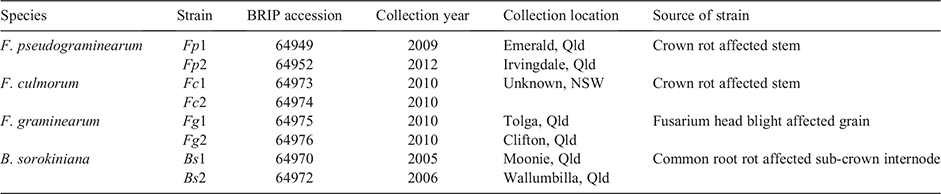
Comparison of disease severity caused by four soil-borne pathogens in winter cereal seedlings
Ahmed Saad A , Bethany Macdonald B , Anke Martin A , Noel L. Knight A C and Cassandra Percy
A , Bethany Macdonald B , Anke Martin A , Noel L. Knight A C and Cassandra Percy  A D
A D
A Centre for Crop Health, University of Southern Queensland, Toowoomba, Qld 4350, Australia.
B Leslie Research Facility, Queensland Department of Agriculture and Fisheries, Toowoomba, Qld 4350, Australia.
C Current address: Centre for Crop and Disease Management, School of Molecular and Life Sciences, Curtin University, Bentley, WA 6102, Australia.
D Corresponding author. Email: cassy.percy@usq.edu.au
Crop and Pasture Science 72(5) 325-334 https://doi.org/10.1071/CP20245
Submitted: 12 July 2020 Accepted: 15 March 2021 Published: 20 May 2021
Journal Compilation © CSIRO 2021 Open Access CC BY
Abstract
In Australia, crown rot of cereals is predominantly caused by Fusarium pseudograminearum and Fusarium culmorum, and common root rot by Bipolaris sorokiniana. Fusarium graminearum is an important pathogen causing Fusarium head blight worldwide and has also been reported to cause crown rot of wheat. The comparative ability of F. pseudograminearum, F. culmorum, F. graminearum and B. sorokiniana to cause crown rot and common root rot across a range of winter cereal species requires investigation. In glasshouse trials, we inoculated one cultivar each of barley, bread wheat, durum wheat, oat and triticale with two strains of each of the four pathogens. At 21 days after inoculation, the sub-crown internode and leaf sheaths of each plant were visually rated for brown discoloration. Shoot length and dry weight of inoculated plants were compared with those of non-inoculated controls. Barley and bread wheat had the highest disease severity ratings on leaf sheaths and sub-crown internode (64.7–99.6%), whereas oat had the lowest disease severity ratings across all pathogens (<5%). The shoot length of all cultivars was significantly reduced (by 12.2–55%, P < 0.05) when exposed to F. pseudograminearum. This study provides a comparison of pathogenicity of crown rot and common root rot pathogens and demonstrates significant variation in visual discoloration and host response across a range of winter cereals.
Keywords: barley, Bipolaris, crown rot, Fusarium, oat, triticale, wheat.
Introduction
Crown rot and common root rot of cereals are of key economic significance worldwide (Kumar et al. 2002; Kazan and Gardiner 2018). In Australia, crown rot results in estimated annual losses of AU$21 million and $79 million for the barley and bread wheat industries, respectively, and common root rot results in estimated annual losses of $13 million and $30 million to these industries (Murray and Brennan 2009, 2010).
Fusarium pseudograminearum is the predominant fungus associated with crown rot of cereals in Australia (Burgess et al. 1975; Backhouse et al. 2004), whereas Bipolaris sorokiniana causes seedling blight and the disease common root rot (Wildermuth 1986). Fusarium culmorum also occurs across the Australian grain belt and has been described as the dominant crown rot pathogen in cooler, high-rainfall areas of South Australia and Victoria (Backhouse and Burgess 2002; Backhouse et al. 2004). Fusarium graminearum has been associated with crown rot in the USA (Dyer et al. 2009), South America (Moya-Elizondo et al. 2015) and China (Zhang et al. 2015). In Australia, F. graminearum has been reported to cause epidemics of Fusarium head blight (Burgess et al. 1975; Obanor et al. 2013), and although F. graminearum strains may cause crown rot of wheat under artificial conditions, they were less aggressive than F. pseudograminearum strains (Akinsanmi et al. 2004). Fusarium graminearum has been divided into 16 phylogenetically distinct species (Aoki et al.2012), with F. graminearum sensu stricto considered the most important Fusarium head blight pathogen worldwide (Gale et al. 2007; Backhouse 2014). Other Fusarium species associated with winter cereals include F. avenaceum, F. crookwellense and F. poae; however, these fungi are infrequently isolated from crown rot diseased tissue (Backhouse et al. 2004; Obanor and Chakraborty 2014). Bipolaris sorokiniana and Fusarium species such as F. pseudograminearum, F. culmorum and F. graminearum have been reported to occur together in the same paddocks (Smiley et al. 2005; Moya-Elizondo et al. 2011).
Crown rot and common root rot pathogens have been isolated from all small grain and winter cereals including barley (Hordeum vulgare), bread wheat (Triticum aestivum), durum wheat (Triticum turgidum var. durum), oat (Avena sativa) and triticale (× Triticosecale) (Burgess et al. 2001; Backhouse and Burgess 2002; Kumar et al. 2002). In Australia, extensive research on crown rot has focused on F. pseudograminearum. Commercial bread wheat and barley cultivars range from moderately susceptible to very susceptible to F. pseudograminearum (Sturgess 2014; Lush et al. 2018). Commercial durum wheat cultivars are considered susceptible to very susceptible to F. pseudograminearum (Lush et al. 2018). Limited research on varietal rankings has been conducted on other winter cereals. Three triticale genotypes (Ningadhu, Hawkeye and Berkshire) have been reported as susceptible to F. pseudograminearum (Klein et al. 1989; Knight and Sutherland 2017), and low or zero levels of disease have been measured in oat cultivars following inoculation with F. pseudograminearum (Nelson and Burgess 1994; Burgess et al. 2001; Percy et al. 2012). Further to varietal rankings, research into F. pseudograminearum has included the infection process of the fungus (Burgess et al. 1975; Wildermuth and McNamara 1994; Percy et al. 2012; Knight and Sutherland 2016), genetic resistance including testing genetic structures, aggressiveness and toxigenicity of F. pseudograminearum populations (Bentley et al. 2008; Obanor and Chakraborty 2014; Khudhair et al. 2019), and yield loss in barley and bread wheat cultivars (Klein et al. 1989; Liu et al. 2012; Kirkegaard et al. 2004).
