Partner notification and retesting for Chlamydia trachomatis and Neisseria gonorrhoeae: a case-note review in New Zealand primary care
Sally B. Rose 1 , Susan M. Garrett 1 , Jane Kennedy 2 , Kim Lund 2 , Deborah Hutchings 2 , Caroline Boyle 2 , Susan R. H. Pullon 11 Department of Primary Health Care and General Practice, University of Otago, Wellington South, New Zealand
2 Wellington Sexual Health Service, Te Aro, Wellington, New Zealand
Correspondence to: Sally B. Rose, Department of Primary Health Care and General Practice, University of Otago, Wellington, PO Box 7343, Wellington South 6242, New Zealand. Email: sally.rose@otago.ac.nz.
Journal of Primary Health Care 10(2) 132-139 https://doi.org/10.1071/HC17025
Published: 26 April 2018
Journal Compilation © Royal New Zealand College of General Practitioners 2018.
This is an open access article licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Abstract
INTRODUCTION: Bacterial sexually transmitted infections (STIs) contribute to a significant burden of ill-health despite being easy to diagnose and treat. STI management guidelines provide clinicians with evidence-based guidance on best-practice case management.
AIM: To determine the extent of adherence to STI management guidelines for partner notification, follow up and testing for reinfection following diagnosis of Chlamydia trachomatis and Neisseria gonorrhoeae.
METHODS: Retrospective review of electronic patient records for individuals diagnosed with chlamydia or gonorrhoea in eight primary care clinics in Wellington, New Zealand. At each clinic, 40 clinical records were reviewed (320 in total). Outcome measures were: overall numbers (%) of cases with documented evidence of reason for testing, sexual history, treatment, advice, partner notification and follow up. Partner notification outcomes were: n (%) with evidence of partner notification discussion and n (%) with partners advised, tested and treated. Proportions retested between 6 weeks and 6 months and n (%) positive on retesting were also determined.
RESULTS: Presenting features and treatment were generally well documented. Recent sexual history including number of partners was documented for half of cases reviewed (159/320). Partner notification discussion was documented for 74% (237/320) of cases, but only 24.4% (78/320) had documentation on numbers of partners notified and 17% (54/320) on numbers of partners treated. Testing for reinfection between 6 weeks and 6 months occurred for 24.7% (79/320), of whom 19% (15/79) re-tested positive.
CONCLUSIONS: This research suggests there are gaps in important aspects of patient care following bacterial STI diagnosis – a factor that may be perpetuating our high rates of infection. A more systematic approach will be needed to ensure people diagnosed with an STI receive the full cycle of care in line with best practice guidelines.
KEYWORDS: Sexually transmitted infections, Chlamydia trachomatis, Neisseria gonorrhoea, Clinical guidelines, Partner notification, Patient care.
| WHAT GAP THIS FILLS |
| What is already known: Most bacterial STIs are diagnosed in primary care in New Zealand. Sexual health guidelines recommend that partner notification, follow up and testing for reinfection are undertaken following diagnosis of Chlamydia trachomatis and Neisseria gonorrhoeae. |
| What this study adds: Gaps were observed between best practice and documented management of partner notification and testing for reinfection. Strategies are needed to ensure primary care practitioners are aware of current sexual health guidelines that outline best practice, and are adequately resourced to more systematically approach these important aspects of patient care. |
Introduction
Sexually transmitted infections (STIs) are one of the main preventable causes of ill-health among young people.1Chlamydia trachomatis continues to be the most commonly diagnosed bacterial STI globally,2 despite the ease with which it can be diagnosed and treated. STI diagnoses may be associated with negative psychosocial impact, and unless treated in a timely manner can also result in serious reproductive health consequences.3,4Chlamydia trachomatis and Neisseria gonorrhoeae infections are most commonly diagnosed and treated in primary care in New Zealand. Because most chlamydia and gonorrhoea infections are asymptomatic, widespread access to opportunistic screening is an essential part of STI control.2 While targeting known higher-risk groups for screening in primary care, together with efforts to improve test coverage are important goals, so too is the need to ensure that people who have been tested and diagnosed with an STI are managed in line with best practice guidelines.
