Effects of inoculation of wheat (Triticum aestivum) with rhizosphere bacteria in Victoria, Australia
Merfat Ben Mahmud A B † , Grant J. Hollaway C D and Ann C. Lawrie
C D and Ann C. Lawrie  A *
A *
A
B
C
D Present address:
† Merfat Ben Mahmud died in Libya in 2022 while this manuscript was being finalised. The manuscript is based on her research for her completed PhD thesis at RMIT University (Ben Mahmud 2008).
Handling Editor: M Denton
Abstract
Wheat (Triticum aestivum) is an important food crop around the world. Its value depends on high grain yield and high protein content, which requires large inputs of nitrogenous fertiliser.
The aim of this study was to test if inoculation with a N2-fixing bacterium could improve wheat yield and/or protein content.
RMBMTa1 bacteria were isolated from soil in a wheat field at Horsham, Victoria, Australia. Wheat was inoculated with this bacterium in pot and field experiments with three factors: (1) one treatment of N2-fixing bacterium with isolate RMBMTa1; (2) two N treatments (ammonium sulfate, AS; urea); and (3) four N fertiliser rates (1, 50, 100, 150 kg ha−1).
RMBMTa1 was closest (93%) to Paraburkholderia species based on 16S sequencing. In the pot trial, inoculated treatments had the greatest grain dry weight with 100 kg ha−1 AS, potentially doubling profit per hectare. In a field trial during a drought year, inoculation reduced grain weight by 9% but increased grain protein by 50%, with a maximum at 100 kg ha−1 AS, thus increasing its grading.
Inoculating with RMBMTa1 increased both grain yield and protein content when rainfall did not limit yield. Larger-scale coordinated trials of inoculation of wheat varieties with other such native bacteria are needed to test if they offer similar benefits across a range of farming conditions.
RMBMTa1 could potentially increase the value of the wheat crop while saving on input costs and pollution from nitrogenous fertiliser.
Keywords: inoculation, N2-fixing, nitrogen, Paraburkholderia, PGPR, protein, wheat, yield.
Introduction
Wheat (Triticum aestivum) is a major crop that occupies the greatest area of any cereal crop in the world, with trade in its grain exceeding that of all other cereals (Curtis 2002; Ghimire et al. 2021). It is used to make manufactured food products such as bread, noodles, pasta, livestock feed, and to a lesser extent, industrial products such as paper coatings and glue. Its economic return for the farmer increases with yield (grain weight per ha) but also with the grain’s protein content (greater protein content is needed for leavened bread). Conversely, this return is reduced by the cost of fertilisers needed to grow it. Both yield and protein content are affected strongly by rainfall; less rain reduces yield but can increase protein content (Ghimire et al. 2021).
Nitrogen (N), a yield-limiting nutrient (Ghimire et al. 2021), is the fertiliser normally used in the greatest quantity and is also the most expensive. It is commonly applied at 50−150 kg ha−1 as an ammonium salt, a nitrate salt or as urea (Angus 2001). Ammonium nitrate is the most expensive form of nitrogenous fertiliser and concerns about its use in explosives have led to ammonium sulfate (AS) being preferred. Urea is cheaper on a weight-for-weight basis but much nitrogen can be lost as urea breaks down in the field into ammonia and carbon dioxide, which volatilise and so are lost to the crop (Lemon 2007). It has been estimated that for each tonne of grain harvested 26–28 kg of N is removed from the soil per hectare (Kennedy et al. 2004).
Grain is graded and priced by percentage of protein, with minima of 13% for Australian prime hard (APH), 11.5% for Australian hard (AH), 10% for Australian premium white (APW), and less than 10% as Australian standard white (ASW), as specialised noodle and cake flour products (AEGIC 2023) or alternatively APH2 > H1/H2 > APW1 > ASW1 > AGP1 > FED1 (GrainCorp Ltd 2021). Therefore, it is a financial incentive for producing higher-protein grain. Since protein content in grain depends on nitrogen content, each additional percentage of N in the grain is estimated to increase protein content by 5.7%, resulting in a 17.53% increase in price (GrainCorp Ltd 2021).
Biofertilisers contain free-living asymbiotic bacteria associated with the rhizosphere that are called plant growth promoting rhizobacteria (PGPR) and that can benefit both non-legume and legume crops (Hasan and Zainal Abidina 2024). Biofertilisers can potentially play a significant role in plant nutrition by improving plant growth through supplying mineral nutrients (Kennedy and Roughley 2002) and/or fixing atmospheric N2 (Kennedy et al. 2004). Thus, biofertilisers could potentially help to reduce the amount and cost of fertilisers for a crop as well as sustain soil productivity and environmental health (O’Connell 1992; Vance 1997). PGPR typically possess traits such as the production of the plant hormone indole acetic acid (IAA), which can increase stem and root growth, and the plant enzyme 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase, which blocks the production of ethylene released from roots under stress (Timofeeva et al. 2023). PGPR can also solubilise phosphorus from sparingly soluble P compounds in standard tests in vitro (Lin et al. 2006; Bulut 2013a; Ahmed et al. 2014), although this does not imply that they can obtain P adsorbed on the variable-charge surfaces in soil (Barrow 2025). In addition, several PGPR can fix atmospheric N2 and could potentially replace or supplement N applied as fertiliser (Kennedy et al. 2004). Several critics claim that the quantities of N2 fixation claimed are over-estimated and responses to inoculation occur only in the presence of fertiliser nitrogen in the rooting medium, suggesting that responses are more probably due to increases in root growth (mediated by IAA and ACC deaminase) and thus increases in uptake of fertiliser N (Kennedy 2003; Giller et al. 2024).
The main PGPR genera associated with improvement of growth, yield and economic return in cereals include Azospirillum, Azotobacter, Azorhizobium, Bacillus, Burkholderia, Herbaspirillum, Klebsiella and Pseudomonas. Burkholderia sensu lato has now been separated into those with human pathogenic genes (Burkholderia sensu stricto) and those without (Paraburkholderia) (Sawana et al. 2014). This means that any previous reports of soil- or plant-borne Burkholderia with plant-growth promoting activity are more likely to be referable to Paraburkholderia. A recent review of 24 Paraburkholderia species concluded that direct and indirect plant growth-promoting mechanisms included improving nutrient uptake, N2 fixation, stimulating growth by phytohormone production, regulation and stimulation of metabolic pathways, induction of abiotic stress tolerance, and disease control by direct pathogen inhibition or induction of systemic resistance in plants (Rojas-Rojas et al. 2025).
Inoculation of cereal crops with several PGPR genera has been associated with increased grain yield and N content in various countries (Kennedy et al. 2004). Inoculation with Burkholderia sensu lato species increased plant biomass and grain weight in rice (Oryza sativa) (Baldani et al. 2000; Trân Van et al. 2000; Nakata 2002), kikuyu grass (Pennisetum clandestinum), and sugarcane (Saccharum officinarum) (Reis et al. 2000; Paungfoo-Lonhienne et al. 2014). In wheat, an early study of co-inoculation with N2-fixing Azotobacter chroococcum and Bacillus megaterium reported a 10–20% increase in yield in field trials (Brown 1974). An estimated gain of 10–30 kg ha−1 of biologically fixed N per wheat crop by asymbiotic N2 fixation was calculated by Kennedy and Islam (2001) from earlier reports by Bohlool et al. (1992) and Peoples et al. (1995). Inoculation with Pseudomonas sp. alone or with Burkholderia caryophylli increased yield of spring wheat (Walley and Germida 1997; Shaharoona et al. 2007) and inoculation of irrigated wheat with Burkholderia phytofirmans PsJN alleviated the effects of drought on wheat grain yield in Pakistan by almost 20% (Naveed et al. 2014). Inoculation of wheat with Pseudomonas or Bacillus increased grain and straw yield and economic returns at intermediate rates of N and P fertilisation in Pakistan (Hussain et al. 2016). In Türkiye, inoculation of wheat with one phosphate-solubilising and two N2-fixing bacteria resulted in 91% of the grain yield and 85% of the grain nitrogen compared with conventional N + P fertilisation (Bulut 2013a, 2013b). Attachment of the PGPR to the rhizoplane may be a key process in PGPR action. As an example, inoculation of wheat with a genetically transformed N2-fixing strain of Pseudomonas protegens increased plant productivity and total grain nitrogen per plant in non-gnotobiotic soil in a pot trial and was ascribed to N2-fixing microcolonies on the rhizoplane (Fox et al. 2016). Also, co-inoculation of wheat failed due to Paenibacillus polymyxa blocking Azospirillum brasilense binding to root hairs (Yegorenkova et al. 2016).
In Australian wheat cropping areas, Alemneh et al. (2022) found that most of the 743 bacteria isolated from the rhizosphere soil during wheat crops belonged to the recognised PGPR genera Bacillus, Burkholderia and Pseudomonas. Many of these strains, especially the 27 (Para)Burkholderia strains, could increase seedling root growth, produce IAA and ACC deaminase and solubilise phosphorus. Most of the 11 closest species to these (Para)Burkholderia strains have also previously been reported to fix atmospheric N2 (Rojas-Rojas et al. 2025). The (Para)Burkholderia strains isolated from sites with relatively low soil fertility and high aridity had greater IAA and ACC deaminase production and P solubilisation, suggesting that wheat in those sites may already benefit from their activities.
In this study, a N2-fixing bacterium (RMBMTa1) was isolated from wheat fields at Horsham in the Wimmera region of the State of Victoria, Australia. It was identified by morphological and physiological characteristics and by 16S DNA sequencing. The aims of controlled environment and field experiments in this study were to test the effects of inoculating wheat with this strain on the growth, yield and %N of the grain. For this site in the south-eastern wheat belt of Australia, the three hypotheses tested were:
Materials and methods
Bacterial isolation and characterisation
Soil was collected from an experimental field (36°42′S, 142°12′E) that had been used previously for wheat trials run by Agriculture Victoria, close to Horsham, Victoria (36°43′S, 142°11′E), Australia. The soil was a grey clay (pH 6). For details on soil composition, see Supplementary Table S1. Soil samples (1 kg each) for isolation of N2-fixing bacteria were collected at random from the rooting zone (0–20 cm) from within the field.
Burk’s N-free medium (Martínez-Toledo et al. 1985) was used to isolate aerobic N2-fixing bacteria. Each soil sample was mixed thoroughly before 2 g samples were added to aliquots of 25 mL of Burk’s liquid medium and incubated on a shaker for 48 h at 30°C. Loopfuls of the suspension were streaked out on Burk’s solidified medium (20 g L−1 agar added) and incubated at 30°C for 2–5 days. Single colonies were sub-cultured by streaking out five times with a 3-day incubation at 30°C each time before analyses.
Bacterial isolates were Gram-stained and tested for motility using a Craigie tube (Harvey and Price 1967). Standard tests were conducted for oxidase, urease, catalase, gelatin hydrolysis, H2S production and nitrate reduction (Seeley and Vandemark 1981). The production of flexirubin pigments was determined by the method of Fautz and Reichenbach (1980). Bacteria were tested for acetoin production by Voges-Proskauer tests and for the ability to grow anaerobically as well as aerobically (Seeley and Vandemark 1981). Carbohydrate utilisation was tested with 0.5% (w/v) of fructose, galactose, glucose, lactose, maltose, mannitol, sucrose, inositol, sorbitol, raffinose and glycerol as sole carbon sources. Starch hydrolysis was tested by growth on starch agar plates and citrate utilisation on Simmon’s citrate agar plates (Seeley and Vandemark 1981). Salt tolerance was tested in nutrient broth with 3% and 5% NaCl (Seeley and Vandemark 1981). Temperature tolerance was tested by growth on Burk’s N-free solid and liquid media at 4°C, 15°C, 21°C, 30°C and 37°C for 3 days. Tolerance of pH was tested by growth in Burk’s N-free liquid medium at pH 4, 5, 6, 7, 8 and 9 for 2 days. Phosphate solubilisation was tested on MIS agar with 2 g L−1 glucose for 7 days (Illmer and Schinner 1992). Antibiotic sensitivity was tested for bacitracin, penicillin G, ampicillin and nalidixic acid, all at 1 mg mL−1, filter-sterilised into Burk’s N-free solidified medium and incubated for up to 72 h at 30°C after inoculation; lack of growth was taken as sensitivity.
DNA was extracted from the isolated bacteria using freeze-thaw cycles with the chloroform-phenol method (Lee and Taylor 1990) or by using a Qiagen DNeasy Blood and Tissue Kit according to the manufacturer’s instructions. The 16S rDNA was amplified using universal bacterial primers fD1 and rP2 according to Weisburg et al. (1991). Amplified rDNA restriction analysis (ARDRA) was performed on the PCR product using the following enzymes: AluI, DdeI, HaeIII, HhaI, HinfI, MspI and RsaI (Fermentas, Progen). A 4-μL aliquot of each PCR product was digested using 0.5 U of the restriction enzyme in 0.5 μL of the corresponding buffer, in a final volume of 20 μL, for 2 h at 37°C or 65°C according to the manufacturer’s instructions. The digestion products were analysed by electrophoresis on 2% agarose gel with TBE buffer (Tris-borate-EDTA), stained with ethidium bromide and examined on a Bio-Rad Gel-doc system. The sizes of the fragments were compared among isolates and sizes calculated relative to a molecular weight marker (GeneRuler 1 kb Plus DNA Ladder, Thermo Fisher Scientific).
