A preliminary phylogeny for the pseudoscorpion family Garypinidae (Pseudoscorpiones: Garypinoidea), with new taxa and remarks on the Australasian fauna
Mark S. Harvey A B *
A B *
A Collections & Research, Western Australian Museum, 49 Kew Street, Welshpool, WA 6106, Australia.
B School of Biological Sciences, University of Western Australia, Crawley, WA 6009, Australia.
Invertebrate Systematics 37(9) 623-676 https://doi.org/10.1071/IS23029
Submitted: 10 June 2023 Accepted: 7 August 2023 Published: 26 September 2023
Abstract
The pseudoscorpion family Garypinidae is globally distributed with 79 species in 21 genera and several species represented by Mesozoic and Eocene fossils. This was recently included with the family Larcidae in a unique superfamily, Garypinoidea but there are no phylogenetic hypotheses for the group. Sequence data were obtained for 14 species in 8 genera and numerous outgroup taxa that formed the basis for a preliminary molecular phylogeny. A new subfamily classification is proposed with Protogarypininae, subfamily nov. comprising five genera mostly found in the southern hemisphere, Amblyolpiinae subfamily nov. comprising two genera and Garypininae for the remaining genera. Several new taxa are described including the first Australian species of Aldabrinus, A. rixi sp. nov., a new genus from South-East Asia, Nobilipinus, comprising Nobilipinus nobilis (With, 1906), N. vachoni (Redikorzev, 1938) (that is removed from the synonymy of G. nobilis) and five new species, N. affinis, N. galeatus, N. karenae, N. kohi and N. tricosus, and Solinus pingrup sp. nov. from south-western Australia. Paraldabrinus Beier, 1966 is newly synonymised with Aldabrinus, and Indogarypinus Murthy and Ananthakrishan, 1977 is newly synonymised with Solinus. The holotype of Garypinus mirabilis With, 1907 from Hawaii is redescribed but found to be a tritonymph, rendering the generic identity uncertain.
ZooBank: urn:lsid:zoobank.org:pub:E15E4705-0697-4208-9338-A778343996CA
Keywords: Aldabrinus, classification, Garypinus, morphology, new taxa, Nobilipinus, Solinus, taxonomy.
Introduction
The pseudoscorpion family Garypinidae is widely distributed globally and currently comprises 81 valid species in 21 genera. Ever since the early 1930s, the group was treated as a subfamily of the Olpiidae (e.g. Chamberlin 1930, 1931a; Beier 1932a; Harvey 1992). However, this was treated as a separate family by Judson (1993, 2005) based on several features that, although not found in all genera, served to separate most taxa from the Olpiidae and other families. This included the divided arolia, the offset chelal pedicel and the basal concentration of trichobothria on the prolateral face of the fixed finger (Judson 2005). A recent transcriptomic analysis that included representatives of most pseudoscorpion families found Garypinidae to group with Larcidae, forming a unique superfamily Garypinoidea that was sister to a clade comprising Sternophoroidea, Cheiridioidea and Cheliferoidea (Benavides et al. 2019). The composition of the Garypinidae is uncontroversial, even though there have never been explicit analyses that have tested for monophyly. However, Larcidae was recovered within the Garypinidae, rendering the latter paraphyletic.
Subgroups of Garypinidae have never been proposed, despite some notable differences between certain garypinine genera. For example, whereas Garypinus Daday, 1889, Amblyolpium Simon, 1898, Serianus Chamberlin, 1930, Solinus Chamberlin, 1930 and several other genera have deeply divided arolia, others such as Protogarypinus Beier, 1954 from Australia have undivided arolia, as found in Olpiinae. This prompted Beier (1954a) to speculate that although this genus was a member of the Garypininae (that was a subfamily of Olpiidae), Protogarypinus was a primitive genus that showed a transition between the Olpiinae and Garypininae. Somewhat surprisingly, Beier (1954a) did not link Protogarypinus with other genera that also lack divided arolia, such as the Neotropical genera Neominniza Beier, 1930, Thaumatolpium Beier, 1931, Teratolpium Beier, 1959 (Beier 1959a, 1964a). Harvey and Šťáhlavský (2010) included these genera, along with Oreolpium Benedict & Malcolm, 1978 from western USA and Tasmania, in the Garypinidae but highlighted the lack of divided arolia as an anomalous feature within the family.
Other garypinids with unusual morphological features include Amblyolpium and Neoamblyolpium Hoff, 1956. Although these possess divided arolia and tergites typical of many other garypinids, venom ducts in the chelal fingers are long and three of the prolateral series of trichobothria that are on the movable chelal finger (isb, ist and it) are situated medially on the finger, compared with the subbasal position found in all other garypinids.
To test the relationships of the genera of Garypinidae, a molecular analysis that included 14 species in 8 genera that were either newly acquired or derived from previous studies (Murienne et al. 2008; Arabi et al. 2012) provided a preliminary molecular phylogeny on which to judge the merits of the morphological features mentioned above. The results of that study are presented here and a new subfamily classification is proposed, with taxa with undivided arolia placed in the new subfamily Protogarypininae, those with long venom ducts and a peculiar trichobothrial pattern placed in the new subfamily Amblyolpiinae, and the remainder in the Garypininae.
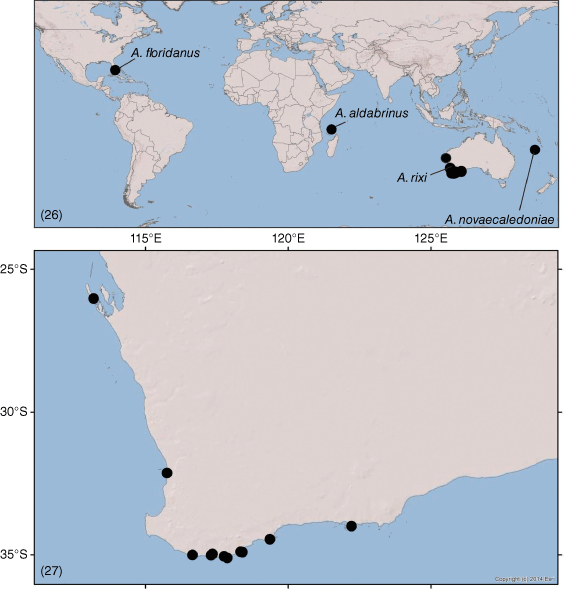
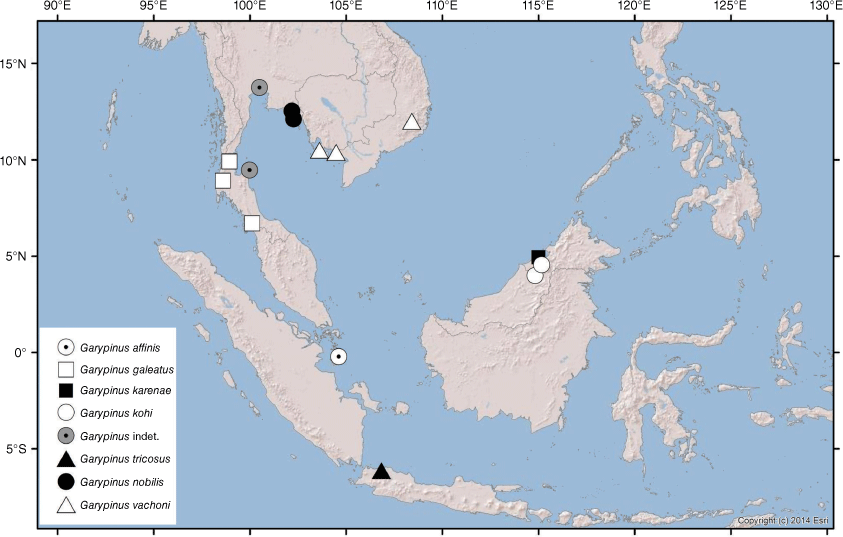
Although there are only 79 described species currently recognised as valid, numerous unnamed species are known from museum collections (M. S. Harvey, unpubl. data), primarily from tropical habitats. The remainder of this study provides new information on several poorly known garypinids from Asia and the Pacific region. The fauna of the Indo-Malayan region is relatively depauperate with only five valid species: Amblyolpium bellum Chamberlin, 1930 from Indonesia (Chamberlin 1930; Harvey 1988), A. biaroliatum (Tömösváry, 1884) from the East Indies (Tömösváry 1884), A. birmanicum (With, 1906) from India, Myanmar and Sumatra (With 1906; Ellingsen 1914; Beier 1930), Caecogarypinus pectinodentatus Dashdamirov, 2007 from Vietnam (Dashdamirov 2007), Garypinus nobilis With, 1906 from Thailand (With 1906) and Indogarypinus minutus Murthy & Ananthakrishnan, 1977 from India (Murthy and Ananthakrishnan 1977). A sixth species, G. vachoni Redikorzev, 1938 was described from the region but later synonymised with G. nobilis by Schawaller (1994). Likewise the fauna of Australasia and Oceania is represented by only 11 species: Amblyolpium ruficeps Beier, 1966 and Paraldabrinus novaecaledoniae Beier, 1966 from New Caledonia (Beier 1966a); Oreolpium semotum Harvey and Šťáhlavský, 2010, Protogarypinus dissimilis Beier, 1975, P. giganteus Beier, 1954 and Solinus australiensis Chamberlin, 1930 from Australia (Chamberlin 1930; Beier 1954a, 1975; Harvey and Šťáhlavský 2010); Nelsoninus maoricus Beier, 1967 from New Zealand (Beier 1967a); Amblyolpium novaeguineae Beier, 1971 and Solinus pusillus Beier, 1971 from Papua New Guinea (Beier 1971a); Amblyolpium salomonense Beier, 1970 and Serianus salomonensis Beier, 1966 from the Solomon Islands (Beier 1966b, 1970a); and Garypinus mirabilis With, 1907 from Hawaii (With 1907).
Materials and methods
Morphological methods
The material examined for this study is lodged in the Natural History Museum, London (BMNH), Brunei Museum, Kota Baru (BMKB), Muséum d’histoire naturelle, Genève (MHNG), Naturhistorisches Museum, Vienna (NHMW), Senckenberg Forschungsinstitut und Naturmuseum, Frankfurt, Germany (SMF) and the Western Australian Museum, Perth (WAM). Specimens were studied using temporary slide mounts prepared by immersion of the specimen in lactic acid at room temperature for several hours to days and mounting these on microscope slides with 10-mm coverslips supported by small sections of 0.25-, 0.35- or 0.5-mm diameter nylon fishing line. After study, specimens were rinsed in distilled water and returned to 75% ethanol with the dissected portions placed in 12 × 3-mm glass genitalia microvials (BioQuip Products, Inc.).
Specimens were examined with a Leica MZ–16A (Wetzlar, Germany) dissecting microscope and an Olympus BH–2 (Shinjuku, Tokyo) compound microscope, and illustrated with the aid of a drawing tube attached to the compound microscopes. Measurements were taken at the highest possible magnification using an ocular graticule.
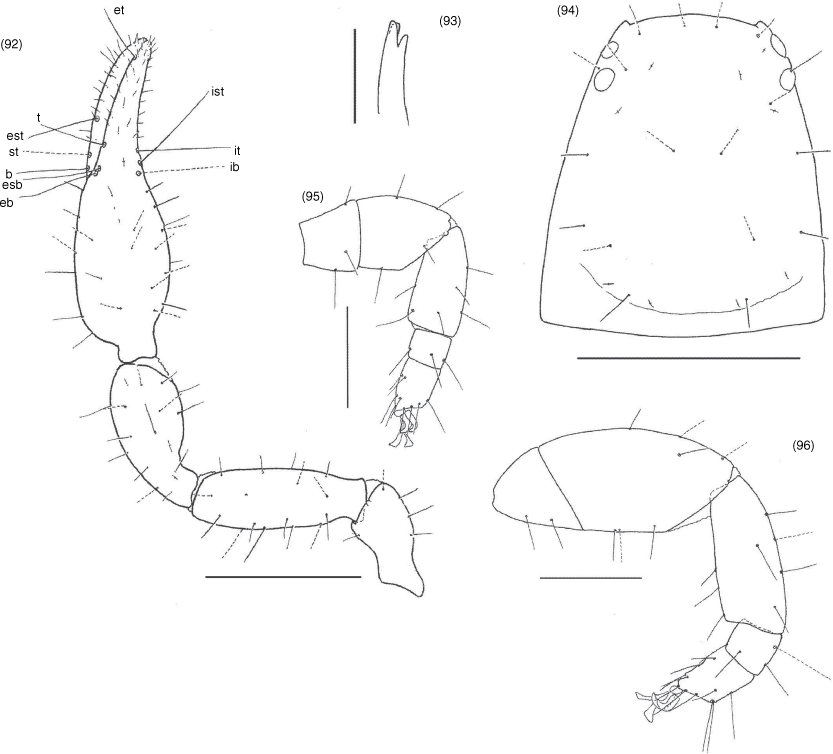
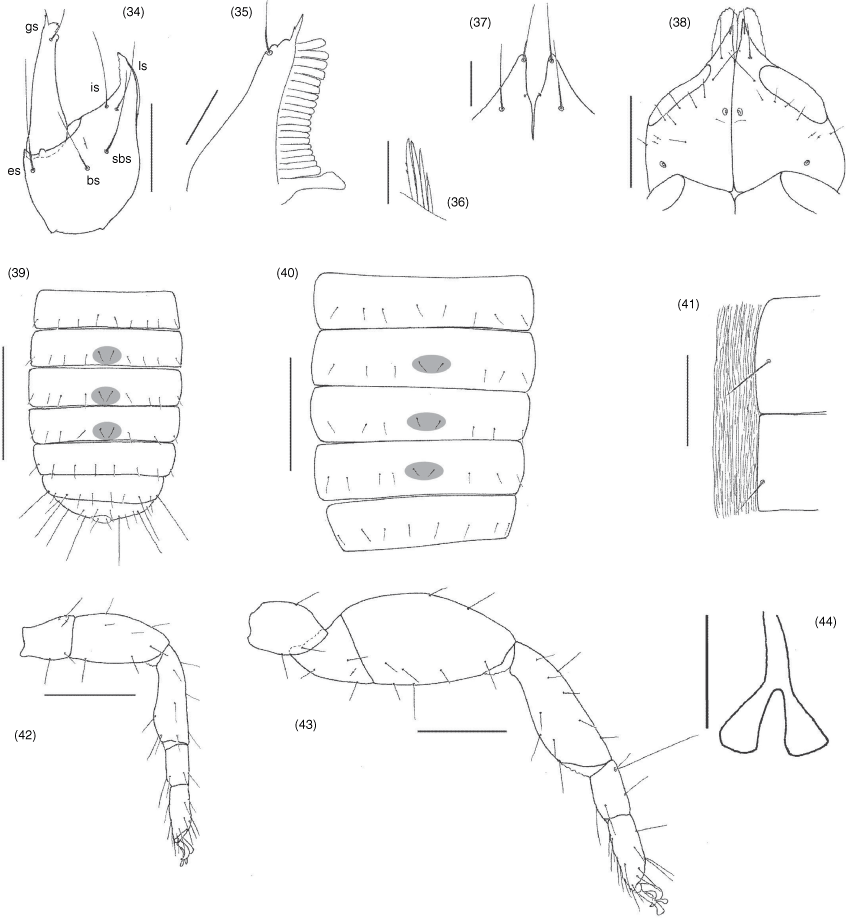
Terminology and mensuration mostly follow Chamberlin (1931a), with the exception of the nomenclature of the pedipalps, legs and with some minor modifications to the terminology of the trichobothria (Harvey 1992), chelicera (Judson 2007) and faces of the appendages (Harvey et al. 2012). Measurements were taken to the nearest 0.005 mm. The following abbreviations are used: chelal trichobothria: fixed finger, eb, externo-basal trichobothrium; esb, externo-subbasal trichobothrium; est, externo-subterminal trichobothrium; et, externo-terminal trichobothrium; ib, interno-basal trichobothrium; isb, interno-subbasal trichobothrium; ist, interno-subterminal trichobothrium; it, interno-terminal trichobothrium; movable finger, b, basal trichobothrium; sb, subbasal trichobothrium; st, subterminal trichobothrium; t, terminal trichobothrium; cheliceral setae: ebs, externobasal seta; es, external seta; gs, galeal seta; is, interior seta; ls, laminal seta; sbs, subbasal seta; male genitalia: dag, dorsal anterior gland; ejca, ejaculatory duct of the genital atrium; lgs, lateral genital sac; vag, ventral anterior gland.
