Translational research in agriculture. Can we do it better?
John B. Passioura
CSIRO Agriculture and Food, PO Box 1700, Canberra, ACT 2601, Australia. Email: john.passioura@csiro.au
Crop and Pasture Science 71(6) 517-528 https://doi.org/10.1071/CP20066
Submitted: 27 February 2020 Accepted: 4 June 2020 Published: 12 June 2020
Journal compilation © CSIRO 2020 Open Access CC BY-NC-ND
Abstract
‘Translational research’ became an increasingly common term when it was realised that much agriculturally inspired basic research failed to contribute to the improvement of crops. Most of the failure has come from laboratory-based attempts to ameliorate abiotic stresses. Dealing with biotic stress has been much more successful; the control of pests and weeds is often enabled by transforming crops with single genes, for such genes have little or no influence on a crop’s metabolism. By contrast, abiotic stress varies with the weather; i.e. crops respond systemically, over a range of levels of organisation (e.g. cells, tissues, organs), with many feedbacks and feedforwards. Drought is the most pervasive form of abiotic stress. There are 4600 papers that have searched, ineffectively, for ‘drought resistance’, a term that usually defies useful definition. By contrast, dealing with a measured, limited water supply (e.g. seasonal rainfall), rather than with ‘drought’, has effectively increased water-limited yield through agronomic innovation based on improving water-use efficiency. ‘Salt tolerance’ has similar difficulties; nevertheless, physiological knowledge has revealed effective single genes, in contrast to the failures of empirical gene prospecting. Another important goal has been to increase potential crop yield by exploring mechanistic opportunities to improve photosynthetic efficiency. These attempts have not, so far, succeeded, perhaps because they have rarely broached physiological responses beyond carbon balance, such as metabolic responses to environmental challenges that may affect meristematic development. A major reason for the predominant failure of translational research from laboratory to field is that the peer-review system is too narrow; i.e. reviewers have the same backgrounds as the authors. Effective translation will require the addition of reviewers who can assess the pathway from laboratory to field.
Additional keywords: genetic transformation, photosynthesis, temperate field crops, water use efficiency.
Introduction
‘Translational research’ is a term in good currency. Its use started to grow rapidly 12 years ago when an issue of Nature devoted 40 pages to it in relation to biomedicine. It has now become common in the plant-based agricultural sciences and refers to the translation of basic scientific discovery into improved, even ‘transformational’, agricultural productivity. Its use is much more common in laboratory-based research than in the field sciences of crop physiology, agronomy and breeding. These field sciences have a compelling aim to improve the productivity of crops. In practice they involve frequent contacts between scientists and farmers which illuminate pathways to adoption of new cultivars or new farming techniques. They cover all influences on productivity, including fertilisers, pests and diseases, grain quality and yield, root and canopy architecture, overall management of farming systems, and sustainability, mainly through protection of soil fertility, but also through a concern for the wellbeing of landscapes (Passioura 1999; Pratley and Kirkegaard 2019). ‘Adoption’ of improved practices and cultivars is the long-standing term for successful change in this arena.
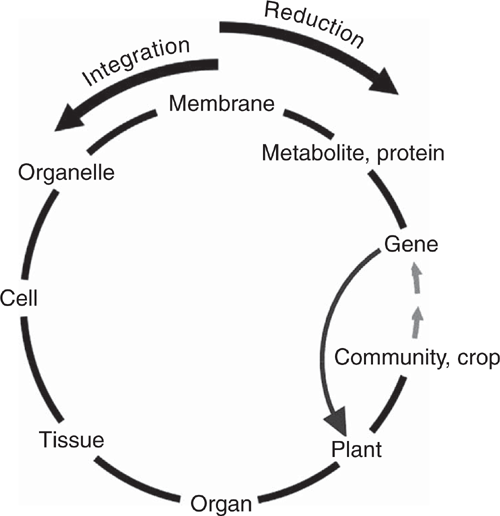
Google Scholar returns about 50 000 items when primed with ‘translational research and crops’. This large number would no doubt be even larger if it included basic mechanistic research that is pursued with an eye to being useful, as Stokes (1997) discussed in his book Pasteur’s quadrant. Ideally, translational research refers to the flow of ideas along the sequence of increasingly complex levels of organisation into which plant and agricultural scientists divide their subject matter (gene, molecules, membrane, organelle, cell, tissue, organ, plant, crop). An important aspect of this sequence is that each level has its own terminology to cover the features and processes peculiar to that level. Table 1 contains examples of different terms used across the nested hierarchy that these levels comprise. It is evident that conversations between specialists working at different levels, even adjacent levels, may not be easy. This sequence can also illustrate how plants transmit their genes, via seeds, into the next generation, by making it circular, as shown in Fig. 1.
|
|
Translation, if it is to progress successfully, usually requires knowledge of the main features of increasingly higher levels of organisation (anti-clockwise movement in Fig. 1) between laboratory-based research and its broad application; major constraints and interactions come into play with the increasing complexity. Gaining that knowledge requires clockwise translation of ideas to provide contextual understanding from ‘above’, i.e. from higher levels of organisation. Such translation is usually needed to identify checkpoints that may need to be passed at each level to avoid the inadvertent study of unrealistic artefacts that can arise from ignorance of the additional conditions that are to be met at higher levels of organisation.
Much laboratory-based transgenic research has bypassed the integrative levels portrayed in anti-clockwise flow around Fig. 1. It has concentrated on direct observation of transgenic phenotypes by taking a shortcut across the circle, from transgene directly to whole plant, thereby ignoring possible artefacts. This is not to say that there have been no successes. There have been. But most of these successes have dealt with useful traits that are largely independent of the fundamental metabolism and the internal feedbacks and feedforwards of higher levels of organisation, as discussed later.
This review has arisen from a workshop held in November 2018 (see Acknowledgements), the papers from which are in Sadras et al. (2020). My aim in this review is to discuss potentially better ways of translating strategic agricultural research into practice. The previous paragraphs imply that there are two ways of thinking about how plants work. One is that they are akin to machines whose parts and products we can improve upon by simple replacements or insertions. The other is that they are hierarchically organised systems (see Table 1) that experience cascades of controls from within. There has been little debate in recent years on these seemingly disparate views, but Weiss (1969, p. 12) wrote a penetrating discussion on this issue 50 years ago which simulated much debate at the time. The debate was centred on this statement:
‘In the system, the structure of the whole determines the operation of the parts; in the machine, the operation of the parts determines the outcome.’ (See: https://www.informationphilosopher.com/solutions/scientists/weiss/)
The following pages explore the circumstances in which viewing plants as hierarchically organised systems is more effective than viewing them as ‘machines’, or vice versa. Each view has its place.
The next section covers the main general features of hierarchical systems and of machines. It is followed by case studies of successes and failures in relation to these differing approaches to translational research in agriculture. These cases deal mainly with abiotic stress and with attempts to increase potential grain yield in ideal growing conditions. The final section ponders agriculturally beneficial conclusions drawn from these case studies.
Hierarchical systems
The essential characteristic of a hierarchical system is illustrated by the Ideal Gas Law
The Ideal Gas Law provides a simple example that deals with a hierarchical system with only two levels to illustrate the control of the higher level over the lower.
The Law is an equation that relates the pressure, P, volume, V, absolute temperature, T, and number of moles, n, of an enclosed ideal gas, namely PV = nRT, where R is the universal gas constant. This relationship explains many everyday phenomena such as the swelling of an inflated balloon when heated. The Law arose from empirical observations of P versus V, V versus T, and P versus T, which were then combined (https://en.wikipedia.org/wiki/Ideal_gas_law).
Twenty years after the publication of the Ideal Gas Law in 1834, it was itself explained by the kinetic theory of gases; it was assumed that the gas consists of myriad molecules (n × Avogadro’s number), each of given mass but of negligible volume, moving randomly in an enclosed space, each with its own velocity which is changed only by perfectly elastic collisions with other molecules or with the enclosing wall, and whose root-mean-square velocity determines the absolute temperature.
