Patient concerns regarding antidepressant drug–drug interactions: a retrospective analysis using data from a medicines call centre
Edgar L. Poon 1 2 3 , Hyang Joo Lim 1 , Samantha A. Hollingworth
1 2 3 , Hyang Joo Lim 1 , Samantha A. Hollingworth  1 , Mieke L. van Driel
1 , Mieke L. van Driel  4 , David M. Pache
4 , David M. Pache  1 2 5 , Geraldine M. Moses 1 2 , Treasure M. McGuire
1 2 5 , Geraldine M. Moses 1 2 , Treasure M. McGuire  1 2 5 *
1 2 5 *
1 School of Pharmacy, The University of Queensland, Brisbane, Qld, Australia.
2 Mater Pharmacy, Mater Health South East Queenland, Brisbane, Qld, Australia.
3 Greenslopes Private Hospital, Ramsay Health, Brisbane, Qld, Australia.
4 Faculty of Medicine, The University of Queensland, Brisbane, Qld, Australia.
5 Faculty of Health Sciences and Medicine, Bond University, Gold Coast, Qld, Australia.
Journal of Primary Health Care 14(2) 99-108 https://doi.org/10.1071/HC21150
Published: 22 April 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of the Royal New Zealand College of General Practitioners. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Introduction: Antidepressant use has increased over the last two decades, with Australia and New Zealand among the highest antidepressant users in Organisation for Economic Co-operation and Development (OECD) countries. Comorbidity and polypharmacy are common in antidepressant users, increasing the likelihood of interaction-related adverse drug events, which are frequently preventable.
Aim: We aimed to identify, profile, and analyse potential antidepressant drug–drug interactions in information-seeking antidepressant users.
Methods: We retrospectively analysed antidepressant-related drug–drug interaction enquiries from patients or carers who contacted a pharmacist-led Australian national medicines call centre over an 8-year period to determine patient characteristics, concomitant drugs involved, prevalence and type of antidepressant-related drug–drug interaction across life stages, and associated risks.
Results: Of 3899 antidepressant drug–drug interaction calls, the most frequent concomitant drugs were antipsychotics, opioids, benzodiazepines, and complementary medicines. Narrative analyses of 2011 calls identified 81.0% of patients with potential drug–drug interactions and 10.4% categorised with worrying symptoms. The most frequent drug–drug interaction risks were excessive sedation, increased anticholinergic effects, serotonin syndrome, and suicidal thoughts. Carers of children aged <15 years and older adults (65–74 years) were more likely to report experiencing worrying symptoms. Although more potential pharmacodynamic than pharmacokinetic interactions were recorded, pharmacokinetic interactions tended to have more significant clinical impact.
Discussion: Antidepressant users often have information gaps and safety concerns regarding drug–drug interactions that motivate help-seeking behaviour. Symptoms and drug–drug interaction consequences may be underestimated in these patients. Primary care health professionals have a role in proactively addressing the risk of drug–drug interactions to support benefit-risk assessment and shared decision-making.
Keywords: Antidepressive agents, call centre, drug information services, drug interactions, help-seeking behaviour, information-seeking behaviour, prescribing, primary care.
| WHAT GAP THIS FILLS |
| What is already known: Antidepressant use has increased globally, with Australia and New Zealand among the highest users of antidepressants in the Organisation for Economic Co-operation and Development (OECD). Comorbidity and polypharmacy are common in antidepressant users, increasing the likelihood of interaction-related adverse drug events. |
| What this study adds: Safety concerns about interactions with concomitant medications or lifestyle drugs motivated antidepressant users to seek information, with carers of children aged <15 years and older adults (aged 65–74 years) more likely to report worrying symptoms related to drug–drug interactions. The most frequently identified risks were excessive sedation, anticholinergic symptoms, serotonin excess and suicidal ideation. |
Introduction
Antidepressant use has increased over the last two decades, with Australia and New Zealand among the top 10 users of antidepressants in the Organisation for Economic Co-operation and Development (OECD).1 Antidepressants represent >5% of prescriptions written in Australian general practice,2 and >29 million antidepressant prescriptions were dispensed on the Australian publicly subsidised Pharmaceutical Benefits Scheme in 2019–20.3 In addition, 12.6% of all New Zealanders were prescribed an antidepressant (16% of females, 9% of males) in 2015, an increase of 21% since 2008.4
Comorbidity and polypharmacy are common in antidepressant users.5 Previous studies have shown that older people, children and adolescents are more likely to experience drug–drug interactions with antidepressants.6,7 However, few studies have determined drug–drug interaction prevalence across life stages. Antidepressant drug–drug interactions increase the likelihood of adverse drug events, which can result in increased morbidity and mortality, loss of drug efficacy, and misdiagnosis of symptoms.8,9 Moreover, nearly half (46.5%) of drug–drug interaction-related adverse events are preventable.10 We aimed to identify, profile, and analyse drugs commonly used concomitantly with antidepressants and potential drug–drug interactions in antidepressant users who sought medicines information from a national medicines call centre.