Molecular methods
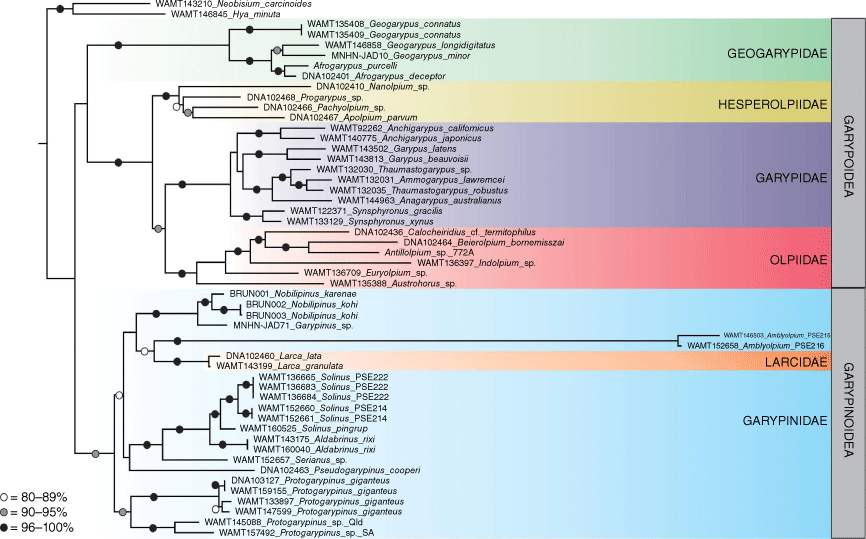
Taxon selection was designed to test for the monophyly of the family Garypinidae, and to test the relationships of the family with other Garypinoidea (i.e. the family Larcidae) and with the morphologically similar Garypoidea (i.e. the families Garypidae, Geogarypidae, Hesperolpiidae and Olpiidae). The analysis was rooted on a species of Chthonioidea, Austrochthonius sp. that is the sister taxon to the remaining pseudoscorpions (Benavides et al. 2019). Other outgroups included a species of the superfamily Feaelloidea (Neopseudogarypus scutellatus Morris, 1948) and the superfamily Neobisioidea (Hya minuta Tullgren, 1907 and Neobisium carcinoides (Hermann, 1803)). For clarity, the phylogram (Fig. 1) omits the chthonioid and feaelloid outgroups.
Maximum likelihood phylogeny of Garypoidea and Garypinoidea, based on alignment of concatenated COI, 18S and 28S. Bootstrap values are presented for nodes greater than 80%.
Fragments of one mitochondrial and two nuclear genes were sequenced using standard Sanger methodology for this study and these sequences have proven to be useful in reconstructing pseudoscorpion phylogeny in a range of different taxa (Murienne et al. 2008; Harvey et al. 2015, 2016a, 2016b, 2020; Harms et al. 2019; Johnson et al. 2022; Hlebec et al. 2023). The present study included fragments of the cytochrome c oxidase subunit I (COI), 18S ribosomal RNA (18S) and 28S ribosomal RNA (28S). Molecular methods used for the polymerase chain reaction (PCR) amplification of each of these genes followed Harvey et al. (2015) and Harvey et al. (2016a, 2020), with PCR purification and Sanger bidirectional sequencing conducted by the Australian Genome Research Facility (AGRF; Perth). Chromatograms were edited using the Geneious software package (ver. 2021.2, Biomatters Ltd, see https://www.geneious.com/; Kearse et al. 2012) and resulting nucleotide sequences for all taxa are deposited in GenBank (Table 1). Sequences were aligned using the MAFFT (ver. 7.490, see https://mafft.cbrc.jp/alignment/software/; Katoh et al. 2002; Katoh and Standley 2013) plug-in within Geneious with the default settings. The concatenated alignments were analysed using maximum likelihood (ML) methodology in the web version of IQ-TREE (ver. 2.2.0, see http://www.iqtree.org/; Trifinopoulos et al. 2016; Minh et al. 2020). The substitution model option was set at Auto and the branch support analysis was performed with 5000 bootstrap alignments.
| Family | Species | Locality | Repository and number | COI | 18S rRNA | 28S rRNA | |
|---|---|---|---|---|---|---|---|
| Superfamily Chthonioidea | |||||||
| Chthoniidae | Austrochthonius sp. | AUSTRALIA: Western Australia: Millstream-Chichester National Park, George River, 2.2 km SE of Mount Montagu | WAM T135835 | OR067318 | OR039602 | OR059142 | |
| Superfamily Feaelloidea | |||||||
| Pseudogarypidae | Neopseudogarypus scutellatus Morris, 1948 | AUSTRALIA: Tasmania: Launceston, Cataract Gorge | WAM T104213 | OR067276 | OR039591 | OR059173 | |
| Superfamily Garypinoidea | |||||||
| Garypinidae | Aldabrinus rixi Harvey, sp. nov. | AUSTRALIA: Western Australia: Torndirrup National Park, Sharp Point at end of Eclipse Island Road | WAM T143175 | OR359471 | OR359931 | OR372788 | |
| AUSTRALIA: Western Australia: Denmark | WAM T160040 | OR359472 | – | – | |||
| Amblyolpium sp. ‘PSE215’ | AUSTRALIA: Western Australia: Paraburdoo Range, ~12.0 km SW of Paraburdoo | WAM T146503 | OR359473 | OR359932 | OR372787 | ||
| Amblyolpium sp. ‘PSE216’ | AUSTRALIA: Western Australia: ~44.4 km SSE of Menzies | WAM T152658 | OR359474 | OR359933 | – | ||
| Garypinus sp. JA-2011 | [Unknown] | MNHN-JAD71 | JN018179 | JN018296 | JN018393 | ||
| Nobilipinus karenae Harvey, sp. nov. | BRUNEI DARUSSALAM: Ulu Temberong National Park, near Kuala Belalong Field Studies Centre | BRUN001 | OR359476 | OR359936 | OR372784 | ||
| Nobilipinus kohi Harvey, sp. nov. | BRUNEI DARUSSALAM: Mengkubau, off Jalan Penghubang mentiri | BRUN002 | OR359477 | OR359934 | – | ||
| BRUNEI DARUSSALAM: Mengkubau, off Jalan Penghubang mentiri | BRUN003 | OR359478 | OR359935 | – | |||
| Protogarypinus giganteus Beier, 1954 | AUSTRALIA: Western Australia: Walpole-Nornalup National Park, The Tingle Tree | DNA103127 | EU559565 | EU559377 | EU559484 | ||
| AUSTRALIA: Western Australia: Fitzgerald River National Park, summit of West Mount Barren | WAM T133897 | OR359479 | OR359937 | – | |||
| AUSTRALIA: Western Australia: Kuch Road | WAM T147599 | OR359480 | OR359938 | OR372783 | |||
| AUSTRALIA: Western Australia: Walpole-Nornalup National Park, Cemetery Road | WAM T159155 | OR359481 | OR359939 | OR372782 | |||
| Protogarypinus sp. ‘QLD’ | AUSTRALIA: Queensland: O’Reilly’s, Wishing Tree Track | WAM T145088 | OR359482 | OR359940 | OR372781 | ||
| Protogarypinus sp. ‘SA’ | AUSTRALIA: South Australia: Burnside Quarry Track | WAMT157492 | OR359483 | OR359941 | OR372780 | ||
| Pseudogarypinus cooperi Muchmore, 1980 | USA: California: Riverside County: James Reserve, Lake Fulor | DNA102463 | EU559566 | EU559423 | EU559485 | ||
| Serianus sp. | CHILE: La Araucania: Comunidad Indigena Quinquén | WAM T152657 | OR359484 | OR359942 | OR372779 | ||
| Solinus pingrup Harvey, sp. nov. | AUSTRALIA: Western Australia: Pingrup | WAM T160525 | OR359490 | – | – | ||
| Solinus sp. ‘PSE214’ | AUSTRALIA: Western Australia: 22 km E of Mundrabilla Roadhouse, Eyre Highway | WAM T152660 | OR359485 | OR359943 | OR372777 | ||
| AUSTRALIA: Western Australia: 24 km SSW of Eucla, Eyre Highway | WAM T152661 | OR359486 | OR359944 | OR372776 | |||
| Solinus sp. ‘PSE222’ | AUSTRALIA: Western Australia: Strelley River | WAM T136665 | OR359487 | OR359945 | OR372778 | ||
| AUSTRALIA: Western Australia: Strelley River | WAM T136683 | OR359488 | OR359946 | – | |||
| AUSTRALIA: Western Australia: Strelley River | WAM T136684 | OR359489 | OR359947 | – | |||
| Larcidae | Larca granulata (Banks, 1891) | USA: New York: E.N. Huyck Preserve, near Research Centre | WAM T143199 | – | OR359930 | OR372789 | |
| Larca lata (Hansen, 1885) | CZECH REPUBLIC: Jihomoravsky Kraj: Lanzhot, Chanov Reserve | DNA102460 | EU559563 | EU559425 | – | ||
| Superfamily Garypoidea | |||||||
| Garypidae | Anchigarypus californicus (Banks, 1909) | USA: California: Marin County: Bolinas Point | WAM T92262 | MN058668 | MN065584 | MN065610 | |
| Anchigarypus japonicus (Beier, 1952) | JAPAN: Ehime Prefecture: O-shima Island, 3 km E of Myakubo | WAM T140775 | MN058687 | MN065603 | MN065628 | ||
| Ammogarypus lawrencei Beier, 1962 | NAMIBIA: Erongo: Gobabeb | WAM T132031 | MN058676 | MN065589 | MN065614 | ||
| Anagarypus australianus Muchmore, 1982 | AUSTRALIA: Northern Territory: Bumtja Beach, Garrthalala | WAM T144963 | MN058692 | MN065607 | MN065632 | ||
| Garypus beauvoisii (Audouin, 1826) | Cyprus: Akanas | WAM T143813 | MN058691 | MN065606 | MN065631 | ||
| Garypus latens Harvey, 2020 | AUSTRALIA: Western Australia: Barrow Island | WAM T143502 | MN058690 | MN065605 | MN065630 | ||
| Synsphyronus gracilis Harvey, 1987 | AUSTRALIA: Western Australia: Mudlark, 92.3 km NW of Newman | WAM T122371 | MN058669 | MN065585 | MN065611 | ||
| Synsphyronus xynus Cullen & Harvey | AUSTRALIA: Western Australia: near Sulfur Springs | WAM T133129 | MN058679 | MN065595 | MN065620 | ||
| Thaumastogarypus robustus Beier, 1947 | NAMIBIA: Hardap: Hardap Dam near Marieantal | WAM T132035 | MN058678 | MN065592 | MN065617 | ||
| Thaumastogarypus sp. | NAMIBIA: Erongo: Uis (on the road C35) | WAM T132030 | MN058675 | MN065588 | MN065613 | ||
| Geogarypidae | Afrogarypus deceptor Neethling & Haddad, 2016 | SOUTH AFRICA: KwaZuluNatal: Ndumo Game Reserve | DNA102401 | EU559560 | EU559385 | EU559436 | |
| Afrogarypus purcelli (Ellingsen, 1912) | SOUTH AFRICA: Western Cape Province: De Hoop Nature Reserve | Afro_purcelli | KP331817 | – | KP297850 | ||
| Geogarypus connatus Harvey, 1986 | AUSTRALIA: South Australia: 14 km WNW of Yalata | WAM T135409 | – | OR359929 | OR372785 | ||
| Geogarypus connatus Harvey, 1986 | AUSTRALIA: South Australia: 14 km WNW of Yalata | WAM T135408 | OR359475 | – | OR372786 | ||
| Geogarypus longidigitatus (Rainbow, 1897) | SINGAPORE: Bukit Timah Nature Reserve, South view path | WAM T146858 | MN058693 | MN065608 | – | ||
| Geogarypus minor (L. Koch, 1873) | [Unknown] | MNHN-JAD10 | JN018180 | JN018297 | JN018394 | ||
| Hesperolpiidae | Apolpium parvum Hoff, 1945 | TRINIDAD & TOBAGO: Mount Saint Benedict | DNA102467 | EU559541 | EU559380 | EU559489 | |
| Nanolpium sp. | ZAMBIA: Kafue National Park, Chibila Camp | DNA102410 | EU559543 | EU559390 | EU559445 | ||
| Pachyolpium sp. | TRINIDAD & TOBAGO: Mount Saint Benedict | DNA102466 | EU559542 | EU559421 | EU559488 | ||
| Progarypus sp. | CHILE: Concepcion: Cerro Caracol | DNA102468 | EU559538 | EU559420 | EU559490 | ||
| Olpiidae | Antillolpium sp. 772A | CUBA: [no further data] | sp. 772A | KX263395 | – | KX263356 | |
| Austrohorus sp. | AUSTRALIA: Western Australia: Madura Pass, 800 m NE of Madura | WAM T135388 | MN058681 | MN065597 | MN065622 | ||
| Beierolpium bornemisszai (Beier, 1966) | AUSTRALIA: Western Australia: Gleneagle State Forest | DNA102464 | EU559545 | EU559378 | EU559486 | ||
| Calocheiridius cf. termitophilus Beier, 1964 | EQUATORIAL GUINEA: Bata District: near Rio Utonde, Bata | MCZIZ-130511 | EU559544 | EU559359 | EU559460 | ||
| Euryolpium sp. | AUSTRALIA: Western Australia: Steep Head Island | DNA102465 | MN058685 | MN065601 | MN065626 | ||
| Indolpium sp. | AUSTRALIA: Western Australia: 79.1 km SW of Tom Price | WAM T136397 | MN058683 | MN065599 | MN065624 | ||
| Superfamily Neobisioidea | |||||||
| Hyidae | Hya minuta (Tullgren, 1905) | BRUNEI DARUSSALAM: Mengkubau, off Jalan Penghubang mentiri | WAM T146845 | OR067360 | OR039622 | OR059105 | |
| Neobisiidae | Neobisium carcinoides (Hermann, 1804) | DENMARK: Stampeskov at Raadvad | WAM T143210 | OR067357 | OR039620 | OR059108 | |
Results
Molecular phylogeny
The phylogeny (Fig. 1) resulting from the molecular analysis recovered a monophyletic Garypinoidea with moderate bootstrap support and a monophyletic Garypoidea with poor support. Relationships of the garypoid families were consistent with the topology recovered with a genomic dataset, i.e. Geogarypidae sister to Hesperolpiidae + Garypidae + Olpiidae and Hesperolpiidae sister to Garypidae + Olpiidae (Benavides et al. 2019).
