How do we support walking prescriptions for type 2 diabetes management? Facilitators and barriers following a 3-month prescription
Andrew N. Reynolds 1 2 4 5 , Ian Moodie 3 , Bernard Venn 2 , Jim Mann 1 2 41 Department of Medicine, University of Otago, Dunedin, New Zealand
2 Department of Human Nutrition, University of Otago, Dunedin, New Zealand
3 Department of English Education, Mokpo National University, Muan, South Korea
4 Edgar Diabetes and Obesity Research Centre, University of Otago, Dunedin, New Zealand
5 Corresponding author. Email: andrew.reynolds@otago.ac.nz
Journal of Primary Health Care 12(2) 173-180 https://doi.org/10.1071/HC20023
Published: 12 June 2020
Journal Compilation © Royal New Zealand College of General Practitioners 2020 This is an open access article licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License
Abstract
INTRODUCTION: Prescribing physical activity is an inexpensive method to promote patients’ long-term health, but determinants of adherence with physical activity prescriptions are seldom considered.
AIM: To identify facilitators and barriers experienced by adults with type 2 diabetes when prescribed regular walking.
METHODS: Participants were prescribed a regular walking routine that met current physical activity guidelines for type 2 diabetes management for a period of 3 months. Pre- and post-intervention questions considered participants’ self-rated health and physical activity amount. Thematic analysis of recorded interviews held after the 3-month prescription identified barriers and facilitators to adherence for participants.
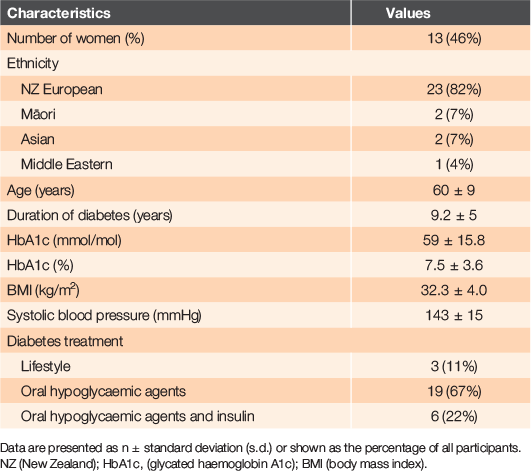
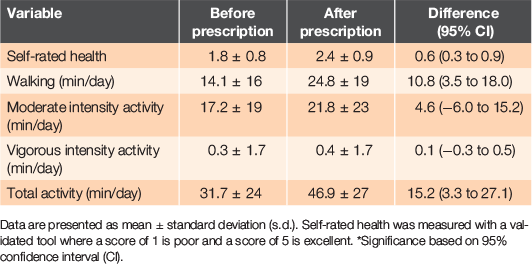
RESULTS: Twenty-eight adults (aged 60 ± 9 years, body mass index 32.3 ± 4.0 kg/m2, HbA1c 59 ± 16 mmol/mol) participated in the 3-month intervention, providing 7 years of lived experience. Self-rated health (14%; 95% confidence interval (CI) 7–22%) and time spent walking (+11 min/day; 95% CI 4–18 min/day) increased following the prescription. Major themes motivating participants were: establishing a walking routine; the support of their family members; observing health benefits; and being monitored by a health professional. The greatest barriers were associated with walking in the evening and included feelings of insecurity in the dark or a preference for sedentary behaviour.
DISCUSSION: A prescription to walk increased time spent in physical activity and self-rated health in adults with type 2 diabetes. Health-care professionals can support walking prescriptions by promoting facilitators and reducing barriers to prescription adherence. Practical solutions to barriers include identifying alternative physical activity opportunities within the house or advice to develop support networks to provide company while walking.
