The relationship between patient characteristics and the development of a multi-resistant healthcare-associated infection in a private South Australian hospital
L. S. Jarratt A B and E. R. Miller AA School of Population Health, The University of Adelaide, SA 5005, Australia.
B Corresponding author. Email: ljarratt@stand.org.au
Healthcare Infection 18(3) 94-101 https://doi.org/10.1071/HI13010
Submitted: 22 February 2013 Accepted: 16 April 2013 Published: 28 May 2013
Journal Compilation © Australasian College for Infection Prevention and Control 2013
Abstract
Background: The prevention of healthcare-associated infections (HAI) and the rise of multi-resistant organisms are significant public health issues. Infections caused by multi-resistant organisms (MRO) can have similar clinical manifestations to infections caused by non-multi-resistant organisms (non-MRO HAI) but antibiotic treatment options are more limited, which can result in treatment failure. This study aimed to reduce the incidence of MRO HAI in a specific South Australian hospital setting by identifying factors that are associated with MRO transmission.
Methods: Using a case-control design, we analysed data from 1017 adult patients who developed an HAI in the 9-year period from 2003 to 2011 in a private South Australian hospital. We compared risk factors in patients who developed MRO HAI (cases) with risk factors in patients who developed non-MRO HAI (controls). Data were collected from the hospital’s patient management database and individual medical records, and analysed using univariate and multivariate techniques.
Results: Independent predictors for the development of MRO HAI were the presence of an indwelling urinary catheter and renal disease. The development of a secondary infection was significantly more likely in MRO relative to non-MRO HAI, as was secondary bloodstream infection following a primary urinary tract infection.
Conclusion: All effective interventions for reducing MRO, specifically in UTI, should be implemented where feasible. Increased healthcare worker education on aseptic non-touch technique, and safe insertion and management of an IDC, particularly important in patients with underlying renal disease, could assist in decreasing the risk of MRO HAI in this setting.
Implications
-
Specifies risk factor for healthcare-associated MRO infection which can be used for risk management in hospitals.
-
Provides evidence to justify targeted interventions including insertion and management of indwelling catheters.
-
Demonstrates the need for future research to explore transmission mechanisms and patients’ comorbidities for specific HAIs.
Introduction
The prevention of healthcare-associated infections (HAI) and the rise of multi-resistant organisms (MRO) are issues of public health importance.1,3 Infections caused by MRO, including methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE) and multi-resistant Gram-negative organisms (MRGN), are more commonly seen in healthcare settings1 and are an increasing concern and challenge to healthcare provision.
In Australia, it is estimated that ~200 000 HAI occur annually.2 Morbidity and mortality are negative outcomes for patients as a result of acquisition of HAI. Infections caused by MRO can contribute to prolonged stays in hospital, increasing occupied bed days,4–11 increased hospital costs,4,8–10,12,13 intensive care treatment,12,14,15 antibiotic therapy,1,3 readmission to hospital,16,17 further surgery,8 severe adverse outcomes,5,8 and MRO related mortality.5,7,8,14,17–19
Increased financial costs to society and the individual can result following the development of HAI with subsequent complications.13 Immeasurable costs causing harm to patients include reduced time spent with family members,3 and pain and suffering, leading to a decrease in quality of life2,3 with physical, emotional and social changes.3,20,21 Diminished worker productivity with loss of income3,20 increases the burden to family members and society.
The development of MRO HAI is known to be associated with a range of patient characteristics such as severe underlying illness and varying comorbid conditions,1,4,6,8,12,19,22–24 older age,4,19,22,23 and pre-existing carriage of multi-resistant organisms.7,17 Hospital exposures have been demonstrated to contribute to the development of MRO HAI and include the presence of medical devices,1,11,18,22,25 surgery,1,12,26 intensive care,1,12,14,15 and longer lengths of stay in hospital.14,15,27
We investigated factors associated with MRO transmission, including hospital treatment and the underlying diseases of patients contributing to the manifestation of MRO HAI, in a private South Australian hospital setting. In identifying these factors, this study aimed to inform targeted prevention activity to reduce the incidence of MRO HAI in a specific South Australian hospital setting. Specific objectives included comparing the characteristics of patients who developed MRO HAI and non-MRO HAI, identifying associations between existing patient comorbidities and the development of MRO HAI and identifying patient related risk factors for MRO HAI.
Methods
In Australia, public healthcare facilities are funded by the government and provide a wide range of healthcare at little or no cost to the patient. Private healthcare facilities are funded by private health insurers supported by the independent contributions of members. Approximately 50% of Australians aged 15 years and over have private health insurance, including 47% of Australians with hospital cover.28
This study was conducted in a private hospital in metropolitan Adelaide, South Australia with more than 200 beds offering elective surgical and medical care. It has an 18 bed critical care unit which includes intensive care beds. The hospital offers a range of different services and medical specialties including general medicine; urology; orthopaedics; colorectal; oncology; vascular; plastic surgery; cardiology; gastroenterology; gynaecology; ear, nose and throat (ENT); neurology; and general surgery.
