Productivity increase, reduction of nitrogen fertiliser use and drought-stress mitigation by inoculation of Marandu grass (Urochloa brizantha) with Azospirillum brasilense
Rubson da C. Leite A D , José G. D. dos Santos A , Eduardo L. Silva A , Cássio R. C. R. Alves A , Mariangela Hungria B , Robson da C. Leite C and Antonio C. dos Santos AA Federal University of Tocantins, Araguaína, TO, Brazil 65907-230.
B Brazilian Agricultural Research Corporation, Embrapa Soja, Londrina, PR, Brazil 86001-970.
C Federal University of Tocantins, Gurupi, TO, Brazil 77402-970.
D Corresponding author. Email: rubsonif@gmail.com
Crop and Pasture Science 70(1) 61-67 https://doi.org/10.1071/CP18105
Submitted: 15 March 2018 Accepted: 30 October 2018 Published: 17 December 2018
Abstract
Among the forage species cultivated in South America, the genus Urochloa is the most used, and the cultivar Marandu of U. brizantha is the most widely planted in Brazil. The objective of this study was to evaluate forage performance in association with Azospirillum brasilense, combined with nitrogen (N) fertilisation. The study was conducted under field conditions in Araguaína, Tocantins, in the central region of Brazil, between March 2016 and March 2017. Four N fertiliser rates (0, 12.5, 25 and 50 kg/ha of N per cutting cycle) were combined with two inoculation treatments (inoculated and non-inoculated), with evaluations carried out in three periods of the year (transition, dry and wet seasons). Marandu grass plants inoculated with A. brasilense had greater plant height, number of tillers and forage production than non-inoculated plants, regardless of the N rate. Inoculation with A. brasilense allowed a 20% reduction in N fertilisation. Our results indicate that inoculation with A. brasilense in Marandu grass, as well as increasing forage production, can help to mitigate the stresses caused by the dry season.
Additional keywords: growth-promoting bacteria, nitrogen fixation, palisade grass, tropical pastures.
Introduction
Brazil has the second-largest herd of cattle in the world, estimated at 219 million, and is responsible for 13.8% of world beef production (ABIEC 2017). Pastures, comprising an area of nearly 190 Mha, are the most economical and usual means for cattle feeding in Brazil (Jank et al. 2014). However, the productivity of these pastures is mostly low because of pasture degradation and inadequate management, with non-replenishment of nutrients to the soil, contrary to practice in cropping areas (Dias Filho 2014).
Although most Brazilian soils are responsive to applied nitrogen (N), its application increases production costs, and its effects are short-lived in tropical soils (Canto et al. 2016). In addition, N fertilisers are manufactured from fossil fuels (de Morais et al. 2012), and there are risks of contamination of soil and water by addition of nitrate, as well as emissions of greenhouse gases (Pedreira et al. 2017; Sá et al. 2017).
In order to reduce the costs of N fertilisation, inoculation with bacteria capable of fixing atmospheric nitrogen (N2) or of promoting plant growth by other mechanisms such as the production of phytohormones is presented as an important strategy for sustainability (Hungria et al. 2016; Leite et al. 2017; Marques et al. 2017).
In the search for positive results resembling those achieved with rhizobia–legume associations in which inoculation with elite strains can partially or fully replace N fertilisation, such as in soybean (Glycine max (L.) Merr.) (Saturno et al. 2017), several studies have been carried out with forage grasses in association with bacteria of the genus Azospirillum. These bacteria, in addition to having the capacity for biological N2 fixation (BNF), can contribute to the production of phytohormones and phosphate solubilisation enzymes (Okon and Labandera-Gonzalez 1994; Baldani et al. 2014; Hungria et al. 2016; Fukami et al. 2017; Marques et al. 2017; Souza et al. 2017). Furthermore, Rubin et al. (2017) and Fukami et al. (2017) highlighted the potential of Azospirillum to promote greater tolerance of plants to biotic and abiotic stresses such as drought. This would be particularly important in several regions of Brazil, where there is a long and well-defined drought period.
Among the forage species cultivated in South America, the genus Urochloa is the most used, and cv. Marandu is the most widely planted in Brazil, with high forage yield and good adaptation to the soils and tropical climatic conditions (de Marchi et al. 2017; Lopes et al. 2017; Rodrigues et al. 2017). The characteristics and importance of this forage species for the Brazilian cattle-production chain justify the need to evaluate its performance in association with Azospirillum brasilense and N fertilisation.