These two descriptions of a gas, one macroscopic and phenomenological, and the other based on particle physics, appear to have little connection with each other. The ideas of volume and number are common to both descriptions, but not those of pressure, temperature and velocity. A molecule does not have a pressure or a temperature, and PV = nRT does not deal with velocity.
The two descriptions are examples of different conceptual layers in a simple system. Each has terms and ideas that are peculiar to it. Yet the two layers are connected, and the apparently disparate terms are related. The connection comes by considering the average properties of a large number of molecules because P and T are both related to the root-mean-square speed of those molecules.
This example of a connection between conceptual layers comes from classical physics, but note that the phenomenological discovery (the Ideal Gas Law) was made before the particulate explanation was available. The reason that we first need to appreciate integral behaviour of the whole system is that the problem of integrating the behaviour of the parts requires knowledge of the boundary conditions. We need to specify at least two constraints when considering the kinetic behaviour of the molecules before we can derive the gas laws: (1) the number of molecules is known, and (2) they are enclosed within a three-dimensional bounding wall. The kinetic theory also applies to Earth’s atmosphere, but then we need different constraints. We replace the constraint of an enclosed space with that of an infinite space bounded internally by Earth’s surface and subject to a gravitational field.
In practice we do not become aware of the extra information we need, the constraints on the behaviour of a subsystem (e.g. a molecule or a cell), until we have recognised the behaviour of a system as a whole (e.g. a gas or a tissue). This is a crucial principle that highlights the importance of exploring plant behaviour at all levels of organisation. Only by articulating connections between all of the layers can we hope to have a comprehensive understanding of how plants work. It is notable that every layer can be viewed as a system or as a subsystem depending on one’s primary interest. For example, crop physiologists working in the field deal mostly with canopies as their system; plants or leaves are for them subsystems. They are well aware that, in a plot experiment, plants in the edge rows behave differently from those in the centre because the latter are more constrained by the proximity of neighbours than are those at the edge.
The information content of a hierarchical system
As we move anticlockwise around Fig. 1, each new level contains additional information that adds to the information contained in the lower levels. For example, a leaf that emerges from a meristem has an epidermis which is punctured by stomata that can control the rate of uptake of carbon dioxide and the rate of loss of water. It contains clearly defined tissues with different functions (e.g. the mesophyll and the vascular system). One can explore the behaviour of a leaf—say, the diurnal pattern of the ratio of the uptake of carbon dioxide to the loss of water—without necessarily needing to think about its vascular system or its mesophyll. The properties of these internal tissues are of course essential but it may not be necessary to think about them, just as when applying the Ideal Gas Law we usually do not need to think about the molecules of a gas.
Thus, in operational terms, the aphorism that the whole is more than the sum of the parts can be replaced by its opposite, that the whole is less than the sum of the parts, less in the sense that we do not always need to consider the parts. This view applies not only operationally (i.e. how do we want to make best use of this information?), but also more fundamentally. Weiss (1969) put it this way:
‘…the complex is a system if the variance of the features of the whole collective is significantly less than the sum of variances of its constituents…. In short, the basic characteristic of a system is its essential invariance beyond the much more variant flux and fluctuations of its elements or constituents.’
What causes what in a hierarchical system? Internal controls and boundary conditions
Returning to the example of a leaf, exogenous diurnal variation in stomatal conductance may control its rates of transpiration and of photosynthesis; for example, low ambient humidities may induce stomata to close. Yet, stomatal conductance may also be influenced endogenously if the demand for photosynthates by the plant as a whole (e.g. respiration, growth of new tissue) is sink-limited (i.e. it is less than the photosynthetic rate). The plant as a whole may respond by reducing the photosynthetic rate and thence stomatal conductance. Any attempt to increase the photosynthetic rates of the leaves may thus be thwarted. The idea of causation has many facets.
The analogy of the Ideal Gas Law exemplifies the constraints of boundary conditions. The boundary condition is that the sample of gas is enclosed within an elastic, three-dimensional wall. For a given amount of gas, its three interconnected properties are its pressure, its volume, and its temperature. The kinetic theory of gases explains how these three variables are connected to produce the Ideal Gas Law, but it can only do so if given the constraining boundary condition. The microscopic is controlled by the macroscopic.
If we consider now the vastly more complicated biological systems that we are interested in, each has several nested layers of increasing complexity. Each layer is defined by a discernible physical boundary: the outer membrane of an organelle; the wall of a cell; the bounding layer of cells that surrounds a tissue (epidermis, endodermis); the junctions that separate organs; the individual plants of a crop that collectively form a canopy.
Each subsystem imposes additional boundary conditions on the subsystems that comprise it. There is a common view that the more one understands about how the minutiae of an organism work, the greater is the chance of improving the whole. However, when we have burrowed through several increasingly detailed levels in our hierarchy to understand what many think of as the absolute fundamental processes of plants, we have done so at the expense of shedding the information contained in the boundary conditions imposed by the higher levels. In trying to retrace our collective steps, it is necessary to regain our understanding of those boundary conditions. To ignore them, as in the shortcut in Fig. 1, can lead to pitfalls.
Plants as ‘machines’: transgenes that escape feedbacks
When we think of trying to improve the performance of a machine, for example a car, we have no difficulty in looking for specific improvements. The introduction of disc brakes to replace drum brakes is a good example. The car will stop faster when we want it to without our needing to change anything else. If we want to put in a more powerful engine, then we may need to make the cars transmission more robust; i.e. we can easily counter the potentially unpleasant response of the drive shaft failing.
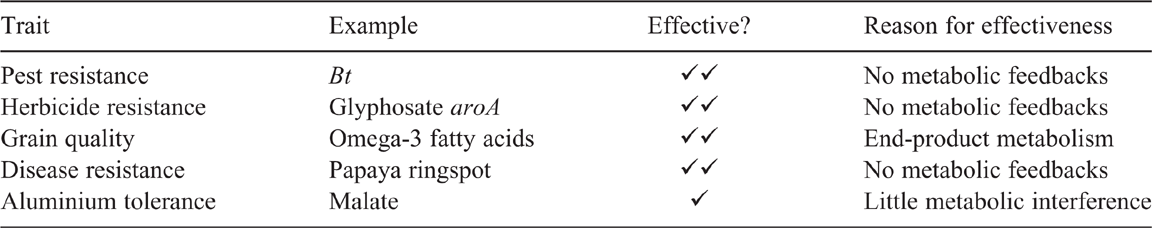
When setting out to improve the performance of agricultural plants, there can be similar circumstances in which there are no systemic feedbacks. Table 2 lists examples of effective genes and the reasons for their effectiveness. The distinguishing feature of these genes is that they are not involved directly in the major metabolic systems of the plant nor in the large-scale processes that determine how crop plants cope productively with highly variable environments. Thus, the shortcut between gene and plant shown in Fig. 1 can work well:
|
-
The Bt gene does its job of killing caterpillars without affecting a plant’s metabolism.
-
The aroA gene from Agrobacterium is not affected by glyphosate, whereas the plant’s version of that gene is, thus enabling glyphosate to be used to kill weeds in a growing crop without affecting the crop itself. (See: https://www.brainkart.com/article/Transgenic-Plants-with-Herbicide-Resistance_13936/; accessed 20 May 2020.)
-
In a growing seed, whatever is loaded into the endosperm stays there until germination and thus has no influence on the rest of the plant; the composition of the endosperm—its starch, its protein, its oils—can thus be improved to increase its nutritional or industrial value. A notable example is the development of a set of transgenes from algae that generate high levels of the especially valuable long-chain fatty acids EPA and DHA in canola seeds (Petrie et al. 2010).
-
Resistance to the Papaya ringspot virus was achieved by bombarding embryonic material with DNA of the coat protein gene of the virus without seeming to affect the plant’s metabolism (Gonsalves 1998).