Using a case-control approach, we analysed data from patients aged at least18 years old who had acquired any HAI (MRO or non-MRO) during the period January 2003 to December 2011. Age below 18 years of age was the only exclusion criterion.
Cases were patients who developed an HAI caused by MRO including methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci and multi-resistant Gram-negative organisms. Controls were patients with an HAI caused by non-MRO. An HAI is here defined as any localised or systemic condition resulting from an infectious agent or toxin for which no evidence was apparent on admission to the acute care setting.29
Infection-control reports are compiled by infection-control staff at the time the infection is confirmed. Hardcopy reports are stored in the infection-control staff office and are used to generate electronic reports for hospital use. Reports dating from January 2003 to December 2011 were reviewed to identify patients who were previously reported as having an HAI. A data collection form was created to record patients’ dates of admission and discharge, lengths of stay in occupied bed days, demographics, medical speciality, types of infection, infecting organism, hospital exposures, complications and comorbidities present on admission.
Data were collected from a range of additional sources including the hospital’s patient management database, which records coding data based on the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, Australian Modification (ICD-10-a.m.). Patients’ medical records and laboratory reports were also reviewed. Unique patient identification numbers were used to link health information and de-identify patients’ details. These identifiers were destroyed at the time of analysis. A separate form for each episode of infection was used to record the data.
The medical records of patients who died, regardless of the cause of death, were archived offsite and were not accessible. While precise cause of death was not available, this outcome was categorised as ‘all cause mortality’.
As well as a range of demographic and health-related data, we collected data on comorbidities and medical speciality on admission. We also collected data on the type of infection including surgical-site infection (SSI), bloodstream infection (BSI), urinary tract infection (UTI), pneumonia, chest infection, skin or soft tissue infection, device-related infection, any medical devices used, MRO carrier status and the specific site of infection. Known patient carrier status was identified through the hospital’s patient management database and alert system or through MRO screening of patients with identified risk factors for MRO on admission. Information on surgical and non-surgical procedures performed, and intensive care treatment were also collected, as well as data on chemotherapy and radiotherapy treatments. Complications of the HAI were recorded including return to theatre, readmissions, development of a secondary infection by type of infection, and mortality.
Data analyses were performed using Stata software version 12 (Stata Corp, College Station, TX, USA). Chi-square, Mann–Whitney tests, and log binomial models formed part of the analysis. Odds ratios were calculated with 95% confidence intervals and all statistical tests were performed at the 0.05 α level. This project received formal approvals from the Medical Records Department and ethics committee of the private hospital concerned, and from the University of Adelaide Human Research Ethics Committee.
Results
During the study period, a total of 1017 HAI were identified in adult patients and were included in the study. This represented ~0.63% of all patients admitted to the hospital during the same period. Of these infections, a total of 103 MRO HAI were identified as cases and a total of 914 non-MRO HAI were identified as controls. Evidence available suggests private hospitals may have a lower infection rate than public hospitals and that patients treated in private hospitals have a lower risk of infection acquisition.30
Descriptive characteristics of cases and controls are presented in Table 1. Cases were slightly older than controls (median age 68 compared with 65 years). Similar proportions of males and females were noted in cases relative to controls, with ~56% males and 44% females in each group. Similar proportions of cases (78%) and controls (76%) were Australian born. The population was homogenous with respect to indigenous status with only one case overall was recorded as being Australian Aboriginal or Torres Strait Islander. More cases resided in ‘metropolitan’ Adelaide than controls (83% and 78% respectively). Metropolitan Adelaide is here defined as South Australian postcode residence 5000 to 5174 as outlined by the boundaries set out in the Atlas of South Australia.31 Of the cases, 7.8% were identified as a resident of an aged care facility (ACF) before admission compared with 1.2% of controls.

|
Cases spent longer in hospital relative to controls with a median occupied bed days of 14 compared to nine. The total comorbidities per category were higher in cases, with a median of four comorbid conditions compared with three in controls. A higher proportion of controls were discharged home following admission (79.7% versus 59.2%) rather than to an aged care facility (3% of controls versus 8.7% of cases). There were 261 patients who died in total, 37 of which were cases and 224 were controls. While it was not possible to access information on cause of death, all cause mortality for cases was estimated at 14.2% in cases and 25.5% in controls.
Relative to controls, a higher proportion of cases developed a UTI (30.1% versus 5.9%) or a skin or soft tissue infection (4.9% compared to 0.2%). A greater proportion of controls developed a surgical-site infection (65.9% compared to 55.3%). The most common secondary infection was a bloodstream infection which occurred in 16.5% of cases and 7.0% of controls.
