Study protocol for an observational study to evaluate an accelerated chest pain pathway using point-of-care troponin in New Zealand rural and primary care populations
Rory Miller 1 12 , Joanna Young 1 , Garry Nixon 2 , John W. Pickering 3 , Tim Stokes 1 , Robin Turner 4 , Gerard Devlin 5 , Antony Watson 6 , Marc Gutenstein 1 7 , Tim Norman 8 , Peter Myles George 9 , Stephen Du Toit 10 , Martin Than 111 Department of General Practice and Rural Health, Dunedin School of Medicine, University of Otago, Dunedin, New Zealand
2 Cardiology, Canterbury DHB, Christchurch Hospital, Christchurch and Department of Medicine, University of Otago – Christchurch, Christchurch, New Zealand
3 Medicine, University of Otago – Christchurch and Emergency Department, Christchurch Hospital and Department of Medicine, University of Otago, Christchurch, New Zealand
4 Centre for Biostatistics, Division of Health Sciences, University of Otago, Dunedin, New Zealand
5 Tairawhiti DHB, Gisborne, New Zealand
6 Emergency Care Foundation, St Albans, Christchurch, New Zealand
7 Rural Health Academic Centre Ashburton, University of Otago and Christchurch and Emergency Department, Nelson Hospital, Nelson, New Zealand
8 Project Office, Midlands Regional Health Network Charitable Trust, Hamilton, New Zealand
9 Chemical pathology, PathoGene, Merivale, Christchurch, New Zealand
10 Biochemistry, Waikato DHB. Biochemistry Department, Waikato Hospital, Hamilton, New Zealand
11 Emergency Department, Canterbury DHB, Christchurch Hospital, Christchurch, New Zealand
12 Corresponding author. Email: Rory.miller@otago.ac.nz
Journal of Primary Health Care 12(2) 129-138 https://doi.org/10.1071/HC19059
Published: 20 May 2020
Journal Compilation © Royal New Zealand College of General Practitioners 2020 This is an open access article licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License
Abstract
INTRODUCTION: Accelerated diagnostic chest pain pathways are used widely in urban New Zealand hospitals. These pathways use laboratory-based troponin assays with good analytical precision. Widespread implementation has not occurred in many of New Zealand’s rural hospitals and general practices as they are reliant on point-of-care troponin assays, which are less sensitive and precise. An accelerated chest pain pathway using point-of-care troponin has been adapted for use in rural settings. A pilot study in a low-risk rural population showed no major adverse cardiac events at 30 days. A larger study is required to be confident that the pathway is safe.
AIMS: To assess the safety and effectiveness of an accelerated chest pain pathway adapted for rural settings and general practice using point-of-care troponin to identify low-risk patients and allow early discharge.
METHODS: This is a prospective observational study of an accelerated chest pain pathway using point-of-care troponin in rural hospitals and general practices in New Zealand. A total of 1000 patients, of whom we estimate 400 will be low risk, will be enrolled in the study.
OUTCOME MEASURES: The primary outcome is the proportion of patients identified by the pathway as low risk for a 30-day major adverse cardiac event. Secondary outcomes include the proportion of low-risk patients who were discharged directly from general practice or rural hospitals, the proportion of patients reclassified as having acute myocardial infarction by the pathway and the proportion of patients with low and intermediate risk safely managed in the rural hospital.
