Responses in growth, yield and cob protein content of baby corn (Zea mays) to amendment of an acid sulfate soil with lime, organic fertiliser and biochar
Loan K. Thi A , Isa A. M. Yunusa B * , M. A. Rab C , Ayalsew Zerihun D and Hoa M. Nguyen E
B * , M. A. Rab C , Ayalsew Zerihun D and Hoa M. Nguyen E
A Kien Giang Agricultural Extension Center, Rach Gia, Kien Giang, Vietnam.
B Graham Centre for Agricultural Innovation, Charles Sturt University, Wagga Wagga, NSW 2650, Australia.
C School of Agriculture and Food, Faculty of Veterinary and Agricultural Science, The University of Melbourne, Parkville, Vic. 3010, Australia.
D Centre for Crop and Disease Management, School of Molecular and Life Sciences, Curtin University, Perth, WA 6845, Australia.
E Faculty of Agronomy, Can Tho University of Agriculture, Can Tho, Vietnam.
Crop & Pasture Science - https://doi.org/10.1071/CP21812
Submitted: 30 June 2021 Accepted: 11 April 2022 Published online: 30 May 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Cropping of acid sulfate soils requires effective treatment of their inherently low pH. We evaluated the efficacy of applications of two levels of lime (0 or 2 Mg/ha), two levels of organic fertiliser (0 or 5 Mg/ha), and three levels of biochar (0, 10 or 30 Mg/ha) in a factorial design for ameliorating acidity in an acid sulfate soil, and measured the subsequent growth and yield of baby corn (Zea mays L.). Lime increased soil pH(H2O) from 3.75 to 4.12, salinity from 1.72 to 1.95 dS/m, and cob yield by 30%. None of the amendments significantly altered total organic carbon or total nitrogen concentrations in the soil. Biochar additions increased cob yields by an average of 28% on both unlimed and limed soil. Addition of organic fertiliser increased cob yield by 45% on unlimed soil but had no significant effect on yields on limed soil. The yields obtained with liming were similar to the highest yields achieved with biochar or organic fertiliser applied either separately or in combinations. Overall, cob yields were increased by 19% with addition of organic fertiliser. The yield increseas from additions of biochar or organic fertiliser were associated with improvements in nutrient supply. However, the increases in cob yield were associated with reduced cob protein, probably resulting from poor availability of nitrogen late in the season. We conclude that biochar and organic fertiliser applied in relatively large quantities can be viable treatments for cropping acid sulfate soils.
Keywords: acid sulfate soils, cob quality, harvest index, liming, organic fertiliser, phenology, rice biochar, Vietnam.
Introduction
Agricultural productivity is generally poor on acid sulfate soils. These soils cover about 12–13 Mha globally, with wide distribution mostly in the coastal tropics of southern and south-eastern Asia (∼10 Mha). Other regions with large areas of acid sulfate soils are found along the coasts of northern West Africa, eastern South America and eastern Australia (Andriesse and Van Mensvoort 2006). The widespread distribution of acid sulfate soils exacerbates the decline in availability of arable lands and poses a significant risk to food security in these densely populated regions of the world (Yunusa et al. 2018). This risk is likely to be increased by the rising sea levels associated with climate change, predicted to increase by 235 cm by 2100 (Milen 2010).
The poor agricultural potential of acid sulfate soils is associated with their low pH and mobilisation of toxic trace elements (e.g. boron, copper, manganese and zinc) along with reduced availability of macronutrients, especially nitrogen (N), phosphorus (P) and potassium (K) (Golez and Kyuma 1997; Fitzpatrick 2003). An acidic rhizosphere damages and restricts growth of plant roots, mobilises toxic trace elements, and interferes with uptake of key macronutrients (Fitzpatrick 2003; Guong and Hoa 2010). For instance, Marschner and Rengel (2012) reported that poor root growth coupled with weak contact of roots with the soil matrix in acid sulfate soils inhibits uptake of N by the plant even in the presence of high concentrations of total N in the bulk soil. Therefore, poor survival, growth and yields have been observed in plants grown on acid sulfate soils; yield reductions of up to 10% have been reported in maize (Zea mays L.) (Sierra et al. 2003). Heavy investments to treat acidity are therefore required for viable cultivation of the vast majority of crops on acid sulfate soils.
Liming is the most common treatment for low pH in acid sulfate soils (Bloomfleld and Coulter 1974; Elisa et al. 2016; Li et al. 2016). Lime addition rates as high as 20 Mg/ha have been used to increase the soil pH by up to 2.0 units in highly acidic soils (Acosta-Martínez and Tabatabai 2000; Hiep 2008). Availability and cost of liming are major constraints on the farmer’s capacity to treat acid sulfate soils sufficiently for profitable cultivation, particularly in developing countries such as Vietnam, where acid sulfate soils account for 6% (2 Mha) of the country’s total land area (Tran 2015). Hence, other materials such as organic matter (Michael and Ian 2005), biochar (Masulili et al. 2010; Manickam et al. 2015) and organic fertilisers (Hati et al. 2008) are often considered as alternative amendments, or as supplements, to lime for ameliorating acid sulfate soils. Halim et al. (2018) reported that organic materials applied in combination with a compound (NPK) fertiliser on an acid sulfate soil raised the soil pH from 3.7 by 0.5–2.0 units within 60 days of treatment and marginally increased rice yield. Organic fertilisers are also viewed as affordable liming agents that have the added benefit of high nutrient contents to benefit crop productivity, especially when used in conjunction with biochar and/or lime to treat acid sulfate soils (Halim et al. 2018).
