Therapeutic errors captured by the New Zealand National Poisons Centre: a retrospective audit
Nicholas Y. Yang 1 , Adam C. Pomerleau 2 , Lucy M. Shieffelbien 2 , Desirée L. Kunac 3 , Rhiannon Braund 31 Student, University of Otago, New Zealand
2 National Poisons Centre, University of Otago, Dunedin, New Zealand
3 New Zealand Pharmacovigilance Centre, University of Otago, Dunedin, New Zealand
4 Corresponding author. Email: rhiannon.braund@otago.ac.nz
Journal of Primary Health Care 13(1) 63-69 https://doi.org/10.1071/HC20066
Published: 23 March 2021
Journal Compilation © Royal New Zealand College of General Practitioners 2021 This is an open access article licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License
Abstract
INTRODUCTION: Medication errors are one important cause of harm to patients. Information about medication errors can be obtained from diverse sources, including databases administered by poisons centres as part of their routine operation.
AIM: The aim of this study was to describe the data regarding therapeutic errors captured by the New Zealand National Poisons Centre (NZNPC).
METHODS: A retrospective study of calls made to the NZNPC between 1 September 2016 and 31 August 2018 was conducted, which involved human patients and were classified as ‘therapeutic error’ in the NZNPC database. Variables extracted and analysed included the demographics of the individual, the substance(s) involved, and site of exposure.
RESULTS: During the study period, a total of 43,578 calls were received by the NZNPC, including 5708 (13%) that were classified as ‘therapeutic error’. Just over half of the exposures occurred in females, 3197 (56%) and 4826 (85%) of the calls involved a single substance. All age groups were affected and 2074 (37%) of the calls were related to children aged <12 years. A residential environment (n = 5568, 97%) was the site of exposure for almost all reported therapeutic errors, most commonly in the patient’s own home (n = 5207, 91%).
DISCUSSION: This study provides insights into therapeutic error-related calls to the NZNPC. Almost all errors occurred in the residential setting. Over one-third of the calls involved children. Enhanced data capture and classification methods are needed to determine the types of errors and their possible causes to better inform prevention efforts.
KEYwords: Medication error; Poisons Centre; therapeutic error
| WHAT GAP THIS FILLS |
| What is already known: There is limited knowledge about therapeutic errors that occur in the general community environment. |
| What this study adds: This study shows that an existing database at the NZNPC routinely collects information about these errors, which have the potential to provide new insights into this area. |
Introduction
Medication errors can be defined as ‘a failure in the treatment process that leads to, or has the potential to lead to, harm to the patient’.1 Medication errors can occur at various stages: the prescribing stage (eg the prescription of an incorrect medication or dose by a doctor or nurse), dispensing stage (eg the dispensing of an incorrect medication, an incorrect dose, or an incorrect formulation by a pharmacist) or administration stage (eg the patient receiving an incorrect dose or receiving the medication by an incorrect method).1
Much of what is known about medication errors in New Zealand comes from the hospital setting,2 with far less known about such errors in the community. A national primary care clinician-based Medication Error Reporting Programme (MERP) provides some information about that care setting. Most errors reported to MERP involve dispensing errors or prescribing errors, whereas administration errors are reported less frequently.3 Internationally, other research has attempted to augment knowledge about the epidemiology of medication errors occurring in the community by using datasets from poisons centres.4,5
The NZNPC (the Poisons Centre, https://poisons.co.nz/) provides a freely accessible 24/7 specialist service for assessment and advice regarding human poisonings that is available to all New Zealanders. Approximately 25,000 calls are handled by the NZNPC annually and callers are both members of the public and health-care professionals. Public callers are provided with a risk assessment for any reported exposure and then given advice on first aid measures and whether further medical care should be sought. Health-care callers are similarly provided risk assessment and specialist treatment advice for patients in their care. The NZNPC handles enquiries of many different types, including some regarding medication errors.