KEYwords: Rural health; Chest pain; Primary Care; rural hospitals; point-of-care troponin
| WHAT GAP THIS FILLS |
| What is already known: Accelerated diagnostic chest pain pathways using laboratory-based troponin assays are used widely in metropolitan emergency departments in New Zealand and have been shown to be safe and effective. There are limited assessments of the safety and efficacy of accelerated diagnostic chest pain pathways in rural hospitals and primary care using point-of-care troponin assays. |
| What this study adds: We describe the protocol for a large (1000 patient) observational trial assessing the safety and efficacy of a chest pain pathway used in New Zealand rural hospitals and primary care locations reliant on point-of-care troponin assays. |
Introduction
Although chest pain is a common reason for patients to seek medical attention, only 10–20% of these patients who present to emergency departments (EDs) will have a final diagnosis of acute myocardial infarction (AMI).1–5 AMI is even less common in patients presenting to general practice.3,4,6,7 Although the true burden of chest pain and AMI is unknown in rural New Zealand, in rural Australia, there are increased rates of AMI, which are associated with higher mortality and morbidity than in metropolitan areas.4,8–10
Clinicians typically take a cautious approach when assessing patients presenting with chest pain due to the substantially increased risk of mortality when AMI is missed.1,3,11 Historically, this led to prolonged investigation, observation and length of stay, adding pressure to crowded EDs.1,3,4,9–13 In an effort to address this, New Zealand’s District Health Boards (DHBs) have adopted Accelerated Diagnostic Chest Pain Pathways.14 These combine an objective scoring system, an electrocardiogram (ECG) and a cardiac bio-marker to stratify patients into low-, intermediate- or high-risk groups.4,14–17 In urban New Zealand, these accelerated chest pain pathways have been shown to safely double the proportion of patients with chest pain discharged from EDs within 6 h of arrival.14
Chest pain pathways currently used in New Zealand incorporate laboratory-based contemporary or high-sensitivity cardiac troponin assays, with good or very good analytical precision.14 However, most rural hospitals rely on point-of-care cardiac troponin assays.18 Most general practitioners (GPs) do not have timely access to any form of troponin assay, which can result in lengthy transfers for patients with chest pain. Current point-of-care troponin tests are less precise and have lower clinical sensitivity than laboratory-based assays.19 Up to 47% of clinically relevant non-ST elevation myocardial infarction (NSTEMI) may be missed if the manufacturer’s recommended cut-off (99th percentile of normal) is used.19,20 Accelerated chest pain pathways that use laboratory troponin, as are used in urban hospitals, are therefore not applicable in rural hospitals and general practices that rely on point-of-care troponin.
The clinical sensitivity of some point-of-care troponin assays can be improved by reducing the cut-off to below the manufacturer’s recommendation.19,20 In the case of the Abbott iSTAT cTnI (iSTAT), reducing the cut-off improves the sensitivity from 34% to 81% and all previously missed AMI were detected.20
Although there are no large studies using point-of-care troponin in rural populations, there is evidence of safety for accelerated diagnostic chest pain pathways that use point-of-care troponin in urban EDs.3,21,22 A pilot study of a chest pain pathway using point-of-care troponin has recently concluded in rural Waikatō general practice. Over 50% of patients were identified as low risk and avoided transfer to hospital with no major cardiac adverse events detected at 30 days.23 A larger study is required to verify the pilot study findings and allow the New Zealand Cardiac Network to endorse incorporating into national policy an accelerated diagnostic chest pain pathway using point-of-care troponin.
The adoption of a validated accelerated chest pain pathway by New Zealand’s rural hospitals and general practices has the potential to reduce health service burden and improve patient outcomes by reduced travel to regional or base hospitals; early reassurance for patients identified as low risk; and improved identification and treatment of patients at intermediate- and high-risk of AMI.
Methods
Study aims
-
To assess the safety and effectiveness of an accelerated diagnostic chest pain pathway using point-of-care troponin to identify low-risk patients and allow their early discharge from rural hospitals or directly from primary care.
-
To determine if the use of the chest pain pathway and a lower Abbott iSTAT cTnI point-of-care troponin cut-off is more sensitive for the detection of NSTEMI than using the manufacturer’s recommended cut-off.
Study design
This is a prospective observational evaluation of an accelerated chest pain pathway adapted for use in rural areas that use point-of-care troponin in the management of suspected cardiac chest pain.23,24 This accelerated chest pain pathway was adapted from a validated metropolitan pathway to be used in rural areas and primary care using point-of-care troponin.24 This pathway is based on expert consensus and available evidence.3,19–23 This pathway has been introduced in some rural hospitals and general practices.
