/or the protozoal populations. Therefore, the observed decrease in methane output can be partially explained by the lower extent of in vitro fermentation.
The extent of the reduction in methane output (CH4/DMi) and in the proportion of butyric acid with increasing concentration of lauric acid was more pronounced for GS+B than for ryegrass, with the opposite effect observed for TGP/DMi. A lower proportion of butyric acid could indicate a decreased number and activity of protozoa (Van Nevel and Demeyer 1988). The greater decline in TGP/DMi for ryegrass than for GS+B may indicate a greater inhibition of cellulolytic bacteria and/or protozoa, evidenced by the numerically lower tVFA/DMi and the more pronounced decrease in aDMd for ryegrass with increasing lauric acid concentration. Lauric acid is known to have a strong inhibitory effect on protozoa (Matsumoto et al. 1991; Hristov et al. 2004) and cellulolytic bacteria (Demeyer 1981; Klevenhusen et al. 2011a), and therefore would be expected to particularly affect the fermentation of more fibrous feeds, as found in the present study. The difference in methane output between the two feeds may reflect the greater decline in pH that occurred for GS+B than that for ryegrass. Although the difference in pH between feeds was not large (pH 0.01 – 0.14) across lauric acid concentrations, the combination of a lower pH and the effect of lauric acid for GS+B may have had a greater effect on methanogens. A decline in rumen pH is known to reduce fibre digestibility (Hoover 1986), alter VFA fermentation profiles towards a lower acetic to propionic (A : P) ratio (Lana et al. 1998) and reduce the activity of rumen methanogens (Van Kessel and Russell 1996; Lana et al. 1998). In the present study, the A : P ratio was numerically lower (data not presented) for GS+B than for ryegrass at each incremental increase in lauric acid. Acetate (and butyrate) promotes methane production while propionate formation can be considered as a competitive pathway for hydrogen use in the rumen (Moss et al. 2000). An alternative explanation for the more pronounced reduction in methane output and in the proportion of butyric acid with increasing concentration of lauric acid for GS+B could be due to the reduced action of lauric acid because of competitive adsorption between the more fibrous ryegrass and rumen microbes (Harfoot et al. 1974; Machmüller et al. 2001, 2003). However, in the present study it is not known whether competition between rumen microbes and feed particles could occur between feeds differing in NDF concentration by ≤100 g/kg DM and what affect milling through a 1-mm screen has on the feed-particle structure of both feeds.
A decline in methane output occurred with the addition of linoleic and linolenic acids for both feeds. CH4/DMi also declined with the addition of oleic acid when incubated with both feeds. These outcomes are most likely to reflect the increased proportions of propionic acid and reduced proportions of acetic acid due to there being less competition for metabolic hydrogen for methanogenesis, which is in agreement with Zhang et al. (2008). Since the amount of metabolisable hydrogen used in the biohydrogenation process of endogenous unsaturated fatty acids is small compared with other processes (Czerkawski 1972), the main reason for the decline in methane output for both feeds, as suggested by Zhang et al. (2008), is likely to have been due to the direct action of unsaturated fatty acids against methanogens and protozoa associated with methanogens. According to earlier research in this area, the toxicity of unsaturated fatty acids towards microorganisms can be explained on a physiochemical basis, namely that fatty acids form adsorption layers around the bacterial cell, which results in altered cell permeability (Kodicek and Worden 1945) and therefore interferes with nutrient uptake (Galbraith and Miller 1973). The inhibitory effect of long-chain fatty acids are not just confined to methanogens, but also to gram negative cellulolytic bacteria (Zhang et al. 2008). Toxicity of fatty acids against cellulolytic bacteria (Zhang et al. 2008) appears to have been greater for linolenic than linoleic acid because TGP/DMi and aDMd were more depressed with the former. With the absence of aDMd data for oleic acid, it is probable that aDMds of both feeds were also adversely affected, as evidenced by the lower proportion of acetic acid and the reduction in TGP in the present study and the known inhibitory effect on cellulolytic bacteria (Nagaraja et al. 1997). In addition, Hristov et al. (2004) reported a severe decrease in protozoal numbers with the addition of oleic, linoleic and linolenic acids, particularly by the two latter fatty acids, which would have contributed to some of the declines in methane output observed.
The extent of reduction in methane output with increasing concentration of oleic, linoleic and linolenic acids was more pronounced for GS+B than for ryegrass, reflecting the greater numerical reduction in A : P ratio (data not presented) with GS+B than with ryegrass when fatty acid concentrations were ≥2.5 ml/L. The proportion of butyric acid was reduced to a greater extent for GS+B than for ryegrass with increasing concentration of linoleic and linolenic acids, which may indicate a decreased number and activity of protozoa (Van Nevel and Demeyer 1988).