Amplified 16S rDNA was purified by gel extraction according to the manufacturer’s instructions (Qiagen QIAquick Gel Extraction Kit) and sequenced by the Sanger method in both directions using Big Dye ver. 3.1 (Applied Biosystems) according to the manufacturer’s instructions. Reaction products were purified using the Applied Biosystems Ethanol Precipitation protocol 1 and sent to Micromon (http://www.micromon.monash.org/) at Monash University for electrophoresis. Sequences were Blast-searched (Altschul et al. 1997) for closest matches in GenBank at NCBI (National Center for Biotechnology Information) (http://www.ncbi.nlm.nih.gov). The closest matches and sequences of type species were downloaded, aligned with the query sequences using ClustalW (Thompson et al. 1994), end-trimmed, analysed using Maximum Likelihood and displayed as a bootstrapped tree in MEGA ver. 7.0.26 (Kumar et al. 2016) (https://www.megasoftware.net/dload_win_gui). The 16S sequence has been deposited in GenBank through the portal at NCBI and given the accession number PP029198.
Pot trial
This experiment was conducted over 23 weeks at RMIT in a controlled temperature room at 25°C and a photoperiod of 12 h at a light level (PAR) of 270 μmol m−2 s−1 with three replicates using a split-split-plot design with three factors: with and without bacterial inoculation, two types of fertiliser (urea 46% N and AS 21% N) and four rates of fertiliser (0, 50, 100, 150 kg N ha−1), giving a total of 16 treatments (2 × 2 × 4) with three replicates per treatment. For details on when samples were taken, see Table S2.
The N2-fixing bacterium (RMBMTa1) isolated from Horsham soil was cultured in 25 mL aliquots of Burk’s N-free medium (pH 7.0) for 5 days on a shaker at 30°C, after which flour was added to make a weak paste and mixed in thoroughly to coat each grain with the paste and give 106 bacteria per grain. The inoculated grain was left for approximately 12 h to air-dry out of direct sunlight in a laminar flow cabinet.
Fresh soil was collected from the same field site at Horsham and used to fill ‘pots’, which were sections of white PVC slotted agricultural drainage pipe (1 m depth × 10 cm diameter). The wheat field soil was a grey clay, pH 6, as before. ‘Pots’ were stood upright in upturned polystyrene boxes inside covered basins to contain the soil at the base (Fig. S1). Each’ pot’ was filled with 6 kg of freshly collected soil to 7 cm below the top and three wheat seeds were sown 25 mm deep in each pot. Pots were irrigated manually to field capacity twice a week. Each pot was fertilised with N (as AS or urea) at 0, 50, 100 or 150 kg ha−1 and with P as superphosphate at 100 kg ha−1 applied at planting. The wheat variety used was ‘Yitpi’ (Code 314), graded as Australian Hard (Grains Australia 2023).
Roots and soil were sampled every 5 weeks to assay nitrogenase activity by acetylene reduction activity (ARA) in the pots. Each ‘pot’ had slits (7 cm long) located 20, 40 and 80 cm from the soil surface (Fig. S1). For the assay, these holes were sealed using grey duct tape. Pots were injected with 10% (v/v) of acetylene (99.99% C2H2, Linde Specialty Gases), puncture holes in the tape were sealed with further tape and the pots were incubated at 25°C for 30 min. After incubation, gas samples were removed and stored by injection into McCartney bottles plugged with Suba-Seal (Crown Scientific) rubber stoppers. Duct tape was removed after gas samples were collected. Ethylene production was measured as peak height on a 2-m stainless steel Porapak T column on a Varian Aerograph (series 1400) gas chromatograph by injecting triplicate samples of 1 mL from each Subasealed bottle. Ethylene was quantified using a calibration curve of peak height versus moles of ethylene injected.
To calculate what percentage of the gain in grain N was attributable to N2 fixed by the RMBMTa1 inoculum, the total N2 fixed throughout the lifetime of the inoculated plants was estimated by curve fitting from the known ARA rates using the conventional rate of 4:1 and compared with the difference in grain N content between inoculated and uninoculated treatments.
‘Pots’ were sampled during the growth cycle at approximately 5 weeks (early heading), 10 weeks (head emergence), 15 weeks (flowering) and 20 weeks (grain filling). Whole plants (both tops and roots) were removed from the pots. Non-rhizosphere soil was removed by shaking off loosely adhering soil. Rhizosphere soil was obtained by removing and mixing tightly adhering soil from the remaining root material. A 10-fold dilution series in sterile 0.8% NaCl solution was prepared from both rhizosphere and non-rhizosphere soil samples and 1 mL of the appropriate dilutions was plated in triplicate on Burk’s N-free medium. After 8 days of incubation at 30°C, some of the colonies grown on the plates were approximately 1 mm diameter and white with flat margins. Two plates of rhizosphere and non-rhizosphere samples with approximately 100 colonies were chosen at random and transferred onto other media to perform further tests to check for purity and their DNA was extracted to test if they were the inoculum. Soil pH in each treatment was determined using a pH electrode at 20 weeks in a 1:1 mixture of soil:water using standard methods (Agriculture Victoria 2020).
To distinguish between the inoculated RMBMTa1 and other N2-fixing bacteria, a pair of specific primers based on the 16S sequence of RMBMTa1 was designed and synthesised at Micromon (Monash University). The forward primer was (BurkF – 5′CTGCGAAAGCCGGAT 3′) and the reverse primer was (BurkR −5′TGCCATACTCTAGCYYGC 3′). DNA was extracted from bacteria grown from rhizosphere and non-rhizosphere soil and amplified using these specific primers by PCR in 25 μL reaction mixtures containing 1 μL of DNA, 0.25 μL of each primer, and Go-Green PCR mix as described previously. Cycling was 95°C (2 min), 35 cycles of: 94°C (30 s), 55°C (30 s) and 72°C (1 min), plus nine additional cycles with 94°C (30 s), 47°C (30 s) and 72°C (90 s) and a final extension at 72°C (5 min). PCR products were electrophoresed, stained and visualised as before. DNA extracts with a single band corresponding to that expected were scored as belonging to the RMBMTa1 inoculum.
At 23 weeks, plants were harvested and dry weight and %N in grain measured. Grain was dried at 65°C for 72 h, ground in a coffee grinder and 100 mg aliquots analysed for total N by standard Kjeldahl methods (AOAC International 2023). Grain protein was estimated by multiplying grain %N by 5.7 (Fowler et al. 1990).
Field trial
Wheat grains were inoculated as in the pot trial by coating with flour moistened with Burk’s liquid medium containing bacteria (106 per grain), left overnight to air-dry out of direct sunlight and stored at 4°C until sown. Seed of the wheat cv. Yitpi was thoroughly mixed with this inoculum (for appropriate treatments) before sowing near Horsham at Agriculture Victoria’s Smart Farm in a fully randomised field experiment during the winter wheat season of 2006 (with supplementary irrigation because of less than average rainfall).
The field experiment was arranged as a randomised block design with three replicates. Treatments were as applied in the pot trial (Table S3). Each plot was 10 m in length (cut back to 7.05 m) and 1.2 m wide with six rows of seed. Seed was sown at a planting rate of 100 kg ha−1 at 70–80 mm deep, without fungicide. At sowing, each plot received one of two types of nitrogen fertiliser and all treatments received phosphate fertiliser as mono-ammonium phosphate (22% P) at 100 kg ha−1 at sowing on 21 July (mid-winter). Inoculated plots received grain with adherent bacteria-containing paste. No pesticide was applied at planting or during growth.
The Horsham field was flood-irrigated using fully treated water from a 700 mL storage pond used by the Horsham Sewerage Treatment Plant (part of Grampians Wimmera Mallee Water Corporation, https://gwmwater.org.au/). The site was watered prior to sowing in April and received two flood irrigations during spring (August–September). Plant emergence was assessed on 11 and 22 August, when the number of plants emerging in two 0.5-m2 areas were counted within each plot and these two counts added to give plants per m2. Grain was harvested on 20 December (early summer) (22 weeks) using a mechanical plot harvester. Data on the rainfall and maximum and minimum temperature for both the year of the field trial and the average for 1966–2006 were downloaded from the Bureau of Meteorology (2024) weather stations 079082 Horsham (rainfall) and Horsham aerodrome 079100 (temperature).
One month before harvest, plants and rhizosphere were sampled on 20 November (late spring) (17 weeks). A random 1-m2 area was selected near the centre of every plot for each replicate of each treatment. Within each 1-m2 area, culms and ears were counted, maximum height measured and all wheat plants within the area uprooted and removed into paper bags for drying and subsequent measurement of dry weight and %N of plants and grain. Plant tops were stored at 4°C until dried at 80°C to constant weight. Harvested grain was ground finely, mixed thoroughly and its nitrogen content measured by Kjeldahl analysis. Grain protein was estimated by multiplying grain %N by 5.7 as for pot trial.
Rhizosphere soil samples were collected from the roots at harvest to determine the population of diazotrophic bacteria. As plants were removed from the 1 m2 random plot, roots were shaken gently to remove the loosely attached soil before tightly attached rhizosphere soil was removed and stored at 4°C for up to a week until used to assess numbers of diazotrophic bacteria in the rhizosphere. Rhizosphere samples from each plot were mixed thoroughly and suspended as 5 g dry soil in 50 mL sterile water. This suspension was shaken for 10 min and left to stand unshaken for 5 min, after which 1 mL of the supernatant liquor was diluted in triplicate to 10−3−10−6 into Burk’s N-free solidified medium and incubated at 30°C for 8 days before colonies were counted to assess colony-forming units (CFU).
Statistical analysis
All statistical analyses were performed using ANOVA in Generalised Linear Modelling mode in various versions of the statistical program Minitab (www.minitab.com). Data were checked for normality and transformed as necessary before analysis. Three-way analysis of variance was performed and the significance of differences between means was determined using Tukey’s family error test. A significance level of P ≤ 0.05 applied throughout. All bars on data in graphs are 2 × s.e..
Results
Bacterial isolation
Colonies of bacteria isolated from soil samples on Burk’s N-free medium were white, glossy and gummy. The results of the biochemical and physiological tests are in Table 1. The bacteria were white, Gram-negative, motile and obligately aerobic. They had variable oxidase activity and all had catalase activity. They utilised fructose, galactose, glucose, lactose, mannitol, sucrose, inositol, sorbitol, raffinose and glycerol but not maltose. They hydrolysed urea, used citrate, reduced nitrate to nitrite but not further and grew in both 3% and 5% NaCl. Tests were negative for production of H2S, starch and gelatin hydrolysis, flexirubin pigmentation, the Voges-Proskauer test and phosphate solubilisation. The bacteria grew better in liquid and on solid medium at 21–37°C than at 4−15°C. They also grew well at pH 5–8 but not at pH 4 or 9. The bacteria were sensitive to penicillin G and nalidixic acid but not to bacitracin and ampicillin.
| Parameter | Horsham soil bacterium RMBMTa1 | Paraburkholderia cabelleronis (Martínez-Aguilar et al. 2013; Rojas- Rojas et al. 2017) | Burkholderia australis (Paungfoo- Lonhienne et al. 2014) | |
|---|---|---|---|---|
| Colony colour | White | White | White | |
| Gram stain | – | – | – | |
| Motility | + | + | + | |
| Oxidase | + | v | + | |
| Catalase | + | + | + | |
| H2S production | – | |||
| Flexirubin pigmentation | – | |||
| Voges-Proskauer test | – | |||
| Growth in glucose | ||||
| - Aerobic | + | + | + | |
| - Anaerobic | – | – | – | |
| Aerobic growth on carbohydrates | ||||
| - Fructose | + | + | + | |
| - Galactose | + | + | ||
| - Glucose | + | + | + | |
| - Lactose | + | – | – | |
| - Maltose | – | – | – | |
| - Mannitol | + | + | ||
| - Sucrose | + | + | – | |
| - Inositol | + | + | ||
| - Sorbitol | + | + | – | |
| - Raffinose | + | + | – | |
| - Glycerol | + | v | ||
| Starch hydrolysis | – | |||
| Citrate utilisation | + | – | + | |
| Urease | + | + | – | |
| Gelatin hydrolysis | – | – | – | |
| N2 fixation | + | + | ||
| Reduction of | ||||
| - Nitrate to nitrite | + | – | – | |
| - Nitrite to gas | – | – | ||
| Growth in NaCl | ≤5% | ≤3% | ||
| Phosphate solubilisation | – | – | ||
| Growth at pH | 5–8 | 4.0–8.5 | ||
| Temperature tolerance (°C) | 21–37 | 15–42 | 28–37 | |
| Antibiotic sensitivity | ||||
| - Bacitracin | + | |||
| - Penicillin G | – | |||
| - Nalidixic acid | – | |||
| - Ampicillin | + | |||
+, Yes; –, No; v, Variable.
