Cefalexin prescribing appropriateness in general practice: an evaluation study
Ibrahim S. Al-Busaidi 1 * , Sarmad Qamar
1 * , Sarmad Qamar  1 , Yao-Min Lin
1 , Yao-Min Lin  1 , Dee Mangin 1 , Ben Hudson
1 , Dee Mangin 1 , Ben Hudson  1
1
1
Abstract
Antibiotic misuse and overuse, among other factors, are the main drivers of increased antimicrobial resistance. Although cefalexin is generally recommended as a second-line agent, recent trends in Aotearoa New Zealand (NZ) indicate increased community use, highlighting the need for closer scrutiny.
This preliminary study aimed to assess the appropriateness and guideline compliance of cefalexin prescribing.
We conducted a cross-sectional study reviewing all cefalexin prescriptions issued at a single urban medical centre in Ōtautahi Christchurch, NZ, during July–August 2023. Retrieved prescriptions were assessed for guideline compliance and clinical appropriateness using a modified audit survey based on national and regional guidelines.
We identified 27 cefalexin prescriptions provided to 25 patients (16 female, 20 NZ European; median age 48.9 years, IQR 49.7). Soft tissue (n = 11, 42.3%) and genito-urinary infections (n = 10, 38.5%) were the most common indications. Of the assessable prescriptions (n = 26), 14 (53.8%) were guideline compliant, and 15 (57.7%) were clinically appropriate. Indications were documented in 22 cases (84.6%) – 6 on the prescription and 19 in the clinical record.
This exploratory study identifies areas for targeted antimicrobial stewardship interventions in general practice to promote improved prescribing practices. A larger multicentre study is planned to further investigate prescribing patterns and appropriateness.
Keywords: antibiotic stewardship, antimicrobial resistance, cefalexin, community prescribing, guideline compliance, general practitioners, primary care, New Zealand.
| WHAT GAP THIS FILLS |
| What is already known: Cefalexin is a broad-spectrum, first-generation cephalosporin that is typically reserved as a second-line or alternative agent for common soft tissue, respiratory, and urinary tract infections, with dosing 2–4 times daily for a duration generally ranging from 3 to 10 days, depending on the indication. Over the past decade, cefalexin dispensing has increased substantially in community and general practice settings in Aotearoa New Zealand (NZ). Although the overuse and misuse of any antibiotic can contribute to antimicrobial resistance, inappropriate use of broad-spectrum agents is particularly associated with the emergence of drug-resistant pathogens. However, data evaluating the indications for, and clinical appropriateness of, cefalexin prescribing remain limited. |
| What this study adds: This study presents the first focused evaluation of cefalexin prescribing appropriateness and guideline compliance in general practice in NZ. It highlights specific areas for targeted antimicrobial stewardship interventions and lays the foundation for future research and broader stewardship initiatives. |
Introduction
Cefalexin is a broad-spectrum first-generation cephalosporin with activity against many Gram-positive and some Gram-negative organisms. It is used to treat soft tissue, respiratory, and urinary tract infections, and as a step-down therapy for serious infections requiring hospitalisation.1 To slow the emergence of resistance, broad-spectrum antibiotics are usually reserved for use when a narrower-spectrum agent cannot be used, for example, due to drug allergy, previous treatment failure, microbiology-directed therapy, diagnostic uncertainty, resistance patterns, patient comorbidities, or the need for broader empirical coverage in more severe or polymicrobial infections. In the case of cefalexin, current antimicrobial guidelines reflect this approach, typically recommending it as a second-line or alternative agent with a treatment duration of 3–10 days, depending on the indication (canterbury.communityhealthpathways.org).2 Despite this, cefalexin use has demonstrated an upward trend in Aotearoa New Zealand (NZ), surpassing doxycycline to become the fourth most frequently prescribed oral antibiotic by 2023.3,4 This growth in use is potentially of concern, as it increases the likelihood of the emergence of antimicrobial resistance (AMR) to cefalexin, which may, in the future, make it a less valuable antimicrobial agent.5
The rise in AMR is mainly driven by the overuse and misuse of antibiotics, particularly broad-spectrum agents, globally.5,6 Other contributing factors include poor infection control, lack of clean water and sanitation, limited access to treatment, and low public awareness.5,6 Optimal antibiotic use is vital for strengthening antimicrobial stewardship (AMS), improving patient outcomes, and reducing healthcare costs.7 NZ has high levels of community antibiotic use compared to other Organisation for Economic Co-operation and Development (OECD) countries, with community use comprising up to 95% of total antibiotic consumption.4,5,7 A recent 10-year analysis of community antibiotic consumption trends in the Waitaha Canterbury region of NZ demonstrated an overall decline in antibiotic consumption. However, the study found a 10-fold rise in cefalexin dispensing, at odds with the overall trend.4 The reasons for, and appropriateness of, cefalexin prescribing remain to be investigated. Although comprehensive data exist on the volume of community antibiotic dispensing in NZ,4 there is no information regarding the appropriateness and guideline adherence for cefalexin.8
This exploratory study aims to assess the appropriateness of community cefalexin prescribing. The primary objective is to evaluate compliance with clinical guidelines and determine the clinical appropriateness of prescribing practices. Secondary objectives are to assess the documentation of indications and to identify the clinical indications for cefalexin use.