Comparative in vitro batch-digestion studies that co-incubated the fatty acids used in the present study with ryegrass or GS+B could not be found in the literature. However, Zhang et al. (2008) reported that the minimum concentration of oleic, linoleic and linolenic acids needed to reduce in vitro methane output when co-incubated with Chinese wild rye meal and cornmeal (50 : 50) was 35 g/kg, which was ~4–8-fold lower than the concentration required to significantly reduce methane output in the present study with either ryegrass or GS+B. Zhang et al. (2008) did not report the final volume of the fermentation medium, the ratio of rumen fluid to buffer or the quantity of feed incubated, making it difficult to conclude whether the discrepancy between the studies was due to the different methodologies used or to inherent differences among feeds.
Halogenated methane analogues (HMA) and pyromellitic diimide (PMDI)
The addition of HMA and PMDI to both feeds caused a reduction in methane output and in the proportion of acetic acid, and an increase in the proportions of propionic and butyric acids and hydrogen output, agreeing with observations reported for BES and BCM by Van Nevel and Demeyer (1995) and Goel et al. (2009), respectively. HMAs caused a decrease in methane output through changes in the direction of fermentation rather than in the extent of fermentation, whereas PMDI appeared to decrease methane output through a combined effect on the extent and direction of fermentation.
BES is a structural bromine analogue of coenzyme M and interferes with methanogenesis by inhibiting the methyl coenzyme-M reductase in methanogens (Balch and Wolfe 1979) and BCM inhibits methane production by reacting with chemically reduced Vitamin B12, which then inhibits the cobamide-dependent methyl transferase step of methanogenesis (Chalupa 1977; Wood et al. 1968). A gradual decrease in methane output was observed with an increasing concentration of BES (1–40 µM), whereas methane output was not detectable at a BCM concentration of ≥5 µM. If there is a connection between this observation and the known persistency of BCM as an inhibitor of methane emissions, as shown in vivo (McCrabb et al. 1997), as compared with a lack of persistency observed for BES (Van Nevel and Demeyer 1995), the development of future inhibitors of enteric methanogenesis would warrant closer attention to the mode of action of BCM. The extent of reduction in methane output with increasing concentration of BES was more pronounced for GS+B than for ryegrass. This is in agreement with Lee et al. (2009), who found a similar interaction for in vitro rumen methane output between feed type (timothy hay vs timothy hay+concentrate, 40 : 60) and the level of BES supplementation (0, 1 and 5 mM). In the present study, the greater reduction in methane output for GS+B with the addition of BES was not due to differences in the extent of fermentation as measured by aDMd or tVFA/DMi, but to the direction of fermentation with a numerically lower A : P ratio (data not presented) for GS+B than for ryegrass.
PMDI is a potent inhibitor of rumen methanogenesis (Martin and Macy 1985). Although not a HMA, in other reports (Van Nevel and Demeyer 1995; Choi et al. 2004) PMDI has been grouped with HMA, primarily because it is thought to share a similar action as BES to inhibit methanogenesis (Martin and Macy 1985). PMDI adversely affected the in vitro fermentation of both feeds to a greater extent than did BES or BCM, as evidenced by the lower aDMd for ryegrass and a lower tVFA/DMi for both feeds with PMDI. It is not known why PMDI affected the aDMd of ryegrass and not GS+B. The reduction in aDMd of ryegrass with the addition of PMDI was somewhat surprising and not observed in previous in vivo studies (Martin and Macy 1985; Eijssen et al. 1990).
Goel et al. (2009) reported the reduction of in vitro methane output using 5 µM BCM with hay as substrate, compared with between 1 and 5 µM BCM required for ryegrass and GS+B in the present study. In the case of BES, 1 µM significantly reduced methane output in timothy hay and timothy hay+concentrate (40 : 60) diets (Lee et al. 2009), which was similar for GS+B in the present study, but not for ryegrass, which required 5–10 µM BES to significantly reduce methane output. There were no comparative studies in the literature for PMDI.
There has been some concern that HMAs are toxic to livestock. For example, chloral hydrate led to liver damage and death in sheep after prolonged feeding (Nagaraja et al. 1997) but there has been no adverse effects on rumen fermentation when BCM was fed to either steers (McCrabb et al. 1997) or goats (Abecia et al. 2012). Therefore, the toxic effects of all HMA need to be assessed in vivo on a compound by compound basis.
Statins
Statins are known to inhibit hydroxmethylglutaryl~SCoA (HMG-CoA) reductase, which prevents the creation of an important precursor, mevalonate, used in the formation of membrane lipids in the methanogenic Archaea cell wall (Miller and Wolin 2001). An in vitro study by Miller and Wolin (2001) reported that 4 nmol of lovastatin per mL of culture medium (= 4 µM) resulted in 50% growth inhibition of a Methanobrevibacter strain, and concentrations ≥10 nmol per mL (= 10 µM) completely inhibited methane formation. Under the conditions of the present study, neither mevastatin (0.25–4 µM) nor lovastatin (0.25–8 µM) elicited a response in methane output. The culture medium used by Miller and Wolin (2001) was synthetic and contained rumen fluid, whereas the medium in the present study contained artificial saliva, rumen fluid and either ryegrass or GS+B as substrate. The lack of response in methane output in the present study could have been due to degradation or inactivation of the statins by a biological or chemical component in the medium, which was not present in the medium prepared by Miller and Wolin (2001). In a recent in vivo trial where sheep were fed 80 mg lovastatin per kg of total dietary DM for 23 days, there was no effect on total daily methane production (Klevenhusen et al. 2011b); however, with an increased concentration of lovastatin (up to 10 g/kg total DM) in a Rusitec experiment, methane formation was decreased by 42% (Soliva et al. 2011). These authors concluded that a dose-dependent effect of lovastatin was possible.