KEYwords: Physical activity prescriptions; motivational behaviours; health promotion; obesity
| WHAT GAP THIS FILLS |
| What is already known: Regular physical activity improves cardio-metabolic health. Walking after eating reduces postprandial glycaemia. Health-care professionals frequently encourage physical activity, although adherence is variable. |
| What this study adds: A prescription to walk each day improved self-reported time spent walking in participants with type 2 diabetes. |
| Establishing regular routines, family support, regular monitoring by a health-care professional and evidence of health benefits were important factors motivating adherence to a physical activity prescription. Walking in the evening, especially in the dark, was a major deterrent to being active late in the day. Exploring facilitators and barriers to increasing physical activity is likely to enhance adherence with physical activity prescriptions. |
Introduction
Regular physical activity is a recognised component of type 2 diabetes management.1 Although physical activity prescriptions delivered in primary care have shown promise as adjunct therapy,2,3 there is relatively little information about determinants of adherence with physical activity prescriptions for people with type 2 diabetes.4–8 Research involving adults with type 2 diabetes has considered the barriers and facilitators to participating in class- or gym-based exercise.4–9 Although structured classes offer social support, participation may be inhibited by cost and travel time to the activity. Furthermore, weekly attendance to these classes on its own is not sufficient to meet physical activity guidelines.10
Not yet investigated are the barriers and facilitators experienced by people with type 2 diabetes who have been prescribed a regular walking routine that would meet current physical activity guidelines, despite knowledge of reduced health-care utilisation and expenses of people with diabetes who are regularly physically active.11 A prescription to walk regularly may be more accessible and reduce inequities for patients as it does not incur the monetary cost of class- or gym-based activities, and it may not require travel time to undertake. Given the benefits observed for postprandial glycaemia by people with type 2 diabetes who walk after eating,12,13 we aimed to identify the barriers and facilitators to adhering with a prescription to walk after each main meal for adults with type 2 diabetes. The findings may provide important context to patients’ successes or failures when prescribed regular physical activity. Results from this study are relevant to health professionals looking to promote physical activity as part of a lifestyle approach to diabetes management.
Methods
The study was conducted between January 2014 and February 2015 in Dunedin, New Zealand. The study protocol was reviewed and approved by the Health and Disability Ethics Committee of the Ministry of Health, New Zealand (Reference H13/039). All participants provided written informed consent. Data were collected following a trial prospectively registered with the Australia New Zealand Clinical Trials Registry (ACTRN12618001285246).
Participants and recruitment
People aged 18–75 years and diagnosed with type 2 diabetes were invited to participate in this study immediately after their participation in a randomised crossover trial, which is described elsewhere.12 Participants were initially recruited through general practices, hospital outpatient clinics, the local diabetes society and services relevant to people with chronic diseases. Presence of comorbidities did not exclude participation. An endocrinologist monitored participants during the study, although this did not preclude them from attending their usual health-care provider.
The walking prescription
To meet current weekly physical activity guidelines,1,14 participants were prescribed to walk for at least 10 min after breakfast, lunch and dinner each day for 3 months. Further details on how long after the meal, or the intensity of walking, were not given. Walking was not objectively monitored. Participants were advised not to change their diet or lifestyle habits beyond complying with the walking prescription. An attempt was made to contact each participant briefly by phone or email every 2 weeks of the 3-month study to remind them of the walking prescription.
Measurements
Participant baseline anthropometry was measured in duplicate and resting blood pressure was measured three times at study commencement. Demographic information was collected and glycated haemoglobin was measured at baseline. Before and after the 3-month prescription, participants were asked about their self-rated health15 and recent physical activity.16 Following the 3-month prescription, audio-recorded interviews of open-ended questions17 enabled participants to share their experiences with the regular walking prescription. These interviews were conducted face-to-face by one researcher (A. N. Reynolds) who had worked with each participant for the preceding 6 months on this study and the previous trial.12 Because of this relationship between researcher and participant, reflexivity is assumed in the qualitative analysis. Findings should be understood as social constructions18 emerging from researcher–participant conversation and researcher engagement with the data. Example questions asked to each participant were: ‘What made it easy to follow the walking prescription?’, ‘What made it hard to follow the walking prescription?’, ‘What was it like being prescribed to walk regularly?’. Interviews lasted 20–60 min and were conducted at the University of Otago, New Zealand.
Data analysis
This study is of a convenience sample of 28 adults with type 2 diabetes from a total of 41 who had just completed participation in a randomised crossover trial.12 Differences in participants’ self-rated health and physical activity before and after the walking prescription were considered, with paired t-tests performed in Stata version 15 (StataCorp, College Station, TX, USA). Data are presented as mean values with 95% confidence intervals (CI).