Barley and wheat cultivars have a wide range of responses to infection with the common root rot pathogen B. sorokiniana (Wildermuth et al. 1992). In Australia, commercial bread wheat cultivars range from moderately resistant to very susceptible (Lush et al. 2018), and significant yield losses have been reported (Wildermuth et al. 1992). The yield loss in susceptible cultivars ranged from 13.9% to 23.9%, whereas yield losses in partially resistant cultivars ranged from 6.8% to 13.6% (Wildermuth et al. 1992). Wildermuth and McNamara (1991) reported an increase in B. sorokiniana populations under wheat, barley and triticale, indicating the potential for these crops to maintain or increase inoculum levels.
The symptoms of crown rot and common root rot disease are similar, and therefore difficult to distinguish without conducting pathogen isolation and identification tests. Crown rot symptoms caused by different Fusarium species are also indistinguishable. The symptoms of crown rot start as small necrotic lesions on the coleoptile, followed by a browning of the sub-crown internode and leaf sheath tissue (Burgess et al. 2001). The first obvious symptom of crown rot in the field is browning of stem bases, which is usually observed after flowering (Burgess et al. 2001). Subsequent discoloration can reach up to the fifth node in stem tissue (Butler 1961; Burgess et al. 2001). Similar to crown rot, common root rot symptoms first appear as small brown necrotic lesions on the coleoptile and roots (Wegulo and Klein 2010). As the disease progresses, lesions also develop on the sub-crown internode and lower parts of the leaf sheaths and the stem (Burrage and Tinline 1960). The sub-crown internode has typically been used for rating common root rot disease, whereas leaf sheaths and stems have been used for rating crown rot (Wildermuth et al. 1992; Burgess et al. 2001). A strong association has been observed between sub-crown internode browning and resistance to common root rot in barley and wheat inoculated with B. sorokiniana (Wildermuth et al. 1992). This has not been demonstrated for F. pseudograminearum crown rot of wheat (Wildermuth and McNamara 1994; Percy et al. 2012).
Although multiple fungal species can be associated with crown rot and common root rot, and barley, bread wheat, durum wheat, oat and triticale have been reported as potential hosts of these fungal species, the comparative ability of these fungi to cause significant crown and common root rot disease across these winter cereal species has not been examined in detail. Knight and Sutherland (2017) and Hollaway et al. (2013) compared the visual discoloration caused by a single strain of each of F. pseudograminearum and F. culmorum across a range of winter cereal species. In these studies, F. culmorum caused discoloration on winter cereals the same as or less than F. pseudograminearum. Furthermore, F. graminearum has not been considered an important pathogen causing crown rot in Australia (Akinsanmi et al. 2004). However, with a changing climate, F. graminearum could be a significant pathogen causing crown rot on hosts such as barley, bread wheat, durum wheat, oat and triticale. Thus, the present study aimed to examine the visual discoloration caused by four crown rot and common root rot pathogens (F. pseudograminearum, F. culmorum, F. graminearum and B. sorokiniana) on leaf sheaths and sub-crown internodes of single, commercially important cultivars of barley, bread wheat, durum wheat, oat and triticale. This study further examined additional host responses to inoculation with the four pathogens through shoot length and dry weight measurements of each cereal species. Knowledge of the disease-causing abilities of each pathogen species informs strategies for disease management in crop rotations and future breeding goals.
Materials and methods
Strains and inoculum preparation
Two strains of each pathogen (F. pseudograminearum, F. culmorum, F. graminearum and B. sorokiniana) were used for inoculations (Table 1). Colonised grain inoculum was produced using a modified method described by Malligan (2009) and Percy et al. (2012). A single spore from each strain was grown on Czapek–Dox agar (CZA) (Leslie and Summerell 2008) and incubated for 7 days at 25°C for the Fusarium species and 22°C for B. sorokiniana. Mycelium was scraped off two CZA plates for each strain and mixed into individual bags (1 kg) of a mix of sterilised (autoclaved twice) grains of bread wheat (650 g) and barley (350 g) and incubated at 25°C in the dark. After 7 days, the bags were shaken manually every 2 or 3 days over 21 days to encourage uniform colonisation of the grain. After the 28-day period, colonised grain was air-dried between sheets of blotting paper and subsequently kept in the dark at 25°C for a further 14 days and stirred every 2 days. Individual inocula were then ground for ~10 s in an electric hammer mill (Christy and Norris 8″ Lab Mill; Christy Turner, Ipswich, UK) to pass through a 2-mm sieve. All inoculum bags were sealed and stored at 4°C for future use.
|
Plant growth and inoculation
Two replicated seedling tests were conducted in a glasshouse at the Leslie Research Facility, Department of Agriculture and Fisheries, Toowoomba, Queensland. The plant growth medium consisted of self-mulching black Vertosol of the Irving clay soil association, obtained from the Darling Downs, Queensland (Thompson and Beckmann 1959), mixed with river sand (50% sand:50% soil). This mixture was steam-sterilised at 80°C for 40 min and air-dried for 7 days. No fertiliser was added to the mix. The two seedling tests were planted on 5 April and 5 May 2016 (Australian autumn). Three replicate pots each of barley (cv. Grimmett, moderately susceptible to F. pseudograminearum) (Sturgess 2014; Lush et al. 2018), bread wheat (cv. Livingston, susceptible to B. sorokiniana and F. pseudograminearum) (Lush et al. 2018), durum wheat (cv. Hyperno, moderately resistant to moderately susceptible to B. sorokiniana and susceptible to very susceptible to F. pseudograminearum) (Lush et al. 2018), oat (cv. Genie) and triticale (cv. Endeavour) were inoculated individually with two strains of each of the four pathogens. A non-inoculated control treatment was also included for each cereal species. The two experiments were arranged as a randomised complete block design, where each treatment (combination of pathogen, strain and cultivar) was randomly allocated to a pot within each replicate block. The seedling inoculation method described by Wildermuth and McNamara (1994) was used with slight modifications. Briefly, moist soil (280 g, 38% moisture content) was added to pots (5 cm by 5 cm by 10 cm). Fifteen seeds were planted at a depth of 5.5 cm from the top of the pot and covered with a layer of sieved dry soil (160 g). Inoculum (0.45 g) was applied in an even layer to the soil surface of all pots except the non-inoculated control. The inoculum was covered with dry soil (40 g). All pots were placed in a water bath in a glasshouse at 25°C with natural daylengths. The inoculum was activated after 7 days by watering each pot to field capacity (38% moisture content by weight), after which the pots were watered daily up to field capacity. Plants were harvested 21 days after planting, and 10 plants from each pot were rated for disease severity and assessed for shoot length and shoot dry weight.