Saccharomyces cerevisiae (SC)
In the present study, SC suppressed CH4/DMd when incubated with ryegrass and a decreasing trend was evident for CH4/DMi. One of the most consistently reported responses to SC supplementation is an increase in the number of total culturable and cellulolytic bacteria recovered from the rumen (Nagaraja et al. 1997). The increase in the proportion of valeric acid with increasing SC concentration was more pronounced for ryegrass than for GS+B. Changes in the molar proportion of valeric acid accompanying the decrease in methane output for ryegrass indicated a small but specific shift in the fermentation pattern. An increase in valeric acid has been observed in other studies with the addition of SC (Carro et al. 1992; Zeleňák et al. 1994). The linear increase in iso-valeric acid with increasing concentrations of SC when incubated with both feeds (data not presented) may indicate that iso-valeric acid is a fermentation product of SC. Since iso-valeric acid is known to be a requirement for fibrolytic bacteria (Allison and Bryant 1963; Bryant 1973), its occurrence will be more beneficial to fibrolytic bacteria in breaking down high-fibre feeds.
Martin et al. (1989) co-incubated bermudagrass with either 0.4 or 1.0 g/L SC with no effect on in vitro methane output. Lila et al. (2004) reported no effect of twin-strains of SC (0.33–1.32 g/L) on in vitro methane output when co-incubated with sudangrass hay and concentrate (1.5 : 1). Lynch and Martin (2002) co-incubated alfalfa hay and coastal bermudagrass hay separately with either a SC culture (0.35 and 0.73 g/L; Diamond VXP) or SC live cells (0.35 and 0.73 g/L; Saf Agri) for 24–48 h and observed a reduction in in vitro methane output only with 0.35 g\L SC live cells with alfalfa hay. In the present study, methane output was reduced with 0.1 g/L SC with ryegrass but not with GS+B. Future studies should examine the potential shifts in the main groups of ruminal microorganisms with the addition of SC when co-incubated separately with ryegrass, GS+B and other feeds under standardised conditions, to explain the discrepancy in methane reduction between different feed types.
Fumaric acid
Fumaric acid is an intermediate in the propionate pathway, in which it is reduced to succinate by fumarate reductase (López et al. 1999). In an in vitro experiment using the Rusitec system, López et al. 1999) demonstrated that the conversion of fumarate via succinate, and thereafter to propionate, was the way by which most (89%) of the added fumarate was fermentated (López et al. 1999). Reducing equivalents are needed in this reaction and therefore fumarate provides an alternative electron sink for hydrogen, resulting in a decline in the availability of hydrogen for methanogenesis in the rumen (López et al. 1999). The findings of López et al. (1999) help explain the reduction in methane output and the increased proportion of propionic acid observed in the present study with increasing concentrations of fumaric acid incubated with ryegrass. Propionic acid also increased with increasing concentrations of fumaric acid with GS+B, but without a corresponding decrease in methane output. Although, the increasing fumaric acid concentration caused a more pronounced decrease in the A : P ratio for GS+B than for ryegrass, this ratio was numerically lower (data not presented) for ryegrass than for GS+B at each incremental increase in fumaric acid, and therefore the increased production of propionate with ryegrass may have reduced the availability of hydrogen for methanogenesis in the present study. García-Martínez et al. (2005) found no significant interactions between fumarate (4 and 8 mM) and a high-, medium- and low-forage diets, but the decrease in methane was greater for the high-forage diet (5.1–5.7%) than for either the medium- or low-forage diets (2.9% and 3.8%, respectively). Newbold et al. (2005) reported that 8 mM fumarate could significantly reduce in vitro methane output in both a high-concentrate and high-forage diet and López et al. (1999) showed in vitro methane output to be significantly reduced with 5 mM fumarate in a grass hay and concentrate diet (50 : 50). Nothwithstanding the different techniques, substrates and form (salt or acid) of the additive, evidence from the literature would support the reduction of methane output with increasing concentrations (0–20 mM) of fumaric acid co-incubated with GS+B.
López et al. (1999) found an increase in DM digestibility and a corresponding increase in cellulolytic bacteria and postulated that one of the beneficial effects of fumarate is an increased fibre digestion. In the current study, the aDMd of both feeds decreased with increasing concentration of fumaric acid, although most of the decline occurred at the highest concentration (20 mM) of fumaric acid added. Newbold et al. (2005) also observed an increase in fibre digestion in batch cultures when the sodium salt (fumarate) was added, but no change was evident with the addition of the free acid (fumaric acid).