Material and methods
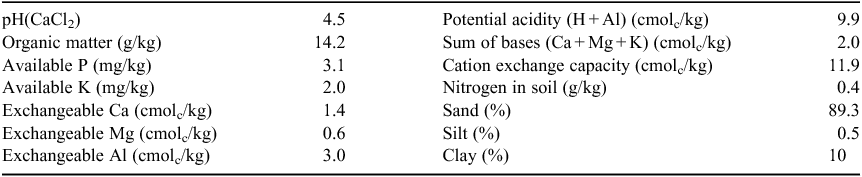
The study was conducted under field conditions in an experimental area of the Federal University of Tocantins, Campus Araguaína, School of Veterinary Medicine and Animal Science (809304.26 and 9213720.68 UTM; elevation 240 m a.m.s.l.) between March 2016 and March 2017. The region is classified as a transition of the biomes Cerrado–Amazônia, with an Aw (hot and humid) climate, according to the Köppen International Classification (Alvares et al. 2013), average annual precipitation 1863 mm and average air humidity 78%. The soil of the experimental area is of sandy texture (Table 1), classified as a Quartzipsamment Entisol (USDA Soil Taxonomy).
The experiment was established in a randomised block design of four N-fertilisation rates (0, 12.5, 25 and 50 kg/ha of N, applied as urea after each forage cut) and two inoculation treatments with A. brasilense (inoculated and non-inoculated, arranged at a distance of 20 m from each other), with four replicates. Each experimental plot had an area of 9.0 m2 (3.0 m by 3.0 m). The inoculant contained elite strains Ab-V5 and Ab-V6 of A. brasilense, commercially used in Brazil for grasses (Hungria et al. 2010, 2016) and co-inoculation of legumes (Hungria et al. 2015).
A basal application of NPK was applied to all plots at 30 days after sowing, according to recommendations for cultivation (Sousa and Lobato 2004) and soil fertility (Table 1) as follows: 20 kg N/ha (ammonium sulfate), 30 kg P/ha (single superphosphate) and 49 kg K/ha (potassium chloride).
Sowing was carried out in March 2016, using 12 kg/ha of viable pure seeds. Subsequently, soil was rolled to improve seed contact. At the time of sowing, seed homogenisation was performed by using inoculant applied at a rate of 200 mL/ha, comprising strains Ab-V5 and Ab-V6 of A. brasilense at the concentration of 2 × 108 colony forming units/mL.
At 62 days after sowing, when plots had achieved a cutting height of at least 40 cm, they were cut to a residual height of 20 cm and the N treatments were applied.
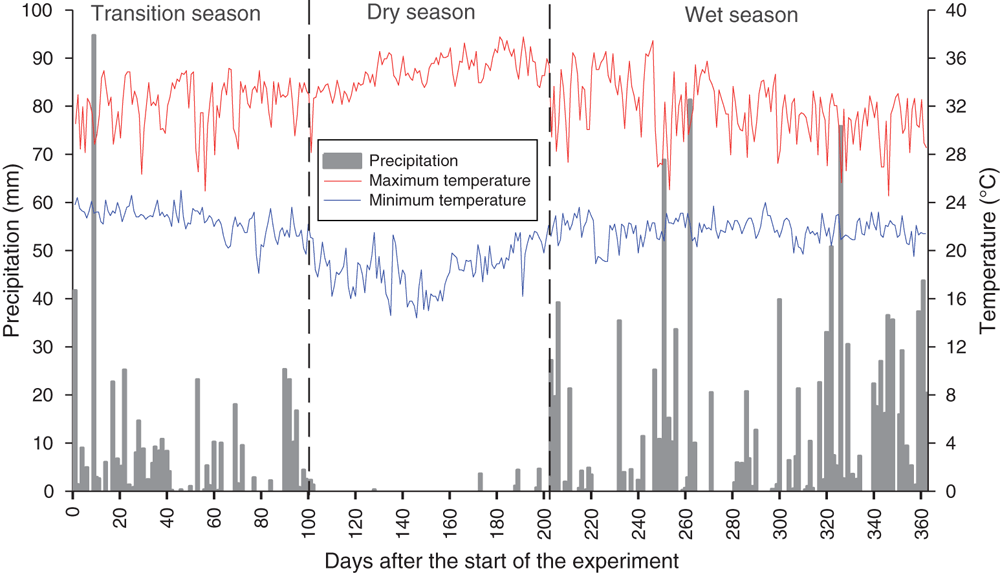
For the experimental period, data from the forage harvests were grouped according to seasons, defined as the transition (0–100 days), dry (100–200 days) and wet (200–365 days), established according to precipitation data collected in the experimental area (Fig. 1).
|
The parameters evaluated were plant height, number of tillers, root mass, daily forage accumulation, forage N concentration and annual forage accumulation. Root mass and forage N concentration were grouped only in the dry season and wet season, and annual forage accumulation was evaluated as the sum of all cuts during the year. All other parameters were evaluated in the three defined periods of the year.