Setting and location
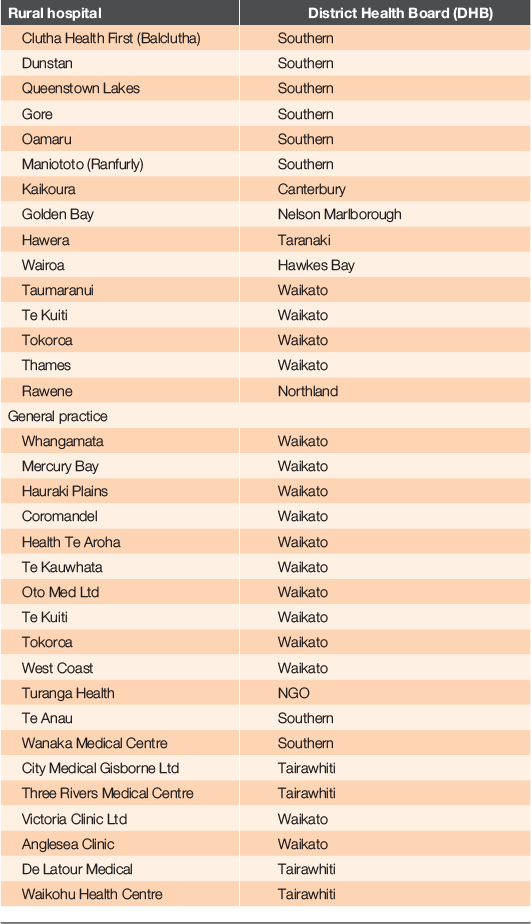
The study will be undertaken in rural hospitals and rural and urban general practices that use the accelerated chest pain pathway. New Zealand rural hospitals are defined as ‘a hospital staffed by suitably trained and experienced generalists (both Medical Officers of Special Scale (MOSSs) and GPs), who take full clinical responsibility for a wide range of clinical presentations. While resident specialists may also work in these hospitals, specialist cover is limited to 24 h/7-day cover in no more than one specialist area.’25–28 These rural hospitals have diverse locations, sizes, patient demographics and resources.25 Rural general practices are identified by the Primary Health Care Organisation (PHO) rural service level alliance team agreement.29
Participants and recruitment
Patient data will be collected at the rural hospital and general practice sites (shown in Table 1) that have implemented the pathway and are reliant on point-of-care troponin at least some of the time. Education sessions will be provided to clinical staff, to ensure that clinical teams are comfortable with the pathway and the data entry platform. Copies of the pathway will be available for reference. Clinical decision support is built into the data collection tool.
|
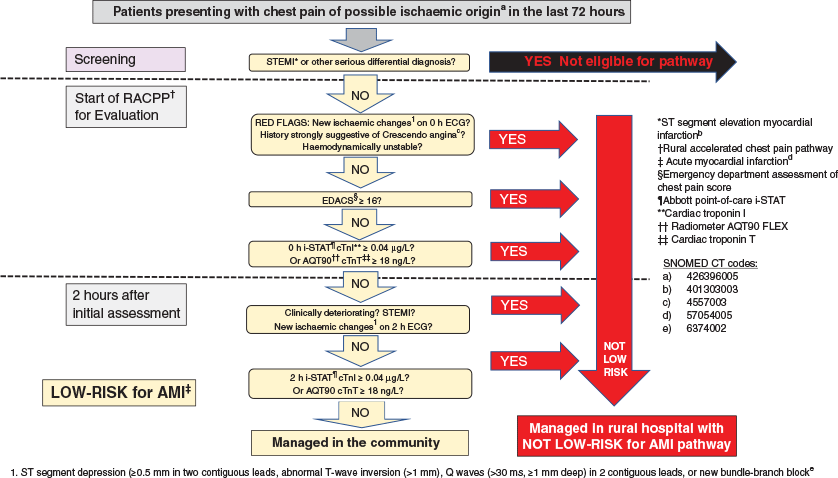
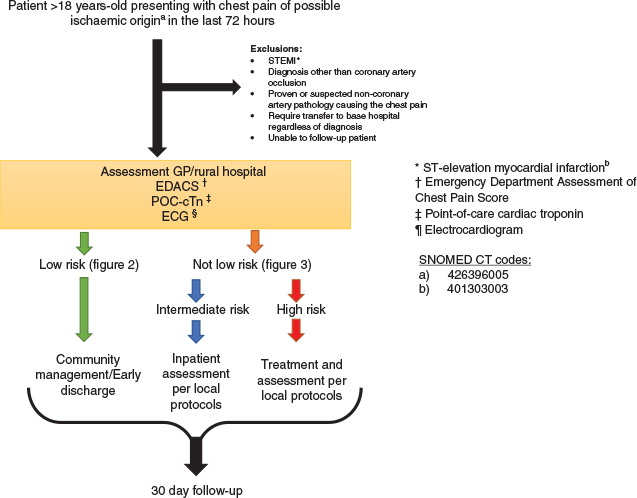
Patients will be identified and progress through the accelerated chest pain pathway as outlined in Figure 1.
|
Inclusion criteria
To be included in this study, patients need to be aged >18 years and present with chest pain of suspected ischaemic (occlusive) origin (beginning within the previous 72 h) to either a general practice or a rural hospital; this includes referred patients. Possible ischaemic symptoms include the presence of acute pain, discomfort or pressure experienced in the chest, epigastric area, neck, jaw or arm without an apparent non-cardiac source.30
Exclusion criteria
Patients will be excluded if they present with any ST-segment elevation myocardial infarction (STEMI), proven or suspected non-coronary artery pathology causing chest pain, requiring transfer to base hospital regardless of negative investigations due to other medical conditions or an anticipated problem with follow up (eg overseas tourists leaving within 30-days).