Although the Garypinoidea was found to be monophyletic, the Larcidae was found to nest deep within the Garypinidae, thereby rendering the latter paraphyletic. The subfamily Protogaryininae, although only represented by a single genus, Protogarypinus, was recovered as sister to the Larcidae + remaining Garypinidae. A well-supported clade consisting of four genera of Garypininae (Aldabrinus, Pseudogarypinus, Serianus and Solinus) was found to be sister to a poorly supported clade with Larcidae (Larca), two genera of Garypininae (Nobilipinus and Garypinus) and the subfamily Amblyolpiinae (Amblyolpium). The bootstrap support values for the clade Nobilipinus + Garypinus + Amblyolpiinae + Larcidae was extremely low (50), casting considerable doubt on the veracity of the result. This seems to be another case of the poor resolving power of the standard genes used in most pseudoscorpion molecular studies, therefore resembling the situation for the Garypidae in which the morphologically distinct subfamilies Garypinae and Synsphyroninae were not reciprocally monophyletic (Harvey et al. 2020); the topology was also recovered in the present study (Fig. 1). The extremely long branch for Amblyolpiinae seems to indicate a very deep divergence from other garypinioids, highlighting the unusual status within the Garypinidae.
Despite the poor resolution of some nodes and the probably spurious position of Larcidae within the Garypinidae, the hypothesis that one of the characteristic features of the clade Amblyolpiinae + Garypininae, the divided arolia, is autapomorphic for this clade is supported. The subfamily Garypininae was found to be paraphyletic with respect to Larcidae + Amblyolpiinae. Therefore, the phylogeny should be regarded as preliminary and additional data should be sought, including additional taxa, more complete gene coverage for the taxa already sequenced and additional markers that provide better resolution at the base of the tree.
Taxonomy
Family GARYPINIDAE Daday, 1889
Garypininae Daday, 1889, p. 123.
Garypinidae: Judson, 1993, p. 697.
Garypinidae differs from all other pseudoscorpion families as follows: from Chthonioidea and Feaelloidea in the presence of venom glands in the chelal fingers (e.g. Fig. 8, 54, 103); from Neobisioidea in the presence of only 1 or 2 distal teeth or lobes on the movable cheliceral finger (Fig. 17, 33); from Garypidae, Geogarypidae and Larcidae in the rectangular carapace and with the eyes situated near the anterior carapaceal margin (e.g. Fig. 17, 49, 99); from Menthidae in the presence of 8 or fewer trichobothria on the fixed chelal finger (e.g. Fig. 10, 54, 102); and from Sternophoroidea, Cheiridioidea and Cheliferoidea in the presence of separate metatarsi and tarsi on the legs (e.g. Fig. 21 42, 43, 105). There are no unambiguous family-level differences with Hesperolpiidae and Olpiidae but Amblyolpiinae and Garypininae differ from these in the presence of divided arolia (Fig. 21, 22, 42–44, 95, 105), and Protogarypininae and Garypininae differ in the basal concentration of trichobothria on the prolateral face of the fixed finger (Fig. 10, 53, 101).
Hand with 4 or 5 (rarely 6) long, acuminate setae; movable finger with 1 long subdistal seta; rallum of 4 blades (rarely 3 or 6); with 2 dorsal and 1 ventral lyrifissures; lamina exterior present or absent; serrula interior modified to form velum.
Femur usually with a single submedial tactile seta; pedicel usually prolaterally offset. Fixed chelal finger with 5, 7 or 8 trichobothria, movable chelal finger with 1, 2, 3 or 4 trichobothria: eb and esb situated at base of fingers; est situated midway between esb and et, sometimes closer to esb than et, or rarely closer to et than esb; et situated subdistally; ib, isb, ist and it situated on prolateral face of chelal finger, with ib, isb and ist situated subbasally adjacent to it, or situated submedially away from it. Venom apparatus present in both chelal fingers, venom ducts either very short, terminating in nodus ramosus near tip of fingers or very long, terminating near middle of finger. Chelal teeth juxtadentate; accessory teeth absent; retrolateral condyle small and rounded.
Subrectangular; usually with 4 eyes situated close to anterior margin of carapace, sometimes with 2 eyes or rarely absent; epistome absent.
Manducatory process distally triangular; 1 suboral seta; maxillary shoulder absent; median maxillary lyrifissure present and situated submedially; all coxae approximately same width.
Femora I and II usually shorter than or same length as patellae I and II, femora rarely longer than patellae; junction between anterior femora and patellae perpendicular; oblique suture line between femora III and IV and patella III and IV; metatarsi and tarsi always separate; subterminal tarsal setae acuminate; arolium longer than claws and either deeply divided or entire; claws slender and simple.
Tergites and sternites usually divided. Pleural membrane longitudinally striate, usually lacking setae, but these are sometimes present. Sternites VI–VIII of male and female usually with median glandular setae. Stigmatic sclerites with 1 or 2 setae; spiracles with spiracular helix. Setae of anterior genital operculum (sternite II) approximately same size as other sternal setae. Posterior tergites and sternites with tactile setae. Anus (tergite XII and sternite XII) without raised rim; situated between tergite XI and sternite XI.
The Garypininae was first established as a subfamily of Garypidae by Daday (1889) for the genus Garypinus Daday, 1889 with the type species G. dimidiatus (L. Koch, 1873). The subfamily Garypininae was later treated as a subfamily of Olpiidae (e.g. Chamberlin 1930, 1931a; Beier 1932b) even though the name was predated by Olpiidae Banks, 1895 (Harvey 1992). The clade was raised to family level by Judson (1993, 2005) based on the divided arolia, the offset chelal pedicel, basal concentration of the prolateral trichobothria on the fixed chelal finger, features that are found in most, but not all, garypinid genera. The transcriptomic study by Benavides et al. (2019) demonstrated that Garypinidae and Larcidae were sister to a clade comprising Sternophoroidea, Cheiridioidea and Cheliferoidea, and proposed the superfamily Garypinoidea for these.
Muchmore (1974) noted the presence in A. aldabrinus and A. floridanus of several thickened setae on the prolateral margin of the chelal fingers that he called ‘spinelike’ setae. Similar setae also occur in A. rixi but although the presence of these in P. novaecaledoniae has not been ascertained, these setae are likely to be present. Dashdamirov (2007) noted two rows of blunt setae in Caecogarypinus pectinodentatus Dashdamirov, 2007 from Vietnam that may be homologous with the setae found in Aldabrinus. He noted that the setae formed a ‘cage’ that allowed the capture of small prey such as springtails and mites.
Reduction of trichobothrial numbers in adult pseudoscorpions tends to follow a neotenic pattern whereby the trichobothria that are usually added incrementally at each moult are not expressed. For example, species with only seven trichobothria on the adult fixed finger have lost trichobothrium isb that is usually expressed at the moult from tritonymph to adult (e.g. Harvey 1992). This has been confirmed for a variety of taxa including Microbisium Chamberlin, 1930 (Neobisiidae) (e.g. Nelson 1982; Sakayori 1989), Microblothrus Mahnert, 1985 (Syarinidae) (Mahnert 1985), Synsphyronus (Garypidae) (Harvey 1987a, Harvey 2010), Geogarypus (Geogarypidae) (Harvey 1986), Cheiridium Menge, 1855 and Cryptocheiridium Chamberlin, 1931 (Cheiridiidae) (Vitali-di Castri 1965; Mahnert 1982a; Harvey 1992) and Afrosternophorus (Sternophoridae) (Murthy and Ananthakrishnan 1977; Harvey 1985). This phenomenon has also been reported in pseudoscorpions with fewer than seven trichobothria. All three species of the African genus Elattogarypus (Garypidae) have five trichobothria on the adult fixed finger (Beier 1964b; Mahnert 1984a, 2007) and est is present along with eb, ist, it and et. Tritonymphs and deutonymphs of E. somalicus Mahnert, 1984 have identical trichobothrial patterns to the adults, whereas the protonymphs have the standard pattern number of three trichobothria on the fixed finger, eb, isb and et (Mahnert 1984a). The retention of the nymphal trichobothrial pattern by adults is a clear example of neoteny. The same pattern of five trichobothria also occurs in adults of Eremogarypus eximius Beier, 1973 from Namibia (Beier 1973) and six trichobothria occur in Meiogarypus mirus Beier, 1955, also from Namibia (Beier 1955a). Synsphyronus ellenae Harvey, 2010 from Australia usually has six trichobothria but occasionally five or seven (Harvey 2010), demonstrating notable intraspecific variation. In all these taxa, the lack of expression of trichobothrium est is the normal condition, most likely resulting from neoteny.
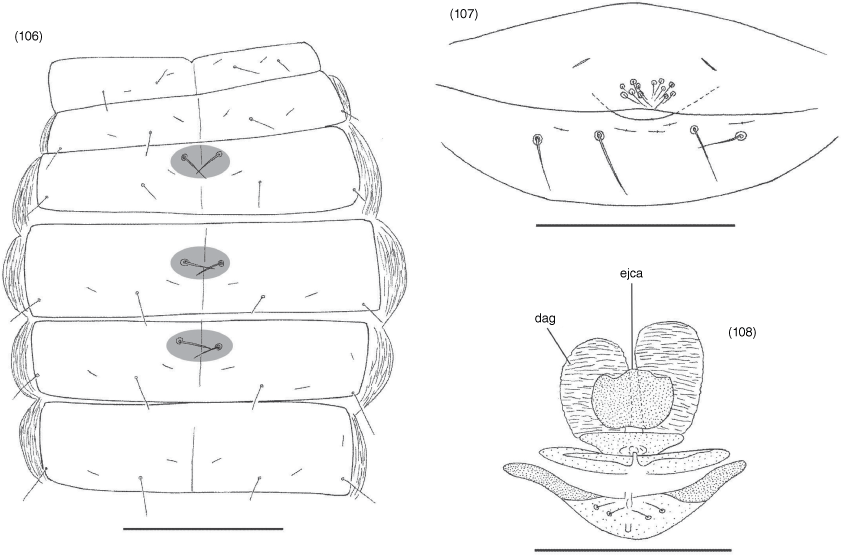
In contrast, those adult garypinids with seven or fewer trichobothria on the fixed finger are lacking trichobothrium est. These taxa, members of the genera Aldabrinus, Galapagodinus, Nelsoninus and Solinellus, represent the only pseudoscorpions with fewer than eight trichobothria on the fixed finger in which est is absent. These observations also include postembryonic data on two species, Galapagodinus franzi (Mahnert 2014) and Aldabrinus rixi (Fig. 14, 15), in which est is absent in the tritonymph and deutonymph. There is no evidence, however, that these four genera form a monophyletic clade.
Another anomalous feature of adult Aldabrinus is the reduction of the trichobothria on the movable chelal finger with A. aldabrinus, A. floridanus and A. rixi possessing two trichobothria (Muchmore 1974; Fig. 9, 11–13) and A. novaecaledoniae possessing only one trichobothrium (Beier 1966a). Ascribing homology statements to the trichobothria of the fixed finger is relatively simple, whereas this is less simple with those of the movable finger. Using the neotenic argument proposed above, the trichobothria not expressed in A. aldabrinus, A. floridanus and A. rixi would be sb and st, as these are also not expressed in deutonymphs of most pseudoscorpion species (e.g. Vachon 1964; Mahnert 1981a; Harvey 1992). Similarly, the sole trichobothrium retained in A. novaecaledoniae would be t. However, comparison of the positions of the trichobothria in all Aldabrinus species suggests that even if b and t are the expressed trichobothria in A. aldabrinus, A. floridanus and A. rixi, t does not appear to be retained in A. novaeclaedoniae as the sole trichobothrium is situated in a basal position.
Species of Aldabrinus have long been known to possess only four setae on the cheliceral hand (Chamberlin 1930), with most other garypinids having the full complement of five setae that represents the standard setal number in garypoid and garypinoid pseudoscorpions. Muchmore (1974) noted the ‘laminal seta located much posterior to interior seta’, suggesting the subbasal seta (sbs) to represent the missing seta. The illustration of the chelicera provided here for A. rixi (Fig. 18) is the first for any species of Aldabrinus and a different conclusion is reached regarding which seta has been lost in this genus. Comparison of the setal patterns of these species with other garypinoids suggests that ls is the missing seta. There is uncertainty about which seta is missing among the other garypinids with only four setae on the cheliceral hand (e.g. Indogarypinus minutus, Serianus elongatus, Solinus africanus, So. corticola and So. pusillus; see Chamberlin 1930, 1931a; Beier 1967b, 1971a; Murthy and Ananthakrishnan 1977; Mahnert 2014), apart from the apparent absence of ls in Serianus elongatus (Mahnert 2014, fig. 38).
Likewise, ls appears to be the missing seta in some Larcidae (the only other family of Garypinoidea, see Benavides et al. 2019), as evidenced by the comparative setal positions in various taxa, including those with four setae, L. bosselaersi Henderickx and Vets, 2002 (Henderickx and Vets 2002), L. cavicola (Muchmore, 1981) (Harvey and Wynne 2014, fig. 7) and L. chamberlini Benedict and Malcolm, 1978 (Benedict and Malcolm 1978, fig. 5), and those with five setae, L. fortunata Zaragoza, 2005 (Zaragoza 2005, fig. 8), L. granulata (Banks, 1891) (Harvey 1992, fig. 137), L. italica Gardini, 1981 (Gardini 1983), L. lata (Hansen, 1884) (Novák 2013, fig. 1A) and L. lucentina Zaragoza, 2005 (Zaragoza 2005, fig. 6). Little is known about the post-embryonic development of the cheliceral setae in garypoid and garypinoid pseudoscorpions and these have been very rarely documented or illustrated. Most garypoids and garypinoids appear to have five setae on the cheliceral hand and one (the galeal seta) on the movable finger in adults, tritonymphs and deutonymphs. Like other pseudoscorpions, the protonymph lacks the galeal seta and has only four setae on the cheliceral hand, as documented in several garypoid and garypinoid taxa, including species of Larca (Larcidae) (Judson and Legg 1996), Geogarypus and Afrogarypus (Geogarypidae) (Mahnert 1982b; Harvey 1986), Garypus, Elattogarypus and Synsphyronus (Garypidae) (Mahnert 1982b, 1984a; Harvey 1987a), Olpium (Olpiidae) (Mahnert 1984a) and Pachyolpium (Mahnert and Schuster 1981). Curiously, Heurtault (1982) provided illustrations of the chelicerae of protonymphs of Beierolpium venezuelense Heurtault, 1982 and Pachyolpium machadoi (Heurtault, 1982) in which only three setae were present. This unusual character state may be more widely distributed in the Garypoidea and protonymphs of other taxa should be documented to explore the phylogenetic relevance.
| 1. | Arolium entire...Protogarypininae, subfam. nov. Arolium deeply divided...2 |
| 2. | Trichobothria isb, ist and it situated basally and grouped with ib; legs I and II either with femur only slightly longer than patella or with patella longer than femur; venom ducts short...Garypininae Daday Trichobothria isb, ist and it situated distally away from ib; legs I and II with femur much longer than patella; venom ducts long...Amblyolpiinae, subfam. nov. |
Subfamily AMBLYOLPIINAE subfam. nov.