Increased availability of macronutrients (NPK) from biochar additions, alone or in combination with other organic amendments, is associated with significant improvements in the yield of maize on fertilised, moderately acidic Red Ferrosol (Agegnehu et al. 2016). The efficacy of organic materials in ameliorating soil acidity depends on their physicochemical properties such as pH, carbon (C):N ratio, alkalinity and mineralisation potential (Xu and Coventry 2003; Gao et al. 2019). For instance, Xu and Coventry (2003) reported that addition of dried and ground straws of lupin (Lupinus alba L.; C:N 10.5) and shoots of wheat (Triticum aestivum L.; C:N 9.0) significantly increased pH in a highly acidic soil (pH <5). The authors attributed the increases in pH to the ash alkalinity and the mineralisation of organic N in the applied plant residues. By contrast, instances of declines in the pH of residue-amended soils were ascribed to nitrification of mineralised N in the applied residues. A meta-analysis by Gao et al. (2019) showed that biochar increased P (both available and microbial) by at least 45% and available N (ammonium and nitrate) by an average of 14%. The analysis revealed that N availability in biochar-amended soil depends on the N content and pH of both the biochar and the receiving substrate. The authors concluded that biochar made from materials having low C:N ratio and generated at low temperatures, or applied at high rates, were quite effective for enhancing soil available P.
Rice biochar is generally considered a viable resource for treating acid sulfate soil in Southeast Asia, where managing the large amounts of rice straw produced every season is a major environmental and economic challenge (Shamshuddin et al. 2017). Use of the rice straw to manufacture biochar is a viable economic end use, being an alternative to traditional burning while combating air pollution and CO2 emissions (Van Hung et al. 2020). Addition of rice biochar improves soil structure and chemical properties by reducing soil bulk density, soil strength, exchangeable aluminium (Al) and soluble iron (Fe) while enhancing soil nutritional and hydrological properties (Masulili et al. 2020). Indeed, Manickam et al. (2015) reported that addition of rice biochar to an acidic soil raised the pH by 1.5 units and significantly increased root growth, but produced only a marginal increase in the yield of maize.
The Mekong Delta is a primary agricultural belt of Vietnam accounting for over half of the country’s total rice production, mostly on acid sulfate soil that is subjected to intrusion of seasonal seawater (Thong et al. 2011). Managing low soil pH and its attendant impacts on soil fertility, crop growth and yield remains the primary constraint on productivity and is a significant impost on farming acid sulfate soils (Guong and Hoa 2010). In addition, it is also not clear whether, or to what extent, addition of rice biochar would effectively relieve the intrinsic acidity of the soil in the short–medium term. Therefore, the present study was undertaken to determine: (i) the relative efficacy of lime, organic fertiliser and rice biochar, applied individually or in combinations, for ameliorating the low pH of acid sulfate soil; and (ii) the impacts of these amendments and their combinations on growth, yield and cob protein content of baby corn.
Materials and methods
Site details
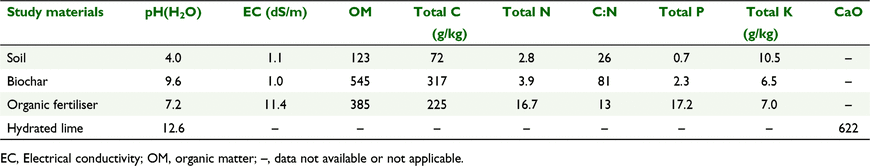
The study was conducted in a glasshouse at the Department of Soil Science, College of Agriculture and Applied Biology, Can Tho University, Can Tho, Vietnam (10°1′N, 105°45′E). We collected soil from the top 20 cm of the profile near the village of Vinh Vien, Long My district, Hau Giang province (9°40′N, 105°26′E). The soil is classified as HypoSali-Umbri-Epi Orthithionic Gleysol according to the World Reference Base System (IUSS Working Group WRB 2007) and is equivalent to Entisols and Inceptisols as per USDA Soil Taxonomy (Soil Survey Staff 1999). These soils are commonly referred to as hydro-morphic (groundwater) soils and are characterised by a shallow clay horizon (55% clay, 35% silt and 15% sand) to a depth of about 1 m (Minh et al. 1998). The soil profile is highly acidic, with total sulfur (as SO42−) 2.0%, actual titratable acidity 2790 mol H+/t, total acidity 8.44 (g/L) , exchangeable Al content 6.6 cmolc/kg, and average pH(H2O) 4.0. Soil total organic matter and total N concentrations were 12% and 0.28%, respectively. Details of soil properties are presented in Table 1. The area experiences transient seawater intrusion between February and April every year.
|
Treatments and experimental design
Soil samples were air-dried, then sieved to pass 2 mm and used to fill plastic pots (32 cm diameter, 25 cm height) at 10 kg per pot. The treatment factors consisted of three types of amendments applied at different rates as follows: hydrated lime at 0 or 2 Mg/ha; organic fertiliser at 0 or 5 Mg/ha; and biochar at 0, 10 or 30 Mg/ha. Treatments were factorially combined generating 12 treatments, which were individually assigned to the potting soil. The experiment was laid out as a randomised block design with four replicates, making a total of 48 pots (experimental units). Within each block, the treatments were arranged randomly. All pots were supplied with basal NPK fertiliser (2.5 N:1 P2O5:1 K2O) at 26 g/pot, which approximated to (per ha) 150 N applied as urea, 360 kg P, and 100 kg K.