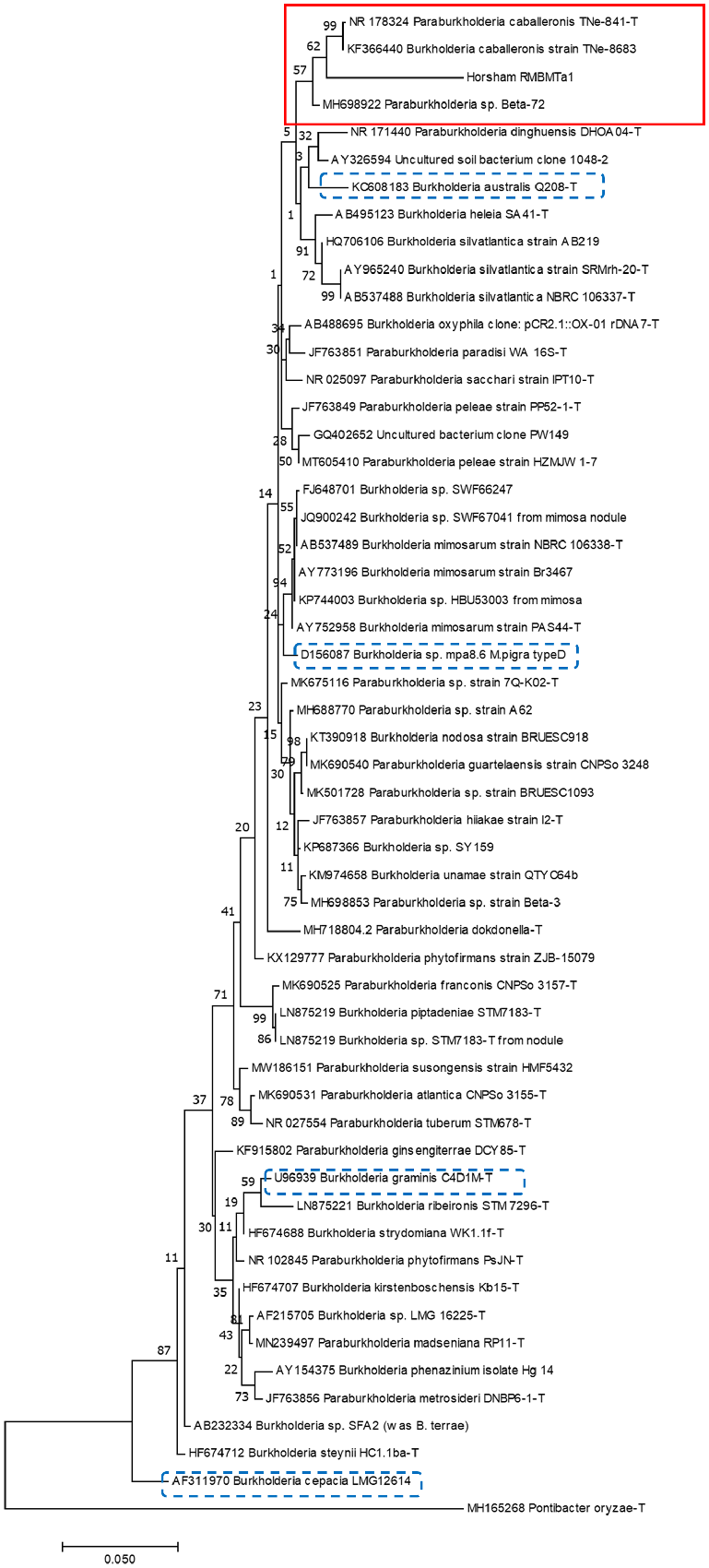
PCR amplification of 16S rDNA from all strains showed a single band of the expected size of approximately 1500 bp. PCR products were digested with DdeI, HhaI and MspI but not with AluI, HaeIII, HinfI or RsaI. RFLP analysis (ARDRA) indicated that all isolates showed identical patterns. The 16S sequences most closely matched those of Paraburkholderia species (Betaproteobacteria) in GenBank but were only 93% similar to a range of species. They most closely matched those of P. cabelleronis and Paraburkholderia sp. Beta-72 in 16S sequence (Fig. 1) but differed from them physiologically (Table 1). As all isolated strains were identical in all features tested, one strain (RMBMTa1) was chosen for pot and field trials.
Relationships between 16S sequences of Horsham bacterium RMBMTa1 and those of species of Burkholderia and Paraburkholderia. The evolutionary history was inferred by using the Maximum Likelihood method based on the Tamura-Nei model (Tamura and Nei 1993). The tree with the highest log likelihood (−6316.40) is shown. The percentages of trees in which the associated taxa clustered together are shown next to the branches. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) approach and then selecting the topology with superior log likelihood value. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. The analysis involved 55 nucleotide sequences. Codon positions included were 1st + 2nd + 3rd. All positions containing gaps and missing data were eliminated. There was a total of 1235 positions in the final dataset. Evolutionary analyses were conducted in MEGA7 (Kumar et al. 2016). Boxed area in red solid line = RMBMTa1 and closest matches, boxed areas in blue dashed lines = other Australian Burkholderia isolates. Sequences are named Burkholderia or Paraburkholderia as in NCBI and GenBank.
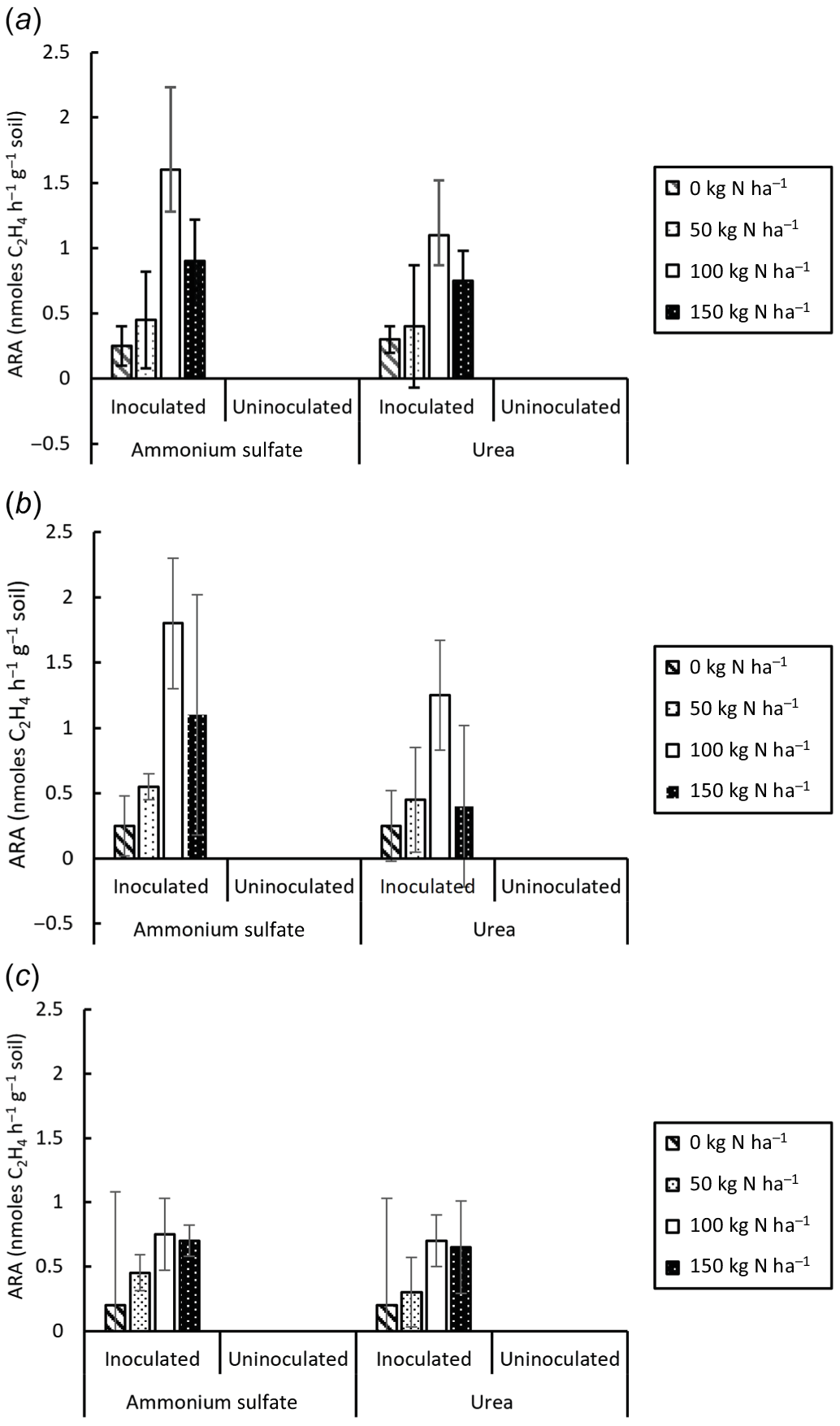
Pot trial
ARA was detected in inoculated but not uninoculated treatments (Fig. 2) and increased with inoculation (F = 727.85, P < 0.001), plant age (F = 3.69, P = 0.029) and N rate (F = 66.61, P < 0.001) but not N type (F = 2.19, P = 0.142). There were significant interactions between inoculation and plant age (F = 3.69, P = 0.029); ARA was greater at 5 weeks and 10 weeks than at 15 weeks and absent at 20 weeks (not shown). There was also a significant interaction between inoculation and N rate (F = 66.61, P = 0.029); ARA was greater with 100–150 kg N ha−1 than with 0–50 kg N ha−1.
N2-fixing bacteria were detected on Burk’s N-free plates at all stages in all treatments and ranged from 1.5 × 102 CFU mL−1 to 1 × 108 CFU mL−1 (Fig. 3). The number of N2-fixing bacteria in the wheat rhizosphere varied with inoculation (F = 115.52, P < 0.001), N rate (F = 102.78, P < 0.001), N type (F = 5.73, P = 0.019) and plant age (F = 8.40, P < 0.001). There were significant interactions between inoculation and plant age (F = 9.24, P < 0.001) and inoculation and N rate (F = 14.15, P < 0.001). In inoculated treatments, bacterial numbers in the rhizosphere increased between early heading (5 weeks) and head emergence (10 weeks) before decreasing at flowering (15 weeks), when the difference between the inoculated and uninoculated treatments narrowed. The maxima were reached in inoculated treatments at head emergence with 100 kg N ha−1 but with lesser maxima at 100–150 kg N ha−1 in uninoculated treatments. Maxima were greater with AS than with urea. By contrast, uninoculated treatments had a smooth increase of bacterial number in the rhizosphere with age and fertiliser dose and the maxima were reached at 100–150 kg N ha−1 at flowering.
Grain weight per pot ranged from 0.70 g to 3.91 g and was affected significantly by inoculation (F = 34.23, P < 0.001) and N rate (F = 75.64, P < 0.001), but not by N type (F = 0.76, P = 0.390) (Fig. 4a). There was a significant interaction between inoculation and N rate (F = 11.76, P < 0.001) whereby both inoculation and N fertilisation increased grain weight per pot, but the maxima differed (at 100 kg N ha−1 in inoculated treatments but at 150 kg N ha−1 in uninoculated treatments). There was also a significant three-way interaction (F = 14.55, P < 0.001) in inoculated treatments with fertiliser at 100 kg N ha−1 whereby grain weight per pot was greater with AS than with urea. The least grain weight was obtained in the uninoculated, unfertilised treatments and the greatest in the inoculated treatment with 100 kg N ha−1 as AS.
Effect of inoculation on (a) grain dry weight, (b) %N in grain and (c) pH of rhizosphere soil at harvest in pot trial. Vertical bars on columns are 2 × s.e. l.s.d.0.05 for (a) 0.4237, (b) 0.0940 and (c) 0.3441.
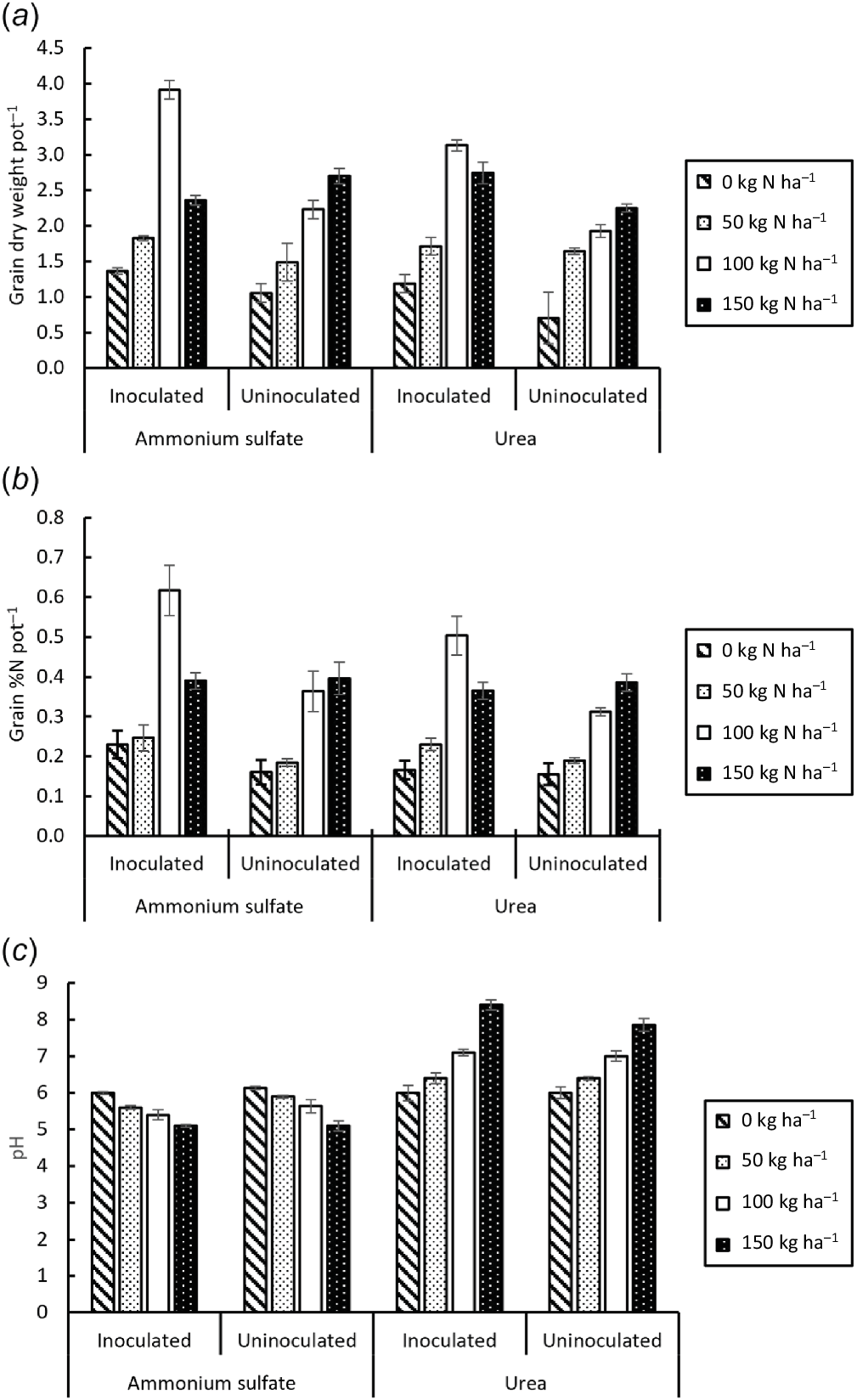
The %N in grain ranged from 0.9% to 3.7% (Fig. 4b) and was affected significantly by inoculation (F = 21.18, P < 0.001), N rate (F = 64.57, P < 0.001) and N type (F = 4.61, P < 0.040). There were significant interactions between inoculation and N rate (F = 97.65, P < 0.001), N rate and N type (F = 10.79, P < 0.001), and a three-way interaction (F = 10.76, P < 0.001). Both inoculation and N fertilisation increased grain %N, with a maximum at 100 kg N ha−1 in inoculated treatments but at 150 kg N ha−1 in uninoculated treatments. Fertilisation with 100 kg N ha−1 AS resulted in greater %N in grain than with the equivalent dose of urea. The maximum %N in grain was reached with inoculation at 100 kg N ha−1 with AS and the minimum in grain %N was recorded without inoculation or N fertiliser.