Methods
We conducted a cross-sectional study of cefalexin prescribing practices and appropriateness at a single urban general medical centre located in Ōtautahi Christchurch, NZ. The practice operates as part of a larger primary health organisation and provides general medical services to approximately 4500 enrolled patients. This setting was selected to reflect prescribing behaviour in routine community general practice.
Cefalexin prescriptions during the 2 months of July and August 2023 were identified using Medtech Evolution prescribing software query builder and assessed using a newly developed survey to evaluate the appropriateness and guideline compliance of cefalexin prescribing in the community. No exclusion criteria were applied; all patient demographics and prescribing encounters involving cefalexin were included for review, regardless of patient age, sex, or clinical indication.
The Cefalexin Prescribing Survey was adapted from the Canterbury District Health Board Hospital Prescribing Survey,9 which itself was based on the multidisciplinary approach recommended by the Australian National Antimicrobial Prescribing Survey (NAPS).10 The survey design was modified to evaluate the appropriateness of prescribing a single oral antibiotic (cephalexin) in a community setting. Variables such as route of administration, specialty, and ward – relevant only in hospital contexts – were excluded, as cefalexin is administered exclusively via the oral route.1 The survey collected data on patient demographics, clinical measurements (estimated glomerular filtration rate (eGFR), height, and weight), prescription characteristics (date, dose, frequency, duration, indication, and documentation), allergy status, microbiology results, empiric antibiotics used, and other relevant history. In cases of incomplete documentation, information was inferred by auditors based on available clinical records (Supplementary Material S1).
Each prescription was assessed by two auditors (ISA and BH) for compliance with national and regional clinical guidelines/guidance, including the bpacnz Primary Care Antibiotic Guide,2 the Waitaha | Canterbury Community HealthPathways (canterbury.communityhealthpathways.org), and additional supplementary criteria developed by the research team. Access to the Waitaha | Canterbury Community HealthPathways is restricted and generally limited to medical practitioners within the Waitaha Canterbury region (canterbury.communityhealthpathways.org).
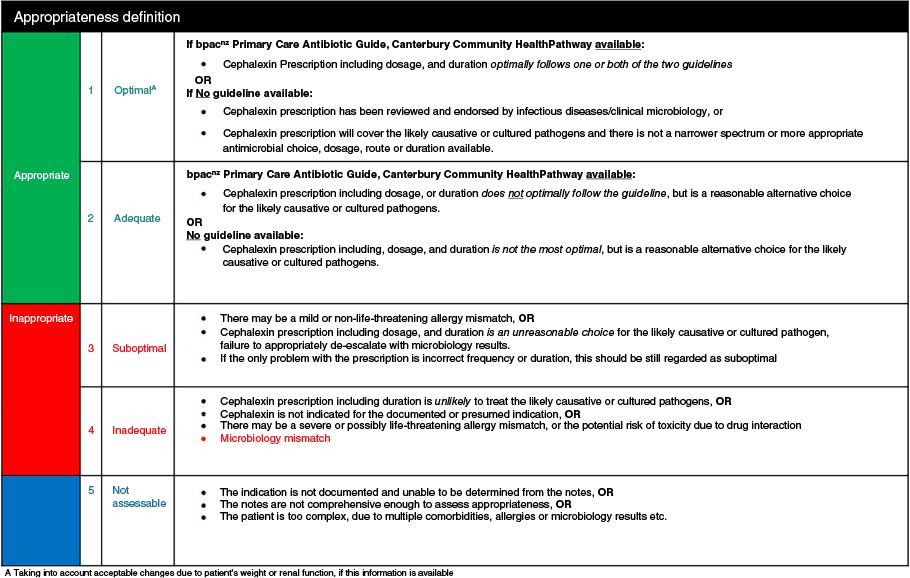
Prescriptions were evaluated for guideline/guidance compliance and were categorised as compliant with either of the two guidelines, non-compliant, directed therapy (the antibiotic was changed from empiric according to culture susceptibilities), recommended by infectious diseases/clinical microbiology, no guideline available for the documented indication, or not assessable (eg medical records are not sufficiently comprehensive to determine the indication or it is difficult to assess if there is compliance). Cefalexin prescriptions that were categorised as directed therapy were determined to be compliant with guidelines. A wider assessment of appropriateness was then evaluated based on this guideline compliance and the documented or presumed justifications for non-compliance, using criteria specific to cefalexin developed following the NAPS process (Fig. 1). These criteria included the presence of allergies, microbiology mismatch, incorrect dosage, frequency or duration, and instances where cefalexin use was deemed unnecessary. Based on these criteria, appropriateness of cefalexin prescribing was classified as appropriate (optimal or adequate), inappropriate (suboptimal or inadequate), or not assessable. Fig. 1 outlines appropriateness criteria and classification for cefalexin prescriptions.