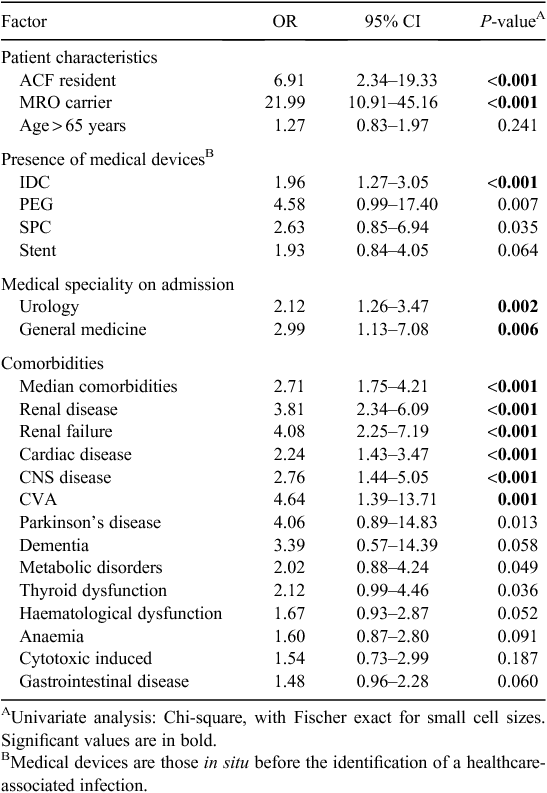
Selected findings of the univariate analyses of MRO HAI risk factors are presented in Table 2. As a dichotomous variable, age greater than 65 years was higher in cases relative to controls but this was not statistically significant. There were no differences in gender between cases and controls and cases were more likely to be a resident of an aged care facility before admission (OR 6.91, CI 2.34–19.33, P < 0.001) or a known carrier of a multi-resistant organism on admission (OR 21.99, CI 10.91–45.16, P < 0.001). There was an increased risk of death from all causes in cases relative to controls as outlined in Table 3.
|
|
Univariate analyses of hospital exposures included medical devices or treatments received by patients before the development of an HAI as outlined in Table 2. The presence of a medical device by device type demonstrated that patients with MRO HAI were significantly more likely to have had an indwelling urinary catheter (IDC) in situ (OR 1.96, CI 1.27–3.05, P = 0.001). The presence of a suprapubic catheter (SPC) and percutaneous endoscopic gastrostomy (PEG) tube both approached, but neither achieved statistical significance. The presence of any of a range of other medical devices was not significantly associated with MRO HAI.
Medical speciality appeared to be an important factor in the development of MRO HAI. Patient admissions based on medical speciality, including urology (OR 2.12, CI 1.26–3.47, P = 0.002) and general medicine (OR 2.99, CI 1.13–7.08, P = 0.006), were significantly associated with MRO HAI. Looking at underlying diseases, patients with MRO HAI were more likely to have underlying renal disease (OR 3.8, CI 2.34–6.09, P < 0.001), cardiac disease (OR 2.24, CI 1.43–3.47, P < 0.001) and CNS disease (OR 2.76, CI 1.44–5.05, P < 0.001).
The association of other treatments or procedures and MRO HAI approached, but did not reach, statistical significance on univariate analyses. This included patients who developed MRO HAI and underwent radiotherapy, chemotherapy, non-surgical diagnostic and/or treatment procedures, operative theatre procedures, or ICU care with or without mechanical ventilation. Due to small numbers, non-surgical procedures were not individually analysed, but collectively did not achieve statistical significance.
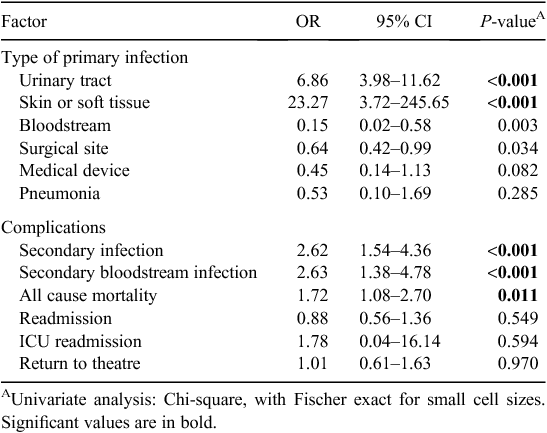
Findings of the univariate analyses of outcomes and/or negative sequelae of MRO HAI are presented in Table 3. Although only small numbers were observed, cases were more likely to develop a UTI (OR 6.86, CI 3.98–11.62, P < 0.001) or a skin or soft tissue infection (OR 23.27, CI 3.72–245.65, P < 0.001) during the period of hospitalisation. MRO HAI was associated with the development of a secondary infection which was related to the primary infection (OR 2.62, CI 1.54–4.36, P < 0.001), either by the same organism isolated at a different infection site or the development of an infection at another without an identified organism following the primary infection. The development of a secondary bloodstream infection (OR 2.63, CI 1.38–4.78, P < 0.001) was associated with MRO HAI. The development of a UTI was associated with a secondary bloodstream infection (OR 16.72, CI 9.51–29.17, P < 0.001). All cause mortality was more strongly associated with MRO HAI relative to controls (OR1.72, CI 1.08–2.70, P = 0.011). No significant associations were found between MRO HAI and return to theatre, ICU readmission and readmission to hospital.