The pH of the rhizosphere soil at harvest ranged from 6.0 to 6.2 without N fertiliser but 4.8–8.5 with N fertiliser. Rhizosphere pH declined by ≤1.2 units with AS but increased by 1–2 units with urea (Fig. 4c). The pH was affected by N fertiliser type (F = 570.6, P < 0.001) and rate (F = 18.54, P < 0.001) but not by inoculation (F = 0.747, P = 0.393). The magnitude of the change increased with the dose of N fertiliser supplied due to the interaction between N rate and N type (F = 124.68, P < 0.01).
Field trial
Rainfall during 2006 at Horsham was 237 mm, only 56% of the long-term average of 448 mm (Bureau of Meteorology 2024). This was within the least 5% of collected data for Horsham weather station 079082 during 1966–2006 (Fig. S2). During the field trial (July–December), rainfall was 46–83% of average in July–September but was only 6–26% in the vital grain-filling months of October–December. Supplementary flood irrigation was applied twice during the critical flowering to grain filling stages to avoid water stress. There were fewer changes in average temperature, although minima were 1–2°C cooler than average during growth.
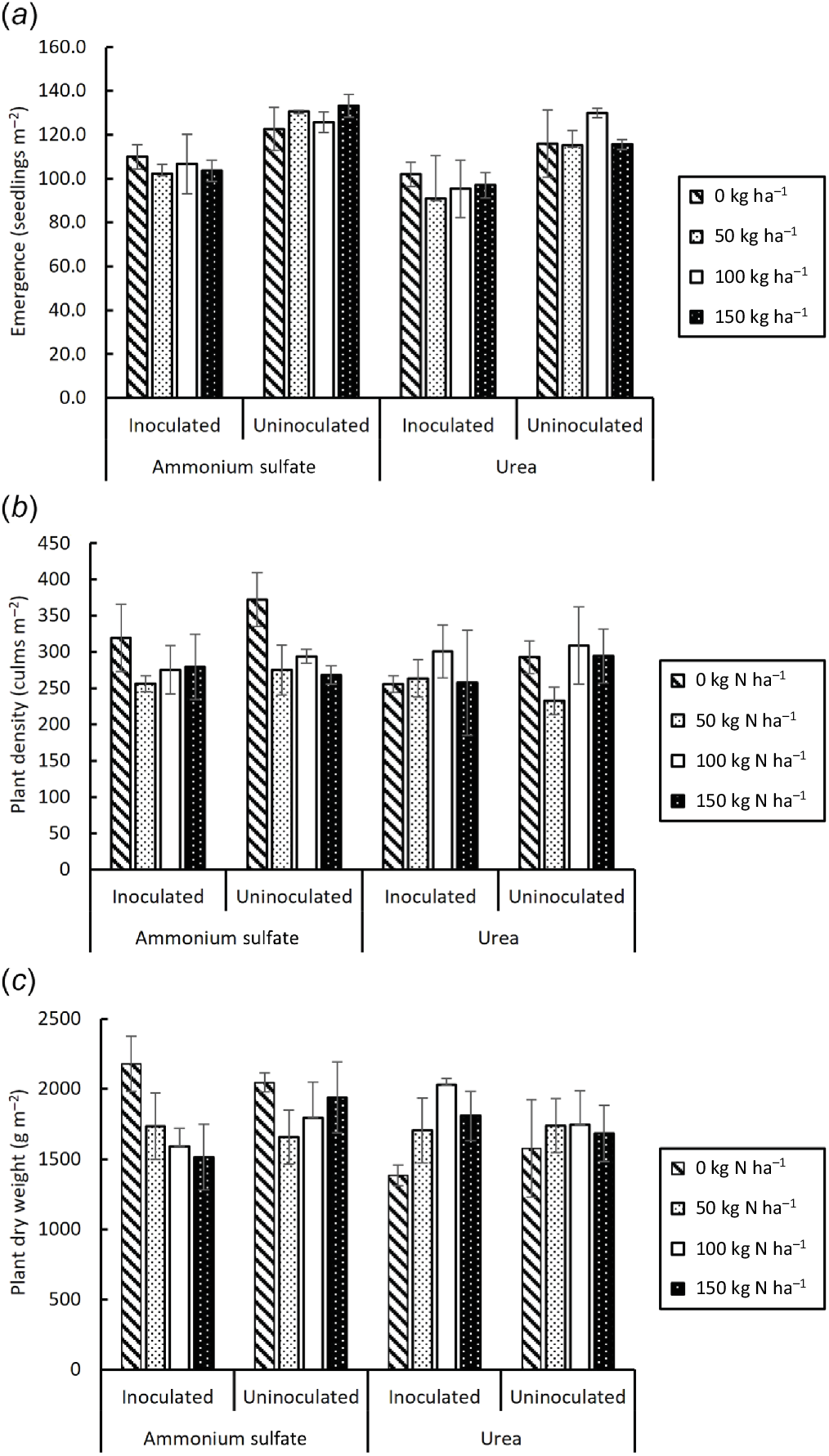
Inoculation reduced emergence (F = 25.268, P < 0.001) (Fig. 5a), had no effect on density of culms or ears (F = 0.815, P = 0.393) (Fig. 5b) or plant dry weight (F = 0.091, P = 0.765) (Fig. 5c) but reduced grain weight per unit area by an average of 8.8% at harvest (F = 7.224, P < 0.011) (Fig. 6a). By contrast, inoculation increased grain %N (F = 37.801, P < 0.001), more with AS than with urea (F = 9.849, P < 0.004), and was affected by N rate (F = 108.782, P < 0.001) (Fig. 6b). There were significant interactions of inoculation with N rate (F = 9.527, P < 0.001) and N source (F = 4.216, P = 0.048). At harvest, grain %N varied from 1.1 to 2.7% and was maximal in inoculated plants with 100 kg N ha−1 AS but at 150 kg N ha−1 in uninoculated plants.
Plant growth parameters at Horsham field site. (a) Seedling emergence 1 month after planting, (b) culm (and ear) density and (c) plant dry weight 1 month before harvest (4 months). Vertical bars on columns are 2 × s.e. l.s.d.0.05 for (a) 25.98, (b) 103.72 and (c) 565.75.
Parameters of grain and inoculum at Horsham field site: (a) dry weight, (b) %N of grain and (c) population of N2-fixing bacteria (CFU). Dashed lines in (b) indicate protein thresholds for wheat classifications: 13%; 11.5%; and 10.5% using standard multiplier 5.7 × %N. Vertical bars on columns are 2 × s.e. l.s.d.0.05 for (a) 23.1928, (b) 0.25442 and (c) 0.2037.
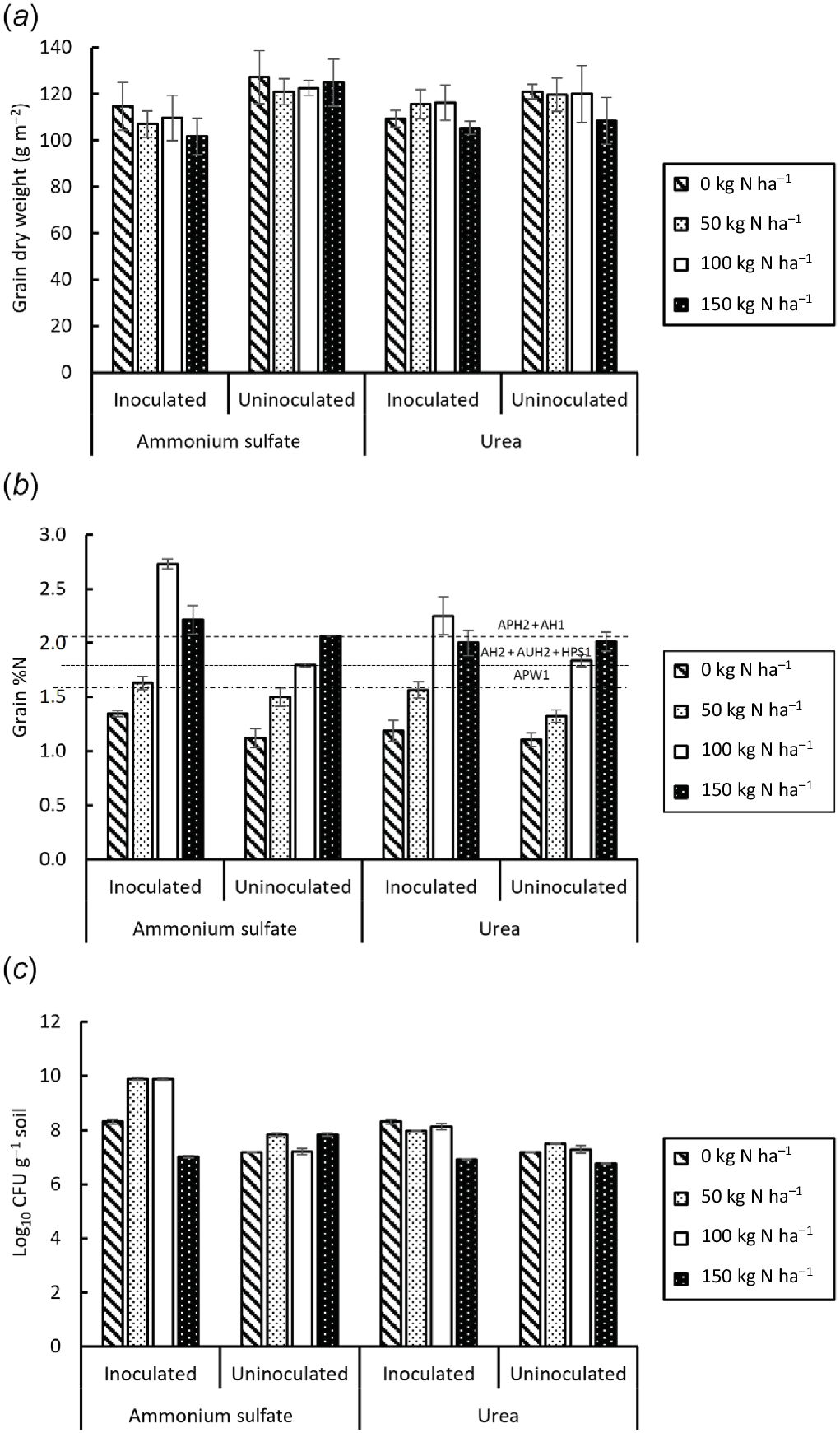
The population of N2-fixing bacteria isolated from the rhizosphere at harvest was 5.79 × 107−7.8 × 1010 CFU g−1 soil and was significantly affected by all factors and their interactions (Fig. 6c). Inoculation increased the N2-fixing bacterial population by 1–2 logs (F = 735.151, P < 0.001), accounting for 25% of total variation, but had significant interactions with N source (F = 72.685, P < 0.001) and N rate (F = 163.503, P < 0.001). Populations in inoculated treatments were greatest at 50–100 kg N ha−1 with AS but at 0–100 kg N ha−1 with urea.
Discussion
The aim of these laboratory and field studies was to test the effects of inoculating wheat with a newly isolated strain of bacteria (RMBMTa1) from soil grown with wheat on the growth, yield and N content of wheat grown in the same soil. The results from the pot trial suggested that inoculation had the potential to increase grain yield and %N but that this was confined to increasing grain %N in this field trial. This suggested that the outcomes of inoculation for the farmer ranged from a potential extra profit of A$281 ha−1 in good growing conditions to an extra loss of A$41 ha−1 in poor growing conditions, as discussed below.
Bacterial isolation
This is the first time that this bacterium has been reported in association with wheat in Australia. Based on molecular and physiological evidence, RMBMTa1 is most closely related to species of Paraburkholderia (Betaproteobacteria) (Kaur et al. 2017). The closest matches to the 16S sequence of RMBMTa1 from Horsham soils were those of Paraburkholderia sp. Beta-72 from South Korea and Paraburkholderia caballeronis from Mexico (Martínez-Aguilar et al. 2013; Rojas-Rojas et al. 2017; Mullins and Mahenthiralingam 2021), but these had only 93% similarity, less than required for conspecificity (Chun et al. 2018). The 16S sequence of RMBMTa1 was not most closely like those of the (Para)Burkholderia species previously isolated from the wheat rhizosphere in Australia such as Blumeria graminis (Viallard et al. 1998), Burkholderia cepacia genomovar III (Balandreau et al. 2001), or the 11 species found by Alemneh et al. (2022). It was also not closely like Paraburkholderia species isolated from the rhizosphere of sugarcane (Paungfoo-Lonhienne et al. 2014), from nodules of two Acacia species (Hoque et al. 2011) or from nodules of the invasive weed Mimosa pigra (Parker et al. 2007) in Australia. Further work to characterise and speciate RMBMTa1 as a new species would require further physiological and biochemical tests (Coenye and Vandamme 2003) and sequencing of several other parts of the genome (Mahenthiralingam et al. 2000).
