Resolving the taxonomy of the Antarctic feather star species complex Promachocrinus ‘kerguelensis’ (Echinodermata: Crinoidea)
Emily L. McLaughlin A B , Nerida G. Wilson
A B , Nerida G. Wilson  A C D and Greg W. Rouse
A C D and Greg W. Rouse  A *
A *
A Scripps Institution of Oceanography, University of California, San Diego, 9500 Gilman Drive, La Jolla, CA 92093, USA.
B The University of Alabama, Campus Box 870344, Tuscaloosa, AL 35487, USA.
C Research and Collections, Western Australian Museum, 49 Kew Street, Welshpool, WA 6106, Australia.
D School of Biological Sciences, University of Western Australia, 35 Stirling Highway, Crawley, WA 6009, Australia.
Invertebrate Systematics 37(7) 498-527 https://doi.org/10.1071/IS22057
Submitted: 2 November 2022 Accepted: 15 June 2023 Published: 14 July 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing.
Abstract
An increasing number of Antarctic invertebrate taxa have been revealed as cryptic species complexes following DNA-based assessments. This ultimately necessitates a morphological reassessment to find traits that will help identify these cryptic or pseudocryptic species without the need for sequencing every individual. This work concerns comatulid crinoid echinoderms long considered to represent a single, circum-Antarctic species, Promachocrinus kerguelensis. The first molecular studies sought to distinguish the diversity in the complex and understand the constituent species distributions but stopped short of formal taxonomic assessment. Here, we continued to increase sample representation around the Southern Ocean and sequenced the mitochondrial COI gene for all new specimens, and additional genes for a few representatives. We also elucidated previously unappreciated features, particularly body pigmentation and morphology of the centrodorsal ossicle in an attempt to diagnose some species morphologically and based on DNA data. The species complex within Promachocrinus is here resolved into P. kerguelensis Carpenter, 1879, P. vanhoeffenianus Minckert, 1905, P. joubini Vaney, 1910, P. mawsoni (Clark, 1937) comb. nov. (transferred from Florometra) and four previously unnamed species, P. fragarius sp. nov., P. unruhi sp. nov., P. uskglassi sp. nov. and P. wattsorum sp. nov. Although most species can be distinguished morphologically, several cannot be reliably separated without DNA data. All sequenced species are essentially circum-Antarctic, with the notable exception of P. wattsorum sp. nov. that is restricted to the Prince Edward Islands in the sub-Antarctic Indian Ocean and P. vanhoeffenianus that is only known from the type locality in the Davis Sea. The vast nature of the Antarctic and Southern Ocean ecosystem dictates large scale sampling to understand the full extent of the biodiversity.
ZooBank: urn:lsid:zoobank.org:pub:F871CDC8-973B-48CE-8A61-33658D4EB4B1
Keywords: Antarctica, Crinoidea, cryptic species, Echinodermata, feather star, phylogeny, species delineation, taxonomy.
Introduction
Molecular methods often reveal cryptic diversity in species previously described using morphology (Brandão et al. 2010; Weber et al. 2015; Berriman et al. 2018; González-Wevar et al. 2019). These revelations of additional diversity need to be followed up with taxonomic revision but a lack of available expertise means this may not occur as often as necessary (see p. 2016). In some cases, the newly revealed species are truly cryptic and cannot be differentiated with morphology (e.g. Halt et al. 2009). However, in most cases, a molecular framework assists a reassessment of character interpretation and subsequently these species can be considered pseudocryptic. Research and management programs rarely account for unnamed taxa, leading to an underrepresentation of diversity and this can have detrimental effects by skewing diversity estimates and conservation efforts (Adams et al. 2014; Nygren 2014; Delić et al. 2017; Larsen et al. 2017).
There is evidence of widespread cryptic biodiversity in Antarctica, as demonstrated in a span of taxa, including bivalves (González-Wevar et al. 2019), cephalopods (Allcock et al. 1997, 2011; Strugnell et al. 2008), pycnogonids (Krabbe et al. 2010; Arango et al. 2011; Dietz et al. 2015; Soler-Membrives et al. 2017), isopods (Held 2003; Raupach and Wägele 2006; Leese et al. 2008; Baird et al. 2011), nudibranchs (Moles et al. 2021; Maroni et al. 2022) and ophiuroids (Jossart et al. 2019). Antarctica and the Southern Ocean have increasingly been shown to be more biodiverse than originally speculated (Brandt et al. 2007; Chown et al. 2015; Brasier et al. 2016). This has raised questions about underlying drivers of biodiversity (Clarke and Crame 1992; di Prisco et al. 1998; Wilson et al. 2013).
The Southern Ocean has unique environmental conditions that may drive biodiversity and influence life history, dispersal and cladogenesis of local fauna (Clarke and Crame 1989; Clarke 2003; Brandt et al. 2007; Fraser et al. 2012). One historical influence, recurrent glaciation, may have caused a great deal of the speciation observed in the Southern Ocean (Clarke and Crame 1992). The cyclical nature of this glaciation that caused barriers to form between regions during glacial maxima with subsequent diminishing of these barriers during glacial minima, is considered to have influenced the evolutionary trajectory of resident populations. Though some areas on the continental shelf are considered to have remained ice-free during the last glacial maximum (LGM) (Bentley et al. 2014; Lau et al. 2020), there is no doubt that the expansion of ice changed the topography throughout the Southern Ocean and created barriers and widespread local extinctions of fauna (Anderson et al. 2002; Convey et al. 2009; Pollard and DeConto 2009; Fraser et al. 2012). During previous glacial maxima, large proportions of the continental slope became uninhabitable (Anderson et al. 2002; Thatje et al. 2005). This forced some Southern Ocean organisms to the deep sea and trapped others on the shelf, potentially forcing these to rely on isolated polynyas (Dayton and Oliver 1977; Thatje et al. 2008). These barriers isolated populations, creating bottlenecks and inhibiting gene flow between populations. As ice later retreated, new areas of seafloor were exposed, creating new environmental niches and decreasing barriers between regions, potentially allowing populations to expand once again. This intermittent connectivity of populations is considered to have promoted adaptive radiations (Clarke and Crame 1989, 1992). Additionally, as the movement of glaciers can cause devastation on communities residing on the shelf, this intermediate disturbance during periods of ice expansion has been hypothesised to be an additional driver for the high biodiversity of Antarctic benthic shelf fauna (Thatje et al. 2005).
One seemingly cryptic species complex endemic to the Southern Ocean is the crinoid taxon Promachocrinus ‘kerguelensis’ Carpenter, 1879. Members of Promachocrinus are large antedonid feather stars, with a wide depth distribution (20–2000 m) that comprise ~70% of crinoid occurrences observed in the Southern Ocean (Marr 1963; Speel and Dearborn 1983). At least some of these crinoids are highly fecund (McClintock and Pearse 1987), with floating eggs and lecithotrophic larvae that are estimated to persist in the water column for 2–3 months before settling to the sea floor (McClintock and Pearse 1987; Bosch and Pearse 1990). Dispersal of Promachocrinus larvae may therefore be heavily influenced by Antarctic currents.
Carpenter (1879) initially used the name Promachocrinus in a brief report and described three species, including P. kerguelensis. These were stated, without explanation, to be nomina nuda by Clark and Clark (1967). However, under article 12 of the current International Code on Zoological Nomenclature (The International Trust for Zoological Nomenclature 1999), Promachocrinus and P. kerguelensis appear to be valid, with authorship as Carpenter (1879). Carpenter (1888) presented a broader study and described the genus and three nominal species. The genus was diagnosed by having 10 rather than the 5 radials characteristic of most other feather stars. Two off the three originally erected species in Promachocrinus had 10 unbranched radials, whereas the third, the type species P. kerguelensis from the Southern Ocean, had radials branching once, resulting in 20 arms. The two taxa with 10 unbranched radials were P. abyssorum Carpenter, 1888 from the Southern Ocean, now accepted as Thaumatocrinus renovatus Carpenter, 1884 and P. naresi Carpenter, 1888 from Indonesia that is currently accepted as T. naresi (see Messing et al. 2023a).
Minckert (1905) and Vaney (1910) described two Antarctic species with 10 radials and 20 arms, Promachocrinus vanhoeffenianus Minckert, 1905 and P. joubini Vaney, 1910. Clark (1915) treated P. vanhoeffenianus and P. joubini as junior synonyms of P. kerguelensis. Though some discussion continued, the validity of synonymising these three names was not challenged over the ensuing decades (Mortensen 1918; John 1938; Clark and Clark 1967). Promachocrinus currently includes only P. kerguelensis, with two synonymised names, P. joubini and P. vanhoeffenianus (Messing et al. 2023a). Several other species, all with 10 arms and described from Antarctic waters, were proposed to be members of Promachocrinus by Clark (1913, 1915), though in subgenera: Promachocrinus (Anthometra) adriani (Bell, 1908), currently accepted as Anthometrina adriani, Promachocrinus (Solanometra) antarctica (Carpenter, 1888), currently accepted as Solanometra antarctica and Promachocrinus (Florometra) magellanica (Bell, 1882), currently accepted as Florometra magellanica (see Messing et al. 2023a).
The application of molecular methods has provided clarity that Promachocrinus kerguelensis contains multiple clades that are arguably separate species (Wilson et al. 2007; Hemery et al. 2012). Wilson et al. (2007) discovered six distinct clades likely representing at least five cryptic species in P. kerguelensis based on DNA sequence data. These were denoted as clades A through F. Hemery et al. (2012) identified a seventh clade (clade E2) after generating over 1000 sequences from seven additional regions. However, Hemery et al. (2012) additionally demonstrated that clade C from Wilson et al. (2007) might not be monophyletic. Later, Eléaume et al. (2014) suggested that although possessing only 5 radials, Florometra mawsoni Clark, 1937 might represent another lineage within Promachocrinus.
To formally revise this genus, sampling was increased to include newly sampled areas (Siple Coast, Diego Ramírez, Prince Edward Islands) and additional samples from previously sampled areas. Molecular and morphological analyses were used to delimit species and assess the distribution and phenotypic variation within a phylogenetic framework.
Materials and methods
Sample collection and morphology
Promachocrinus specimens were collected using trawls conducted by the R/V Yuzhmorgeologiya (Leg II of the 2008–2009 US Antarctic Marine Living Resources field season; hard-bottom snapper trawl) to the South Orkney Islands, R/V Nathaniel B. Palmer (NBP11-05 and NBP13-03) to the Scotia Arc, R/V Polarstern (cruise PS79 (ANT-XXVIII/4); 140′ bottom trawl) to the South Shetland Islands and Akademik Treshnikov (2016–2017 ACE circumnavigation cruise), the latter of which extended the sampling of sub-Antarctic Islands. Specimens from the Scotia Arc cruises were deposited at the Scripps Institution of Oceanography, Benthic Invertebrate Collection (SIO-BIC), La Jolla, CA, USA. Specimens from the ACE cruise were deposited in the Western Australian Museum (WAM), Perth, WA, Australia. Whole specimens and tissue subsamples were fixed in 95% ethanol. Supplementary Table S1 lists further details of collection localities, voucher numbers, and GenBank accession numbers (MT264097–MT264730).
Type specimens examined and the associated institution are as follows: USNM E378 (syntype, P. vanhoeffenianus) and USNM E3052 (paratype, F. mawsoni) from the Smithsonian National Museum of Natural History (USNM), Washington, DC, USA. Photographs of type specimen and the institution at which these were taken are as follows: AMS J5569 (holotype, F. mawsoni from the Australian Museum, Sydney (AMS), Australia; NHM 1888.11.9.12 (syntype, P. kerguelensis) from the Natural History Museum (NHM), London, UK; and MNHN-IE-2014-734 (holotype, P. joubini) from Muséum National D’Histoire Naturelle (MNHN), Paris, France. Morphological analysis was based on feather star features described in Messing et al. (2000).
DNA extraction, amplification and sequencing
Total genomic DNA was extracted from ethanol-preserved subsamples using the Zymo Research DNA-Tissue Miniprep kit or the Qiagen DNeasy blood and tissue kit, following the manufacturer’s protocol. Extracted DNA was stored at −20°C. Partial mitochondrial cytochrome c oxidase subunit I (COI) DNA sequences were obtained from specimens for species delimitation (Supplementary Table S1). Some additional specimens were also sequenced for mitochondrial cytochrome B (CytB), 16S rRNA (16S) and nuclear 28S rRNA (28S), and ITS1+5.8 rRNA+ITS2 (ITS) genes. All new sequences obtained were deposited in GenBank (Supplementary Tables S1 and S3). Amplifications were conducted using a PCR mixture of 12.5 µL of Apex 2.0× Taq Red DNA Polymerase Master Mix (Genesee Scientific), 1 µL of each of the appropriate forward and reverse primers (10 µM), 8.5 µL of ddH2O and 2 µL of eluted DNA or when amplification using this mixture failed, 12.5 µL of Conquest PCR 2.0× Master Mix 1 (Lamda Biotech) was substituted. DNA sequencing was completed with the following PCR primers and temperature profiles, performed in a thermal cycler (Eppendorf). Final PCR products were purified with the ExoSAP-IT (USB Affimetrix, Cleveland, OH, USA), and Sanger sequencing was performed by Eurofins Genomics (Louisville, KY, USA) or the Australian Genome Research Facility (Perth, WA, Australia). A 1000-bp barcode region of COI was amplified using the Helgen and Rouse (2006) primer pair FsCOI (5′-AGT CGT TGG TTG TTT TCT AC-3′) and COI3’R (5′-CAA TGA GTA AAA CCA GAA-3′) with the reaction protocol: 95°C for 180 s, (94°C for 45 s, 48°C for 45 s and 72°C for 60 s) × 35 cycles and 72°C for 300 s. Alternatively, some samples were sequenced for the shorter standard COI barcode using the primers LCO/HCO (Folmer et al. 1994) (5′-GGTCAACAAATCATAAAGATATTGG-3′) and (5′-CTAAACTTCAGGGTGACCAAAAAATCA-3′) under similar conditions. Up to 630 bp of CytB, 720 bp of 28S, 766 bp of ITS and 630 bp of 16S were amplified using published primer pairs and reaction protocols (Hemery et al. 2012). Consensus sequences were created using De Novo Assembly on Geneious (ver. 11.0.5, see www.geneious.com; Kearse et al. 2012) with default settings.
Species delimitation
For assessing species delimitation, newly generated Promachocrinus COI sequences (n = 634, Supplementary Table S1), along with published COI sequences (Supplementary Table S2) from Wilson et al. (2007; 57 unique sequences), Lanterbecq et al. (2010, 1 sequence), Hemery et al. (2012; 1307 sequences) and Summers et al. (2014, 5 sequences) were gathered. Additionally, 160 Promachocrinus mawsoni comb. nov. COI sequences (56 newly generated for this study and 104 from GenBank) were included. One of the P. mawsoni comb. nov. sequences from GenBank, as Florometra mawsoni, was taken from Hemery et al. (2013), and the other 103 have not been associated with a publication. In total, 62 of these 103 unpublished sequences are listed as Florometra mawsoni and 41 were incorrectly identified as Solanometra antarctica (Carpenter, 1880) on GenBank (see Supplementary Table S2 sequences marked with an asterisk, *). The entire 2133-sequence COI file is available in the Supplementary Material (see File S1).
A Maximum likelihood (ML) analysis was conducted on a COI dataset of the unique haplotypes (826 ingroup sequences), aligned using Muscle (ver. 3.6, see http://www.drive5.com/muscle/; Edgar 2004) with default settings. The COI file is available in the Supplementary Material (see Files S1–S3). The data were analysed under the HKY+I+Γ model selected with AIC and BIC using ModelTest-NG (ver. 0.1.7, https://github.com/ddarriba/modeltest/; Darriba et al. 2020). The ML analysis was performed using RaxML-NG (ver. 1.1.0, see https://github.com/amkozlov/raxml-ng; Kozlov et al. 2019) implemented with raxmlGUI (ver. 2.1.10, see https://antonellilab.github.io/raxmlGUI/; Edler et al. 2021) with 50 random addition searches and using a COI sequence from Notocrinus virilis Mortenson, 1918 to root the results. Node support was assessed using the transfer bootstrapping expectation option (Lemoine et al. 2018) with 1000 pseudoreplicates. Species delimitation was assessed for COI data using Assemble Species by Automatic Partitioning (ASAP) (Puillandre et al. 2021). The COI dataset of unique haplotypes was analysed using the ASAP webserver (see https://bioinfo.mnhn.fr/abi/public/asap/) implementing uncorrected distances, and the models Jukes–Cantor (JC69) and Kimura (K80) TS/TV (=2). Two of our newly generated sequences, MT264193 P. kerguelensis s.s. and MT264558 P. fragarius sp. nov., were excluded from the ASAP analysis since these were too short to overlap with some previously published sequences and hence provide any distances. Species delimitation was also assessed using the multi-rate Poisson Tree Processes method (Kapli et al. 2017). This was implemented at the mPTP webserver (see https://mptp.h-its.org/#/tree) with default settings after uploading the COI tree generated in RAxML-NG and designating Notocrinus virilis as the outgroup and excluding it.