Plant height was determined with a graduated ruler, from the soil to the top of the plant. The number of tillers was counted by using a 1.0 m by 0.15 m metal frame. For root mass evaluation, two samples per plot were collected, using steel cylinders at depth 0–20 cm with 5 cm of distance of the cut clumps for the evaluation of productivity. After sampling, the material was placed in plastic bags for later washing and separation of roots from soil. The separated roots were weighed and oven-dried at 55°C for determination of dry root mass, in kg/ha. Annual forage accumulation was evaluated with a 1.0 m by 0.5 m metal frame, with a cut height of the residue of 20 cm, followed by drying in an oven at 55°C for 72 h and subsequent weighing. Forage samples, after pre-drying and grinding in a 1-mm sieve, were digested with sulfuric acid and immediately distilled (Kjeldahl) for the percentage of N. Daily forage accumulation was estimated by dividing the yield of each cut by the number of days passed since the previous cut.
Data were initially tested for normality (Shapiro–Wilk test) and homoscedasticity. Inoculation treatment were analysed by analysis of variance. The N-rate treatments were submitted to regression analysis, by evaluating the significance of slopes and determination coefficients to obtain the appropriate regression model, adopting a significance level of P = 0.05. All statistical procedures were performed with SISVAR 5.3 software (Ferreira 2011).
Results
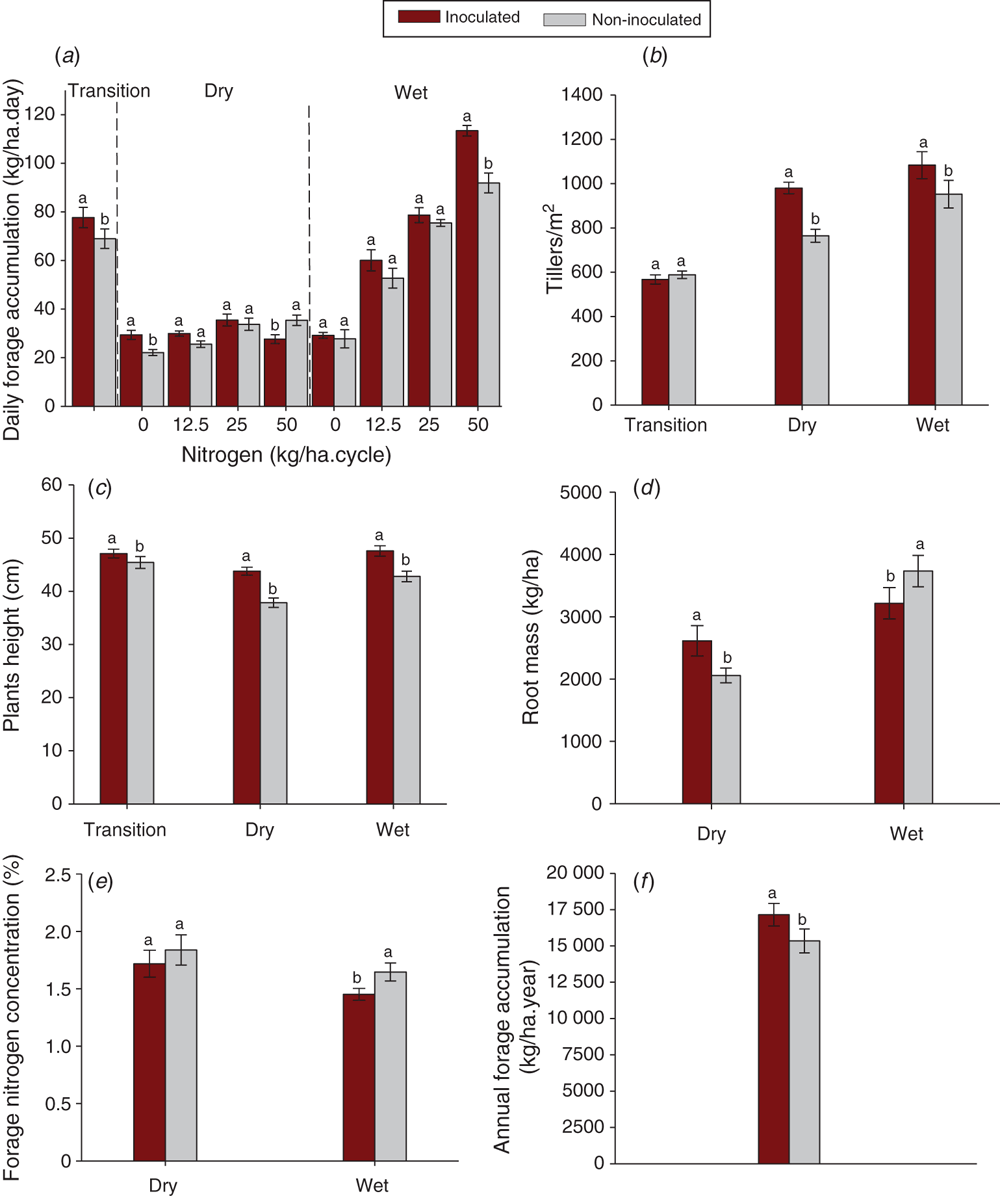
Analysis of variance demonstrated the effect of inoculation with A. brasilense on all parameters evaluated (Fig. 2). The only significant N rate × inoculation interaction was for daily forage accumulation (Fig. 2a). For this parameter, there was a positive effect of inoculation in the transition season, when the inoculated plants accumulated 77 kg/ha.day of forage, whereas the non-inoculated plants accumulated 69 kg/ha.day. These values represent an increase of 12.5% for average daily yield provided by the inoculation. In the dry season, there was a positive response of the inoculation in the absence of nitrogen fertilisation, at the dose of 50 kg/ha of N non-inoculated plants presented greater daily accumulation. These results indicate that, in the dry season, inoculation favoured growth only in the absence of N fertilisation. In the wet season, inoculation resulted in an increase in daily forage accumulation only at the N rate of 50 kg/ha. The additional forage accumulation due to inoculation was 21.5 kg/ha.day, corresponding to a daily contribution of 23.4% to accumulation of forage (Fig. 2a).