The New Zealand Sexual Health Society (NZSHS) provides evidence-based guidelines (accessible at: www.nzshs.org/guidelines) detailing step-by-step management of specific STIs.5 Partner notification (or ‘contact tracing’) is a key part of patient care and an important secondary prevention process by which health-care professionals explain to STI-positive patients the risk of infection in their sexual partner(s) who are then identified, notified, counselled, tested and treated.6 The importance of telling all sexual partners and contacts within the past 3 months should be emphasised during STI consultations, and a plan for notifying partners discussed and documented. Patients most often choose to tell partners themselves (known as ‘patient referral’), so verbal and written advice should be offered to support individuals in this process.5,7
Effective partner notification identifies and treats people with infection who may not otherwise have presented for health care (often males), reduces the chances that index cases become reinfected by untreated partners and thus can potentially reduce onward population transmission.7 Chlamydia reinfection rates are high; 18% of people retested within 6 months of diagnosis were positive on retesting in one region of New Zealand,8 and 19% of people aged 16–29 years retested positive in an Australian general practice population.9 Untreated and repeat infection may be associated with more serious long-term reproductive health complications,10 so timely detection and treatment are vital. NZSHS guidelines recommend routinely testing for reinfection 3 months post-treatment,5 and New Zealand Ministry of Health Chlamydia management guidelines recommend retesting at 3–6 months.6 Follow up (by phone or in person) at 1-week post-treatment is also recommended to determine treatment compliance, risk of reinfection, the subsequent need for re-treatment and to support partner notification efforts.5
There are recognised provider and patient-related challenges associated with effective partner notification and follow up,11–14 and research in New Zealand suggests that this aspect of STI management is not generally well carried out.15,16 Before initiating a pilot study to trial new approaches to partner notification management and testing for reinfection (Trial number: ACTRN12616000837426), we undertook baseline data collection reviewing the documented management of chlamydia and gonorrhoea cases diagnosed in primary care to determine the extent of partner notification, follow up and testing for reinfection undertaken according to NZSHS guidelines.
Methods
Study design
This retrospective review of electronic patient records took place between November 2015 and April 2016. Ethical approval was granted by the Southern Health and Disability Ethics Committee (Ref. 15/STH/109). Approval was granted for clinical members of the research team to review patient records within clinics (no names or contact details were recorded on audit forms).
Participants and setting
Eight primary health-care clinics participated, including two youth health clinics, a student health clinic and five general practice clinics (including two not-for-profit clinics for high-needs patients and a Māori health provider) situated within the greater Wellington region (includes Wellington, Hutt and Porirua cities). A laboratory data extract was used to identify clinics diagnosing the highest numbers of chlamydia and gonorrhoea cases in the 12 months to June 2015. The 121 clinics with one or more positive results during that period were ranked from the highest to lowest count, and 10 of the top 20 were invited to participate in the study (excluding Family Planning and Sexual Health Services). We selected the participants to include a range of clinic types with a diversity of patient populations including high proportions of Māori and Pacific. Two clinics declined participation due to lack of time. During 2014, 442 cases of chlamydia or gonorrhoea were diagnosed at the eight participating clinics (16.5% of the 2674 cases in the region).
Data collection
A case-note review list was generated for each clinic using the laboratory data, which included National Health Index (NHI) numbers, date of birth, date of test and results for 40 consecutive, unique individuals diagnosed with chlamydia or gonorrhoea between September 2013 and February 2015. The review period in clinics varied from 13 to 18 months depending on annual case numbers (range 27–129 cases diagnosed per clinic in 2014) and attempts to ensure sufficient numbers of males were included from clinics with higher annual caseloads. We adapted an existing audit template to collect data relating to diagnosis, treatment, advice given (including partner notification discussion), follow up, partner notification outcomes and retesting, as documented in electronic patient notes.15 The form was completed by a clinical member of the research team (in six practices) or by a member of the participating practice team (two practices requested that their own nurses complete the review).
Data analysis
The number (and percentage) of individuals for whom details were documented about key aspects of STI management according to best practice care were collated. Proportions retested between 6 weeks and 6 months post-treatment and with a positive result on retesting were also calculated.