Phylogenetic analysis
For a more robust phylogenetic analysis of Promachocrinus, a subset of previously used terminals from Hemery et al. (2012) was supplemented with two terminals each of F. mawsoni and a newly recovered species from our sampling. Simple protein coding sequences (COI, CytB) were aligned in Muscle and those with loop regions (16S, 28S and ITS) were aligned with MAFFT (ver. 7.505, see https://mafft.cbrc.jp/alignment/software/; Katoh and Standley 2013). Aligned partitions were subsequently concatenated using Sequence Matrix (ver. 1.8.0_361, see https://github.com/gaurav/taxondna/releases/tag/1.8; Vaidya et al. 2011), resulting in an alignment of 3883 base pairs. Notocrinus virilis Mortensen, 1918 and Anthometrina adriani (Bell, 1908) were chosen as the most appropriate outgroups based on previous phylogenetic results (Hemery et al. 2013). This data set was partitioned by gene and models selected by ModelTest-NG before applying Maximum likelihood analysis (50 random addition searches) with RAxML-NG. Models used were TVM+I+Γ (COI), TPM2uf+I+Γ (CytB and 16S) and TIM2+I (28S) TPM3uf (ITS). Node support was assessed using the ‘thorough’ bootstrapping (with 1000 pseudoreplicates). A Bayesian inference (BI) analysis of the concatenated data, partitioned by gene, was conducted using MrBayes (ver. 3.2.7a, see https://github.com/NBISweden/MrBayes/; Ronquist and Huelsenbeck 2003). Models used for the partitions were adjusted from those used in RAxML (since these are not available in MrBayes) to GTR+I+Γ (COI, 16S, CytB), GTR+I (28S, ITS). Default priors in MrBayes were used and data partitions were unlinked for parameter estimations. Two iterations of four Markov chain Monte Carlo (MCMC) were run for 50 million generations, sampling every 1000 generations. A majority rule consensus tree was made from the trees remaining after discarding 25% of trees as burn-in, after checking with Tracer (ver. 1.7.1, see https://github.com/beast-dev/tracer/releases/tag/v1.7.1; Rambaut et al. 2018). A Maximum parsimony (MP) analysis was also conducted with PAUP* (ver. 4.0a166, see http://phylosolutions.com/paup-test/; Swofford 2002), using heuristic searches with the tree-bisection-reconnection (TBR) branch-swapping algorithm and stepwise addition, with all characters unordered and equally weighted. Support values were determined using 100 jackknife replicates each with 100 random addition searches and heuristic search with tree-bisection-reconnection.
Taxonomic order
In the taxonomy section the following order is used for the treatment of taxa within Promachocrinus: P. kerguelensis as type species, followed by the four new species P. fragarius sp. nov., P. unruhi sp. nov., P. uskglassi sp. nov. and P. wattsorum sp. nov., the 10-armed P. mawsoni comb. nov. and the species for which no molecular data were available but that we accept as valid, P. vanhoeffenianus.
Results
Species delimitation and phylogeny
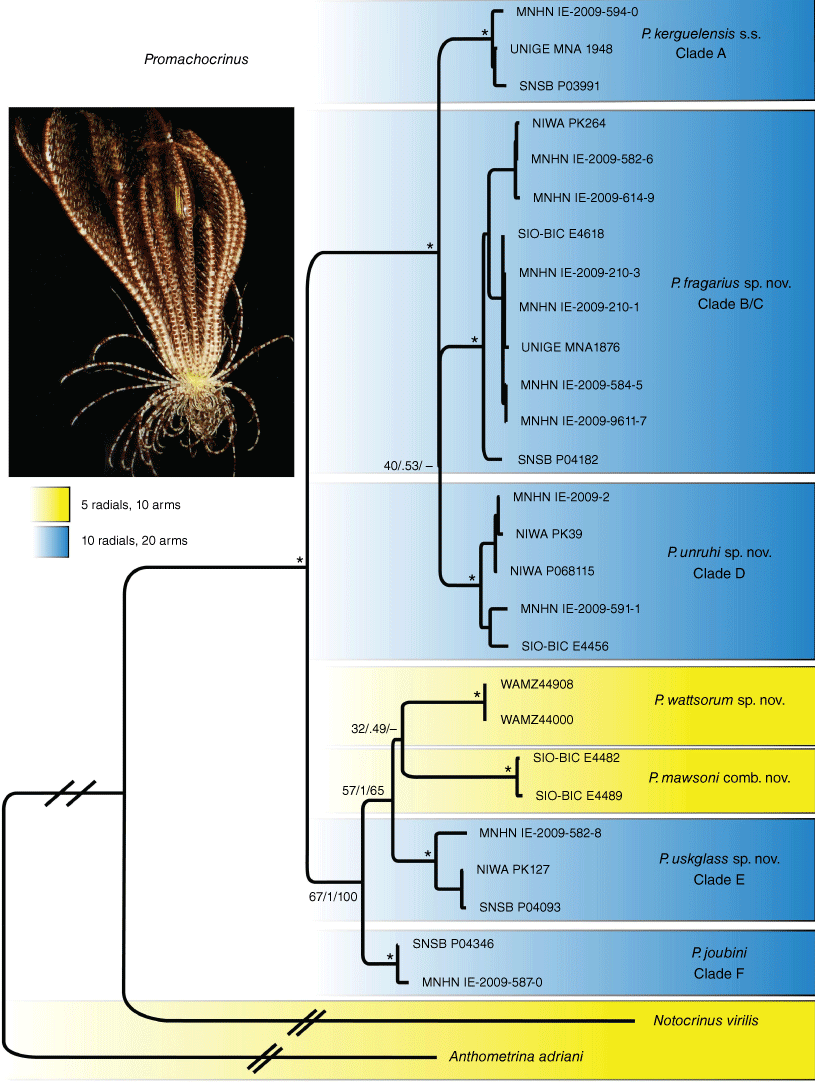
The ML analysis (Fig. 1) of unique COI sequences recovered the previously reported (Wilson et al. 2007, Hemery et al. 2012) clades (A–F) and one novel clade, P. wattsorum sp. nov.thatcomprised four new COI sequences from the sub-Antarctic Prince Edward Islands, a previously unsampled locality. This new species was the sister group to all other Promachocrinus species. Most major clades regarded as species had moderate support. The name Promachocrinus kerguelensis is restricted to Clade A for reasons explained below. Promachocrinus fragarius sp. nov. (Clades B + C) also formed three subclades as previously reported, one of which was sister to clade B, making Clade C paraphyletic. Promachocrinus uskglassi sp. nov. (clade E) contained two subclades (E1 and E2). Clade F is referred to the previously described species P. joubini. Promachocrinus mawsoni comb. nov. formed a well-supported clade that was nested well within Promachocrinus, as sister taxon to P. uskglassi sp. nov. (clade E). The ASAP analysis recovered the species partitions as outlined above. mPTP found the same partitions as ASAP but also showed the two well-supported subclades (E1 and E2) of clade E (Promachocrinus uskglassi sp. nov.) as species (Fig. 1). We adopt a conservative approach here and treat Clade E as one species, P. uskglassi sp. nov. Using these delimitations (see Fig. 1), we accepted seven species-level taxa of Promachocrinus and P. vanhoeffenianus for which morphological evidence was available (see below). The three phylogenetic analyses (ML, BI and MP) on the multigene data set all recovered generally congruent tree topologies (Fig. 2). These differed notably from the COI-only data (Fig. 1) in that the ML and BI analyses placed P. mawsoni comb. nov. as sister to P. wattsorum sp. nov., creating a clade of crinoids with only 5 radials. However, the support for this topology was very low (Fig. 2). The MP analysis also placed P. wattsorum sp. nov. nested well within Promachocrinus as the sister group to P. uskglassi sp. nov. (Clade E), though also with low support. All analyses placed P. joubini (Clade F) as sister taxon to the clade comprising P. uskglassi sp. nov. (clade E), P. wattsorum sp. nov. and P. mawsoni comb. nov. Both BI and MP analyses gave good support for this relationship, however the bootstrap support from ML was low (Fig. 2). In all analyses, P. kerguelensis sensu stricto (s.s., Clade A), P. fragarius sp. nov. (Clade B + C) and P. unruhi sp. nov. (clade D) formed well-supported clades, though relationships among the three varied depending on the analysis and none were well supported. The nesting of the 5-rayed P. mawsoni comb. nov. as sister to P. wattsorum sp. nov. (Fig. 2) in a grade of 10-rayed Promachocrinus suggests, on a most parsimonious reconstruction, that the 5-rayed condition is a reversal to the outgroup condition observed in Antedonidae. This also suggests that having 10 rays and 20 arms is the plesiomorphic condition for Promachocrinus.
Maximum likelihood (ML) tree generated with RaxML-NG using all unique Promachocrinus COI sequences available from GenBank, along with new sampling, totalling 826 sequences, rooted with Notocrinus virilis (outgroup not shown). Secondary clade names, Clade A–F, refer to previous phylogenetic studies of Promachocrinus (Wilson et al. 2007; Hemery et al. 2012; Eléaume et al. 2014). The variation within each species is collapsed. Node values are transfer bootstrap expectation scores. Summary of species delimitations using ASAP and mPTP are shown on the right-hand side. Species names are applied using the most conservative species delimitation therefore the splitting of P. uskglassi sp. nov. inferred by mPTP is ignored.
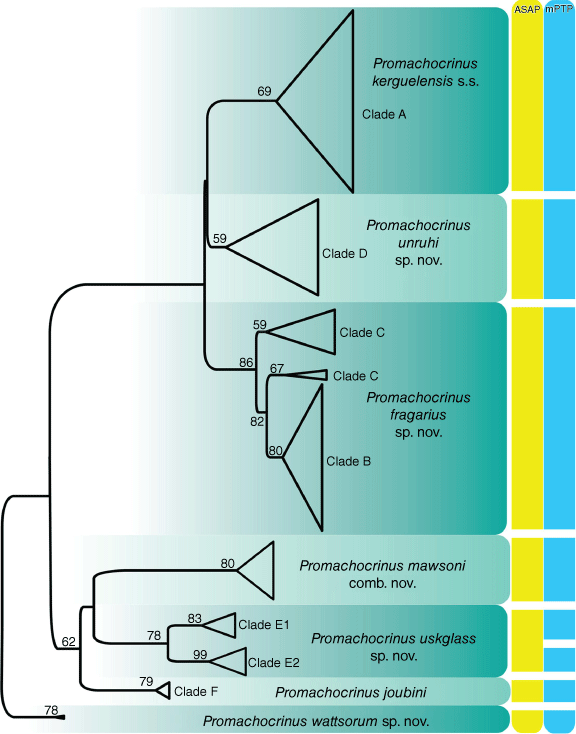
Maximum likelihood (ML) tree from concatenated five-gene dataset (COI, 16S, 28S, ITS and CytB) with Anthometrina adriani and Notocrinus virilis as outgroups. Values near nodes refer to bootstrap or posterior probability scores, respectively supporting Maximum likelihood (ML) and Bayesian inference (BI), or Maximum parsimony (MP). Asterisks indicate nodes with >90% bootstrap support and >0.99 posterior probability. A dash is given for nodes not recovered from the MP analysis. Blue boxes around clades indicate 10 radials, yellow boxes around clades indicate 5 radials. The nesting of the 5-radial clade (P. mawsoni comb. nov. and P. wattsorum sp. nov.) inside a grade of the other Promachocrinus species suggests that the 10-ray state represents the plesiomorphic condition for Promachocrinus. Promachocrinus mawsoni comb. nov. and P. wattsorum sp. nov. would therefore appear to show a reversal to the 5-radial state that occurs in the outgroups. The photo is of a specimen of Promachocrinus kerguelensis s.s. from South Georgia (Scotia Arc) (SIO-BIC E6340).
Biogeography and depth distributions
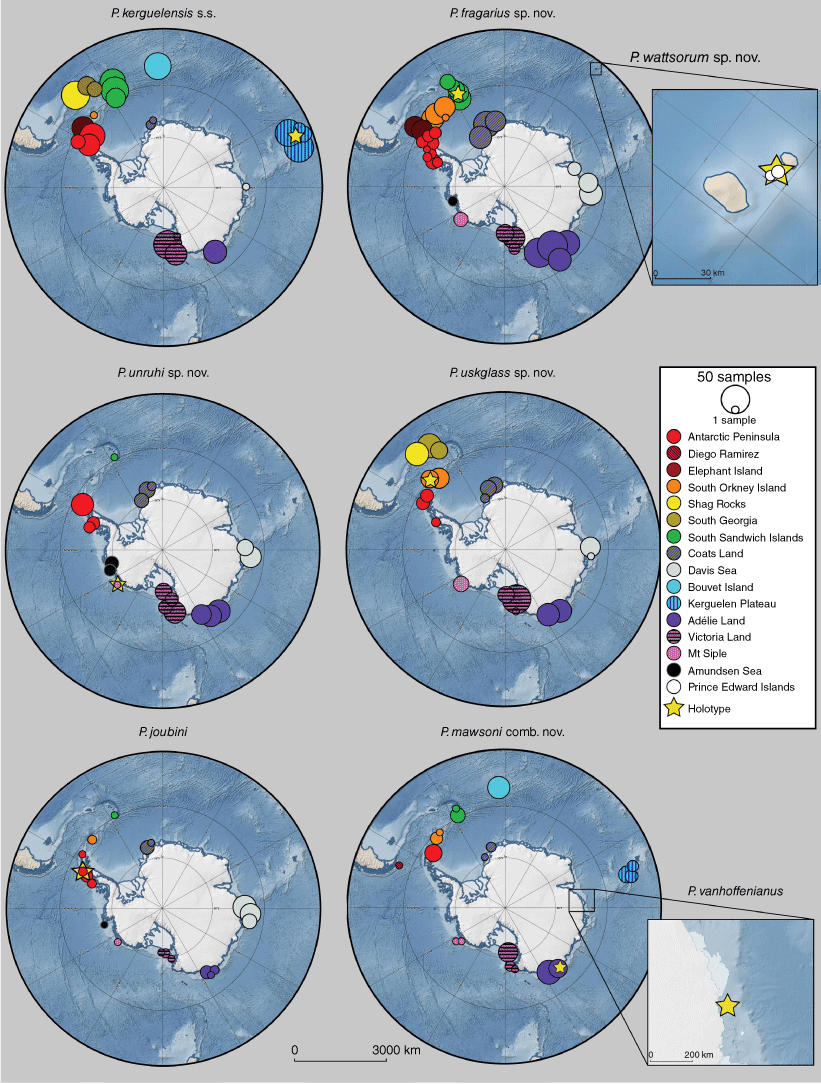
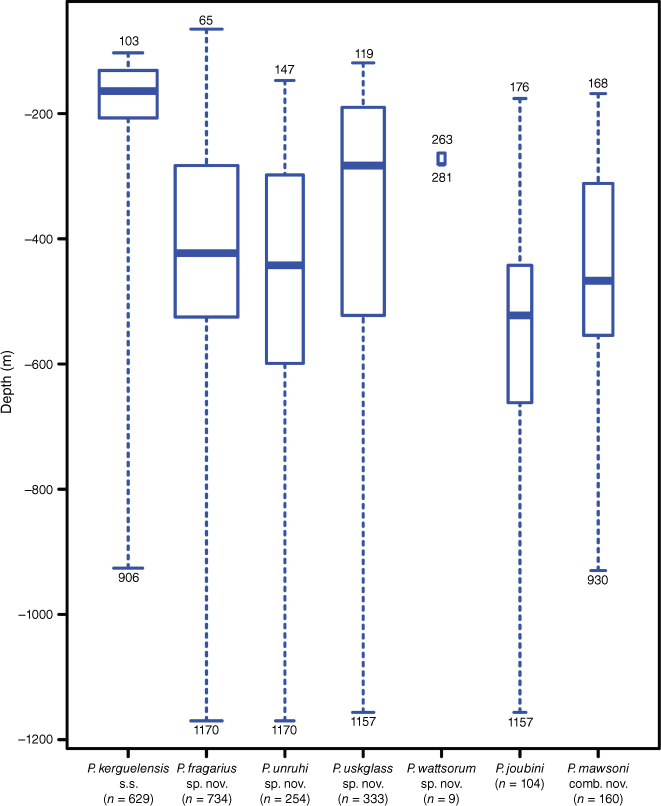
All Promachocrinus species, except for P. wattsorum sp. nov. and P. vanhoeffenianus, were demonstrated to have a wide-ranging distribution throughout the Southern Ocean (Fig. 3). Distribution overlap was common between species, with 4 of the 16 regions being home to all six widely distributed species. However, at each region, different proportions of each species were found. Around the Scotia Arc, higher proportions of P. kerguelensis s.s. and P. fragarius sp. nov. were sampled than for other clades. Only one P. kerguelensis s.s. specimen was sampled for the Davis Sea, whereas P. joubini and P. fragarius sp. nov. were relatively common. Only P. kerguelensis s.s. (Clade A) and P. mawsoni comb. nov. occurred on the Kerguelen Plateau (the type locality for P. kerguelensis) and Bouvet Island. Promachocrinus mawsoni comb. nov. was the only species sampled from near the tip of South America, off the Diego Ramírez Islands. Promachocrinus wattsorum sp. nov. was the only species found on the Prince Edward Islands. The type locality for P. vanhoeffenianus (Davis Sea) is shown in Fig. 3. Five other species of Promachocrinus were recovered from the Davis Sea but no specimens could be attributed to P. vanhoeffenianus. Bathymetric distributions overlapped and all species showed a similar mean sampled depth and maximum depth (Fig. 4). The exception to this is P. wattsorum that was only collected from less than a 300-m depth.