-
In a wide range of crops, especially cereals, the defence of roots against aluminium toxicity is boosted by incorporating specific genes that result in root tips releasing small amounts of organic anions (malate, citrate, oxalate) that can counter the toxic effects of aluminium with seemingly little requirements for photosynthate. The genes are activated if there is enough aluminium in the soil to be toxic (Delhaize et al. 2012).
Case studies of successes and failures in translational research on crop plants
The search for ‘drought resistance’
‘Drought resistance’ is a nebulous term. It has no defined units and is therefore not quantifiable. However, it does have a useful statistical meaning: some genotypes perform better in water-limited environments than do others. To breeders, this means, justifiably, that they are more drought resistant.
Nevertheless, there has been much laboratory research, a form of prospecting, aimed to find specific genes for drought resistance, whether from genes expressed in a drought treatment, or by best guesses for useful genetic transformations.
Searching the Core Collection of the Web of Science using the criteria (‘drought resistance’ or ‘drought tolerance’) AND (transgenic or molecular) AND (wheat or barley or maize or rice or canola or soybean) NOT (field) returned about 1800 papers (April 2020). Exclusion of field aimed to restrict the papers to laboratory research. Adding Arabidopsis to the list of species increased the number of papers by 50%, to 2600. Further, there is a similar, overlapping, genre that explores the responses of plants to drought, perhaps in the spirit of Pasteur’s quadrant (Stokes 1997). Most of these papers deal with variation in gene expression, metabolites and hormones in drought-stressed plants, accompanied by discussion on ways of beneficially manipulating the variation. Adding response to transgenic and molecular in the search increased the number of papers selected by a further 2000, to 4600.
What has all this activity achieved? So far, only one transgenic cultivar has been released to farmers, namely, Monsanto’s DroughtGard, maize transformed with the gene for cold shock protein B (cspB). It was reported to have about a 6% increase in water-use efficiency in field trials before its release in 2013, although information about it is hard to find since then. This increase of about 6% divided by the time taken to produce the genotype (more than 10 years?) is likely to be outpaced by conventional breeding, especially when there are large delays arising from bureaucratic requirements for release of genetically modified (GM) crops, thereby adding another few years to the release date (Hall and Richards 2013; Araus et al. 2019; González et al. 2020).
That said, González et al. (2020) have shown in extensive field trials over several years in Argentina that wheat and soybean transformed with a variant of the transcription factor HaHB4 yielded substantially better than the wild types. This was especially so when water was scarce, seemingly because of better growth during floral development leading to more grains per unit area. Their paper describes the large multidisciplinary effort that was put into reaching this stage of development, the time-consuming breeding of the transgenic lines, and then the years of agronomic testing. Their GM lines of soybean have now been approved for release. This remarkable success highlights the need for the basic research to be augmented by engaging with crop physiologists, breeders and agronomists. Without that engagement, which may require 10 times the effort of that already spent in the laboratory, the work has no chance of practical success.
The initial criterion for drought tolerance in these two popular genres (gene prospecting and responses to drought) is usually the survival of transgenic plants, grown in pots, after rapid depletion of their water supply. This approach typically uses the shortcut shown in Fig. 1. There are two problems with this criterion. The first is that the survival of crop plants is essentially irrelevant in the real world. The second is that transgenic plants frequently grow more slowly than the wild types. Thus, the wild types often use up their water supply earlier and thence die whereas the transgenics are still alive (Morran et al. 2011). Such results are artefacts.
By contrast, the work of Boyer and colleagues on the mechanisms underlying drought-induced abortion of maize ovaries involved following a sequence of hypotheses starting with intact plants at the time of pollination. The fertility could be substantially recovered by feeding sucrose into the xylem of the stem, thereby restoring the supply of photosynthate to the plant as a whole, a supply which the drought treatment had greatly reduced. A brilliant paper by Boyer and McLaughlin (2007) introduced the term ‘functional reversion’ to describe this technique, in which the phenotype is restored by external means.
Water stress changes the expression of a multitude of genes, so many that it is hard to identify those that might control fertility. Restoring the phenotype enabled Boyer and McLaughlin to discover a handful of fertility-controlling genes and the interactions among them, including the activation of senescence genes. These genes can therefore be targets for preventing, or at least moderating, abortion.
The conceptual shift from ‘drought resistance’ to ‘water use’ and ‘water-use efficiency’
Agronomically, it is much more effective to think not of ‘drought resistance’ but of ‘resource economics’, i.e. by asking how to make best use of a given, growth-limiting, water supply:
This successful example starts with the work of Nix and Fitzpatrick (1969) who, in setting out to find a simple model for predicting the grain yield of water-limited wheat in Queensland, noticed that the yield was approximately proportional to the amount of available water in the soil at about the time of late floral development and anthesis.
In response to this observation, crop physiologists (Passioura 1972, 1977; Fischer 1979) started exploring how the pattern of seasonal water use affected yield in water-limited environments. The term ‘harvest index’ (the ratio of grain yield to standing dry mass at harvest), which Donald and Hamblin (1976) explored in an influential review, began to have a new operational meaning: the relationship between grain yield and water in the soil at anthesis stimulated agronomic thoughts about improved grain set and the potential filling of those grains, the twin drivers of grain yield and higher harvest index.
The scene was set for the subsequent work of French and Schultz (1984a), who stunned agriculturalists by pointing out that the yield of wheat was rarely limited by water, for the prevailing view at that time was that yield was almost always limited by water. Rather, other prominent factors were to blame, especially weeds, diseases and nutrition (French and Schultz 1984b). French and Schultz (1984a) showed that the maximal yield from a given limited water supply could be estimated by subtracting about 100 mm from the seasonal rainfall, to account for direct evaporation from the soil, and then multiplying the remaining seasonal rainfall (mm) by 20 kg ha–1.
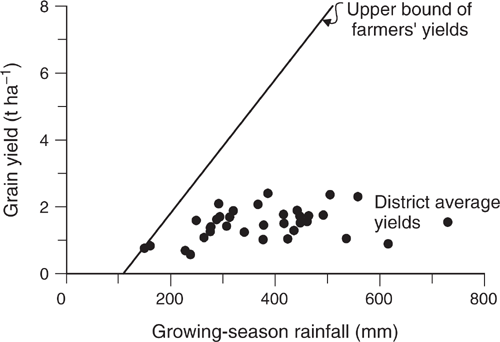
Then came Cornish and Murray (1989), who astonished us all by showing that the yield of wheat in the Wagga Wagga Shire between 1950 and 1983 was almost completely independent of the seasonal rainfall, which ranged from 100 to 700 mm during this period. The data were later extended by Angus and van Herwaarden (2001) to 1992 (Fig. 2). The low yields in the early years reflected the low-input systems of that era (except for the greater use of superphosphate in the mid-1950s to stimulate the growth of subterranean clover pastures, which were rotated with crops), but in the later years, the main limiter was probably implicit risk management. Farmers had become aware that crops that grew too much during their vegetative phase (as a result of excessive nitrogen supply) tended to produce low yields because of ‘haying-off’, a syndrome in which crops produce a large biomass, low yield and pinched grain (van Herwaarden et al. 1998). The farmers therefore avoided adding nitrogen fertiliser, in effect aiming for yields of ~2 t, which is what they got. Agronomists subsequently realised that they had been unaware of endemic root diseases, and that haying-off was typically triggered by impoverished root systems that failed to take up available water during grain-filling. Once the root diseases were controlled, the crops responded well to fertiliser.
|
These two papers by French and Schultz from the 1980s inspired farmers. The term ‘drought’ has a somewhat fatalistic ring to it. The term ‘water supply’ defines a resource that can be made use of. The term ‘water-use efficiency’ is a challenge to make best use of that water supply. There was an empirical maximum water-use efficiency that became a commonly attainable benchmark on farm: 15 kg grain ha–1 mm–1 seasonal rainfall. (The maximum identified by French and Schultz (1984a), 20 kg grain ha–1 mm–1, required meticulous, probably overly expensive, management and luck with the weather.) The large proportion of farmers who found that their water-use efficiency was well under this benchmark strove to reach it by improving various aspects of their management, such as nitrogen management or disease control. A conceptual example of the change in attitude comes from a group of leading farmers in Western Australia who belonged to the Three Tonne Club (Perry 1980) and then later talked of the ‘15 kg per hectare millimetre club’.