The lime and organic fertiliser were obtained from a commercial supplier (BioPro; MSUN, Ha Noi City, Vietnam). The organic fertiliser was a composted mixture of sugarcane filter cake and fish manure. The biochar was produced from rice husk using a top-lit updraft stove at the Department of Soil Science, College of Agriculture and Applied Biology, Can Tho University, following the procedures described by Hoa et al. (2014) and Luong et al. (2012). This involved air-drying the rice husks in the sun to ensure efficient combustion when fed into the stove, using burning paper as a starter. The husk combustion lasted about 90 min, during which time the temperatures rose to 500–550°C.
Planting and maintenance
The baby corn variety used in the study was Pacific 421 (Pacific Seeds, Toowoomba, Qld, Australia), known for its high-quality seed and tolerance of diseases (Guong and Hoa 2010). Lime was applied to the relevant pots 14 days before planting, and organic fertiliser, biochar and basal fertiliser (superphosphate, 1/5 urea and 1/5 KCl) were applied 1 day before planting. Four maize seeds were sown in each pot on 17 October 2014. The pots were each placed on trays and arranged in rows 0.7 m long, with pots spaced 0.25 m apart within the row. After germination, the four plants in each pot were gradually thinned to one by cutting a plant at the base and removing it with minimal rhizosphere disturbance at 7, 14 and 28 days after sowing (DAS). The pots were watered every morning to ensure adequate water supply while avoiding drainage by checking for any leakage from the perforation at the bottom of the pots. On the rare occasions when leakage occurred, the drained water was poured back into the respective pots during watering. Fertiliser application was repeated for all pots during the final plant thinning at 28 DAS.
Measurements
Nutritional analysis of soil and amendments
Bulk samples of the soil and the three amendments were analysed to determine their pH, EC, total organic C, total N, total P and total K content. The CaO content of the lime was estimated following the procedures compiled by Rayment and Higginson (1992). Chemical analysis of the potting soil was performed on finely ground samples twice during the trial: first, before sowing soon after adding soil amendments, and then immediately after completion of harvest. Soil pH was determined in deionised water (1:2.5 soil:water) and measured with a pH meter (Metrohm 744; Metrohm, Herisau, Switzerland) (Thomas et al. 1996). The soil-water preparations were also used to determine electrical conductivity (EC) with a handheld meter (EC-Lab 960; SCHOTT Instruments, Mainz, Germany). Total organic C and total N were determined by using a C:N analyser (LECO, St. Joseph, MI, USA).
Plant measurements
Plant height, leaf area and leaf chlorophyll contents were determined at 14, 21, 28, 35 and 42 DAS; measurements at 14 and 28 DAS were made on the thinned plants. Plant height was measured from the base of the plant to the base of the topmost fully expanded leaf, using a measuring tape. Leaf area was determined from the length and width of fully expanded leaves as: leaf length × leaf maximum width × 0.75 (Yunusa 1989). Chlorophyll content was determined on the uppermost fully expanded leaves, using a chlorophyll meter (SPAD-502; Konica Minolta, Osaka, Japan) (Coste et al. 2010).
Dates of tassel and first silk emergence were recorded, and harvesting was undertaken 2 days after silk emergence between 53 and 64 DAS depending on the treatment. Final plant growth and yield variables at harvest were made on the one plant left in each pot. The cobs were harvested during the 5-day period following silking (i.e. 58–70 DAS) as per established practice in Vietnam. These plants were cut at the soil surface and the ears (consisting of cob, husk and silk) were removed and counted. These plant samples were then dried in an oven at 80°C for 72 h and weighed to determine their total dry matter. The ears were then dehusked and the cob weighed to calculate the harvest index as: cob weight/total plant weight (i.e. shoot + ear). The cobs were then analysed for total N content (%) and crude protein content was estimated as 6.25 × total N (Mariotti et al. 2008).
Statistical analyses
All data were evaluated first for normality and homogeneity of variances using the Kolmogorov–Smirnov test and Levene’s test, respectively, and then a three way analysis of variance (ANOVA) was performed on the data using SPSS 16 (SPSS, Chicago, IL, USA). When treatment effects were significant (P < 0.05), both the main effects and interaction effects of treatments factors were evaluated using a post hoc multiple comparison for observed means with Duncan’s multiple range test.
Principal component analysis (PCA) was performed to identify the most critical variables and their interrelations, using FactoMineR (Lê et al. 2008) package in R software (R Foundation for Statistical Computing, Vienna, Austria). PCA transforms the observed variables linearly into orthogonal uncorrelated variables known as principal components (PC), which maintain the total variance in the original data. This analysis was performed on the correlation matrix, standardising data measured on different scales to unit variance. Therefore, the PCs become independent of the scale and units of the observed variables.