Using the two-sample Wilcoxon rank-sum (Mann-Whitney) test, differences associated with the median total number of occupied bed days during admission were significant (P < 0.001), as was the total number of comorbidities noted in patients per category (P < 0.001) between cases and controls. Increasing median age was also significantly associated (P < 0.001). No differences were noted between the number of patient readmissions and the number of times a patient returned to theatre during the study period.
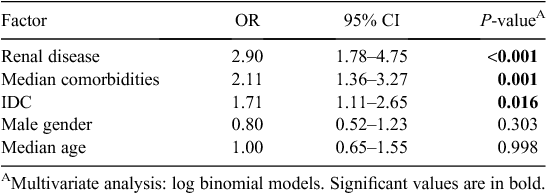
Variables associated with MRO HAI in univariate analysis at α level 0.05, as well as those known to be clinically important were included in multivariate models. Independent predictors for MRO HAI are presented in Table 4. These were renal disease (OR 2.90, CI 1.78–4.75, P < 0.001), median number of comorbidities (OR 2.11, CI 1.36–3.27, P = 0.001) and the presence of an IDC (OR 1.71, CI 1.11–2.65, P = 0.016). Adjusted sex and age were not significant in this model. In a model including IDC and risk factors, the presence of an IDC and male gender were independently associated with the development of a UTI when adjusted for age (data not shown). The median number of comorbidities was not significant in this model.
|
Discussion
Several studies have investigated the adverse outcomes and risk factors for MRO HAI.4–19 These studies have focussed on increased costs, readmission, length of stay, morbidity and mortality and explained these adverse events due to the presence of underlying patient conditions. In contrast, we used a case-control design to specifically investigate excess risk factors for MRO relative to non-MRO HAI. To our knowledge, this is the first study investigating MRO HAI conducted in an Australian setting of this kind. The findings are likely to be of increasing importance in Australia, which is experiencing sustained increases in the number of private hospitals nationwide that are covering an increased proportion of the population.32 Our study has confirmed the findings from other studies that age was associated with MRO HAI.4,19,22,23 This is also supported by the association of aged care facility residence with MRO HAI where most residents of such facilities are older. As reported elsewhere,7,11 known carriers of MRO on admission were more likely to develop MRO HAI. The present study found that cases spent longer total time in hospital and had an increased risk of death due to all causes, and this is consistent with previously published studies.4–11,14,17 Longer lengths of hospital stay can contribute to greater exposure to pathogens that may be persistent in the environment, opportunities for infection acquisition through hospital care and treatment,6 and require more complex management. Subsequent mortality is more likely to arise in patients in an already compromised state.3
Patients with MRO HAI had a greater number of comorbid illnesses than those with non-MRO HAI, which also confirms findings in current literature.4,6,8,9,12 In particular, renal, cardiac and CNS disease were univariately associated with the development of MRO HAI in our study.
In contrast to previously published findings,12,14,15,26 no significant univariate associations were found between MRO HAI and several medical procedure and treatment exposures. These included theatre, non-surgical diagnostic and/or treatment procedures, chemotherapy, radiotherapy and ICU with or without mechanical ventilation, although the relationship between radiotherapy and MRO HAI approached significance. Hospital-specific differences that might impact on patient susceptibility – such as the range of medical and surgical services offered, particular infection-control policies and differences in the demographic characteristics of patients – may help to explain the differences in findings. Patients returning to theatre and those requiring readmission to the hospital were also not associated with MRO HAI although these outcomes are described in the literature as negative outcomes of MRO HAI.8,16,17
Patients admitted to the medical specialties of urology and general medicine were more likely to develop MRO HAI. This is a particularly interesting finding considering infection-control procedures are likely to have been be similarly applied across the specialities within a single hospital.