Visual discoloration ratings
The severity of lesions on the sub-crown internode and the first three leaf sheaths was assessed using a 0–100% rating scale based on the visual brown to black discoloration. Rating of each tissue occurred in 5% increments where 0 is no discoloration and 100% is completely discoloured tissue. Following visual discoloration ratings, all roots and the sub-crown internode were removed, and shoot length of each plant was measured from the base of the crown to the tip of the longest leaf. Individual shoots were placed in paper bags and dried in a 65°C oven (UF160; Memmert, Schwabach, Germany) for 48 h, after which dry weights were recorded.
Data analyses
The percentage values of visual discoloration on the first three leaf sheaths were added together and divided by three to give a combined leaf sheath percentage. In order to ensure homogeneity of variance, an arcsine square root transformation was applied to the sub-crown internode and the combined leaf sheath rating data. Analysis of each variable was performed using a linear mixed model. The model included fixed effects for pathogen, strain within pathogen, cultivar, experiment, and their interactions. Terms to account for the replicate blocks, pots, and plants within plots were included as random effects, with these variances estimated separately for each experiment. Estimates of variance parameters were generated using residual maximum likelihood (REML) estimation (Patterson and Thompson 1971). Predictions for each trait were generated from their respective models as empirical best linear unbiased estimators (eBLUEs). Where a transformation had been used, predicted means were back-transformed to the original scale, and approximate standard errors were calculated by using the Taylor series approximation. All analyses were performed with ASReml-R (Butler et al. 2009) in the R software environment (The R Foundation, Vienna). Significance of fixed effects was assessed using a Wald test with a significance level of 0.05.
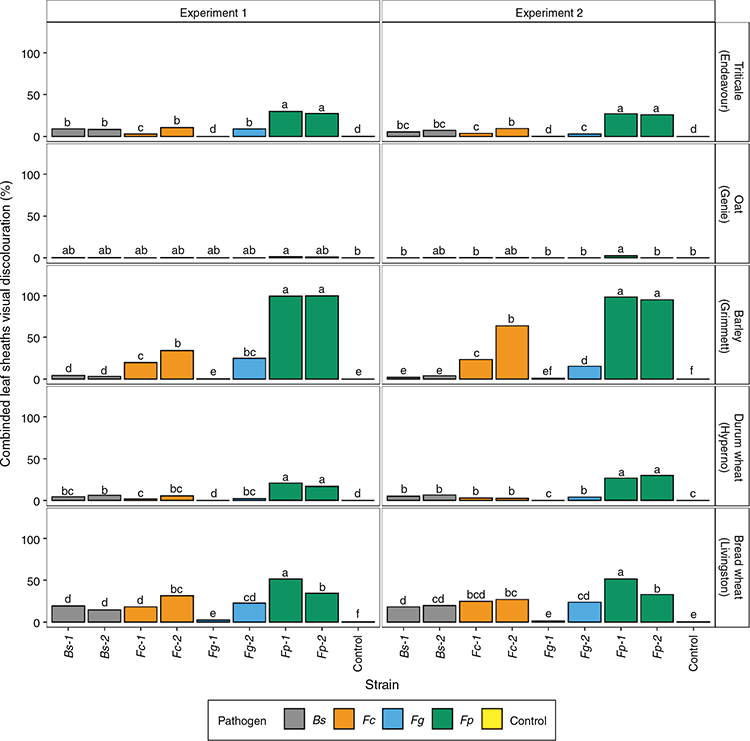
Data for visual discoloration of the leaf sheaths and the sub-crown internode have each been presented graphically in two ways to allow comparison of significant differences detected among cultivars and strains.
Results
Comparison of leaf sheath visual discoloration
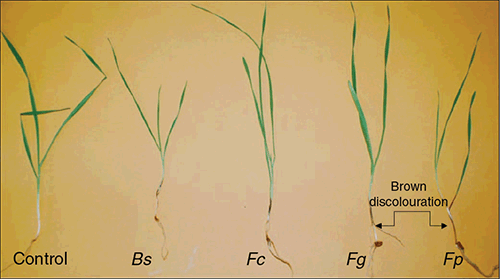
The appearance of visual symptoms caused by the four pathogens on the first three leaf sheaths of each symptomatic cultivar was similar (Fig. 1). A significant interaction of experiment, pathogen, strain and cultivar (P = 0.017) was observed in the leaf sheath ratings (Supplementary Materials, Table S1, available at the journal’s website); therefore, the results of the two experiments could not be combined for analysis and are presented individually. Fusarium pseudograminearum caused significantly greater visual discoloration than F. culmorum, F. graminearum and B. sorokiniana in barley (cv. Grimmett), triticale (cv. Endeavour) and durum wheat (cv. Hyperno) in both experiments (Fig. 2). The greatest visual discoloration rating was observed for F. pseudograminearum in Grimmett (99.6%) in both experiments. Some significant differences were observed between strains of the same pathogen; for example, strains Fc2 and Fg2 had significantly higher leaf sheath ratings than strain Fc1 and Fg1, respectively, for Grimmett, Livingston and Endeavour, and strain Fp1 had a greater leaf sheath rating than Fp2 for Livingston (Fig. 2) in both experiments.
|
For most of the Fusarium spp. strains, the visual discoloration on Grimmett leaf sheath tissue was significantly greater than on all other cereals (Fig. S1). Hyperno (0.09–30%) and Endeavour (0.002–30%) had less visual discoloration than Grimmett (0.33 to 99.6%) and Livingston (1.14–51.53%) when infected by all pathogens, whereas oat cv. Genie exhibited significantly (P < 0.05) less visual discoloration across all pathogens (0.03–2.5%) (Fig. S1). The B. sorokiniana strains caused low levels of visual discoloration (<20%) across the cultivars, with Livingston exhibiting significantly greater visual discoloration than the other cultivars.