In the context of a crop being part of a system, this example deals firstly with the crop level in Fig. 1, then goes beyond that level to the farmer (for every system is somebody else’s subsystem). From an agronomist’s point of view, details concerning the behaviour of leaves (a level of organisation below that of the crop), their water potential, their stomatal conductance and their photosynthetic rate, were of little interest. It was the specific constraint of water supply at an important time that mattered. No deeper understanding was needed at this time when yield increases of 50–100% became feasible.
The translation was upwards, to the farmers, and was enabled by the deepening agronomic understanding, over a period of >30 years, of the water relations of dryland crops, a developing understanding that many farmers kept pace with; i.e. there was much exchange of ideas between these two levels. Average Australian wheat yields increased by about 30% during the 1990s (Kirkegaard and Hunt 2010), owing to the new appreciation of water-use efficiency and to the introduction of canola into farming systems, which helped to deal with the previous endemic root disease (Kirkegaard et al. 1994).
Advances in water productivity have continued (although challenged by reduced rainfall; Hochman et al. 2017), largely through early sowing, which, in the southern Australian environment, enables crops to capture extra water conserved in the soil from summer rain (e.g. Kirkegaard et al. 2014) accompanied by greater root depth from a combination of warmer subsoils and the longer growing season. The opportunity to sow early was embraced by many desperate farmers during Australia’s Millennium drought (2001–10) when rainfall declined sharply during the usual sowing period in late autumn and early winter. The farmers experimented, successfully, with sowing their crops into dry soil. An important advantage of dry sowing is that haste is not an issue as it is with sowing after rain, when it can take 3 weeks or more to complete the sowing.
This opportunity was enabled by better herbicides, the previous adoption of direct drilling (which softened untilled soil) and other innovations that together enabled transformational agronomic changes (Hunt et al. 2019a). The most important of these innovations has been the development of slow-maturing cultivars that can be sown early while still flowering at optimal times to minimise the combined risks of damage from frost, heat and drought (Flohr et al. 2017). Such cultivars were sparse during the Millennium drought, but continuing development has shown their value in increasing yields (Hunt et al. 2019b). Essentially, the idea of water-use efficiency, which in the 1990s focused on making the best use of rain during the growing season, is being replaced by the idea of making best use of the annual rainfall. This innovation is a brilliant example of adapting agriculture to climatic challenges in real time.
Salinity tolerance
The parallel story on laboratory-based search for specific genes that confer salinity tolerance is closely similar to that for drought tolerance (see above). There were 2800 papers and citations returned from the Web of Science when the search criteria were (‘salt resistance’ or ‘salt tolerance’) AND (transgenic or molecular or response) AND (wheat or barley or maize or rice or canola or soybean) NOT (field) (April 2020). Adding Arabidopsis to the list of species increased this number to ~4600.
Little has been learnt from all this activity in relation to practical salt tolerance. Experimental artefacts have been rife. Many of these papers have involved exposing the roots suddenly (instead of gradually) to strongly saline solutions. This is shock treatment. The very low osmotic potentials of the saline solutions rapidly suck water out of the root cells, thereby causing them to plasmolyse, meaning that the plasma membranes detach from the cell walls, thereby creating a major artefact accompanied by much irrelevant gene expression (irrelevant, that is, to salinity tolerance). This and a range of other artefacts that can occur when trying to translate controlled-environment experiments towards practicality are discussed in Passioura (2010).
Another and more important problem is that most of these 4600 papers deal only with short-term responses to salinity, 1–3 days, whereas useful variation in salt tolerance may take weeks to become evident across a range of genotypes. This is because salt tolerance requires the almost complete exclusion of salt by the roots as they take up water. Bread wheat, which is salt tolerant, excludes ~98% of the salt from the water that passes across the roots to the xylem. Durum wheat, which is more salt sensitive, excludes only ~96%. Barley, which is able to sequester high concentrations of sodium in the vacuoles of the leaves and uses salts as a cheap form of osmotic adjustment, excludes ~93% (Munns et al. 2020). Salt that is not excluded or sequestered slowly builds up in the leaves, eventually causing irreversible damage. Ignorance of these processes, the first at the cellular level and the second at the leaf level, has resulted in little of practical worth coming out of most of the published research purporting to be about salinity tolerance. Nevertheless, the influence of specific genes in conferring salt tolerance is much greater than with drought tolerance, and much progress has been made, largely stimulated by whole-plant physiology:
Success with a different approach to salinity tolerance
A good understanding of the physiology of salt-affected crop plants has led to simple techniques for measuring the rate of accumulation of salt in young leaves as a predictor of salt tolerance, and, from that, the successful identification of salt-tolerant genotypes (having slow rates of accumulation) and of the genes that control this trait. It is informative to see how these genes were discovered and how they were shown to confer salt tolerance in durum wheat by reducing the rate at which salt entered the roots on its way to the leaves. This journey has been amply described by James et al. (2012) but is briefly summarised below.
The journey started with observations in the field that hitherto unrecognised soil salinity was restricting the yield of durum wheat. A durum breeder (Ray Hare) working with a physiologist (Rana Munns) found a line with low concentrations of sodium in leaves that they thought would be salt tolerant. That line was crossed with a commercial cultivar; the properties of the progeny revealed two Mendelian genes, which were then identified and cloned. One gene retrieved sodium that had entered the xylem in the roots, and the other retrieved more sodium from the xylem flowing through the leaf sheaths, thereby further reducing the amounts reaching the leaves themselves. Salt-tolerant durum lines were crossbred and shown to yield about 25% more in saline fields (Munns et al. 2012). Seeds from this cross, together with relevant molecular markers, have been provided to breeding companies in many countries to enable crossing into locally adapted cultivars; nevertheless, no cultivar has yet been released, owing perhaps to the long lead time needed for breeding and field testing in a range of environments (Gilliham et al. 2017). Another issue is that competitive breeding companies prefer to sow their tens of thousands of plots on good soil, understandingly because of wanting to avoid the large spatial variation that is common in saline areas.
Prospecting in the laboratory for genes that might confer salt tolerance in plants has, with rare exceptions, been unsuccessful. The successful journey just described started in the field, moved clockwise around Fig. 1, identified the competent genes, and was then able to make effective use of the shortcut shown in Fig. 1. This process, based as it was on a deep understanding of the processes involved, set the scene for a more focused search for other useful genes (Xue et al. 2004).
Heat tolerance
Heat as an abiotic stress has attracted much less interest than has drought or salinity; there are only about 600 papers from laboratory studies on this topic in the Core Collection of the Web of Science, about 15% of those concerning drought or salinity.
Yet a major quantitative analysis by Telfer et al. (2013) of 600 rainfed field trials of wheat in southern Australia between 2005 and 2010 showed large decreases in yield, both with chronic increases in temperature and with transient hot days. For example, every increase in average temperature of 1°C during flowering resulted in a 20% decrease in yield, and during grain-filling, a 10% decrease in yield. A temperature exceeding 30°C during any single day during flowering resulted in a decrease in yield of 15%, double that if the temperature exceeded 35°C.
These are undoubtedly large effects, and it is unclear why this topic has not been more attractive. Perhaps this is just as well, for Parent and Tardieu (2012) showed that breeding over hundreds of generations in a wide range of climates had not changed the developmental response of 17 crop species to temperature; all lines within a species had the same optimal temperature. Evidently, the genetics of growth processes in relation to temperature has been strongly conserved.