The development of a UTI was univariately associated with MRO HAI. A UTI is a common infection in the community and also often a consequence of healthcare provision which can result in complicated infections where structural and functional urinary tract abnormalities and the presence of an IDC can be implicated.33 Elderly patients are more likely than younger patients to have a complicated UTI due to comorbid disease and the presence of an IDC.34 We also found the presence of an IDC and underlying renal disease in infected patients with UTI independently predicted MRO HAI. The insertion of an IDC into the bladder increases the susceptibility of a patient to develop a UTI where opportunistic organisms are introduced into the urinary tract including faecal contaminants, endogenous skin flora or transient bacteria from the hospital environment resulting in colonisation of the periurethral area.35 Given that antimicrobial resistance is evolving and increasing in many pathogens responsible for infections in healthcare facilities,1,3 the association of MRO HAI with the presence of an IDC could reflect the changing epidemiology of endogenous and transient bacteria introduced into the urinary tract in patients with more comorbid disease resulting in the manifestation of MRO HAI in our study.
The significance of IDC insertion with the development of a UTI is an important clinical predictor of MRO HAI in our study. This is especially true in regard to the development of a secondary infection following a primary infection which was a significant adverse outcome of MRO HAI. As others have reported,36 catheter-associated UTI can result in the development of a bloodstream infection. The most common secondary infection in our study was bloodstream infection which was univariately associated with both the development of MRO HAI and UTI. As bloodstream infections are systemic infections as opposed to a localised UTI, this results in further concerns for treatment and morbidity and mortality especially when the infecting organism is multi-resistant.
That our study was conducted in a single, private hospital where a relatively homogenous population of patients are admitted for elective care and treatment is an important limitation of our study, as it impacts on the generalisability of the findings. Nonetheless, it is reasonable to expect similar findings may be observed in other private hospitals offering similar medical services in Australia. There are nearly 600 private hospitals in Australia reporting ~3.5 million patient separations per year.32 Our confirmation of increased risk in urological patients underscores the need for further investigation in an Australian public hospital setting where important differences in demographic characteristics of patients and services offered might be expected. For instance, public hospitals have larger proportions of patient in older age groups and/or lower socioeconomic status relative to patients in private hospitals.37
We were unable to assess antibiotic exposure data in our study, including any antibiotic treatment patients received before the development of any HAI. Given the changing susceptibilities of microorganisms to antimicrobial treatments that have resulted from their widespread use,3 patient antibiotic exposure over time may influence both MRO acquisition and outcome of infection. While this is unlikely to have impacted on our consistent univariate and multivariate findings in relation to the presence of an IDC in patients with renal disease in the short term, it does remain a consideration in the longer term. Patients admitted to the private hospital in our study are likely to differ in their demographic and clinical profiles compared to Australian public hospital patients. Further differences in medical services and patient admission criteria, including emergency and trauma admissions, are likely to exist. More research should be considered to determine whether antibiotic exposure may influence the development of MRO HAI in both settings.
Medical records were not accessible for patients who had died during their hospital admission or died out of hospital. While this meant it was not possible to analyse cause-specific death, this represented only a very small proportion of the patients. Comorbidity and procedure codes were collected from the hospital’s patient management system but could not be verified by medical record review. All public and private hospitals in Australia code to ICD-10-a.m. Procedure and diagnosis codes are recorded relevant to each inpatient episode of care.38 Therefore, not all patient comorbidity data are recorded for each patient admission. This was not considered to be a major concern as each patient admission episode of care is coded and most patients had multiple admissions recorded on the hospital’s patient management database, allowing for comorbidity data collection.
Strengths of the study included the time period of nine years of data collection which allowed for a greater number of patients with HAI to be included for comparison. The study was also conducted across all hospital services without focusing on a particular clinical area such as ICU. This increased the size of the sample and allowed for the analysis of intra-population differences where infection-control practices could be expected to be very similar in line with hospital policy.
Conclusion
This study identified patient-related risk factors for the development of MRO HAI including renal, cardiac, CNS disease and MRO carriage on admission where the presence of an IDC and renal disease were independent predictors for MRO HAI.
Patient-related factors, including the underlying comorbidities of patients, would be difficult to modify in a hospital population, yet identification of these factors should help to promote increased awareness of the associated MRO risk. Regardless of patient-related characteristics, including those with renal disease, the insertion of an IDC is a practice which can be modified. Healthcare worker education on aseptic non-touch technique and safe insertion and management of an IDC through competency-based and theoretical assessment could assist in decreasing the risk of MRO HAI in this setting. Other practices including screening of patients for MRO carriage on admission with implementation of isolation precautions as required and patient education on invasive device management including an IDC will aid in preventing the incidence of MRO HAI.
The development of secondary bloodstream infection following UTI in patients with MRO HAI was the most common secondary infection associated with MRO HAI and UTI. Greater understanding of the mechanisms, patient factors and the epidemiology of gut colonisation of MRO common to these infections could assist in minimising this risk. The susceptibilities of microorganisms have been changing as a consequence of widespread antimicrobial use.3 Further research on the role of prior antibiotic exposure in the development, and consequences, of any HAI caused by specific microorganisms in healthcare settings is warranted.