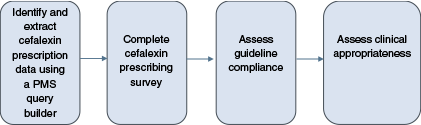
The process followed to identify cefalexin prescriptions and evaluate guideline compliance and appropriateness is summarised in Fig. 2.
Process of evaluating cefalexin guideline compliance and appropriateness. PMS; Practice Management System.
Ethics
The study was approved by the Otago Human Research Ethics Committee (reference number H24/0091) and the Ngāi Tahu Research Consultation Committee.
Statistical analysis
Descriptive statistics were used to summarise patient characteristics and prescription data. Categorical variables – including gender, ethnicity, guideline compliance, and clinical appropriateness – were reported as counts and percentages. Continuous variables were summarised using means and standard deviations (s.d.) or medians and interquartile ranges (IQRs), depending on data distribution.
As there were no prior published data available on the appropriateness of cefalexin prescribing in community general practice settings in NZ, this study was considered exploratory in nature. Accordingly, no formal sample size or power calculations were performed. The focus was on generating preliminary insights into prescribing behaviour and identifying areas for potential improvement or further investigation. All data collation and analysis were conducted using Microsoft Excel (Version 2411, Redmond, WA, USA), which was used to calculate descriptive statistics and support data visualisation.
Results
Patient demographics
A total of 27 cefalexin prescriptions were issued to 25 patients during the 2-month study period. Of these, 16 patients (64%) were female, 6 (24%) were <18 years old, and 20 (80%) identified as NZ European. The median age was 48.9 years (IQR 49.7). Table 1 summarises patients’ characteristics.
| Characteristic | Value | |
|---|---|---|
| Gender, n (%) | ||
| Female | 16 (64%) | |
| Male | 9 (36%) | |
| Ethnicity, n (%) | ||
| NZ European | 20 (80%) | |
| European other | 3 (12%) | |
| Māori | 2 (8%) | |
| Age, years, median (IQR) | 48.9 (49.7) | |
| Range | 1.3–90.2 | |
Cefalexin prescription characteristics
Across the 27 cefalexin prescriptions reviewed, the total median dose was 500 mg, with a mean ± s.d. prescribed dose of 472.2 (138.9) mg (range, 250–1000 mg). The median prescribing frequency and duration were 3 times daily (ter die sumendus, TDS) and 7 days (IQR 2), respectively. The most common dose, frequency, and treatment duration were 500 mg, twice daily, and 5–7 days, respectively. Four prescriptions (14.8%) exceeded a duration of 7 days, including courses of 10 days (acute lower urinary tract infection), 14 days (indication not determinable), 28 days (acute exacerbation of bronchiectasis), and 90 days (prophylaxis for recurrent urinary tract infections).
For children under 18 years (n = 6 [24%]; median age: 5.3 years, IQR 3.3–10.1; range 1.3–12.9), the median prescribed dose was 425 mg (IQR 350–500 mg), with a mean ± s.d. of 416.7 ± 93.1 mg (range 300–500 mg). Twice-daily dosing was most common (n = 5), with a median treatment duration of 6 days (IQR 5–7; range 3–7). The mean prescribed cefalexin dose and treatment duration were comparable across the total sample (n = 27; 500 mg and 7 days), adult (n = 21; 500 mg and 7 days), and paediatric (n = 6; 425 mg and 6 days) subgroups.