The qualitative analysis adapted grounded theory methods.19 First, the audio-recorded interviews and field notes were uploaded into a qualitative data analysis program for analysis (MAXQDA12, VERBI Software, Berlin, Germany). An initial coding scheme was developed on first review of the data (A. N. Reynolds) and then refined as the analysis progressed (A. N. Reynolds and B. Venn). This first-cycle coding covered the audio recordings with 782 descriptive and in-vivo codes. In this stage, the analysis also made use of questionnaire data, field notes and analytic memos made during the prescription timeframe, as participants had been contacted each fortnight for the 3-month prescription and had often commented on their walking during these brief conversations. Next, second-cycle coding entailed reorganising, categorising and refining the code system to look across and within cases for patterns and themes. As an example, individual codes (e.g. ‘doesn’t like dark’, ‘scared of dark’, ‘won’t go out at night because it is dark’) were identified as reoccurring themes (e.g. ‘darkness as an inhibitor to walking’). Each recording of a post-intervention interview was listened to at least twice, with key excerpts accessed several more times. Analysis was regarded as complete when saturation of codes was reached, with new data not altering the analysis and code system in any meaningful way.19 To increase credibility of the results, member checking was undertaken where a researcher (A. N. Reynolds) checked the study findings against the participants’ experiences. Debriefing occurred separately with two further researchers; one was a consultant physician with clinical experience in diabetes management (J. Mann) and the other was an independent qualitative researcher who helped address researcher bias and ensured that the analysis was reflective of the data (I. Moodie). To strengthen the results, findings were applied to the Theoretical Domains Framework,20 providing an opportunity to interpret the inductive findings from this study within an established framework. The identified themes are presented as facilitators and barriers to adherence with a regular walking prescription.
Results
Twenty-eight participants agreed to participate in this study and all attended an interview after the 3-month walking prescription, providing 7 years of collective lived experience. Participant baseline characteristics are shown in Table 1. Self-reported measures obtained before and after the 3-month prescription are shown in Table 2. Participants’ self-rated health improved by a mean of 14% (95% CI: 7–22%) over the duration of the prescription, as did the daily time spent walking, by a mean increase of 11 min/day (95% CI: 4–18 min/day). Three adverse events were reported during the study; two participants fell while walking and one participant attributed a hypoglycaemic event to brisk walking.
|
|
Facilitators
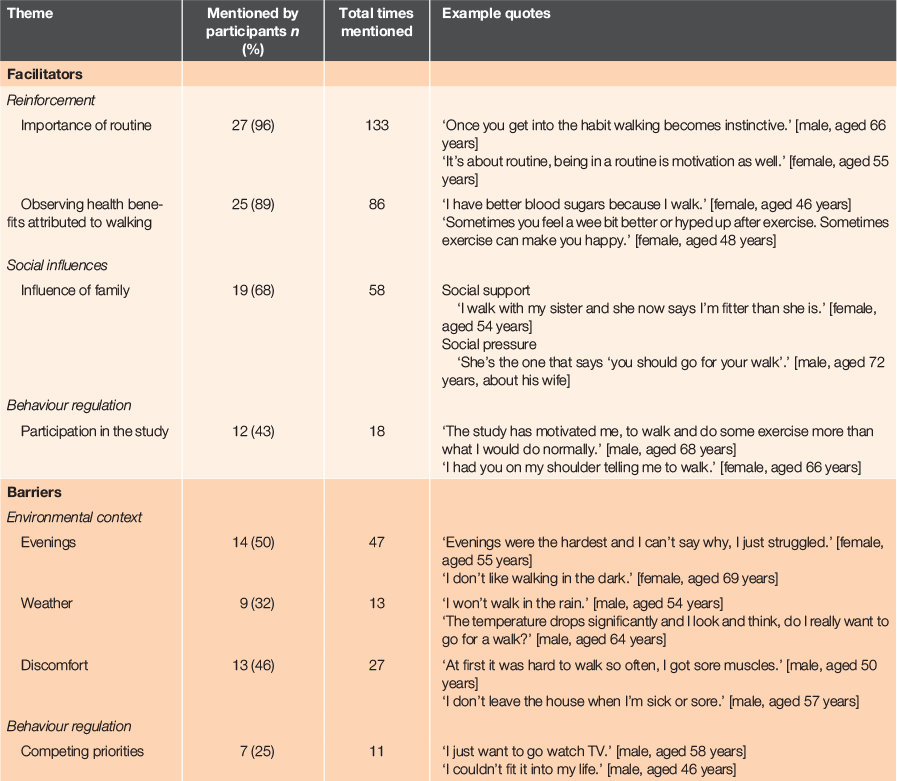
The main facilitators to emerge from the interviews are shown in Table 3. The most prominent facilitators fell under two Theoretical Domains Framework categories: reinforcement and social influences. To a lesser degree, participation in the study fell under a third category, behaviour regulation.