The development of MRO HAI has important clinical outcomes for patients. The prevention, control and surveillance of HAI are important to reduce their incidence and subsequent morbidity and mortality to patients. Prevention of such infections is important to ensure good patient outcomes, maintain patient safety and decrease costs to patients, society and the health sector.
Conflicts of interest
None declared.
Funding
No funding has been received.
Acknowledgements
The authors would like to acknowledge the support provided by executive management of the hospital and the assistance provided by the medical records and coding department staff.
References
[1] Siegel J, Rhinehart E, Jackson M, Chiarello L. Management of multidrug-resistant organisms in healthcare Settings, 2006. Healthcare Infection Control Practices Advisory Committee. USA: Centres for Disease Control and Prevention; 2006. Available from: http://www.cdc.gov/hicpac/pdf/MDRO/MDROGuideline2006.pdf [verified April 2013].[2] Cruickshank M, Ferguson J, eds. Reducing harm to patients from health care associated infection: the role of surveillance. Australian Commission on Safety and Quality in Health Care (ACSQHC). Canberra: Biotext Pty Ltd; 2008.
[3] World Health Organization (WHO). The evolving threat of antimicrobial resistance. Options for action. Geneva: Switzerland; 2012. Available from: http://apps.who.int/iris/bitstream/10665/44812/1/9789241503181_eng.pdf [verified April 2013].
[4] Cosgrove S, Youlin Q, Kaye K, Harbarth S, Karchmer A, Carmeli Y. The impact of methicillin resistance in Staphylococcus aureus bacteraemia on Patient Outcomes: mortality, length of stay and hospital charges. Infect Control Hosp Epidemiol 2005; 26 166–74.
| The impact of methicillin resistance in Staphylococcus aureus bacteraemia on Patient Outcomes: mortality, length of stay and hospital charges.Crossref | GoogleScholarGoogle Scholar | 15756888PubMed |
[5] Cosgrove S. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis 2006; 42 S82–9.
| The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs.Crossref | GoogleScholarGoogle Scholar | 16355321PubMed |
[6] Graffunder E, Venezia R. Risk factors associated with nosocomial methicillin-resistant Staphylococcus aureus (MRSA) infection including previous use of antimicrobials. J Antimicrob Chemother 2002; 49 999–1005.
| Risk factors associated with nosocomial methicillin-resistant Staphylococcus aureus (MRSA) infection including previous use of antimicrobials.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XltVyrtr8%3D&md5=c9a0f2135bb4ecf9054dab9320b35fabCAS | 12039892PubMed |
[7] Shukla S, Nixon M, Acharya M, Korim M, Pandey R. Incidence of MRSA surgical – site infection in MRSA carriers in an orthopaedic trauma unit. J Bone Joint Surg Br 2009; 91-B 225–8.
| Incidence of MRSA surgical – site infection in MRSA carriers in an orthopaedic trauma unit.Crossref | GoogleScholarGoogle Scholar |
[8] Carmeli Y, Eliopoulos G, Mozaffari E, Samore M. Health and economic outcomes of vancomycin-resistant enterococci. Arch Intern Med 2002; 162 2223–8.
| Health and economic outcomes of vancomycin-resistant enterococci.Crossref | GoogleScholarGoogle Scholar | 12390066PubMed |
[9] Lautenbach E, Patel J, Bilker W, Edelstein P, Fishman N. Extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae: risk factors for infection and impact of resistance on outcomes. Clin Infect Dis 2001; 32 1162–71.
| Extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae: risk factors for infection and impact of resistance on outcomes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXjslKnsLo%3D&md5=5fc2a8373b1f2f2fd4f887ed28c848b2CAS | 11283805PubMed |
[10] Engemann J, Carmeli Y, Cosgrove S, Fowler V, Bronstein M, Trivette S, et al Adverse clinical and economic outcomes attributable to methicillin resistance among patients with Staphylococcus aureus surgical site infection. Clin Infect Dis 2003; 36 592–8.
| Adverse clinical and economic outcomes attributable to methicillin resistance among patients with Staphylococcus aureus surgical site infection.Crossref | GoogleScholarGoogle Scholar | 12594640PubMed |
[11] Huang S, Platt R. Risk of methicillin-resistant Staphylococcus aureus infection after previous infection or colonisation. Clin Infect Dis 2003; 36 281–5.
| Risk of methicillin-resistant Staphylococcus aureus infection after previous infection or colonisation.Crossref | GoogleScholarGoogle Scholar | 12539068PubMed |
[12] Roberts R, Hota B, Ahmad I, Scott D, Foster S, Abbasi F, et al Hospital and societal costs of antimicrobial-resistant infections in a Chicago teaching hospital: implications for antibiotic stewardship. Clin Infect Dis 2009; 49 1175–84.