Results
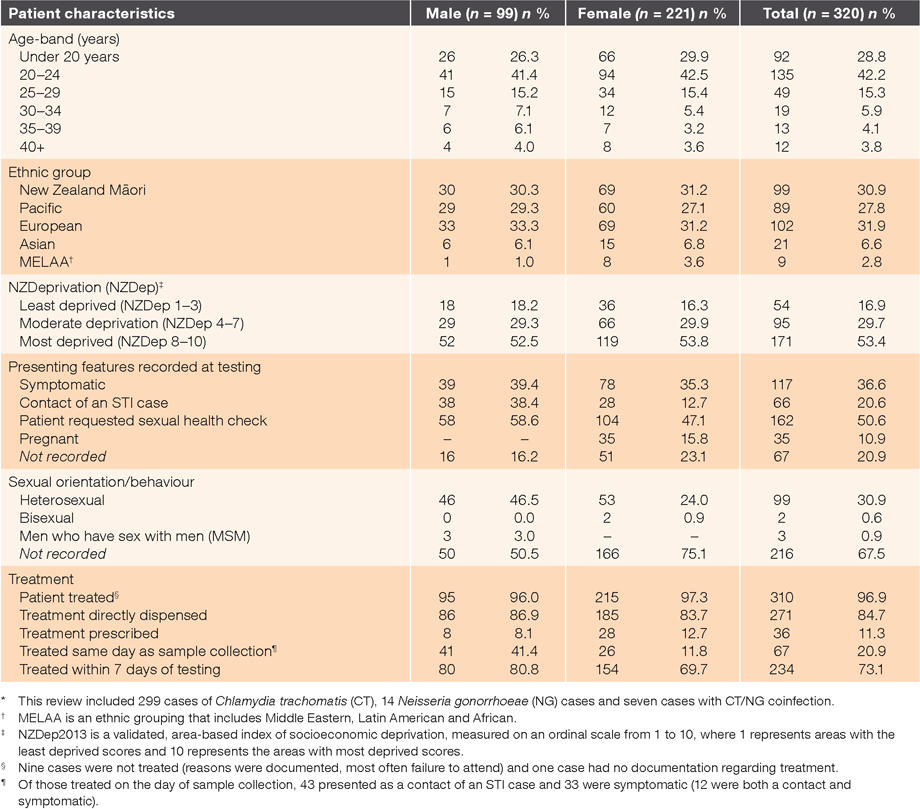
In the 12 months to June 2015, 9.8% of samples submitted for chlamydia and gonorrhoea testing returned positive results at the eight clinics collectively (470/4818). Table 1 presents the demographic characteristics of the 320 cases reviewed (40 per clinic), together with documentation on presenting features and treatment. Māori and Pacific people were over-represented, as were people living in areas of higher deprivation (due to inclusion of clinics with high proportions of enrolled Māori and Pacific, and two low-fee clinics).
|
Testing and treatment
Overall, there was generally good documentation about reasons for testing and treatment; 79% had a record of one or more presenting features or reason(s) for testing (clinic range 70–90%). Documentation regarding treatment was present for 97% (310/320) of cases treated (clinic range 90–100%). The median time to treatment was 6 days, and 95% were treated within 3 weeks of diagnosis. Of the 21 individuals with gonorrhoea, 71% (15/21) received the recommended dual therapy (ceftriaxone and azithromycin), but in five cases, only ceftriaxone appeared to have been given.