Conclusions
With increasing concentrations of fatty acids, BES and PMDI, there was a dose-dependent decline in methane output (CH4/DMi, CH4/DMd) with ryegrass and GS+B. Methane output could not be detected with a BCM concentration of ≥5 µM for both feeds. The statins mevastatin and lovastatin did not reduce methane output with either feed. S. cerevisiae reduced methane output (CH4/DMd) when incubated with ryegrass but not with GS+B. Fumaric acid reduced methane output (CH4/DMi, CH4/DMd) only when incubated with ryegrass. The extent of reduction in methane output with increasing concentration of lauric, oleic, linoleic and linolenic acids and BES was more pronounced for GS+B than for ryegrass.
The results presented in the current study are from experiments of a relatively short exposure to the inhibitory additives, and studies of a longer duration are required to find out whether the effects can be sustained, particularly in vivo.
The effectiveness of some additives to inhibit in vitro rumen fermentation was both diet and dose dependent. Future in vitro and in vivo studies using these additives should include diet type as an important component in the experimental design. To identify an effective dose level of each additive, a review of previous dose levels administered in vivo should be first consulted, as in vivo trials can provide additional information on the effect of an additive dose(s) on feed intake, animal performance, animal health and adaptation effects, which is not available from this or other in vitro experiments. Failing to find relevant in vivo studies, the minimum concentrations of each additive identified in the present study to reduce methane may prove useful.
Owing to the differing modes of action for many additives to inhibit rumen methanogenesis as shown in this and other studies, incorporating different feed types for the initial in vitro screening of all new additives is strongly recommended.
Acknowledgements
Funding for this study was provided under the National Development Plan through the Research Stimulus Fund administered by the Department of Agriculture, Fisheries and Food (RSF No. 06 361). The authors thank the Grange Laboratories staff for undertaking chemical analyses.
References
Abecia L, Toral PG, Martín-García AI, Martínez G, Tomkins NW, Molina-Alcaide E, Newbold CJ, Yánez-Ruiz DR (2012) Effect of bromochloromethane on methane emission, rumen fermentation pattern, milk yied, and fatty acid profile in lactating dairy goats. Journal of Dairy Science 95, 2027–2036.| Effect of bromochloromethane on methane emission, rumen fermentation pattern, milk yied, and fatty acid profile in lactating dairy goats.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xksleitrw%3D&md5=ba75a19e351b7eda8d0c719a02b97451CAS | 22459848PubMed |
Allison MJ, Bryant MP (1963) Biosynthesis of branched-chain amino acids from branched-chain fatty acids by rumen bacteria. Archives of Biochemistry and Biophysics 101, 269–277.
| Biosynthesis of branched-chain amino acids from branched-chain fatty acids by rumen bacteria.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF3sXktlSgurk%3D&md5=523a1a93bcf26b23f8e7cd258636d48dCAS | 14012183PubMed |
AOAC (1990) ‘Official methods of analysis. Vol. 2.’ 15th edn. (Association of Official Analytical Chemists: Arlington, VA)
Balch WE, Wolfe RS (1979) Transport of coenzyme M (2-mercaptoethanesulfonic acid) in Methanobacterium ruminantium. Journal of Bacteriology 137, 264–273.
Beare-Rogers J, Dieffenbacher A, Holm JV (2001) Lexicon of lipid nutrition (IUPAC Technical Report). Pure and Applied Chemistry 73, 685–744.
| Lexicon of lipid nutrition (IUPAC Technical Report).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXltVyks78%3D&md5=a77f4e035e3e8a31e7f18a251acd483bCAS |
Beauchemin KA, McGinn SM (2005) Methane emissions from feedlot cattle fed barley or corn diets. Journal of Animal Science 83, 653–661.
Bodas R, López S, Fernández M, García-González R, Rodríguez AB, Wallace RJ, González JS (2008) In vitro screening of the potential of numerous plant species as antimethanogenic feed additives for ruminants. Animal Feed Science and Technology 145, 245–258.
| In vitro screening of the potential of numerous plant species as antimethanogenic feed additives for ruminants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXpsVKrsb0%3D&md5=955b876361f158f85b788659e7f950ebCAS |
Bryant MP (1973) Nutritional requirements of the predominant rumen cellulolytic bacteria. Federation Proceedings 32, 1809–1813.
Carro MD, Lebzien P, Rohr K (1992) Influence of yeast culture on the in vitro fermentation (Rusitec) of diets containing variable portions of concentrates. Animal Feed Science and Technology 37, 209–220.
| Influence of yeast culture on the in vitro fermentation (Rusitec) of diets containing variable portions of concentrates.Crossref | GoogleScholarGoogle Scholar |
Chalupa W (1977) Manipulating rumen fermentation. Journal of Animal Science 46, 585–599.
Choi NJ, Lee SY, Sung HG, Lee SC, Ha JK (2004) Effects of halogenated compounds, organic acids and unsaturated fatty acids on in vitro methane production and fermentation characteristics. Asian-Australasian Journal of Animal Sciences 17, 1255–1259.