ZooBank: urn:lsid:zoobank.org:act:3101B7F4-15C7-427F-A86E-21F9BA12D555
Members of the Amblyolpiinae differ from other garypinids in the positions of trichobothria isb, ist and it that are situated distally away from ib, by the morphology of legs I and II where the femur is much longer than the patella and the long venom ducts.
As for family, except as follows.
Hand with 5 long, acuminate setae; rallum of 4 blades; lamina exterior absent or present but very thin.
Pedicel not prolaterally offset. Fixed chelal finger with 8 trichobothria, movable chelal finger with 4 trichobothria: est situated midway between esb and et; isb, ist and it situated submedially on prolateral face of chelal finger. Venom apparatus present in both chelal fingers, venom ducts very long, terminating near middle of finger.
Amblyolpium Simon, 1898, Neoamblyolpium Hoff, 1956 and †Baltamblyolpium Stanczak, Harvey, Harms, Hammel, Kotthoff and Loria, 2023.
The Amblyolpiinae is a very distinct group of garypinids that differs from Protogarypinae and Garypininae in several ways, as outlined in the key and diagnosis. Neoamblyolpium differs from Amblyolpium in the position of trichobothrium ib thatis situated posterior to the level of eb and esb (Hoff 1956; Muchmore 1980), whereas ib is situated on approximately the same level as, or slightly distal to the level of eb and esb in Amblyolpium (e.g. Beier 1932b, 1959a, 1966a, 1966c, 1970a, 1970b, 1971a; Heurtault 1970; Mahnert 1976; Harvey 1988; Tooren 2002a; Nassirkhani et al. 2016; Hernández-Corral et al. 2018).
An Eocene genus from Europe, Baltamblyolpium, that closely resembles Amblyolpium and Neoamblyolpium but differs in the positions of the chelal trichobothria and the sternal glandular setae (Stanczak et al., in press) has recently been described.
The 16 Holocene species of Amblyolpium occur in many parts of the world, including the Palaearctic region (A. anatolicum Beier, 1967, A. atropatesi Nassirkhani and Doustaresharaf, 2019, A. dollfusi Simon, 1898, A. franzi Beier, 1970, A. goldastehae Nassirkhani, Shoushtari and Abadi, 2016, A. graecum Mahnert, 1976 and A. simoni Heurtault, 1970), Indo-Malayan region (A. biaroleatum (Tömösváry, 1884), A. birmanicum (With, 1906), A. bellum Chamberlin, 1930), Japan (A. japonicum Morikawa, 1960), Australasia (A. ruficeps Beier, 1966, A. novaeguineae Beier, 1971 and A. salomonense Beier, 1970) and the Neotropical region (A. martinense Tooren, 2002 and A. ortonedae (Ellingsen, 1902)) (Simon 1898; e.g. Beier 1932b, 1966b, 1967c, 1970a, 1971a; Morikawa 1960; Heurtault 1970; Mahnert 1976; Harvey 1988; Tooren 2002a; Nassirkhani et al. 2016; Hernández-Corral et al. 2018; Nassirkhani and Doustaresharaf 2019). Amblyolpium burmiticum (Cockerell, 1920) is also recorded from Mesozoic amber fossils from Myanmar (Cockerell 1920; Judson 1997).
Only two species of Neoamblyolpium have been described, both from western USA: N. alienum Hoff, 1956 from Colorado and New Mexico (Hoff 1956, 1961), and N. giulianii Muchmore, 1980 from California (Muchmore 1980). The differences between Amblyolpium and Neoamblyolpium are rather slight with the primary character cited by Hoff (1956) being the position of trichobothrium ib that is situated posterior to the level of eb and esb in Neoamblyolpium and at approximately the same level as eb and esb in Amblyolpium.
Subfamily GARYPININAE Daday, 1889
Garypininae Daday, 1889, p. 123.
Members of the Garypininae differ from those of Protogarypininae in the divided arolia, and from Amblyolpiinae in the positions of trichobothria ib, isb, ist and it that are situated at the base of the chelal finger and the short venom ducts.
As for family, except as follows.
Aldabrinus Chamberlin, 1930 (junior synonym Paraldabrinus Beier, 1966), †Ajkagarypinus Novák, Harvey, Szabó, Hammel, Harms, Kotthoff, Hörweg, Brazidec & Ősi, 2023, Caecogarypinus Dashdamirov, 2007, Galapagodinus Beier, 1978, Garypinidius Beier, 1955, Garypinus Daday, 1889, Haplogarypinus Beier, 1959, Hemisolinus Beier, 1977, Nelsoninus Beier, 1967, Paraldabrinus Beier, 1966, Pseudogarypinus Beier, 1931, Serianus Chamberlin, 1930 (junior synonym Paraserianus Beier, 1939), Solinellus Muchmore, 1979 and Solinus Chamberlin, 1930 (junior synonym Indogarypinus Murthy and Anantkarishnan, 1977).
We define the Garypininae as a rather homogenous group of genera that possess the features mentioned in the diagnosis. Some genera are not very well supported and characterised by the loss of individual trichobothria on the chelal fingers. Mahnert (1988) showed that this character may be labile, even within a single species and must be used with caution when defining genera of garypinids.
Differentiating between the genera Haplogarypinus with the type and only species H. pauperatus Beier, 1959 from Central Africa (Beier 1959b) and Hemisolinus with the type and only species H. helenae from the St Helena Islands (Beier 1977), both of which possess virtually identical trichobothrial patterns, five setae on the cheliceral hand and single pairs of glandular setae on sternites VI–VIII has not been possible.
The recent redescription of Garypinus dimidiatus, the type species of Garypinus, by Gardini and Gavalas (2021) has allowed for a much better understanding of the genus.
A Mesozoic garypinid, Ajkagarypinus, has recently been described from Ajkaite amber collected in Central Europe (Novák et al. 2023).
Garypinines have been recorded from most regions of the world, including some that are found in more than one biogeographical realm: Palaearctic (Garypinus, Serianus and Solinus), Nearctic (Pseudogarypinus and Serianus), Indomalayan (Caecogarypinus and Nobilipinus), Neotropic (Aldabrinus, Galapagodinus, Pseudogarypinus and Solinellus), Afrotropic (Aldabrinus, Garypinidius, Haplogarypinus, Hemisolinus and Solinus) and Australasia (Nelsoninus, Paraldabrinus, Serianus and Solinus).
| 1. | Legs I and II with femur slightly longer than patella; sternites VI–VIII without glandular setae...Pseudogarypinus Beier (USA, Costa Rica) Legs I and II with femur shorter than patella (Fig. 42) or with patella approximately same size as femur; sternites VI–VIII usually with at least one pair of glandular setae (Fig. 23, 39, 40, 106)...2 |
| 2. | Cheliceral setae ls and is situated very close to each other (Fig. 34)...3 Cheliceral setae ls and is situated apart from each other (Fig. 18, 100)...5 |
| 3. | Abdominal pleural membrane with 1 or 2 setae per segment...Garypinus Daday (eastern Mediterranean region, Asia, South Africa) Abdominal pleural membrane without setae (Fig. 41)...4 |
| 4. | Eyes present (e.g. Fig. 49); galea of female (occasionally male) deeply trifid (e.g. Fig. 51); chelal fingers with flat dental row (e.g. Fig. 54, 55)...Nobilipinus gen. nov. (South-East Asia) Eyes absent; galea with small, terminal rami; movable chelal finger with basal teeth raised into a comb-shaped structure...Caecogarypinus Dashdamirov (South-East Asia) |
| 5. | Sternites VI–VIII of male with one pair of glandular setae (Fig. 23, 106)...6 Sternites VI–VII of male with more than one pair of glandular setae, sternite VIII with one pair of glandular setae...Serianus Chamberlin (North & South America, North Africa, Asia, Solomon Islands) |
| 6. | Fixed chelal finger with 8 trichobothria, est present (Fig. 102)...7 Fixed chelal finger with 5 or 7 trichobothria, est absent (Fig. 9–13)...9 |
| 7. | Trichobothrium est situated closer to et than esb...Garypinidius Beier (South Africa) Trichobothrium est situated subbasally, much closer to esb than to et...8 |
| 8. | Movable chelal finger with 3 trichobothria...Haplogarypinus Beier (Central Africa) and Hemisolinus Beier (St Helena Islands) Movable chelal finger with 2 trichobothria (Fig. 102)...Solinus Chamberlin (cosmopolitan) |
| 9. | Fixed chelal finger with 7 trichobothria, trichobothrium est absent (Fig. 9–13)...10 Fixed chelal finger with 5 trichobothria, with esb, est and isb absent...Solinellus Muchmore (USA) |
| 10. | Chela cylindrical, with depth and breadth approximately the same...11 Chela much deeper than broad (Fig. 9–13)...Aldabrinus Chamberlin (Aldabra Islands, Australia, USA) |
| 11. | All rami situated distally...Nelsoninus Beier (New Zealand) At least one ramus situated at base of galea...Galapagodinus Beier (Galapagos Islands) |
Genus Aldabrinus Chamberlin, 1930
Aldabrinus Chamberlin, 1930, p. 597.
Paraldabrinus Beier, 1966a, pp. 367–368. New synonymy.
Aldabrinus: Aldabrinus aldabrinus Chamberlin, 1930, by original designation.
Paraldabrinus: Paraldabrinus novaecaledoniae Beier, 1966, by original designation.
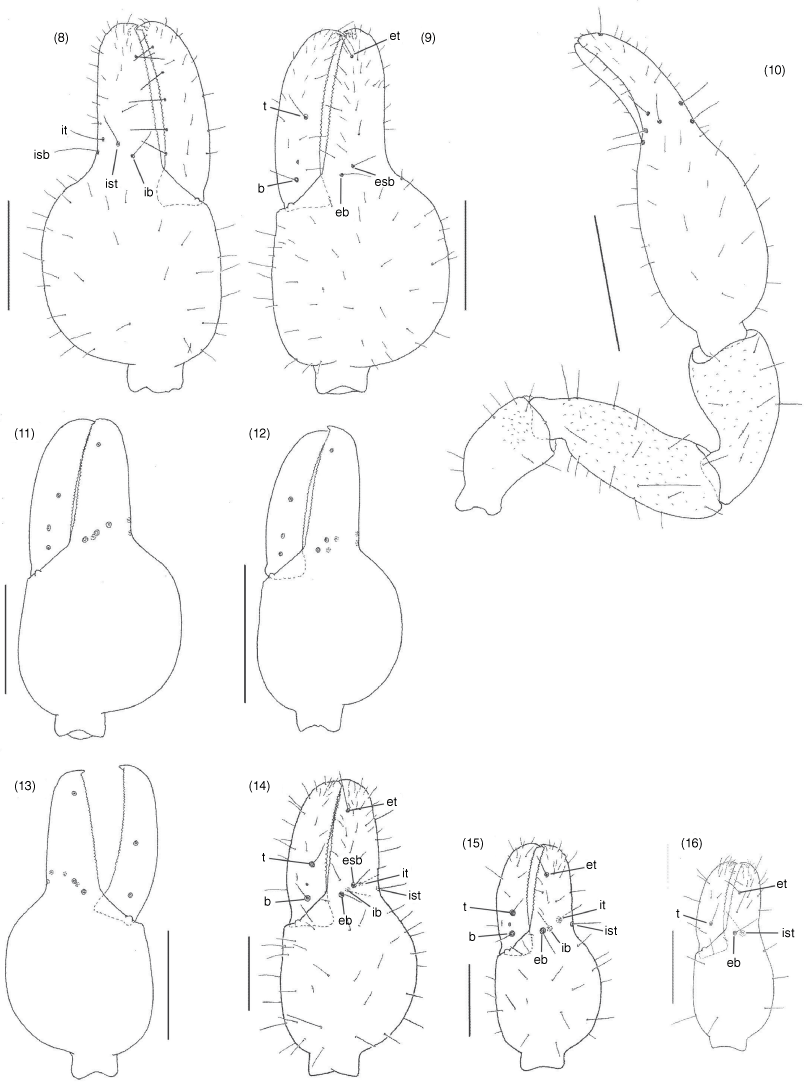
The genus Aldabrinus differs from all other garypinid genera except Galapagodinus, Nelsoninus and Solinellus by the absence of trichobothrium est on the fixed chelal finger (Fig. 9, 11–13). Aldabrinus differs from these genera in the presence of only 4 setae on the cheliceral hand (Fig. 18) and the chelal hand is much deeper than broad (Fig. 8, 9, 11–13).
As described by Muchmore (1974) except that the movable chelal finger may have one or two trichobothria.
The genus Aldabrinus was established by Chamberlin (1930) for A. aldabrinus Chamberlin, 1930, an unusual pseudoscorpion from Il Esprit, Aldabra Islands. No further specimens of Aldabrinus were reported in the literature until Muchmore (1974) described a second species, A. floridanus Muchmore, 1974, from Key Largo, Florida and presented a redescription of the holotype of A. aldabrinus. Muchmore (1979) added illustrations of the male genital region of A. floridanus, noting that this appeared very similar to that of Solinellus simberloffi Muchmore, 1979, another garypinid found in Florida.
Chamberlin (1930) cited several features to separate the five genera that he included in the subfamily Garypininae, that was considered a subfamily of the Olpiidae at that time. The genera Garypinus and Amblyolpium had a ‘submobile’ articulation between the femur and patella of the anterior legs, and of the remaining genera, Serianus had all four trichobothria on the movable chelal finger, whereas Aldabrinus and Solinus were missing trichobothria st and t. Aldabrinus was distinguished from Solinus by the presence of prominent chelal teeth (reduced in Solinus), the short tactile seta of metatarsus IV (long in Solinus), the absence of basal seta of the chelicera (present in Solinus) and the absence of trichobothrium est (present in Solinus). The lack of the basal setae was contradicted later in the paper where a species of Solinus was recorded with the basal seta present. Four other garypinid genera have since been described that also lack trichobothrium est: Galapagodinus from the Galapagos Islands (Beier 1978; Mahnert 2014), Nelsoninus from New Zealand (Beier 1967a), Paraldabrinus from New Caledonia (Beier 1966a) and Solinellus from Florida (Muchmore 1979). Representatives of four genera lack cheliceral seta ls (see below): Indogarypinus minutus Murthy and Ananthakrishnan, 1977 from India (Murthy and Ananthakrishnan 1977), Paraldabrinus novaecaledoniae Beier, 1966 from New Caledonia (Beier 1966a), one species of Serianus (Mahnert 2014) and three species of Solinus (Chamberlin 1930; Beier 1967b, 1971a). The only species of Serianus that has been recorded with four cheliceral setae is S. elongatus Mahnert, 2014 from the Galapagos Islands (Mahnert 2014), however this species may likely be misplaced as all other species possess five setae, have trichobothrium st situated dorsal to sb (distal to sb in S. elongatus, and S. salomonensis Beier, 1966, see Beier 1966a), the presence of two enlarged setae on the pedipalpal femur (one in other species of Serianus) or a simple galea (many Serianus have been recorded with one ramus situated subbasally or submedially rather than distally with the other rami, e.g. Muchmore 1968; Tooren 2002b; Mahnert 2014). Although most species of Solinus have the full complement of five setae on the cheliceral hand (e.g. Chamberlin 1930; Morikawa 1953a; Beier 1966c; Dashdamirov 1996), three species have four setae: S. africanus Beier, 1967 from Kenya (Beier 1967b), S. corticola (Chamberlin, 1923) from Mexico (Chamberlin 1930, 1931a) and S. pusillus Beier, 1971 from Papua New Guinea (Beier 1971a).