Documented advice, partner notification and follow up
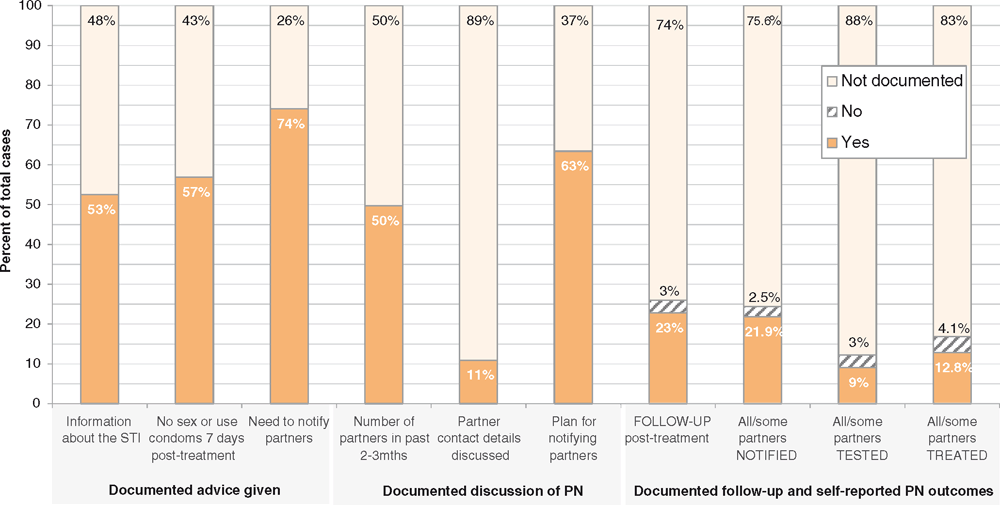
Figure 1 presents the percentage of cases for whom key aspects of patient care were documented as having been provided, and partner notification outcomes. Partner notification was documented as having been discussed with 74% of cases (clinic range 53–100%), most often at the time of diagnosis or treatment, and more often discussed by nurses than GPs. The plan for notifying partners was recorded for 63% of cases (clinic range 40–90%), with most indicating patient referral as the approach. Ten individuals were given a prescription for their partners (this occurred in five of the settings reviewed). Follow up by phone or at a subsequent appointment was carried out for 23% of cases (73/320, clinic range 18–33%), but the nature of follow up was not always documented. Treatment compliance and partner notification outcomes were most commonly documented as having been discussed (for approximately half those followed up).
|
Best practice care continuum
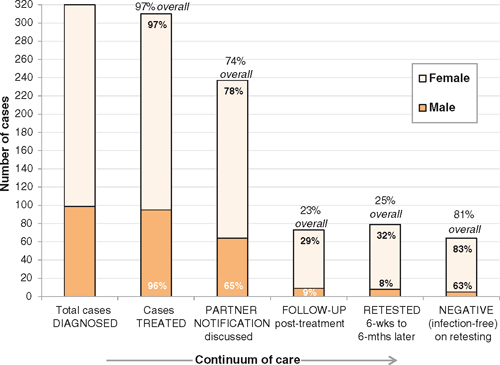
Figure 2 presents the number of cases for whom ‘best practice’ was documented as having been provided at each step on the continuum of care from diagnosis to retesting. Overall, 25% of cases (79/320) were retested between 6 weeks and 6 months post-treatment, with retesting rates varying from 13% to 53% between clinics. While one-third of females were retested (17% positive, 12/71), only 8% of males were retested (38% positive, 3/8). Eighteen cases were retested sooner than 6 weeks (none were positive), but reasons for early retesting were not clear.
|
Discussion
Frequent gaps in documentation limited our ability to assess all aspects of patient management (including partner notification and follow up) for individuals diagnosed with chlamydia or gonorrhoea in primary care practices, but suggest gaps exist between recommended and actual care. Three-quarters of cases had documented evidence that telling partners had been advised, but follow up on treatment compliance and assessment of partner notification outcomes occurred in just under one-quarter of cases, so actual partner notification outcomes were difficult to accurately assess. Only one-quarter of cases were retested within 6 months of treatment, and among those retested, reinfection rates were high. Marked variation was observed in the extent of best practice care documented as having been provided to patients both within and between clinics.
Our findings are consistent with research conducted in the Waikato region that reviewed 415 chlamydia cases diagnosed across 20 clinical settings in 2008.15,16 Details related to diagnosis and treatment of the STI were generally well documented in both studies, but less well with respect to sexual history taking and partner notification. Approximately half of case records in both studies had no detail about recent sexual history. While 41% of cases in the Waikato study had no record of partner notification discussion, 26% of cases were missing this documentation in our study. The planned method of contacting partners was recorded for 63% of cases in the present study but only for 31% in the Waikato study. Small proportions of individuals had a record of partner notification outcomes in both studies. We found 24.4% of cases had a record of the numbers of partner(s) advised (vs. 21% in Waikato), and 16.9% had a record of number of partners treated as reported by the index patient (vs. 12% in Waikato). Low rates of follow up and lack of knowledge about partner outcomes for some cases who were followed up mean that these figures likely underestimate the extent of actual partner treatment. These results also reflect challenges faced when undertaking partner notification and follow up, identified in our survey of primary health-care clinicians.14
This review revealed deficiencies in recorded sexual history and lack of documentation on advice given and follow up on treatment adherence, risk-reduction education and partner notification. It was not therefore clear from this review what advice or patient information is routinely given. Multiple visits are typically needed for the full cycle of STI care, and patients will often be seen by different members of the primary care team. The patient record not only acts as a memoir to the clinician providing care at a given visit, but also as a way to provide continuity of care. Taking (and documenting) a sexual history, together with documentation regarding advice and the partner notification plan, is key to ensuring patients receive appropriate care when diagnosed with an STI.17 Use of a sexual health template (see NZ Doctor, Nov 2014 for an example)18 could act as a prompt for discussion and facilitate record-keeping.