Cole NA, McCroskey JE (1975) Effects of hemiacetal of chloral and starch on the performance of beef steers. Journal of Animal Science 41, 1735–1741.
Czerkawski JW (1972) Fate of metabolic hydrogen in the rumen. The Proceedings of the Nutrition Society 31, 141–146.
| Fate of metabolic hydrogen in the rumen.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE3sXmvFeqtA%3D%3D&md5=9edecd8ae894fe9c18a54441a00ee9f7CAS | 4563287PubMed |
Demeyer DI (1981) Rumen microbes and digestion of plant cell walls. Agriculture and Environment 6, 295–337.
| Rumen microbes and digestion of plant cell walls.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL38XjsFOq&md5=de41d212ad554f131afd3c1036a3f15aCAS |
Eijssen AFMM, Barry TN, Brookes IM (1990) The effect of pyromellitec diimide upon the rumen fermentation of sheep fed a forage diet. Animal Feed Science and Technology 28, 145–153.
| The effect of pyromellitec diimide upon the rumen fermentation of sheep fed a forage diet.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXltlWjtbc%3D&md5=1416080672a80e41e532caf5cd263493CAS |
Finneran E, Crosson P, O’Kiely P, Shalloo L, Forristal D, Wallace M (2012) Stochastic simulation of the cost of home-produced feeds for ruminant livestock systems. The Journal of Agricultural Science 150, 123–139.
| Stochastic simulation of the cost of home-produced feeds for ruminant livestock systems.Crossref | GoogleScholarGoogle Scholar |
Galbraith H, Miller TB (1973) Effect of long chain fatty acids on bacterial respiration and amino acid uptake. Journal of Applied Microbiology 36, 659–675.
| Effect of long chain fatty acids on bacterial respiration and amino acid uptake.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE2cXht1yktbw%3D&md5=f3d25cf01dfeabb598af5d8b0aa90a14CAS |
García-Martínez R, Ranilla MJ, Tejido ML, Carro MD (2005) Effects of disodium fumarate on in vitro rumen microbial growth, methane production and fermentation of diets differing in their forage : concentrate ratio. The British Journal of Nutrition 94, 71–77.
| Effects of disodium fumarate on in vitro rumen microbial growth, methane production and fermentation of diets differing in their forage : concentrate ratio.Crossref | GoogleScholarGoogle Scholar | 16115335PubMed |
Goel G, Makkar HPS, Becker K (2009) Inhibition of methanogens by bromochloromethane: effects on microbial communities and rumen fermentation using batch and continuous fermentations. The British Journal of Nutrition 101, 1484–1492.
| Inhibition of methanogens by bromochloromethane: effects on microbial communities and rumen fermentation using batch and continuous fermentations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXnslSlsLo%3D&md5=c2f4ac970821655084ff1a795981f656CAS | 19243639PubMed |
Goodrich RD, Garrett JE, Gast DR, Kirick MA, Larson DA, Meiske JC (1984) Influence of monensin on the performance of cattle. Journal of Animal Science 58, 1484–1498.
Harfoot CG, Crouchman ML, Noble RC, Moore JH (1974) Competition between food particles and rumen bacteria in the uptake of long-chain fatty acids and triglycerides. Journal of Applied Microbiology 37, 633–641.
| Competition between food particles and rumen bacteria in the uptake of long-chain fatty acids and triglycerides.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE2MXht1WntLw%3D&md5=0d1ee1c418f320dc4a8e39e1e703afa6CAS |
Hoover WH (1986) Chemical factors involved in ruminal fibre digestion. Journal of Dairy Science 69, 2755–2766.
| Chemical factors involved in ruminal fibre digestion.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2sXhsleitw%3D%3D&md5=12fa3b0add6d641d537bde7db90c1f6bCAS | 3027148PubMed |
Hristov AN, Ivan M, McAllister TA (2004) In vitro effects of individual fatty acids on protozoal numbers and on fermentation products in ruminal fluid from cattle fed a high-concentrate, barley-based diet. Journal of Animal Science 82, 2693–2704.
Jouany J (1994) Manipulation of microbial activity in the rumen. Archiv fur Tierernahrung 46, 133–153.
| Manipulation of microbial activity in the rumen.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXhsFSnu74%3D&md5=2cd3374b4067dc31162a2226eced76e5CAS | 7717843PubMed |
Klevenhusen F, Bernasconi SM, Kreuzer M, Soliva CR (2008) The methanogenic potential and C-isotope fractionation of different diet types represented by either C3 or C4 plants as evaluated in vitro and in dairy cows. Australian Journal of Experimental Agriculture 48, 119–123.
| The methanogenic potential and C-isotope fractionation of different diet types represented by either C3 or C4 plants as evaluated in vitro and in dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXovVOh&md5=9e3c59548b8ab23e1846972fea64fcfbCAS |
Klevenhusen F, Meile L, Kreuzer M, Soliva CR (2011a) Effects of monolaurin on ruminal methanogens and selected bacterial species from cattle, as determined with the rumen simulation technique. Anaerobe 17, 232–238.