Identification and management of low-risk patients
Low-risk patients are defined as patients (Figure 2) with all of the following features:
-
No ‘red flags’ (aortic dissection, pulmonary embolus, pancreatitis, history strongly suggestive of crescendo angina, haemodynamically unstable).
-
The absence of potentially significant ECG changes suggestive of cardiac ischaemia: ST-segment depression (≥0.5 mm in two contiguous leads), abnormal T-wave inversion (>1 mm), Q waves (>30 ms, ≥1 mm deep) in two contiguous leads or new bundle-branch block in an ECG at 0 and 2 h.
-
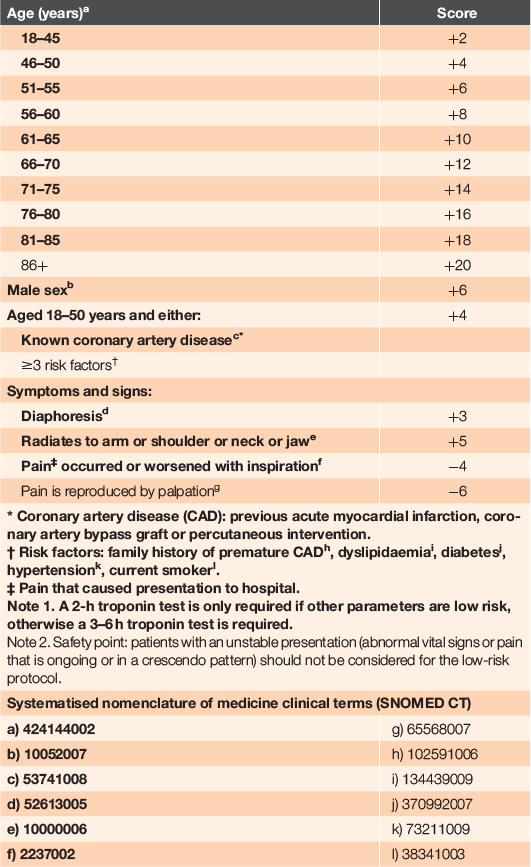
Emergency Department Assessment of Chest Pain Score (EDACS) <1631, as shown in Table 2.
-
Non-diagnostic point-of-care troponin at 0 and 2 h defined as:19,20
-
-
Abbott i-STAT cTnI (iSTAT) <0.04 ug/L (99th percentile of normal = 0.08 ug/L, Limit of blank = 0.02).
-
Radiometer AQT-90 FLEX cTnT (AQT90) <18 ng/L (99th percentile of normal = 17 ng/L, Limit of detection = 10 ng/L).
-
-
No further chest pain or ECG changes and remains clinically stable.
|
If the patient fails to meet any of these criteria, they exit the low-risk arm to enter the not-low-risk arm. If all these criteria are met, then the patient can be discharged home. Consideration will be given to outpatient provocative testing or cardiology referral on an individual basis.
Identification and management of not-low-risk patients
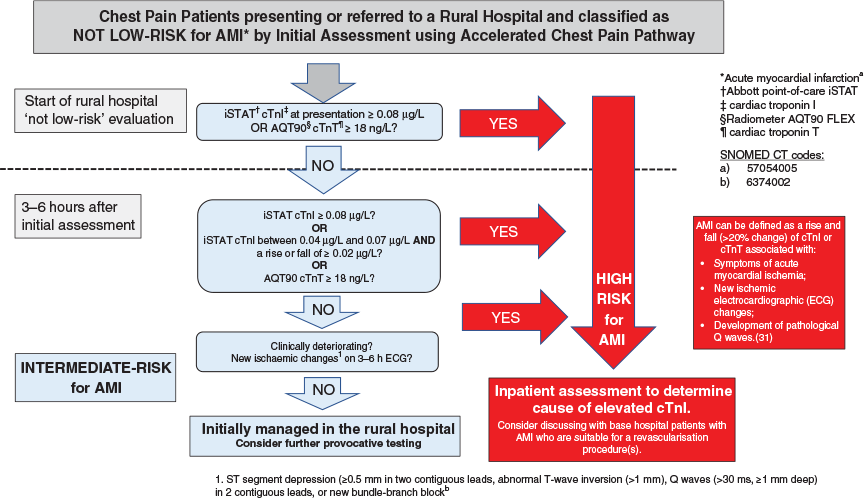
Patients who do not fulfil the criteria for the low-risk pathway or exit due to a change in clinical condition enter the not-low-risk arm of the pathway (Figure 3).