Implications for clinical practice
The need to prioritise and address New Zealand’s high rates of STIs is indisputable. In the absence of any national-level strategies to address STIs, clinician time invested in undertaking more effective partner notification and follow up has real potential to reduce the overall prevalence of these infections and reduce associated health burden (including infertility and serious infection) and overall provider workload in the long term. Clinicians should ensure partner notification is always addressed at the time of treatment in a way that increases the likelihood that individuals feel willing and able to talk with their partner(s) about possible exposure to an STI. Clinicians discussing partner notification should be familiar with evidence-based guidelines relating to STI and partner notification management (produced by NZSHS,19 ASHM20). Use of printed or online resources regarding partner notification, such as those available on the Sexual Health Society website (www.nzshs.org) and the consumer-based website, www.justthefacts.co.nz, could help facilitate the process. The Australian website (www.letthemknow.org.au) that allows for notification of partners electronically (anonymously if preferred) was deemed an important tool by Australian Family Planning clinicians, and regarded as useful by individuals who find contacting partners in person difficult.21 Funding to provide a similar tool would likely be of value to clinicians, patients and their partners in New Zealand.
Although a relatively small proportion of cases were retested in this study, the reinfection rate was high, consistent with local and international research.8,9,22,23 Low overall retesting rates and early retesting (within 6 weeks) may be indicative of lack of awareness of guideline recommendations regarding the timing of retesting.14 Ensuring patients are educated about the importance of retesting is an important component of STI-related patient advice.2 Opportunities for retesting may have been missed due to a lack of systems in place to facilitate this aspect of patient care. Research in Australia has demonstrated improved re-attendance for retesting following the use of text message (SMS) reminders for a test of reinfection,24,25 with the added provision of a postal home sample collection kit (as well as an SMS) further improving retesting rates.23 Suggesting patients add a reminder to their mobile phone, drop-in clinics for asymptomatic patients to provide a self-sample,26 and use of electronic chart prompts or flags to facilitate opportunistic testing when patients next present for care,9,10 have all been suggested as strategies that might facilitate retesting.
Strengths and limitations
This research adds to work carried out previously in the Waikato region,15,16 further emphasising the need for a more systematic approach to STI case management (and documentation) in primary care. We reviewed only a modest number of cases overall, as our review served as the preliminary data collection phase for a planned pilot study designed to trial new approaches to partner notification and follow up that required participation by only six clinics. Data could not therefore be analysed in detail at the level of gender, age or ethnicity. Participating clinics were among clinics diagnosing the highest numbers of bacterial STIs in the region and agreed to take part having recognised they were facing challenges with partner notification (eg lack of guidance on what to discuss and document, lack of time and resources to follow up, difficulties getting hold of patients). Findings might not therefore be generalisable to all primary care settings, but are likely to reflect challenges faced in many practices. We were only able to review outcomes documented in patient notes, so our results might underestimate the extent to which some aspects of best practice occurred.
Conclusion
This study suggests that for individuals diagnosed with chlamydia or gonorrhoea in New Zealand, some aspects of patient care may not always be addressed in line with best practice guidelines. If we are to make progress in reducing STIs and reinfection rates, every stage of the STI care pathway needs adequate attention. Strategies will be needed to ensure primary care practitioners are familiar with, and adequately supported and resourced to follow STI management guidelines.
COMPETING INTERESTS
None.
ACKNOWLEDGEMENTS
We thank all primary care teams allowing us to undertake this clinical records review in their clinics, and the staff who assisted with the review process. This project was funded by a 2015 Lottery Health Research grant (Application number 353074). The funding body played no role in the design, implementation or interpretation of the research.