| Hospital and societal costs of antimicrobial-resistant infections in a Chicago teaching hospital: implications for antibiotic stewardship.Crossref | GoogleScholarGoogle Scholar | 19739972PubMed |
[13] Beigi R, Bunge K, Song Y, Lee B. Epidemiologic and economic effect of methicillin-resistant Staphylococcus aureus in obstetrics. Obstet Gynecol 2009; 113 983–91.
| 19384112PubMed |
[14] Sunenshine R, Wright M, Maragakis L, Harris A, Song X, Hebden J, et al Multidrug-resistant Acinetobacter infection mortality rate and length of hospitalisation. Emerg Infect Dis 2007; 13 97–103.
| Multidrug-resistant Acinetobacter infection mortality rate and length of hospitalisation.Crossref | GoogleScholarGoogle Scholar | 17370521PubMed |
[15] Maragakis L, Perl T. Acinetobacter baumanii: epidemiology, antimicrobial resistance, and treatment options. Clin Infect Dis 2008; 46 1254–63.
| Acinetobacter baumanii: epidemiology, antimicrobial resistance, and treatment options.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXlsVCjs7w%3D&md5=8a459b39bb32160fcf40a9897e5f468eCAS | 18444865PubMed |
[16] Emerson C, Eyzaguirre L, Albrecht J, Comer A, Harris A, Furuno J. Healthcare associated infection and hospital readmission. Infect Control Hosp Epidemiol 2012; 33 539–44.
| Healthcare associated infection and hospital readmission.Crossref | GoogleScholarGoogle Scholar | 22561707PubMed |
[17] Huang S, Hinrichsen V, Datta R, Spurchise L, Miroshnik I, Nelson K, et al Methicillin-resistant Staphylococcus aureus infection and hospitalisation in high-risk patients in the year following detection. PLOS ONE 2011; 6 e24340
| Methicillin-resistant Staphylococcus aureus infection and hospitalisation in high-risk patients in the year following detection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXht1yqsrjE&md5=ea8139d63ded68d31977c1461d789509CAS | 21949707PubMed |
[18] Tumbarello M, Repetto E, Trecharichi E, Bernardini C, De Pascale G, Parisini A, et al Multidrug-resistant Pseudomonas aeruginosa bloodstream infections: risk factors and mortality. Epidemiol Infect 2011; 139 1740–9.
| Multidrug-resistant Pseudomonas aeruginosa bloodstream infections: risk factors and mortality.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC38zhsVKmtw%3D%3D&md5=f3073c0a9a6363992d22b156f9d7da35CAS | 21226988PubMed |
[19] Shurland S, Zhan M, Bradham D, Roghmann M. Comparison of mortality risk associated with bacteraemia due to methicillin-resistant and methicillin- susceptible Staphylococcus aureus. Infect Control Hosp Epidemiol 2007; 28 273–9.
| Comparison of mortality risk associated with bacteraemia due to methicillin-resistant and methicillin- susceptible Staphylococcus aureus.Crossref | GoogleScholarGoogle Scholar | 17326017PubMed |
[20] Andersson A, Bergh I, Karlsson J, Nilsson K. Patients’ experiences of acquiring a deep surgical site infection: An interview study. Am J Infect Control 2010; 38 711–7.
| Patients’ experiences of acquiring a deep surgical site infection: An interview study.Crossref | GoogleScholarGoogle Scholar | 21034980PubMed |
[21] Mutsonziwa G, Green J. Colonised and isolated: a qualitative metasynthesis of patients’ experiences of being infected with multiple drug resistant organisms and subsequent isolation. Healthc Infect 2011; 16 147–55.
| Colonised and isolated: a qualitative metasynthesis of patients’ experiences of being infected with multiple drug resistant organisms and subsequent isolation.Crossref | GoogleScholarGoogle Scholar |
[22] Safdar N, Maki D. The commonality of risk factors for nosocomial colonisation and infection with antimicrobial- resistant Staphylococcus aureus, Enterococcus, gram-negative bacilli, Clostridium difficile and Candida. Ann Intern Med 2002; 136 834–44.
| The commonality of risk factors for nosocomial colonisation and infection with antimicrobial- resistant Staphylococcus aureus, Enterococcus, gram-negative bacilli, Clostridium difficile and Candida.Crossref | GoogleScholarGoogle Scholar | 12044132PubMed |
[23] Hayden M. Insights into the epidemiology and control of infection with vancomycin- resistant enterococci. Clin Infect Dis 2000; 31 1058–65.
| Insights into the epidemiology and control of infection with vancomycin- resistant enterococci.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3M7htVagsg%3D%3D&md5=8037cc339381e929e0bc8a1be4882466CAS | 11049790PubMed |
[24] Lautenbach E, Bilker W, Brennan P. Enterococcal bacteraemia: Risk factors for vancomycin resistance and predictors of mortality. Infect Control Hosp Epidemiol 1999; 20 318–23.