Patients who present to primary care are transferred to their usual referral centre. Patients who present to a rural hospital are admitted. A point-of-care troponin is drawn and an ECG performed at presentation and then again 3 to 6 h later. It may be clinically appropriate to repeat the ECG sooner than this. Patients will be classified as high risk if there is an elevated troponin without a non-occlusive cardiac cause:
iSTAT >0.04 ug/L but <0.08 ug/L with a difference between the first and second value of >0.02 ug/L or
Any iSTAT >0.07 ug/L or
Any AQT90 >17 ng/L (regardless of any rise or fall of troponin).
These patients will be investigated for the cause of their elevated troponin, and any patient who may be suitable for a coronary revascularisation procedure should be discussed with the cardiology service at the base hospital. Patients who are not low-risk and have two non-diagnostic point-of-care troponin concentrations are classified intermediate risk and inpatient monitoring and investigations are recommended according to local guidelines.
Protocol deviation
There are many reasons for an elevated troponin and we expect deviations from this pathway.32 If patient care does deviate from the pathway due to a clinician or system error or through patient choice, then they will be identified and followed up by a research nurse. Further assessment and investigations may be recommended to the patient’s GP or the rural hospital they attended.
Point-of-care cardiac troponin testing
Venous blood samples will be collected and analysed using the point-of-care troponin device according to manufacturer’s instructions. A new generation high precision point-of-care troponin assay is likely to be released for clinical use in the near future.33 Where possible, a duplicate blood sample will be sent to a central laboratory and stored, so that testing with the new assay can occur when it becomes available. This will future proof the study as point-of-care technology evolves.
Follow up
Included patients will be followed up 30 days after they present with chest pain to the rural hospital or general practices. Health events, based on International Classification of Diseases, 10th Revision (ICD-10) codes, using National Health Index identifier event searches will be collected from patients’ clinical records and the National Minimal Dataset of public hospital admissions, Ministry of Health (MOH). Mortality data will be retrieved from the National Mortality Collection, MOH.
Outcome measures
Primary outcome
The primary outcome is 30-day major adverse cardiac events in patients identified as low risk by the pathway. A major adverse cardiac event is defined as ‘death, cardiac arrest, emergency revascularisation procedure, cardiogenic shock, ventricular arrhythmia, ventricular fibrillation, high-degree atrio-ventricular block needing intervention, or acute myocardial infarction’.9 Relevant ICD-10 codes are shown in Appendix 1.
Secondary outcomes
Secondary outcomes are the proportion of patients reclassified as NSTEMI as a result of adopting the lower point-of-care troponin cut-offs, the proportion of patients avoiding referral to hospital from general practice, the proportion of low-risk patients discharged from rural hospital care within 6 h and the proportion of patients with low and intermediate risk, whose care is managed in rural hospitals without occurrence of a 30-day major adverse cardiac event.
Data collection
From participating general practices, data will be collected via a bespoke electronic template built into the practice management system. At other sites data will be inputted into the Research Electronic Data Capture (REDCap) database.34 REDCap is a web-based database that enables secure data collection, storage and maintenance, and is recommended for recording data, including personal information, that are covered by health information privacy principles, The Privacy Act or ethics committee specifications that require a secure tool.34 The collection form was designed to also provide basic clinical decision-support. All REDCap data are securely stored in the University of Otago servers.
Data from both sources and follow-up health event data will be combined using a translation table and analysed. De-identified data will be stored in a secure cloud-based storage solution and kept for 10 years.
Bias
Selection bias due to patient data not being entered can be minimised with education and continued engagement from the investigative group with the clinical environment to ensure that data are collected for all eligible participants. Internal validity is improved by having a data collection tool with inbuilt decision support. External validity is addressed by having multiple sites throughout New Zealand.
Sample size
Primary endpoint
A sample size of 1000 patients is estimated to provide at least 400 patients identified as low risk by the accelerated chest pain pathway.4,14,24 A 30-day major adverse cardiac event rate of <1% is required to show acceptable safety.35
Assuming a major adverse cardiac event rate of 1%, 410 low-risk patients will generate a 95% confidence interval between 0.27% and 2.5%. The Cardiac Network has indicated that this sample size would be satisfactory for them to endorse this pathway as national policy. It is estimated that it will take a maximum of 24 months to enrol 1000 patients into the study.
Secondary endpoints
Assuming there will be 200 patients with NSTEMI included in the sample and that ~30% of patients will be reclassified using the accelerated chest pain pathway and a lower troponin cut-off,3–5,11,20 the 95% confidence interval for patients re-classified will be 24–37%. Even the lower value of 24% will be considered clinically relevant.
Statistical analyses
Descriptive statistics will be presented, including self-determined ethnicity.