Sampling frequency and geographical distribution for each Promachocrinus clade, with size relative to the number of samples. Holotype locality for each is designated with a yellow asterisk. Base maps created in Quantarctica (Matsuoka et al. 2021) (see https://www.npolar.no/en/quantarctica/).
Comparison of sampled depth (m) for each clade using box and whisker plots. The box spans the interquartile range, with the inner line indicating the median measurement. Whiskers extend to the full range of what was sampled for each clade. The width of each box is proportional to the number of data points.
Taxonomy
Genus Promachocrinus Carpenter, 1879
Heliometrinae usually with 10 radials and 20 arms; 5 radials and 10 arms occur in some taxa. Centrodorsal rounded to conical. Arms rounded dorsally; brachials with spinous distal ends. Pinnule P1, similar to P2, with 40–50 segments; longest slightly broader than long.
Type species: Promachocrinus kerguelensis Carpenter, 1879
Promachocrinus vanhoeffenianus Minckert, 1905; P. joubini Vaney, 1910; Promachocrinus mawsoni (Clark, 1937) comb. nov.; Promachocrinus fragarius sp. nov.; Promachocrinus unruhi sp. nov., Promachocrinus uskglassi sp. nov.; and Promachocrinus wattsorum sp. nov.
65–1170 m based on this study (Fig. 4).
Circum-Antarctic (Fig. 3).
This diagnosis is amended from Clark and Clark (1967) to reflect the morphological variation present within this clade. Two species now included in Promachocrinus, P. mawsoni comb. nov. and P. wattsorum sp. nov., have 5 radials and 10 arms. The other six accepted species have 10 radials and 20 arms.
Promachocrinus kerguelensis Carpenter, 1879 sensu stricto
Promachocrinus kerguelensis – Carpenter (1879)
Promachocrinus kerguelensis – Carpenter (1888)
Promachocrinus kerguelensis – Clark and Clark (1967)
Clade A – Wilson et al. (2007)
Clade A – Hemery et al. (2012)
Haplogroup A– Eléaume et al. (2014)
Syntypes. NHM 1888.11.9.132, larger of the two specimens collected from Christmas Harbour (now Baie de l’Oiseau), Kerguelen Island, −48.685°, 69.054°; Challenger expedition, station number 149H, depth 232 m; NHM 1888.11.9.133, smaller of the two specimens.
Additional examined material. SIO-BIC E4807 (GenBank COI accession number = MT264165) from the South Georgia Islands at −55.081°, −35.173°, depth 222 m; SIO-BIC E4808 (GenBank COI accession number MT264154) from the South Sandwich Islands at −59.394°, −27.323°, depth 188 m; SIO-BIC E4822 (GenBank COI accession number MT264720) from the South Georgia islands at −53.801°, −37.219°, depth 145 m; SIO-BIC E4838 (GenBank COI accession number MT264182) from the South Georgia Islands at −53.517°, −41.629°, depth 135 m; SIO-BIC E4844 (GenBank COI accession number MT264211) from Shag Rocks at −53.534°, −41.634, depth 132 m; SIO-BIC E4849 (GenBank COI accession number MT264169) from the South Georgia Islands at −55.08°, −35.173, depth 222 m; SIO-BIC E4852 (GenBank COI accession number MT264194) from Shag Rocks at −53.424°, −42.018°, depth 149 m; SIO-BIC E4874 (GenBank COI accession number MT264222) and SIO-BIC E4878 (GenBank COI accession number MT264281) from Elephant Island at −61.339°, −55.625°; depth 143 m; SIO-BIC E4880 (GenBank COI accession number MT264251) from Elephant Island at −62.753°, −57.322°, depth 292 m; SIO-BIC E4896 (GenBank COI accession number MT264224) from Elephant Island at −63.339°, −55.625°, depth 143 m; SIO-BIC E4901 (GenBank COI accession number MT264234) from Elephant Island at −61.276°, −55.777°, depth 149 m; SIO-BIC E4907 (GenBank COI accession number MT264276) from Elephant Island at −63.325°, −59.625°, depth 197 m; SIO-BIC E4908 (MT264296) from the South Sandwich Islands at −59.394°, −27.327°, depth 130 m; SIO-BIC E4915 (GenBank COI accession number MT264243) from the Bransfield Strait at −62.872°, −57.1924°, depth 247 m; SIO-BIC E4931 (GenBank COI accession number MT264290) from Shag Rocks at −53.424°, −42.018°, depth 149 m; SIO-BIC E4936 (GenBank COI accession number MT264292) from Shag Rocks at −60.127°, −34.903°, depth 128 m, SIO-BIC E4941 (GenBank COI accession number MT264198), SIO-BIC E4943 (GenBank COI accession number MT264197), SIO-BIC E4945 (GenBank COI accession number MT264311), SIO-BIC E4949 (GenBank COI accession number MT264199), and SIO-BIC E4957 (GenBank COI accession number MT264393) from Shag Rocks at −53.424°, −42.018, depth 149; SIO-BIC E4965 (GenBank COI accession number MT264192) from Shag Rocks at −53.529°, −41.634°, depth 128 m; SIO-BIC E4966 (GenBank COI accession number MT264312) from Shag Rocks at −53.424°, −42.018°, depth 149 m; SIO-BIC E4968 (GenBank COI accession number MT264214) from Shag Rocks at −53.534°, −41.634°, depth 132 m; SIO-BIC E4971 (GenBank COI accession number MT264204) from Shag Rocks at −53.529°, −41.634°, depth 128 m; SIO-BIC E4973 (GenBank COI accession number MT264196) from Shag Rocks at −53.424°, −42.018°, depth 149 m; SIO-BIC E4975 (GenBank COI accession number MT264203) from Shag Rocks at −53.529°, −41.634°, depth 128 m; SIO-BIC E4976 (GenBank COI accession number MT264183) and SIO-BIC E4977 (GenBank COI accession number MT264185) from Shag Rocks at −53.517, −41.629, depth 136 m; SIO-BIC E4978 (GenBank COI accession number MT264190) and SIO-BIC E4979 (GenBank COI accession number MT264207) from Shag Rocks at −53.529°, −41.634°, depth 128 m; SIO-BIC E4980 (GenBank COI accession number MT264213) from Shag Rocks at −53.534°, −41.634°, depth 132 m; SIO-BIC E4981 (GenBank COI accession number MT264187) from Shag Rocks at −53.529°, −41.634°, depth 128 m; SIO-BIC E4985 (GenBank COI accession number MT264212) from Shag Rocks −53.534°, −41.634°, depth 132 m; SIO-BIC E4993 (GenBank COI accession number MT264205) from Shag Rocks at −53.529°, −41.634°, depth 128 m; SIO-BIC E5005 (GenBank COI accession number MT264155) from the South Sandwich Islands at −59.394°, −27.323°, depth 188 m. For other records and GenBank accession numbers see Supplementary Tables S1 and S2.
Radials 10. Arms 20, with dark pigmented spots, 1–2 mm in diameter scattered along arms, sometimes lacking. Centrodorsal rounded to rounded conical. Cirral sockets rounded, extend to ~1 mm from aboral pole. Cirri up to 4 cm long with 40 cirrals. Proximal and distal three cirrals have length half that of width, other cirrals often reasonably uniform, usually only as long as wide, sometimes two or three times the width; penultimate cirral with small opposing spine, terminal claw tends to be hooked.
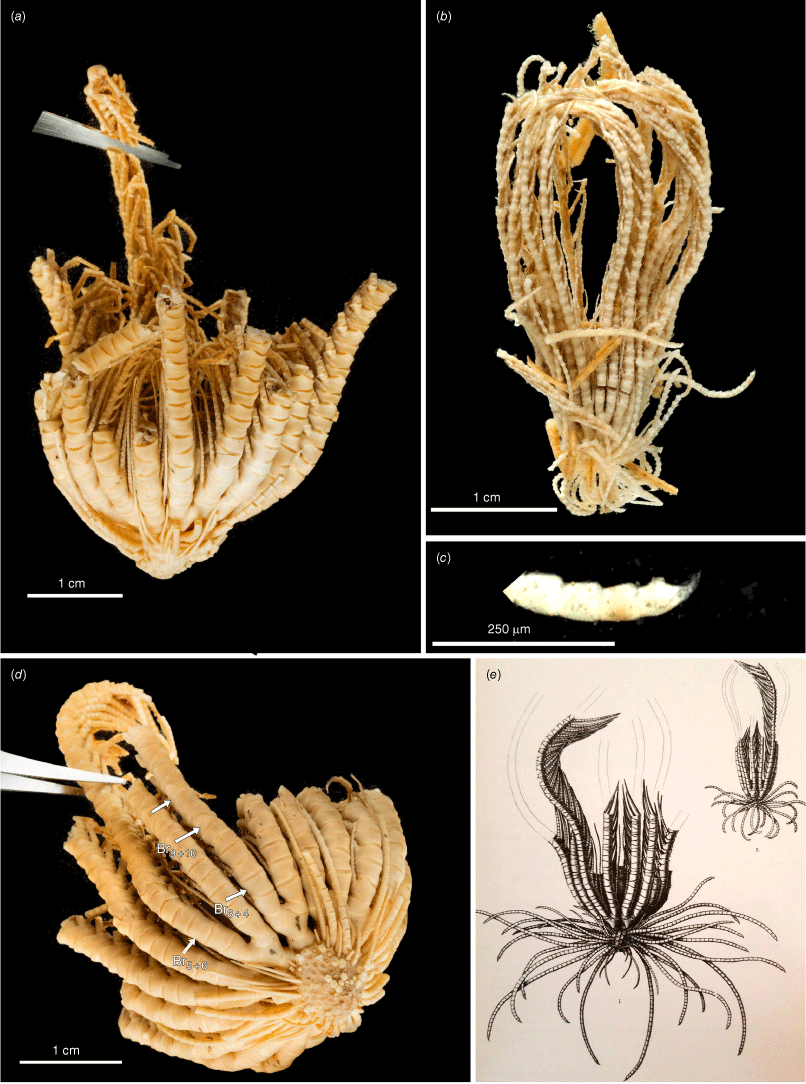
Larger syntype (Fig. 5a, d), smaller syntype (Fig. 5b, c). Centrodorsal rounded on large syntype, length 7 mm, width 9 mm (Fig. 5a, d); centrodorsal obscured by cirri in smaller syntype (Fig. 5b). Lack of notable pigment pattern on either syntypes in present state or as drawn (Fig. 5e) in Carpenter (1888). Cirri of larger syntype covering full centrodorsal in columns of 8–10 rows. Cirral sockets near centrodorsal pole rounded, diameter 0.4 mm; towards arms, sockets larger, diameter ~0.7 mm (Fig. 5a, d). Cirri broken on larger syntype, though original figure (Fig. 5e) shows some complete. Smaller syntype with intact cirri bearing terminal claw and small opposing spine (Fig. 5c). Cirri widths vary from 0.3 to 0.9 mm; C1 length at 0.3–0.7 mm, C2 length 0.4–0.9 mm, C5+ ~0.9–1.4 mm. Larger syntype IBr1 length 0.9 mm; axil length 3.0 mm and width at narrowest point 2 mm; Br1 length ~1.2 mm; Br2 length 2.3 mm. First syzygy at Br3+4, width 2.8 mm. Second syzygy usually at Br9+10, but one arm at Br5+6 (Fig. 5d). Larger syntype length, centrodorsal pole to end of least broken arm ~5.2 cm.
103–926 m (Fig. 4).
Throughout the Southern Ocean (Fig. 3). Recorded on the Antarctic continent from the coast of Victoria Land (Ross Sea), Adélie Land, Queen Mary Land (Davis Sea) and Coats Land (Weddell Sea). Found around Pike Bank, Kerguelen Island and Heard Island on the Kerguelen Plateau. Also on Bouvet Island, and throughout the Scotia Arc including Shag Rocks, South Georgia, the South Sandwich Islands, the South Orkney Islands, Elephant and Clarence Islands, and in the Bransfield Strait. See Supplementary Tables S1 and S2.
Among the non-type samples available for study, two centrodorsal shapes were apparent (Fig. 6a, c). Pigment patterns also ranged from distinctly spotted specimens (Fig. 2, 6b, d) to minimal pigment (Fig. 6e).
The diagnosis is amended from Carpenter (1888). Clark and Clark (1967) provided an extensive description of P. kerguelensis but no diagnosis and included a large variety of specimens representing a range of the 20-armed Promachocrinus species based on current evidence. Our diagnosis and molecular data delineate Promachocrinus kerguelensis in a restricted sense referred to as sensu stricto.
DNA data were not available from the original Challenger specimens, therefore associating the original name with one of the clades in the study was reliant on morphology and specimens collected from near the type locality. Fortunately, only one clade with 10 radials has been found on the Kerguelen Plateau (110 individuals sequenced from Kerguelen Island and 20 from Pike Bank belonging to Clade A, Fig. 1). This allows for high confidence when assigning the name Promachocrinus kerguelensis s.s. to Clade A of Wilson et al. (2007), Hemery et al. (2012) and Eléaume et al. (2014).
Variation in pigmentation and centrodorsal shape common. Carpenter (1888) noted that preserved specimens from Kerguelen ranged from ‘light yellowish-brown to greyish-white with dark red spots.’ No distinct pigmentation remains on the syntypes but many of the P. kerguelensis s.s. specimens used for this study have dark spots (Fig. 2, 6e) and these were not observed in any other Promachocrinus species. Promachocrinus kerguelensis s.s. is the only Promachocrinus species where many, though not all, members may have a centrodorsal with a large, rounded shape. Though P. kerguelensis s.s. tends to have more rounded and smaller cirral sockets than P. fragarius sp. nov., there is a great deal of variation in P. kerguelensis s.s. (Fig. 6a, b). Some specimens, particularly ones with minimal pigment and a rounded, conical centrodorsal are not distinguishable from P. fragarius sp. nov. without DNA data.