The genus Paraldabrinus was described by Beier (1966a) for P. novaecaledoniae Beier, 1966 from New Caledonia that was distinguished from Aldabrinus by the presence of only a single trichobothrium (b) on the movable chelal finger, the longer tactile seta of metatarsus IV and only two setae in the posterior setal row of the carapace. Many other features are identical to Aldabrinus, such as the absence of trichobothrium est, the presence of only four setae on the cheliceral hand and the presence of only one tactile seta on the pedipalpal femur. Muchmore (1974) noted that A. floridanus possessed only two setae in the posterior carapaceal row and the two new species described below from Australia also possess only two setae. Therefore this feature cannot be used to segregate Paraldabrinus from Aldabrinus. The major diagnostic difference used by Beier (1966a), the presence of only one trichobothrium on the movable chelal finger, also seems difficult to defend. Although numerous pseudoscorpion genera have been defined primarily on the presence or absence of one or more trichobothria, some have subsequently been found to be synonymous with genera with different trichobothrial numbers. For example, species of Synsphyronus Chamberlin, 1930 (Garypidae) range from five to eight trichobothria on the fixed finger and one to three on the movable finger (e.g. Chamberlin 1943; Harvey 1987a, 2010). Species of Anagarypus Chamberlin, 1930 (Garypidae) have one or two trichobothria on the movable finger (Muchmore 1982). Species of Eremogarypus Beier, 1955 (Garypidae) have either five or eight trichobothria on the fixed finger and one, two or three trichobothria on the movable finger (Beier 1955a, 1962, 1973). Most species of Thaumastogarypus Beier, 1947 (Garypidae) have eight trichobothria on the fixed finger and four on the movable finger (Beier 1947, 1955a, 1958, 1964b) but T. mancus Mahnert, 1982 has seven and two trichobothria respectively (Mahnert 1982b). Most species of Geogarypus Chamberlin, 1930 (Geogarypidae) have eight trichobothria on the fixed finger (e.g. Beier 1932b; Harvey 1986, 2000; Neethling and Haddad 2016; Gardini et al. 2017; Novák and Harvey 2018) but two species have only seven trichobothria in which isb is absent (Harvey 1986, 1987b). The genus Larca (Larcidae) includes species with two, three or four trichobothria on the movable finger (e.g. Hoff 1961; Muchmore 1981a; Gardini 1983; Henderickx and Vets 2002; Zaragoza 2005; Harvey and Wynne 2014). The majority of Serianus Chamberlin, 1930 species have four trichobothria on the movable finger (e.g. Chamberlin 1930; Feio 1945; Hoff 1956, 1964; Beier 1959a; Muchmore 1968; Lee 1979; Muchmore 1981b; Mahnert 1991) but S. bolivianus Beier, 1939 has three or four (Mahnert 1988) and S. argentiniae Muchmore, 1981 has two, three of four (Hoff 1950). Most species of Apocheiridium Chamberlin, 1924 (Cheiridiidae) have seven trichobothria on the fixed finger and one on the movable finger (e.g. Chamberlin 1924, 1932; Beier 1936, 1964c, 1967a, 1967c, 1976; Hoff 1952; Morikawa 1953b; Murthy and Ananthakrishnan 1977; Benedict 1978; Mahnert 1982a, 1993; Muchmore 1992), but some species have only six on the fixed finger (Vitali-di Castri 1962) and some have two on the movable finger (Beier 1964b). Species of Neocheiridium Beier, 1932 (Cheiridiidae) have five or seven trichobothria on the fixed finger (Vitali-di Castri 1962). Species of Afrosternophorus Beier, 1967 and Idiogaryops Hoff, 1963 (Sternophoridae) have two or three trichobothria on the movable finger (Harvey 1985). The type species of Pseudochernes Beier, 1954 (Withiidae), P. crassimanus Beier, 1954, has eight trichobothria on the fixed finger (Beier 1954b), whereas P. arabicus Mahnert, 1991 has seven trichobothria (Mahnert 1991). Two species of Anaperochernes Beier, 1964 (Chernetidae) have eight trichobothria on the fixed finger but the other two species have only seven trichobothria, with isb absent (Beier 1964a, 1964d; Mahnert 1985). The vast majority of the many species of Parachernes Chamberlin, 1931 (Chernetidae) have four trichobothria on the movable finger (e.g. Chamberlin 1931b; Beier 1932a) but P. bisetus Muchmore & Alteri, 1974 from Florida has only two trichobothria in which sb and st apparently represent the missing trichobothria (Muchmore and Alteri 1974). Finally, most species of Americhernes Muchmore, 1976 have four trichobothria on the movable finger (Muchmore 1976; Harvey 1990) but A. neboissi Harvey, 1990 from south-eastern Australia has only two trichobothria (Harvey 1990). This list does not include the genera in which all species have the same reduced number of trichobothria.
Among the more remarkable trichobothrial patterns are the increase of adult trichobothria from the standard pattern of 8/4. The most extreme situations are all members of the family Ideoroncidae that have 17–31 on the fixed chelal finger and 9–14 on the movable chelal finger (e.g. Mahnert 1981b, 1984b; Harvey and Muchmore 2013; Harvey and Du Preez 2014; Harvey 2016), and Menthidae that have 11 on the fixed chelal finger and 4 on the movable chelal finger (e.g. Harvey 1989). The holotype of Ectromachernes mirabilis Beier, 1944 (Withiidae) was reported to have an extra trichobothrium in the et region (Beier 1944) but this is likely to represent a teratological duplication rather than a species-specific apomorphy.
Therefore, regarding P. novaecaledoniae as a species of Aldabrinus, that renders Paraldabrinus as a junior objective synonym, seems more informative. Aldabrinus is here defined as a garypinid that lacks trichobothrium est and has only four setae on the cheliceral hand. The only other garypinids that lack est are Galapagodinus franzi Beier, 1978 from the Galapagos Islands, Nelsoninus maoricus Beier, 1967 from New Zealand and Solinellus simberloffi Muchmore, 1979 from Florida, each of which belong to monotypic genera. Unlike Aldabrinus, these have five setae on the cheliceral hand and lack the robust pedipalps and deep chelal hand found in Aldabrinus (Beier 1967a, 1978; Muchmore 1979; Mahnert 2014). Solinellus further differs from the others in having only five trichobothria on the fixed chelal finger.
| 1. | Movable chelal finger with 2 trichobothria (Fig. 9, 11–13)...2 Movable chelal finger with 1 trichobothrium...A. novaecaledoniae (Beier) |
| 2. | Trichobothrium et situated distally...A. floridanus Muchmore Trichobothrium et situated subdistally (Fig. 9–13)...3 |
| 3. | Carapace with 5 setae in the posterior setal row; female carapace smooth...A. aldabrinus Chamberlin Carapace with 2 setae in the posterior setal row (Fig. 17); female carapace coarsely granulated (Fig. 17)...A. rixi sp. nov. |
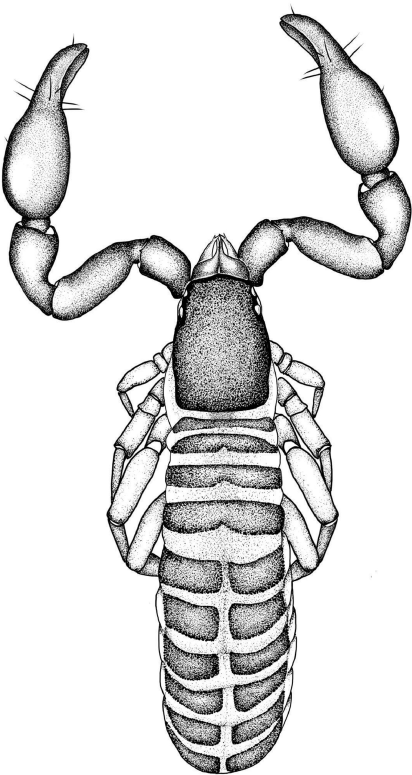
Aldabrinus rixi, sp. nov.
ZooBank: urn:lsid:zoobank.org:act:f14f153e-7ece-4015-bff8-79f6c560cb57
Holotype. AUSTRALIA: Western Australia: ♀, Torndirrup National Park, Sharp Point at end of Eclipse Island Road, 35°06′22″S, 117°52′04″E, 14 March 2008, sifting/beating, M.G. Rix, M.S. Harvey (WAM T143175).
Paratypes. AUSTRALIA: Western Australia: 1 ♂, Albany Windfarm, 35°02′56″S, 117°45′30″E, 14 November 2007, R. Teale (WAM T95085); 1 ♂, Cape Le Grand National Park, Lucky Bay, near Flinders monument, 33°59′37.6″S, 122°13′16.3″E, 20 June 2014, sifting, hanging litter, J.M. Waldock, C.A. Car (WAM T133035); 1 ♂, Waychinicup Nature Reserve, S. of Mount Manypeaks, Surprise Gully, 34°54′11″S, 118°23′57″E, 13 March 2007, beating reeds and grasses, M.L. Moir, M.S. Harvey, S. Comer, M.G. Rix (WAM T79640); 1 tritonymph, Mount Hallowell, 35°00′34″S, 117°18′04″E, 6 November 2006, beat, plants, M.L. Moir, D. Jolly (WAM T78899); 1 deutonymph, same data except 34°53′25″S, 118°20′04″E (WAM T78961).
Other material. AUSTRALIA: Western Australia: 1 tritonymph, 1 deutonymph, Bremer Bay, Native Dog Beach, 34°27′17″S, 119°21′43″E, 21 November 2006, beating plants, M.L. Moir (WAM T78821); 1 protonymph, Denmark, 35°01′07″S, 117°13′40″E, 117.366°E, 22–29 March 2022, Malaise trap, Kwoorabup Nature School (formally Spirit of Play Community School) students (Insect Investigators BIOUG82737-E05) (WAM T160040); 1 tritonymph, Denmark area, ~34°58′S, 117°21′E, 10 February 2010, under jarrah bark, P. Hennig (WAM T100325); 1 ♀, Dirk Hartog Island National Park, ~2.6 km SW of homestead (Eco Lodge), 26°01′20.5″S, 113°11′06.6″E, 17 May 2018, S.J. John (WAM T156157); 1 ♂, Walpole, 348 Tinglewood Road, 35°00′S, 116°39′E, 12 April 2017, yellow sticky trap, DAFWA staff (WAM T143494); 1 ♀, Woodman Point Nature Reserve, 32°07′50″S, 115°45′28″E, 1 September–4 November 1994, wet pitfall trap, J.M. Waldock, A.F. Longbottom (WAM T63240); 1 tritonymph, same data except 28 June–1 September 1994 (WAM T63242); 1 ♀, same data except 4 November 1994–19 January 1995, J.M. Waldock, M.S. Harvey (WAM T63241).
This species is named for Mike Rix, collector of the holotype and many other fascinating arachnids, and for his contributions to arachnid systematics.
Aldabrinus rixi differs from A. novaecaledoniae in the possession of two trichobothria on the movable chelal finger (Fig. 9, 11–13) (only one in A. novaecaledoniae), from A. floridanus in the subdistal position of trichobothrium et (Fig. 8–13) (distal in A. floridanus), from A. aldabrinus in the presence of only two setae in the posterior setal row of the carapace (Fig. 17) (five setae in A. aldabrinus) and from all species in the coarsely granulated female carapace (Fig. 17) (smooth in others).
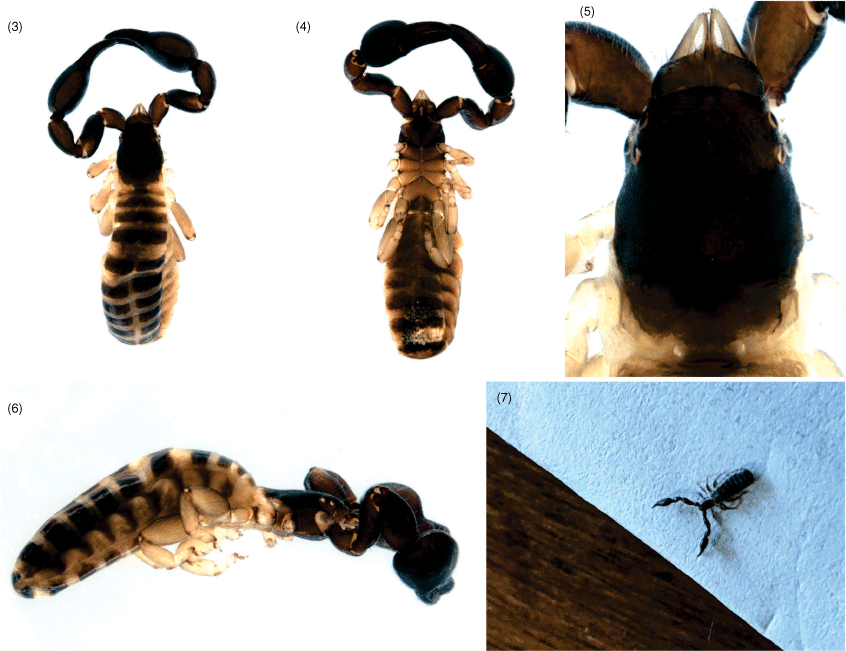
Setae long, mostly straight and acicular (Fig. 8–10, 17, 23); most cuticular surfaces smooth and glossy, except for carapace of female that is granulated (Fig. 17).
Aldabrinus rixi sp. nov., female holotype (WAM T143175), unless stated otherwise: 8, left chela, prolateral; 9, left chela, retrolateral; 10, right pedipalp, dorsal; 11, left chela, retrolateral, without setae, male paratype (WAM T79640); 12, left chela, retrolateral, without setae, male paratype (WAM T133035); 13, right chela, retrolateral, without setae, male paratype (WAM T95085); 14, left chela, retrolateral, tritonymph paratype (WAM T78899); 15, left chela, retrolateral, deutonymph paratype (WAM T78961); 16, left chela, retrolateral, protonymph paratype (WAM T160040). Scale lines: 0.5 mm (10, 12); 0.2 mm (8, 9, 11, 13–15).