Method
Between September 2002 and June 2010, we conducted a retrospective observational study of consumer questions concerning antidepressant interactions, using data from National Prescribing Service (NPS) MedicineWise (formerly the NPS Medicines Line), operated by pharmacists at Mater Health Services, Brisbane, Queensland, Australia. Although there was a change in service provider after this time, the consistency of caller demographics and types of enquiries generating consumer concerns over the 8 years of service supports the current relevance of our study aims.11 Few new antidepressants have emerged in the last decade, so the drugs in this study continue to have prominent use. Eight specific antidepressants comprised 84% of all antidepressants used in Australia between 2006 and 2018.12
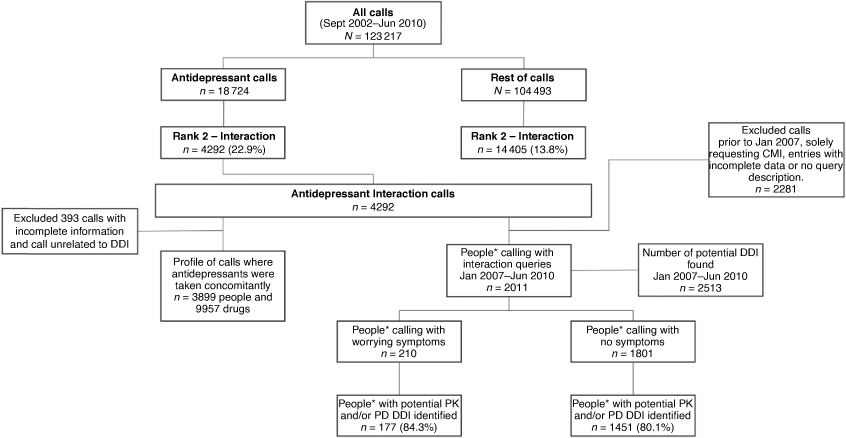
For the first part of our analysis, we extracted all calls about antidepressant medicines from the database, based on their Anatomical Therapeutic Chemical (ATC) Level 3 (therapeutic class).13 Call characteristics were compared with their respective ‘rest of calls’ (Fig. 1). We explored antidepressant drug–drug interaction queries as this was a more highly ranked enquiry type, (22.9%) for antidepressants than the rest of the calls (13.8%, P < 0.001). A potential drug–drug interaction was defined as any concomitant drug use that could modify a person’s response, potentially affecting the therapeutic intent or causing undesired effects, based on pharmacokinetic (PK), pharmacodynamic (PD) and clinical evidence. We excluded entries with incomplete data or those that were not drug–drug related.
For the second part of our analysis, we focused on antidepressant users (patients) who were at potential drug–drug interaction risk. Patient demographics, including patients’ age, gender, and their medication use profile, were extracted from calls where the patient was a caller ringing for themself (80.4%) or the data were provided by the caller (19.6%), a family member, carer or friend, where the patient was prescribed an antidepressant, had questions or concerns regarding potential interactions with their concomitant drug use (January 2007–June 2010), and where the database held detailed narratives from each call. All concomitant patients’ medicines (prescribed or self-medicated) were classified by their ATC therapeutic class (Fig. 1).13
Pharmacists answering the call categorised interaction queries into two patient groups: patients having an information gap or concern but not experiencing symptoms – the ‘no symptoms’ group; and patients presenting with symptoms of concern to themselves and to the pharmacist answering the call – the ‘worrying symptoms’ group. All calls classified as associated with ‘worrying symptoms’ were referred to patients' designated primary healthcare professional. We excluded calls solely requesting consumer medicines information and entries with incomplete data, including no description of their query. We compared patient characteristics to profile and differentiate between the two patient groups.
To obtain an objective picture of the likelihood of a drug–drug interaction, we used a drug–drug interaction database (YouScript)14 for primary interaction analysis. We also used Stockley’s Interactions Checker,15 the Australian Medicine Handbook,16 AccessPharmacy,17 and Natural Medicines18 as secondary resources when a medication was not listed on YouScript. Each call was analysed separately for potential pharmacokinetic and pharmacodynamic interactions. The significance of a potential pharmacokinetic interaction was expressed by severity grade and estimated percentage change in serum drug concentrations as predicted on YouScript: 0% = nil, ~20% = minimal, ~60% = minor, ~100% = major, and >100% = contraindicated. This pharmacokinetic drug–drug interaction scale estimates potential patient impact, taking into consideration the many factors that can influence the clinical impact of drug exposure including dose, therapeutic use, inter-patient and intra-patient variabilities in drug disposition.19 Pharmacodynamic interactions were classified by potential severity of an interaction-induced adverse event using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE):20 no adverse event (due to a drug–drug interaction); mild: asymptomatic or mild symptoms – no intervention indicated; moderate – symptoms with minimal intervention indicated; severe – medically significant symptoms but not life- threatening – intervention indicated; life-threatening or disabling symptoms – drug use contraindicated. In cases where more than one drug–drug interaction was identified, the highest level was chosen. A team including pharmacists and a general practitioner (EP, GM, DP and MVD) assessed cases where the drug–drug interaction evidence was ambiguous or conflicting.
As data were originally collected for routine service without specific a priori research goals, this research was conducted and reported in accordance with the REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) guidelines.21
Statistical analysis
All variables are categorical, and descriptive results are presented using numbers and percentages. Evidence for a difference between patients with worrying symptoms and no symptoms was investigated using Pearson’s chi-squared test of independence. Statistical analysis was performed using R Statistical Software (version 4.0.3; R CoreTeam 2020).
Ethics approval
This study was approved by the Human Research Ethics Committee of Mater Health Services, Brisbane (LNR submission 2012-68).