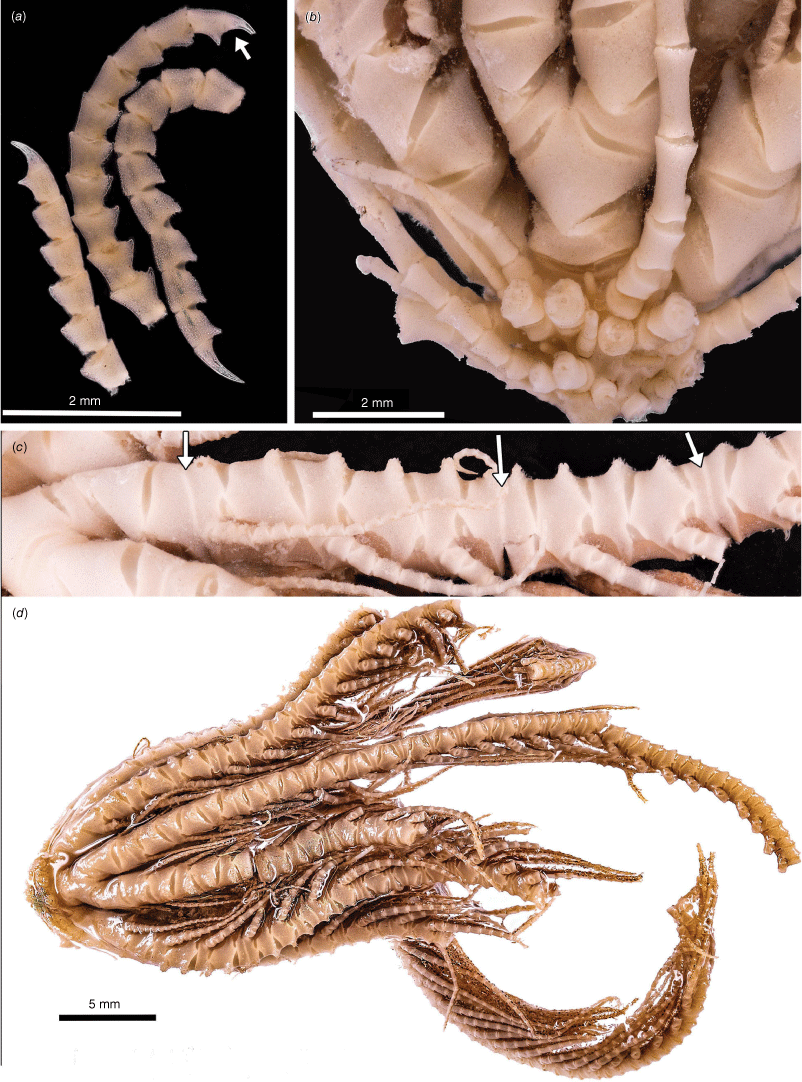
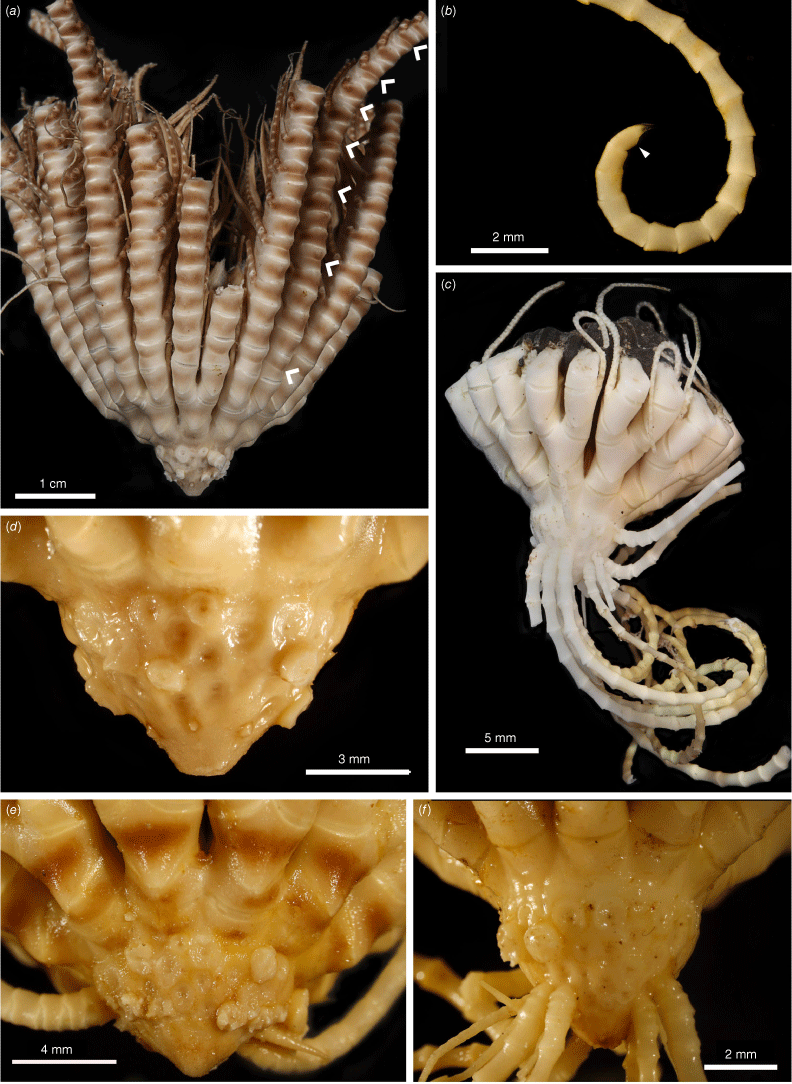
Promachocrinus kerguelensis Carpenter, 1888 syntypes NHM 1888.11.9.132 (a, d, e) and syntype NHM 1888.11.9.133 (b, c, e). (a) Lateral view of centrodorsal and rays. (b) Lateral view of whole specimen. (c) Terminal region of a cirrus showing hooked claw and small opposing spine. (d) Aboral view of centrodorsal and rays. (e) Illustration of Promachocrinus kerguelensis from Carpenter (1888). Photographs: Harry Taylor of the Natural History Museum UK (London) (a–d).
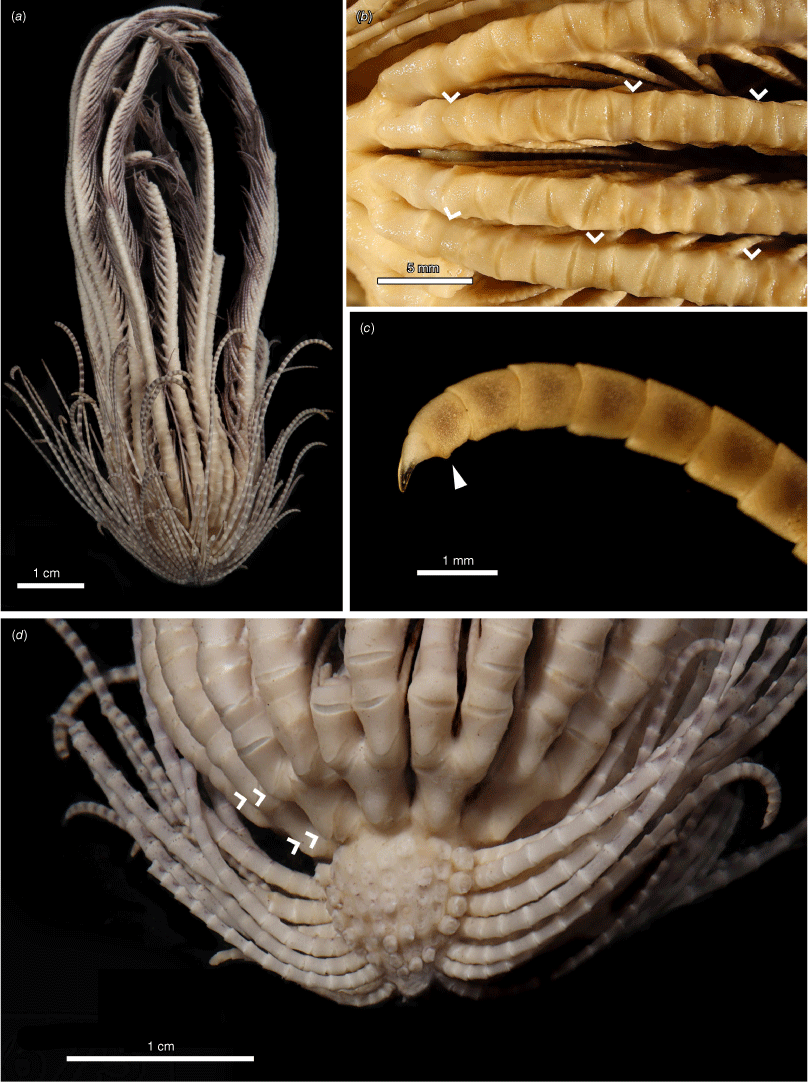
Promachocrinus kerguelensis specimens demonstrating morphological variation. (a) Rounded centrodorsal form (SIO-BIC E4993) from Shag Rocks (Scotia Arc). (b) Preserved specimen (SIO-BIC E4979) from Shag Rocks (Scotia Arc) showing distinctive spots retained on arms after preservation seen only on specimens of P. kerguelensis s.s. Arrow indicates first syzygy at Br3+4 (c). Conical centrodorsal form (SIO-BIC E4979). (d) Live specimen (SIO-BIC E4979) from Shag Rocks (Scotia Arc) showing distinctive spots on arms found only on specimens of P. kerguelensis s.s. (e) Live specimen (SIO-BIC E4908) from the South Sandwich Islands (Scotia Arc) showing reasonably unpigmented form of P. kerguelensis s.s.

Promachocrinus fragarius, sp. nov.
(Fig. 7.)
ZooBank: urn:lsid:zoobank.org:act:B7D9CA38-E404-42CF-A14E-9A7F4A4868F5
Clade B + C – Wilson et al. (2007)
Clade B + C – Hemery et al. (2012)
Haplogroup B + C – Eléaume et al. (2014)
Holotype. SIO-BIC E5002 (GenBank COI accession number MT264437) from South Sandwich Islands, −59.383°, 27.345°, depth 800 m, on October 7, 2011, on the Nathanial Palmer by trawl, fixed in 95% ethanol.
Other examined material. SIO-BIC E4821 (GenBank COI accession number MT264439), SIO-BIC E4830 (GenBank COI accession number MT264705), SIO-BIC E4856 (GenBank COI accession number MT264715), SIO-BIC E4867 (GenBank COI accession number MT264395) from Bransfield Strait at −62.753°, −57.322°, depth 292 m; SIO-BIC E4951 (GenBank COI accession number MT264440) and SIO-BIC E4953 (GenBank COI accession number MT264438) from the South Sandwich Islands −59.383°, −27.345°, depth 926 m. For other records and GenBank accession numbers see Supplementary Tables S1 and S2.
Radials 10. Arms 20. Centrodorsal shape rounded–conical, slightly increases in width before curving back to rounded tip, strawberry-like. Cirral sockets mostly in columns, taller than wide, covering centrodorsal to aboral pole, occasionally aboral pole tip bare. Pinnules may have pigmentation, purplish, extending to corresponding brachials. Cirri pigmentation paler if present.
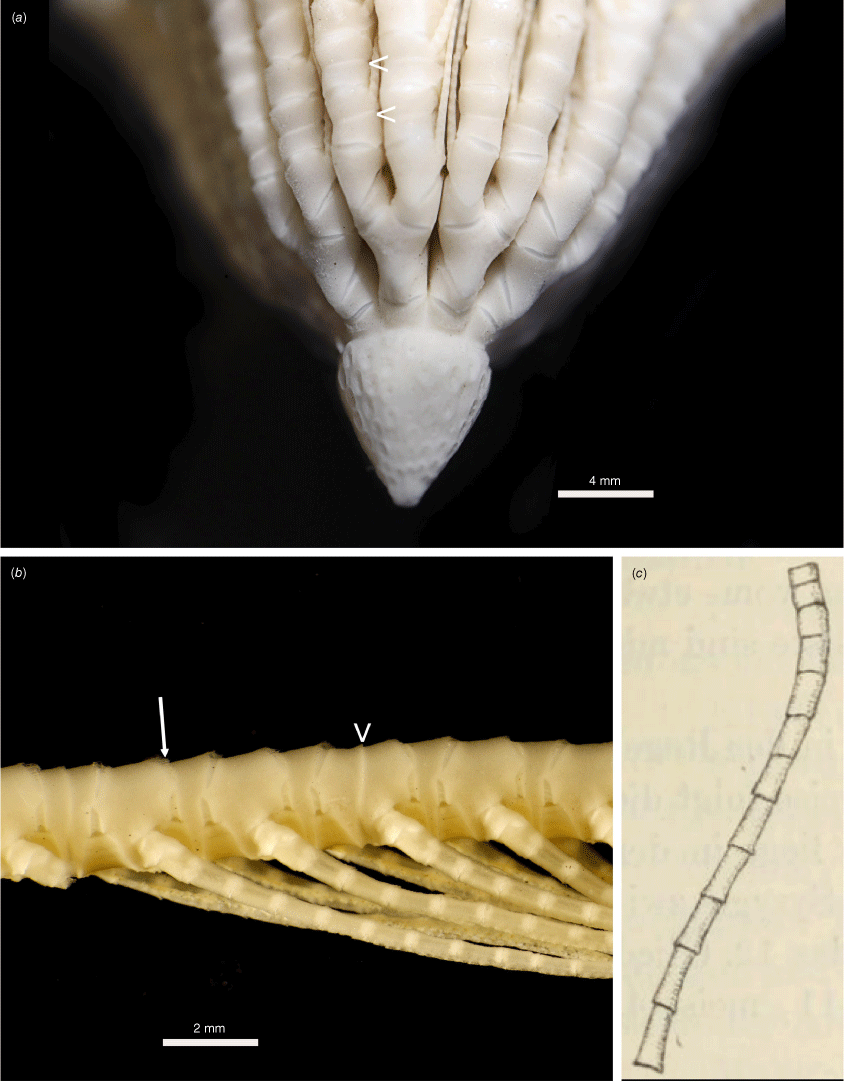
Holotype intact with some distal regions of arms broken (Fig. 7a). Centrodorsal width at meeting of arms 8.2 mm; maximum width of centrodorsal 8.7 mm, length 8.1 mm. Aboral pole 0.7 mm wide without cirri (Fig. 7d). Cirral sockets form 6–7 rows, generally in columns (Fig. 7d). Cirral sockets oblong, long axis 0.8 mm at aboral pole to 1.3 mm, short axis ~0.7–1.0 mm. Cirri shortest at aboral pole, 2 cm long, 2 mm wide. Larger cirri up to 4 cm long, 6 mm wide. Proximal cirrals short, C1 ~0.5 mm, lengthening distally to 1.4–2.0 mm until halfway when cirrals shorten to ~0.6 mm (Fig. 7a). Distalmost cirrals with terminal claw; penultimate cirrals with small opposing spine (Fig. 7c), opposing spine is slightly larger than aboral spines on other cirrals (Fig. 7c). First syzygy ~2.5 mm wide at Br3+4; second syzygy generally at Br9+10 but some at Br8+9 to Br10+11; syzygies continue at intervals of 3–5 muscular junctions (Fig. 7b, d). Synarthries at IBr1+2 and IIBr1+2 form minor aboral wedge (Fig. 7d). Cirri with pigment stripes primarily on peripheral side. Pinnules with similar tint but less uniform, darker pigmented pinnules often in groups of 3–7, pigmented ossicles correspond to darker pinnules. Pinnules darker distally, fading proximally (Fig. 7a).
This name is derived from the Latin word fragum meaning strawberry, named for the resemblance of the shape of the centrodorsal to a strawberry.
65–1170 m (Fig. 4).
Throughout the Southern Ocean (Fig. 3). Recorded on the Antarctic continent from the Marie Byrd Land (Amundsen Sea), Siple Coast, Victoria Land (Ross Sea), Adélie Land, Queen Mary Land (Davis Sea), Prdyz Bay, east of Joinville Island and Coats Land (Weddell Sea). Throughout the southern Scotia Arc including the South Sandwich Islands, Discovery Bank, the South Orkney Islands, Elephant Island, South Shetland Islands, through the Bransfield Strait and west Antarctic Peninsula. See Supplementary Tables S1 and S2.
The number of rows of cirri varies based on specimen size. Pigmented pinnules are common. Some individuals of P. fragarius belonging to ‘Clade B’ display dark reddish pigmentation along the arms, starting after the first syzygy and darkening until the second syzygy, remaining constant distally (not shown).