The lack of diversity in N2-fixing bacteria isolated from the Horsham wheatfield site was also noted in South Australian isolates (Balandreau et al. 2001) and may be due to the high competitiveness of RMBMTa1. Other bacteria may have been out-competed during liquid culturing before plating and colony picking, as Tago et al. (2006) observed that microcosms rapidly became dominated by Paraburkholderia species. Beukes et al. (2021) noted that soils with low pH, low available N and periodic drought were associated with dominance by Paraburkholderia species. This would apply to Horsham wheatfield soils, which had a pH of 6, relatively low nutrients, including low N, and are subject to summer drought, with a major drought occurring approximately every 10 years (Bureau of Meteorology 2024). Such characteristics (low mineral nutrients and high aridity) were also found by Alemneh et al. (2022) to increase characteristics contributing to increased root growth (IAA and ACC deaminase production and P solubilisation) in (Para)Burkholderia strains isolated from the wheat rhizosphere in Australian wheat-growing areas.
RMBMTa1 may benefit wheat plants through mechanisms other than N2 fixation. There are currently 96 validly published and correct species of Paraburkholderia (https://lpsn.dsmz.de/genus/paraburkholderia), which are >98% similar in their 16S sequences (Sawana et al. 2014; Oren and Garrity 2015; Dobritsa and Samadpour 2016; Paulitsch et al. 2021). Paraburkholderia species are found in soils, plant rhizospheres and nodules (Sawana et al. 2014) and are frequently associated with improved plant growth (Fan et al. 2015; Chhetri et al. 2023; Rojas-Rojas et al. 2025). Paraburkholderia sp. has also been found previously in the wheat rhizosphere in China after mulching with straw and fertilisation with 120 kg N ha−1 (Chen et al. 2021). The actions of Paraburkholderia strains are diverse: some fix N2 from the surrounding air; some reduce plant pathogenesis (Azcón 1989, 1993; Liu et al. 2022); and some improve plant growth under both biotic and abiotic stress (Fan et al. 2015; Esmaeel et al. 2018; Alemneh et al. 2022). Some make nutrients more available to plants through solubilisation of P (Gao et al. 2019; Beukes et al. 2021, Alemneh et al. 2022) or sequestration of Fe through production of siderophores (Gopalkrishnan et al. 2018; Hermenau et al. 2018, 2019). Others invade plants (Compant et al. 2008) and even form nodules on legume roots, including in Australia (Frommel et al. 1991; Coenye et al. 2001; Vandamme et al. 2002; Sessitsch et al. 2005; Compant et al. 2008). Many Paraburkholderia strains isolated from agricultural soils have shown degradation of aromatics (Wilson et al. 2003; Iwaki and Hasegawa 2007; Sakai et al. 2007; Beukes et al. 2021; Eze et al. 2022) and methanotrophy in peat (Too et al. 2021). This suggests that RMBMTa1 may be capable of degrading more complex substances, such as lignified crop residues. Such abilities would help make nutrients from crop residues available for wheat growth, both directly and indirectly, by providing energy for the metabolism of the inoculant, thereby helping to explain how the bacterium improves wheat yield and grain quality. It would also be essential to test RMBMTa1 for PGPR qualities, such as its ability to produce IAA and ACC deaminase, solubilise P and increase root growth as well as other traits (Rojas-Rojas et al. 2025).
Pot trial
This is the first demonstration that inoculation with RMBMTa1, a Paraburkholderia-like bacterium, increased grain yield and %N in a pot trial of wheat in Australian soil. In uninoculated plants, grain dry weight, %N and estimated protein content increased linearly with fertiliser dose for both AS and urea, as found in field trial sites in the USA (Ghimire et al. 2021). However, this was not the case in inoculated plants, in which these maxima were reached with less than the maximum N fertiliser dose.
Could the increase in %N in grain in inoculated treatments in this pot trial be only or mainly ascribed to N2 fixation by the inoculant bacteria? ARA was present only in inoculated pots and the parallel patterns in the magnitudes of ARA and plant and grain N with fertiliser N dose may suggest this. Also, the benefit of inoculation declined at 150 kg ha−1 of nitrogenous fertiliser, as noted in maize (Zea mays) with N2-fixing bacteria (Dobbelaere et al. 2001), presumably because of a declining C:N ratio in the soil, which mitigates against N2 fixation (Kennedy and Islam 2001). However, the measured increase in grain N in wheat in this pot trial was up to 179% yet only up to 19% of this increase could be accounted for by estimates from ARA using a conventional 4:1 ratio of C2H2 reduction:N2 fixation. Also, little or no benefit of inoculation was observed until 50–100 kg ha−1 of nitrogenous fertiliser had been added, suggesting that the main benefit of the inoculant was to increase plant growth and uptake of fertiliser N, as found in other crops (Kennedy et al. 2004; Bulut 2013a, 2013b; Paungfoo-Lonhienne et al. 2014; Gallart et al. 2021, 2022). A critical examination of N2 fixation by the bacteria using 15N uptake in situ is needed to quantify potential nitrogenase activity, as well as other tests specified by Giller et al. (2024). Furthermore, the benefits observed are not necessarily the action of live bacteria. More extensive experimentation, such as including control treatments of grain coated with starch alone and of grain inoculated with dead bacteria, is required to exclude other influences.
The amount of grain N gained in this pot test was comparable with the estimated 10–30 kg N ha−1 (Kennedy and Islam 2001) and the 27–33 kg ha−1 (Ladha et al. 2016) gained in wheat, though these estimates need to be applied with caution (Unkovich et al. 2020; Herridge et al. 2022; Giller et al. 2024). The types of effects of RMBMTa1 in wheat are like those of other N2-fixing Paraburkholderia and Burkholderia species from cereal-growing soils; e.g. Burkholderia vietnamiensis (Gillis et al. 1995; Trân Van et al. 1996), Paraburkholderia kururiensis (Estrada-De los Santos et al. 2001), Paraburkholderia unamae (Caballero-Mellado et al. 2004), Paraburkholderia tropica (Reis et al. 2004; Bernabeu et al. 2018), Burkholderia brasilensis (Reis et al. 2000) and Burkholderia australis (Paungfoo-Lonhienne et al. 2014). Burkholderia inoculants as biofertilisers have also increased yield of other cereal crops (Omar et al. 1992; Baldani et al. 2000; Guimarães et al. 2000; Trân Van et al. 2000; Ciccillo et al. 2002) and other grasses (Paungfoo-Lonhienne et al. 2019). However, inoculation of wheat with Paraburkholderia tropici (isolated from maize and sugarcane) in Argentina resulted in good endophytic and rhizoplane colonisation but no significant differences in wheat yield with or without N and P fertilisation (Bernabeu et al. 2018). Further experimentation is needed, including critical evaluation of the magnitude of any N2 fixation by the inoculant bacteria relative to any N gains in crops. This would involve feeding with 15N2 and quantifying the amount taken up by the plants relative to that acquired from N fertiliser.
There was a greater response in wheat grain yield and %N to AS than urea. This could be partly because of the greater breakdown and volatilisation of urea as NH3 and CO2 (Parr and Papendick 1965). AS also sticks to clay particles and so is much less likely than urea to be leached down past the plant roots, especially over pH 7.0 (Holford et al. 1992). The greater response to AS than to urea could also be due to their differing their effects on soil pH. Adding AS caused pH to decline slightly but it was still well within the pH 5–8 tolerance of the inoculant whereas adding urea increased pH up to pH 9, outside the pH tolerance of the inoculant, and effects were more pronounced with greater urea doses. Nitrogenous fertiliser type affected the inoculant directly as ARA was greater and inoculant populations in the rhizosphere and soil were 1–2 logs greater with AS than with urea. This high pH would be expected to decrease the concentrations of NH4+ and NO3− while increasing rates of urea volatilisation and denitrification as soil pH increases up to pH 8 (Parr and Papendick 1965). The high pH would also be expected to affect leakage of C-containing exudates from wheat roots, changing the food source for the bacteria (Salles et al. 2002; Nelson and Mele 2006; Bezrukova et al. 2008).
The specific primers designed for the Horsham isolates seemed to be specific enough to identify only the inoculum, as they did not detect the inoculum in uninoculated samples, which possibly contained small numbers of RMBMTa1. Such a marker could be useful to trace the location of the bacteria in the rooting zone in the field, especially if it can be used quantitatively in qPCR or in situ PCR is used to find their location on the roots and in the rhizosphere in the field. It could also be useful to compare the ratio of the inoculant with other soil bacteria, as in avocado (Persea americana) seedlings (Gallart et al. 2021).
Inoculation with such large numbers of RMBMTa1 may allow the inoculum to occupy all available sites in the rhizosphere and on the rhizoplane (Pereg Gerk et al. 2000), which are typically enriched by high C:N exudates from the roots (Huang and Germida 2002; Hayes et al. 2021). It may also change the pattern of rhizodeposition of carbohydrates, especially sugars, in the wheat rhizosphere, resulting in large changes in microbial community structure (Nelson and Mele 2006). In such a high C:N ratio, N2 fixation would be favoured, which is consistent with the initial concentration of RMBMTa1 in the rhizosphere. Similarly, Baldani et al. (2000) and Sessitsch et al. (2005) found greater numbers of Burkholderia associated with the rhizosphere than with bulk soil in rice and Donn et al. (2015) found dominance of betaproteobacteria (including Burkholderiaceae) in the tightly bound fraction of the wheat rhizosphere. Populations of RMBMTa1 may need to be in these larger numbers to invoke greater N2 fixation, due to interaction between microorganisms within the rhizosphere (Sarita et al. 2008). The high competitiveness of Paraburkholderia species (Tago et al. 2006) may also be linked to quorum sensing (Ulrich 2004; Singh and Singh 2025), as many (Para)Burkholderia strains produce the lactones necessary to invoke a quorum sensing approach to dominating the rhizosphere of several crop plants, in addition to producing IAA, antibiotics and ACC deaminase, traits advantageous for plant growth (Poonguzhali et al. 2007; Alemneh et al. 2022). Further study is needed to elucidate if quorum sensing plays a part in the dominance of RMBMTa1 in the wheat rhizosphere studied here.
Field trial
Water was the overriding limiting factor for growth during the field trial despite three irrigations. Symptoms of water stress noted at harvest were the relatively small size of the plants and poor ear development in both inoculated and uninoculated wheat. Late planting may also have affected plant size and grain yield. However, a comparable non-irrigated trial at Horsham had only one-third of the culm/ear density, and half the plant height of these in the irrigated trial (data not shown). Grain yield (1−1.2 tonnes ha−1) in the irrigated trial was less than the average for the Wimmera (2.4 tonnes ha−1) but greater than the average for the year of the field trial (0.7 tonnes ha−1) (Agriculture Victoria 2023). Numerous authors have reported that water stress reduces wheat grain yield; e.g. Ghimire et al. (2021). It was unfortunate that a season that began well with good rain in autumn and dried up later, which made 2006 one of the worst drought years recorded in the Wimmera region (Bureau of Meteorology 2006; Australian Bureau of Statistics 2008) and many farmers were unable to harvest a crop.
Relative dryness probably affected the inoculant in the rhizosphere directly, by reducing survival and N2 fixation, as well as by increasing salinity, both inherent and from unused fertiliser salts and irrigation water, as well as root growth. The inoculant was tolerant to up to 5% NaCl in vitro, a tolerance probably resulting from natural selection, as semi-arid soils regularly experience drought. Lack of rainfall was suggested as a major limiting factor to N gains from inoculation during and following wheat crops in southern Australia, ranging from 10 kg ha−1 in more southerly regions with Mediterranean climates to 40 kg ha−1 in more northerly regions with summer-dominant rainfall (Gupta et al. 2006).
By contrast with yield, grain %N did show differences among treatments, suggesting that the availability and uptake of nitrogen varied among treatments even though plants were affected by drought (Viets 1972). Moreover, the trends in grain %N were the same as in the pot trial, with maxima in grain %N achieved using 100 kg N ha−1 of AS, although the absolute figures for grain %N were less in the field trial than in the pot trial. The promising results achieved in the pot trial suggest that greater responses to inoculation might be possible in a wetter year, although grain %N tends to increase as yield decreases (Lemon 2007; Walsh et al. 2020; Ghimire et al. 2021). Walsh et al. (2020) suggested that optima in grain yield and protein could be obtained with 75% of the maximum irrigation in spring wheat in semi-arid states in the USA. It would therefore be desirable to evaluate the effects of inoculation with RMBMTa1 on grain yield and nitrogen content on field-grown wheat in an average season, however difficult that might be to predict.
Inoculation reduced grain yield by an average of 8.8% and was probably the consequence of inoculation reducing plant emergence by 10%. The flour used to coat the seed grain hardened during 4°C storage and there was some mechanical damage during sowing. The flour also would have been an attractive food source for many common fungi found in soil (Brown and Ogle 1997). However, culm and ear density, plant height and overall biomass measured soon before harvest were not affected by the reduction in emergence and suggest that this early setback was overcome during growth. Wheat can compensate for lower seeding rates by increasing the number of tillers and spikes per plant, resulting in little or no effect on yield (Bavec et al. 2002; Moreno-Ramos et al. 2004; Beavers et al. 2008). In future studies, alternative carriers such as peat or kaolin (Kennedy et al. 2004; Naveed et al. 2014) or zeolite (Paungfoo-Lonhienne et al. 2019; Gallart et al. 2021) should be tested to deliver this inoculant, as inoculation with RMBMTa1 showed potential to increase grain %N despite the dry conditions.