The calls received by the NZNPC are grouped into broad categories reflecting the reasons underlying an exposure, as well as the type and setting. Reason categories used to classify exposures include: unintentional, intentional, child exploratory, abuse, other, unknown and ‘therapeutic error’. Therapeutic errors are defined as ‘an unintentional deviation from a proper therapeutic regimen that results in the wrong dose, incorrect route of administration, administration to the wrong person or administration of the wrong substance’.6 Coding by NZNPC staff broadly follows this definition when classifying reasons for exposures, with a notable extension that any non-pharmaceutical substance being used with therapeutic intent is also included. For example, someone intending to treat their daughter with an herbal, traditional, or homeopathic remedy, but mistakenly giving it to their son instead would be classified as a therapeutic error in the NZNPC database.
The purpose of this study was to describe the therapeutic errors captured by the NZNPC to determine whether this information could be used to identify areas for error reduction and to assess the utility of this dataset for further investigation.
Methods
This was a retrospective chart review study of calls made to the NZNPC. Call records meeting inclusion criteria were extracted from the NZNPC electronic exposure database and de-identified before analysis. Inclusion criteria were: the call occurred between 1 September 2016 and 31 August 2018, involved a human patient (as the NZNPC also receives calls about animals) and was coded with the call type ‘therapeutic error’.
Data included: relationship of the caller to the patient; incident date and time; substance name, type and ingredient; incident details; site of exposure; treatment classification; symptoms; assessment and advice; and demographic information about patient gender, age and ethnicity. These variables were extracted into an electronic spreadsheet, where data could be aggregated, manipulated, and filtered for analysis. Most variables included in the extraction were coded fields following standard NZNPC practices, but some were recorded as free text; these were incident details, symptoms, and assessment and advice. Treatment classification is a coded variable routinely used by the NZNPC that encapsulates the treatment advice that was given during the call and indicates the recommended management site for the patient. The available options for treatment classification were:
medical referral (active investigation or treatment) – assigned when patients are advised to seek medical attention and specific investigations or treatments were indicated based on the risk assessment at the time of the call.
medical referral (assessment and observation) – assigned when patients are advised to seek medical attention for assessment and observation, but specific investigations or treatments are not necessarily indicated based on the risk assessment at the time of the call.
medical referral (psychiatric assessment) – assigned when patients are advised to seek medical attention out of concern for the intent (e.g. suicidal intent) behind the exposure, but the substances of exposure do not pose substantial risk of harm.
medical referral (unrelated) – assigned when patients are advised to seek medical attention out of concern for reported symptoms and the reported exposure is not feasibly the cause of the symptoms.
referral to other service – assigned when callers are directed to another service (eg a manufacturer, governmental organisation, etc) to answer their inquiry.
no treatment required – assigned when the NZNPC risk assessment is that the patient’s reported exposure does not require referral for medical assessment or any other specific treatment.
self-treatment – assigned when the NZNPC risk assessment is that the patient’s reported exposure does not require referral for medical assessment, but first aid or other specific measures are recommended as part of management at the patient’s current location.
further information required – assigned when there is insufficient information from the history of the exposure to make a definite recommendation at the time of the call.
In this study, the NZNPC treatment classifications were categorised into three groups as follows: (1) Medical referral, which comprised the classifications: medical referral (active investigation and treatment), medical referral (assessment and observation), medical referral (psychiatric assessment) and medical referral (unrelated); (2) No medical referral, which comprised no treatment required and self-treatment; and (3) Other, which comprised referral to other service and further information required.
Patients were grouped by age based on international categorisation frameworks as neonatal (age <1 month), infant (ages 1–23 months, inclusive), child (ages 2–11 years, inclusive), adolescent (ages 12–17 years, inclusive), adult (ages 18–64 years, inclusive), older person (ages 65–79 years inclusive) and elderly (ages ≥80 years).3 As the NZNPC records age data in years and not months, the neonatal and infant age bands were treated as a single group for the analysis. Patient ethnicity was determined using the ethnicity entered first for the recorded call (as multiple ethnicities may be listed).
The list of substances involved in included call records contained product, generic, and trade names, and these were converted (where appropriate) into generic names. Medications were classified according to the World Health Organization’s Anatomical Therapeutic Classification (ATC) for medications, to facilitate analysis.