The centrodorsal shape (Fig. 7d) is distinctive for P. fragarius sp. nov. relative to most specimens of the sister species P. kerguelensis s.s. However, some specimens of P. kerguelensis s.s. with minimal pigment and a rounded conical centrodorsal are notably not distinguishable from P. fragarius sp. nov. without DNA data.
Promachocrinus fragarius sp. nov. Preserved holotype (SIO-BIC E5002): (a) Whole specimen showing numerous elongate intact cirri and arms variously broken. (b) One ray, indicating syzygies of two different arms (arrows). (c) Distal end of a cirrus. Arrow indicates opposing spine on penultimate cirral. (d) Centrodorsal with some cirri removed and base of several rays. Arrows indicating synarthries at IBr1+2 and IIBr1+2.
Promachocrinus unruhi, sp. nov.
(Fig. 8.)
ZooBank: urn:lsid:zoobank.org:act:EE8DECFD-92AE-439D-AF0E-E0D24528CBAC
Clade D – Wilson et al. (2007)
Clade D – Hemery et al. (2012)
Haplogroup D – Eléaume et al. (2014)
Holotype. SIO-BIC E4455 (GenBank COI accession number JN640423) from the Bransfield Strait at −63.232°, −59.458°, depth 759 m.
Other material examined. SIO-BIC E4454 (GenBank COI accession number MT264526), SIO-BIC E4456 (GenBank COI accession number JN640422), SIO-BIC E4457 (GenBank COI accession number JN640421), SIO-BIC E4458 (GenBank COI accession number JN640420), SIO-BIC E4460 (GenBank COI accession number JN460418), SIO-BIC E4461 (GenBank COI accession number JN640417), SIO-BIC E4462 (GenBank COI accession number JN640416), SIO-BIC E4463 (GenBank COI accession number JN640415), SIO-BIC E4464 (GenBank COI accession number JN640414), SIO-BIC E4466 (GenBank COI accession number JN640413), SIO-BIC E4459 (GenBank COI accession number JN640414) from the Bransfield Strait at −63.232°, −59.458°, depth 759 m; and WAM Z44803 (GenBank COI accession number MT264527) from Siple Coast −73.203°, −127.25°, depth 300 m. For other records and GenBank accession numbers see Supplementary Tables S1 and S2.
Radials 10. Arms 20. Centrodorsal reasonably small, shallow, rounded to conical. Cirral sockets large relative to centrodorsal length, shape rounded, becoming ovoid towards aboral pole; in 2–4 rows, not in columns. Large area of aboral pole lacking cirri. Cirri shape wider laterally. Long axillary, synarthries at IBr1+2 and IIBr1+2 extend laterally slightly.
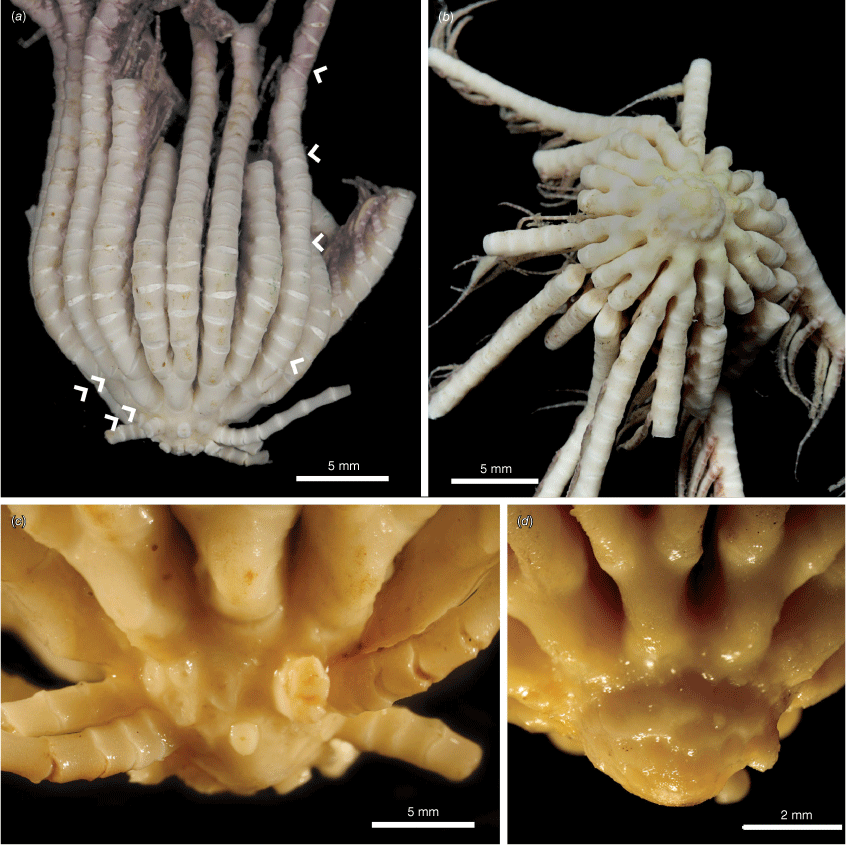
Centrodorsal width 4.1 mm, length 2.4 mm; 0.7 mm of aboral pole bare (Fig. 8a, c). Centrodorsal shallow rounded with length two-thirds of width. Cirral sockets rounded, diameter 0.4–0.6 mm; in three rows, few cirri present, none complete. First syzygy on Br3+4, width 1.3 mm. Length of IBr1 0.5 mm; axil 2.1 mm, IIBr1 0.4 mm long, IIBr2 is 1.3 mm long.
This species was named in honour of Matthew Unruh for his friendship and support to the first author.
147–1170 m (Fig. 4).
Found in most areas around continental Antarctica (Fig. 3). Present on Marie Byrd Land (Amundsen Sea), the Siple Coast, Victoria Land (Ross Sea), Adélie Land, Queen Mary Land (Davis Sea) and Coats Land (Weddell Sea). Also recorded from the west Antarctic Peninsula, South Shetland Islands, Bransfield Strait and the South Sandwich Islands. See Supplementary Tables S1 and S2.
Though none of the specimens examined in this study had less than 10 radials, Hemery et al. (2012) noted some members of this species (as Clade D) with only 6 radials rather than 10. No specimens studied here showed this. Some specimens showed an even lower and more rounded centrodorsal than the holotype (Fig. 8b, d).
The specimens examined for this species were all in poor condition, making distinguishing some characteristics difficult. The centrodorsal was the only region available for most specimens. However, Promachocrinus unruhi sp. nov. is notable for the very short centrodorsal that is unique among Promachocrinus species. Although this has the same rounded centrodorsal shape as in some P. kerguelensis s.s. specimens, the size disparity makes differentiation between the two simple. Additionally, whereas P. kerguelensis s.s. tends to have the smallest diameter of cirri and cirral sockets, P. unruhi sp. nov. has one of the largest. Unlike P. kerguelensis s.s. and P. fragarius sp. nov., the centrodorsal of P. unruhi sp. nov. only contains 2–4 rows of cirri, like that of P. uskglassi sp. nov.
Promachocrinus unruhi sp. nov. (a) Holotype (SIO-BIC E4455), lateral view indicating syzygies with single arrows, double arrows indicating synarthries at IBr1+2 and IIBr1+2. (b) Centrodorsal, aboral view of specimen WAM Z44803. (c) Lateral view of centrodorsal, holotype (SIO-BIC E4455). (d) Lateral view of bare centrodorsal (WAM Z44803).
Promachocrinus uskglassi, sp. nov.
(Fig. 9.)
ZooBank: urn:lsid:zoobank.org:act:0794E92F-3E74-42B8-B1D7-E2124E308BEF
Clade E – Wilson et al. (2007)
Clade E1 and E2 – Hemery et al. (2012)
Haplogroup E1 and 2 – Eléaume et al. (2014)
Holotype. SIO-BIC E4516 (GenBank COI accession number JN640430). South Orkney Island, −60.832°, −44.254°, depth 182 m.
Other material examined. SIO-BIC E4508 (GenBank COI accession number JN640438), SIO-BIC E4509 (GenBank COI accession number JN640437), SIO-BIC E4510 (GenBank COI accession number JN640436) from South Orkney Islands at −60.841°, −44.260°, depth 182 m; SIO-BIC E4499 (GenBank COI accession number JN640473), SIO-BIC E4494 (GenBank COI accession number JN640477), and SIO-BIC E4496 (GenBank COI accession number JN640475) from South Orkney Islands at −60.411°, −46.521°, depth 220 m; SIO-BIC E4526 (GenBank COI accession number JN640452) from South Orkney Islands at −60.797, −46.513, depth 210 m; SIO-BIC E4615 (GenBank COI accession number JN640463) and SIO-BIC E4614 (GenBank COI accession number JN640464) from South Orkney Islands at −60.952°, −45.329°, depth 236 m; SIO-BIC E4621 (JN640457) from South Orkney Islands at −60.841°, −44.260°, depth 182 m; SIO-BIC E4495 (GenBank COI accession number JN640476) from South Orkney Islands at −60.841°, −44.260°, depth 182 m; SIO-BIC E4468 (GenBank COI accession number JN640441) from South Orkney Islands at −60.841°, −44.260°, depth 182 m; SIO-BIC E4500 (GenBank COI accession number JN640473), SIO-BIC E4613 (GenBank COI accession number JN640465) from South Orkney Islands at −60.841, −44.260°, depth 182 m; SIO-BIC E4518 (GenBank COI accession number JN640428) from South Orkney Islands at −60.841°, −44.260°, depth 182 m; SIO-BIC E4616 (GenBank COI accession number JN640462) from South Orkney Islands at −60.841°, −44.260°, depth 182 m; SIO-BIC E4516 (GenBank COI accession number JN640430) from South Orkney Islands at −60.841°, −44.260°, depth 182 m. For other records and GenBank accession numbers see Supplementary Tables S1 and S2.
Radials 10. Arms 20. Centrodorsal conical, aboral pole pointed or flattened. Cirri in 3–5 rows, covering 70% of centrodorsal, aboral pole region bare. Synarthries IB1+2 and IIB1+2 with medial regions extending laterally as bumps. Light horizontal stripes along centre of brachials often present.
Centrodorsal 8.6 mm wide, 5.4 mm long; conical, pole flattened. Cirral sockets round, diameter 0.7–1.1 mm, in 3–4 rows, not aligned in columns; aboral pole lacks cirral sockets (Fig. 9a, d). Cirri missing. Brachials with central line of light pigment, pigmentation darker on distal segments. First syzygy at Br3+4, 3.1 mm wide. Most second syzygies occur on Br9+10, though once on Br10+11 and once at Br14+15; additional syzygies at 3–5 muscular articulations. IBr1 1.4–1.7 mm long (Fig. 9a). Axils 3.9–4.2 mm long; IIBr2 3.5–4.0 mm long. Synarthries of IBr1+2 and IIBr1+2 protrude slightly.
Named for the character John Uskglass from the book Jonathan Strange and Mr Norell by Susanna Clarke (2004). John Uskglass is the human name of the Raven King who brought magic to England and this name reflects the otherworldly appearance of the swimming motions of feather stars.
119–1157 m (Fig. 4).
Found in most areas around continental Antarctica (Fig. 3). Present on the Siple Coast, Victoria Land (Ross Sea), Adélie Land, Queen Mary Land and Wilhelm II land (Davis Sea), and Coats Land (Weddell Sea). Also recorded from the west Antarctic Peninsula, South Shetland Islands, through the Bransfield Strait, the South Orkney Islands, and Shag Rocks and South Georgia. See Supplementary Tables S1 and S2.
Most specimens available for study, including the holotype, belonged to subclade E1. Not all P. uskglassi sp. nov. specimens have the same pigment pattern as the holotype (Fig. 9a), with many lacking substantial pigment when preserved. Also, a second shape of centrodorsal pole can occur in subclade E1, with the centrodorsal coming down to a conical point (Fig. 9e). Specimens that have cirri showed distal cirrals with a terminal claw and the penultimate cirral a minimal opposing spine (Fig. 9b). Only one specimen from clade E2 was available for examination in this study (WAM Z44774) and this had a more rounded conical centrodorsal like P. fragarius sp. nov. and some P. kerguelensis s.s., with cirri covering most of centrodorsal, leaving the apical 1.0 mm bare (Fig. 9c, f).
Hemery et al. (2012) found two clades, denoted E1 and E2 that we refer to as P. uskglassi sp. nov. The morphology of the single E2 specimen studied differs from that seen in Clade E1 specimens that have a much shorter centrodorsal with relatively larger cirral sockets that do not extend to the centrodorsal pole. Based on specimens analysed, any specimens with full stripes that traverse across the brachials can be referred to as P. uskglassi sp. nov., though not all P. uskglassi sp. nov. specimens have this pigment. The striped pigmentation was generally much lighter than the pigment spots found on P. kerguelensis s.s. specimens, however a few specimens displayed darker bands. Owing to the lack of E2 specimens, these were restricted to Clade E1. Based on the species delimitation of COI data (Fig. 1) we accept P. uskglassi sp. nov. as both clades E1 and E2 pending further data, with the holotype in clade E1 (SIO-BIC E4516). Members of clade E2 may need sequencing to be distinguished from some P. kerguelensis s.s. and P. fragarius sp. nov. but examination of further specimens is needed. Clades E1 and E2 have different distribution trends but there is overlap between these two clades. Clade E1 occurs more commonly in the western hemisphere, whereas clade E2 is more commonly found in the eastern hemisphere. Both have been sampled in some of the same localities however, such as the Antarctic Peninsula, Victoria Land and Adélie Land.
Promachocrinus uskglassi sp. nov. (a) Lateral view of whole holotype (SIO-BIC E4516) showing arms broken and cirri missing. Arrows indicate syzygies. (b) Cirrus of specimen SIO-BIC E4615. Arrow indicates the limited opposing spine on the penultimate segment. (c) Specimen from subclade E2, WAM Z44774. (d) Centrodorsal of holotype. (e) Centrodorsal of preserved specimen SIO-BIC E4510, showing centrodorsal with a ‘pointed’ centrodorsal pole. (f) Centrodorsal specimen from subclade E2 (WAM Z44774).
Promachocrinus joubini Vaney, 1910
(Fig. 10.)
Clade F – Wilson et al. (2007)
Clade F – Hemery et al. (2012)
Haplogroup F – Eléaume et al. (2014)
Holotype. MNHN-IE-2014-734. Collected on the French Antarctic Expedition at Biscoe Bay, −64.8°, −63.833°, depth 110 m.
Other material examined. WAM Z44794 (GenBank COI accession number MT264637) from the Siple Coast at −73.2034°, −127.25°, depth 300 m; NIWA36557 (pictures only) (GenBank COI accession GU804565) from Victoria Land at −76.591°, 176.883°, depth 360 m. For other records and GenBank accession numbers see Supplementary Tables S1 and S2.
Radials 10. Arms 20. Arms extend from body nearly horizontally (Fig. 10e). Centrodorsal 5.4 mm long, 7.4 mm wide; semispherical (Fig. 10c, e). Cirral sockets, in three rows, diameter 0.8–1.3-mm; aboral pole lacks cirral sockets. Cirri not present, though drawn for description (Fig. 10c), proximal segments wider than long, fourth segment as wide as long, and most thereafter are 1.5 times longer than wide, with distal cirral pointed (Fig. 10c). Oral disc region 24.7 mm wide. Radial ossicle 1.2 mm long; axil 3.6 mm long. First syzygy occurs at Br3+4, 2.4 mm wide; second syzygy at Br9+10. IBr1+2 protrudes slightly, unlike IIBr1+2.
Yellow-brown body with pale cirri (Fig. 10a). Wide body, arms spread, 20 cm in diameter. Approximately 100 cirri; aboral pole lacking cirral sockets (Fig. 10a).
110–1157 m (Fig. 4).
Found in most areas around continental Antarctica (Fig. 3). Present on Marie Byrd Land (Amundsen Sea), the Siple Coast, Victoria Land (Ross Sea), Adélie Land, Queen Mary Land and Wilhelm II land (Davis Sea), and Coats Land (Weddell Sea). Also recorded from the west Antarctic Peninsula, near Elephant Island, the South Orkney Islands and the South Sandwich Islands. See Supplementary Tables S1 and S2.