Results
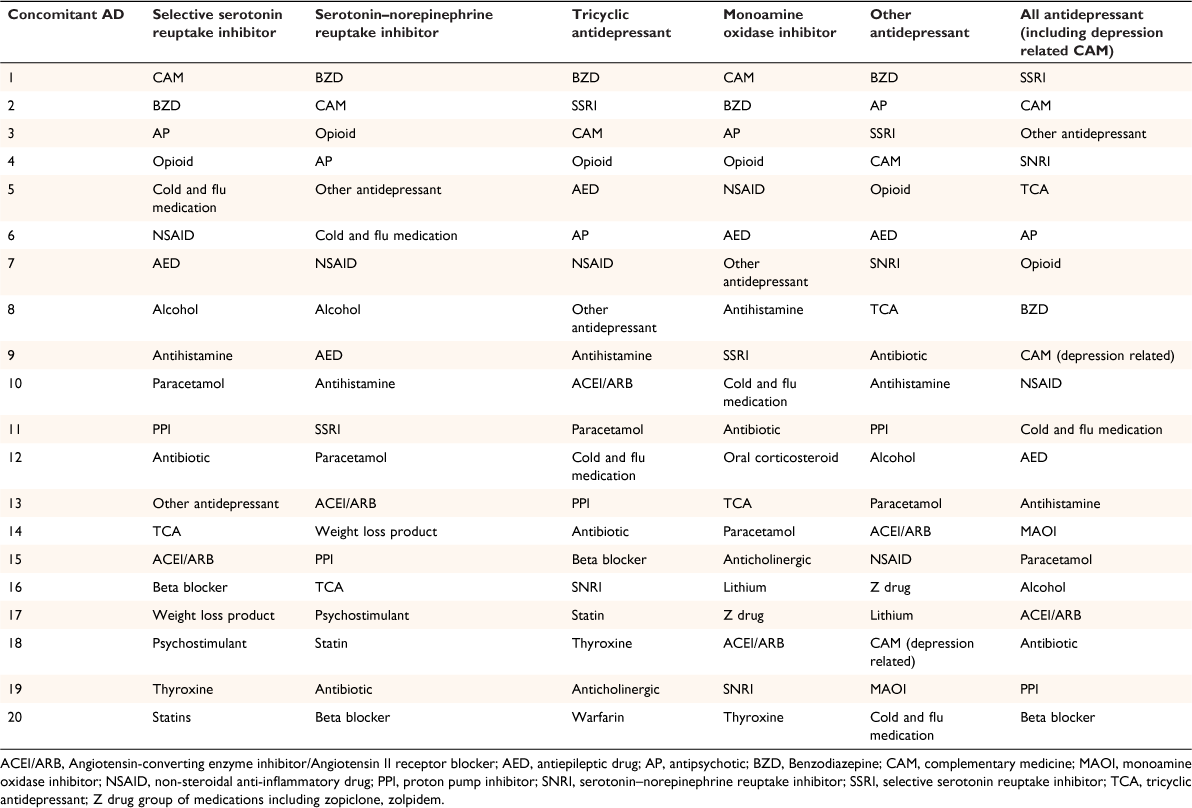
Of 123 217 calls received from Australian consumers between September 2002 and June 2010, 18 724 (15.2%) involved questions about antidepressants, of which one in four (4292 calls, 22.9%) focused on potential interactions. After applying the inclusion criteria, we analysed 3899 calls for concomitant medicines by antidepressant class and summarised the 20 most common concomitant therapeutic classes. In rank order, antipsychotics, opioid analgesics, benzodiazepines, and complementary medicines constituted more than half of medicines taken concomitantly with antidepressants (Table 1).
|
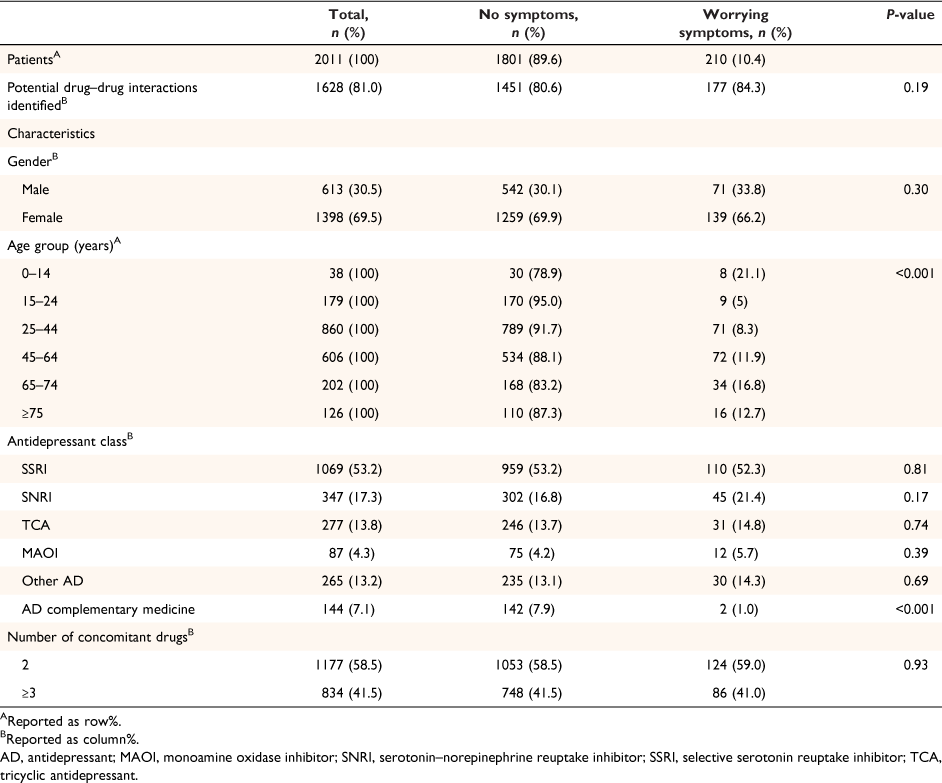
Of 2011 drug–drug interaction calls with a recorded narrative, 1801 (89.6%) patients with interaction queries were categorised as presenting with no symptoms and 210 (10.4%) presenting with worrying symptoms (Table 2). Irrespective of symptom causality, a review of drug–drug interaction resources showed good correlation between consumer help-seeking behaviours for concerns about antidepressant interactions, with a potential drug–drug interaction identified in 81.0% (1628) of these individuals.