Aldabrinus rixi sp. nov., female holotype (WAM T143175), unless stated otherwise: 17, carapace, dorsal; 18, left chelicera, dorsal, male paratype (WAM T133035); 19, left galea, lateral; 20, left rallum, lateral, male paratype (WAM T133035); 21, left leg IV, prolateral; 22, arolium, left leg IV, dorsal; 23, sternites VI–VIII, ventral, glandular setae highlighted in grey; 24, male genitalia, ventral, male paratype (WAM T133035); 25, female genitalia, ventral. Scale lines: 0.2 mm (17, 18, 19, 21, 25); 0.1 mm (24); 0.05 mm (19, 20, 22).
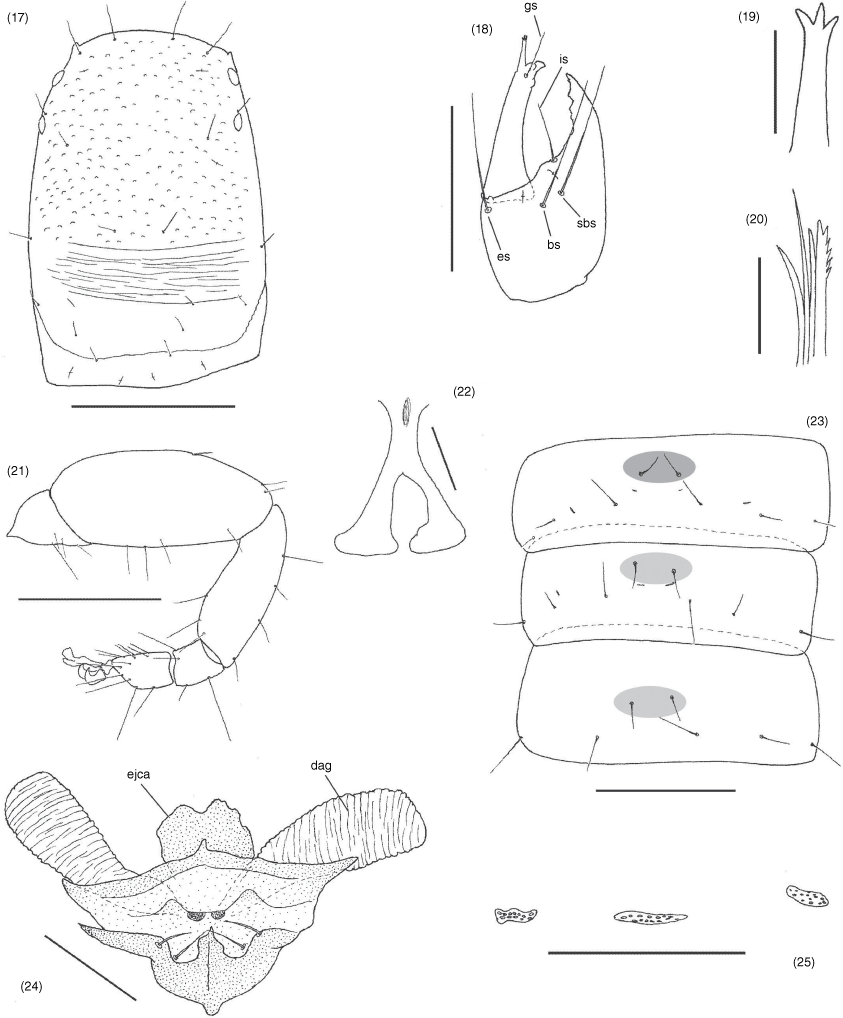
Surface smooth; cheliceral hand with 4 setae, ls absent; movable finger with 1 subdistal seta; all setae acuminate; galea long and slender, with 4 (♂) or 3 (♀) distal rami (Fig. 19); fixed finger with 7 small teeth, each approximately same size; movable finger with 1 subdistal tooth; with 2 dorsal and 1 ventral lyrifissures; serrula interior modified to form velum; serrula interior with 16 (♂, ♀) blades; lamina exterior absent; rallum with 4 blades, anterior blade with anterior spinules, remainder smooth (Fig. 20).
Very stout, robust (Fig. 3, 4, 6, 10); trochanter with low granulations in distal half, most of femur and patella with low granulations, prolateral face of chela weakly granulated at base of fingers; setae acicular, straight or nearly so; trochanter 1.73–1.80× (♂), 1.85–1.86× (♀); femur cylindrical, with 1 tactile seta on dorsal surface, situated near middle of segment, 2.57–2.72× (♂), 2.44–2.74× (♀); patella robust, with several small lyrifissures situated basally on dorsal surface, 2.03–2.21× (♂), 1.81–2.16× (♀); chelal hand robust, retrolateral chelal condyle small and rounded, chela (with pedicel) 2.75–3.03× (♂), 2.88–2.97× (♀), chela (without pedicel) 2.48–2.81× (♂), 2.63–2.73× (♀), hand (without pedicel) 1.18–1.28× (♂), 1.19–1.42× (♀) all longer than broad, movable finger 1.17–1.23× (♂), 1.05–1.19× (♀) longer than hand. Fixed finger with 7 trichobothria, est absent, movable finger with 2 trichobothria, sb and st absent, eb and esb situated at base of fixed finger on retrolateral margin, ib, isb ist and it situated prolaterally at base of fixed finger, et subdistal (Fig. 8–13); chelal fingers straight in lateral view (Fig. 8, 9, 11–13). Chelal teeth juxtadentate; fixed finger with 37 (♂), 40 (♀) triangular, retrorse chelal teeth; movable finger with 33 (♂), 37 (♀) triangular, retrorse chelal teeth; accessory teeth absent; fixed chelal finger with 1 spine-like setae on prolateral margin, situated distally; movable chelal finger with 5 spine-like setae on prolateral margin (Fig. 8). Venom teeth very broad; venom apparatus present in both chelal fingers, duct very short, terminating in nodus ramosus near tip of fingers (Fig. 8, 9), nodus ramosus not inflated.
Carapace subrectangular (Fig. 5, 17); anterior margin slightly convex; epistome absent; completely smooth in male, granulated in female (Fig. 17); lateral margins evenly convex; posterior margin irregularly convex; 1.40–1.57× (♂), 1.28–1.57× (♀) longer than broad, ♂ with 18 setae arranged 4: 2: 2: 4: 4: 2; ♀ with 20 setae arranged 4: 2: 2: 4: 6: 2; all setae subequal in length; with 1 shallow median furrow; with two pairs of large, corneate eyes, situated near anterior margin of carapace, not on tubercle. Manducatory process distally triangular; with 1 seta situated distally, another situated medially; with 9 (♂), 8 (♀) additional setae; suboral seta present, reduced to a small nubbin. Maxillary shoulder absent; median maxillary lyrifissure situated medially. Coxa I approximately same width as coxa IV; coxae setal formula: ♂, 6: 6: 5: 4; ♀, 6: 6: 3: 4.
Femora I and II shorter than patellae I and II respectively; femora I and II with primary slit sensillum directed transversely; junction between anterior femora and patellae perpendicular; junction between posterior femora and patellae strongly oblique; femora III and IV much smaller than patellae III and IV respectively; femur + patella IV 2.55× (♂), 2.88× (♀) longer than deep; metatarsi IV with long basal tactile seta (Fig. 21); metatarsi shorter than tarsi; claws smooth; arolium much longer than claws, deeply divided (Fig. 22).
Tergites straight, divided (Fig. 2, 3); setal formula ♂, 4: 4: 6: 6: 5: 6: 6: 6: 6: 10 (including 4 tactile setae): 6 (including 2 tactile setae): 2; ♀, 6: 6: 6: 6: 6: 6: 6: 6: 7: 12 (including 4 tactile setae): 6 (including 2 tactile setae): 2; setae arranged in single rows; sternites, setal formula ♂, 6: (2) 4 [2 + 2] (2): (2) 4 (2): 6: 6 + 2 gls: 6 + 2 gls: 6 + 2 gls: 6: 10 (including 4 tactile setae): 6 (including 4 tactile setae): 2; ♀, 8: (2) 4 (2): (2) 4 (2): 6: 6 + 2 gls: 6 + 2 gls: 6 + 2 gls: 8: 8 (including 4 tactile setae): 8 (including 4 tactile setae): 2; setae of anterior genital operculum (sternite II) of ♀ approximately same size as other sternal setae; posterior tergites and sternites with several tactile setae; 1 pair of glandular setae (gls) situated on sternites VI, VII and VIII (Fig. 23) near mid-line, slightly in advance of regular setae, each seta slightly longer than other sternal setae; most sternites with broad median suture line; pleural membrane uniformly longitudinally striate; without setae; stigmatic helix present; anus (tergite XII and sternite XII) without raised rim; situated between tergite XI and sternite XI.
Dorsal anterior glands greatly enlarged; lateral genital sacs and median genital sacs absent; with 2 pairs of setae within genital atrium (Fig. 24).
With single large median cribriform plate and a pair of lateral cribriform plates (Fig. 25).
Paratype male (WAM T133035) (followed by 3 other males, when measured, in parentheses): Body length (excluding chelicerae) 2.78 (3.57–3.73). Pedipalp: trochanter 0.390/0.225 (0.395–0.415/0.220–0.240); femur 0.640/0.235 (0.670–0.695/0.250–0.270); patella 0.575/0.26 (0.580–0.600/0.275–0.295); chela (with pedicel) 1.115/0.380 (1.120–1.170/0.370–0.425); chela (without pedicel) length 1.030 (1.040–1.055); chelal hand (without pedicel) length 0.475 (0.475–0.500), depth 0.525 (0.530–0.585); movable finger length 0.585 (0.575–0.585). Carapace 0.770/0.550 (0.830–0.855/0.530–0.595); anterior eye diameter 0.050; posterior eye diameter 0.045. Leg I: femur 0.110/0.135; patella 0.255/0.150; tibia 0.245/0.110; metatarsus 0.100/0.085; tarsus 0.180/0.075. Leg IV: femur + patella 0.600/0.235; tibia 0.400/0.165; metatarsus 0.120/0.100; tarsus 0.145/0.085.
Holotype female (followed by 2 other females, when measured, in parentheses). Body length (excluding chelicerae) 3.68 (3.34). Pedipalp: trochanter 0.475/0.255 (0.480/0.260); femur 0.795/0.290 (0.610–0.720/0.250); patella 0.670/0.310 (0.550–0.560/0.280–0.310); chela (with pedicel) 1.310/0.450 (1.100–1.265/0.370–0.440); chela (without pedicel) 1.230 (1.010–1.155); hand (without pedicel) 0.640 (0.465–0.525), 0.645 (0.545–0.635) deep; movable finger length 0.670 (0.465–0.625). Carapace 0.840/0.655 (0.960-/0.585–0.610); anterior eye 0.055; posterior eye 0.055. Leg I: femur 0.130/0.140; patella 0.295/0.175; tibia 0.280/0.130; metatarsus 0.110/0.090; tarsus 0.135/0.085. Leg IV: femur + patella 0.750/0.260; tibia 0.480/0.165; metatarsus 0.145/0.110; tarsus 0.165/0.095.
Most cuticular surfaces yellow–brown, pedipalps and especially chela darker, posterior portion of carapace paler.
Trochanter 1.97×, femur 2.77×, patella 1.82×, chela (with pedicel) 3.18×, chela (without pedicel) 2.88×, hand (without pedicel) 1.38× all longer than broad, movable finger 1.06× longer than hand (without pedicel). Fixed chelal finger with 6 trichobothria, eb, esb, et, ib, ist and it present; movable finger with 2 trichobothria, b and t present (Fig. 14); trichobothria rather short. Fixed chelal finger with 30 pointed, juxtadentate teeth; movable chelal finger 29 pointed, juxtadentate teeth.
1.25× longer than broad; with 18 setae; arranged 4: 2: 2: 4: 4: 2; smooth, without furrows; 2 pair of eyes.
Trochanter 1.78×, femur 2.37×, patella 1.64×, chela (with pedicel) 2.93×, chela (without pedicel) 2.69×, hand (without pedicel) 1.45× all longer than broad, movable finger 0.84× longer than hand (without pedicel). Fixed chelal finger with 5 trichobothria, eb, et, ib, ist and it present; movable finger with 2 trichobothria, b and t present (Fig. 15); trichobothria rather short; chelal finger with 24 teeth; chelal finger pointed, juxtadentate, basal teeth slightly enlarged; movable chelal finger with 25 teeth; teeth mostly pointed, juxtadentate.
1.16× longer than broad; with 18 setae, arranged 4: 2: 2: 4: 4: 2; without furrows; with 2 pairs of eyes.
Trochanter 1.74×, femur 2.04×, patella 1.46×, chela (with pedicel) 3.01×, chela (without pedicel) 2.88×, hand (without pedicel) 1.27× all longer than broad, movable finger 1.26× longer than hand (without pedicel). Fixed finger with 3 trichobothria: eb situated at base of fixed finger on retrolateral margin, ist situated prolaterally at base of fixed finger, et subdistally; movable finger with 1 trichobothrium, t, situated subbasally (Fig. 16); chelal finger with 20 teeth; chelal finger pointed, juxtadentate, basal teeth slightly enlarged; movable chelal finger with 20 teeth; teeth mostly pointed, juxtadentate.
1.24× longer than broad; with 16 setae, arranged 4: 2: 2: 4: 2: 2; without furrows; with 2 pairs of eyes.
Most specimens of A. rixi have been collected by beating low vegetation, including ‘hanging’ litter that forms when twigs, leaves and other detritus accumulates among low vegetation above ground level. One specimen was taken from a malaise trap. The species is widespread across southern Western Australia and rarely found more than a few hundred metres from the coast (Fig. 27). In addition to the specimens listed above, an adult specimen was found in Albany by Sheila Murray who kindly supplied an image (Fig. 7).
Aldabrinus novaecaledoniae (Beier, 1966), comb. nov.
(Fig. 26.)
ZooBank: urn:lsid:zoobank.org:act:B4FC69DE-BA0A-4CE9-B037-39EAC3256962
Paraldabrinus novaecaledoniae Beier, 1966a, pp. 368–370, fig. 4.
Holotype. NEW CALEDONIA: Province Nord: ♂, Gorge de Ndokoa, between Pic Adio and Dent de Poya [21°19′S, 165°19′E], unter Niaouli-Rinde, 11 August 1965, A. Kaltenbach (NHMW 22766).
Paratypes. NEW CALEDONIA: Province Nord: 2 ♀, collected with holotype (NHMW 22767, 22768).
Aldabrinus novaecaledoniae differs from all other species of the genus in the presence of only a single trichobothrium on the movable chelal finger.
See Beier (1966a).
Paraldabrinus novaecaledoniae was described by Beier (1966a) from a male and female from Gorge de Ndokoa, between Pic Adio and Dent de Poya, New Caledonia (Fig. 26). As discussed above, Paraldabrinus is here synonymised with Aldabrinus, thereby necessitating the transfer of P. novaecaledoniae to Aldabrinus.
The specimens were collected from under bark of the Niaouli, Melaleuca quinquenervia, a tree that occurs in New Caledonia, Papua New Guinea and coastal regions of eastern Australia, in subtropical and tropical habitats (see Atlas of Living Australia, https://bie.ala.org.au/species/https://id.biodiversity.org.au/node/apni/2887514, accessed 1 July 2021).