Most common prescription clusters
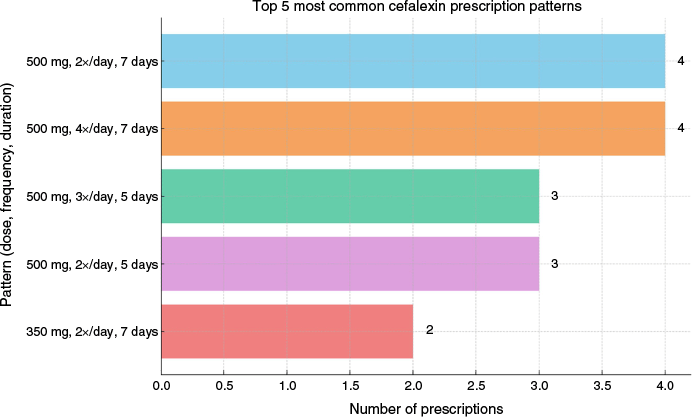
The two most common prescription patterns were 500 mg twice daily for 7 days and 500 mg 4 times daily for 7 days, each observed 4 times (Fig. 3). Prescriptions tended to cluster tightly around a 500 mg dose, with variation largely in frequency and duration.
Indications
A total of 26 prescriptions were able to be assessed. Soft tissue infections were the most common indication (n = 11, 42.3%), followed by genito-urinary infections (n = 10, 38.5%), lower respiratory tract infections (n = 3, 11.5%), and upper respiratory tract infections (n = 2, 7.7%).
Indication documentation
Indications for cefalexin use were documented in 22 (84.6%) instances. However, only 6 (27.3%) of these were recorded directly on the prescription, and 19 (86.4%) were recorded in the clinical notes. Only three prescriptions had indications documented in both the prescription and the clinical notes (13.6%). Sole documentation of indications was more common in the clinical notes (16/22, 72.7%), with a minority (3/22, 13.6%) documented solely on the prescription.
Guideline/guidance compliance and appropriateness
Compliance with guidelines/guidance was observed in 14 out of 26 prescriptions (53.8%), while 11 prescriptions (42.3%) were non-compliant. Of the compliant prescriptions, one was classified as directed therapy and one was recommended by infectious diseases/microbiology. When assessed for appropriateness, 57.7% of prescriptions (n = 15) were considered appropriate (optimal or adequate), whereas 34.6% (n = 9) were classified as inappropriate (suboptimal or inadequate), and 7.7% (n = 2) were not assessable.
Of the nine prescriptions that were deemed inappropriate, three were for soft tissue infections, three for genito-urinary infections, two for lower respiratory tract infections, and one for an upper respiratory tract infection.
Table 2 provides a detailed summary of the three quality indicators of cefalexin prescribing.
| Category | N | % | |
|---|---|---|---|
| Guideline/guidance compliance | |||
| Compliant A | 14 | 53.8 | |
| Non-compliant | 11 | 42.3 | |
| Not assessable | 1 | 3.8 | |
| Appropriateness | |||
| Appropriate (optimal/adequate) B | 15 | 57.7 | |
| Inappropriate (suboptimal/inadequate) | 9 | 34.6 | |
| Not assessable | 2 | 7.7 | |
| Indication documentation | |||
| Documented (anywhere) | 22 | 84.6 | |
| Prescription | 6 | 23.1 | |
| Clinical record | 19 | 73.1 | |
Discussion
Summary of main findings
This exploratory study offers an initial insight into cefalexin prescribing patterns in NZ general practice and highlights potential targets for AMS initiatives. Around half of the cefalexin prescriptions reviewed were guideline compliant and deemed clinically appropriate. Soft tissue and genito-urinary tract infections were the most common indications for cefalexin prescriptions. Although indication documentation was relatively high, most indications were found in clinical notes rather than on the prescription itself.
Comparison with existing literature
Around one-third of cefalexin prescriptions (n = 9, 34.6%) were deemed inappropriate. These were for infections where narrower-spectrum agents are considered first-line options with comparable efficacy. Specifically, flucloxacillin is preferred for soft tissue infections (n = 3), nitrofurantoin for uncomplicated lower urinary tract infections (n = 3), and amoxicillin for respiratory tract infections (2 lower, 1 upper; n = 3).