ACKNOWLEDGEMENTS
We thank all primary care teams allowing us to undertake this clinical records review in their clinics, and the staff who assisted with the review process. This project was funded by a 2015 Lottery Health Research grant (Application number 353074). The funding body played no role in the design, implementation or interpretation of the research.
References
[1] Ministry of Health. Sexual and reproductive health. A resource book for New Zealand health care organisations. Wellington, New Zealand: Ministry of Health; 2003 [cited 2017 March 1]. Available from: https://www.health.govt.nz/system/files/documents/publications/sexualreprohealthresource.pdf[2] Centers for Disease Control and Prevention (CDC) CDC Grand Rounds: Chlamydia prevention: Challenges and strategies for reducing disease burden and sequelae. MMWR Morb Mortal Wkly Rep. 2011; 60 370–73.
[3] Cates W, Wasserheit JN. Genital chlamydial infections: Epidemiology and reproductive sequelae. Am J Obstet Gynecol. 1991; 164 1771–81.
| Genital chlamydial infections: Epidemiology and reproductive sequelae.Crossref | GoogleScholarGoogle Scholar |
[4] Gottlieb SL, Stoner BP, Zaidi AA, et al. A prospective study of the psychosocial impact of a positive Chlamydia trachomatis laboratory test. Sex Transm Dis. 2011; 38 1004–11.
| A prospective study of the psychosocial impact of a positive Chlamydia trachomatis laboratory test.Crossref | GoogleScholarGoogle Scholar |
[5] New Zealand Sexual Health Society. NZSHS STI Management Guidelines for use in primary care 2017. NZSHS; 2017 [cited 2017 November 11]. Available from: http://nzshs.org/guidelines
[6] Ministry of Health. Chlamydia management guidelines. Wellington, New Zealand: Ministry of Health; 2008 [cited 2017 February 22]. Available from: http://www.moh.govt.nz/notebook/nbbooks.nsf/0/edfd568777f16125cc2574b600749353/$FILE/chlamydia-management-guidelines.pdf
[7] Ward H, Bell G. Partner notification. Medicine. 2014; 42 314–7.
| Partner notification.Crossref | GoogleScholarGoogle Scholar |
[8] Rose SB, Garrett SM, Stanley J, Pullon SRH. Retesting and repeat positivity following diagnosis of Chlamydia trachomatis and Neisseria gonorrhoea in New Zealand: a retrospective cohort study. BMC Infect Dis. 2017; 17 526
| Retesting and repeat positivity following diagnosis of Chlamydia trachomatis and Neisseria gonorrhoea in New Zealand: a retrospective cohort study.Crossref | GoogleScholarGoogle Scholar |
[9] Bowring AL, Gouillou M, Guy R, et al. Missed opportunities - low levels of chlamydia retesting at Australian general practices, 2008–2009. Sex Transm Infect. 2012; 88 330–4.
| Missed opportunities - low levels of chlamydia retesting at Australian general practices, 2008–2009.Crossref | GoogleScholarGoogle Scholar |
[10] California Department of Public Health (CDPH). Sexually Transmitted Diseases (STD) Control Branch. Best practices for the prevention and early detection of repeat chlamydial and gonococcal infections: effective partner treatment and patient retesting strategies for implementation in California health care settings. CDPH; updated 2016 [cited 2017 November 24]. Available from: https://www.cdph.ca.gov/Programs/CID/DCDC/CDPH%20Document%20Library/Best_Practices_for_Preventing_RepeatCT_Inf.pdf
[11] Hocking JS, Parker RM, Pavlin N, et al. What needs to change to increase chlamydia screening in general practice in Australia? The views of general practitioners. BMC Public Health. 2008; 8 425
| What needs to change to increase chlamydia screening in general practice in Australia? The views of general practitioners.Crossref | GoogleScholarGoogle Scholar |
[12] Bangor-Jones RD, McCloskey J, Crooks AML, et al. Attitudes of WA GPs to chlamydia partner notification: A survey. Aust Fam Physician. 2009; 38 448–52.