In the dry season, the number of tillers in the inoculated treatments was 28% higher than in non-inoculated treatments, and in the wet season it was 12% higher. There was no significant effect in the transition season (Fig. 2b).
Plant height was positively influenced by inoculation in all evaluated periods (Fig. 2c). In the transition season, inoculation resulted in an increase of 4% in relation to the non-inoculated treatment regardless of the rate of N. For the dry season, the increase was 16% and in the wet season 11%.
From the start of the dry season, daily pasture accumulation rates on non-inoculated treatments decreased by 17% relative to the transition season, whereas inoculated treatments decreased by only 7%. The recovery in the wet period was 13% for the non-inoculated plants and 9% for the inoculated plants. Consequently, the greater plant height of Marandu grass indicates the beneficial effects provided by inoculation in mitigating the effects of water stress (Fig. 2c).
At the start of the dry season the root mass of inoculated plants was 27% higher than of non-inoculated plants (Fig. 2d). At the end of the wet season, this ranking was reversed, with the inoculated plants showing root mass 15% lower than the non-inoculated plants.
In relation to the forage N concentration, plants responded differently to inoculation in the evaluated periods (Fig. 2e). In the dry season, inoculated and non-inoculated plants were similar, whereas in the wet season, the inoculated plants had lower N concentration (Fig. 2e).
Annual forage accumulation was increased by inoculation (Fig. 2f). Inoculated plants had total annual forage accumulation of 17 kg/ha.year, and non-inoculated 15 kg/ha.year, averaged across N rates.
Plants showed different responses to N in each period (Table 2). In the transition season, the highest daily accumulation rate for inoculated plants was at 50 kg N/ha, which provided an accumulation of 99 kg/ha.day, representing an increase of 60% compared with nil N fertiliser. For non-inoculated plants, there was a significant effect of N fertiliser, and plants accumulated, on average, 68 kg/ha.day. In the absence of N fertiliser, inoculated plants showed 32% higher accumulation than non-inoculated plants.
During the dry season, the non-inoculated plants showed higher daily accumulation rates at the highest N rate, producing 37 kg/ha.day. In the absence of N, inoculation resulted in an increase of 33% in forage daily accumulation.