The study protocol was approved by the ethics committee at the University of Otago (reference number HD18/090) and Māori consultation was provided by the Ngāi Tahu Research Consultation Committee.
Results
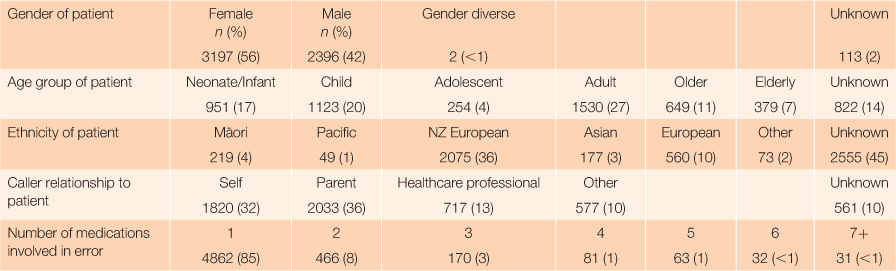
From 1 September 2016 to 31 August 2018, a total of 43,578 calls were received by the NZNPC, of which 5708 (13%) were classified as ‘therapeutic error’. Table 1 summarises the descriptive features of therapeutic error calls during the study period.
|
Of all therapeutic error calls, 56% of exposures occurred in females and 85% of the calls involved a single substance or supplement. All age groups were affected and 37% of the calls were related to children aged <12 years. For most calls, the caller’s relationship to the patient who experienced the therapeutic error was either a parent (36%) or the patient themselves (32%). Health-care professionals accounted for 13% of calls. The caller relationship was unknown or missing for 10% of calls. A total of 7398 substances were involved in the 5708 therapeutic error calls in the study. The number of substances involved in the event was a single agent in 85% of the calls. Multiple substances were involved in a lower number of calls; this occurred often when one patient was given another patient’s medication pack.
Analgesics were the class of medications most commonly involved in errors. There were 2750 analgesic medications involved in errors, representing 37% (2750/7398) of all substances involved in reported medication errors. These included 1503 paracetamol-based (both paracetamol and paracetamol combinations), 683 opioid-based, and 565 non-steroidal anti-inflammatory-based medications. Anti-hypertensive medications were involved in 958 errors (13%), antidepressants in 398 (5%), cough and cold products in 211 (3%), and vitamins or supplements in 130 (2%).
A residential environment (n = 5568, 97%) was the site of exposure for almost all reported therapeutic errors; most commonly this was the patient’s own home (n = 5207, 91%). Other residential environments included residential care (n = 239, 4%) and homes other than the patient’s home (n = 122, 2%). Other sites of exposure that contributed <1% of all medication errors each were preschools, primary schools, secondary schools, public areas, medical centres, workplaces, hospitals, outdoor environments, other environments, and unknown.
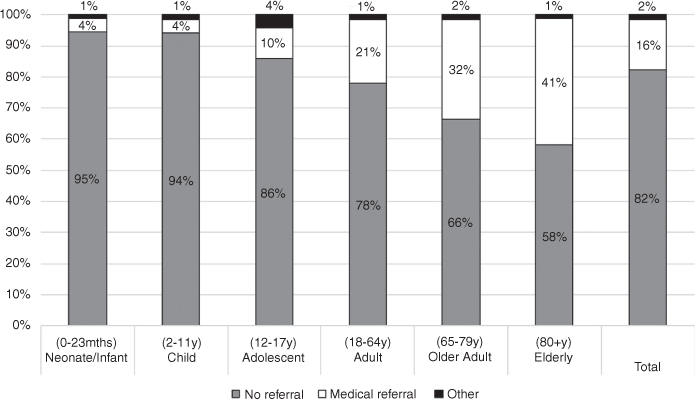
Of the total 5708 calls, only 4886 (86%) had full information for both age and treatment classification. Figure 1 shows the referrals made relative to the age bands.
Discussion
This study found that nearly all therapeutic errors captured by the NZNPC occurred in a residential environment. This is in contrast to most other studies of medication errors, which describe errors occurring in medical settings. This study therefore describes a group of therapeutic errors (those happening in people’s homes every day) that little is known about. The primary role of the NZNPC is to provide assessment and advice for potentially harmful exposures in a timely manner, but the data routinely collected by the NZNPC has additional value because it can provide information about therapeutic errors that occur commonly and are not often captured by other datasets or reporting systems.