| Effects of monolaurin on ruminal methanogens and selected bacterial species from cattle, as determined with the rumen simulation technique.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXht1ens7jM&md5=0cb21f9ef3d7a2242a6eecab4fa34101CAS | 21787874PubMed |
Klevenhusen F, Duval S, Zeitz JO, Kreuzer M, Soliva CR (2011b) Diallyl disulphide and lovastatin: effects on energy and protein utilisation in, as well as methane emission from, sheep. Archives of Animal Nutrition 65, 255–266.
| Diallyl disulphide and lovastatin: effects on energy and protein utilisation in, as well as methane emission from, sheep.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXptFKnu74%3D&md5=279747f6d7767b7d5ec2bfa678073d33CAS |
Kodicek E, Worden AN (1945) The effect of unsaturated fatty acids on Lactobacillus helveticus and other Gram-positive micro-organisms. Biochemical Journal 39, 78–85.
Lana RP, Russell JB, Van Amburgh ME (1998) The role of pH in regulating ruminal methane and ammonia production. Journal of Animal Science 76, 2190–2196.
Lee SY, Yang SH, Lee WS, Kim HS, Shin DE, Ha JK (2009) Effect of 2-bromoethanesulfonic acid on in vitro fermentation characteristics and methanogen population. Asian-Australasian Journal of Animal Sciences 22, 42–48.
Lila ZA, Mohammed N, Yasui T, Kurokawa Y, Kanda S, Itabashi H (2004) Effects of a twin strain of Saccharomyces cerevisiae live cells on mixed ruminal microorganism fermentation in vitro. Journal of Animal Science 82, 1847–1854.
López S, Valdés C, Newbold CJ, Wallace RJ (1999) Influence of sodium fumarate addition on rumen fermentation in vitro. The British Journal of Nutrition 81, 59–64.
López S, Dhanoa MS, Dijkstra J, Bannink A, Kebreab E, France J (2007) Some methodological and analytical considerations regarding application of the gas production technique. Animal Feed Science and Technology 135, 139–156.
| Some methodological and analytical considerations regarding application of the gas production technique.Crossref | GoogleScholarGoogle Scholar |
Lovett D, Lovell S, Stack L, Callan J, Finlay M, Conolly J, O’Mara FP (2003) Effect of forage/concentrate ratio and dietary coconut oil level on methane output and performance of finishing beef heifers. Livestock Production Science 84, 135–146.
| Effect of forage/concentrate ratio and dietary coconut oil level on methane output and performance of finishing beef heifers.Crossref | GoogleScholarGoogle Scholar |
Lynch HA, Martin SA (2002) Effects of Saccharomyces cerevisiae culture and Saccharomyces cerevisiae live cells on in vitro mixed ruminal microorganism fermentation. Journal of Dairy Science 85, 2603–2608.
| Effects of Saccharomyces cerevisiae culture and Saccharomyces cerevisiae live cells on in vitro mixed ruminal microorganism fermentation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XotlCmsrY%3D&md5=4a922c4b5f80c7afb80414e42481f20eCAS | 12416814PubMed |
Machmüller A, Dohme F, Soliva CR, Wanner M, Kreuzer M (2001) Diet composition affects the level of ruminal methane suppression by medium-chain fatty acids. Australian Journal of Agricultural Research 52, 713–722.
| Diet composition affects the level of ruminal methane suppression by medium-chain fatty acids.Crossref | GoogleScholarGoogle Scholar |
Machmüller A, Soliva CR, Kreuzer M (2003) Methane-suppressing effect of myristic acid in sheep as affected by dietary calcium and forage proportion. The British Journal of Nutrition 90, 529–540.
| Methane-suppressing effect of myristic acid in sheep as affected by dietary calcium and forage proportion.Crossref | GoogleScholarGoogle Scholar | 13129458PubMed |
Martin SA, Macy JM (1985) Effects of monensin, pyromellitic diimide and 2-bromoethanesulfonic acid on rumen fermentation in vitro. Journal of Animal Science 60, 544–550.
Martin SA, Nisbet DJ, Dean RG (1989) Influence of a commercial yeast supplement on the in vitro ruminal fermentation. Nutrition Reports International 40, 395–403.
Matsumoto M, Kobayahsi T, Takenaka A, Itabashi H (1991) Defaunation effects of medium-chain fatty acids and their derivatives on goat rumen protozoa. The Journal of General and Applied Microbiology 37, 439–445.
| Defaunation effects of medium-chain fatty acids and their derivatives on goat rumen protozoa.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XhvFejtbg%3D&md5=4b3f8a2d4f24481ecca9f2114486fec6CAS |
Mauricio RM, Mould FL, Dhanoa MS, Owen E, Channa KS, Theodorou MK (1999) A semi-automated in vitro gas production technique for ruminant feedstuff evaluation. Animal Feed Science and Technology 79, 321–330.