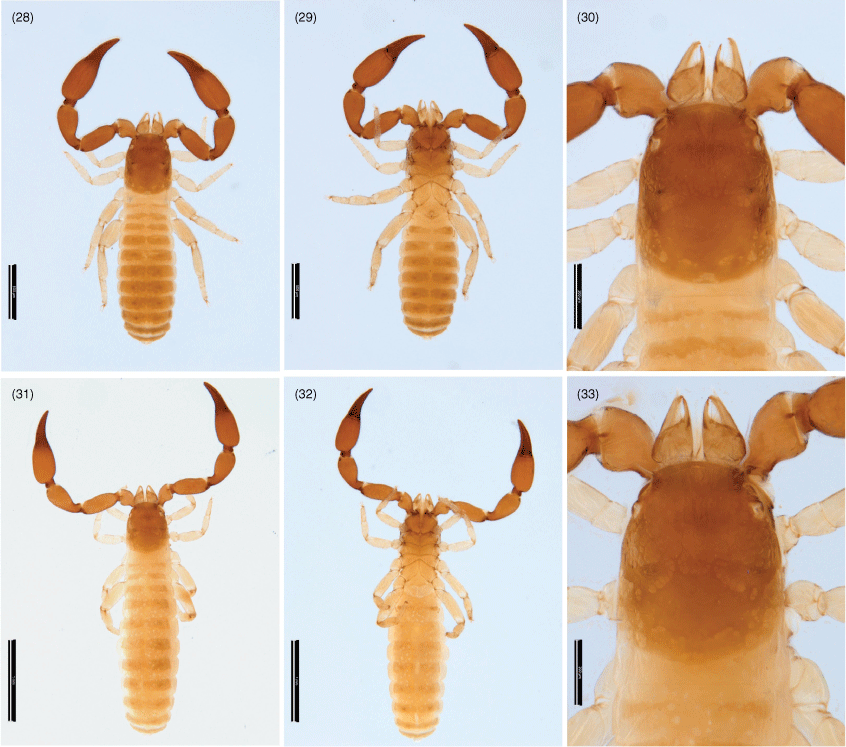
Genus Nobilipinus, gen. nov.
ZooBank: urn:lsid:zoobank.org:act:DE430F15-64AE-48B2-B234-5EA9BD07D016
The genus name is a partial anagram of the name of the type species, Garypinus nobilis and is to be treated as masculine.
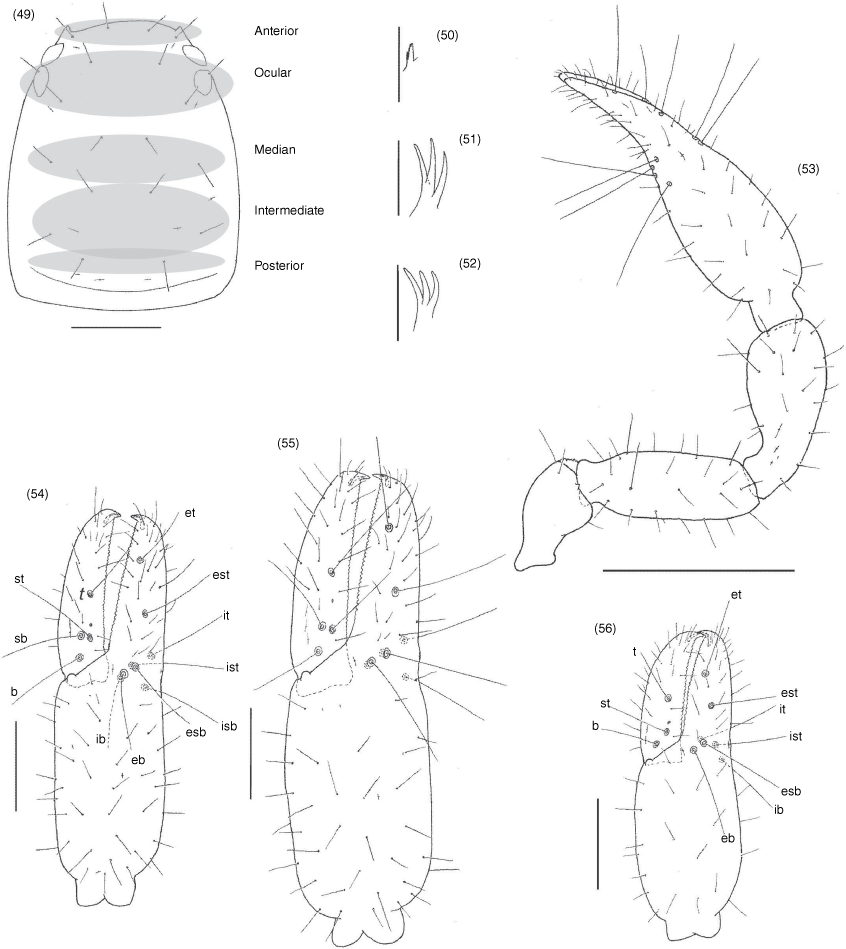
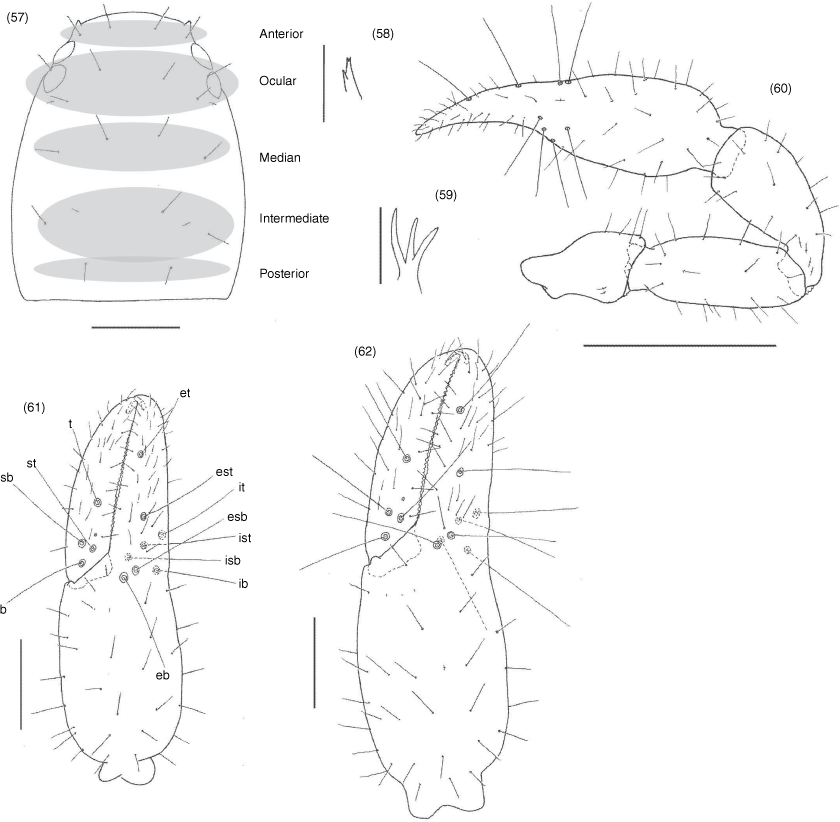
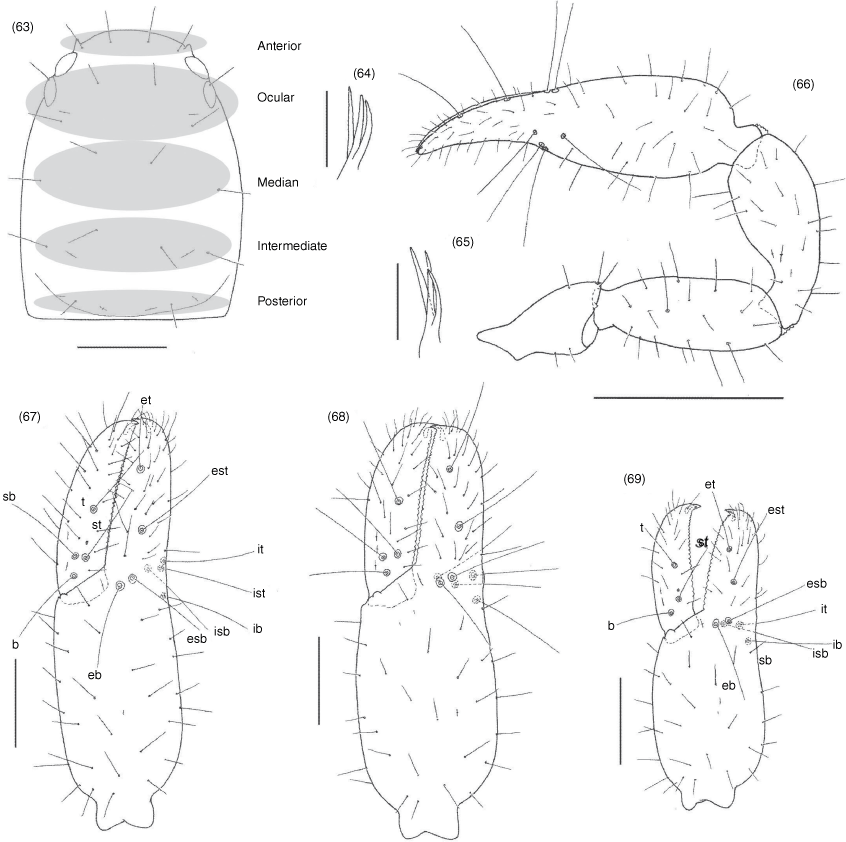
Nobilipinus differs from all other genera of Garypinidae in the presence of a deeply trifid galea in females, nymphs (Fig. 51, 52, 58, 59, 65, 71, 76, 77, 85, 86) and the males of N. galeatus (Fig. 64). The genus most closely resembles Garypinus and Caecogarypinus as all three genera have cheliceral setae ls and is situated very close to each other (Fig. 34). Further resemblance with Caecogarypinus is shown in both genera having trichobothrium st situated dorsal or basi-dorsal to sb (Fig. 54, 55, 61, 62, 67, 68, 73, 79, 80, 88, 89) but the genus differs in the presence of eyes (Fig. 30, 31, 49, 57, 63, 70, 74, 84) (absent in Caecogarypinus) and the lack of strong guard setae on the prolateral faces of the chelal fingers. Nobilipinus differs from Garypinus in the lack of setae in the pleural membrane (Fig. 41) (present in Garypinus).
Hand with 5 long, acuminate setae, ls and is situated very close to each other (Fig. 34); movable finger with 1 long subdistal seta; rallum of 4 blades, distal blade with several spinules on anterior margin, others smooth (Fig. 36); with 2 dorsal and 1 ventral lyrifissures; lamina exterior present; serrula interior modified to form velum; galea of ♀ comprising 3 long rami on a very short stalk (Fig. 51, 59, 65, 76, 86), of ♂ either like ♀ (Fig. 64) or short with 3 small distal rami (Fig. 50, 58, 71, 75, 85).
Femur with a single submedial tactile seta (Fig. 53, 60, 66, 72, 78, 87); patella without disto-prolateral excavation; chelal hand ovate; pedicel prolaterally offset. Fixed chelal finger with 8 trichobothria, movable chelal finger with 4 trichobothria (Fig. 54, 55, 61, 62, 67, 68, 73, 79, 80, 88, 89): eb and esb situated at base of fingers; est situated submedially, usually midway between esb and et but sometimes closer to esb than et; et situated subdistally; ib, isb, ist and it situated subbasally, with ib separated from the others; b, sb and st situated subbasally, with st situated dorsal to sb; t situated submedially. Venom apparatus present in both chelal fingers, venom ducts very short, terminating in nodus ramosus near tip of fingers. Chelal teeth juxtadentate; accessory teeth absent; retrolateral condyle small and rounded.
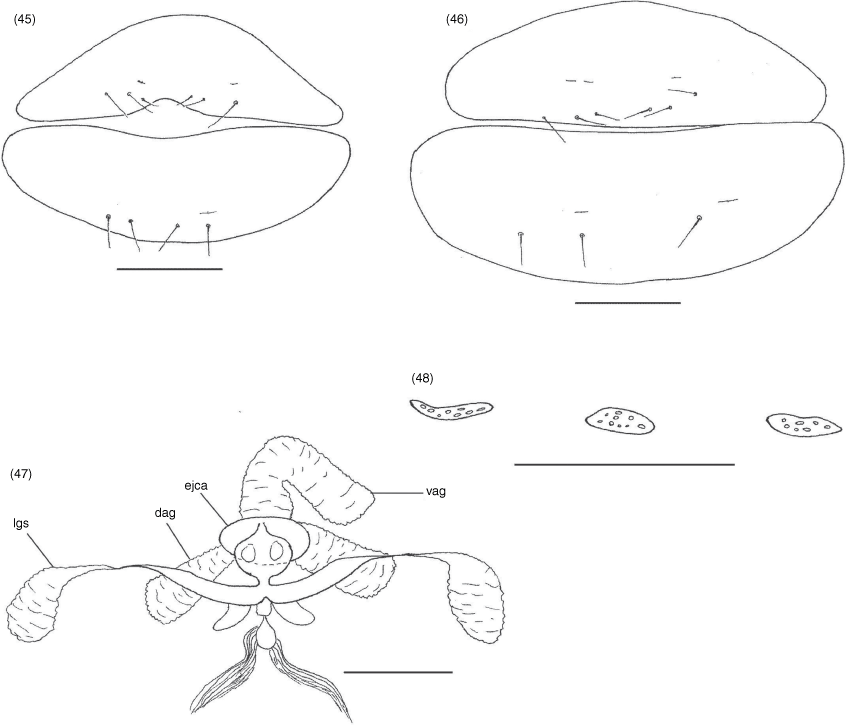
Subrectangular (Fig. 49, 57, 63, 70, 74, 84), anterior margin slightly convex; with 20–22 setae arranged with 4 in anterior row, 6 (occasionally with 7 or 8) in ocular row, 4 in median row, 4 in intermediate row and 2 (rarely 3) in posterior row; with 4 large eyes situated close to anterior margin of carapace; epistome absent; with shallow median transverse furrow present or absent.
Manducatory process distally triangular (Fig. 37), with 1 seta situated distally, another situated medially and 1 suboral seta that is reduced to a nubbin; maxillary shoulder absent; median maxillary lyrifissure present and situated submedially (Fig. 38); all coxae approximately same width (Fig. 29, 32).
Femora I and II shorter than patellae I and II; junction between anterior femora and patellae perpendicular (Fig. 42); oblique suture line between femora III and IV and patella III and IV (Fig. 43); tarsi slightly longer than metatarsi (Fig. 42, 43); metatarsi III and IV with a long basal tactile seta; tibia III and IV with a long medial tactile seta; subterminal tarsal setae acuminate; arolium deeply divided (Fig. 44) and longer than claws; claws slender and simple.