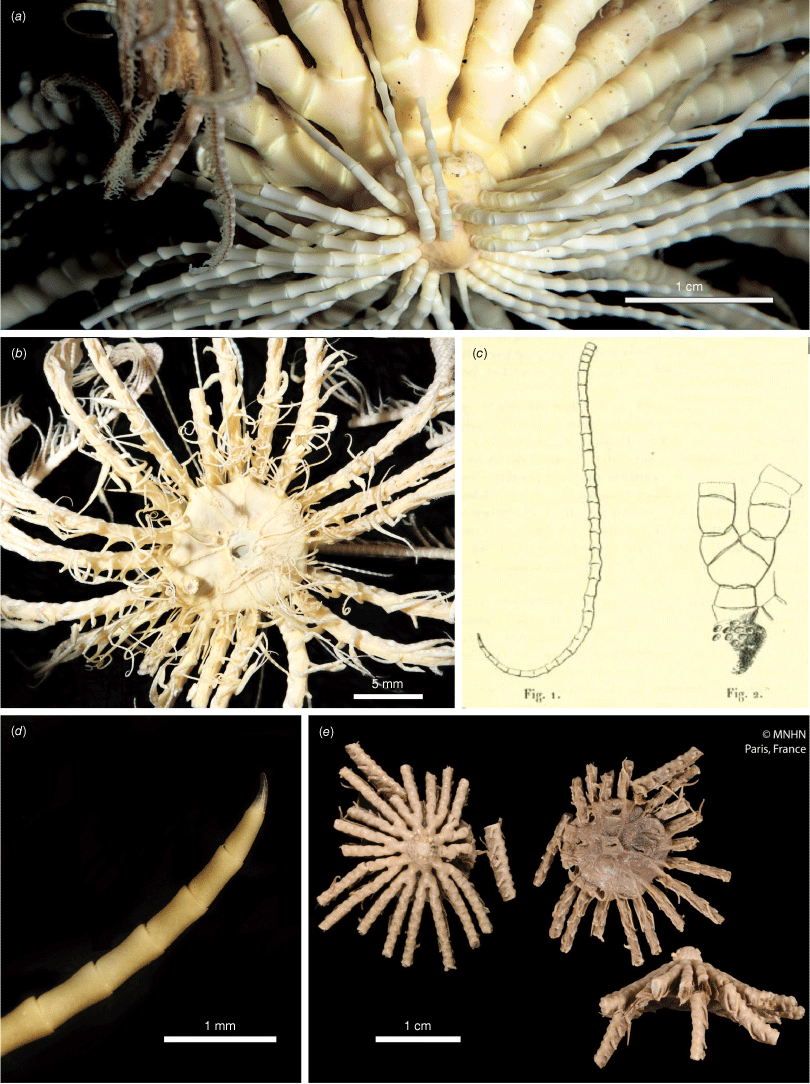
We were unable to obtain a tissue sample of the holotype to attempt to extract DNA. When assessing the application of the name P. joubini, a wide diversity of Promachocrinus specimens with 10 radials were notably sampled from the vicinity of Biscoe Bay, the type locality of P. joubini. The anatomical features of specimens from Clade F (Fig. 1) match those of P. joubini reasonably well (Fig. 10a, b, d). The body structure of this species is wider than that of other Promachocrinus, at 3.4 times the width of the centrodorsal at the widest point (Fig. 10a, b, e). Also, the arms extend outwards and from Vaney’s (1910) fig. 1 (on Fig. 10c) and other specimens such as WAM Z44794, the distal cirrals reach a clear point rather than a hook (Fig. 10d). Such features have only been observed in the holotype of P. joubini and the specimens in Clade F. Based on this we chose to apply the name P. joubini to Clade F.
Promachocrinus joubini Vaney, 1910. (a) Specimen WAM Z44794 aboral view of centrodorsal and proximal arms. (b) Oral disc and arms of specimen WAM Z44794 showing wide oral disc and spread posture of the arms. (c) Drawings of holotype from Vaney (1910). Note distal end of cirrus comes to a point. (d) Distal end of cirrus of specimen WAM Z44794 with a single tooth. (e) Preserved holotype (MNHN-IE-2014-734) showing splayed arms; photo credit MNHN- Marie Hennonion 2018.
Promachocrinus wattsorum, sp. nov.
(Fig. 11.)
ZooBank: urn:lsid:zoobank.org:act:541906FB-DCDE-4961-803D-C103D0DFAEBE
Holotype. WAM Z44000 (GenBank COI accession number MT264692), Prince Edward Islands, −46.726°, −37.894°, depth 281 m. Collected on the ACE 16/17 cruise on December 28, 2016, by Agassiz trawl, fixed in ethanol.
Other material examined. WAM Z44906 (GenBank COI accession number MT264695), WAM Z44907 (GenBank COI accession number MT264694), WAM Z44908 (GenBank COI accession number MT264700), WAM Z44909 (GenBank COI accession number MT264696), and WAM Z44910 (GenBank COI accession number MT264698) from the Prince Edward Islands at −46.726°, −37.894°, depth 281 m; WAM Z44016 (GenBank COI accession number MT264693), WAM Z44925 (GenBank COI accession number MT264699), and WAM Z44926 (GenBank COI accession number MT264697) from the Prince Edward Islands at −46.717°, −37.894°, depth 263 m.
Body small relative to other Promachocrinus species. Radials 5. Arms 10. Centrodorsal conical, pole rounded, wider than long. Cirral sockets rounded, 5–6 rows, in columns, covering centrodorsal. Cirri with light pigment, starting at C5 to C10, continuing distally. Radials lacking pigment, gradient of pigment on arms, begin at second syzygy, becoming darker distally. Pinnule pigmentation similar to or darker than attached arm ossicle. Synarthries protrude where IBr1+2 and IIBr1+2 meet (Fig. 11a).
Length 8.1 cm from aboral pole (Fig. 11a) to tip of most complete arm (Fig. 11b). Radials 5. Arms 10 (Fig. 11b). Centrodorsal conical, pole rounded, 4.5 mm wide, 3.6 mm long (Fig. 11a). Cirral sockets round, 5–6 rows, in columns. Cirri extend to apical pole. Cirri elliptical in cross-section. Cirrals proximally short, length approximately half of width, distally cirrals lengthen to three times width, shorten, flare distally (Fig. 11a, d); penultimate cirral with opposing spine, most distal containing curved claw. Cirri curled toward aboral pole, creating 1 cm ball (Fig. 11b). Cirri to 2.1 cm length, 22–28 segments. Cirri white or with pigmented gradient starting at cirral six to eight, darkening distally. Arm pairs fuse at axillary (Fig. 11a–c). At IBr1+2 and IIBr1+2, synarthries protrude laterally; distal to Br2, arms extend straight upwards (Fig. 11a). First syzygy at Br3+4, second syzygy chiefly at Br9+10, others at Br8+9 to Br12+13; subsequent syzygies occur every 3–6 articulations, third articulation primarily five after second; remaining articulations primarily four with a range of 3–5 (Fig. 11c). Synarthries after first syzygy are angular, alternating directions, creating wedge-shaped brachials.
This species is named to honour Mel Watts and family for supporting the Western Australian Museum.
263–281 m (Fig. 4).
Only known from the Prince Edward Islands (Fig. 3).
Not all specimens have such angular brachials as the holotype, some are more subtle, though all have synarthries that protrude laterally at IBr1+2 and IIBr1+2. Aboral portions of the brachials on the arms of some specimens have spines bordering articulations, though small these tend to be more significant on proximal brachials.
This is one of two species within Promachocrinus that has five radials and is morphologically quite like P. mawsoni comb. nov., though the two are distinct based on DNA data and the status as sister taxa is not well supported (Fig. 1, 2). The two species differ in the shape of the terminal claw of the cirri and the centrodorsal is less conical in P. mawsoni comb. nov.
Promachocrinus wattsorum sp. nov. holotype (WAM Z44000). (a) Centrodorsal lateral view, double arrowheads indicating ‘protruding’ synarthries at IBr1+2 and IIBr1+2. (b) Whole holotype. (c) Arms, arrows indicating syzygies on two different arms. (d) Cirri, arrows indicating opposing spines on two penultimate segments.

Promachocrinus mawsoni, comb. nov. (AH Clark, 1937)
(Fig. 12.)
Florometra mawsoni – Clark (1937)
Florometra mawsoni – Eléaume et al. (2014)
Holotype. AMS J5569; collected during the Australian Antarctic Expedition 1911–1914, at station 2, Adélie Land, −66.92°, 145.35°, depth 581 m (∼318 fathoms).
Paratype. USNM E3052. Obtained during the Australian Antarctic Expedition, same details as holotype.
Other material examined. SIO-BIC E4442 (GenBank COI accession number MT264639), SIO-BIC E4443 (GenBank COI accession number MT264640), SIO-BIC E4444 (GenBank COI accession number MT264641), SIO-BIC E4445 (GenBank COI accession number MT264642), SIO-BIC E4446 (GenBank COI accession number MT264644), SIO-BIC E4447 (GenBank COI accession number MT264645), SIO-BIC E4448 (GenBank COI accession number MT264646), SIO-BIC E4449 (GenBank COI accession number MT264647), SIO-BIC E4450 (GenBank COI accession number MT264648), SIO-BIC E4451 (GenBank COI accession number MT264649), SIO-BIC E4452 (GenBank COI accession number MT264650), SIO-BIC E4487 (GenBank COI accession number MT264656), SIO-BIC E4488 (GenBank COI accession number MT264657), SIO-BIC E4489 (GenBank COI accession number MT264658), all off Joinville Island, leg. Nerida Wilson, R/V Yuzhmorgeologiya, hard-bottom snapper trawl, Station 101-76, 4 Mar 2009, from −63.01817°, −52.36567° to −63.01783°, −52.44567°, mean depth 623 m; SIO-BIC E6859 (GenBank COI accession number MT264638), Station 103-77, 5 Mar 2009, from −62.58717°, −53.77283° to −62.56333°, −53.81983°, mean depth 731 m; SIO-BIC E4474 (GenBank COI accession number MT264651), SIO-BIC E4486 (GenBank COI accession number MT264653), South Orkney Islands, leg. Nerida Wilson, R/V Yuzhmorgeologiya, hard-bottom snapper trawl, Station 30-30, 17 Feb 2009, from −60.9285°, −44.86383° to −60.91233°, −44.85467°, mean depth 235 m; SIO-BIC E4597 (GenBank COI accession number MT264654), SIO-BIC E4598 (GenBank COI accession number MT264655), Station 31-28, 16 Feb 2009, from −61.044°, −44.71383° to −61.0295°, −44.70317°, mean depth 254 m; SIO-BIC E4482 (GenBank COI accession number MT264652), Station 33-26, 16 Feb 2009, from −61.13383°, −44.233° to −61.145°, −44.26033°, mean depth 337 m; SIO-BIC E4990, South Orkney Islands, leg. Nerida Wilson and Greg Rouse, R/V Nathaniel B. Palmer, Blake trawl, Station SO1a-78, 20 Oct 2011, from −60.55086°, −45.17611° to −60.54543°, −45.19013°, depth 222 – 278 m; SIO-BIC E5487, SIO-BIC E5488, South Sandwich Islands, leg. Nerida Wilson and Greg Rouse, R/V Nathaniel B. Palmer, Blake trawl, Station SS3a-43, 7 Oct 2011, from −59.3831°, −27.34542° to −59.37918°, −27.367°, depth 701–926 m; WAM Z44234 (GenBank COI accession number MT264661), Mertz Glacier Tongue at −67.0926°, 144.0128°, depth 930 m; WAM Z44410 (GenBank COI accession number MT264662) Diego Ramírez Islands at −56.7246°, −68.6333°, depth 380 m; WAM Z44428 (GenBank COI accession number MT264663), Siple Coast at −72.7408°, −124.9667°, depth 480 m; WAM Z44671 (GenBank COI accession number MT264664), Bouvet Island −54.4065°, 3.5833°, depth 500 m. For other records and GenBank accession numbers see Supplementary Tables S1 and S2.
Radials 5. Arms 10. Centrodorsal small, conically pointed, with 40–77 cirri in 4–5 rows, irregular columns, stopping short of aboral pole. Cirri with up to 34 cirrals; longest cirrals L/W ratio up to three. Distal cirrals with terminal claw, slender and curved, opposing spine conical. Proximal brachials spinous, everted distally, or less so in larger specimens. Third syzygy usually at Br14+15. P1 with up to 50 segments; distal pinnulars slightly modified as rudimentary comb, producing scalloped profile. Lack of notable pigment.
Radials 5. Arms 10. Body length to tip of most complete arm 44.6 mm. Centrodorsal length 2.4 mm, width 2.9 mm, cirri stopping 0.5 mm short of dorsal pole. Round cirral sockets ~0.4–0.7 mm in diameter. Short proximal cirrals, C1 length 0.3 mm, C3 length 0.8 mm, C4+ length 1.4 mm. Distalmost cirral a terminal claw, penultimate cirral a large opposing spine. First syzygy occurs at Br3+4 width 1.7 mm, additional syzygies at Br9+10, Br14+15 or Br16+17, Br21+22 continue distally at intervals of 4–6 brachials (Fig. 12c, d). Radial ossicle 0.2 mm long; axil length 2.1 mm, width 2.2 mm; Br2 length 1.4 mm. Synarthries angular after first syzygy, alternating directions, creating wedge-shaped brachials. Arms gently curved, minimal medial protrusion from synarthries. Short brachials, most between 0.5 and 1.2 mm, shorter than long (Fig. 12d).
168–930 m (Fig. 4).
Recorded on the Antarctic continent from the Siple Coast, Victoria Land (Ross Sea), Adélie Land and Coats Land (Weddell Sea). Throughout the southern Scotia Arc including the South Sandwich Islands, the South Orkney Islands, east of Joinville Island, through the Antarctic Peninsula. Also known from the Diego Ramírez islands, Bouvet Island and the Kerguelen Plateau (Fig. 3). This is the only Promachocrinus species record confirmed by DNA data from the South American continent. See Supplementary Tables S1 and S2.
The diagnosis is based on Messing et al. (2023b). Along with P. wattsorum sp. nov., P. mawsoni comb. nov. is one of two species within Promachocrinus with 5 radials and 10 arms (Fig. 2). For this reason, the species has traditionally been placed in Florometra (Clark and Clark 1967). Apart from clear distinction based on DNA (Fig. 1, 2), the two species differ morphologically only in minor ways and share similar distributions of syzygies and synarthries. Promachocrinus wattsorum sp. nov. has thinner arms and cirri, with cirri present on the centrodorsal pole. Notably the two species differ in the shape of the terminal claw of the cirri and the centrodorsal is less conical in P. mawsoni comb. nov. than in P. wattsorum. Promachocrinus mawsoni comb. nov. is the only other known Promachocrinus taxon sampled from Bouvet Island and Kerguelen Plateau apart from P. kerguelensis s.s.
Promachocrinus vanhoeffenianus Minckert, 1905
(Fig. 13.)
Promachocrinus vanhoeffenianus – Minckert (1905)
Holotype. USNM E378. Collected during the Gauss expedition at −65.835°, 89.541°. Depth between 350 and 400 m.
Centrodorsal conical. Cirri to aboral pole in ordered columns of 6–7 rows (Fig. 13a). Cirral sockets oval, largest near arms at 1.0 mm by 0.7 mm, at aboral pole 0.4 mm by 0.3 mm. Cirri all missing. First syzygy at Br3+4, 1.8 mm wide; second syzygy typically at Br5+6 (Fig. 13a), syzygies thereafter sporadic (Fig. 13b). Radial length is 0.7 mm; Br1 length ~0.7 mm; axil length ~3.7 mm, maximum width 3.0 mm (Fig. 13a); IIBr1 length ~0.9 mm; IIBr2 length 2.7 mm. Minimal protrusion of synarthries. Brachials with small spines along margin (Fig. 13b arrow). Arms broken at ~Br20.
From Minckert’s (1905) description: Larger cirri 30–40 segments, 4–5 cm long. Proximal and distal cirrals short, middle cirrals long (Fig. 13c). Most distal cirral a terminal claw; penultimate cirral an opposing spine.
Unfortunately, though attempted with ancient DNA protocols (TrEnD Lab, Curtin University), DNA was not successfully extracted. The holotype specimen arguably represents a unique species with no other specimens attributable to this from the available samples. Promachocrinus vanhoeffenianus has a centrodorsal that is much longer than that of any other Promachocrinus species and is longer than the widest point. The radial ossicles are also very apparent and much longer than those of all other Promachocrinus species apart from clade E2 of P. uskglassi sp. nov. The arms of P. vanhoeffenianus are slender and the muscular articulations relatively smooth compared to those of other species.