|
More than two in three people who called about potential drug–drug interactions were female. This proportion was similar whether the antidepressant user was experiencing symptoms or not (P = 0.30). Whether or not people were identified as having worrying symptoms was associated with age (P < 0.001, Table 2). Carers of children aged <15 years and older adults (aged 65–74 years) were more likely than other age groups to report experiencing worrying symptoms. The antidepressant drug use profiles were also similar across different antidepressant classes, except for complementary medicines commonly used for depression: for example, St. John’s wort, S-adenosyl-l-methionine (SAMe) (worrying symptoms 1.0% vs no symptoms 7.9%, P < 0.001). The most commonly implicated antidepressants were selective serotonin reuptake inhibitors (SSRI; n = 1069, 53.2%), serotonin noradrenaline reuptake inhibitors (SNRI; n = 347, 17.3%) and tricyclic antidepressants (TCA; n = 277, 13.8%), and depression-related complementary medicines (n = 142, 7.1%).
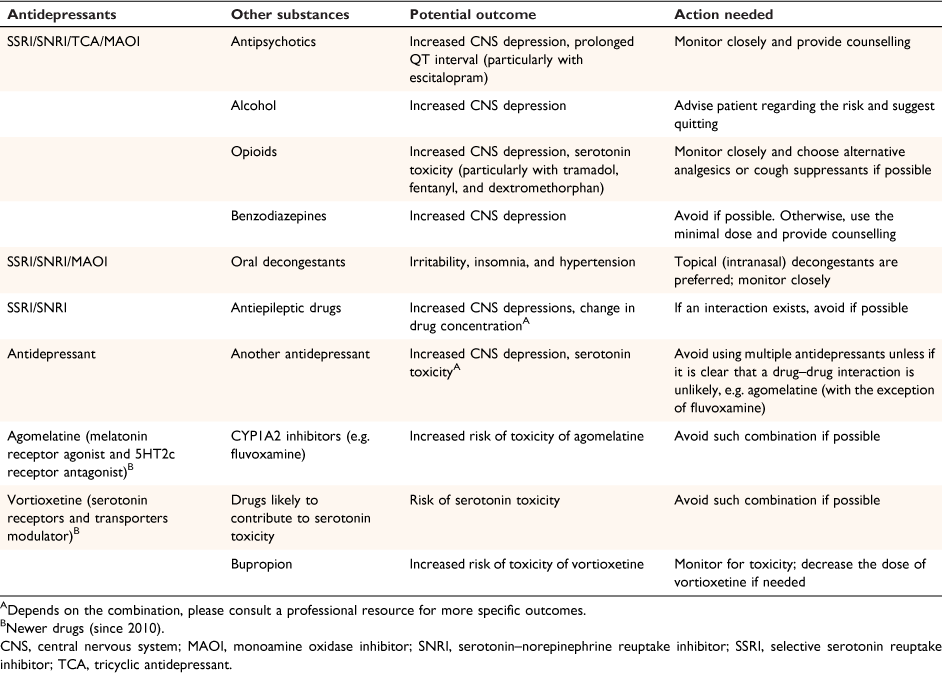
We identified 2513 interactions in the 2011 patients in our dataset (Table 3). In general, more potential PD interactions were recorded compared to potential PK interactions, but when comparing the distribution of the significance of interaction, PK interactions tended to have a higher significance of clinical impact than PD interactions, with 48.0% of patients experiencing a potential drug level change between 61 and >100%) (Table 3). We describe the frequency of potential PD effects in Table 3; risk of central nervous system (CNS) depression, increased anticholinergic effects, serotonin syndrome and suicidal thoughts were more commonly identified from patients’ concurrent medication use. We compiled a resource of key and common potential interactions that clinicians should be aware of before prescribing an antidepressant, derived from our data and for antidepressants marketed since 2010 (Table 4).

|
|
Discussion
Our real-world medicines call-centre data demonstrated help-seekers who take antidepressants have drug–drug interaction concerns about concomitantly used medicines or lifestyle products including complementary medicines.22 There is a bidirectional relationship between mental health disorders and comorbid chronic physical conditions.23–25 The guidelines from the National Institute for Health and Care Excellence indicate that one in five (20%) patients with a chronic physical condition also experience depression;23 whereas World Mental Health surveys from the World Health Organization reported that almost three in four (72%) patients with a major depressive disorder also have a chronic physical condition, increasing the probability of a clinically relevant adverse medication event relating to a drug–drug interaction.26
In our study, CNS-active drugs of dependence; for example, benzodiazepines, opioids, and alcohol, were commonly used concomitantly with antidepressants, consistent with previous research.27 Patients with depression and a substance use disorder; for example, in the form of dependence, were more likely to seek help, but their needs were often unmet due to barriers accessing mental health care.28 It is therefore important to closely monitor patients concurrently using antidepressants and CNS-active drugs to address perceived barriers to mental health care.