The improvements in wheat grain %N (and protein content) noted by others on inoculation with (Para)Burkholderia strains (e.g. B. phytofirmans PsJN; Naveed et al. 2014) are potentially possible in better field conditions in Victoria. Despite the reduction in yield, the grain %N and therefore estimated %protein of field-harvested grain increased with 100 kg ha−1 AS and so its quality improved. This shows that RMBMTa1 has some potential to increase grain protein content and hence profit, at less than the greatest level of nitrogenous fertiliser (100 kg ha−1 rather than 150 kg ha−1), but that lack of water in the normal dryland cultivation of wheat inevitably reduces yield and thus the potential benefits of inoculation (Kennedy et al. 2004).
The trend of significant increases in %N in grain reaching a maximum at 100 kg ha−1 nitrogenous fertiliser, with AS greater than with urea, were strikingly similar in both pot and field trials. The reduction in response to inoculation at greater levels of fertiliser N parallels the reduction in N2 fixation by rhizobia with supra-optimal N fertiliser (Lemon 2007). The apparent need for >50 kg ha−1 nitrogenous fertiliser for the maximum response may indicate that the inoculant improved the assimilation of applied nitrogen or the efficiency of transfer to the grain from other parts of the plant. Increased root growth may also occur as a reaction to bacterial infection at the root interface (Kapulnik et al. 1985), as the inoculant could also affect crop growth through mechanisms such as production of phytohormones; e.g. IAA, that improve root development (Tien et al. 1979; Hecht-Buchholz 1998). These are important because they would increase absorptive area and volume of soil substrate available to the plant, with subsequent increase in the rates of water and mineral uptake (Haahtela et al. 1988).
The presence of greater populations of RMBMTa1 bacteria in the rhizosphere in inoculated treatments suggests that the bacteria survived even in these relatively dry conditions and that inoculation could have a long-lasting effect on the soil microflora. This would be consistent with the observation that the soil microflora in seeded pasture was closely associated with the seeded species in their original rows (Hayes et al. 2021). There is a problem, however, in distinguishing between RMBMTa1 that was in the soil originally and that added as the inoculant. The specific primers used in the pot trial could be used in qPCR to distinguish the inoculant from other resident strains and would arguably be preferable to genetically modifying the inoculum bacteria by inserting genetic markers such as gus (Yates and Sparks 2008) or gfp (Elliott et al. 2007).
Limitations of this research
This preliminary study of RMBMTa1 shows that it has some promise as an inoculant in wheat but the study had limitations of finance and season that led to the results from only one bacterium being reported in only one field site; these need to be overcome in future studies. The identification of RMBMTa1 should be pursued by performing both traditional and molecular tests, including sequencing of further areas of its genome, as its 16S sequence is only a 92% match for the closest Paraburkholderia species and it may belong to a new genus. RMBMTa1 should be studied to clarify if it possesses PGPR traits such as the production of IAA and ACC deaminase, siderophores for Fe and other ions, and signalling lactones for quorum sensing. Attachment of RMBMTa1 to the rhizoplane should be studied by qPCR and in situ PCR using the specific primers designed here at different doses of inoculum, with and without other rhizosphere microorganisms. Further field trials are needed in a range of wheat varieties and cropping environments to test if inoculation with RMBMTa1 benefits wheat grain yield and nitrogen content, and if other PGPRs isolated from wheatfield soils; e.g. Alemneh et al. (2022) have the same effects on wheat yield and quality.
Conclusion
The most remarkable result due to inoculation by RMBMTa1 was the consistent maximum in grain %N nitrogen with N fertiliser at 100 kg N ha−1. This maximum was highly correlated with maxima in ARA and numbers of the inoculant in the rhizosphere. It is important to maximise the efficient use of applied nitrogenous fertiliser to maximise wheat yield and quality, as well as profitability. Inoculation has the potential benefit of increasing wheat yield while decreasing inputs of nitrogenous fertilisers when yield is not limited by lack of water, as it was in this field trial. There are many questions raised from these results that need answers before inoculation with PGPR could be accepted as a routine procedure in wheat growing, but it is important to continue to explore its potential, especially in realistic field trials.
Data availability
The data used to generate the results in this paper are available on reasonable request from the corresponding author.
Declaration of funding
The authors declare that there was no specific external funding for this research.
Acknowledgements
This paper forms part of the PhD thesis of Merfat Ben Mahmud (2008). The authors thank Mr Graham Exell, technical officer, for support associated with the conduct of the field experiment.
References
AEGIC (2023) Australian grain industry facts. Available at https://www.aegic.org.au/ [accessed 8 July 2024]
Agriculture Victoria (2020) Measuring and interpreting soil pH. Available at https://vro.agriculture.vic.gov.au/dpi/vro/vrosite.nsf/pages/soilhealth_measure_ph [accessed 7 January 2024]
Agriculture Victoria (2023) Growing wheat in Victoria. Available at https://agriculture.vic.gov.au/crops-and-horticulture/grains-pulses-and-cereals/growing-grains-pulses-and-cereals/growing-wheat-in-victoria [accessed 8 July 2024]
Ahmed MF, Kennedy IR, Choudhury ATMA (2014) Evaluation of phosphorus mobilization potentials of six bacterial strains from four insoluble phosphorus sources. Communications in Soil Science and Plant Analysis 45(17), 2341-2362.
| Crossref | Google Scholar |
Alemneh AA, Zhou Y, Ryder MH, Denton MD (2022) Soil environment influences plant growth-promotion traits of isolated rhizobacteria. Pedobiologia 90, 150785.
| Crossref | Google Scholar |
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Research 25(17), 3389-3402.
| Crossref | Google Scholar | PubMed |
Angus JF (2001) Nitrogen supply and demand in Australian agriculture. Australian Journal of Experimental Agriculture 41(3), 277-288.
| Crossref | Google Scholar |
AOAC International (2023) Official methods of analysis of AOAC International. (Oxford University Press) Available at https://academic.oup.com/officialmethodsofanalysis-aoac [accessed 8 July 2024]
Australian Bureau of Statistics (2008) 7123.2.55.001 – Agricultural state profile, Victoria, 2006-07. Available at https://www.abs.gov.au/ausstats/abs@.nsf/mf/7123.2.55.001 [accessed 7 January 2024]
Azcón R (1989) Selective interaction between free-living rhizosphere bacteria and vesiculararbuscular mycorrhizal fungi. Soil Biology and Biochemistry 21(5), 639-644.
| Crossref | Google Scholar |
Azcón R (1993) Growth and nutrition of nodulated mycorrhizal and non-mycorrhizal Hedysarum coronarium as a result of treatment with fractions from a plant growth-promoting rhizobacteria. Soil Biology and Biochemistry 25(8), 1037-1042.
| Crossref | Google Scholar |
Balandreau J, Viallard V, Cournoyer B, Coenye T, Laevens S, Vandamme P (2001) Burkholderia cepacia Genomovar III is a common plant-associated bacterium. Applied and Environmental Microbiology 67(2), 982-985.
| Crossref | Google Scholar | PubMed |
Baldani VLD, Baldani JI, Döbereiner J (2000) Inoculation of rice plants with the endophytic diazotrophs Herbaspirillum seropedicae and Burkholderia spp. Biology and Fertility of Soils 30, 485-491.
| Crossref | Google Scholar |
Barrow NJ (2025) Phosphate solubilizing microorganisms; the modern philosopher’s stone. Plant and Soil 508, 21-28.
| Crossref | Google Scholar |
Bavec M, Bavec F, Varga B, Kovačević V (2002) Relationships among yield, its quality and components, in winter wheat (Triticum aestivum L.) cultivars affected by seeding rates. Die Bodenkultur 53(3), 143-151.
| Google Scholar |
Beavers RL, Hammermeister AM, Frick B, Astatkie T, Martin RC (2008) Spring wheat yield response to variable seeding rates in organic farming systems at different fertility regimes. Canadian Journal of Plant Science 88(1), 43-52.
| Crossref | Google Scholar |
Ben Mahmud M (2008) Effect of Burkholderia as biofertiliser on cereal productivity. PhD Thesis, RMIT University, Melbourne, Vic, Australia. Available at https://research-repository.rmit.edu.au/articles/thesis/Effect_of_Burkholderia_as_biofertiliser_on_cereal_productivity/27340674?file=50728917
Bernabeu PR, García SS, López AC, Vio SA, Carrasco N, Boiardi JL, Luna MF (2018) Assessment of bacterial inoculant formulated with Paraburkholderia tropica to enhance wheat productivity. World Journal of Microbiology and Biotechnology 34, 81.
| Crossref | Google Scholar | PubMed |
Beukes CW, Venter SN, Steenkamp ET (2021) The history and distribution of nodulating Paraburkholderia, a potential inoculum for Fynbos forage species. Grass and Forage Science 76, 10-32.
| Crossref | Google Scholar |
Bezrukova M, Kildibekova A, Shakirova F (2008) WGA reduces the level of oxidative stress in wheat seedlings under salinity. Plant Growth Regulation 54, 195-201.
| Crossref | Google Scholar |
Bohlool BB, Ladha JK, Garrity DP, George T (1992) Biological nitrogen fixation for sustainable agriculture: a perspective. Plant and Soil 141, 1-11.
| Crossref | Google Scholar |
Brown ME (1974) Seed and root bacterization. Annual Review of Phytopathology 12, 181-197.
| Crossref | Google Scholar |
Bulut S (2013a) Evaluation of yield and quality parameters of phosphorous-solubilizing and N-fixing bacteria inoculated in wheat (Triticum aestivum L.). Turkish Journal of Agriculture and Forestry 37(5), 545-554.
| Crossref | Google Scholar |
Bulut S (2013b) Evaluation of efficiency parameters of phosphorous-solubilizing and N-fixing bacteria inoculations in wheat (Triticum aestivum L.). Turkish Journal of Agriculture and Forestry 37(6), 734-743.
| Crossref | Google Scholar |
Bureau of Meteorology (2006) Drought intensifies over eastern and southern Australia as spring rains fail. Available at http://www.bom.gov.au/climate/drought/archive/20061103.shtml [accessed 8 July 2024]
Bureau of Meteorology (2024) Climate data online. Available at http://www.bom.gov.au/climate/data/ [accessed 8 July 2024]
Caballero-Mellado J, Martínez-Aguilar L, Paredes-Valdez G, Estrada-De los Santos P (2004) Burkholderia unamae sp. nov., an N2-fixing rhizospheric and endophytic species. International Journal of Systematic and Evolutionary Microbiology 54(4), 1165-1172.
| Crossref | Google Scholar | PubMed |
Chen S, Xiang X, Ma H, Penttinen P, Zhao J, Li H, Gao R, Zheng T, Fan G (2021) Straw mulching and nitrogen fertilization affect diazotroph communities in wheat rhizosphere. Frontiers in Microbiology 12, 658668.
| Crossref | Google Scholar | PubMed |
Chhetri G, Kim I, Kim J, So Y, Park S, Jung Y, Seo T (2023) Paraburkholderia tagetis sp. nov., a novel species isolated from roots of Tagetes patula enhances the growth and yield of Solanum lycopersicum L. (tomato). Frontiers in Microbiology 14, 1140484.
| Crossref | Google Scholar | PubMed |
Chun J, Oren A, Ventosa A, Christensen H, Ruiz Arahal D, Da Costa MS, Rooney AP, Yi H, Xu X-W, De Meyer S, Trujillo ME (2018) Proposed minimal standards for the use of genome data for the taxonomy of prokaryotes. International Journal of Systematic and Evolutionary Microbiology 68(1), 461-466.
| Crossref | Google Scholar | PubMed |
Ciccillo F, Fiore A, Bevivino A, Dalmastri C, Tabacchioni S, Chiarini L (2002) Effects of two different application methods of Burkholderia ambifaria MCI 7 on plant growth and rhizospheric bacterial diversity. Environmental Microbiology 4(4), 238-245.
| Crossref | Google Scholar | PubMed |
Coenye T, Vandamme P (2003) Diversity and significance of Burkholderia species occupying diverse ecological niches. Environmental Microbiology 5(9), 719-729.
| Crossref | Google Scholar | PubMed |
Coenye T, Vandamme P, Govan JRW, LiPuma JJ (2001) Taxonomy and identification of the Burkholderia cepacia complex. Journal of Clinical Microbiology 39(10), 3427-3436.
| Crossref | Google Scholar | PubMed |
Compant S, Kaplan H, Sessitsch A, Nowak J, Ait Barka E, Clément C (2008) Endophytic colonization of Vitis vinifera L. by Burkholderia phytofirmans strain PsJN: from the rhizosphere to inflorescence tissues. FEMS Microbiology Ecology 63(1), 84-93.
| Crossref | Google Scholar |
Curtis BC (2002) Wheat in the world. In ‘Bread wheat: improvement and production’. (Eds BC Curtis, S Rajaram, H Gomez Macpherson). (Food and Agriculture Organization of the United Nations: Rome, Italy) Available at https://www.fao.org/3/y4011e/y4011e04.htm#bm04
Dobbelaere S, Croonenborghs A, Thys A, Ptacek D, Vanderleyden J, Dutto P, Labandera-Gonzalez C, Caballero-Mellado J, Aguirre JF, Kapulnik Y, Brener S, Burdman S, Kadouri D, Sarig S, Okon Y (2001) Responses of agronomically important crops to inoculation with Azospirillum. Australian Journal of Plant Physiology 28(9), 871-879.
| Crossref | Google Scholar |
Dobritsa AP, Samadpour M. (2016) Transfer of eleven species of the genus Burkholderia to the genus Paraburkholderia and proposal of Caballeronia gen. nov. to accommodate twelve species of the genera Burkholderia and Paraburkholderia. International Journal of Systematic and Evolutionary Microbiology 66(8), 2836-2846.