|
A key motivation that enabled patients to follow the prescription was reinforcement from establishing a routine, which fell under the themes of the importance of routine, mentioned by 27 (96%) participants and observing health benefits attributed to walking, mentioned by 25 (86%) participants. Improved self-esteem and feelings of wellbeing (stated 22 times) were associated with the physical act of walking as frequently as other benefits reported such as weight loss, improved digestion, improved sleep quality or improved blood glucose control (stated 25 times). Twelve (43%) participants recognised that study participation instilled a sense of obligation to follow the regular walking prescription, thus regulating their behaviour. Study participation raised awareness for four (14%) participants about dietary factors in blood glucose control and helped two (8%) others change their eating behaviours. Behavioural changes associated with participation were eating less at meals to feel more comfortable walking or skipping second helpings. Conversely, three (11%) participants experienced guilt for not walking regularly, and nine (32%) participants reported losing their routine during the 3-month prescription due to going on holiday (3), injury or illness (4), loss of a family member (1) or loss of their dog (1). These cases represent situations where reinforcement or behaviour regulation was unsuccessful or incomplete and thus underscore the importance for sustaining a walking routine.
Social influences were most prominently stated as the influence of family (19 participants (68%)), which was realised as either social support or social pressure. The support of partners was often integral to the success of both male and female participants. Socially, regular walking provided opportunities to spend quality time with family members; for example, by walking with children or grandchildren. The importance of family was accentuated by seven (25%) negative cases with participants living alone or lacking the normative and social influences provided by family.
Barriers
The dominant barriers to emerge from the interviews are also shown in Table 3. As per the Theoretical Domains Framework, the most frequent barriers related to the environmental context, most prominently with challenges for walking in the evening, as stated by 14 (50%) participants, 47 times in the dataset. When prompted further, the 10 female participants who considered evenings a barrier all stated being outside in darkness as an obstacle. People in the dark, dogs in the dark, uneven pavement in the dark and general unease were all stated. However, an aversion to walking in the dark was not stated by any male participants. Physical discomfort or pain to an extent that it affected their walking were experienced by 13 (46%) participants. Three (11%) participants became sick during the 3 months. Six (21%) experienced pain from existing injuries. Two (7%) participants had conditions that were exacerbated by regular walking. One (4%) participant attributed pain as a side-effect to his medication. One (4%) attributed sore muscles to the increase in physical activity. Conversely, two (7%) participants noted that following the regular walking prescription relieved physical discomfort.
Nine (32%) participants expressed reservations about walking in bad weather. Solutions provided by participants for both poor weather and evening walks were the use of gym equipment inside the house (3 (11%)) or household chores that require movement and lifting (3 (11%)), such as cleaning floors or manually washing dishes. Finally, adhering to the prescription was seen as a low priority for seven (25%) participants who faced challenges with behavioural regulation during the study. For them, competing interests or demands took precedence over following the walking prescription.