Short-term suicidal ideation was reported as a potential concern. There is a correlation between antidepressant dose and suicidal ideation risk;29 a high antidepressant concentration in the blood may be associated with increased suicidality. This highlights the importance of identifying and monitoring for a potential PK drug–drug interaction involving antidepressants.30 If a patient is prescribed a treatment that reduces antidepressant metabolism and/or elimination, it will manifest as an increased antidepressant dose, thereby raising suicidal ideation risk. In contrast, if the new treatment induces antidepressant clearance, symptoms of depression may return, or the patient may experience withdrawal effects. Suicidal ideation generated by antidepressant use continues to court controversy.31 Our results suggest that monitoring patient progress during a severe mental health disorder may be suboptimal, and that appropriate, regular, counselling remains essential to ensure the patient comprehends their symptoms and how they can be appropriately managed. For many, this includes reassurance that these symptoms will be temporary, but that they need to seek help should they persist.
Worrying symptoms related to antidepressant drug–drug interactions were more likely to be reported by carers of children aged <15 years and older adults (65–74 years). This would be expected in an older age group who use multiple medicines, as polypharmacy is a predictor of adverse drug reactions,32 but it is less well recognised that children may have a similar rate of experiencing worrying symptoms or adverse drug events.33 Children and adolescents are increasingly being prescribed antidepressants, with at least 101 174 Australians aged 0–17 years (1.8%) having an antidepressant dispensed between July 2017 to June 2018.31 Over an 11- year period (2008–18), Australian antidepressant dispensing (0–27 years of age, per capita) increased 66% and suicide rates (0–24 years) increased 49%.31 It is unclear whether suicidality is correlated with antidepressant use or the limited efficacy of antidepressants in suicidal children and adolescents.34 This issue warrants early identification and monitoring for PK drug–drug interactions involving antidepressants, with patients reassured that most symptoms will be temporary, but to seek help should they persist.30
In our study, people using a complementary medicine for depression, for example St John’s wort, were more likely to seek information in the absence of worrying symptoms. This parallels our previous medicines call centre research demonstrating general consumer concerns about complementary medicine risks, where 34% of calls asked about possible interactions with complementary medicines versus only 13% for conventional medicines.35 Mainstream and social media have also increased consumer awareness of the dangers of ‘mixing medicines’. As complementary medicines are often self-initiated without input from health practitioners, it places the onus on the consumer to seek information about potential interactions. This is supported by a study indicating that complementary medicine consumers consult more clinical resources than patients who are on conventional medicines.36 Widespread use of complementary medicines carries increased drug–drug interaction risk.35,37 This is an opportunity for prescribers to counsel patients that ‘natural’ is not synonymous with ‘safe’, and highlights the importance of gathering information regarding complementary medicine use, especially when first prescribing antidepressants.
We found that potential PD interactions were more prevalent than potential PK interactions, with CNS depression and anticholinergic effects commonly reported as potential symptoms. Clinicians may also underestimate the extent to which less overt drug–drug interaction effects such as daytime somnolence, dizziness, or constipation may contribute to treatment discontinuation or poor adherence.38 When comparing the distribution of interaction severity, a higher proportion of people with no symptoms (49.8%) than people with worrying symptoms (33.6%) were estimated to have a percentage PK drug change between 61 and >100%. However, intrinsic patient characteristics and a drug’s therapeutic window would contribute to interaction risk.39 In contrast, the severity of PD interaction between patients with and without worrying symptoms were similar. This suggests that the clinical impact of PK and PD interactions cannot be directly compared, with current interaction checkers being poor predictors of patients likely to experience actual drug–drug interaction symptoms. They do, however, serve as a flag to explore individual patient characteristics and drug dose as contributors to drug–drug interaction risk. Clearly, patients who are taking antidepressants, with their inherent diverse pharmacological profiles, together with other drugs, are at increased drug–drug interaction risk that can negatively impact treatment outcomes. This highlights the value of a trusted healthcare professional with drug–drug interaction expertise assessing the drug–drug interaction risk of individual patients.
We did not identify any predictor that would indicate which patient group was more likely to experience symptomatic antidepressant drug–drug interactions. Nine in ten patients or carers in our study commonly sought help for their drug–drug interaction concerns despite a lack of worrying symptoms. This may suggest antidepressant users overestimate their medication risk. Maladaptive risk perception is a cornerstone of cognitive models of anxiety disorders where worried individuals generally overestimate negative outcomes,40 and emotional reactions to perceived risk or uncertainty often drive behaviour such as help-seeking.41 As four in five patients had at least one identified drug–drug interaction of potential clinical significance, this validates healthcare professionals being receptive to patients’ drug–drug interaction concerns.
The main strength of this study was that we used real-world, routinely collected health service data and demonstrated remarkable consistency in consumer safety concerns, particularly about antidepressant drug–drug interactions, over an 8-year period. Consistent with information behaviour theories, callers used the medicines call centre to seek information in response to uncertainty associated with worrying symptoms or multiple interpretations of information (inadequate, conflicting, or overload).11,35
We note two main limitations. First, although the data analysed were collected a decade ago, and have not captured some of the newer antidepressants, the individual antidepressants reported in our study continue to be widely used. A recent analysis comparing antidepressant use in Australia and Sweden demonstrated that SSRIs and SNRIs remain the most commonly used antidepressants over a 13-year period (2006–18).12 We developed a guide (Table 4) for primary healthcare professionals to identify and monitor common antidepressant drug–drug interactions, their potential outcomes and actions needed. It includes newer antidepressants approved since 2010.