Results
Plant growth and development
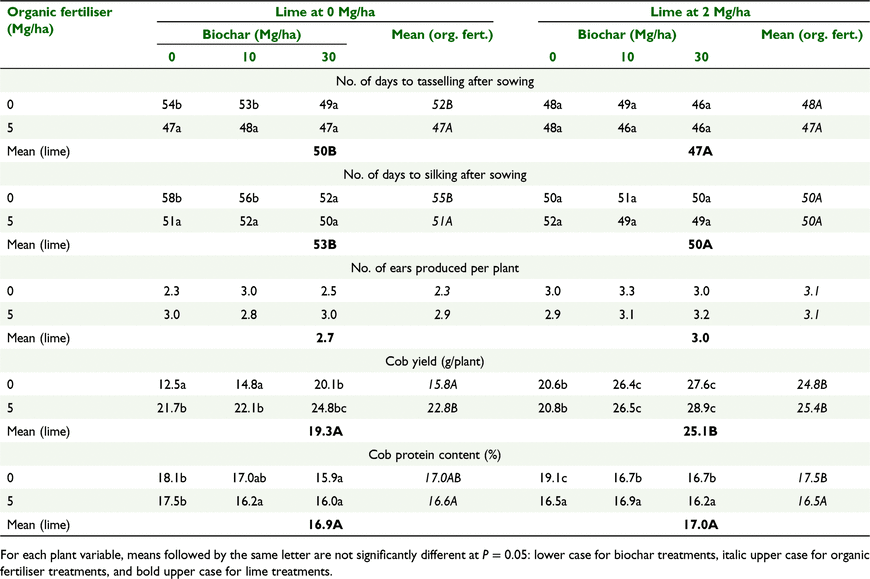
The patterns of treatment effects (i.e. liming, organic fertiliser and biochar) on plant height, stem thickness, leaf area and chlorophyll concentrations were consistent at all four sampling dates. Therefore, for brevity we present data for the last sampling only (Table 2). Plants on limed soil and amended with organic fertiliser and/or biochar were taller and thicker, had higher chlorophyll concentration, and developed faster (earlier tasselling and silking) than those on unlimed soils without organic fertiliser; these responses marginally increased with biochar addition at 30 Mg/ha. None of the amendments had a significant effect on the number of ears produced per plant, but the weight of ears was significantly higher for plants grown on limed soils and was increased further with additions of organic fertiliser and biochar (Table 3). There was no significant difference in cob weight between biochar applications at 10 and 30 Mg/ha.

|
|
Responses in growth variables
Biochar applied at 30 Mg/ha increased plant height, leaf area and stem diameter on unlimed soil irrespective of organic fertiliser addition (Table 2). Chlorophyll concentration was not significantly affected by biochar addition on unlimed soil but was increased when biochar was applied at either 10 or 30 Mg/ha on limed soil with or without organic fertiliser. Organic fertiliser increased plant height, leaf area and stem diameter only on unlimed soil, but had no significant effect on chlorophyll concentration. Lime addition significantly increased all growth variables except chlorophyll concentration.
Responses in phenology, yield and yield variables
Addition of biochar at 30 Mg/ha reduced the time to tasselling and silking by as much as 4 days on unlimed soil, but not on limed soil irrespective of organic fertiliser treatment (Table 3). Organic fertiliser addition hastened tasselling and silking by up to 5 days on unlimed soil but not on limed soil. Plants on limed soil also attained tasselling and silking earlier than those on unlimed soil.
The number of ears produced per plant was not affected by the treatments, in contrast to the yield response (total weights of the cob produced) (Table 3). Biochar applied at 30 Mg/ha increased cob yield on both unlimed and limed soil with or without organic fertiliser addition. Organic fertiliser addition increased cob yield by almost 16% on unlimed soil but not on limed soil, where its impact was modest. Overall, liming of the soil significantly increased cob yield by about 30%.
Biochar applied at 30 Mg/ha significantly reduced cob protein content by up to 13% on all lime and organic fertiliser treatment combinations except on limed soil treated with organic fertiliser, where there was no significant impact (Table 3). Organic fertiliser addition reduced the cob protein significantly only on limed soil, and liming had no significant effect on cob protein.
Shoot weight was significantly increased by biochar addition at 30 Mg/ha with addition of organic fertiliser on unlimed soil (Fig. 1). Liming significantly increased shoot weight and decreased harvest index (Fig. 1).
Soil variables
Before sowing, biochar applied at 30 Mg/ha increased the soil pH on unlimed soil, although not significantly, whereas on limed soil, biochar effects were less evident (Fig. 2). Addition of organic fertiliser marginally increased pH on limed soil. On the other hand, addition of lime significantly increased soil pH by 0.37 units (from 3.75 to 4.12). Soil EC was not significantly affected by the addition of biochar on limed soil with or without organic fertiliser, whereas applying biochar at 30 Mg/ha significantly lowered the EC in both fertilised and unfertilised soils (Fig. 2). Soil EC ranged from 1.60 dS/m in unlimed and unfertilised soil to 2.04 dS/m in limed and fertilised soil, but these values were not statistically different. Total organic C was not affected by any treatment, whereas total N was significantly higher in fertilised and limed soil when biochar was added at 30 Mg/ha (Fig. 2).
There was an almost universal decline in the values of all soil variables measured after plant harvest (Supplementary material Table S1) compared with initial measurements. Reductions in soil pH after plant harvest were larger with biochar addition, except on limed soil that was supplied with organic fertiliser. Changes in the pH were similar on both unlimed and unfertilised soils. The magnitude of reduction in soil EC was significantly lower with biochar addition on unlimed soil and without organic fertiliser, whereas it was similar on the other soils irrespective of lime and organic fertiliser addition. The magnitude of reduction in EC was greater on limed than on unlimed soil. Neither total organic C nor total N was significantly affected after harvest by the additions of any of the three soil amendments or their combinations.
Multivariate analysis
The results of PCA are presented in Fig. 3. PC1 accounted for almost 40% of the total variances and was mostly associated with the pH gradient. PC2, which represented the fertility (total organic C and total N) spectrum, accounted for just over 11% (Fig. 3a). All plant variables (growth and yield) and soil pH measured at both times (before sowing and after harvest) were positively correlated (PC1). However, plant variables were inversely correlated with total soil N and organic C both at sowing and harvest (PC2). The vegetative growth variables (dry matter, leaf area, stem diameter) were inversely correlated with yield and yield component variables (ear number and cob yield) on PC2 axis.
Addition of organic fertiliser generally improved soil and plant variables, but its impact was greater when it was applied in combination with lime as illustrated by the response clusters for the soil treated with organic fertiliser, which shifted further to the right of those for lime-only treatments on the horizontal axis (Fig. 3b). A similar response was observed with the biochar treatment, which shifted the response clusters to the right (Fig. 3c) on the PC1 axis. Liming alone shifted the response clusters to the right of the vertical reference line (0), which was not achieved with either organic fertiliser or biochar alone.