| Crossref | Google Scholar |
Donn S, Kirkegaard JA, Perera G, Richardson AE, Watt M (2015) Evolution of bacterial communities in the wheat crop rhizosphere. Environmental Microbiology 17(3), 610-621.
| Crossref | Google Scholar |
Elliott GN, Chen W-M, Bontemps C, Chou J-H, Young JPW, Sprent JI, James EK (2007) Nodulation of Cyclopia spp. (Leguminosae, Papilionoideae) by Burkholderia tuberum. Annals of Botany 100(7), 1403-1411.
| Crossref | Google Scholar | PubMed |
Esmaeel Q, Miotto L, Rondeau M, Leclère V, Clément C, Jacquard C, Sanchez L, Barka EA (2018) Paraburkholderia phytofirmans PsJN-plants interaction: from perception to the induced mechanisms. Frontiers in Microbiology 9, 2093.
| Crossref | Google Scholar |
Estrada-De Los Santos P, Bustillos-Cristales R, Caballero-Mellado J (2001) Burkholderia, a genus rich in plant-associated nitrogen fixers with wide environmental and geographic distribution. Applied and Environmental Microbiology 67(6), 2790-2798.
| Crossref | Google Scholar | PubMed |
Eze MO, Thiel V, Hose GC, George SC, Daniel R (2022) Bacteria-plant interactions synergistically enhance biodegradation of diesel fuel hydrocarbons. Communications Earth & Environment 3, 192.
| Crossref | Google Scholar |
Fan X, Hu H, Huang G, Huang F, Li Y, Palta J (2015) Soil inoculation with Burkholderia sp. LD-11 has positive effect on water-use efficiency in inbred lines of maize. Plant and Soil 390, 337-349.
| Crossref | Google Scholar |
Fautz E, Reichenbach H (1980) A simple test for flexirubin-type pigments. FEMS Microbiology Letters 8(2), 87-91.
| Crossref | Google Scholar |
Fowler DB, Brydon J, Darroch BA, Entz MH, Johnston AM (1990) Environment and genotype influence on grain protein concentration of wheat and rye. Agronomy Journal 82(4), 655-664.
| Crossref | Google Scholar |
Fox AR, Soto G, Valverde C, Russo D, Lagares A, Jr, Zorreguieta Á, Alleva K, Pascuan C, Frare R, Mercado-Blanco J, Dixon R, Ayub ND (2016) Major cereal crops benefit from biological nitrogen fixation when inoculated with the nitrogen-fixing bacterium Pseudomonas protegens Pf-5 X940. Environmental Microbiology 18(10), 3522-3534.
| Crossref | Google Scholar | PubMed |
Frommel MI, Nowak J, Lazarovits G (1991) Growth enhancement and developmental modifications of in vitro grown potato (Solanum tuberosum spp. tuberosum) as affected by a nonfluorescent Pseudomonas sp. Plant Physiology 96(3), 928-936.
| Crossref | Google Scholar | PubMed |
Gallart M, Paungfoo-Lonhienne C, Gonzalez A, Trueman SJ (2021) Nitrogen source influences the effect of plant growth-promoting rhizobacteria (PGPR) on Macadamia integrifolia. Agronomy 11(6), 1064.
| Crossref | Google Scholar |
Gallart M, Paungfoo-Lonhienne C, Trueman SJ (2022) Effects of a growth-promoting Paraburkholderia species on nitrogen acquisition by avocado seedlings. Scientia Horticulturae 295, 110767.
| Crossref | Google Scholar |
Gao Z-H, Ruan S-L, Huang Y-X, Lv Y-Y, Qiu L-H (2019) Paraburkholderia phosphatilytica sp. nov., a phosphate-solubilizing bacterium isolated from forest soil. International Journal of Systematic and Evolutionary Microbiology 69(1), 196-202.
| Crossref | Google Scholar | PubMed |
Ghimire D, Das S, Mueller ND, Creech CF, Santra D, Beanziger PS, Easterly AC, Mausti B, Maharjani B (2021) Effects of cultivars and nitrogen management on wheat grain yield and protein. Agronomy Journal 113(5), 4348-4368.
| Crossref | Google Scholar |
Giller KE, James EK, Ardley J, Unkovich MJ (2024) Science losing its way: examples from the realm of microbial N2-fixation in cereals and other non-legumes. Plant Soil 2024,.
| Crossref | Google Scholar |
Gillis M, Tran Van V, Bardin R, Goor M, Hebbar P, Willems A, Segers P, Kersters K, Heulin T, Fernandez MP (1995) Polyphasic taxonomy in the genus Burkholderia leading to an emended description of the genus and proposition of Burkholderia vietnamiensis sp. nov. for N2-fixing isolates from rice in Vietnam. International Journal of Systematic and Evolutionary Microbiology 45(2), 274-289.
| Crossref | Google Scholar |
Gopalkrishnan S, Srinivas V, Vemula A, Samineni S, Rathore A (2018) Influence of diazotrophic bacteria on nodulation, nitrogen fixation, growth promotion and yield traits in five cultivars of chickpea. Biocatalysis and Agricultural Biotechnology 15, 35-42.
| Crossref | Google Scholar |
GrainCorp Ltd (2021) Wheat standards 2021–2022. Available at https://grains.graincorp.com.au/wp-content/uploads/2021/08/Wheat-Standards-2021-2022.pdf [accessed 8 July 2024]
Grains Australia (2023) 2024-2025 Wheat Variety Master List. Available at https://grainsaustralia.com.au/master-lists/wheat-variety-list#wheat-master-list/?view_4_page=1&view_4_filters=%5B%7B%22field%22%3A%22field_94%22%2C%22operator%22%3A%22in%22%2C%22value%22%3A%5B%22AH%22%5D%7D%2C%7B%22field%22%3A%22field_95%22%2C%22operator%22%3A%22in%22%2C%22value%22%3A%5B%22AH%22%5D%7D%2C%7B%22field%22%3A%22field_310%22%2C%22operator%22%3A%22in%22%2C%22value%22%3A%5B%22all%22%5D%7D%5D [accessed 6 June 2025]
Guimarães SL, Silva RA, Baldani JI, Baldani VLD, Döbereiner J (2000) Effects of the inoculation of endophytic diazotrophic bacteria on grain yield of two rice varieties (Guarani and CNA 8305) grown under field conditions. In ‘Nitrogen fixation: from molecules to crop productivity’. (Eds FO Pedrosa, M Hungria, G Yates, WE Newton) p. 431. (Kluwer: Dordrecht)
Gupta VVSR, Roper MM, Roget DK (2006) Potential for non-symbiotic N2-fixation in different agroecological zones of southern Australia. Australian Journal of Soil Research 44, 343-354.
| Crossref | Google Scholar |
Haahtela K, Laakso T, Nurmiaho-Lassila E-L, Korhonen TK (1988) Effects of inoculation of Poa pratensis and Triticum aestivum with root-associated, N2-fixing Klebsiella, Enterobacter and Azospirillum. Plant and Soil 106, 239-248.
| Crossref | Google Scholar |
Harvey RWS, Price TH (1967) The isolation of salmonellas from animal feedingstuffs. Journal of Hygiene 65(2), 237-244.
| Crossref | Google Scholar | PubMed |
Hasan N, Zainal Abidina NH (2024) Plant growth-promoting rhizobacteria and sustainable agriculture. In ‘Soil bacteria’. (Eds S Dheeman, MT Islam, D Egamberdieva, MN Siddiqui) pp. 253–287. (Springer: Singapore). 10.1007/978-981-97-3473-3_9
Hayes RC, Gupta VVSR, Li GD, Peoples MB, Rawnsley RP, Pembleton KG (2021) Contrasting soil microbial abundance and diversity on and between pasture drill rows in the third growing season after sowing. Renewable Agriculture and Food Systems 36(2), 163-172.
| Crossref | Google Scholar |
Hecht-Buchholz C (1998) The apoplast-habitat of endophytic dinitrogen-fixing bacteria and their significance for the nitrogen nutrition of nonleguminous plants. Zeitschrift fur Pflanzenernahrung und Bodenkunde 161(5), 509-520.
| Crossref | Google Scholar |
Hermenau R, Ishida K, Gama S, Hoffmann B, Pfeifer-Leeg M, Plass W, Mohr JF, Wichard T, Saluz H-P, Hertweck C (2018) Gramibactin is a bacterial siderophore with a diazeniumdiolate ligand system. Nature Chemical Biology 14, 841-843.
| Crossref | Google Scholar | PubMed |
Hermenau R, Mehl JL, Ishida K, Dose B, Pidot SJ, Stinear TP, Hertweck C (2019) Genomics-driven discovery of NO-donating diazeniumdiolate siderophores in diverse plant-associated bacteria. Angewandte Chemie International Edition 58(37), 13024-13029.
| Crossref | Google Scholar | PubMed |
Herridge DF, Giller KE, Jensen ES, Peoples MB (2022) Quantifying country-to-global scale nitrogen fixation for grain legumes II. Coefficients, templates and estimates for soybean, groundnut and pulses. Plant and Soil 474, 1-15.
| Crossref | Google Scholar |
Holford ICR, Doyle AD, Leckie CC (1992) Nitrogen response characteristics of wheat protein in relation to yield responses and their interactions with phosphorus. Australian Journal of Agricultural Research 43(5), 969-986.
| Crossref | Google Scholar |
Hoque MS, Broadhurst LM, Thrall PH (2011) Genetic characterization of root-nodule bacteria associated with Acacia salicina and A. stenophylla (Mimosaceae) across south-eastern Australia. International Journal of Systematic and Evolutionary Microbiology 61(2), 299-309.
| Crossref | Google Scholar | PubMed |
Hussain M, Asgheri Z, Tahir M, Ijaz M, Shahid M, Ali H, Sattar A (2016) Bacteria in combination with fertilizers improve growth, productivity and net returns of wheat (Triticum aestivum L.). Pakistan Journal of Agricultural Science 53(3), 633-645.
| Crossref | Google Scholar |
Illmer P, Schinner F (1992) Solubilization of inorganic phosphates by microorganisms isolated from forest soils. Soil Biology and Biochemistry 24(4), 389-395.
| Crossref | Google Scholar |
Iwaki H, Hasegawa Y (2007) Degradation of 2-nitrobenzoate by Burkholderia terrae strain KU-15. Bioscience, Biotechnology, and Biochemistry 71(1), 145-151.
| Crossref | Google Scholar | PubMed |
Kapulnik Y, Okon Y, Henis Y (1985) Changes in root morphology of wheat caused by Azospirillum inoculation. Canadian Journal of Microbiology 31(10), 881-887.
| Crossref | Google Scholar |
Kaur C, Selvakumar G, Ganeshamurthy AN (2017) Burkholderia to Paraburkholderia: the journey of a plant-beneficial-environmental bacterium. In ‘Recent advances in applied microbiology’. (Ed P Shukla) pp. 213–228. (Springer Nature: Singapore) 10.1007/978-981-10-5275-0_10
Kennedy IR (2003) Symposium summary and future perspectives. Symbiosis 35(1–3), 271-278.
| Google Scholar |
Kennedy IR, Islam N (2001) The current and potential contribution of asymbiotic nitrogen fixation to nitrogen requirements on farms: a review. Australian Journal of Experimental Agriculture 41(3), 447-457.
| Crossref | Google Scholar |
Kennedy IR, Choudhury ATMA, Kecskés ML (2004) Non-symbiotic bacterial diazotrophs in crop-farming systems: can their potential for plant growth promotion be better exploited? Soil Biology and Biochemistry 36(8), 1229-1244.
| Crossref | Google Scholar |
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33(7), 1870-1874.
| Crossref | Google Scholar | PubMed |
Ladha JK, Tirol-Padre A, Reddy CK, Cassman KG, Verma S, Powlson DA, van Kessel C, Richter DdB, Chakraborty D, Pathak H (2016) Global nitrogen budgets in cereals: a 50-year assessment for maize, rice and wheat production systems. Scientific Reports 6, 19355.
| Crossref | Google Scholar | PubMed |
Lemon J (2007) Nitrogen management for wheat protein and yield in the Esperance port zone. Department of Agriculture and Food Bulletin 4707, Western Australia, Perth. Available at https://researchlibrary.agric.wa.gov.au/bulletins
Lin T-F, Huang H-I, Shen F-T, Young C-C (2006) The protons of gluconic acid are the major factor responsible for the dissolution of tricalcium phosphate by Burkholderia cepacia CC-A174. Bioresource Technology 97(7), 957-960.
| Crossref | Google Scholar | PubMed |
Liu M, Philp J, Wang Y, Hu J, Wei Y, Li J, Ryder M, Toh R, Zhou Y, Denton MD, Wu Y, Yang H (2022) Plant growth-promoting rhizobacteria Burkholderia vietnamiensis B418 inhibits root-knot nematode on watermelon by modifying the rhizosphere microbial community. Scientific Reports 12, 8381.
| Crossref | Google Scholar | PubMed |
Mahenthiralingam E, Bischof J, Byrne SK, Radomski C, Davies JE, Av-Gay Y, Vandamme P (2000) DNA-based diagnostic approaches for identification of Burkholderia cepacia complex, Burkholderia vietnamiensis, Burkholderia multivorans, Burkholderia stabilis, and Burkholderia cepacia genomovars I and III. Journal of Clinical Microbiology 38(9), 3165-3173.
| Crossref | Google Scholar | PubMed |
Martínez-Aguilar L, Salazar-Salazar C, Méndez RD, Caballero-Mellado J, Hirsch AM, Vásquez-Murrieta MS, Estrada-De Los Santos P (2013) Burkholderia caballeronis sp. nov., a nitrogen fixing species isolated from tomato (Lycopersicon esculentum) with the ability to effectively nodulate Phaseolus vulgaris. Antonie van Leeuwenhoek 104, 1063-1071.