| A semi-automated in vitro gas production technique for ruminant feedstuff evaluation.Crossref | GoogleScholarGoogle Scholar |
Mc Geough EJ, O’Kiely P, Hart KJ, Maloney AP, Boland TM, Kenny DA (2010) Methane emissions, feed intake, performance, digestibility, and rumen fermentation of finishing beef cattle offered whole-crop wheat silages differing in grain content. Journal of Animal Science 88, 2703–2716.
| Methane emissions, feed intake, performance, digestibility, and rumen fermentation of finishing beef cattle offered whole-crop wheat silages differing in grain content.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXpvVOrt74%3D&md5=b392b27d5d0cf09b18654e759a8ae2d3CAS | 20382872PubMed |
Mc Geough EJ, O’Kiely P, O’Brien M, Kenny DA (2011) An evaluation of the methane output associated with high-moisture grains and silages using the in vitro total gas production technique. Animal Production Science 51, 627–634.
| An evaluation of the methane output associated with high-moisture grains and silages using the in vitro total gas production technique.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXnvF2hsb0%3D&md5=84b9d3971e330a0925bb47e1920eadecCAS |
McAllister TA, Okine EK, Mathison GW, Cheng K-J (1996) Dietary, environmental and microbiological aspects of methane production in ruminants. Canadian Journal of Animal Science 76, 231–243.
| Dietary, environmental and microbiological aspects of methane production in ruminants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XkslKjsrg%3D&md5=e00b0e66c4050574c82268e544eae972CAS |
McCleary BV, Gibson TS, Mugford DC (1997) Measurement of total starch in cereal products by amyloglucosidase-α-amylase method: collaborative study. Journal of the Official Analytical Chemists 80, 571–579.
McCrabb GJ, Berger KT, Magner T, May C, Hunter RA (1997) Inhibiting methane production in Brahman cattle by dietary supplementation with a novel compound and the effects on growth. Australian Journal of Agricultural Research 48, 323–329.
| Inhibiting methane production in Brahman cattle by dietary supplementation with a novel compound and the effects on growth.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXislals7Y%3D&md5=324de9ba6135e490417b6b7333559db9CAS |
McDougall EI (1948) Studies on ruminant saliva. 1. The composition and output of sheep’s saliva. Biochemical Journal 43, 99–109.
Miller TL, Wolin MJ (2001) Inhibition of growth of methane-producing bacteria of the ruminant forestomach by hydroxymethyglutaryl~SCoA reductase inhibitors. Journal of Dairy Science 84, 1445–1448.
| Inhibition of growth of methane-producing bacteria of the ruminant forestomach by hydroxymethyglutaryl~SCoA reductase inhibitors.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXktlKgs7k%3D&md5=ec1ab68555661ab4cf4a0d82a45c5791CAS | 11417704PubMed |
Moss AR, Jouany J, Newbold J (2000) Methane production by ruminants: its contribution to global warming. Annales de Zootechnie 49, 231–253.
| Methane production by ruminants: its contribution to global warming.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXnt12msrk%3D&md5=48844f2b1bda0b62dd154aafe7c43c59CAS |
Nagaraja TG, Newbold CJ, Van Nevel CJ, Demeyer DI (1997) Manipulation of ruminal fermentation. In ‘The rumen microbial ecosystem’. (Eds PJ Hobson, CS Stewart) pp. 523–632. (Blackie Academic and Professional: London)
Navarro-Villa A, O’Brien M, López S, Boland TM, O’Kiely P (2011) Modifications of a gas production technique for assessing in vitro rumen methane production from feedstuffs. Animal Feed Science and Technology 166–167, 163–174.
| Modifications of a gas production technique for assessing in vitro rumen methane production from feedstuffs.Crossref | GoogleScholarGoogle Scholar |
Newbold CJ, Lassalas B, Jouany JP (1995) The importance of methanogens associated with ciliate protozoa in ruminal methane production in vitro. Letters in Applied Microbiology 21, 230–234.
| The importance of methanogens associated with ciliate protozoa in ruminal methane production in vitro.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK28%2FjtVartw%3D%3D&md5=ed1f70db141e55580d81e16b93c399dfCAS | 7576513PubMed |
Newbold CJ, López S, Nelson N, Ouda JO, Wallace RJ, Moss AR (2005) Propionate precursors and other metabolic intermediates as possible alternative electron acceptors to methanogenesis in ruminal fermentation in vitro. The British Journal of Nutrition 94, 27–35.
| Propionate precursors and other metabolic intermediates as possible alternative electron acceptors to methanogenesis in ruminal fermentation in vitro.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtFantr7J&md5=c113f8a428efa5677dc70cdc59b45009CAS | 16115329PubMed |
Nikolich MP, Hong G, Shoemaker NB, Salyers AA (1994) Evidence for natural horizontal transfer of tetQ between bacteria that normally colonize humans and bacteria that normally colonize livestock. Applied and Environmental Microbiology 60, 3255–3260.
O’Riordan E, O’Kiely P (1996) Potential for beef production systems based on grass. Irish Grassland and Animal Production Association Journal 30, 185–217.