In the wet season, the inoculation treatments showed similar positive linear responses to increased N fertilisation, but with different magnitudes (Table 2). For the inoculated plants, at the rate of 50 kg/ha of N, the daily forage accumulation was 116.1 kg/ha.day, which represented an increase of 241% compared with nil N. Non-inoculated plants at the same N rate produced 97 kg/ha.day, 181% higher than in the absence of N fertilisation.
With regard to tiller density in response to N fertiliser in the dry season, the non-inoculated treatment had 905 tillers/m2 at the highest N rate, whereas the inoculated treatment had an average of 980 tillers/m2 and was not significantly affected by N rate. Even in the absence of N fertiliser, the inoculated plants had a higher tiller density than the non-inoculated plants at 50 kg N/ha. In the wet season in the absence of N, tiller densities were 758 and 641 tillers/m2 for inoculated and non-inoculated treatments, respectively. When 50 kg N/ha was applied, tiller densities were 1500 tillers/m2 for inoculated and 1352 tillers/m2 for non-inoculated treatments.
Plant height in the transition season was not significantly affected by N fertiliser for non-inoculated plants (mean 45 cm), but there was a significant effect in the inoculated treatment, which produced plants up to 51 cm in height at 50 kg N/ha (Table 2). In the dry season, inoculated plants were not significantly affected by N-fertiliser rate, with mean plant height of 47.5 cm. However, there was a significant effect for non-inoculated plants, with 41 cm height at 50 kg N/ha (Table 2). Inoculated plants in the dry season were therefore taller than non-inoculated plants at the highest N rate. In the wet season, in the absence of N fertilisation, inoculated plants were 15% taller than non-inoculated plants, and at 50 kg N/ha, they were 6% taller. Plants that were inoculated and receiving 25 kg N/ha had similar height to those without inoculation and receiving 50 kg N/ha.
Forage N concentration was decreased by inoculation but increased by N fertilisation. In the dry season, inoculated plants had 6% lower N concentration at nil N and 19% lower at 50 kg N/ha. In the wet season, the respective values were 9% and 6%.
For annual forage accumulation in the absence of inoculation, maximum yield was 23 t/ha at 50 kg N/ha, which represented a productivity increase of 13 t/ha relative to the treatment without N. Similar results were observed for the inoculated plants, but with an even higher maximum yield of 26 t/ha, an increase of 10 t/ha relative to the treatment without N.
Discussion
Increases in plant height and number of tillers in grasses inoculated with A. brasilense have been mainly attributed to the production of phytohormones (Hungria et al. 2016; Pedreira et al. 2017). Auxins, the main phytohormones released by A. brasilense to the host plants, promote root and shoot growth and have the capacity to regulate plant height (Dobbelaere et al. 2003; Taiz and Zeiger 2009; Fukami et al. 2017). According to Souza et al. (2017), the auxin IAA (indole-3-acetic acid) promotes root growth and stimulates the differentiation in the meristematic tissues that depends on hormonal concentration. Among the benefits of Azospirillum, apparently IAA production is quantitatively the most important for grass growth (Vande Broek and Vanderleyden 1995; Fukami et al. 2017).
In our study, plant height, number of tillers and root mass of plants inoculated with A. brasilense were significantly increased under drought conditions; consequently, inoculation is presented as an alternative to minimise impacts in Brazilian pastures under these conditions.
The greater development of roots allows better water and nutrient absorption, causing an increase in biomass production and chlorophyll concentration, and promoting tolerance to environmental stresses such as drought (Souza 2014; Souza et al. 2017; Fukami et al. 2018). In addition to these benefits, plant-growth-promoting bacteria can provide increased water retention in the soil through production of an extracellular matrix containing oligosaccharides and polysaccharides that increase water-retention capacity (Rubin et al. 2017).
According to Pedreira et al. (2017), the dry season in Brazil is characterised by an intense period of water deficit (Fig. 1), which reduces growth and increases plant mortality. Those authors suggest inoculation with A. brasilense in pastures to minimise the effects of climatic constraints, attributing the increased stress tolerance to improved root development. Indeed, this was confirmed by our results.
In the dry season, the non-inoculated seedlings showed variation in N concentration of 1.37–1.97, similar to values found for Urochloa brizantha (1.3–2.0) (Sousa and Lobato 2004). The inoculated plants showed a range of 1.29–1.65% N, with the minimum value being slightly below adequate for the grass. However, use of stable isotopes (15N) would be necessary to confirm the contribution of BNF. We did not perform 15N analyses because we assumed that the main benefit of strains Ab-V5 and Ab-V6 of A. brasilense would be attributed the production of phytohormones (Hungria et al. 2016; Fukami et al. 2017).