Comparison of sub-crown internode visual discoloration
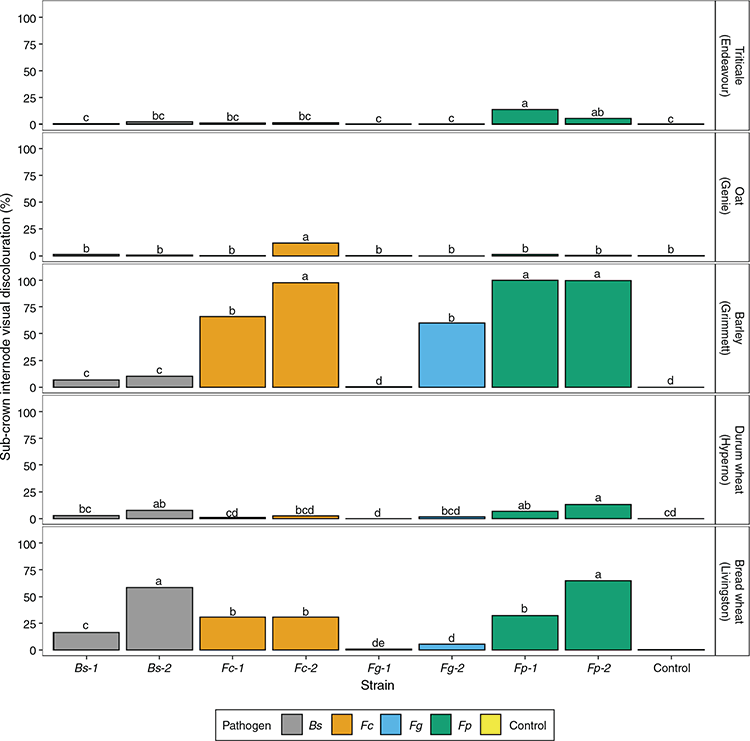
A significant (P < 0.001) pathogen × strain × cultivar interaction for the sub-crown internode rating was observed (Table S2). The greatest visual discoloration ratings were observed in Grimmett inoculated with F. pseudograminearum strains Fp1 and Fp2 (100%), F. culmorum strain Fc2 (97.6%), and F. graminearum strain Fg2 (59.8%) (Fig. 3). Sub-crown internode visual discoloration in Livingston inoculated with F. pseudograminearum strains ranged from 32.3% to 64.7%, with B. sorokiniana strains from 16.5% to 58.2%, and a 31% visual discoloration rating was observed with the F. culmorum strains (Fig. 3). Significant variation between strains was observed in sub-crown internode visual discoloration of Grimmett inoculated with F. culmorum and F. graminearum, Livingston inoculated with F. pseudograminearum and B. sorokiniana, and Genie inoculated with F. culmorum (Fig. 3).
Differences among cultivars varied depending on the pathogen and the strain. Grimmett (0.5–100%) and Livingston (0.9–64.7%) exhibited the greatest visual discoloration on the sub-crown internode, whereas Genie (0–12%) and Endeavour and Hyperno (0–13.7%) had low levels of sub-crown internode visual discoloration (Fig. S2).
Shoot length
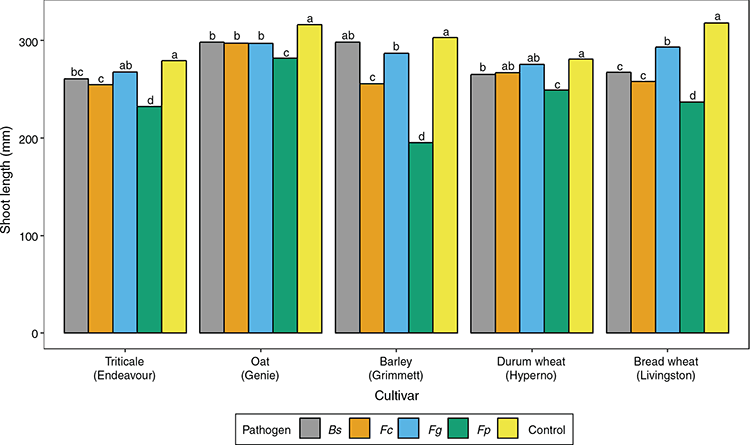
The shoot length of cultivars varied significantly (P < 0.001; Table S3) in response to pathogen inoculation (Fig. 4). In most instances, the inoculated treatments had a reduced shoot length compared with the controls. The greatest reduction in shoot length occurred in all cultivars inoculated with F. pseudograminearum, where shoot length was reduced by 12% for Genie, 13% for Hyperno, 20% for Endeavour, 34.3% for Livingston and 55% for Grimmett compared with the control (Fig. 4). Oat cultivar Genie had the lowest levels of reduction in shoot length across all pathogens (6–12%) (Fig. 4).
Shoot dry weight
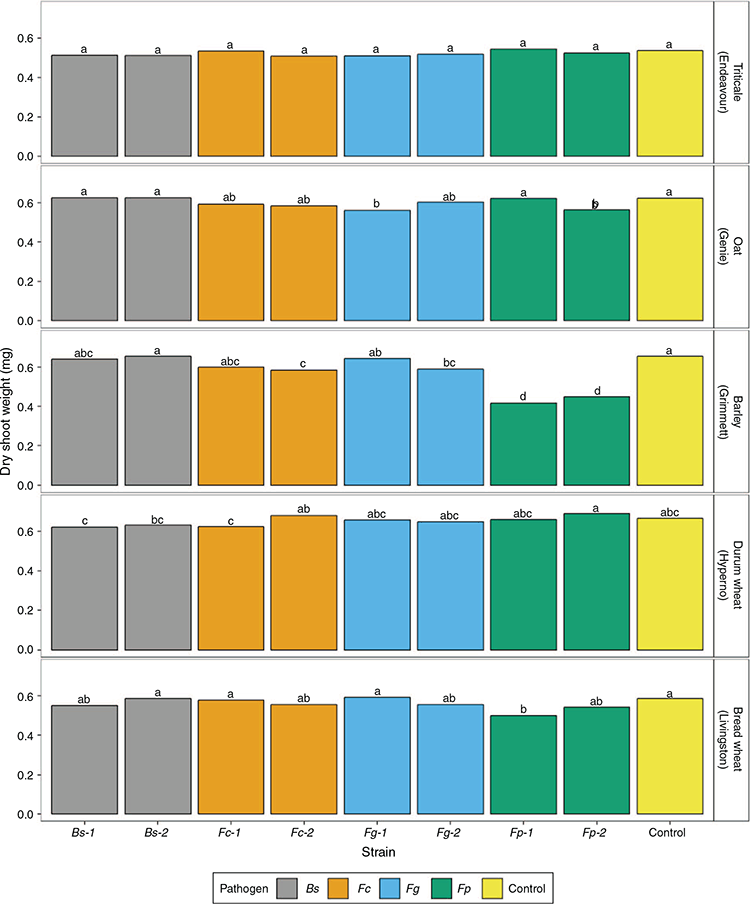
A significant interaction of cultivars and strains within the pathogens was observed for shoot dry weight (P = 0.035; Table S4). The reduction in dry shoot weight between control and inoculated plants was significant for Grimmett inoculated with both F. pseudograminearum strains (45.1–57%), Fc2 (11.6%) and Fg2 (10.7%) (Fig. 5). Genie seedlings inoculated with strains Fg1 and Fp2 also had a significant decreases of 11% and 9%, respectively, in dry shoot weight compared with the control. The dry shoot weight of Livingston was significantly lower than the control when inoculated with Fp1 (17.2%).