Although it is common to associate ‘heat tolerance’ with destructively high temperatures, for example the massive loss of green leaf area of a temperate crop in response to one hot day with a temperature in the high 30s, the inexorable rise in Earth’s temperature may affect crops at many developmental stages. Hunt et al. (2018) discuss examples that affect wheat before anthesis. In brief:
-
High soil temperatures at sowing accelerate the drying of the seedbed and reduce the maximum length of the short coleoptiles (50–60 mm) of common semi-dwarf wheats, thereby endangering emergence once the temperature exceeds ~22°C. Lines with alternative dwarfing genes are available in which the coleoptiles are long, but commercial breeders have been wary of using these as parents, perhaps because of a concern that the pedigree of the alternative dwarfing genes may have resulted in the presence of other, deleterious, genes.
-
Accelerated vegetative development makes flowering earlier than optimal.
-
Spike development between flag-leaf emergence and anthesis requires ~300 degree-days above a base temperature of 0°C. The higher the temperature the shorter is the time to develop the spike, and thence the lower is the grain number unless the photosynthetic rate is remarkably high. There is a robust linear relationship between yield and photothermal quotient, which is the ratio of photosynthetic radiation received by a crop and the mean temperature during this period.
A major opportunity for protecting leaves from scorching during grain-filling is to modify the structure of the canopy and the reflectance of the leaf by selecting for erect leaves with glaucous surfaces and the propensity to roll. Such leaves avoid the scorching that floppy leaves are prone to. Although alleles for these traits are present in current Australian cultivars, their frequency is probably too low for them to become pyramided during empirical selection. However, visual selection for glaucousness and canopy structure is effective and quick and can be made in early generations (Hunt et al. 2018).
It remains to be seen whether breeders see this as a significant opportunity. There has been some interest in finding quantitative trait loci (QTLs) for heat tolerance, but interest by breeders in pursuing these has not been evident. There is also a substantial literature on ‘heat-shock factors’ in plants, although it does seem to be predominantly descriptive rather than prescriptive.
Attempts to increase potential yield
Potential yield (PY) is usefully defined as ‘the measured yield of the best cultivar, grown with optimal agronomy and without manageable biotic and abiotic stresses, under natural resource and cropping system conditions representative of the target area’ (Fischer 2015).
The many attempts to increase PY by manipulating the biochemistry and physiology of photosynthesis and respiration have so far been unsuccessful. However, the reasons for this lack of success differ from those underlying the failure to ameliorate abiotic stress. The latter has predominantly involved prospecting for specific genes, creating transgenic plants using (arbitrarily?) selected genes, and then directly phenotyping those plants for salt or drought tolerance, as illustrated by the shortcut shown in Fig. 1. By contrast, research on photosynthesis has approached the challenge of increasing PY by looking for possible impediments in the cascade of processes occurring across the complete range of levels of organisation from within chloroplasts to whole plants. The difference is that research on photosynthesis has been driven by mechanistic hypotheses rather than by the empirical black-box search for associations that typifies naive transgenic research on abiotic stress.
Why then has the fundamental work on ‘improving’ photosynthesis been unsuccessful, at least so far? Two recent papers offer arguments that could explain this lack of success:
Sinclair et al. (2019) comment, in relation to grain yield, that (1) there is a substantial literature, spanning several decades, that has shown no correlation between grain yield and the photosynthetic rate of leaves; and (2) substantial increases in grain yield have depended historically on the availability and uptake of water and nutrients and their roles in the partitioning of nutritionally important metabolic products of photosynthesis (carbohydrates, proteins, oils) into the grain.
Körner (2015) has argued that growth is typically limited by the activity of meristems rather than by the availability of photosynthate. He supports this view from the influences of temperature and of drought on rates of growth and of photosynthesis; in both cases, growth falls before photosynthetic rate and is marked by an increase in non-structural carbohydrates in the plant.
To these arguments can be added:
-
The large disparities in behaviour between plants in pots and those in the field.
-
The current paucity of micrometeorological expertise for testing the behaviour of crop canopies. Tantalisingly, Richards et al. (2019) have shown, using 4-way and 8-way MAGIC populations of spring wheats grown in moderate field environments, that lines with erect leaves averaged 11% more biomass, with little effect on harvest index, than lines with floppy leaves.
-
The complexities of source–sink interactions (Sonnewald and Fernie 2018).
-
A wide range of inevitable trade-offs, as described with many examples by Sadras and Denison (2016).
-
The general lack of credibility of claims of transgenic improvement of yield, at least in wheat (Araus et al. 2019).
-
The influence of water deficits on reducing growth much more than photosynthesis (Muller et al. 2011).
-
The large influence of various soil conditions on growth, independent of changes on the water status of the leaves, and often associated with inhibitory signals from the roots (Passioura 2002).
-
The thought-provoking arguments of Thomas and colleagues that plants, including crop plants, typically have more photosynthate than they can make use of (e.g. Thomas and Sadras 2001).
How valuable is ‘big data’?
Major technological innovations have major consequences. Their inventors often develop them to solve problems that are otherwise intractable—such are their motives. However, as the appreciation of the power of these technologies spreads, fascination with them can change their role. They become seen as powerful tools to try in all sorts of novel ways. They may then become the (often inept) drivers of research, rather than the means to solve specific important problems.
What of ‘big data’ in agriculture? Is it a distracting driver or a solver? It is, of course, both. It will undoubtedly be useful in many ways, given, for example, the availability of increasingly cheap sensors for monitoring environmental variables that can inform better management. It may help breeders to select better genotypes through, for example, the use of machine learning for selecting optimal combinations of flowering genes for targeted environments, or by making use of a variety of sensors in the field to monitor a wide diversity of breeding lines from which desirable alleles may be accrued (Rebetzke et al. 2019).
In relation to translational research from controlled environments, its main attraction is in facilitating high-throughput phenotyping in the search for desirable traits. However, as Sadras (2019) has pointed out, phenotyping in controlled environments rarely leads to new cultivars because (he writes): ‘phenotyping in an unnatural context, i.e. where correlations between environmental variables have been unrealistically altered, are often of little agronomic relevance because biased relationships among the states of different environmental variables disturb the information decoded by the plant, and hence the phenotype’. Put another way, much automated phenotyping treats plants as machines rather than as systems, as discussed earlier. Little cognisance is given to how the performance of a crop, and ultimately its yield and profitability, depend on many environmental influences during the crop’s life history, influences which in turn stimulate important feedbacks, feedforwards, and requirements for trade-offs.
Concluding remarks
Major improvements in the performance of crops typically start with observations in the field. Such observations can trigger ideas which eventually lead to better agronomic techniques and better genotypes. Often, implementing these ideas needs the involvement of crop and plant physiologists who can provide better mechanistic understanding of pertinent processes. The successful improvement in salt tolerance of durum wheat, described earlier, is a good example of this. Similarly, the use of ‘functional reversion’ by Boyer and McLaughlin (2007) to identify genes responsible for floral abortion in maize is remarkable. They have not only identified the responsible genes, but have also uncovered mechanistic interactions among those genes.
By contrast, transgenic research to ameliorate abiotic stress, which begins in the laboratory rather than in the field, has been an almost complete failure. Yet, as an industry, it is large and growing rapidly, currently by ~10% per year. Why then do we remain fascinated with its possibilities? Is this because it is attractively easy to expose plants to salinity or drought in controlled environments? Is it because the glamour of increasingly powerful molecular biological techniques generates romantic enthusiasm about our being useful?