Second, our study findings might not represent all antidepressant users, but rather those concerned enough to contact a medicines call centre. A previous longitudinal analysis of over 125 000 calls to the same medicines call centre found call demographics were remarkably consistent. Callers originated from all Australian states and territories, and importantly, when caller location was grouped by the Accessibility/Remoteness Index of Australia (ARIA),42 those from ‘rural and remote’ areas approached the relative population frequency.11 This provides reassurance that a medicines call centre model has utility for the Australian public, particularly those with less access to health services. Moreover, in a study of 2348 calls to a Finnish national consumer medicines call centre surveying use of the different medicines information sources, people with mental disorders were more frequent users of telephone- and internet-based medicines information sources and patient information leaflets than people without mental disorders.43 Call centre popularity is cited as primarily due to their low consumer cost, ease of access, immediacy of help, provision of caller anonymity and protection from stigma.44,45 Furthermore, some patient characteristics such as ethnicity were not recorded by the medicines call centre, although previous research has identified it can impact the prevalence of antidepressant use.46 Finally, the study design precluded factors such as adherence and under-reporting being explored and thus, requires future research to address these factors.
In conclusion, antidepressant users who information seek commonly take combinations of medicines and lifestyle drugs, and often have drug–drug interaction safety concerns. Prescribers may underestimate the risk of excessive sedation, anticholinergic symptoms, serotonin syndrome, and suicidal ideation in people using antidepressants together with other drugs. Primary healthcare professionals, especially general practitioners and pharmacists, are ideally placed to identify clinically relevant drug–drug interactions when a patient commences an antidepressant or if there is a medicine change. They can proactively address any potential drug–drug interaction risk or patient interaction concerns to encourage shared decision-making.
Data availability
The data that support this study were obtained through a shared agreement between NPS MedicineWise and Mater Health Services, Brisbane by permission/licence. Data will be shared upon reasonable request to the corresponding author with permission from both parties.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This study did not receive any specific funding.
Author contributions
TM, GM, MvD, SH, DP and EP contributed to the study design. EP and HL contributed to data collection and management. EP, DP, GM and TM contributed to analysis. All authors contributed to interpretation of results and the final manuscript.
Acknowledgements
We would like to acknowledge NPS MedicineWise (formerly National Prescribing Service, Australia), funder of NPS Medicines Line and service provider since July 2010. We would also like to thank Mater Health Services, Brisbane, for providing the raw service data from September 2002 to 30 June 2010; and Gabrielle Hartley, Pharmacy, and Laura Deckx, University of Queensland, for database assistance; and Alison Griffin, QIMR Berghofer Medical Research Institute, for her advice regarding statistical analysis.
References
[1] Organisation for Economic Co-operation and Development. Health at a glance 2019. Paris: OECD Publishing; 2019.[2] Britt H, Miller GC, Henderson J, et al. General practice activity in Australia 2015–16. Sydney University Press; 2016.
[3] Australian Institute of Health and Welfare. Mental health services in Australia. Canberra: Australian Institute of Health and Welfare; 2021. Available at https://www.aihw.gov.au/reports/mental-health-services/mental-health-services-in-australia [Accessed 21 August 2021]
[4] Wilkinson S, Mulder RT. Antidepressant prescribing in New Zealand between 2008 and 2015. N Z Med J 2018; 131 52–9.
| 30408818PubMed |
[5] Caughey GE, Roughead EE, Shakib S, et al. Comorbidity of chronic disease and potential treatment conflicts in older people dispensed antidepressants. Age Ageing 2010; 39 488–94.
| Comorbidity of chronic disease and potential treatment conflicts in older people dispensed antidepressants.Crossref | GoogleScholarGoogle Scholar | 20511245PubMed |
[6] Hanlon JT, Wang X, Castle NG, et al. Potential underuse, overuse, and inappropriate use of antidepressants in older veteran nursing home residents. J Am Geriatr Soc 2011; 59 1412–20.
| Potential underuse, overuse, and inappropriate use of antidepressants in older veteran nursing home residents.Crossref | GoogleScholarGoogle Scholar | 21824120PubMed |
[7] Lai LL, Alvarez G, Dang L, et al. Prevalence and trend of potential drug–drug interaction among children with depression in U.S. outpatient settings. J Pharm Health Serv Res 2019; 10 393–9.
| Prevalence and trend of potential drug–drug interaction among children with depression in U.S. outpatient settings.Crossref | GoogleScholarGoogle Scholar |
[8] Calderón-Larrañaga A, Poblador-Plou B, González-Rubio F, et al. Multimorbidity, polypharmacy, referrals, and adverse drug events: Are we doing things well? Br J Gen Pract 2012; 62 e821–e6.
| Multimorbidity, polypharmacy, referrals, and adverse drug events: Are we doing things well?Crossref | GoogleScholarGoogle Scholar | 23211262PubMed |
[9] Coupland C, Hill T, Morriss R, et al. Antidepressant use and risk of adverse outcomes in people aged 20-64 years: Cohort study using a primary care database. BMC Med 2018; 16 36
| Antidepressant use and risk of adverse outcomes in people aged 20-64 years: Cohort study using a primary care database.Crossref | GoogleScholarGoogle Scholar | 29514662PubMed |
[10] Doucet J, Jego A, Noel D, et al. Preventable and non-preventable risk factors for adverse drug events related to hospital admissions in the elderly. Clin Drug Investig 2002; 22 385–92.