Discussion
Our study identified significant variation among visual discoloration ratings caused by crown rot and common root rot pathogens on leaf sheath and sub-crown internode tissues of five winter cereals. To the best of our knowledge, this is the first direct comparison of disease caused by these four pathogens across major winter cereal species. This information will be important for predicting the disease risk across these cereal species, and the potential implications of crop rotations in the presence of these pathogens.
Fusarium pseudograminearum strains caused greater visual discoloration than any of the other pathogens on Grimmett, Livingston, Hyperno and Endeavour. Similar results were observed by Knight and Sutherland (2017), who reported a comparison of visual disease symptoms on the leaf sheaths and fungal biomass of a single strain of each of F. pseudograminearum and F. culmorum in seedlings of six winter cereals (barley, durum wheat, oat, rye (Secale cereale), spring wheat and triticale) and three summer cereals (maize (Zea mays), rice (Oryza sativa) and sorghum (Sorghum bicolor)). Fusarium pseudograminearum caused greater discoloration than F. culmorum in all of the cereals except oat, rye, maize and rice, for which only minimal disease was reported.
In our study, F. graminearum caused brown discoloration on the sub-crown internode and leaf sheaths of Grimmett and Livingston at levels similar to F. culmorum but less than F. pseudograminearum. Fusarium graminearum has not historically been considered an important crown rot pathogen in Australia (Obanor and Chakraborty 2014). However, Dyer et al. (2009) and Obanor and Chakraborty (2014) suggested that the ability of F. graminearum to cause crown rot might increase in areas where Fusarium head blight is more common. In Australia, the fungus has been isolated from the crowns in areas where head blight occurred in northern New South Wales and in warm to subtropical areas with moderate to high rainfall in Queensland (Backhouse and Burgess 2002; Akinsanmi et al. 2004). The results support the potential for F. graminearum to contribute to crown rot disease in Australia under favourable conditions. However, only F. graminearum strains isolated from Fusarium head blight were available in the present study, and along with the difference in visual discoloration caused by each isolate, it suggests that further assessment with strains from crown rot infections should be investigated.
Low visual discoloration ratings were observed on the leaf sheaths of most hosts inoculated with B. sorokiniana strains, except for Livingston, which also had the greatest visual discoloration on the sub-crown internode. This finding confirms previous studies suggesting that B. sorokiniana is more effective at causing disease on the lower part of the plant (Burrage and Tinline 1960; Wildermuth et al. 1992).
Variation between strains within a pathogen species was observed for combined leaf sheath and sub-crown internode ratings in some of the cultivars. Each of the F. culmorum and F. graminearum strains caused different levels of visual discoloration across the cultivars. Fusarium pseudograminearum has been described to vary in aggressiveness between strains, depending on several factors including farming system, geographical factors, and the genetic diversity of each strain (Akinsanmi et al. 2004). A similar scenario is suggested to occur in the other Fusarium species assessed.
Low levels of visual discoloration were observed in durum wheat cv. Hyperno on the leaf sheaths and the sub-crown internode after inoculation with each of the pathogens. These results contrast with the findings of Knight and Sutherland (2017), where durum wheat cvv. EGA Bellaroi and Jandaroi had visual discoloration ratings similar to the most diseased cultivars of barley. The low level of disease visual discoloration on durum wheat in our study could be due to some cultivars exhibiting resistance in the early stages of growth, with the disease symptoms becoming more pronounced in the advanced stages. Yang et al. (2010) suggested that different genes can be responsible for crown rot resistance at early developmental stages of wheat and barley cultivars, and this resistance might disappear throughout growing stages. Liu et al. (2012) estimated the fungal biomass in barley, bread wheat and durum wheat cultivars at various times (3, 7, 14, 21, 28 and 35 days) post inoculation with F. pseudograminearum. The fungal quantities measured at the six time points of infection were characterised by three distinct phases in each of the cereals. During all stages, the durum wheat cultivar (Wollaroi, highly susceptible) exhibited late and slow fungal accumulation compared with barley cultivars, indicating in the early stages of infection, less disease may also develop. Lush et al. (2018), in ‘2018 wheat varieties, Queensland’ reported that durum wheat cultivars, including Hyperno, are considered susceptible to very susceptible to F. pseudograminearum. Data in this guide are from disease assessed at maturity (Lush et al. 2018). Although differences in the age of plants were minimal between the present study and that of Knight and Sutherland (2017), the growth conditions and cultivars did vary. The inoculation techniques could also have had an impact on disease progression. Knight and Sutherland (2017) applied 6 μL of an inoculum of 106 conidia/mL to the coleoptile at 14 days after planting, whereas we applied the inoculum as a layer above the seeds, which required activation by moisture 7 days after planting. Thus, in the present study, the coleoptile grew through the soil and the inoculum, similar to a natural field infection, compared with direct application of a conidial suspension.
Triticale cv. Endeavour had low levels of visual symptoms on the leaf sheaths and sub-crown internode compared with Grimmett and Livingston. Knight and Sutherland (2017) reported that triticale cvv. Hawkeye and Berkshire had a high level of visual discoloration, with responses to a single strain of each of F. pseudograminearum and F. culmorum similar to barley, durum wheat and spring wheat cultivars. This difference may be due to the different inoculation methods used or genetic variation among cultivars.