A major difficulty is that we are comfortable with the pervasive idea that translational research is linear and one-way, that we think of ourselves as engineers whose aim is to improve a ‘machine’. The rarity of reality checks in translational research is testimony to that. Common terms in this language that we use are: ‘extension’; ‘technology transfer’; ‘input’, ‘output’, ‘outcome’, ‘impact’; ‘delivering outcomes’; ‘translational research’; and ‘transformational research’. This dominant language is firmly set in our minds so that we are prone to think linearly, in big steps, from laboratory directly to field if we are ‘agricultural’ scientists, from proposals to products if we are funders. All imply that R&D produces solutions to agricultural problems, which are then ‘delivered’ to receptive farmers. Yet wide experience shows that pertinent agricultural research requires us to proceed clockwise around the sequence shown in Fig. 1, but only as far as is needed to gives us enough mechanistic understanding to translate the research into improved productivity.
This linear language that we are usually comfortable with gives no hint of the innovative richness of conversations across levels. Conversations between farmers and field scientists are especially important in fostering new ideas (Fig. 3). The two-way flow of information alerts scientists to potential problems that may need tackling, and to operational constraints that may render the scientists’ new ideas impractical. At the same time, it helps to train the intuition of the farmers by giving them a deeper understanding of the processes going on in their crops and pastures, and in their soil—for it is the activity of well-informed, inventive farmers that leads to many agricultural innovations.
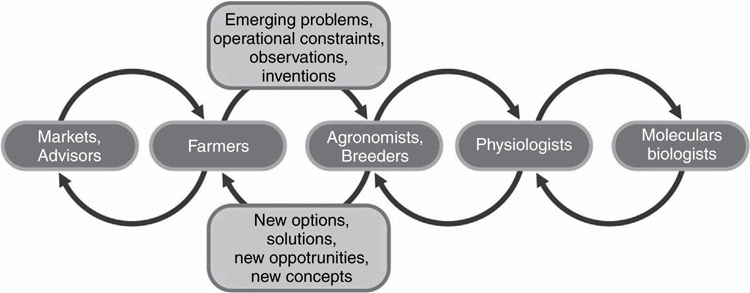
|
An outstanding example of the worth of such conversations is that of a major, 5-year initiative sponsored by the Australian Grains Research and Development Corporation (GRDC) for improving the water productivity of wheat in southern Australia by at least 10% (Kirkegaard et al. 2014). This initiative involved 16 regionally focused groups of farmers and of scientists working together on a diverse range of agronomic topics linked together by a coordinating project that enabled conversations among the 16 groups. The initiative easily exceeded its goal of a 10% increase in water productivity at the field scale, partly through making better use of water stored from summer rainfall. One of the reasons for its success is that the ideas of the scientists were monitored for practicality by the farmers, so that the proposed agronomic techniques had already been field-tested by the end of the project. An initial period of extension was therefore not needed, and the continued dissemination of the techniques is likely accelerated because of the experience of hundreds of farmers involved in the initiative, for farmers like learning from farmers.
How are major funding bodies placed against this backdrop of successes and failures? They are under pressure to encourage proposals that aim to solve major problems. This pressure is reflected in the increasing frequency of papers in the plant sciences that have an introductory paragraph on improving food security, even though there is usually no discernible connection (to an agricultural scientist) between the results shown in such papers and food security. Cassman (2016) and Cassman and Grassini (2020) have thoroughly discussed what is needed if we are to maintain food security for the next 30 years, and have lamented the poor focus in many aspects of agricultural R&D.
Research proposals that promise utility attract money from naive funders who believe that they are fostering useful research. Even the Australian Research Council (ARC) and the GRDC have been prone to such naivety, though the ARC’s new Centre of Excellence for Plant Success in Nature and Agriculture (https://www.arc.gov.au/2020-arc-centre-excellence-plant-success-nature-and-agriculture) looks to be well focused. Nevertheless, the idea of the reality check has not penetrated far into the funding process. The many plant scientists who are unused to conversing across organisational levels in search of reality checks will not spontaneously start doing so while they find it easy to attract grants.
What lies behind the rapid growth of the large global academic industry that has been pursuing, with almost no success, the use of transgenes to induce tolerance of abiotic stress in crops? Is it the positive feedback that comes from the research being welcomed in ‘high-impact’ journals? Peer review is defined strictly, in that it resides only within this industry, which is thereby protected from critical reviews by plant and crop physiologists who should also qualify as ‘peers’ if claims of utility are made. The positive feedback comes because it is common for universities to reward scientists who publish in such journals. Such behaviour exemplifies some penetrating comments by Neff (2020) on the behaviour of the publishing industry.
The way ahead must be for the major funding bodies to augment their selection panels, where necessary, with people who can effectively judge claims of utility. To do so would have a double benefit. It would select proposals with much better chances of practical success. And it would free up many other scientists across all levels of biological organisation to ask questions that are more penetrating of the materials that interest them, questions that are driven by mechanistic hypotheses rather than by empirical searches for associations. Deepening mechanistic understanding at every level remains important in agricultural research, provided that it has clear implications for higher levels of organisation in the hierarchy illustrated in Fig. 1
Conflict of Interest
The author declares no conflict of interest.
Acknowledgements
An early, short version of this article was presented at the workshop ‘Making Science Useful to Agriculture’, held in Adelaide, 26–29 November 2018 (https://msua.aweb.net.au/), with financial contributions by OECD Co-Operative Research Program Biological Resource Management for Sustainable Agricultural Systems; South Australian Grain Industry Trust Fund; and the Grains Research and Development Corporation. I am indebted to Tony Fischer, John Kirkegaard, Rana Munns, and Victor Sadras for their perceptive comments on the manuscript and to the reviewers for their valuable comments.
References
Angus JF, van Herwaarden AF (2001) Increasing water use and water use efficiency in dryland wheat. Agronomy Journal 93, 290–298.| Increasing water use and water use efficiency in dryland wheat.Crossref | GoogleScholarGoogle Scholar |
Araus JL, Serret MD, Lopes MS (2019) Transgenic solutions to increase yield and stability in wheat: shining hope or flash in the pan? Journal of Experimental Botany 70, 1419–1424.
| Transgenic solutions to increase yield and stability in wheat: shining hope or flash in the pan?Crossref | GoogleScholarGoogle Scholar | 30856274PubMed |
Boyer JS, McLaughlin JE (2007) Functional reversion to identify controlling genes in multigenic responses: analysis of floral abortion. Journal of Experimental Botany 58, 267–277.
| Functional reversion to identify controlling genes in multigenic responses: analysis of floral abortion.Crossref | GoogleScholarGoogle Scholar | 17105969PubMed |
Cassman KG (2016) Long-term trajectories: crop yields, farmland, and irrigated agriculture. Economic Review, Special Issue 2016, pp. 21–46. Federal Reserve Bank of Kansas City, MO, USA. Available at: https://www.kansascityfed.org/~/media/files/publicat/econrev/econrevarchive/2016/si16cassman.pdf/
Cassman KG, Grassini P (2020) A global perspective on sustainable intensification research. Nature Sustainability 3, 262–268.
| A global perspective on sustainable intensification research.Crossref | GoogleScholarGoogle Scholar |
Cornish P, Murray G (1989) Low rainfall rarely limits wheat yields in southern New South Wales. Australian Journal of Experimental Agriculture 29, 77–83.
| Low rainfall rarely limits wheat yields in southern New South Wales.Crossref | GoogleScholarGoogle Scholar |
Delhaize E, Ma JF, Ryan PR (2012) Transcriptional regulation of aluminium tolerance genes. Trends in Plant Science 17, 341–348.
| Transcriptional regulation of aluminium tolerance genes.Crossref | GoogleScholarGoogle Scholar | 22459757PubMed |
Donald CM, Hamblin J (1976) The biological yield and harvest index of cereals as agronomic and plant breeding criteria. Advances in Agronomy 28, 361–405.
| The biological yield and harvest index of cereals as agronomic and plant breeding criteria.Crossref | GoogleScholarGoogle Scholar |
Fischer RA (1979) Growth and water limitations to dryland wheat yield in Australia; a physiological framework. Journal of the Australian Institute of Agricultural Science 45, 83–95.