Discussion
Our primary purpose in this study was to test the liming potentials of biochar and organic fertiliser, which are more affordable and available than agricultural lime in Vietnam. Neither biochar nor organic fertiliser significantly raised the pH of the acid sulfate soil, whereas lime addition raised the soil pH by almost 0.4 units (Fig. 2). The high organic matter contents of acid sulfate soils provide a large buffering capacity, making it difficult to increase soil pH significantly without liming. Both the biochar and organic fertiliser contain high amounts of organic matter, exceeding 35% (Table 1) and their additions to the soil would have further enhanced the soil’s buffering capacity and subsequently stabilised its pH. It was only on unlimed soil that biochar applied at the rate of 30 Mg/ha increased the pH by 0.24 units, not dissimilar to the rise of 0.32–1.04 units in pH after 30 days of incubation of an acid sulfate soil amended with rice residues including husk biochar (Masulili et al. 2010).
In all treatments, the pH declined from pre-planting values such that the pH at 60 DAS (around the time of final harvest) was lower than at the start of the trial (Table S1). This response was consistent with an earlier observation (Jayalath et al. 2016) involving incubation of drained acid sulfate soils with plant residues. Those authors reported declines in pH by >2 units (from 6.5 to 4.0) of the drained hypersulfuric acid soil (pH 4.1, C:N 15) when amended with residues of common reeds (Phragmites australis) that have low C:N (28), over a dry period of 11 weeks. By contrast, the pH decline was more gradual (∼1.0 unit) when the same soil was treated with pea (Pisum sativum) straws (C:N = 50) during the same period. Irrespective of treatment, the authors reported that the decline in pH of treated soil stabilised within 8 weeks of treatment applications. Both the pH and C:N ratio of the acid sulfate soil in that study were similar to those in the present study (Table 1). The basis for differential amelioration outcome is not entirely clear. However, Xu and Coventry (2003) suggested that declines in soil pH following addition of plant residues are the result of nitrification of ammonium-N to nitrate-N in the substrate and release of hydrogen ions in the process. They further argued that this process was especially strong where the receiving substrate has a low pH and more than compensated for any alkalinising effect of the applied amendment. Similar arguments were presented by Jayalath et al. (2016) for the decreases in pH of acid sulfate soils amended with plant residues. Furthermore, both the biochar and organic fertiliser in the present study had relatively high organic matter content (Table 1) to have increased the pH buffering capacity of the soil, as noted by Jayalath et al. (2016).
Neither biochar nor organic fertiliser addition had significant impact on soil pH (Fig. 2). This could be the result, at least in part, of enhanced pH buffering capacity of the soil by the additional inputs of organic matter from these two amendments (Table 1). Several previous studies reported relative stability of pH in highly acidic soils due organic matter input from the plant residues added to the soils (Xu and Coventry 2003; Jayalath et al. 2016). However, additions of biochar and organic fertilisers increased organic matter input to the soil and would have improved supply of nutrients, especially P and K (Table 1), to account for the significant increases in plant growth and yield (Tables 2 and 3). The yield of 26.4 g/plant obtained with liming alone was similar to that obtained with highest doses of biochar or organic fertiliser applied separately or in combination to soil (Table 3). This demonstrates that because of their high nutrient contents, applications of biochar at 10–30 Mg/ha and organic fertiliser at 5 Mg/ha were as effective as 2 Mg/ha of lime in unlocking the productivity potential of the acid sulfate soils in this study.
Liming had the largest impact on the soil pH and growth and yield of baby corn, whereas the effects of either organic fertiliser or biochar on plant growth characteristics and cob yield were marginal. This is clearly revealed in the PCA, which shows positive correlations of soil pH (both initial and at harvest) with plant growth and yield variables on the PC1 axis. These accounted for almost 40% of the total variability (Fig. 3a). PC1 effectively represents a liming threshold that demonstrates improvement in plant performance with rising pH, and plant growth variables being strongly correlated with soil pH. It also shows the inverse correlations of yield variables (ear number and weight) with total N and organic carbon, which was consistent with the suggested occurrence of impaired nutrient availability late in the growing season. Influence of the fertility spectrum, primarily N, is expressed along the PC2 axis, and accounted for a further 11% of the total variance. PC2 reveals a positive association between soil organic C and total N, on the one hand, and plant growth variables such as shoot dry matter, shoot diameter and thickness, on the other. The impacts of lime on both soil and plant variables are demonstrated by the large separation between unlimed and limed treatments in their clusters of response variables (Fig. 3b, c). Additions of either biochar or organic fertiliser shifted the response clusters positively on both axes, demonstrating that these amendments further enhanced the benefits of liming.
Furthermore, vegetative growth (plant height, plant diameter and shoot weight) were negatively correlated with yield variables on the PC2 axis, suggesting that the early rapid growth was at the expense of the development of yield variables such as the number and weight of ears toward the end of the growing period. This has been commonly observed in such environments with limited growth resources where rapid expansion of the canopy and dry matter accumulation (biological yield) occur early in the season when growth resources are adequate in soil, but the result is poor grain yield due to limited availability of resources later in the season (Donald and Hamblin 1976; Yunusa and Sedgley 1992). It was probable that the baby corn in our study experienced an inadequate supply of available N during the reproductive phase due to persistent low pH, which is consistent with the negative correlation between total N and yield variables on the PC2 axis (Fig. 3a). Nugroho and Kuwatsuka (1990) reported that an initial rapid rate of decomposition and nitrification stabilised within 20 days of incubating a mildly acidic soil amended with rice residues. However, they further observed that concurrent with nitrification, a moderate level of denitrification (N loss) started from Day 10. In another study, increased N loss through ammonia volatilisation from soil amended with rice biochar was observed (Huang et al. 2018). Both of these studies suggest that continued N losses will deplete N availability. It is therefore likely that only some of the N present at the start of our study was used by the plant to support early growth, and availability might have been limited during the reproductive phase.