Oat is considered a resistant or an asymptomatic host of F. pseudograminearum (Percy et al. 2012; Knight and Sutherland 2017). Low levels of visual discoloration, still significantly greater than the controls, were observed on the sub-crown internode of Genie when inoculated with one strain of F. culmorum and on the leaf sheaths when inoculated with one strain of F. pseudograminearum. Although disease levels were low, the capacity of oats to exhibit discoloration after inoculation with F. pseudograminearum and F. culmorum supports recommendations that oat should not be used as a rotational crop for crown rot caused by F. pseudograminearum (Nelson and Burgess 1994) or for common root rot management (Wildermuth and McNamara 1991).
The physiological impact of disease caused by these four crown rot and common root rot pathogens has not been extensively detailed. Fusarium pseudograminearum resulted in the greatest reduction of shoot length across all cultivars. Grimmett and Livingston had significant shoot length reductions across all pathogens, except in the case of B. sorokiniana with Grimmett. Oat cv. Genie had low or no symptoms; however, there was a significant reduction in the shoot length of Genie across all pathogens (P < 0.05). The reduction in shoot length indicates that each pathogen had a negative effect on the development of the host. Similarly, Smiley et al. (2005) indicated that visual discoloration was negatively correlated with plant height for F. pseudograminearum, F. culmorum and F. graminearum but not for B. sorokiniana.
Differences in host shoot dry weight were observed but varied according to pathogen or strain. Strain Fp1 significantly reduced dry shoot weight in Grimmett and Livingston, whereas Fg1 significantly decreased dry shoot weight in Genie (P < 0.05). This outcome contrasts with the findings of Knight et al. (2012), who indicated a significant increase in dry weight of the individual leaf sheath up to the fourth leaf for four bread wheat cultivars colonised by F. pseudograminearum, compared with non-inoculated controls. This difference between the two studies could be due to the dry shoot weight of the entire seedling, including leaf blade, being included in our study. A negative impact on the dry shoot weight of plant tissue indicates that each pathogen has a detrimental effect on plant growth; however, this level in reduction was not as significant as that for shoot length. Further assessment of plant height and weight in the field is crucial for investigating the physiological impact of these pathogens.
This study identified significant differences in visual discoloration caused by infection with F. pseudograminearum, F. culmorum, F. graminearum and B. sorokiniana in five winter cereals species. Fusarium pseudograminearum caused the greatest visual discoloration on both the sub-crown internode and leaf sheaths, followed by F. culmorum, B. sorokiniana and F. graminearum. The most severe disease symptoms were observed on Grimmett and Livingston, whereas Genie showed low or no symptoms. Significant differences were observed in the host response (shoot dry weight and shoot length) to all pathogens, with the reduction in shoot length being more significant than the reduction in shoot weight. The reactions observed across the cereal hosts demonstrate the comparative disease impacts of each of these fungi, which will inform improved management strategies for crown rot and common root rot diseases by crop rotation. A field test will facilitate further investigation of the impact of these four pathogens on the five winter cereals at different stages of plant growth.
Conflicts of interest
The authors declare no conflict of interest.
Funding statement
This research was partly funded by the Grains Research and Development Corporation project DAN00175. The first author was supported by a USQ Postgraduate Research Scholarship and the Queensland Government.
Acknowledgements
The authors thank Dr Alison Kelly, DAF Qld Biometry (SAGI), Toowoomba, for statistical guidance; Mr Tim Clewett for pasteurising the soil used in these experiments; Ms Tina Sarmon, Ms Prue Bottomley, Ms Eliza Kelly and Mr Darren Wrigley for technical assistance with laboratory and glasshouse experiments; and Dr David Backhouse from the University of New England for providing F. culmorum strains.
References
Akinsanmi OA, Mitter V, Simpfendorfer S, Backhouse D, Chakraborty S (2004) Identity and pathogenicity of Fusarium spp. isolated from wheat fields in Queensland and northern New South Wales. Australian Journal of Agricultural Research 55, 97–107.| Identity and pathogenicity of Fusarium spp. isolated from wheat fields in Queensland and northern New South Wales.Crossref | GoogleScholarGoogle Scholar |
Aoki T, Ward TJ, Kistler HC, O’Donnell K (2012) Systematics, phylogeny and trichothecene mycotoxin potential of Fusarium head blight cereal pathogens. Mycotoxins 62, 91–102.
| Systematics, phylogeny and trichothecene mycotoxin potential of Fusarium head blight cereal pathogens.Crossref | GoogleScholarGoogle Scholar |
Backhouse D (2014) Global distribution of Fusarium graminearum, F. asiaticum and F. boothii from wheat in relation to climate. European Journal of Plant Pathology 139, 161–173.
| Global distribution of Fusarium graminearum, F. asiaticum and F. boothii from wheat in relation to climate.Crossref | GoogleScholarGoogle Scholar |
Backhouse D, Burgess L (2002) Climatic analysis of the distribution of Fusarium graminearum, F. pseudograminearum and F. culmorum on cereals in Australia. Australasian Plant Pathology 31, 321–327.
| Climatic analysis of the distribution of Fusarium graminearum, F. pseudograminearum and F. culmorum on cereals in Australia.Crossref | GoogleScholarGoogle Scholar |
Backhouse D, Abubakar A, Burgess L, Dennis J, Hollaway G, Wildermuth G (2004) Survey of Fusarium species associated with crown rot of wheat and barley in eastern Australia. Australasian Plant Pathology 33, 255–261.
| Survey of Fusarium species associated with crown rot of wheat and barley in eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Bentley AR, Leslie JF, Liew EC, Burgess LW, Summerell BA (2008) Genetic structure of Fusarium pseudograminearum populations from the Australian grain belt. Phytopathology 98, 250–255.
| Genetic structure of Fusarium pseudograminearum populations from the Australian grain belt.Crossref | GoogleScholarGoogle Scholar | 18943202PubMed |
Burgess L, Wearing A, Toussoun T (1975) Surveys of Fusaria associated with crown rot of wheat in eastern Australia. Australian Journal of Agricultural Research 26, 791–799.
| Surveys of Fusaria associated with crown rot of wheat in eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Burgess LW, Backhouse D, Summerell BA, Swan LJ (2001) Crown rot of wheat. In ‘Fusarium: Paul E. Nelson Memorial Symposium’. (Eds BA Summerell, JF Leslie, D Backhouse, WL Nryden, LW Burguess) pp. 271–294. (American Phytopathological Society: St. Paul, MN, USA)
Burrage R, Tinline R (1960) Common root rot and plant development following treatments of wheat seed with aldrin, gamma BHC, and heptachlor, with and without mercury fungicides. Canadian Journal of Plant Science 40, 672–679.
| Common root rot and plant development following treatments of wheat seed with aldrin, gamma BHC, and heptachlor, with and without mercury fungicides.Crossref | GoogleScholarGoogle Scholar |
Butler F (1961) Root and foot rot diseases of wheat. Science Bulletin NSW Department of Agriculture 77, 1–98.