Discussion
Adults with type 2 diabetes identified facilitators that provided purpose, reward and incentive to follow a walking prescription. Perhaps the most relevant to clinical practice is the consistent finding in this and other studies6 that the researcher–participant relationship is a motivator for adherence to physical activity prescriptions. These findings highlight the need for health-care providers to understand their own role in regulating patient behaviour through promoting regular physical activity. Formally documenting physical activity prescriptions, enquiring about the prescription at follow up, encouraging family participation, positive reinforcement of health benefits due to regular activity and achievable goal setting with patients may all be appropriate methods to promote regular physical activity to patients.
The barriers to following a regular walking prescription may be largely modifiable for many patients. The primary barrier led to avoiding evening physical activity. Attempts to overcome this barrier might include the prescription of physical activity that can be undertaken within the home, such as the use of personal gym equipment, household chores that require multiple muscle groups or encouraging family members or friends to join the activity and provide support.6 Reducing barriers to evening activity is directly relevant to providing clinical support to patients with type 2 diabetes, as the greatest improvement in postprandial blood glucose have been observed when walking after an evening meal.12
Previous studies of factors associated with physical activity implementation in other settings4–8,21 have reported some findings similar to this study. A consistent observation has been the extent to which positive feelings and improved self-esteem can motivate and sustain physical activity routines.4,6–9 Exploring and reinforcing such feelings systematically within the health professional–patient relationship could aid the development of physical activity routines and patient ownership of their health, addressing the ongoing challenge of translating short-term interventions into permanent lifestyle changes. Importantly, the regular walking prescription produced a broader awareness of blood glucose control and the need to address other issues, notably dietary factors, as has been observed in a study of young adults.22
Many of the strengths and weaknesses of this research also apply to the trial on which this study is based.12 The current study is a novel consideration of a prescription for achievable activity undertaken in a free-living environment over 3 months. An important novelty of our work when compared with studies of gym- or class-based interventions was that participants did not express boredom with walking, or a need for greater variety in physical activity. Furthermore, regular walking does not involve the cost and inconvenience often regarded as inhibitors of gym- or class-based activities. The modality of physical activity chosen for this prescription is a further strength, given the accessibility of regular walking for most patients. Much of the current literature on blood glucose control and physical activity modality or effectiveness has focused on high-intensity interval training,23 due to its potential to mitigate an observed barrier to activity commencement, which is the time that physical activity requires.24 Far less attention has been given to the long-term achievability of high-intensity activity or its suitability for clinical populations when not under direct supervision. In the current study, lack of time did not emerge as an inhibitor to following a regular walking prescription, despite it being prescribed at a duration that meets current physical activity guidelines for adults with type 2 diabetes1,14 and at fixed times of the day. Following the prescription did not require the clinical supervision needed for high-intensity interval training.
The study limitations include the possibility that findings were influenced by the relationship between participants and researchers, and the status of health professionals as a source of credible information overcame further barriers to commencing and maintaining activity.25 Therefore many of our findings are limited to the context of prescribed physical activity. As it was not the intent of the study to quantify a change in physical activity volume (rather it was to identify facilitators and barriers to adherence), an objective marker of physical activity over the 3-month prescription was not used. Although the self-reported measure of activity indicated participants did increase their time spent walking, an objective measure would have provided further insight on the use of prescriptions for regular walking. Finally, the generalisability of these findings to other settings or other lifestyle prescriptions is unknown. Although clear themes emerged from the analysis, participants were a convenience sample identified following their participation in a research trial.12 Future research should measure the effect on adherence with physical activity prescriptions that may be achieved by enhancing facilitators and reducing the barriers observed in this research.
Although physical activity is an established component of diabetes management1 and health-care professionals already encourage their patients to be physically active, adherence is variable. Our findings indicate that this variability may result from patients experiencing a range of barriers and facilitators. We have shown that a walking prescription can increase self-reported time spent walking. Given the extent to which even modest postprandial physical activity can improve glycaemic control,12,13 daily physical activity should be a regular topic in consultations with patients with diabetes. Our findings are relevant to all health-care professionals in health-care teams, providing support to increase patient success when following a physical activity prescription.
Competing interests
The authors declare no competing interests.
Funding
This research was funded by the University of Otago and the New Zealand Artificial Limb Service. Andrew Reynolds is supported by the National Heart Foundation of New Zealand. Jim Mann is supported by the Healthier Lives National Science Challenge. These entities were not involved with the study design or use of data produced.