A probable loss and/or immobilisation of N late in the season was consistent with the marginal or negative impacts, respectively, of organic fertiliser and biochar on cob protein (Table 3). The reduction in grain protein in baby corn supplied with biochar likely arose from the dilution of cob N content by the continued accumulation of biomass. The plants supplied with biochar attained the reproductive phase early (Table 3), when most well-watered crops attain peak demand for N, often exceeding the capacity of the soil to supply the nutrient through mineralisation or fertilisation (Angus 2001). Without supply of additional N, the tissue N concentration will be diluted by the continued accumulation of biomass; hence, the often-reported reciprocal relationship between grain yield and grain protein in resource-limited environments (Yunusa and Rashid 2007). The remaining principal components (PC3 to PC16) in the PCA individually explained <8% of the total variance (Table S2). These are not considered in detail here but could account for the impacts of other soil characteristics such as C:N ratios, alkalinity and mineralisation potentials (Xu and Coventry 2003; Gao et al. 2019).
Conclusions
Additions of organic fertiliser and biochar to acid sulfate soil increased cob yield to a value similar to that obtained with addition of 2 Mg/ha of lime. However, the three amendments reduced cob protein because of limited soil N supply, especially during the reproductive phase, potentially contributing to the dilution of N concentration in the cob tissue. We conclude that biochar and organic fertilisers that are high in key nutrients, such as used in the present study, can be as effective as liming in supporting viable cropping of acid sulfate soils.
Supplementary material
Supplementary material is available online.
Data availability
The data that support this study are available in the article and accompanying online supplementary material.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
Acknowledgements
The first author received an AusAID scholarship that supported this research and thanks Alex Pearce and Christine Fyfe for their assistance during the program at University of New England, Armidale, NSW, Australia. We thank Huynh Khanh Tran for her technical assistance at Can Tho University, Can Tho, Vietnam, during the trial. We acknowledge the comments of the anonymous referees and the associate editors on the manuscript. We thank Phil Newton for his comments and suggestions, which improved the manuscript.
References
Acosta-Martínez V, Tabatabai MA (2000) Enzyme activities in a limed agricultural soil. Biology and Fertility of Soils 31, 85–91.| Enzyme activities in a limed agricultural soil.Crossref | GoogleScholarGoogle Scholar |
Agegnehu G, Bass AM, Nelson PN, Bird MI (2016) Benefits of biochar, compost and biochar–compost for soil quality, maize yield and greenhouse gas emissions in a tropical agricultural soil. Science of the Total Environment 543, 295–306.
| Benefits of biochar, compost and biochar–compost for soil quality, maize yield and greenhouse gas emissions in a tropical agricultural soil.Crossref | GoogleScholarGoogle Scholar | 26590867PubMed |
Andriesse W, Van Mensvoort MEF (2006) Acid sulfate soils: distribution and extent. In ‘Encyclopedia of soil science. Vol. 1’. (Ed. R Lal) pp. 14–19. (CRC Press: Boca Raton, FL, USA)
Angus JF (2001) Nitrogen supply and demand in Australian agriculture. Australian Journal of Experimental Agriculture 41, 277–288.
| Nitrogen supply and demand in Australian agriculture.Crossref | GoogleScholarGoogle Scholar |
Bloomfleld C, Coulter JK (1974) Genesis and management of acid sulfate soils. In ‘Advances in agronomy. Vol. 25’. (Ed. NC Brady) pp. 265–326. (Academic Press: Cambridge, MA, USA)
Coste S, Baraloto C, Leroy C, Marcon E, Renaud A, Richardson AD, Roggy J-C, Schimann H, Uddling J, Hérault B (2010) Assessing foliar chlorophyll contents with the SPAD-502 chlorophyll meter: a calibration test with thirteen tree species of tropical rainforest in French Guiana. Annals of Forest Science 67, 607
| Assessing foliar chlorophyll contents with the SPAD-502 chlorophyll meter: a calibration test with thirteen tree species of tropical rainforest in French Guiana.Crossref | GoogleScholarGoogle Scholar |
Donald CM, Hamblin J (1976) The biological yield and harvest index of cereals as agronomic and plant breeding criteria. In ‘Advances in agronomy. Vol. 28’. (Ed. NC Brady) pp. 361–405. (Academic Press: Cambridge, MA, USA)
Elisa AA, Ninomiya S, Shamshuddin J, Roslan I (2016) Alleviating aluminum toxicity in an acid sulfate soil from Peninsular Malaysia by calcium silicate application. Solid Earth 7, 367–374.
| Alleviating aluminum toxicity in an acid sulfate soil from Peninsular Malaysia by calcium silicate application.Crossref | GoogleScholarGoogle Scholar |
Fitzpatrick RW (2003) Overview of acid sulfate soil properties, environmental hazards, risk mapping and policy development in Australia Advances in Regolith. In ‘Proceedings of the CRC LEME regional regolith symposia’. pp. 122–125. (Cooperative Research Centre for Landscape Environments and Mineral Exploration: Perth, WA, Australia)
Gao S, DeLuca TH, Cleveland CC (2019) Biochar additions alter phosphorus and nitrogen availability in agricultural ecosystems: a meta-analysis. Science of the Total Environment 654, 463–472.
| Biochar additions alter phosphorus and nitrogen availability in agricultural ecosystems: a meta-analysis.Crossref | GoogleScholarGoogle Scholar | 30447585PubMed |
Golez NV, Kyuma K (1997) Influence of pyrite oxidation and soil acidification on some essential nutrient elements. Aquacultural Engineering 16, 107–124.
| Influence of pyrite oxidation and soil acidification on some essential nutrient elements.Crossref | GoogleScholarGoogle Scholar |
Guong TV, Hoa NM (2010) Results from research on use and management of acid sulfate soil in Mekong Delta Vietnam. Can Tho University, Vietnam.