Butler D, Cullis BR, Gilmour A, Gogel B (2009) ‘ASReml-R reference manual.’ (Department of Primary Industries and Fisheries: Brisbane, Qld)
Dyer AT, Johnston RH, Hogg AC, Johnston JA (2009) Comparison of pathogenicity of the Fusarium crown rot (FCR) complex (F. culmorum, F. pseudograminearum and F. graminearum) on hard red spring and durum wheat. European Journal of Plant Pathology 125, 387–395.
| Comparison of pathogenicity of the Fusarium crown rot (FCR) complex (F. culmorum, F. pseudograminearum and F. graminearum) on hard red spring and durum wheat.Crossref | GoogleScholarGoogle Scholar |
Gale LR, Ward TJ, Balmas V, Kistler HC (2007) Population subdivision of Fusarium graminearum sensu stricto in the upper Midwestern United States. Phytopathology 97, 1434–1439.
| Population subdivision of Fusarium graminearum sensu stricto in the upper Midwestern United States.Crossref | GoogleScholarGoogle Scholar | 18943513PubMed |
Hollaway GJ, Evans ML, Wallwork H, Dyson CB, McKay AC (2013) Yield loss in cereals, caused by Fusarium culmorum and F. pseudograminearum, is related to fungal DNA in soil prior to planting, rainfall, and cereal type. Plant Disease 97, 977–982.
| Yield loss in cereals, caused by Fusarium culmorum and F. pseudograminearum, is related to fungal DNA in soil prior to planting, rainfall, and cereal type.Crossref | GoogleScholarGoogle Scholar | 30722534PubMed |
Kazan K, Gardiner DM (2018) Fusarium crown rot caused by Fusarium pseudograminearum in cereal crops: recent progress and future prospects. Molecular Plant Pathology 19, 1547–1562.
| Fusarium crown rot caused by Fusarium pseudograminearum in cereal crops: recent progress and future prospects.Crossref | GoogleScholarGoogle Scholar | 29105256PubMed |
Khudhair M, Thatcher L, Gardiner D, Kazan K, Roper M, Aitken E, Obanor F (2019) Comparative analysis of genetic structures and aggressiveness of Fusarium pseudograminearum populations from two surveys undertaken in 2008 and 2015 at two sites in the wheat belt of Western Australia. Plant Pathology 68, 1337–1349.
| Comparative analysis of genetic structures and aggressiveness of Fusarium pseudograminearum populations from two surveys undertaken in 2008 and 2015 at two sites in the wheat belt of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Kirkegaard JA, Simpfendorfer S, Holland J, Bambach R, Moore KJ, Rebetzke GJ (2004) Effect of previous crops on crown rot and yield of durum and bread wheat in northern NSW. Australian Journal of Agricultural Research 55, 321–334.
| Effect of previous crops on crown rot and yield of durum and bread wheat in northern NSW.Crossref | GoogleScholarGoogle Scholar |
Klein T, Burgess L, Ellison F (1989) The incidence of crown rot in wheat, barley and triticale when sown on two dates. Australian Journal of Experimental Agriculture 29, 559–563.
| The incidence of crown rot in wheat, barley and triticale when sown on two dates.Crossref | GoogleScholarGoogle Scholar |
Knight NL, Sutherland MW (2016) Histopathological assessment of Fusarium pseudograminearum colonization of cereal culms during crown rot infections. Plant Disease 100, 252–259.
| Histopathological assessment of Fusarium pseudograminearum colonization of cereal culms during crown rot infections.Crossref | GoogleScholarGoogle Scholar | 30694128PubMed |
Knight NL, Sutherland MW (2017) Assessment of Fusarium pseudograminearum and F. culmorum biomass in seedlings of potential host cereal species. Plant Disease 101, 2116–2122.
| Assessment of Fusarium pseudograminearum and F. culmorum biomass in seedlings of potential host cereal species.Crossref | GoogleScholarGoogle Scholar | 30677367PubMed |
Knight NL, Sutherland MW, Martin A, Herde DJ (2012) Assessment of infection by Fusarium pseudograminearum in wheat seedling tissues using quantitative PCR and a visual discoloration scale. Plant Disease 96, 1661–1669.
| Assessment of infection by Fusarium pseudograminearum in wheat seedling tissues using quantitative PCR and a visual discoloration scale.Crossref | GoogleScholarGoogle Scholar | 30727460PubMed |
Kumar J, Schäfer P, Hückelhoven R, Langen G, Baltruschat H, Stein E (2002) Bipolaris sorokiniana, a cereal pathogen of global concern: cytological and molecular approaches towards better control. Molecular Plant Pathology 3, 185–195.
| Bipolaris sorokiniana, a cereal pathogen of global concern: cytological and molecular approaches towards better control.Crossref | GoogleScholarGoogle Scholar | 20569326PubMed |
Leslie JF, Summerell BA (2008) ‘The Fusarium laboratory manual.’ Vols 2 and 10. (John Wiley & Sons: New York)
Liu Y, Ma J, Yan W, Yan G, Zhou M, Wei Y, Zheng Y, Liu C (2012) Different tolerance in bread wheat, durum wheat and barley to Fusarium crown rot disease caused by Fusarium pseudograminearum. Journal of Phytopathology 160, 412–417.
| Different tolerance in bread wheat, durum wheat and barley to Fusarium crown rot disease caused by Fusarium pseudograminearum.Crossref | GoogleScholarGoogle Scholar |
Lush D, Forknall C, Neate S, Sheedy J (2018) 2018 wheat varieties, Queensland. Crop Variety Guides. Grains Research and Development Corporation, and Queensland Department of Agriculture and Fisheries, Brisbane, Qld.