References
[1] American Diabetes Association Lifestyle management: standards of medical care in diabetes. Diabetes Care. 2019; 42 S46–60.| Lifestyle management: standards of medical care in diabetes.Crossref | GoogleScholarGoogle Scholar | 30559231PubMed |
[2] Swinburn BA, Walter LG, Arroll B, et al. The green prescription study: a randomized controlled trial of written exercise advice provided by general practitioners. Am J Public Health. 1998; 88 288–91.
| The green prescription study: a randomized controlled trial of written exercise advice provided by general practitioners.Crossref | GoogleScholarGoogle Scholar | 9491025PubMed |
[3] Orrow G, Kinmonth A-L, Sanderson S, Sutton S. Effectiveness of physical activity promotion based in primary care: systematic review and meta-analysis of randomised controlled trials. BMJ. 2012; 344 e1389
| Effectiveness of physical activity promotion based in primary care: systematic review and meta-analysis of randomised controlled trials.Crossref | GoogleScholarGoogle Scholar | 22451477PubMed |
[4] Wycherley TP, Mohr P, Noakes M, et al. Self‐reported facilitators of, and impediments to maintenance of healthy lifestyle behaviours following a supervised research‐based lifestyle intervention programme in patients with type 2 diabetes. Diabet Med. 2012; 29 632–9.
| Self‐reported facilitators of, and impediments to maintenance of healthy lifestyle behaviours following a supervised research‐based lifestyle intervention programme in patients with type 2 diabetes.Crossref | GoogleScholarGoogle Scholar | 21916973PubMed |
[5] Wanko NS, Brazier CW, Young-Rogers D, et al. Exercise preferences and barriers in urban African Americans with type 2 diabetes. Diabetes Educ. 2004; 30 502–13.
| Exercise preferences and barriers in urban African Americans with type 2 diabetes.Crossref | GoogleScholarGoogle Scholar | 15208848PubMed |
[6] Penn L, Dombrowski SU, Sniehotta FF, White M. Participants’ perspectives on making and maintaining behavioural changes in a lifestyle intervention for type 2 diabetes prevention: a qualitative study using the theory domain framework. BMJ Open. 2013; 3 e002949
| Participants’ perspectives on making and maintaining behavioural changes in a lifestyle intervention for type 2 diabetes prevention: a qualitative study using the theory domain framework.Crossref | GoogleScholarGoogle Scholar | 24227871PubMed |
[7] Helmink JHM, Kremers SPJ, Van Boekel LC, et al. The BeweegKuur programme: a qualitative study of promoting and impeding factors for successful implementation of a primary health care lifestyle intervention for overweight and obese people. Fam Pract. 2012; 29 i68–i74.
| The BeweegKuur programme: a qualitative study of promoting and impeding factors for successful implementation of a primary health care lifestyle intervention for overweight and obese people.Crossref | GoogleScholarGoogle Scholar |
[8] Casey D, De Civita M, Dasgupta K. Understanding physical activity facilitators and barriers during and following a supervised exercise programme in Type 2 diabetes: a qualitative study. Diabet Med. 2010; 27 79–84.
| Understanding physical activity facilitators and barriers during and following a supervised exercise programme in Type 2 diabetes: a qualitative study.Crossref | GoogleScholarGoogle Scholar | 20121893PubMed |
[9] Advika TS, Idiculla J, Kumari SJ. Exercise in patients with Type 2 diabetes: facilitators and barriers – a qualitative study. J Family Med Prim Care. 2017; 6 288
| Exercise in patients with Type 2 diabetes: facilitators and barriers – a qualitative study.Crossref | GoogleScholarGoogle Scholar | 29302534PubMed |
[10] Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016; 39 2065–79.
| Physical activity/exercise and diabetes: a position statement of the American Diabetes Association.Crossref | GoogleScholarGoogle Scholar | 27926890PubMed |
[11] Wu J, Davis-Ajami ML, Lu ZK. Real-world impact of ongoing regular exercise in overweight and obese US adults with diabetes on health care utilization and expenses. Prim Care Diabetes. 2019; 13 430–40.