| Crossref | Google Scholar |
Martínez-Toledo MV, Gonzalez-Lopez J, de la Rubia T, Ramos-Cormenzana A (1985) Isolation and characterization of Azotobacter chroococcum from the roots of Zea mays. FEMS Microbiology Ecology 1(4), 197-203.
| Crossref | Google Scholar |
Moreno-Ramos OH, Rodriguez-Casas J, Johnson D, Thompson TL (2004) Wheat response to population density and bed spacing in northwest Mexico. Cereal Research Communications 32, 273-279.
| Crossref | Google Scholar |
Mullins AJ, Mahenthiralingam E (2021) The hidden genomic diversity, specialized metabolite capacity, and revised taxonomy of Burkholderia sensu lato. Frontiers in Microbiology 12, 726847.
| Crossref | Google Scholar | PubMed |
Nakata PA (2002) The generation of a transposon-mutagenized Burkeholderia glumae library to isolate novel mutants. Plant Science 162(2), 267-271.
| Crossref | Google Scholar |
Naveed M, Hussain MB, Zahir ZA, Mitter B, Sessitsch A (2014) Drought stress amelioration in wheat through inoculation with Burkholderia phytofirmans strain PsJN. Plant Growth Regulation 73, 121-131.
| Crossref | Google Scholar |
Nelson DR, Mele PM (2006) The impact of crop residue amendments and lime on microbial community structure and nitrogen-fixing bacteria in the wheat rhizosphere. Australian Journal of Soil Research 44(4), 319-329.
| Crossref | Google Scholar |
Omar MNA, Berge O, Hassanein EE, Shalan SN (1992) In vitro and in situ effects of herbicide thiobencarb on rice-Azospirillum association. Symbiosis 13, 55-63.
| Google Scholar |
Oren A, Garrity GM (2015) List of new names and new combinations previously effectively, but not validly, published. International Journal of Systematic and Evolutionary Microbiology 65, 2017-2025.
| Crossref | Google Scholar |
O’Connell PF (1992) Sustainable agriculture-a valid alternative. Outlook on Agriculture 21(1), 5-12.
| Crossref | Google Scholar |
Parker MA, Wurtz AK, Paynter Q (2007) Nodule symbiosis of invasive Mimosa pigra in Australia and in ancestral habitats: a comparative analysis. Biological Invasions 9, 127-138.
| Crossref | Google Scholar |
Parr JR, Papendick RI (1965) Retention of ammonia in soils. In ‘Agricultural anhydrous ammonia technology and use’. (Eds MH McVicker, WP Martin, IE Miles, HH Tucker) pp. 213–236. (Agricultural Ammonia Institute: Memphis, American Society of Agronomy: Madison, Soil Science Society of America: Madison) 10.2134/1966.nh3agricultural
Paulitsch F, Bueno dos Reis F, Jr, Hungria M (2021) Twenty years of paradigm-breaking studies of taxonomy and symbiotic nitrogen fixation by beta-rhizobia, and indication of Brazil as a hotspot of Paraburkholderia diversity. Archives of Microbiology 203, 4785-4803.
| Crossref | Google Scholar | PubMed |
Paungfoo-Lonhienne C, Lonhienne TGA, Yeoh YK, Webb RI, Lakshmanan P, Chan CX, Lim PE, Ragan MA, Schmidt S, Hugenholtz P (2014) A new species of Burkholderia isolated from sugarcane roots promotes plant growth. Microbial Biotechnology 7(2), 142-154.
| Crossref | Google Scholar | PubMed |
Paungfoo-Lonhienne C, Redding M, Pratt C, Wang W (2019) Plant growth promoting rhizobacteria increase the efficiency of fertilisers while reducing nitrogen loss. Journal of Environmental Management 233, 337-341.
| Crossref | Google Scholar | PubMed |
Peoples MB, Herridge DF, Ladha JK (1995) Biological nitrogen fixation: an efficient source of nitrogen for sustainable agricultural production? Plant and Soil 174, 3-28.
| Crossref | Google Scholar |
Pereg Gerk L, Gilchrist K, Kennedy IR (2000) Mutants with enhanced nitrogenase activity in hydroponic Azospirillum brasilense-wheat associations. Applied and Environmental Microbiology 66(5), 2175-2184.
| Crossref | Google Scholar | PubMed |
Poonguzhali S, Madhaiyan M, Sa T (2007) Quorum-sensing signals produced by plant-growth promoting Burkholderia strains under in vitro and in planta conditions. Research in Microbiology 158(3), 287-294.
| Crossref | Google Scholar | PubMed |
Reis VM, Baldani JI, Baldani VLD, Döbereiner J (2000) Biological dinitrogen fixation in Gramineae and palm trees. Critical Reviews in Plant Science 19(3), 227-247.
| Crossref | Google Scholar |
Reis VM, Estrada-De los Santos P, Tenorio-Salgado S, Vogel J, Stoffels M, Guyon S, Mavingui P, Baldani VLD, Schmid M, Baldani JI, Balandreau J, Hartmann A, Cabellero-Mellado J (2004) Burkholderia tropica sp. nov., a novel nitrogen-fixing, plant-associated bacterium. International Journal of Systematic and Evolutionary Microbiology 54(6), 2155-2162.
| Crossref | Google Scholar |
Rojas-Rojas FU, Tapia-García EY, Maymon M, Humm E, Huntemann M, Clum A, Pillay M, Palaniappan K, Varghese N, Mikhailova N, Stamatis D, Reddy TBK, Markowitz V, Ivanova N, Kyrpides N, Woyke T, Shapiro N, Hirsch AM, Estrada-De los Santos P (2017) Draft genome of Paraburkholderia caballeronis TNe-841T, a free-living, nitrogen-fixing, tomato plant-associated bacterium. Standards in Genomic Sciences 12, 80.
| Crossref | Google Scholar | PubMed |
Rojas-Rojas FU, Gómez-Vázquez IM, Estrada de los Santos P, Shimade-Beltrán H, Vega-Arreguín JC (2025) The potential of Paraburkholderia species to enhance crop growth. World Journal of Microbiology and Biotechnology 41, 62.
| Crossref | Google Scholar | PubMed |
Sakai Y, Ogawa N, Fujii T, Sugahara K, Miyashihta K, Hasebe A (2007) 2,4-dichrolophenoxyacetic acid-degrading genes from bacteria isolated from soil in Japan: spread of Burkholderia cepacia RASC-type degrading genes harbored on large plasmids. Microbes and Environments 22(2), 145-156.
| Crossref | Google Scholar |
Salles JF, De Souza FA, Van Elsas JD (2002) Molecular method to assess the diversity of Burkholderia species in environmental samples. Applied and Environmental Microbiology 68(4), 1595-1603.
| Crossref | Google Scholar | PubMed |
Sarita S, Priefer UB, Prell J, Sharma PK (2008) Diversity of nifH gene amplified from rhizosphere soil DNA. Current Science 94(1), 109-115 Available at https://eurekamag.com/research/018/767/018767147.php.
| Google Scholar |
Sawana A, Adeolu M, Gupta RS (2014) Molecular signatures and phylogenomic analysis of the genus Burkholderia: proposal for division of this genus into the emended genus Burkholderia containing pathogenic organisms and a new genus Paraburkholderia gen. nov. harboring environmental species. Frontiers in Genetics 5, 429.
| Crossref | Google Scholar | PubMed |
Sessitsch A, Coenye T, Sturz AV, Vandamme P, Ait Barka E, Salles JF, Van Elsas JD, Faure D, Reiter B, Glick BR, Wang-Pruski G, Nowak J (2005) Burkholderia phytofirmans sp. nov., a novel plant-associated bacterium with plant-beneficial properties. International Journal of Systematic and Evolutionary Microbiology 55(3), 1187-1192.
| Crossref | Google Scholar | PubMed |
Shaharoona B, Jamro GM, Zahir ZA, Arshad M, Memon KS (2007) Effectiveness of various Pseudomonas spp. and Burkholderia caryophylli containing ACC-deaminase for improving growth and yield of wheat (Triticum aestivum L.). Journal of Microbiology and Biotechnology 17(8), 1300-1307.
| Google Scholar | PubMed |
Singh AA, Singh AK (2025) Role of bacterial quorum sensing in plant growth promotion. World Journal of Microbiology and Biotechnology 41, 18.
| Crossref | Google Scholar |
Tago K, Sekiya E, Kiho A, Katsuyama C, Hosshito Y, Yamada N, Hirano K, Sawada H, Hayatsu M (2006) Diversity of fenitrothion-degrading bacteria in soils from distant geographical areas. Microbes and Environments 21(1), 58-64.
| Crossref | Google Scholar |
Tamura K, Nei M (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Molecular Biology and Evolution 10(3), 512-526.
| Crossref | Google Scholar | PubMed |
Thompson JD, Higgins DG, Gibson TJ (1994) Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research 22(22), 4673-4680.
| Crossref | Google Scholar | PubMed |
Tien TM, Gaskins MH, Hubbell DH (1979) Plant growth substances produced by Azospirillum brasilense and their effect on the growth of pearl millet (Pennisetum americanum L.). Applied and Environmental Microbiology 37(5), 1016-1024.
| Crossref | Google Scholar | PubMed |
Timofeeva AM, Galyamova MR, Sedykh SE (2023) Plant growth-promoting soil bacteria: nitrogen fixation, phosphate solubilization, siderophore production, and other biological activities. Plants 12(24), 4074.
| Crossref | Google Scholar | PubMed |
Too CC, Ong KS, Yule CM, Keller A (2021) Putative roles of bacteria in the carbon and nitrogen cycles in a tropical peat swamp forest. Basic and Applied Ecology 52, 109-123.
| Crossref | Google Scholar |
Trân Van V, Berge O, Balandreau J, Heulin T, Ngô Kê S (1996) Isolation and nitrogenase activity of Burkholderia vietnamiensis, a nitrogen-fixing bacterium associated with rice (Oryza sativa L.) on an acid sulphate soil of Vietnam. Agronomie 16(8), 479-491.
| Crossref | Google Scholar |
Trân Van V, Berge O, Ngô Kê S, Balandreau J, Heulin T (2000) Repeated beneficial effects of rice inoculation with a strain of Burkholderia vietnamiensison early and late yield components in low fertility sulphate acid soils of Vietnam. Plant and Soil 218, 273-284.
| Crossref | Google Scholar |
Ulrich RL (2004) Quorum quenching: enzymatic disruption of N-acylhomoserine lactone-mediated bacterial communication in Burkholderia thailandensis. Applied and Environmental Microbiology 70(10), 6173-6180.
| Crossref | Google Scholar | PubMed |
Unkovich M, Herridge D, James EK, Giller K, Peoples MB (2020) Reliable quantification of N2 fixation by non-legumes remains problematic. Nutrient Cycling in Agroecosystems 118, 223-225.
| Crossref | Google Scholar |
Vance CP (1997) Enhanced agricultural sustainability through biological nitrogen fixation. In ‘Biological fixation of nitrogen for ecology and sustainable agriculture’. NATO ASI Series, Vol. 39. (Eds A Legocki, H Bothe, A Puhler) pp. 179–185. (Springer-Verlag: Berlin, Germany) Available at https://link.springer.com/chapter/10.1007/978-3-642-59112-9_36
Vandamme P, Goris J, Chen W-M, Vos PD, Willems A (2002) Burkholderia tuberum sp. nov. and Burkholderia phymatum sp. nov., nodulate the roots of tropical legumes. Systematic and Applied Microbiology 25(4), 507-512.
| Crossref | Google Scholar |
Viallard V, Poirier I, Cournoyer B, Haurat J, Wiebkin S, Ophel-Keller K, Balandreau J (1998) Burkholderia graminis sp. nov., a rhizospheric Burkholderia species, and reassessment of [Pseudomonas] phenazinium, [Pseudomonas] pyrrocinia and [Pseudomonas] glathei as Burkholderia. International Journal of Systematic and Evolutionary Microbiology 48, 549-563.
| Crossref | Google Scholar | PubMed |
Walley FL, Germida JJ (1997) Response of spring wheat (Triticum aestivum) to interactions between Pseudomonas species and Glomus clarum NT4. Biology and Fertility of Soils 24, 365-371.
| Crossref | Google Scholar |
Walsh OS, Torrion JA, Liang X, Shafian S, Yang R, Belmont KM, McClintick-Chess JR (2020) Grain yield, quality, and spectral characteristics of wheat grown under varied nitrogen and irrigation. Agrosystems, Geosciences & Environment 3(1), e20104.
| Crossref | Google Scholar |
Weisburg WG, Barns SM, Pelletier DA, Lae DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. Journal of Bacteriology 173(2), 697-703.
| Crossref | Google Scholar | PubMed |
Wilson MS, Herrick JB, Jeon CO, Hinman DE, Madsen EL (2003) Horizontal transfer of phnAc dioxygenase genes within one of two phenotypically and genotypically distinctive naphthalene-degrading guilds from adjacent soil environments. Applied and Environmental Microbiology 69(4), 2172-2181.
| Crossref | Google Scholar | PubMed |
Yates IE, Sparks D (2008) Fusarium verticillioides dissemination among maize ears of field-grown plants. Crop Protection 27(3–5), 606-613.
| Crossref | Google Scholar |
Yegorenkova IV, Tregubova KV, Burygin GL, Matora LY, Ignatov VV (2016) Assessing the efficacy of co-inoculation of wheat seedlings with the associative bacteria Paenibacillus polymyxa 1465 and Azospirillum brasilense Sp245. Canadian Journal of Microbiology 62(3), 279-285.
| Crossref | Google Scholar | PubMed |