Fischer RA (2015) Definitions and determination of crop yield, yield gaps and of rates of change. Field Crops Research 182, 9–18.
| Definitions and determination of crop yield, yield gaps and of rates of change.Crossref | GoogleScholarGoogle Scholar |
Flohr BM, Hunt JR, Kirkegaard JA, Evans JR (2017) Water and temperature stress define the optimal flowering period for wheat in south-eastern Australia. Field Crops Research 209, 108–119.
| Water and temperature stress define the optimal flowering period for wheat in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
French RJ, Schultz JE (1984a) Water use efficiency of wheat in a Mediterranean type environment. I. The relation between yield, water use and climate. Australian Journal of Agricultural Research 35, 743–764.
| Water use efficiency of wheat in a Mediterranean type environment. I. The relation between yield, water use and climate.Crossref | GoogleScholarGoogle Scholar |
French RJ, Schultz JE (1984b) Water use efficiency of wheat in a Mediterranean type environment. II. Some limitations to efficiency. Australian Journal of Agricultural Research 35, 765–775.
| Water use efficiency of wheat in a Mediterranean type environment. II. Some limitations to efficiency.Crossref | GoogleScholarGoogle Scholar |
Gilliham M, Able JA, Roy SJ (2017) Translating knowledge about abiotic stress tolerance to breeding programmes. The Plant Journal 90, 898–917.
| Translating knowledge about abiotic stress tolerance to breeding programmes.Crossref | GoogleScholarGoogle Scholar | 27987327PubMed |
Gonsalves D (1998) Control of papaya ringspot virus in papaya: a case study. Annual Review of Phytopathology 36, 415–437.
| Control of papaya ringspot virus in papaya: a case study.Crossref | GoogleScholarGoogle Scholar | 15012507PubMed |
González FG, Rigalli N, Miranda PV, Romagnoli M, Otegui ME, Ribichich KF, Trucco F, Portapila M, Otegui ME, Chan RL (2020) An interdisciplinary approach to study the performance of second-generation genetically modified crops in field trials: a case study with soybean and wheat carrying the sunflower HaHB4 transcription factor. Frontiers in Plant Science 11, 178
| An interdisciplinary approach to study the performance of second-generation genetically modified crops in field trials: a case study with soybean and wheat carrying the sunflower HaHB4 transcription factor.Crossref | GoogleScholarGoogle Scholar | 32210989PubMed |
Hall AJ, Richards RA (2013) Prognosis for genetic improvement of yield potential and water-limited yield of major grain crops. Field Crops Research 143, 18–33.
| Prognosis for genetic improvement of yield potential and water-limited yield of major grain crops.Crossref | GoogleScholarGoogle Scholar |
Hochman Z, Gobbett DL, Horan H (2017) Climate trends account for stalled wheat yields in Australia since 1990. Global Change Biology 23, 2071–2081.
| Climate trends account for stalled wheat yields in Australia since 1990.Crossref | GoogleScholarGoogle Scholar | 28117534PubMed |
Hunt JR, Hayman PT, Richards RA, Passioura JB (2018) Opportunities to reduce heat damage in rain-fed wheat crops based on plant breeding and agronomic management. Field Crops Research 224, 126–138.
| Opportunities to reduce heat damage in rain-fed wheat crops based on plant breeding and agronomic management.Crossref | GoogleScholarGoogle Scholar |
Hunt J, Kirkegaard JA, Celestina C, Porker K (2019a) Transformational agronomy: restoring the role of agronomy in modern agricultural research. In ‘Australian agriculture in 2020, from conservation to automation’. Ch. 23. (Eds J Pratley, J Kirkegaard) pp. 373–388. (Agronomy Australia and Charles Sturt University: Wagga Wagga, NSW) Available at: http://agronomyaustraliaproceedings.org/index.php/special-publications
Hunt JR, Lilley JM, Trevaskis B, Flohr BM, Peake A, Fletcher A, Zwart AB, Gobbett D, Kirkegaard JA (2019b) Early sowing systems can boost Australian wheat yields despite recent climate change. Nature Climate Change 9, 244–247.
| Early sowing systems can boost Australian wheat yields despite recent climate change.Crossref | GoogleScholarGoogle Scholar |
James RA, Blake C, Zwart AB, Hare RA, Rathjen AJ, Munns R (2012) Impact of ancestral wheat sodium exclusion genes Nax1 and Nax2 on grain yield of durum wheat on saline soils. Functional Plant Biology 39, 609–618.
| Impact of ancestral wheat sodium exclusion genes Nax1 and Nax2 on grain yield of durum wheat on saline soils.Crossref | GoogleScholarGoogle Scholar | 32480813PubMed |
Kirkegaard JA, Hunt JR (2010) Increasing productivity by matching farming system management and genotype in water-limited environments. Journal of Experimental Botany 61, 4129–4143.
| Increasing productivity by matching farming system management and genotype in water-limited environments.Crossref | GoogleScholarGoogle Scholar | 20709725PubMed |
Kirkegaard JA, Gardner PA, Angus JF, Koetz E (1994) Effect of brassica break crops on the growth and yield of wheat. Australian Journal of Agricultural Research 45, 529–545.
| Effect of brassica break crops on the growth and yield of wheat.Crossref | GoogleScholarGoogle Scholar |
Kirkegaard JA, Hunt JR, McBeath TM, Lilley JM, Moore A, Verburg K, Robertson M, Oliver Y, Ward PR, Milroy S, Whitbread AM (2014) Improving water productivity in the Australian grains industry—a nationally coordinated approach. Crop & Pasture Science 65, 583–601.
| Improving water productivity in the Australian grains industry—a nationally coordinated approach.Crossref | GoogleScholarGoogle Scholar |
Körner C (2015) Paradigm shift in plant growth control. Current Opinion in Plant Biology 25, 107–114.
| Paradigm shift in plant growth control.Crossref | GoogleScholarGoogle Scholar | 26037389PubMed |
Morran S, Eini O, Pyvovarenko T, Parent B, Singh R, Ismagul A, Eliby S, Shirley N, Langridge P, Lopato S (2011) Improvement of stress tolerance of wheat and barley by modulation of expression of DREB/CBF factors. Plant Biotechnology Journal 9, 230–249.
| Improvement of stress tolerance of wheat and barley by modulation of expression of DREB/CBF factors.Crossref | GoogleScholarGoogle Scholar | 20642740PubMed |
Muller B, Pantin F, Genard M, Turc O, Freixes S, Piques M, Gibon Y (2011) Water deficits uncouple growth from photosynthesis, increase C content, and modify the relationships between C and growth in sink organs. Journal of Experimental Botany 62, 1715–1729.
| Water deficits uncouple growth from photosynthesis, increase C content, and modify the relationships between C and growth in sink organs.Crossref | GoogleScholarGoogle Scholar | 21239376PubMed |
Munns R, James RA, Xu B, Athman A, Conn SJ, Jordans C, Byrt CS, Hare RA, Tyerman SD, Tester M, Plett D, Gilliham M (2012) Wheat grain yield on saline soils is improved by an ancestral transporter gene. Nature Biotechnology 30, 360–364.
| Wheat grain yield on saline soils is improved by an ancestral transporter gene.Crossref | GoogleScholarGoogle Scholar | 22407351PubMed |
Munns R, Passioura JB, Colmer TD, Byrt CS (2020) Osmotic adjustment and energy limitations to plant growth in saline soil. Tansley Insight. New Phytologist 225, 1091–1096.
| Osmotic adjustment and energy limitations to plant growth in saline soil. Tansley Insight.Crossref | GoogleScholarGoogle Scholar | 31006123PubMed |
Neff MW (2020) How academic science gave its soul to the publishing industry. Issues in Science and Technology 36, 35–43.