NZ has one of the highest rates of community antibiotic dispensing among OECD countries. In 2018, the national dispensing rate was approximately 22 defined daily doses per 1000 population per day (DID), comparable to countries with the highest rates such as France, Poland, and Spain, and more than double the rates observed in Sweden, Austria, and the Netherlands.5 Notably, the dispensing rate for beta-lactam antibiotics (penicillins and cephalosporins) was 12.9 DIDs, which is over twice the rate in northern European countries such as Sweden (5.8), Norway (5.6), and the Netherlands (2.9).5
Although we identified no studies that specifically assessed cefalexin prescribing appropriateness in community settings, recent evidence continues to demonstrate suboptimal rates of appropriate antibiotic prescribing in general across both community and hospital settings.11,12 A cross-sectional study in South Africa found that overall adherence to national guidelines for antibiotic prescribing in primary care was 45.1%.13 Similarly, a study conducted in the Netherlands analysing 248,896 episodes of respiratory infection in general practice showed high rates of inappropriate antibiotic prescribing. These included 57% for acute tonsillitis and 51% for acute bronchitis when antibiotics were not clinically indicated.14 These trends align with our study, which found suboptimal performance in quality indicators of cefalexin prescribing in general practice, including guideline compliance and clinical appropriateness.
Documentation of the indication was generally adequate, particularly within the clinical notes. Although only a minority of prescriptions included the indication directly, this is not a mandatory requirement in NZ. Clear documentation facilitates assessment of prescribing quality and supports safer and more transparent clinical decision-making. Interventions aimed at improving prescriber awareness of guideline recommendations, promoting documentation, and supporting reflective prescribing may help to improve overall prescribing quality.
Strengths and limitations of the study
To our knowledge, this is the first evaluation of the appropriateness and guideline compliance of cefalexin prescribing in general practice using a standardised approach. However, several limitations should be noted. This was a cross-sectional study with a small sample size drawn from a single, urban-based medical centre in Christchurch, which provides initial insight but limits the strength of the conclusions and their generalisability to other community settings. The data collection period – July to August 2023, corresponding to the winter season, which is associated with higher rates of inappropriate antibiotic prescribing, particularly for viral upper respiratory tract infections – may also have introduced seasonal bias (though only three of the prescriptions were for respiratory illness). This could affect the representativeness of the findings in terms of actual guideline adherence, prescribing appropriateness, and broader prescribing patterns. As the data were collected retrospectively, the study relied on the interpretation of pre-existing clinical records, which may have been missing, incomplete, or inaccurate. Finally, the study did not examine the reasons for non-compliant or inappropriate prescriptions, which could provide valuable insights for targeted AMS interventions.
Implications for clinical practice and future research
These findings underscore a need for enhanced AMS in the community. Educational initiatives, such as AMS programmes and clinical decision-support tools targeting prescribers, may help to improve adherence to guidelines and reduce inappropriate prescribing. Encouraging clinicians to document indications directly on prescriptions may also enhance communication and promote appropriate antibiotic use.15,16
Future research should involve a larger, multicentre study to validate these findings and explore prescribing trends across different populations and primary care settings (solo vs group practices, general practitioner vs nurse practitioner, urban vs rural, etc). Practices could be sampled from different regions across NZ and stratified by practice type and geographical location. The survey tool developed in this study could be standardised and refined for broader application. Data collection over an extended time frame (a full calendar year) would help mitigate seasonal variation in infection patterns and prescribing. In addition to quantitative analysis, such studies could incorporate structured interviews or surveys with prescribers to capture their perspectives and explore barriers to guideline compliance.
Conclusion
Although the documentation of prescribing indications was consistently high, only about half of cefalexin prescriptions in general practice were clinically appropriate and aligned with guidelines. These findings highlight the need to strengthen AMS efforts in community settings, particularly by enhancing guideline adherence and fostering more transparent documentation. Reinforcing AMS at the community level is essential to preserving the long-term efficacy of antibiotics for future generations.
Data availability
Data supporting the findings of this study are available from the corresponding author upon reasonable request.
Declaration of funding
This research received funding from Pegasus Health (Charitable) Ltd to support YML’s participation in the 2023 Future Health Researcher programme.
Acknowledgements
We thank Pegasus Health (Charitable) Ltd for providing funding to support YML’s participation in the Medical Student Future Health Researcher Programme. We also thank Dr Sharon Gardiner, Antimicrobial Pharmacist at the Canterbury District Health Board and Clinical Pharmacology, Department of Medicine, University of Otago, Christchurch, for providing the Canterbury District Health Board Hospital Prescribing Survey and sharing her expertise.