Halim SNA Halim SNA (2018)) Influence of soil amendments on the growth and yield of rice in acidic soil. Agronomy 8, 165
Hati KM, Swarup A, Mishra B, Manna MC, Wanjari RH, Mandal KG, Misra AK (2008) Impact of long-term application of fertilizer, manure and lime under intensive cropping on physical properties and organic carbon content of an Alfisol. Geoderma 148, 173–179.
| Impact of long-term application of fertilizer, manure and lime under intensive cropping on physical properties and organic carbon content of an Alfisol.Crossref | GoogleScholarGoogle Scholar |
Hiep TT (2008) Research on potential release of Aluminium (Al), Copper (Cu), Manganse (Mn) and Zinc (Zn) of some kinds of acid sulfate soils in Long Xuyen Quadrangle and solution by using of lime under laboratory conditions. Master of Soil Science Thesis, Can Tho University, Can Tho, Vietnam.
Hoa NM, Luong PT, Nghi NQ, Tinh NT (2014) New technology in burning agricultural waste and potential use of biochar in improving acid sulfate soils. In ‘Agricultural extension the 4th, 2014’. Can Tho University, Hau Giang Province, Vietnam. pp. 125–135.
Huang M, Fan L, Chen J, Jiang L, Zou Y (2018)) Continuous applications of biochar to rice: effects on nitrogen uptake and utilization. Scientific Reports 8, 11461
| Continuous applications of biochar to rice: effects on nitrogen uptake and utilization.Crossref | GoogleScholarGoogle Scholar | 30061619PubMed |
IUSS Working Group WRB (2007) World Reference Base for Soil Resources 2006. First update 2007. World Soil Resources Report No. 103. Food and Agriculture Organization of the United Nations, Rome, Italy.
Jayalath N, Fitzpatrick RW, Mosley L, Marschner P (2016) Type of organic carbon amendment influences pH changes in acid sulfate soils in flooded and dry conditions. Journal of Soils and Sediments 16, 518–526.
| Type of organic carbon amendment influences pH changes in acid sulfate soils in flooded and dry conditions.Crossref | GoogleScholarGoogle Scholar |
Lê S, Josse J, Husson F (2008) FactoMineR: an R package for multivariate analysis. Journal of Statistical Software 25, 1–18.
| FactoMineR: an R package for multivariate analysis.Crossref | GoogleScholarGoogle Scholar |
Li Z, Qi X, Fan X, Du Z, Hu C, Zhao Z, Isa Y, Liu Y (2016) Amending the seedling bed of eggplant with biochar can further immobilize Cd in contaminated soils. Science of the Total Environment 572, 626–633.
| Amending the seedling bed of eggplant with biochar can further immobilize Cd in contaminated soils.Crossref | GoogleScholarGoogle Scholar | 27567319PubMed |
Luong PT, Nghi NQ, Hoa NM (2012) Applied of burning technique of TLUD stove in burning agricultural waste and producing of biochar in household. In ‘Proceedings of scientific conference CAAB 2012: sustainable agriculture’. Can Tho University. pp. 435–446. (Can Tho University: Can Tho, Vietnam)
Manickam T, Cornelissen G, Bachmann RT, Ibrahim IZ, Mulder J, Hale SE (2015) Biochar application in Malaysian sandy and acid sulfate soils: soil amelioration effects and improved crop production over two cropping seasons. Sustainability 7, 16756–16770.
| Biochar application in Malaysian sandy and acid sulfate soils: soil amelioration effects and improved crop production over two cropping seasons.Crossref | GoogleScholarGoogle Scholar |
Mariotti F, Tomé D, Mirand PP (2008) Converting nitrogen into protein: beyond 6.25 and Jones’ factors. Critical Review in Food Science and Nutrition 48, 177–184.
| Converting nitrogen into protein: beyond 6.25 and Jones’ factors.Crossref | GoogleScholarGoogle Scholar | 18274971PubMed |
Marschner P, Rengel Z (2012) Nutrient availability in soils. In ‘Marschner’s mineral nutrition of higher plants’. 3rd edn. (Ed. P Marschner) pp. 315–330. (Academic Press: Cambridge, MA, USA)
Masulili A, Utomo WH, Syechfani MS (2010) Rice husk biochar for rice based cropping system in acid soil 1. The characteristics of rice husk biochar and its influence on the properties of acid sulfate soils and rice growth in West Kalimantan, Indonesia. Journal of Agricultural Science 2, 39
| Rice husk biochar for rice based cropping system in acid soil 1. The characteristics of rice husk biochar and its influence on the properties of acid sulfate soils and rice growth in West Kalimantan, Indonesia.Crossref | GoogleScholarGoogle Scholar |
Masulili A, Suyanto A, Youlla SD (2020) Reducing soil strength and increase growth and results of paddy (Oryza Sativa l.) on acid sulphate soil. In ‘International Conference on Community Development (ICCD 2020)’. pp. 30–34. (Atlantis Press)
Michael DM, Ian W (2005) Acid sulfate soils. In ‘Encyclopedia of soil science’. 2nd edn. (Ed. R Lal) pp. 11–13. (CRC Press: Boca Raton, FL, USA)
Milen D (2010) Vulnerability, risk reduction, and adaptation to climate change for Vietnam. Climate Risk and Adaptation Country Profile, April 2011. (The World Bank Group) Available at https://data.opendevelopmentmekong.net/dataset/da812872-0e52-4b4d-964f-106f7fded300/resource/72bbc1f6-3be5-41f9-aef6-4604fd8fc8ef/download/wb_gfdrr_climate_change_country_profile_for_vnm.pdf (accessed 15 August 2022)
Minh LQ, Tuong TP, Van Mensvoort MEF, Bouma J (1998) Soil and water table management effects on aluminum dynamics in an acid sulphate soil in Vietnam. Agriculture, Ecosystems & Environment 68, 255–262.