These errors are common, as this study found 13% of the all exposure calls to the NZNPC during the study period were related to therapeutic errors. It is important to recognise that this is only the tip of the iceberg, as errors reported to the NZNPC are self-reported by callers and likely to describe only a subset of errors occurring in the community. Although these data are not representative of all therapeutic errors occurring in the community, they are useful for describing errors that have actually occurred and this database is a source of data that is readily available for monitoring.
Our results show more than one-third of all therapeutic errors occurred in children aged <12 years, suggesting that a high number of children are exposed to therapeutic errors in their home environment. Research from the National Poison Centre of Ireland showed children aged <18 years were involved in 52% of therapeutic errors reported to that poison centre, and the most commonly reported therapeutic errors were administration errors such as double dosing, use of an incorrect medication other than intended, and the administration of an incorrect dose of medication.4 Similarly, results from a study of therapeutic error occurring outside health-care facilities and reported to the Israel National Poison Information Centre found 59% of errors occurring among children aged <6 years and the same types of errors (administration errors involving incorrect dose, incorrect medication, and extra doses).5 We hypothesise that similar findings for types of errors would be likely on further examination of the NZNPC data.
This study found a variety of medication classes involved in errors, with analgesics, antihypertensives, antidepressants, and cold and cough products being most common. In a 13-year study of US poison centres, analgesics were involved in the highest proportion of medication error calls at 18%.7 The NZNPC data found a much higher proportion of medication error calls related to analgesics at 37%. Although many of these calls are likely related to low-risk scenarios (eg a double dose of paracetamol), any medication error is a preventable event and significant harms do occur when paracetamol is administered incorrectly.8 In addition, antihypertensive medications have potential for harm because of their mechanism of action, a small therapeutic index for many specific drugs and the prevalence of use particularly in older adults.
Our data showed a 32% rate of medical referral for adults aged 65–79 years and 41% for adults aged ≥80 years, whereas only 4% of children aged <12 years were referred for medical assessment. This is not surprising as older adults comprise a key subpopulation for medication errors, as polypharmacy is a well-known issue for older patients, and the medications involved often have increased potential for harm (eg cardiovascular, psychotropic, etc) and narrow therapeutic windows.9
Any retrospective poison centre data have some inherent limitations, which include self-selection bias as all calls are made voluntarily by the callers, incomplete or missing information, and inability to objectively confirm what has occurred in the reported situation.10 As the primary role of the NZNPC is to provide real-time advice and recommendations to individuals who have had a ‘poisoning’ event or potentially harmful exposure to a substance, in some instances, there was unknown information including the age, ethnicity and the caller relationship. A further limitation is that there is no mechanism to follow patients to an outcome so we do not know what ultimately happened and whether harms occurred. For many cases, the call to the NZNPC happens soon after the exposure (when the person realises the error) and before significant drug absorption has occurred. The risk assessment conducted by the NZNPC is reflected in the treatment classification, which is a proxy for the recommended level of care (stay at home or referral for medical assessment) indicated by the risk of the scenario.
This study has provided some useful insights, but further work is needed to determine the circumstances that cause therapeutic errors to occur and to further refine and develop ways to reduce therapeutic error. Its ongoing collection and availability means that NZNPC data could become a component of a sentinel or signal monitoring system for therapeutic errors, or be regularly examined to provide a more complete picture of the epidemiology of therapeutic errors.
Paediatric patients were commonly involved in NZNPC data; this is a population group where limited data exist about therapeutic errors and further study would be useful. In particular, it would be helpful to know more about the types of errors occurring in this population. An analysis of the types of errors reported to the NZNPC was outside the scope of the current study, but is planned for future work. The high prevalence of errors involving analgesics in this study suggests that further research specifically regarding analgesics will also be an important area for future work, considering the high number of children affected by double dosing and incorrect dosing, which can have important implications for their health. NZNPC data could also provide further insights into therapeutic errors occurring in the home environment of older adults where there is likely to be greater risk of significant harms.