Nix HA, Fitzpatrick EA (1969) An index of crop water stress related to wheat and grain sorghum yields. Agricultural Meteorology 6, 321–337.
| An index of crop water stress related to wheat and grain sorghum yields.Crossref | GoogleScholarGoogle Scholar |
Parent B, Tardieu F (2012) Temperature responses of developmental processes have not been affected by breeding in different ecological areas for 17 crop species. New Phytologist 194, 760–774.
| Temperature responses of developmental processes have not been affected by breeding in different ecological areas for 17 crop species.Crossref | GoogleScholarGoogle Scholar | 22390357PubMed |
Passioura JB (1972) Effect of root geometry on the yield of wheat growing on stored water. Australian Journal of Agricultural Research 23, 745–752.
| Effect of root geometry on the yield of wheat growing on stored water.Crossref | GoogleScholarGoogle Scholar |
Passioura JB (1977) Grain yield, harvest index, and water use of wheat. Journal of the Australian Institute of Agricultural Science 43, 117–121.
Passioura JB (1999) Can we bring about a perennially peopled and productive countryside? Agroforestry Systems 45, 411–421.
| Can we bring about a perennially peopled and productive countryside?Crossref | GoogleScholarGoogle Scholar |
Passioura JB (2002) Soil conditions and plant growth. Plant, Cell & Environment 25, 311–318.
| Soil conditions and plant growth.Crossref | GoogleScholarGoogle Scholar |
Passioura JB (2010) Scaling up, the essence of effective agricultural research. Functional Plant Biology 37, 585–591.
| Scaling up, the essence of effective agricultural research.Crossref | GoogleScholarGoogle Scholar |
Perry MW (1980) The Three Tonne Club, pioneering a new cropping era? Journal of the Department of Agriculture, Western Australia 21, 113–114.
Petrie JR, Shrestha P, Mansour MP, Nichols PD, Liu Q, Singh SP (2010) Metabolic engineering of omega-3 long-chain polyunsaturated fatty acids in plants using an acyl-CoA Δ6-desaturase with ω3-preference from the marine microalga Micromonas pusilla. Metabolic Engineering 12, 233–240.
| Metabolic engineering of omega-3 long-chain polyunsaturated fatty acids in plants using an acyl-CoA Δ6-desaturase with ω3-preference from the marine microalga Micromonas pusilla.Crossref | GoogleScholarGoogle Scholar | 20004733PubMed |
Pratley J, Kirkegaard J (2019) From conservation to automation in the search for sustainability. In ‘Australian agriculture in 2020: from conservation to automation’. Ch. 26. (Eds J Pratley, J Kirkegaard) pp. 419–435. (Agronomy Australia and Charles Sturt University: Wagga Wagga, NSW) Available at: http://agronomyaustraliaproceedings.org/index.php/special-publications
Rebetzke GJ, Jimenez-Berni J, Fischer RA, Deery DM, Smith DJ (2019) High-throughput phenotyping to enhance the use of crop genetic resources. Plant Science 282, 40–48.
| High-throughput phenotyping to enhance the use of crop genetic resources.Crossref | GoogleScholarGoogle Scholar | 31003610PubMed |
Richards RA, Cavanagh CR, Riffkin P (2019) Selection for erect canopy architecture can increase yield and biomass of spring wheat. Field Crops Research 244, 107649
| Selection for erect canopy architecture can increase yield and biomass of spring wheat.Crossref | GoogleScholarGoogle Scholar |
Sadras VO (2019) Effective phenotyping applications require matching trait and platform and more attention to theory. Frontiers in Plant Science 10, 1339
| Effective phenotyping applications require matching trait and platform and more attention to theory.Crossref | GoogleScholarGoogle Scholar | 31695718PubMed |
Sadras VO, Denison RF (2016) Neither crop genetics nor crop management can be optimised. Field Crops Research 189, 75–83.
| Neither crop genetics nor crop management can be optimised.Crossref | GoogleScholarGoogle Scholar |
Sadras VO, Alston J, Aphalo P, Connor D, Denison DR, Fischer T, Gray R, Hayman P, Kirkegaard J, Kirchmann H, Kropff M, Lafitte R, Langridge P, Lenne J, Mínguez MI, Passioura JB, Porter JR, Reeves T, Rodriguez D, Ryan M, Villalobos FJ, Wood D (2020) Making science more useful for agriculture. Advances in Agronomy,
| Making science more useful for agriculture.Crossref | GoogleScholarGoogle Scholar | In press
Sinclair TR, Rufty TW, Lewis RS (2019) Increasing photosynthesis, unlikely solution for world food problem. Trends in Plant Science 24, 1032–1039.
| Increasing photosynthesis, unlikely solution for world food problem.Crossref | GoogleScholarGoogle Scholar | 31488354PubMed |
Sonnewald U, Fernie AR (2018) Next-generation strategies for understanding and influencing source–sink relations in crop plants. Current Opinion in Plant Biology 43, 63–70.
| Next-generation strategies for understanding and influencing source–sink relations in crop plants.Crossref | GoogleScholarGoogle Scholar | 29428477PubMed |
Stokes DE (1997) ‘Pasteur’s quadrant: basic science and technological innovation.’ (Brookings Institution Press: Washington, DC, USA)
Telfer P, Edwards J, Kuchel H, Reinheimer J, Bennett D (2013) Heat stress tolerance of wheat. Update Papers 7 February 2013. GRDC, Barton, ACT. Available at: https://grdc.com.au/resources-and-publications/grdc-update-papers/tab-content/grdc-update-papers/2013/02/heat-stress-tolerance-of-wheat (accessed 20 May 2020)
Thomas H, Sadras VO (2001) The capture and gratuitous disposal of resources by plants. Functional Ecology 15, 3–12.
| The capture and gratuitous disposal of resources by plants.Crossref | GoogleScholarGoogle Scholar |
van Herwaarden AF, Farquhar GD, Angus JF, Richards RA, Howe GN (1998) ‘Haying-off’ the negative grain yield response of dryland wheat to nitrogen fertiliser I. Biomass grain yield and water use. Australian Journal of Agricultural Research 49, 1067–1082.
| ‘Haying-off’ the negative grain yield response of dryland wheat to nitrogen fertiliser I. Biomass grain yield and water use.Crossref | GoogleScholarGoogle Scholar |
Weiss PA (1969) The living system, determinism stratified. In ‘Beyond reductionism’. (Eds A Koestler, JR Smythies) (Hutchinson: London)
Xue Z-Y, Zhi D-Y, Xue G-P, Zhang H, Zhao Y-X, Xia G-M (2004) Enhanced salt tolerance of transgenic wheat (Triticum aestivum L.) expressing a vacuolar Na+/H+ antiporter gene with improved grain yields in saline soils in the field and a reduced level of leaf Na+. Plant Science 167, 849–859.
| Enhanced salt tolerance of transgenic wheat (Triticum aestivum L.) expressing a vacuolar Na+/H+ antiporter gene with improved grain yields in saline soils in the field and a reduced level of leaf Na+.Crossref | GoogleScholarGoogle Scholar |
John Passioura graduated with a bachelor’s degree in agricultural science (1958) followed by a Ph.D. in soil chemistry (1963) from Melbourne University, Australia. He joined CSIRO in 1966 after 3 years as a Postdoc in Europe. He currently holds an emeritus appointment at CSIRO Agriculture in Canberra, and was formerly Leader of the Crop Adaptation Program there. His research has ranged over: soil chemistry and physics (transport of water and nutrients in soil and uptake by roots); plant physiology (water relations, drivers of growth rate and adaptation to abiotic stresses); and wheat pre-breeding and agronomy directed at improving water-limited productivity of dryland crops. He was elected Fellow of the Australian Academy of Science in 1994. He spent 6 years on partial secondment to the Australian Grains Research and Development Organization (GRDC) where he oversaw a portfolio of projects on soil and water management which aimed at improving both the productivity and environmental performance of Australian grain farms. Since then he has written several reviews relating to crop productivity and the pursuit of effective agricultural research. He has also worked as a consultant to the CGIAR, undertaking high-level reviews of several of their programs, existing or prospective.