| Enterococcal bacteraemia: Risk factors for vancomycin resistance and predictors of mortality.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1M3nslCmsg%3D%3D&md5=2a794e1f6dcbd1b5a5c2a2c1d04b7bc7CAS | 10349947PubMed |
[25] Pena C, Gudiol C, Tubau F, Saballs M, Pujol M, Dominguez M, et al Risk-factors for acquisition of extended-spectrum β lactamase-producing Escherichia coli among hospitalised patients. Clin Microbiol Infect 2006; 12 279–84.
| Risk-factors for acquisition of extended-spectrum β lactamase-producing Escherichia coli among hospitalised patients.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XjsFOrtbk%3D&md5=2929c135c1f9380bf3a7c1d6de247e98CAS | 16451416PubMed |
[26] Zaas A, Song X, Tucker P, Perl T. Risk factors for development of vancomycin-resistant enterococcal bloodstream infection in patients with cancer who are colonised with vancomycin-resistant enterococci. Clin Infect Dis 2002; 35 1139–46.
| Risk factors for development of vancomycin-resistant enterococcal bloodstream infection in patients with cancer who are colonised with vancomycin-resistant enterococci.Crossref | GoogleScholarGoogle Scholar | 12410472PubMed |
[27] Kim P, Harris A, Roghmann M, Morris J, Strinivasan A, Perencevich E. Epidemiological risk factors for isolation of ceftriaxone-resistant versus- susceptible Citrobacter freundii in hospitalised Patients. Antimicrob Agents Chemother 2003; 47 2882–7.
| Epidemiological risk factors for isolation of ceftriaxone-resistant versus- susceptible Citrobacter freundii in hospitalised Patients.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXntFegsb0%3D&md5=27919db5467ed9e1d31d139b80837344CAS | 12936989PubMed |
[28] Australian Bureau of Statistics. Private Health Insurance: A snapshot, 2004–05. Australian Bureau of Statistics; 2006. Available from: http://www.abs.gov.au/ausstats/abs@.nsf/mf/4815.0.55.001/ [verified April 2013].
[29] Definition of HAI and criteria for specific types of infections. USA: Centres for Disease Control (CDC); 2012. Available from: http://www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf [verified April 2013].
[30] Public and Private Hospitals: Productivity Commission Research Report. Melbourne, Victoria: Productivity Commission; 2009. Available from: http://www.pc.gov.au/projects/study/hospitals/report [verified April 2013].
[31] Government of South Australia. Atlas of South Australia. Metropolitan Adelaide Boundary [Internet]. Government of South Australia; 2006. Available from: http://www.atlas.sa.gov.au/go/resources/metropolitan-adelaide-boundary [verified April 2013].
[32] Australian Bureau of Statistics. Private Hospitals, Australia, 2010–2011. Australian Bureau of Statistics; 2012. Available from: http://www.abs.gov.au/AUSSTATS/abs@.nsf/Lookup/4390.0Main+Features12010-11?OpenDocument [verified April 2013].
[33] Pallett A, Hand K. Complicated urinary tract infections: practical solutions for the treatment of multiresistant gram-negative bacteria. J Antimicrob Chemother 2010; 65 iii25–33.
| Complicated urinary tract infections: practical solutions for the treatment of multiresistant gram-negative bacteria.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXht1GjsbzE&md5=f1f1537990a5fb7d20ab249d369ae008CAS | 20876625PubMed |
[34] Engel L. Multidrug- resistant gram negative bacteria: trends, risk factors, and treatments. Emerg Med; 2009. Available from: http://www.emedmag.com/PDF/041110018.pdf [verified April 2013].
[35] Jacobsen S, Stickler D, Mobley H, Shirtliff M. Complicated catheter-associated urinary tract infections due to Escherichia coli and Proteus mirabilis. Clin Microbiol Rev 2008; 21 26–59.
| Complicated catheter-associated urinary tract infections due to Escherichia coli and Proteus mirabilis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXitlGrtL8%3D&md5=e06b15578a703f01d96aa06046c1c526CAS | 18202436PubMed |
[36] Maki D, Tambyah P. Engineering out the risk for infection with urinary catheters. Emerg Infect Dis 2001; 7 342–7.
| Engineering out the risk for infection with urinary catheters.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3MzpsFSlsA%3D%3D&md5=b22d00f8e2c7260edc555fe22b2c2479CAS | 11294737PubMed |
[37] Australian Institute of Health and Welfare. Australian hospital statistics 2010–11. Health services series no. 43. Cat. no. HSE 117. Canberra: AIHW; 2012.
[38] Australian Government. Department of Health and Ageing. Casemix Classifications. Canberra: Australian Government; 2010. Available from: http://www.health.gov.au/internet/main/publishing.nsf/Content/Classificationshub.htm [verified April 2013].