| Real-world impact of ongoing regular exercise in overweight and obese US adults with diabetes on health care utilization and expenses.Crossref | GoogleScholarGoogle Scholar | 30808561PubMed |
[12] Reynolds AN, Mann JI, Williams S, Venn BJ. Advice to walk after meals is more effective for lowering postprandial glycaemia in type 2 diabetes mellitus than advice that does not specify timing: a randomised crossover study. Diabetologia. 2016; 59 2572–8.
| Advice to walk after meals is more effective for lowering postprandial glycaemia in type 2 diabetes mellitus than advice that does not specify timing: a randomised crossover study.Crossref | GoogleScholarGoogle Scholar | 27747394PubMed |
[13] Aqeel M, Forster A, Richards EA, et al. The effect of timing of exercise and eating on postprandial response in adults: a systematic review. Nutrients. 2020; 12 221
| The effect of timing of exercise and eating on postprandial response in adults: a systematic review.Crossref | GoogleScholarGoogle Scholar |
[14] Mendes R, Sousa N, Almeida A, et al. Exercise prescription for patients with type 2 diabetes: a synthesis of international recommendations: narrative review. Br J Sports Med. 2016; 50 1379–81.
| Exercise prescription for patients with type 2 diabetes: a synthesis of international recommendations: narrative review.Crossref | GoogleScholarGoogle Scholar | 26719499PubMed |
[15] Lorig K, Stewart A, Ritter P, et al. Outcome Measures for Health Education and Other Health Care Interventions. Thousand Oaks: Sage Publications; 1996.
[16] McLean G, Tobias M. The New Zealand physical activity questionnaires: report on the validation and use of the NZPAQ-LF and NZPAQ-SF self-report physical activity survey instruments. Wellington: SPARC; 2004.
[17] Cohen D, Crabtree B. Qualitative Research Guidelines Project. Princeton: Robert Wood Johnson Foundation; 2006.
[18] Lincoln YS, Guba EG. Naturalistic Inquiry. Thousand Oaks: Sage Publications; 1985.
[19] Corbin J, Strauss A. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. Thousand Oaks: Sage Publications; 2014.
[20] Atkins L, Francis J, Islam R, et al. A guide to using the Theoretical Domains Framework of behaviour change to investigate implementation problems. Implement Sci. 2017; 12 77
| A guide to using the Theoretical Domains Framework of behaviour change to investigate implementation problems.Crossref | GoogleScholarGoogle Scholar | 28637486PubMed |
[21] Ahlin K, Billhult A. Lifestyle changes - a continuous, inner struggle for women with type 2 diabetes: a qualitative study. Scand J Prim Health Care. 2012; 30 41–7.
| Lifestyle changes - a continuous, inner struggle for women with type 2 diabetes: a qualitative study.Crossref | GoogleScholarGoogle Scholar | 22324486PubMed |
[22] Joo J, Williamson SA, Vazquez AI, et al. The influence of 15-week exercise training on dietary patterns among young adults. Int J Obes. 2019; 43 1681–1690.
| The influence of 15-week exercise training on dietary patterns among young adults.Crossref | GoogleScholarGoogle Scholar |
[23] Jelleyman C, Yates T, O’Donovan G, et al. The effects of high‐intensity interval training on glucose regulation and insulin resistance: a meta‐analysis. Obes Rev. 2015; 16 942–61.
| The effects of high‐intensity interval training on glucose regulation and insulin resistance: a meta‐analysis.Crossref | GoogleScholarGoogle Scholar | 26481101PubMed |
[24] Gillen JB, Gibala MJ. Is high-intensity interval training a time-efficient exercise strategy to improve health and fitness? Appl Physiol Nutr Metab. 2014; 39 409–12.
| Is high-intensity interval training a time-efficient exercise strategy to improve health and fitness?Crossref | GoogleScholarGoogle Scholar | 24552392PubMed |
[25] Nutting PA. Health promotion in primary medical care: problems and potential. Prev Med. 1986; 15 537–48.
| Health promotion in primary medical care: problems and potential.Crossref | GoogleScholarGoogle Scholar | 3774783PubMed |