Livestock carrying capacity is related, among other factors, to the dry-matter-production capacity of the pastures (Hungria et al. 2016). In the present study, inoculation with Azospirillum resulted in an increase in forage yield per year of 8–14%, depending on N-application rate, or 11% if 15 kg N/ha is taken as standard. Therefore, to produce an amount of forage similar to the inoculated plants, it would be necessary to have a larger area, of 1.1 ha. It would be possible to reduce the current pasture area in Brazil by inoculation with A. brasilense from 190 Mha (Jank et al. 2014) to 169 Mha, without any reduction to animal production.
Inoculation of Marandu grass plants provided greater annual forage accumulation (Fig. 2f), reaching an increase of 11% in relation to non-inoculated plants during the year. Because degraded pastures show drastically reduced productive capacity (Dias-Filho 2014), we suggest that the inoculation practice could represent an important component in efforts to reverse the degradation of Brazilian pastures.
By evaluating the inoculation of native pastures with A. brasilense, Itzigsohn et al. (2000) concluded that the practice of inoculation with these bacteria has the potential to increase forage production and reduce environmental damage caused by the use of fertilisers. Similarly, from inoculation of A. brasilense on Coastcross grass (Cynodon dactylon (L.) Pers.), Aguirre et al. (2018) found an increase in forage yield and better pasture establishment. Overall, our results for U. brizantha support the results of Itzigsohn et al. (2000) and Aguirre et al. (2018).
In our study, considering the regression analyses for all evaluated parameters, inoculation with Azospirillum allowed an estimated reduction of 20% in the need for N fertiliser. Therefore, considering the area of Brazil planted with Marandu grass (50.0 Mha), the inoculation would represent an economy of 505 000 t N, considering the average fertilisation rate of 50 kg/ha (Sousa and Lobato 2004).
Finally, it should be considered that the residual benefits of pasture inoculation with A. brasilense can be observed in subsequent or associated crops, for example, as reported for maize (Zea mays L.) grown in no-till, inoculated pasture (Leite et al. 2017). The benefits reported in our study suggest that the inoculation of pastures as a practice, in addition to reducing costs and increasing productivity, contributes to environmental sustainability.
Conflict of interests
The authors declare no conflicts of interest.
Acknowledgements
The authors gratefully thank the Coordination of Improvement of Higher Education Personnel (CAPES) for granting the scholarship to the first author, the National Council of Scientific and Technological Development (CNPq) for research grants to the third and fourth authors, and the company Stoller Brasil for providing Azospirillum inoculant and financial assistance.
References
ABIEC (2017) Livestock profile in Brazil: annual report. [in Portuguese]. Associação Brasileira das Indústrias Exportadoras de Carnes, São Paulo. Available at: http://abiec.com.br/Sumario.aspx (accessed 14 February 2018)Aguirre PF, Olivo CJ, Rodrigues PF, Falk DR, Adams CB, Schiafino HP (2018) Forage yield of Coastcross-1 pastures inoculated with Azospirillum brasilense. Acta Scientiarum. Animal Sciences 40, 1–8.
Alvares CA, Stape JL, Sentelhas PC, Goncalves JLM, Sparovek G (2013) Koppen’s climate classification map for Brazil. Meteorologische Zeitschrift 22, 711–728.
| Koppen’s climate classification map for Brazil.Crossref | GoogleScholarGoogle Scholar |
Baldani JI, Reis VM, Videira SS, Boddey LH, Baldani VLD (2014) The art of isolating nitrogen-fixing bacteria from non-leguminous plants using N-free semi-solid media: a practical guide for microbiologists. Plant and Soil 384, 413–431.
| The art of isolating nitrogen-fixing bacteria from non-leguminous plants using N-free semi-solid media: a practical guide for microbiologists.Crossref | GoogleScholarGoogle Scholar |
Canto MW, Almeida GM, Costa ACS, Barth Neto A, Scaliante Júnior JR, Orlandini CF (2016) Seed production of ‘Mombasa’ grass subjected to different closing cut dates and nitrogen rates. Pesquisa Agropecuária Brasileira 51, 766–775.