This study has highlighted an important, but often unseen source of therapeutic errors; errors that occur within the home. Although much research has explored medication errors in the prescribing and dispensing aspects of medication supply, the administration phase is more challenging to monitor. Primary health-care professionals need to continue to remind patients of the appropriate use and storage of all medication and therapeutic products. Patient and caregiver health literacy also needs to be considered when explaining dosing schedules, particularly for paediatric patients.
Conclusion
A notable proportion of all calls received by the NZNPC are in relation to therapeutic errors, most of which occur in the patient’s own home. Much attention has been given to medication errors occurring in medical settings, but we found that a high number of errors occur in the home. The NZNPC is a valuable source for identifying these errors in NZ. Additional resources to enhance data capture and classification methods are needed to determine the types of errors and their possible causes to better inform prevention efforts.
Competing interests
The authors declare no competing interests.
Funding
Financial assistance for this study was provided by the Dunedin School of Medicine Summer Student Scholarship.
References
[1] Aronson JK. Medication errors: what they are, how they happen, and how to avoid them. QJM. 2009; 102 513–21.| Medication errors: what they are, how they happen, and how to avoid them.Crossref | GoogleScholarGoogle Scholar | 19458202PubMed |
[2] Robb G, Loe E, Maharaj A, et al. Medication-related patient harm in New Zealand hospitals. N Z Med J. 2017; 130 21–32.
| 28796769PubMed |
[3] Kunac DL, Tatley MV, Seddon ME. A new web-based Medication Error Reporting Programme (MERP) to supplement pharmacovigilance in New Zealand: findings from a pilot study in primary care. N Z Med J. 2014; 127 69–81.
| 25225758PubMed |
[4] Cassidy N, Duggan E, Williams DJP, Tracey JA. The epidemiology and type of medication errors reported to the National Poisons Information Centre of Ireland. Clin Toxicol (Phila). 2011; 49 485–91.
| The epidemiology and type of medication errors reported to the National Poisons Information Centre of Ireland.Crossref | GoogleScholarGoogle Scholar | 21824059PubMed |
[5] Lavon O, Ben-Zeev A, Bentur Y. Medication errors outside healthcare facilities: a national poison centre perspective. Basic Clin Pharmacol Toxicol. 2014; 114 288–92.
| Medication errors outside healthcare facilities: a national poison centre perspective.Crossref | GoogleScholarGoogle Scholar | 24330094PubMed |
[6] Mowry JB, Spyker DA, Cantilena LR, et al. 2012 Annual report of the American Association of Poison Control Centres Poison Data System. Clin Toxicol (Phila). 2013; 51 949–1229.
| 2012 Annual report of the American Association of Poison Control Centres Poison Data System.Crossref | GoogleScholarGoogle Scholar | 24359283PubMed |
[7] Brophy TJ, Spiller HA, Casavant MJ, et al. Medication errors reported to U.S. Poison Control Centers, 2000–2012. Clin Toxicol (Phila). 2014; 52 880–8.
| Medication errors reported to U.S. Poison Control Centers, 2000–2012.Crossref | GoogleScholarGoogle Scholar | 25175900PubMed |
[8] Medsafe. Paracetamol dangerous when not used correctly. Wellington: Ministry of Health; 2019. [cited 2020 January 20]. Available from: https://www.medsafe.govt.nz/profs/PUArticles/September2019/Paracetamol-dangerous-when-not-used-correctly.htm
[9] Healthcare Quality and Safety Commission New Zealand. Atlas of Healthcare – Polypharmacy. Wellington: Health Quality and Safety Commission; 2019. [cited 2019 December 23]. Available from: https://www.hqsc.govt.nz/our-programmes/health-quality-evaluation/projects/atlas-of-healthcare-variation/polypharmacy/
[10] Hoffman RS. Understanding the limitations of retrospective analyses of poison center data. Clin Toxicol (Phila). 2007; 45 943–5.
| Understanding the limitations of retrospective analyses of poison center data.Crossref | GoogleScholarGoogle Scholar | 18163236PubMed |