Primary endpoint
The proportion of patients identified as low risk with a 30-day major adverse cardiac event from presentation will be estimated and a 95% confidence interval for the estimate will be calculated. Sub-group analysis will be performed based on the type of point-of-care troponin assay used. Sensitivity, specificity and positive and negative predictive values presented with 95% confidence intervals will be derived for the rate of major adverse cardiac events in the low-risk arm of the pathway.
Secondary endpoints
From the proportion of patients reclassified as having AMI by the accelerated chest pain pathway using a lower cut-off, patients discharged within 6 h from a rural hospital and the proportion of patients with low and intermediate risk safely managed in a rural hospital will be estimated and presented with a 95% confidence interval. Sensitivity, specificity and positive and negative predictive values presented with 95% confidence intervals will be derived for the pathway’s ability to detect a major adverse cardiac event in not-low risk populations.
Ethics and dissemination
Ethical approval
The Health and Disability Ethics Committee approved the protocol as an Audit and Audit-related activity (16/CEN/107/AM03). Consultation was undertaken with the Ngāi Tahu Research Consultation Committee.
Dissemination
Study findings will be disseminated widely among key stakeholders, including the New Zealand Cardiac Network, New Zealand Heart Foundation, MOH and DHBs. Results will be shared with relevant professional agencies, including the Royal New Zealand College of General Practitioners, Division of Rural Hospital Medicine New Zealand and the Australasian College of Emergency Medicine. Findings will also be communicated directly to participating rural hospitals and general practices. Results will be submitted for publication in relevant peer-reviewed journals and presented at New Zealand and international conferences.
Competing interests
Dr Joanna Young is supported by a New Zealand Heart Foundation Research Fellowship. Associate Professor John Pickering has undertaken statistical consultancy for Abbott Diagnostics and his group has undertaken independent analysis of assay performance using kits supplied free-of-charge by Abbott Point of Care. Dr Martin Than has received funding for clinical trials and education and payment for speaking from Abbott and Alere. He has also received funding for clinical trials and education from Beckman Coulter and funding for clinical trials and payment for speaking from Roche.
Funding
This study is supported by a grant awarded by the Heart Foundation of New Zealand (1770). Further study funding was provided by an investigator grant from Abbott Point-of-Care. The funding bodies have had no role in the design of the study.
Acknowledgements
The authors would like to acknowledge all rural participating sites that are, as a rule, currently understaffed and underfunded, but continue to donate their time to contribute to this study.
References
[1] Goodacre S, Cross E, Arnold J, et al. The health care burden of acute chest pain. Heart. 2005; 91 229–30.| The health care burden of acute chest pain.Crossref | GoogleScholarGoogle Scholar | 15657244PubMed |
[2] Ellis C, Gamble G, Devlin G, et al. The management of acute coronary syndrome patients across New Zealand in 2012: results of a third comprehensive nationwide audit and observations of current interventional care. N Z Med J. 2013; 126 36–68.
| 24362734PubMed |
[3] Than M, Cullen L, Reid CM, et al. A 2-h diagnostic protocol to assess patients with chest pain symptoms in the Asia-Pacific region (ASPECT): a prospective observational validation study. Lancet. 2011; 377 1077–84.
| A 2-h diagnostic protocol to assess patients with chest pain symptoms in the Asia-Pacific region (ASPECT): a prospective observational validation study.Crossref | GoogleScholarGoogle Scholar | 21435709PubMed |
[4] Roche T, Jennings N, Clifford S, et al. Diagnostic accuracy of risk stratification tools for patients with chest pain in the rural emergency department: a systematic review. Emerg Med Australas. 2016; 28 511–24.
| Diagnostic accuracy of risk stratification tools for patients with chest pain in the rural emergency department: a systematic review.Crossref | GoogleScholarGoogle Scholar | 27469348PubMed |
[5] Bruins Slot MHE, van der Heijden GJMG, Stelpstra SD, et al. Point-of-care tests in suspected acute myocardial infarction: a systematic review. Int J Cardiol. 2013; 168 5355–62.
| Point-of-care tests in suspected acute myocardial infarction: a systematic review.Crossref | GoogleScholarGoogle Scholar |
[6] Andersson PO, Karlsson J-E, Landberg E, et al. Consequences of high-sensitivity troponin T testing applied in a primary care population with chest pain compared with a commercially available point-of-care troponin T analysis: an observational prospective study. BMC Res Notes. 2015; 8 210
| Consequences of high-sensitivity troponin T testing applied in a primary care population with chest pain compared with a commercially available point-of-care troponin T analysis: an observational prospective study.Crossref | GoogleScholarGoogle Scholar | 26036786PubMed |
[7] Bösner S, Becker A, Haasenritter J, et al. Chest pain in primary care: epidemiology and pre-work-up probabilities. Eur J Gen Pract. 2009; 15 141–6.