| Preventable and non-preventable risk factors for adverse drug events related to hospital admissions in the elderly.Crossref | GoogleScholarGoogle Scholar |
[11] Pache DM, Hollingworth SA, van Driel ML, McGuire TM. Does consumer medicines interest reflect medicines use? An observational study comparing medicines call center queries with medicines use. Res Social Adm Pharm 2019; 15 440–7.
| Does consumer medicines interest reflect medicines use? An observational study comparing medicines call center queries with medicines use.Crossref | GoogleScholarGoogle Scholar | 29935855PubMed |
[12] Eek E, van Driel M, Falk M, et al. Antidepressant use in Australia and Sweden—a cross-country comparison. Pharmacoepidemiol Drug Saf 2021; 30 409–17.
| Antidepressant use in Australia and Sweden—a cross-country comparison.Crossref | GoogleScholarGoogle Scholar | 33098321PubMed |
[13] World Health Organization. WHO collaborating centre for drug statistics methodology. Guidelines for ATC classification and DDD assignment. Norwegian Institute of Public Health; 2021. Available at https://www.whocc.no/atc_ddd_index_and_guidelines/guidelines/
[14] Youscript. United States: YouScript Inc, 2021. Available at: https://www.youscript.net/ [Accessed 20 March 2021]
[15] Baxter K, Preston CL (eds). Stockley’s interactions checker. London: Pharmaceutical Press; 2021. Available at http://www.medicinescomplete.com/publication/stockleys-drug-interactions/ [Accessed 20 March 2021]
[16] Rossi S (ed). Australian Medicines Handbook Adelaide: Australian Medicines Handbook Pty Ltd. 2021. Available at https://amhonline.amh.net.au/ [Accessed 20 March 2021]
[17] Accesspharmacy. United States: McGraw Hill LLC; 2021. Available at https://accesspharmacy.mhmedical.com/ [Accessed 20 March 2021]
[18] Natural Medicines [database on the internet]. Somerville (MA): Therapeutic Research Center LLC; 2021. Available at https://naturalmedicines.therapeuticresearch.com/ [Accessed 20 March 2021]
[19] Abbasi Nazari M, Khanzadeh Moqhadam N. Evaluation of pharmacokinetic drug interactions in prescriptions of intensive care unit (ICU) in a teaching hospital. Iran J Pharm Res 2010; 5 215–8.
| Evaluation of pharmacokinetic drug interactions in prescriptions of intensive care unit (ICU) in a teaching hospital.Crossref | GoogleScholarGoogle Scholar |
[20] U.S. Department of Health and Human Services. Common terminology criteria for adverse events (CTCAE) version 5.0. U.S. Department of Health and Human Services; 2017.
[21] Benchimol EI, Smeeth L, Guttmann A, et al. The reporting of studies conducted using observational routinely-collected health data (record) statement. PLoS Med 2015; 12 e1001885
| The reporting of studies conducted using observational routinely-collected health data (record) statement.Crossref | GoogleScholarGoogle Scholar | 26440803PubMed |
[22] Freeman MP. Complementary and alternative medicine (CAM): Considerations for the treatment of major depressive disorder. J Clin Psychiatry 2009; 70 4–6.
| Complementary and alternative medicine (CAM): Considerations for the treatment of major depressive disorder.Crossref | GoogleScholarGoogle Scholar | 19909686PubMed |
[23] National Institute for Health and Care Excellence. Clinical guidelines. Depression in adults: recognition and management [CG90]. London: National Institute for Health and Care Excellence (UK); 2009. Available at https://www.nice.org.uk/guidance/cg90 [Accessed 29 May 2021]
[24] Zhang T, Carleton BC, Prosser RJ, et al. The added burden of comorbidity in patients with asthma. J Asthma 2009; 46 1021–6.
| The added burden of comorbidity in patients with asthma.Crossref | GoogleScholarGoogle Scholar | 19995140PubMed |
[25] Beurel E, Toups M, Nemeroff CB. The bidirectional relationship of depression and inflammation: double trouble. Neuron 2020; 107 234–56.
| The bidirectional relationship of depression and inflammation: double trouble.Crossref | GoogleScholarGoogle Scholar | 32553197PubMed |
[26] Kessler RC, Birnbaum HG, Shahly V, et al. Age differences in the prevalence and co-morbidity of dsm-iv major depressive episodes: results from the WHO world mental health survey initiative. Depress Anxiety 2010; 27 351–64.
| Age differences in the prevalence and co-morbidity of dsm-iv major depressive episodes: results from the WHO world mental health survey initiative.Crossref | GoogleScholarGoogle Scholar | 20037917PubMed |
[27] Bushnell GA, Stürmer T, Gaynes BN, et al. Simultaneous antidepressant and benzodiazepine new use and subsequent long-term benzodiazepine use in adults with depression, United States, 2001-2014. JAMA Psychiatry 2017; 74 747–55.
| Simultaneous antidepressant and benzodiazepine new use and subsequent long-term benzodiazepine use in adults with depression, United States, 2001-2014.Crossref | GoogleScholarGoogle Scholar | 28593281PubMed |
[28] Chen LY, Crum RM, Martins SS, et al. Service use and barriers to mental health care among adults with major depression and comorbid substance dependence. Psychiatr Serv 2013; 64 863–70.