Malligan CD (2009) Crown rot (Fusarium pseudograminearum) symptom development and pathogen spread in wheat genotypes with varying disease resistance. PhD thesis, University of Southern Queensland, Qld, Australia.
Moya-Elizondo EA, Jacobsen BJ, Hogg AC, Dyer AT (2011) Population dynamics between Fusarium pseudograminearum and Bipolaris sorokiniana in wheat stems using real-time qPCR. Plant Disease 95, 1089–1098.
| Population dynamics between Fusarium pseudograminearum and Bipolaris sorokiniana in wheat stems using real-time qPCR.Crossref | GoogleScholarGoogle Scholar | 30732056PubMed |
Moya-Elizondo E, Arismendi N, Castro MP, Doussoulin H (2015) Distribution and prevalence of crown rot pathogens affecting wheat crops in southern Chile. Chilean Journal of Agricultural Research 75, 78–84.
| Distribution and prevalence of crown rot pathogens affecting wheat crops in southern Chile.Crossref | GoogleScholarGoogle Scholar |
Murray GM, Brennan JP (2009) Estimating disease losses to the Australian wheat industry. Australasian Plant Pathology 38, 558–570.
| Estimating disease losses to the Australian wheat industry.Crossref | GoogleScholarGoogle Scholar |
Murray G, Brennan J (2010) Estimating disease losses to the Australian barley industry. Australasian Plant Pathology 39, 85–96.
| Estimating disease losses to the Australian barley industry.Crossref | GoogleScholarGoogle Scholar |
Nelson K, Burgess L (1994) Reaction of Australian cultivars of oats and barley to infection by Fusarium graminearum Group 1. Australian Journal of Experimental Agriculture 34, 655–658.
| Reaction of Australian cultivars of oats and barley to infection by Fusarium graminearum Group 1.Crossref | GoogleScholarGoogle Scholar |
Obanor F, Chakraborty S (2014) Aetiology and toxigenicity of Fusarium graminearum and F. pseudograminearum causing crown rot and head blight in Australia under natural and artificial infection. Plant Pathology 63, 1218–1229.
| Aetiology and toxigenicity of Fusarium graminearum and F. pseudograminearum causing crown rot and head blight in Australia under natural and artificial infection.Crossref | GoogleScholarGoogle Scholar |
Obanor F, Neate S, Simpfendorfer S, Sabburg R, Wilson P, Chakraborty S (2013) Fusarium graminearum and Fusarium pseudograminearum caused the 2010 head blight epidemics in Australia. Plant Pathology 62, 79–91.
| Fusarium graminearum and Fusarium pseudograminearum caused the 2010 head blight epidemics in Australia.Crossref | GoogleScholarGoogle Scholar |
Patterson HD, Thompson R (1971) Recovery of inter-block information when block sizes are unequal. Biometrika 58, 545–554.
| Recovery of inter-block information when block sizes are unequal.Crossref | GoogleScholarGoogle Scholar |
Percy CD, Wildermuth GB, Sutherland MW (2012) Symptom development proceeds at different rates in susceptible and partially resistant cereal seedlings infected with Fusarium pseudograminearum. Australasian Plant Pathology 41, 621–631.
| Symptom development proceeds at different rates in susceptible and partially resistant cereal seedlings infected with Fusarium pseudograminearum.Crossref | GoogleScholarGoogle Scholar |
Smiley RW, Gourlie JA, Easley SA, Patterson LM (2005) Pathogenicity of fungi associated with the wheat crown rot complex in Oregon and Washington. Plant Disease 89, 949–957.
| Pathogenicity of fungi associated with the wheat crown rot complex in Oregon and Washington.Crossref | GoogleScholarGoogle Scholar | 30786628PubMed |
Sturgess J (2014) Barley: planting and disease guide 2014 Queensland and NSW. AgriScience Queensland, Department of Agriculture Fisheries and Forestry, Brisbane, Qld.
Thompson C, Beckmann GG (1959) Soils and land use in the Toowoomba area, Darling Downs, Queensland. Soil and Land Use Series No. 28. Division of Soils, Commonwealth Scientific and Industrial Research Organization, Melbourne, Vic.
Wegulo S, Klein R (2010) Common root rot and Fusarium foot rot of wheat. NebGuide. University of Nebraska–Lincoln Extension, Institute of Agriculture and Natural Resources, Lincoln, NE, USA.
Wildermuth G (1986) Geographic distribution of common root rot and Bipolaris sorokiniana in Queensland wheat soils. Australian Journal of Experimental Agriculture 26, 601–606.
| Geographic distribution of common root rot and Bipolaris sorokiniana in Queensland wheat soils.Crossref | GoogleScholarGoogle Scholar |
Wildermuth G, McNamara R (1991) Effect of cropping history on soil populations of Bipolaris sorokiniana and common root rot of wheat. Australian Journal of Agricultural Research 42, 779–790.
| Effect of cropping history on soil populations of Bipolaris sorokiniana and common root rot of wheat.Crossref | GoogleScholarGoogle Scholar |
Wildermuth G, McNamara R (1994) Testing wheat seedlings for resistance to crown rot caused by Fusarium graminearum group 1. Plant Disease 78, 949–953.
| Testing wheat seedlings for resistance to crown rot caused by Fusarium graminearum group 1.Crossref | GoogleScholarGoogle Scholar |
Wildermuth G, Tinline R, McNamara R (1992) Assessment of yield loss caused by common root rot in wheat cultivars in Queensland. Australian Journal of Agricultural Research 43, 43–58.
| Assessment of yield loss caused by common root rot in wheat cultivars in Queensland.Crossref | GoogleScholarGoogle Scholar |
Yang X, Ma J, Li H, Ma H, Yao J, Liu C (2010) Different genes can be responsible for crown rot resistance at different developmental stages of wheat and barley. European Journal of Plant Pathology 128, 495–502.
| Different genes can be responsible for crown rot resistance at different developmental stages of wheat and barley.Crossref | GoogleScholarGoogle Scholar |
Zhang XX, Sun HY, Shen CM, Li W, Yu HS, Chen HG (2015) Survey of Fusarium spp. causing wheat crown rot in major winter wheat growing regions of China. Plant Disease 99, 1610–1615.
| Survey of Fusarium spp. causing wheat crown rot in major winter wheat growing regions of China.Crossref | GoogleScholarGoogle Scholar | 30695959PubMed |