| Chest pain in primary care: epidemiology and pre-work-up probabilities.Crossref | GoogleScholarGoogle Scholar | 19883149PubMed |
[8] Baker T, McCoombe S, Mercer-Grant C, Brumby S. Chest pain in rural communities; balancing decisions and distance: farmers with chest pain: a pilot study. Emerg Med Australas. 2011; 23 337–45.
| Chest pain in rural communities; balancing decisions and distance: farmers with chest pain: a pilot study.Crossref | GoogleScholarGoogle Scholar | 21668721PubMed |
[9] Kinsman LD, Rotter T, Willis J, et al. Do clinical pathways enhance access to evidence-based acute myocardial infarction treatment in rural emergency departments? Aust J Rural Health. 2012; 20 59–66.
| Do clinical pathways enhance access to evidence-based acute myocardial infarction treatment in rural emergency departments?Crossref | GoogleScholarGoogle Scholar | 22435765PubMed |
[10] Blokker BM, Jannsen JH, van Beeck E. Referral patterns of patients presenting with chest pain at two rural emergency departments in Western Australia. Rural Remote Health. 2010; 10 1558
| 20815655PubMed |
[11] Pope JH, Aufderheide TP, Ruthazer R, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med. 2000; 342 1163–70.
| Missed diagnoses of acute cardiac ischemia in the emergency department.Crossref | GoogleScholarGoogle Scholar | 10770981PubMed |
[12] Bingisser R, Cairns C, Christ M, et al. Cardiac troponin: a critical review of the case for point-of-care testing in the ED. Am J Emerg Med. 2012; 30 1639–49.
| Cardiac troponin: a critical review of the case for point-of-care testing in the ED.Crossref | GoogleScholarGoogle Scholar | 22633720PubMed |
[13] Goodacre S, Thokala P, Carroll C, et al. Systematic review, meta-analysis and economic modelling of diagnostic strategies for suspected acute coronary syndrome. Health Technol Assess. 2013; 17 1–177.
| Systematic review, meta-analysis and economic modelling of diagnostic strategies for suspected acute coronary syndrome.Crossref | GoogleScholarGoogle Scholar |
[14] Than MP, Pickering JW, Dryden JM, et al. ICare-ACS (Improving Care Processes for Patients With Suspected Acute Coronary Syndrome): a study of cross-system implementation of a national clinical pathway. Circulation. 2018; 137 354–63.
| ICare-ACS (Improving Care Processes for Patients With Suspected Acute Coronary Syndrome): a study of cross-system implementation of a national clinical pathway.Crossref | GoogleScholarGoogle Scholar | 29138293PubMed |
[15] Huis in ’t Veld MA, Cullen L, Mahler SA, et al. The fast and the furious: low-risk chest pain and the rapid rule-out protocol. West J Emerg Med. 2017; 18 474–8.
| The fast and the furious: low-risk chest pain and the rapid rule-out protocol.Crossref | GoogleScholarGoogle Scholar | 28435499PubMed |
[16] Christiansen J. Less is more: chest pain pathways in clinical care. Med J Aust. 2017; 207 193–4.
| Less is more: chest pain pathways in clinical care.Crossref | GoogleScholarGoogle Scholar | 28987131PubMed |
[17] Munro AR, Jerram T, Morton T, Hamilton S. Use of an Accelerated Diagnostic Pathway allows rapid and safe discharge of 70% of chest pain patients from the emergency department. N Z Med J. 2015; 128 62–71.
| 25662380PubMed |
[18] Miller R, Stokes T, Nixon G. Point-of-care troponin use in New Zealand rural hospitals: a national survey. N Z Med J. 2019; 132 25–37.
| 30973857PubMed |
[19] Simpson P. Recommendations for use of point-of-care (POC) troponin assays in assessment of acute coronary syndrome. Australasian Association of Clinical Biochemists; 2016. [cited 2018 November 6]. Available from: https://www.aacb.asn.au/documents/item/4483
[20] Schneider HG, Ablitt P, Taylor J. Improved sensitivity of point of care troponin I values using reporting to below the 99th percentile of normals. Clin Biochem. 2013; 46 979–82.