[13] European Centre for Disease Prevention and Control (ECDC). Public health benefits of partner notification for sexually transmitted infections and HIV. Technical report. Stockholm: ECDC; 2013 [cited 2017 March 1]. Available from: http://ecdc.europa.eu/en/publications/Publications/Partner-notification-for-HIV-STI-June-2013.pdf
[14] Rose SB, Garrett SM, Pullon SRH. Overcoming challenges associated with partner notification following chlamydia and gonorrhoea diagnosis in primary care: a postal survey of doctors and nurses. J Prim Health Care. 2017; 9 136–44.
[15] Morgan J, Donnell A, Bell A. Is everyone treated equally? Management of genital Chlamydia trachomatis infection in New Zealand. Int J STD AIDS. 2010; 21 595–600.
| Is everyone treated equally? Management of genital Chlamydia trachomatis infection in New Zealand.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cbitFaqug%3D%3D&md5=fa2b7f25b52873599111b336bfd8caeaCAS |
[16] Morgan J, Donnell A, Bell A. A multi-setting audit of the management of genital Chlamydia trachomatis infection. N Z Med J. 2010; 123 42–54.
[17] Best Practice Advocacy Centre New Zealand (BPAC) A “how-to guide” for a sexual health check-up. BPAC NZ. 2013; 52
[18] Claydon L. . Taking sexual histories in the techno-sexual era. NZ Doctor. 2014; 5
[19] New Zealand Sexual Health Society. Partner notification/contact tracing management summary, 2017. NZSHS; 2017 [cited 2017 November 15]. Available from: http://www.nzshs.org/docman/guidelines/principles-of-sexual-health-care/144-partner-notification-guideline/file
[20] Australasian Society for HIV Viral Hepatitis and Sexual Health Medicine (ASHM). Australasian contact tracing guidelines. ASHM; 2016 [cited 2017 November 11]. Available from: http://contacttracing.ashm.org.au
[21] Guy RJ, Micallef JM, Mooney-Somers J, et al. Evaluation of chlamydia partner notification practices and use of the “Let Them Know” website by family planning clinicians in Australia: cross-sectional study. J Med Internet Res. 2016; 18 e173
| Evaluation of chlamydia partner notification practices and use of the “Let Them Know” website by family planning clinicians in Australia: cross-sectional study.Crossref | GoogleScholarGoogle Scholar |
[22] LaMontagne SD, Baster K, Emmett L, et al. Incidence and reinfection rates of genital chlamydial infection among women aged 16–24 years attending general practice, family planning and genitourinary medicine clinics in England: a prospective cohort study by the Chlamydia Recall Study Advisory Group. Sex Transm Infect. 2007; 83 292–303.
| Incidence and reinfection rates of genital chlamydial infection among women aged 16–24 years attending general practice, family planning and genitourinary medicine clinics in England: a prospective cohort study by the Chlamydia Recall Study Advisory Group.Crossref | GoogleScholarGoogle Scholar |
[23] Smith KS, Hocking JS, Chen MY, et al. Dual intervention to increase Chlamydia retesting: A randomized controlled trial in three populations. Am J Prev Med. 2015; 49 1–11.
| Dual intervention to increase Chlamydia retesting: A randomized controlled trial in three populations.Crossref | GoogleScholarGoogle Scholar |
[24] Downing SG, Cashman C, McNamee H, et al. Increasing chlamydia test of re-infection rates using SMS reminders and incentives. Sex Transm Infect. 2013; 89 16–9.
| Increasing chlamydia test of re-infection rates using SMS reminders and incentives.Crossref | GoogleScholarGoogle Scholar |
[25] Guy R, Wand H, Knight V, et al. SMS reminders improve re-screening in women and heterosexual men with chlamydia infection at Sydney Sexual Health Centre: a before-and-after study. Sex Transm Infect. 2013; 89 11–5.
| SMS reminders improve re-screening in women and heterosexual men with chlamydia infection at Sydney Sexual Health Centre: a before-and-after study.Crossref | GoogleScholarGoogle Scholar |
[26] Nakatsukasa-Ono W, Howard H. Practical Strategies for Improving Chlamydia and Gonorrhea Retesting. Expert Commentary. National Chlamydia Coalition (NCC) Research Translation Committee: Oct. 2012, No. 14. NCC Research Translation Committee; 2012 [cited 2018 March 13]. Available from: http://www.hopkinsmedicine.org/medicine/std/downloads/ncc_expert_commentary_april2012.pdf