Author contributions
I. S. A. conceived the idea, supervised the study, developed the study survey, collected and analysed the data, and wrote the final manuscript. S. Q. co-wrote the initial manuscript and contributed to data analysis. Y.-M. L co-designed the study survey, interpreted the data, and provided feedback on the manuscript. D. M. provided critical guidance throughout the research process and reviewed versions of the manuscript. B. H. assisted in study design, collected the data, and provided feedback and guidance during the analysis and writing process. All authors have read and approved the final manuscript.
References
1 New Zealand Formulary (NZF). NZF version 154; 2025. Available at https://nzf.org.nz/nzf_3048?searchterm=cephalosporins [accessed April 2025].
2 bpacNZ Primary Care Antibiotic Guide. bpacNZ. November 2024. Available at https://bpac.org.nz/antibiotics/guide.aspx [accessed January 2025].
3 bpacnz. Revisiting antibiotic use in New Zealand: how does your prescribing compare?. Bpac.org.nz. June 2024. Available at https://bpac.org.nz/2024/antibiotic-use.aspx [accessed April 2025].
4 Hagedoorn NN, Al-Busaidi I, Bridgford P, et al. Longitudinal trends in community antibiotic consumption in the Waitaha Canterbury Region of Aotearoa New Zealand over 10 years (2012-2021): an observational study. N Z Med J 2023; 136(1571): 49-64.
| Crossref | Google Scholar | PubMed |
5 Thomas MG. Improving community antibiotic prescribing to keep antibiotics working in Aotearoa New Zealand. N Z Med J 2024; 137(1592): 90-99.
| Crossref | Google Scholar | PubMed |
6 Antimicrobial Resistance Collaborators.. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 2022; 399: 629-655.
| Crossref | Google Scholar | PubMed |
7 Duffy E, Ritchie S, Metcalfe S, et al. Antibacterials dispensed in the community comprise 85%-95% of total human antibacterial consumption. J Clin Pharm Ther 2018; 43(1): 59-64.
| Crossref | Google Scholar | PubMed |
8 Le G, Ivy M, Dickey S, et al. A study review of the appropriateness of oral antibiotic discharge prescriptions in the Emergency Department at a rural hospital in Mississippi, USA. Antibiotics 2023; 12(7): 1186-6.
| Crossref | Google Scholar |
9 Gardiner SJ, Basevi AB, Hamilton NL, et al. Point prevalence surveys of antimicrobial use in adult inpatients at Canterbury District Health Board Hospitals. N Z Med J 2020; 133(1525): 18-33.
| Google Scholar | PubMed |
10 National Antimicrobial Prescribing Survey. National Centre for Antimicrobial Stewardship. Available at https://www.naps.org.au/Default.aspx [accessed April 2025].
11 Thilly N, Pereira O, Schouten J, et al. Proxy indicators to estimate appropriateness of antibiotic prescriptions by general practitioners: a proof-of-concept cross-sectional study based on reimbursement data, north-eastern France 2017. Euro Surveill 2020; 25(27): 1900468.
| Crossref | Google Scholar | PubMed |
12 Wushouer H, Yu J, Du K, et al. Evaluation of appropriateness of antibiotic prescribing in primary healthcare institutions in China using proxy indicator. Lancet Reg Health West Pac 2024; 49: 101132.
| Crossref | Google Scholar | PubMed |
13 Gasson J, Blockman M, Willems B. Antibiotic prescribing practice and adherence to guidelines in primary care in the Cape Town Metro District, South Africa. S Afr Med J 2018; 108(4): 304-310.
| Crossref | Google Scholar | PubMed |
14 Hek K, van Esch TEM, Lambooij A, et al. Guideline adherence in antibiotic prescribing to patients with respiratory diseases in primary care: prevalence and practice variation. Antibiotics 2020; 9(9): 571.
| Crossref | Google Scholar | PubMed |
15 Ciarkowski C, Vaughn VM. Antibiotic documentation: death by a thousand clicks. BMJ Qual Saf 2022; 31: 773-775.
| Crossref | Google Scholar | PubMed |
16 He Ako Hiringa. Antimicrobial stewardship: Primary care can build on recent gains. Available at https://www.akohiringa.co.nz/education/antimicrobial-stewardship-primary-care-can-build-on-recent-gains#GOOD [accessed April 2025].