| Seed production of ‘Mombasa’ grass subjected to different closing cut dates and nitrogen rates.Crossref | GoogleScholarGoogle Scholar |
de Marchi SR, Bellé JR, Foz CH, Ferri J, Martins D (2017) Weeds alter the establishment of Brachiaria brizantha cv. Marandu. Tropical Grasslands - Forrajes Tropicales 5, 85–93.
| Weeds alter the establishment of Brachiaria brizantha cv. Marandu.Crossref | GoogleScholarGoogle Scholar |
de Morais RF, Quesada DM, Reis VM, Urquiaga S, Alves BJR, Boddey RM (2012) Contribution of biological nitrogen fixation to elephant grass (Pennisetum purpureum Schum.). Plant and Soil 356, 23–34.
| Contribution of biological nitrogen fixation to elephant grass (Pennisetum purpureum Schum.).Crossref | GoogleScholarGoogle Scholar |
Dias-Filho MB (2014) Diagnosis of pastures in Brazil. Documentos 402. Embrapa Amazônia Oriental, Belém, Brazil. [in Portuguese]
Dobbelaere S, Vanderleyden J, Okon Y (2003) Plant growth-promoting effects of diazotrophs in the rhizosphere. Critical Reviews in Plant Sciences 22, 107–149.
| Plant growth-promoting effects of diazotrophs in the rhizosphere.Crossref | GoogleScholarGoogle Scholar |
Ferreira DF (2011) Sisvar: A computer statistical analysis system. Ciência e Agrotecnologia 35, 1039–1042.
| Sisvar: A computer statistical analysis system.Crossref | GoogleScholarGoogle Scholar |
Fukami J, Ollero FJ, Megías M, Hungria M (2017) Phytohormones and induction of plant-stress tolerance and defense genes by seed and foliar inoculation with Azospirillum brasilense cells and metabolites promote maize growth. AMB Express 7, 153
| Phytohormones and induction of plant-stress tolerance and defense genes by seed and foliar inoculation with Azospirillum brasilense cells and metabolites promote maize growth.Crossref | GoogleScholarGoogle Scholar |
Fukami J, Cerezin P, Hungria M (2018) Azospirillum: benefits that go far beyond biological nitrogen fixation. AMB Express 8, 73
| Azospirillum: benefits that go far beyond biological nitrogen fixation.Crossref | GoogleScholarGoogle Scholar |
Hungria M, Campo RJ, Souza EM, Pedrosa FO (2010) Inoculation with selected strains of Azospirillum brasilense and A. lipoferum improves yields of maize and wheat in Brazil. Plant and Soil 331, 413–425.
| Inoculation with selected strains of Azospirillum brasilense and A. lipoferum improves yields of maize and wheat in Brazil.Crossref | GoogleScholarGoogle Scholar |
Hungria M, Nogueira MA, Araujo RS (2015) Soybean seed co-inoculation with Bradyrhizobium spp. and Azospirillum brasilense: A new biotechnological tool to improve yield and sustainability. American Journal of Plant Sciences 6, 811–817.
| Soybean seed co-inoculation with Bradyrhizobium spp. and Azospirillum brasilense: A new biotechnological tool to improve yield and sustainability.Crossref | GoogleScholarGoogle Scholar |
Hungria M, Nogueira MA, Araujo RS (2016) Inoculation of Brachiaria spp. with the plant growth-promoting bacterium Azospirillum brasilense: An environment-friendly component in the reclamation of degraded pastures in the tropics. Agriculture, Ecosystems & Environment 221, 125–131.
| Inoculation of Brachiaria spp. with the plant growth-promoting bacterium Azospirillum brasilense: An environment-friendly component in the reclamation of degraded pastures in the tropics.Crossref | GoogleScholarGoogle Scholar |
Itzigsohn R, Burdman S, Okon Y (2000) Plant-growth promotion in natural pastures by inoculation with Azospirillum brasilense under suboptimal growth conditions. Arid Soil Research and Rehabilitation 14, 151–158.
| Plant-growth promotion in natural pastures by inoculation with Azospirillum brasilense under suboptimal growth conditions.Crossref | GoogleScholarGoogle Scholar |
Jank LAB, Barrios SC, Valle CB, Simeão RM, Alves GF (2014) The value of improved pastures to Brazilian beef production. Crop & Pasture Science 65, 1132–1137.
| The value of improved pastures to Brazilian beef production.Crossref | GoogleScholarGoogle Scholar |
Leite RC, Soares GOS, Leite RC, Santos JGD, André TB, Santos AC (2017) Cultivo de milho em sistema de plantio direto em pastagem inoculada com Azospirillum brasilense. Revista Brasileira de Agropecuária Sustentável 7, 43–49.