| Improved sensitivity of point of care troponin I values using reporting to below the 99th percentile of normals.Crossref | GoogleScholarGoogle Scholar | 23628595PubMed |
[21] Aldous S, Richards MA, George PM, et al. Comparison of new point-of-care troponin assay with high sensitivity troponin in diagnosing myocardial infarction. Int J Cardiol. 2014; 177 182–6.
| Comparison of new point-of-care troponin assay with high sensitivity troponin in diagnosing myocardial infarction.Crossref | GoogleScholarGoogle Scholar | 25499373PubMed |
[22] Meek R, Braitberg G, Cullen L, et al. Outcome at 30 days for low-risk chest pain patients assessed using an accelerated diagnostic pathway in the emergency department. Emerg Med Australas. 2016; 28 279–86.
| Outcome at 30 days for low-risk chest pain patients assessed using an accelerated diagnostic pathway in the emergency department.Crossref | GoogleScholarGoogle Scholar | 26998819PubMed |
[23] Norman T, Devlin G, Than M, et al. Measured implementation of an accelerated chest pain diagnostic pathway in primary care. Heart Lung Circ. 2018; 27 S4–5.
| Measured implementation of an accelerated chest pain diagnostic pathway in primary care.Crossref | GoogleScholarGoogle Scholar |
[24] Miller R, Nixon G. The assessment of acute chest pain in New Zealand rural hospitals utilising point-of-care troponin. J Prim Health Care. 2018; 10 90–2.
| The assessment of acute chest pain in New Zealand rural hospitals utilising point-of-care troponin.Crossref | GoogleScholarGoogle Scholar | 30068457PubMed |
[25] Williamson M, Gormley A, Dovey S, Farry P. Rural hospitals in New Zealand: results from a survey. N Z Med J. 2010; 123 20–9.
| 20581927PubMed |
[26] The Royal New Zealand College of General Practitioners. Division of Rural Hospital Medicine New Zealand: training handbook. Wellington, NZ: Division of Rural Hospital Medicine; 2017. [cited 2018 September 15]. Available from: https://oldgp16.rnzcgp.org.nz/assets/2018DRHM-Handbook.pdf
[27] Medical Council of New Zealand. Types of vocational scope. Wellington, NZ: Medical Council of New Zealand; 2019. [cited 2020 January 5]. Available from: https://www.mcnz.org.nz/get-registered/scopes-of-practice/vocational-registration/types-of-vocational-scope/
[28] Medical Council of New Zealand. Rural hospital medicine. Wellington, NZ: Medical Council of New Zealand; 2019. [cited 2020 January 5]. Available from: https://www.mcnz.org.nz/get-registered/scopes-of-practice/vocational-registration/types-of-vocational-scope/rural-hospital-medicine/
[29] Ministry of Health. PHO Services Agreement. Wellington: Ministry of Health; 2018.
[30] Luepker RV. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation 2003; 108 2543–9.
| Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute.Crossref | GoogleScholarGoogle Scholar | 14610011PubMed |
[31] Than M, Flaws D, Sanders S, et al. Development and validation of the Emergency Department Assessment of Chest pain Score and 2 h accelerated diagnostic protocol: Emergency Department Assessment of Chest pain Score. Emerg Med Australas. 2014; 26 34–44.
| Development and validation of the Emergency Department Assessment of Chest pain Score and 2 h accelerated diagnostic protocol: Emergency Department Assessment of Chest pain Score.Crossref | GoogleScholarGoogle Scholar | 24428678PubMed |
[32] Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. 2018; 72 2231–64.
| Fourth universal definition of myocardial infarction (2018).Crossref | GoogleScholarGoogle Scholar | 30153967PubMed | 30153967PubMed |
[33] Pickering JW, Young JM, George PM, et al. Validity of a novel point-of-care Troponin assay for single-test rule-out of acute myocardial infarction. JAMA Cardiol. 2018; 3 1108–12.
| Validity of a novel point-of-care Troponin assay for single-test rule-out of acute myocardial infarction.Crossref | GoogleScholarGoogle Scholar | 30347004PubMed | 30347004PubMed |
[34] Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009; 42 377–81.
| Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support.Crossref | GoogleScholarGoogle Scholar |
[35] Than M, Herbert M, Flaws D, et al. What is an acceptable risk of major adverse cardiac event in chest pain patients soon after discharge from the Emergency Department? Int J Cardiol. 2013; 166 752–4.
| What is an acceptable risk of major adverse cardiac event in chest pain patients soon after discharge from the Emergency Department?Crossref | GoogleScholarGoogle Scholar | 23084108PubMed | 23084108PubMed |
Appendix 1. International Classification of Diseases, 10th Revision (ICD-10) codes for a major adverse cardiac event (MACE)

|