The original name for this species was spelled P. vanhöffenianus, though in the subsequent literature, this has primarily been spelled with a normal ‘o’ replacing the ‘ö’ (e.g. Messing et al. 2023a). However, the International Code on Zoological Nomenclature, 4th edition (The International Trust for Zoological Nomenclature 1999, section 32.5.2) states that letters with an umlaut, such as ö, need to have the umlaut removed and an ‘e’ added after the vowel. In the case of P. vanhöffenianus, the correct spelling would be P. vanhoeffenianus.
Promachocrinus vanhoeffenianus Minckert, 1905. (a) Holotype (USNM E378), lateral view of centrodorsal and several arms. (b) Lateral view of an arm, arrow indicates spines on brachial, arrowhead a syzygy. (c) Proximal portion of a cirrus, from Minckert (1905).
Discussion
Species delimitation and the application of species names
The molecular analyses and morphological revision conducted in this study support increasing the total number of Promachocrinus species from one to eight. The circumscription of five previously identified clades as species-level taxa is mainly concordant with previous delimitations as clades A, B + C, D, E and F (Wilson et al. 2007; Hemery et al. 2012; Eléaume et al. 2014). We were conservative in designating clades as species by combining what had been regarded as separate phylogroups in some cases (B + C, E1 + E2, Hemery et al. 2012). In each of these cases, monophyly and species delimitation results (ASAP and mPTP) (Fig. 1), and morphology were considered.
Despite the common sympatry of species, the only 20-armed lineage present at the type locality (Kerguelen Island) was Clade A that has morphology matching the original description, therefore we were able to assign this to be P. kerguelensis s.s. Promachocrinus fragarius sp. nov. is named for clades B + C, P. unruhi sp. nov. for clade D, P. uskglassi sp. nov. for clades E1 + E2 and P. joubini is resurrected for clade F. The 10-armed Promachocrinus wattsorum sp. nov. had not been recovered in previous sequencing and is a new and potentially geographically restricted species of Promachocrinus. The analyses also corroborated the proposal by Eléaume et al. (2014) to place the 10-armed Florometra mawsoni in Promachocrinus as P. mawsoni comb. nov. Six of the eight species show circum-Antarctic distributions, with the two exceptions being P. wattsorum (Prince Edward Islands) and P. vanhoeffenianus (near the Davis Sea).
DNA was not obtained from the holotype of Promachocrinus vanhoeffenianus. Assigning this name to a clade was subsequently reliant on the combination of morphological characteristics and collection data, as was the case with P. kerguelensis s.s. and P. joubini. All clades had representative specimens sampled from near the type locality of P. vanhoeffenianus but none of the clades had all the distinguishing morphological characteristics found in the holotype of P. vanhoeffenianus. Therefore, we conclude that P. vanhoeffenianus does not seem to have been recollected since description.
Morphology
Although some species delineated here can be easily distinguished based on morphology, problems with separating some taxa without DNA remain, as indicated in various Remarks sections above. Significant morphological has been noted within Promachocrinus kerguelensis in past studies (Carpenter 1888; John 1938; Clark and Clark 1967; Speel and Dearborn 1983). Speel and Dearborn (1983) attempted to note trends corresponding to locality but found marked variation at single locations. Given the sympatry of most of the Promachocrinus species currently recognised and high amount of variation within clades, these observations are not unexpected. Previous Promachocrinus DNA-based studies made little mention of the morphological variation in relation to the various clades. Wilson et al. (2007) mentioned that colour variation was present but noted that this did not appear to be restricted to certain lineages.
Several of the 20-armed Promachocrinus species showed distinctive pigmentation patterns. The most common pigment pattern that was found in several clades (P. kerguelensis s.s., P. fragarius sp. nov. and P. unruhi sp. nov.) had dark pinnules without notable pigment on the arms or centrodorsal. However, no single distinctive pigmentation pattern was present on all specimens in a species, so these are unfortunately not fully diagnostic. For example, many P. kerguelensis s.s. specimens exhibited dark spots (Fig. 6b, d) and many P. uskglassi sp. nov. specimens demonstrate pigmented bands across the 20 arms, with the darkest pigment on the narrowest portion of the brachials (Fig. 9a). In both cases other members of the respective species lacked pigmentation. Molecular methods are needed to confidently identify 20-armed Promachocrinus specimens that have minimal pigmentation, or centrodorsal features or cirral shapes that are not clearly diagnostic.
Three main shapes of centrodorsal were observed in Promachocrinus: round, rounded conical and pointed cone. A round centrodorsal occurred in two species, P. kerguelensis s.s. (Fig. 5a) and P. unruhi sp. nov. (Fig. 8) but was much lower and had fewer cirri in P. unruhi sp. nov. so they can be separated. However, many P. kerguelensis s.s. had a rounded conical centrodorsal that was most common inthe Promachocrinus species sampled in this study and descriptions from Promachocrinus literature (Minckert 1905; John 1938; Clark 1941). This centrodorsal shape occurs in most Promachocrinus clades: P. kerguelensis s.s., P. fragarius sp. nov., P. wattsorum sp. nov., P. vanhoeffenianus and within clade E2 of P. uskglassi sp. nov. Three species had a pointed conical centrodorsal: P. joubini sp. nov., P. mawsoni comb. nov. and clade E1 of P. uskglassi sp. nov. Cirri are present down to the centrodorsal pole on P. kerguelensis s.s., P. fragarius sp. nov., P. wattsorum sp. nov., P. vanhoeffenianus and clade E2 of P. uskglassi sp. nov. Conversely, P. unruhi sp. nov., P. joubini, P. mawsoni comb. nov. and clade E1 of P. uskglassi sp. nov. havecirri that stop short of the centrodorsal pole.
Promachocrinus was originally erected based on the presence of 10 radials and 10 or 20 arms (Carpenter 1879). Results (Fig. 1, 2) allowed for the inclusion of two species with 5 radials (10 arms), P. mawsoni comb. nov. and P. wattsorum sp. nov. This clearly represents a reversal to the ancestral condition found across Antedonidae. Promachocrinus mawsoni comb. nov. and P. wattsorum sp. nov. are easily distinguished from other Promachocrinus species by the number of arms and from each other by the terminal claw of the cirri and centrodorsal shape. P. wattsorum sp. nov. is only known only from the Prince Edward Islands, whereas P. mawsoni comb. nov. is widely distributed.
Distributions and biogeography
All species except for P. wattsorum sp. nov. and P. vanhoeffenianus have a circum-Antarctic distribution and were each found from at least half of the 16 general regions in this study (Fig. 3). However, not all species were found at each site and not all species were found in the same proportions. There were only four locations, the Antarctic Peninsula, Coats Land, Victoria Land and Adélie Land, from which all six circumpolar species were sampled. These four locations are situated on the continental shelf or slope. The island locations however, seemed to have fewer species present and this may indicate barriers to dispersal in these areas. The isolated Kerguelen Plateau and Bouvet Island were both locations where only P. kerguelensis s.s. and P. mawsoni comb. nov. were found. On the other hand, the nearest continental locations to these islands, Coats Land and the Davis Sea, were uncommon for P. kerguelensis s.s. and P. mawsoni comb. nov. compared to those of the other four species. In the northern part of the Scotia Arc (Shag Rocks and South Georgia) only P. kerguelensis s.s. and P. uskglassi sp. nov. were found. Additionally, P. mawsoni comb. nov. was the only Promachocrinus found at the southern tip of South America, at Diego Ramírez islands.
All sequenced species had a wide and an approximately overlapping depth distribution of approximately 100 to over 1000 m (excepting P. wattsorum, with limited sampling). Promachocrinus kerguelensis s.s. had a shallower mean depth (150 m) than all other species. The maximum depth occurrence of P. kerguelensis s.s. (926 m) was not much shallower than that of other species. Apart from P. wattsorum sp. nov. (and P. vanhoeffenianus that was not recollected), all species were observed in geographical and bathymetric sympatry.
Promachocrinus wattsorum sp. nov. is a biological outlier within Promachocrinus. This species has only 5 radials, compared with the 10 radials of most Promachocrinus species and has been collected from only one location, the Prince Edward Islands. Additionally, no other Promachocrinus species have been sampled from these islands, raising questions about dispersal capabilities and existing barriers in this location. These islands lie between the Sub-Antarctic Front and the Antarctic Polar Front. The Polar Front is a known dispersal barrier, creating both physical and physiological barriers to larvae. Apart from P. mawsoni comb. nov., Promachocrinus is notably absent from most locations north of the Polar Front (although at some point in time Kerguelen Island may have been north of this boundary, Park et al. 2014). There is one report of Promachocrinus ‘kerguelensis’ being collected from the Straits of Magellan at two localities east of Punta Arenas (Mutschke and Rios 2006). Our subsequent trawling efforts in that area (in 2013, NGW and GWR) could not confirm this. The estimated larval life for a Promachocrinus species was based on specimens from the Ross Sea (McClintock and Pearse 1987) that hosts at least six species. Therefore larval life and hence dispersal ability differ among species.
The application of molecular data often reveals that current taxonomy does not accurately reflect biodiversity but there is a lag time between this discovery and resolution of the situation (also see Fontaine et al. 2012). The recognition of these unnamed taxa by sequence data refers to these as ‘dark taxa’ (Page 2016). The resolution of dark taxa from Antarctica can take longer than usual because of constraints on the scale of sampling necessary. Understanding which taxa are truly cryptic and only recognisable with molecular data, and those that are pseudocryptic and can be identified once characters have been revised in a molecular framework is important. Monitoring biodiversity requires robust identification of taxa and this can be very complicated when taxa are truly cryptic. This study suggests that some species in Promachocrinus can be determined on the basis of morphology, however the ambiguity among some species suggests that sequencing (minimally COI) should remain the primary tool, if species-level resolution is required.
Supplementary material
Supplementary Table S1 provides sample information and GenBank accession numbers for new COI sequences generated for this study, Supplementary Table S2 includes sample information and COI sequences for previously available Promachocrinus species from GenBank and Supplementary Table S3 provides samples and GenBank accession numbers for the concatenated gene phylogeny of Promachocrinus. The supplementary material includes three sequence files: Supplementary File S1 provides a Promachocrinus COI dataset of 2133 available sequences in nexus format, Supplementary File S2 includes a Promachocrinus COI dataset of 826 unique sequences including the outgroup in nexus format, and Supplementary File S3 provides a concatenated 5-gene Promachocrinus dataset in nexus format. Supplementary material is available online.
Data availability
Newly generated sequences, with details in the Supplementary Table, are available through GenBank (https://www.ncbi.nlm.nih.gov/).
Conflicts of interest
The authors declare that authors Nerida Wilson and Greg Rouse are also Editors of Invertebrate Systematics but did not at any stage have editor-level access to this manuscript while in peer review, as is the standard practice when handling manuscripts submitted by an editor to this journal. Invertebrate Systematics encourages editors to publish in the journal and editors are kept totally separate from the decision-making processes for manuscripts. The authors have no further conflicts of interest to declare.
Acknowledgements
The authors are grateful for the efforts of many expedition participants in making these samples available. Avery Hiley, Jose Carvajal, Alex Hickling, Ekin Tilic and Charlotte Seid are thanked for support with sequencing or cataloguing. We thank the two anonymous reviewers for the valuable comments on the original submission.
References
Adams M, Raadik TA, Burridge CP, Georges A (2014) Global biodiversity assessment and hyper-cryptic species complexes: more than one species of elephant in the room? Systematic Biology 63, 518-533.
| Crossref | Google Scholar |
Allcock AL, Brierley AS, Thorpe JP, Rodhouse PG (1997) Restricted gene flow and evolutionary divergence between geographically separated populations of the Antarctic octopus Pareledone turqueti. Marine Biology 129, 97-102.
| Crossref | Google Scholar |
Allcock AL, Barratt I, Eléaume M, Linse K, Norman MD, Smith PJ, Steinke D, Stevens DW, Strugnell JM (2011) Cryptic speciation and the circumpolarity debate: a case study on endemic Southern Ocean octopuses using the COI barcode of life. Deep-Sea Research – II. Topical Studies in Oceanography 58, 242-249.
| Crossref | Google Scholar |
Anderson JB, Shipp SS, Lowe AL, Wellner JS, Mosola AB (2002) The Antarctic Ice Sheet during the Last Glacial Maximum and its subsequent retreat history: a review. Quaternary Science Reviews 21, 49-70.
| Crossref | Google Scholar |
Arango CP, Soler-Membrives A, Miller KJ (2011) Genetic differentiation in the circum-Antarctic sea spider Nymphon australe (Pycnogonida; Nymphonidae). Deep-Sea Research – II. Topical Studies in Oceanography 58, 212-219.
| Crossref | Google Scholar |
Baird HP, Miller KJ, Stark JS (2011) Evidence of hidden biodiversity, ongoing speciation and diverse patterns of genetic structure in giant Antarctic amphipods. Molecular Ecology 20, 3439-3454.
| Crossref | Google Scholar |
Bentley MJ, Ó Cofaigh C, Anderson JB, Conway H, Davies B, Graham AGC, Hillenbrand CD, Hodgson DA, Jamieson SSR, Larter RD, Mackintosh A, Smith JA, Verleyen E, Ackert RP, Bart PJ, Berg S, Brunstein D, Canals M, Colhoun EA, Crosta X, Dickens WA, Domack E, Dowdeswell JA, Dunbar R, Ehrmann W, Evans J, Favier V, Fink D, Fogwill CJ, Glasser NF, Gohl K, Golledge NR, Goodwin I, Gore DB, Greenwood SL, Hall BL, Hall K, Hedding DW, Hein AS, Hocking EP, Jakobsson M, Johnson JS, Jomelli V, Jones RS, Klages JP, Kristoffersen Y, Kuhn G, Leventer A, Licht K, Lilly K, Lindow J, Livingstone SJ, Massé G, McGlone MS, McKay RM, Melles M, Miura H, Mulvaney R, Nel W, Nitsche FO, O’Brien PE, Post AL, Roberts SJ, Saunders KM, Selkirk PM, Simms AR, Spiegel C, Stolldorf TD, Sugden DE, van der Putten N, van Ommen T, Verfaillie D, Vyverman W, Wagner B, White DA, Witus AE, Zwartz D (2014) A community-based geological reconstruction of Antarctic Ice Sheet deglaciation since the Last Glacial Maximum. Quaternary Science Reviews 100, 1-9.
| Crossref | Google Scholar |
Berriman JS, Ellingson RA, Awbrey JD, Rico DM, Valdés ÁA, Wilson NG, Aguilar A, Herbert DG, Hirano YM, Trowbridge CD, Krug PJ (2018) A biting commentary: integrating tooth characters with molecular data doubles known species diversity in a lineage of sea slugs that consume “killer algae”. Molecular Phylogenetics and Evolution 126, 356-370.
| Crossref | Google Scholar |
Bosch I, Pearse JS (1990) Developmental types of shallow-water asteroids of McMurdo Sound, Antarctica. Marine Biology 104, 41-46.
| Crossref | Google Scholar |
Brandão SN, Sauer J, Schön I (2010) Circumantarctic distribution in Southern Ocean benthos? A genetic test using the genus Macroscapha (Crustacea, Ostracoda) as a model. Molecular Phylogenetics and Evolution 55, 1055-1069.
| Crossref | Google Scholar |
Brandt A, Gooday AJ, Brandão SN, Brix S, Brökeland W, Cedhagen T, Choudhury M, Cornelius N, Danis B, De Mesel I, Diaz RJ, Gillan DC, Ebbe B, Howe JA, Janussen D, Kaiser S, Linse K, Malyutina M, Pawlowski J, Raupach M, Vanreusel A (2007) First insights into the biodiversity and biogeography of the Southern Ocean deep sea. Nature 447, 307-311.
| Crossref | Google Scholar |
Brasier MJ, Wiklund H, Neal L, Jeffreys R, Linse K, Ruhl H, Glover AG (2016) DNA barcoding uncovers cryptic diversity in 50% of deep-sea Antarctic polychaetes. Royal Society Open Science 3, 160432.
| Crossref | Google Scholar |
Carpenter PH (1879) II. Preliminary report upon the Comatulœ of the ‘Challenger’ Expedition. Proceedings of the Royal Society of London 28, 383-395.