Lopes MJS, Dias-Filho MB, Castro THR, Silva GB (2017) Light and plant growth-promoting rhizobacteria effects on Brachiaria brizantha growth and phenotypic plasticity to shade. Grass and Forage Science 73, 493–499.
Marques ACR, Oliveira LB, Nicoloso FT, Jacques RJS, Giacomini SJ, Quadros FLF (2017) Biological nitrogen fixation in C4 grasses of different growth strategies of South America natural grasslands. Applied Soil Ecology 113, 54–62.
| Biological nitrogen fixation in C4 grasses of different growth strategies of South America natural grasslands.Crossref | GoogleScholarGoogle Scholar |
Okon Y, Labandera-Gonzalez CA (1994) Agronomic applications of Azospirillum: an evaluation of 20 years worldwide field inoculation. Soil Biology & Biochemistry 26, 1591–1601.
| Agronomic applications of Azospirillum: an evaluation of 20 years worldwide field inoculation.Crossref | GoogleScholarGoogle Scholar |
Pedreira BC, Barbosa PL, Pereira LET, Mombach MA, Domiciano LF, Pereira DH, Ferreira A (2017) Tiller density and tillering on Brachiaria brizantha cv. Marandu pastures inoculated with Azospirillum brasilense. Arquivo Brasileiro de Medicina Veterinária e Zootecnia 69, 1039–1046.
| Tiller density and tillering on Brachiaria brizantha cv. Marandu pastures inoculated with Azospirillum brasilense.Crossref | GoogleScholarGoogle Scholar |
Rodrigues LF, Santos AC, Silveira O, Santos JGD (2017) Productivity of Urochloa brizantha ‘Marandu’ influenced by strategic rest periods and nitrogen levels. Semina. Ciências Agrárias 38, 3203–3214.
| Productivity of Urochloa brizantha ‘Marandu’ influenced by strategic rest periods and nitrogen levels.Crossref | GoogleScholarGoogle Scholar |
Rubin RL, Van Groenigen KJ, Hungate BA (2017) Plant growth promoting rhizobacteria are more effective under drought: a meta-analysis. Plant and Soil 416, 309–323.
| Plant growth promoting rhizobacteria are more effective under drought: a meta-analysis.Crossref | GoogleScholarGoogle Scholar |
Sá JCM, Lal R, Cerri CC, Lorenz K, Hungria M, Carvalho PCF (2017) Low-carbon agriculture in South America to mitigate global climate change and advance food security. Environment International 98, 102–112.
| Low-carbon agriculture in South America to mitigate global climate change and advance food security.Crossref | GoogleScholarGoogle Scholar |
Saturno DF, Cerezini P, Moreira SP, Oliveira AB, Oliveira MCN, Hungria M, Nogueira MA (2017) Mineral nitrogen impairs the biological nitrogen fixation in soybean of determinate and indeterminate growth types. Journal of Plant Nutrition 40, 1690–1701.
| Mineral nitrogen impairs the biological nitrogen fixation in soybean of determinate and indeterminate growth types.Crossref | GoogleScholarGoogle Scholar |
Sousa DMG, Lobato E (2004) Cerrado: Correção do solo e adubação. Embrapa Informação Tecnológica, Brasília.
Souza PT (2014) Inoculação com Azospirillum brasilense e adubação nitrogenada em Brachiaria brizantha cv. Marandu. Dissertação de mestrado em Produção Vegetal pela Universidade Federal de Goiás, Jataí, Goiás, Brazil.
Souza MST, Baura VA, Santos SA, Fernandes-Júnior PI, Reis FB, Marques MR, Paggi GM, Silva BM (2017) Azospirillum spp. from native forage grasses in Brazilian Pantanal floodplain: biodiversity and plant growth promotion potential. World Journal of Microbiology & Biotechnology 33, 81
| Azospirillum spp. from native forage grasses in Brazilian Pantanal floodplain: biodiversity and plant growth promotion potential.Crossref | GoogleScholarGoogle Scholar |
Taiz L, Zeiger E (2009) ‘Fisiologia vegetal.’ 4th edn. (Artmed: Porto Alegre, Brazil)
Vande Broek A, Vanderleyden J (1995) Review: Genetics of the Azospirillum–plant root association. Critical Reviews in Plant Sciences 14, 445–466.
| Review: Genetics of the Azospirillum–plant root association.Crossref | GoogleScholarGoogle Scholar |