| Service use and barriers to mental health care among adults with major depression and comorbid substance dependence.Crossref | GoogleScholarGoogle Scholar | 23728427PubMed |
[29] Courtet P, Lopez-Castroman J, Jaussent I, et al. Antidepressant dosage and suicidal ideation. JAMA Intern Med 2014; 174 1863–5.
| Antidepressant dosage and suicidal ideation.Crossref | GoogleScholarGoogle Scholar | 25243608PubMed |
[30] Perroud N, Bondolfi G, Uher R, et al. Clinical and genetic correlates of suicidal ideation during antidepressant treatment in a depressed outpatient sample. Pharmacogenomics 2011; 12 365–77.
| Clinical and genetic correlates of suicidal ideation during antidepressant treatment in a depressed outpatient sample.Crossref | GoogleScholarGoogle Scholar | 21449676PubMed |
[31] Whitely M, Raven M, Jureidini J. Antidepressant prescribing and suicide/self-harm by young Australians: regulatory warnings, contradictory advice, and long-term trends. Front Psychiatry 2020; 11 478
| Antidepressant prescribing and suicide/self-harm by young Australians: regulatory warnings, contradictory advice, and long-term trends.Crossref | GoogleScholarGoogle Scholar | 32587531PubMed |
[32] Mangoni AA. Predicting and detecting adverse drug reactions in old age: Challenges and opportunities. Expert Opin Drug Metab Toxicol 2012; 8 527–30.
| Predicting and detecting adverse drug reactions in old age: Challenges and opportunities.Crossref | GoogleScholarGoogle Scholar | 22512705PubMed |
[33] Temple ME, Robinson RF, Miller JC, et al. Frequency and preventability of adverse drug reactions in paediatric patients. Drug Saf 2004; 27 819–29.
| Frequency and preventability of adverse drug reactions in paediatric patients.Crossref | GoogleScholarGoogle Scholar | 15350153PubMed |
[34] Dragioti E, Solmi M, Favaro A, et al. Association of antidepressant use with adverse health outcomes: a systematic umbrella review. JAMA Psychiatry 2019; 76 1241–55.
| Association of antidepressant use with adverse health outcomes: a systematic umbrella review.Crossref | GoogleScholarGoogle Scholar | 31577342PubMed |
[35] Kreijkamp-Kaspers S, McGuire T, Bedford S, et al. Your questions about complementary medicines answered. Aust Fam Physician 2015; 44 373–4.
| 26209986PubMed |
[36] Busato A, Dönges A, Herren S, et al. Health status and health care utilisation of patients in complementary and conventional primary care in Switzerland - an observational study. Fam Pract 2006; 23 116–24.
| Health status and health care utilisation of patients in complementary and conventional primary care in Switzerland - an observational study.Crossref | GoogleScholarGoogle Scholar | 16115833PubMed |
[37] Moses GM, McGuire TM. Drug interactions with complementary medicines. Aust Prescr 2010; 33 177–80.
| Drug interactions with complementary medicines.Crossref | GoogleScholarGoogle Scholar |
[38] van Geffen ECG, van Hulten R, Bouvy ML, et al. Characteristics and reasons associated with nonacceptance of selective serotonin-reuptake inhibitor treatment. Ann Pharmacother 2008; 42 218–25.
| Characteristics and reasons associated with nonacceptance of selective serotonin-reuptake inhibitor treatment.Crossref | GoogleScholarGoogle Scholar |
[39] Palleria C, Di Paolo A, Giofrè C, et al. Pharmacokinetic drug-drug interaction and their implication in clinical management. J Res Med Sci 2013; 18 601–10.
| 24516494PubMed |
[40] Hengen KM, Alpers GW. What’s the risk? Fearful individuals generally overestimate negative outcomes and they dread outcomes of specific events. Front Psychol 2019; 10 1676
| What’s the risk? Fearful individuals generally overestimate negative outcomes and they dread outcomes of specific events.Crossref | GoogleScholarGoogle Scholar | 31417450PubMed |
[41] Loewenstein GF, Weber EU, Hsee CK, et al. Risk as feelings. Psychol Bull 2001; 127 267–86.
| Risk as feelings.Crossref | GoogleScholarGoogle Scholar | 11316014PubMed |
[42] Australian Bureau of Statistics. Families in regional, rural and remote Australia 2006 census data. Canberra: Australian Government; 2010. Available at https://www.abs.gov.au/AUSSTATS/abs@.nsf/Lookup/1379.0.55.001Explanatory%20Notes12002-2006?OpenDocument [Accessed 7 December 2021]
[43] Pohjanoksa-Mäntylä M. Medicines information sources and services for consumers: A special focus on the internet and people with depression (dissertation). Helsinki: University of Helsinki; 2010. p. 142.
[44] Coman GJ, Burrows GD, Evans BJ. Telephone counselling in Australia: applications and considerations for use. Br J Guid Counc 2001; 29 247–58.
| Telephone counselling in Australia: applications and considerations for use.Crossref | GoogleScholarGoogle Scholar |
[45] Burgess N, Christensen H, Leach LS, et al. Mental health profile of callers to a telephone counselling service. J Telemed Telecare 2008; 14 42–7.
| Mental health profile of callers to a telephone counselling service.Crossref | GoogleScholarGoogle Scholar | 18318929PubMed |
[46] Arroll B, Goodyear-Smith F, Kerse N, et al. The prevalence of depression among Maori patients in Auckland general practice. J Prim Health Care 2009; 1 26
| 20690483PubMed |