| Crossref | Google Scholar |
Carpenter PH (1880) On the Genus Solanocrinus Goldfuss, and its relations to recent Comatulae. The Zoological Journal of the Linnean Society 15, 187-217.
| Crossref | Google Scholar |
Carpenter PH (1888) Report on the Crinoidea collected during the voyage of H.M.S. Challenger, during the years 1873–76 Part II. The Comatulae. Reports of the Scientific Results of the Voyage of H.M.S. Challenger, Zoology 26(60), 1-402.
| Google Scholar |
Chown SL, Clarke A, Fraser CI, Cary SC, Moon KL, McGeoch MA (2015) The changing form of Antarctic biodiversity. Nature 522, 431-438.
| Crossref | Google Scholar |
Clark AH (1913) Notes on the recent crinoids in the British Museum. Smithsonian Miscellaneous Collections 61, 1-89.
| Google Scholar |
Clark AH (1915) Die Crinoiden Der Antarktis. [The crinoids of Antarctica.]. Deutsche Südpolar-Expedition 1901–1903 16, 101-210 [In German].
| Google Scholar |
Clark AH (1937) Crinoidea. Scientific Reports of the Australasian Antarctic Expedition 1911–14, under the Leadership of Sir Douglas Mawson Series C Zoology and Botany 8(4), 1-18.
| Google Scholar |
Clark AH (1941) A monograph of the existing crinoids, The comatulids. Vol. 1. Part 4a. Bulletin of the United States National Museum 82, 1-603.
| Google Scholar |
Clark AH, Clark AM (1967) A monograph of the existing crinoids. Vol. 1. Part 5. Bulletin of the United States National Museum 82, 1-860.
| Google Scholar |
Clarke A, Crame JA (1989) The origin of the Southern Ocean marine fauna. Geological Society, London, Special Publications 47, 253-268.
| Crossref | Google Scholar |
Clarke A, Crame JA (1992) The Southern Ocean benthic fauna and climate change: a historical perspective. Philosophical Transactions of the Royal Society of London – B. Biological Sciences 338, 299-309.
| Crossref | Google Scholar |
Convey P, Stevens MI, Hodgson DA, Smellie JL, Hillenbrand CD, Barnes DKA, Clarke A, Pugh PJA, Linse K, Cary SC (2009) Exploring biological constraints on the glacial history of Antarctica. Quaternary Science Reviews 28, 3035-3048.
| Crossref | Google Scholar |
Darriba D, Posada D, Kozlov AM, Stamatakis A, Morel B, Flouri T (2020) ModelTest-NG: a new and scalable tool for the selection of DNA and protein evolutionary models. Molecular Biology and Evolution 37, 291-294.
| Crossref | Google Scholar |
Dayton PK, Oliver JS (1977) Antarctic soft-bottom benthos in oligotrophic and eutrophic environments. Science 197, 55-58.
| Crossref | Google Scholar |
Delić T, Trontelj P, Rendoš M, Fišer C (2017) The importance of naming cryptic species and the conservation of endemic subterranean amphipods. Scientific Reports 7, 3391.
| Crossref | Google Scholar |
Dietz L, Arango CP, Dömel JS, Halanych KM, Harder AM, Held C, Mahon AR, Mayer C, Melzer RR, Rouse GW, Weis A, Wilson NG, Leese F (2015) Regional differentiation and extensive hybridization between mitochondrial clades of the Southern Ocean giant sea spider Colossendeis megalonyx. Royal Society Open Science 2, 140424.
| Crossref | Google Scholar |
Edgar RC (2004) MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 19, 113.
| Crossref | Google Scholar |
Edler D, Klein J, Antonelli A, Silvestro D (2021) raxmlGUI 2.0: a graphical interface and toolkit for phylogenetic analyses using RAxML. Methods in Ecology and Evolution 12, 373-377.
| Crossref | Google Scholar |
Eléaume M, Hemery LG, Ameziane N, Roux M (2014) Chapter 10.7. Phylogeographic patterns of the Southern Ocean crinoids. In ‘SCAR-Marine Biodiversity Information Network, biogeographic atlas of the Southern Ocean’. (Eds C De Broyer, P Koubbi, H Griffiths, B Raymond, C D’Udekem d’Acoz, A Van De Putte, B Danis, B David, S Grant, J Gutt, C Held, G Hosie, F Huettmann, A Post, Y Ropert-Coudert) pp. 448–455. (The Scientific Committee on Antarctic Research)
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3, 294-299.
| Google Scholar |
Fontaine B, Perrard A, Bouchet P (2012) 21 years of shelf life between discovery and description of new species. Current Biology 22(22), R943-R944.
| Crossref | Google Scholar |
Fraser CI, Nikula R, Ruzzante DE, Waters JM (2012) Poleward bound: biological impacts of southern hemisphere glaciation. Trends in Ecology & Evolution 27, 462-471.
| Crossref | Google Scholar |
González-Wevar CA, Gérard K, Rosenfeld S, Saucède T, Naretto J, Díaz A, Morley SA, Brickle P, Poulin E (2019) Cryptic speciation in Southern Ocean Aequiyoldia eightsii (Jay, 1839): Mio-Pliocene trans-Drake Passage separation and diversification. Progress in Oceanography 174, 44-54.
| Crossref | Google Scholar |
Halt MN, Kupriyanova EK, Cooper SJB, Rouse GW (2009) Naming species with no morphological indicators: species status of Galeolaria caespitosa (Annelida: Serpulidae) inferred from nuclear and mitochondrial gene sequences and morphology. Invertebrate Systematics 23, 205-222.
| Crossref | Google Scholar |
Held C (2003) Molecular evidence for cryptic speciation within the widespread Antarctic crustacean Ceratoserolis trilobitoides (Crustacea, Isopoda). In ‘Antarctic Biology in a Global Context’. (Eds AHL Huiskes, WWC Gieskes, J Rozema, RML Schorno, SM van der Vies, WJ Wolff) pp. 135–139. (Backhuys Publishers: Leiden, Netherlands)
Helgen LE, Rouse GW (2006) Species delimitation and distribution in Aporometra (Crinoidea: Echinodermata): endemic Australian featherstars. Invertebrate Systematics 20(3), 395-414.
| Crossref | Google Scholar |
Hemery LG, Eléaume M, Roussel V, Améziane N, Gallut C, Steinke D, Cruaud C, Couloux A, Wilson NG (2012) Comprehensive sampling reveals circumpolarity and sympatry in seven mitochondrial lineages of the Southern Ocean crinoid species Promachocrinus kerguelensis (Echinodermata). Molecular Ecology 21, 2502-2518.
| Crossref | Google Scholar |
Hemery LG, Roux M, Ameziane N, Eléaume M (2013) High-resolution crinoid phyletic inter-relationships derived from molecular data. Cahiers de Biologie Marine 54, 511-523.
| Google Scholar |
John DD (1938) Crinoidea. Discovery Reports 18, 121-222.
| Google Scholar |
Jossart Q, Sands CJ, Sewell MA (2019) Dwarf brooder versus giant broadcaster: combining genetic and reproductive data to unravel cryptic diversity in an Antarctic brittle star. Heredity 123, 622-633.
| Crossref | Google Scholar |
Kapli P, Lutteropp S, Zhang J, Kobert K, Pavlidis P, Stamatakis A, Flouri T (2017) Multi-rate poisson tree processes for single-locus species delimitation under maximum likelihood and Markov Chain Monte Carlo. Bioinformatics 33, 1630-1638.
| Crossref | Google Scholar |
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30, 772-780.
| Crossref | Google Scholar |
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A (2012) Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28, 1647-1649.
| Crossref | Google Scholar |
Kozlov AM, Darriba D, Flouri T, Morel B, Stamatakis A (2019) RAxML-NG: a fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics 35, 4453-4455.
| Crossref | Google Scholar |
Krabbe K, Leese F, Mayer C, Tollrian R, Held C (2010) Cryptic mitochondrial lineages in the widespread pycnogonid Colossendeis megalonyx Hoek, 1881 from Antarctic and Subantarctic waters. Polar Biology 33, 281-292.
| Crossref | Google Scholar |
Lanterbecq D, Rouse GW, Eeckhaut I (2010) Evidence for cospeciation events in the host-symbiont system involving crinoids (Echinodermata) and their obligate associates, the myzostomids (Myzostomida, Annelida). Molecular Phylogenetics and Evolution 54, 357-371.
| Crossref | Google Scholar |
Larsen BB, Miller EC, Rhodes MK, Wiens JJ (2017) Inordinate fondness multiplied and redistributed: the number of species on Earth and the new pie of life. The Quarterly Review of Biology 92(3), 229-265.
| Crossref | Google Scholar |
Lau SCY, Wilson NG, Silva CNS, Strugnell JM (2020) Detecting glacial refugia in the Southern Ocean. Ecography 43(11), 1639-1656.
| Crossref | Google Scholar |
Leese F, Kop A, Wägele JW, Held C (2008) Cryptic speciation in a benthic isopod from Patagonian and Falkland Island waters and the impact of glaciations on its population structure. Frontiers in Zoology 5, 19.
| Crossref | Google Scholar |
Lemoine F, Domelevo Entfellner JB, Wilkinson E, Correia D, Dávila Felipe M, De Oliveira T, Gascuel O (2018) Renewing Felsenstein’s phylogenetic bootstrap in the era of big data. Nature 556(7702), 452-456.
| Crossref | Google Scholar |
Maroni PJ, Baker BJ, Moran AL, Woods HA, Avila C, Johnstone GJ, Stark JS, Kocot KM, Lockhart S, Saucède T, Rouse GW, Wilson NG (2022) One Antarctic slug to confuse them all: the underestimated diversity of Doris kerguelenensis. Invertebrate Systematics 36(5), 419-435.
| Crossref | Google Scholar |
Marr JWS (1963) Unstalked crinoids of the Antarctic Continental Shelf, notes on their natural history and distribution. Philosophical Transactions of the Royal Society of London Series – B. Biological Sciences 246, 327-379.
| Crossref | Google Scholar |
Matsuoka K, Skoglund A, Roth G, de Pomereu J, Griffiths H, Headland R, Herried B, Katsumata K, Le Brocq A, Licht K, Morgan F, Neff PD, Ritz C, Scheinert M, Tamura T, Van de Putte A, van den Broeke M, von Deschwanden A, Deschamps-Berger C, Van Liefferinge B, Tronstad S, Melvær Y (2021) Quantarctica, an integrated mapping environment for antarctica, the Southern Ocean, and sub-Antarctic islands. Environmental Modelling & Software 140, 105015.
| Crossref | Google Scholar |
McClintock JB, Pearse JS (1987) Reproductive biology of the common Antarctic crinoid Promachocrinus kerguelensis (Echinodermata: Crinoidea). Marine Biology 96, 375-383.
| Crossref | Google Scholar |
Messing CG, Ameziane N, Eleaume M (2000) Echinodermata Crinoidea: comatulid crinoids of the KARUBAR Expedition to Indonesia. The families Comasteridae, Asterometridae, Calometridae and Thalassometridae. Memoires du Museum National d’Histoire Naturelle 184, 627-702.
| Google Scholar |
Messing C, Gondim AI, Taylor K (2023a) Promachocrinus Carpenter, 1879. In ‘World List of Crinoidea’. (World Register of Marine Species) Available at https://www.marinespecies.org/aphia.php?p=taxdetails&id=173828
Messing C, Gondim AI, Taylor K (2023b) Florometra mawsoni AH Clark, 1937. In ‘World List of Crinoidea’. (World Register of Marine Species) Available at https://www.marinespecies.org/aphia.php?p=taxdetails&id=173812
Minckert VW (1905) Das Genus Promachocrinus, zugleich ein Beitrag zur Faunistik der Antarktis. [The genus Promachocrinus, at the same time a contribution to the faunistics of the Antarctic.]. Zoologischer Anzeiger 28, 490-501 [In German].
| Google Scholar |
Moles J, Berning MI, Hooker Y, Padula V, Wilson NG, Schrödl M (2021) Due south: the evolutionary history of sub-Antarctic and Antarctic Tritoniidae nudibranchs. Molecular Phylogenetics and Evolution 162, 107209.
| Crossref | Google Scholar |
Mortensen T (1918) The Crinoidea of the Swedish Antarctic Expedition. Wissenschaftliche Ergebnisse der Schwedischen Südpolar-Expedition 1901–1903 4(8), 1-23.
| Google Scholar |
Mutschke E, Rios C (2006) Spatial distribution and relative abundance of echinoderms from the Strait of Magellan, Chile. Ciencia y Tecnología del Mar 29, 91-102.
| Google Scholar |
Nygren A (2014) Cryptic polychaete diversity: a review. Zoologica Scripta 43, 172-183.
| Crossref | Google Scholar |
Page RDM (2016) DNA barcoding and taxonomy: dark taxa and dark texts. Philosophical Transactions of the Royal Society of London – B. Biological Sciences 371, 20150334.
| Crossref | Google Scholar |
Park Y-H, Durand I, Kestenare E, Rougier G, Zhou M, d’Ovidio F, Cotté C, Lee JH (2014) Polar front around the Kerguelen Islands: an up-to-date determination and associated circulation of surface/subsurface waters. Journal of Geophysical Research: Oceans 119, 6575-6592.
| Crossref | Google Scholar |
Pollard D, DeConto RM (2009) Modelling West Antarctic ice sheet growth and collapse through the past five million years. Nature 458, 329-332.
| Crossref | Google Scholar |
Puillandre N, Brouillet S, Achaz G (2021) ASAP: assemble species by automatic partitioning. Molecular Ecology Resources 21, 609-620.
| Crossref | Google Scholar |
Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA (2018) Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Systematic Biology 67, 901-904.
| Crossref | Google Scholar |
Raupach MJ, Wägele JW (2006) Distinguishing cryptic species in Antarctic Asellota (Crustacea: Isopoda) – a preliminary study of mitochondrial DNA in Acanthaspidia drygalskii. Antarctic Science 18, 191-198.
| Crossref | Google Scholar |
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572-1574.
| Crossref | Google Scholar |
Soler-Membrives A, Linse K, Miller KJ, Arango CP (2017) Genetic signature of last glacial maximum regional refugia in a circum-Antarctic sea spider. Royal Society Open Science 4, 170615.
| Crossref | Google Scholar |
Strugnell JM, Rogers AD, Prodöhl PA, Collins MA, Allcock AL (2008) The thermohaline expressway: the Southern Ocean as a centre of origin for deep-sea octopuses. Cladistics 24, 853-860.
| Crossref | Google Scholar |
Summers MM, Al-Hakim II, Rouse GW (2014) Turbo-taxonomy: 21 new species of Myzostomida (Annelida). Zootaxa 3873, 301-344.
| Crossref | Google Scholar |
Swofford DL (2002) Phylogenetic analysis using parsimony. Options 42, 294-307.
| Google Scholar |
Thatje S, Hillenbrand CD, Larter R (2005) On the origin of Antarctic marine benthic community structure. Trends in Ecology & Evolution 20, 534-540.
| Crossref | Google Scholar |
Thatje S, Hillenbrand CD, Mackensen A, Larter R (2008) Life hung by a thread: endurance of Antarctic fauna in glacial periods. Ecology 89, 682-692.
| Crossref | Google Scholar |
Vaidya G, Lohman DJ, Meier R (2011) Sequence Matrix: concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics 27, 171-180.
| Crossref | Google Scholar |
Vaney C (1910) Une nouvelle espèce de Promachocrinus. [A new species of Promachocrinus.]. Bulletin du Museum Paris 16, 158-162 [In French].
| Google Scholar |
Weber AAT, Mérigot B, Valière S, Chenuil A (2015) Influence of the larval phase on connectivity: strong differences in the genetic structure of brooders and broadcasters in the Ophioderma longicauda species complex. Molecular Ecology 24, 6080-6094.
| Crossref | Google Scholar |
Wilson NG, Hunter RL, Lockhart SJ, Halanych KM (2007) Multiple lineages and absence of panmixia in the ‘circumpolar’ crinoid Promachocrinus kerguelensis from the Atlantic sector of Antarctica. Marine Biology 152, 895-904.
| Crossref | Google Scholar |
Wilson NG, Maschek JA, Baker BJ (2013) A species flock driven by predation? Secondary metabolites support diversification of slugs in Antarctica. PloS One 8(11), e80277.
| Crossref | Google Scholar |