| Soil and water table management effects on aluminum dynamics in an acid sulphate soil in Vietnam.Crossref | GoogleScholarGoogle Scholar |
Nugroho SG, Kuwatsuka S (1990) Concurrent observation of several processes of nitrogen metabolism in soil amended with organic materials. Soil Science and Plant Nutrition 36, 215–224.
| Concurrent observation of several processes of nitrogen metabolism in soil amended with organic materials.Crossref | GoogleScholarGoogle Scholar |
Rayment GE, Higginson FR (1992) ‘Australian laboratory handbook of soil and water chemical methods’, (Inkata Press: Melbourne, Vic., Australia)
Shamshuddin J, Panhwar QA, Alia FJ, Shazana MARS, Radziah O, Fauziah CI (2017) Formation and utilization of acid sulfate soils in Southeast Asia for sustainable rice cultivation. Pertanika Journal of Tropical Agricultural Science 40, 225–246.
Sierra J, Noël C, Dufour L, Ozier-Lafontaine H, Welcker C, Desfontaines L (2003) Mineral nutrition and growth of tropical maize as affected by soil acidity. Plant and Soil 252, 215–226.
| Mineral nutrition and growth of tropical maize as affected by soil acidity.Crossref | GoogleScholarGoogle Scholar |
Soil Survey Staff (1999) Soil Taxonomy: a basic system of soil classification for making and interpreting soil surveys.. Agricultural Handbook No. 436. United States Department of Agriculture, Natural Resources Conservation Service, Washington, DC, USA. Available at https://www.nrcs.usda.gov/Internet/FSE_DOCUMENTS/nrcs142p2_051232.pdf (accessed 11 August 2021)
Thomas G, Sparks D, Page A, Helmke P, Loeppert R, Soltanpour P, Tabatabai M, Johnston C, Sumner M (1996) Soil pH and soil acidity. In ‘Methods of soil analysis: Part 3 - chemical methods’. (Ed. DL Sparks) pp. 475–490. (Soil Science Society of America: Madison, WI, USA)
Thong LP, Xuan HTD, Duyen TTT (2011) Compare economic efficiency between summer rice and autumn–winter rice in Mekong Delta, Vietnam. Journal of Science 18a, 267–276.
Tran MT (2015) Country report: Vietnam soil resources. In ‘Asian soil partnership consultation workshop on sustainable management and protection of soil resources’. (Food and Agriculture Organization of the United Nations: Rome, Italy) Available at https://www.fao.org/fileadmin/user_upload/GSP/docs/asia_2015/Vietnam.pdf (accessed 24 March 2022)
Van Hung N, Maguyon-Detras MC, Migo MV, Quilloy R, Balingbing C, Chivenge P, Gummert M (2020) Rice straw overview: availability, properties, and management practices. In ‘Sustainable rice straw management’. (Eds M Gummert, N Hung, P Chivenge, B Douthwaite) (Springer: Cham, Switzerland)
| Crossref |
Xu RK, Coventry DR (2003) Soil pH changes associated with lupin and wheat plant materials incorporated in a red–brown earth soil. Plant and Soil 250, 113–119.
| Soil pH changes associated with lupin and wheat plant materials incorporated in a red–brown earth soil.Crossref | GoogleScholarGoogle Scholar |
Yunusa IAM (1989) Effects of planting density and plant arrangement pattern on growth and yields of maize (Zea mays L.) and soya bean (Glycine max (L.) Merr.) grown in mixtures. The Journal of Agricultural Science 112, 1–8.
| Effects of planting density and plant arrangement pattern on growth and yields of maize (Zea mays L.) and soya bean (Glycine max (L.) Merr.) grown in mixtures.Crossref | GoogleScholarGoogle Scholar |
Yunusa IAM, Rashid MA (2007) Productivity and rotational benefits of grass, medic pastures and faba beans in a rainfall limited environment. Soil and Tillage Research 97, 150–161.
| Productivity and rotational benefits of grass, medic pastures and faba beans in a rainfall limited environment.Crossref | GoogleScholarGoogle Scholar |
Yunusa IAM, Sedgley RH (1992) Reduced tillering spring wheats for heavy textured soils in a semi-arid Mediterranean environment. Journal of Agronomy and Crop Science 168, 159–168.
| Reduced tillering spring wheats for heavy textured soils in a semi-arid Mediterranean environment.Crossref | GoogleScholarGoogle Scholar |
Yunusa IAM, Zerihun A, Gibberd MR (2018) Analysis of the nexus between population, water resources and Global Food Security highlights significance of governance and research investments and policy priorities. Journal of the Science of Food and Agriculture 98, 5764–5775.
| Analysis of the nexus between population, water resources and Global Food Security highlights significance of governance and research investments and policy priorities.Crossref | GoogleScholarGoogle Scholar |