Porter MG, Murray RS (2001) The volatility of components of grass silage on oven drying and the inter-relationship between dry-matter content estimated by different analytical methods. Grass and Forage Science 56, 405–411.
| The volatility of components of grass silage on oven drying and the inter-relationship between dry-matter content estimated by different analytical methods.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XhvF2qtro%3D&md5=d859253a384ae3ef62cc3c1a63daa848CAS |
Prins RA, Van Nevel CJ, Demeyer DI (1972) Pure culture studies of inhibitors for methanogenic bacteria. Antonie van Leeuwenhoek 38, 281–287.
| Pure culture studies of inhibitors for methanogenic bacteria.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE3sXhtlKqtQ%3D%3D&md5=51d970537517e75c49cf3d73a6f417b2CAS | 4538621PubMed |
Ranfft K (1973) Determination by gas chromatography of short chain fatty acids in ruminal fluids. Archiv fur Tierernahrung 23, 343–352.
| Determination by gas chromatography of short chain fatty acids in ruminal fluids.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE2cXlslWiug%3D%3D&md5=22e2e1ce266c96b8623d8d45e28000d4CAS | 4770711PubMed |
Soliva CR, Amelchanka SL, Duval SM, Kreuzer M (2011) Ruminal methane inhibition potential of various pure compounds in comparison with galic oil as determined with a rumen simulation technique (Rusitec). The British Journal of Nutrition 106, 114–122.
| Ruminal methane inhibition potential of various pure compounds in comparison with galic oil as determined with a rumen simulation technique (Rusitec).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXosVOmu7k%3D&md5=a7173da321bf6c17f0ed7dd3ce89efd8CAS | 21554814PubMed |
Theodorou MK, Williams BA, Dhanoa MS, McAllan AB, France J (1994) A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Animal Feed Science and Technology 48, 185–197.
| A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds.Crossref | GoogleScholarGoogle Scholar |
Thomas TA (1977) An automated procedure for the determination of soluble carbohydrates in herbage. Journal of the Science of Food and Agriculture 28, 639–642.
| An automated procedure for the determination of soluble carbohydrates in herbage.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE2sXltlagu70%3D&md5=fa4547922585dc8e891c6dc4bc1ba07bCAS |
Tilley JMA, Terry RA (1963) A two-stage technique for the in vitro digestion of forage crops. Journal of the British Grassland Society 18, 104–111.
| A two-stage technique for the in vitro digestion of forage crops.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF3sXks1Cmurk%3D&md5=92d24cc5f65c2fea9e8e124a75b5df21CAS |
Van Kessel JAS, Russell JB (1996) The effect of pH on ruminal methanogenesis. FEMS Microbiology Ecology 20, 205–210.
| The effect of pH on ruminal methanogenesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XkslGiur4%3D&md5=316d80697c9c02a4bea10dd6f2437b0dCAS |
Van Nevel CJ, Demeyer DI (1988) Manipulation of rumen fermentation. In ‘The rumen microbial ecosystem’. (Ed. PN Hobson) pp. 387–443. (Elsevier Applied Science: London)
Van Nevel C, Demeyer D (1995) Feed additives and other interventions for decreasing methane emissions. In ‘Biotechnology in animal feeds and animal feeding’. (Eds RJ Wallace, A Chesson) pp. 329–349. (VCH: Weinheim, Germany)
Van Soest PJ, Robertson JB, Lewis BA (1991) Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. Journal of Dairy Science 74, 3583–3597.
| Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK38%2FnvVCltA%3D%3D&md5=83605a987aed1cab5024d20cbdabf9b5CAS | 1660498PubMed |
Windholz M, Budavari S, Stroumtsos LY, Fertig MN (1976) ‘The Merck index: an encyclopedia of chemicals and drugs.’ (Merck and Co.: Rahway, NJ)
Wood JM, Kennedy FS, Wolfe RS (1968) The reaction of multihalogenated hydrocarbons with free and bound reduced vitamin B12. Biochemistry 7, 1707–1713.
| The reaction of multihalogenated hydrocarbons with free and bound reduced vitamin B12.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF1cXktFKhtbw%3D&md5=6b6c1169dace7dc6dbba954c13c0156aCAS | 4870333PubMed |
Zeleňák I, Jalč D, Kmet V, Siroka P (1994) Influence of diet and yeast supplement on in vitro ruminal characteristics. Animal Feed Science and Technology 49, 211–221.
| Influence of diet and yeast supplement on in vitro ruminal characteristics.Crossref | GoogleScholarGoogle Scholar |
Zhang CM, Guo YQ, Yuan ZP, Wu YM, Wang JK, Liu JX, Zhu WY (2008) Effect of octadeca carbon fatty acids on microbial fermentation, methanogenesis and microbial flora in vitro. Animal Feed Science and Technology 146, 259–269.
| Effect of octadeca carbon fatty acids on microbial fermentation, methanogenesis and microbial flora in vitro.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtFCku7fL&md5=8dcefc7c52a3a9c67a3feb6408cc3c34CAS |