Nobilipinus nobilis (With), specimens from Ko Chang (MHNG): 34, left chelicera, dorsal, male; 35, left movable finger of chelicera, lateral, male; 36, left rallum, lateral, male; 37, pedipalpal coxae, ventral, male; 38, manducatory processes, ventral, male; 39, sternites V–XII, ventral, male; 40, sternites V–IX, ventral, female; 41, pleural membrane adjacent to segments VI–VII, dorsal, male; 42, right leg I, retrolateral, male; 43, right leg IV, retrolateral, male; 44, arolium from left leg III, dorsal, male. Scale lines: 0.5 mm (39, 40), 0.2 mm (37, 41–43), 0.1 mm (34), 0.05 mm (35, 36, 38, 44).
Tergites and sternites (Fig. 28, 29, 31, 32) sometimes divided but sometimes undivided. Pleural membrane longitudinally striate and lacking setae (Fig. 41). Sternites VI–VIII of male and female with a pair of median glandular setae (Fig. 39, 40). Stigmatic sclerites with 1 or 2 setae; spiracles with spiracular helix. Setae of anterior genital operculum (sternite II) approximately same size as other sternal setae (Fig. 45, 46). Posterior tergites and sternites with tactile setae. Anus (tergite XII and sternite XII) without raised rim; situated between tergite XI and sternite XI.
With prominent pair of dorsal anterior glands, a single ventral anterior gland, paired lateral genital sacs and small, ovoid median genital sac; with 1 pair of setae within genital atrium.
Hand with 5 long, acuminate setae; movable finger 1 subdistal seta; galea with 3 long rami (Fig. 52).
Nobilipinus nobilis (With), specimens from Ko Chang (MHNG): 49, carapace, dorsal, male; 50–52, left galea: 50, dorsal, male; 51, ventral, female; 52, ventral, tritonymph; 53, right pedipalp, dorsal, male; 54–56, left chelae, retrolateral: 54, male; 55, female; 56, tritonymph. Scale lines: 0.5 mm (53), 0.2 mm (49, 54–56), 0.05 mm (50–52).
Fixed finger and hand with 7 trichobothria, movable finger with 3 trichobothria (Fig. 56, 69, 81); eb, esb, est, et, ib, ist, it, b, st and t present, isb and sb absent.
Nobilipinus affinis sp. nov., holotype male and paratype female (MHNG): 57, carapace, dorsal, male; 58, left galea, ventral, male; 59, right galea, dorsal, female; 60, right pedipalp, dorsal, male; 61, 62, left chelae, retrolateral: 63, male; 64, female. Scale lines: 0.5 mm (60), 0.2 mm (57, 62, 62), 0.05 mm (58, 59).
Nobilipinus galeatus sp. nov., holotype male, paratype female and tritonymph (MHNG): 63, carapace, dorsal, male; 64, right galea, ventral, male; 65, left galea, ventral, female; 66, right pedipalp, dorsal, male; 67–69, left chelae, retrolateral: 67, male; 68, female; 69, tritonymph. Scale lines: 0.5 mm (63), 0.2 mm (67–69), 0.05 mm (64, 65).
Hand with 4 or 5 long, acuminate setae; movable finger 1 subdistal seta; galea with 3 long rami (Fig. 77).
Hand with 4 long, acuminate setae, sbs absent; movable finger without seta; galea with 3 long rami.
Fixed finger and hand with 3 trichobothria, movable finger with 1 trichobothrium (Fig. 83); eb and isb subbasal; et subdistal; t submedial; all others absent.
Nobilipinus is readily diagnosed from all other garypinid genera by the deeply trifid galea in females and nymphs of all species (Fig. 51, 52, 58, 59, 65, 71, 76, 77, 85, 86) and in the males of N. galeatus (Fig. 64), a characteristic feature that was illustrated by With (1906, fig. 8c) and later mentioned by Beier (1932b) in a brief redescription of G. nobilis. The other noteworthy diagnostic feature is the position of trichobothrium st that is situated directly dorsally to sb in all species of Nobilipinus (Fig. 54, 55, 61, 62, 67, 68, 73, 79, 80, 88, 89). This morphology is quite different from most other species of Garypinidae in which st is situated noticeably distally to sb, at least in the species in which sb and st are present. The only other garypinids in which st is directly dorsal to sb are species of Caecogarypinus (C. pectinodentatus Dashdamirov, 2007 from Vietnam) (Dashdamirov 2007) and Protogarypinus (P. giganteus Beier, 1954 and P. dissimilis Beier, 1975 from Australia) (Beier 1954a, 1975). This also occurs in the type species of Garypinidius, G. mollis Beier, 1955, from South Africa (Beier 1955a) but not in the only other species, G. capensis (Ellingsen, 1912) (Beier 1958), in one of the species of Garypinus, G. asper Beier, 1955 from Syria (Beier 1955b) and most species of Serianus (Chamberlin 1931a; Feio 1945; Hoff 1950, 1956, 1964; Beier 1959a, 1964a, 1971b, 1978; Muchmore 1968; Lee 1979; Muchmore 1981b; Mahnert 1988, 1991, 2014; Dashdamirov and Schawaller 1993; Tooren 2002b; Bedoya-Roqueme et al. 2017; Piedra-Jiménez et al. 2019). The species of Serianus that have st situated distinctly distally to sb are S. elongatus Mahnert, 2014 from the Galapagos Islands (Mahnert 2014) and S. salomonensis Beier, 1966 from the Solomon Islands (Beier 1966b) and the systematic positions require verification. Only a single female specimen of S. elongatus was available to Mahnert (2014) and males are required to assess whether these have more than one pair of glandular setae on sternites VI, VII and sometimes VIII as is characteristic of Serianus. Another peculiar modification of S. elongatus is the presence of only four setae on the cheliceral hand (Mahnert 2014). Although most garypinids have five setae, several other garypinid species are known to have only four setae: the species of Aldabrinus, Indogarypinus and Paraldabrinus (Beier 1966a; Muchmore 1974; Murthy and Ananthakrishnan 1977) and three species of Solinus, S. africanus Beier, 1967, S. corticola (Chamberlin, 1923) and S. pusillus Beier, 1971 (Chamberlin 1930; Beier 1967b, 1971a). Apart from the anomalous position of st, the male of S. salomonensis is reported to have only one pair of glandular setae (Beier 1966b) that would also seem to preclude this from Serianus. The position of st is unknown for S. serianus (Chamberlin, 1923) and S. solus (Chamberlin, 1923) that is especially unfortunate as S. serianus from Baja California is the type species of the genus. Although the other two species of Serianus described by Chamberlin (1923) from Baja California, S. arboricola (Chamberlin, 1923) and S. litoralis (Chamberlin, 1923), have since been found to have st dorsal to sb (Chamberlin 1930; Lee 1979), this cannot be assumed.
Nobilipinus karenae sp. nov., holotype male (MHNG): 70, carapace, dorsal; 71, left galea, ventral; 72, right pedipalp, dorsal; 73, left chela, retrolateral. Scale lines: 0.5 mm (72), 0.2 mm (70, 73), 0.05 mm (71).
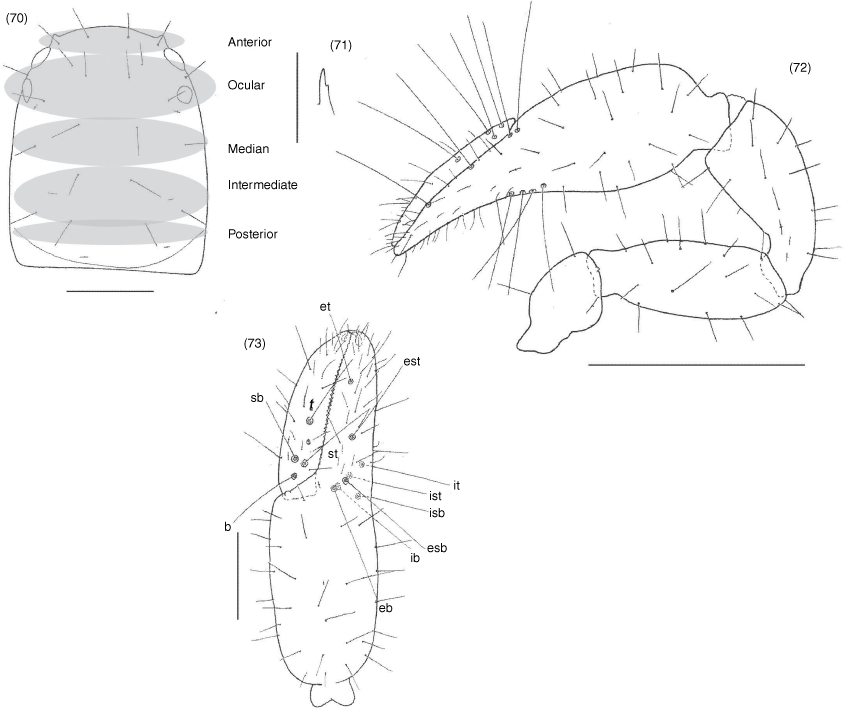
Nobilipinus kohi sp. nov., holotype male, paratype female, tritonymph and protonymph (MHNG) and paratype deutonymph (BMKB): 74, carapace, dorsal, male; 75–77, left galeae, ventral: 75, male; 76, female; 77, deutonymph; 78, right pedipalp, dorsal, male; 79–83, left chelae, retrolateral: 79, male; 80, female; 81, tritonymph; 82, deutonymph; 83, protonymph. Scale lines: 0.5 mm (78), 0.2 mm (74, 79–83), 0.05 mm (75–77).
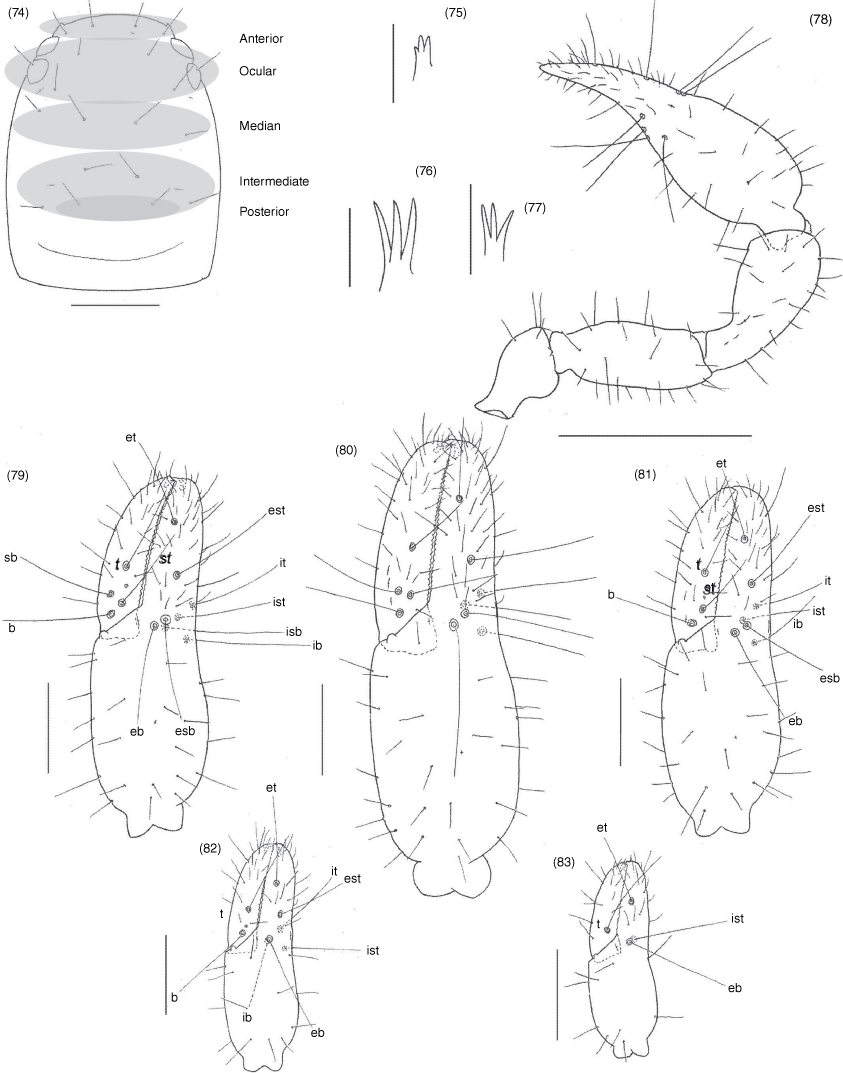
Nobilipinus tricosus sp. nov., holotype male, paratype female, deutonymph (SMF): 84, carapace, dorsal, male; 85, 86, galeae, dorsal: 85, left galea, male; 86, right galea, female; 87, right pedipalp, dorsal, male; 88–90, left chelae, retrolateral: 88, male; 89, female; 90, deutonymph. Scale lines: 0.5 mm (87), 0.2 mm (84, 88–90), 0.05 mm (85, 86).
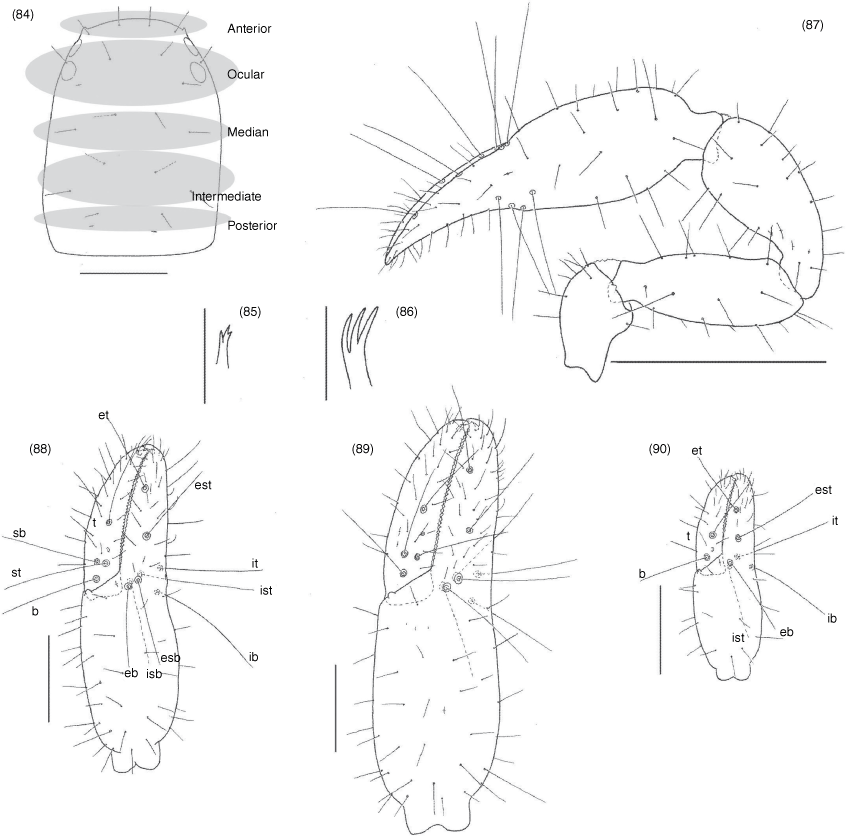
The male genitalia of garypinids appear to be of significant value and may hold the key in establishing a stable subfamilial classification. Regrettably, only a handful of genera have had the genital morphology described or illustrated in detail (e.g. Chamberlin 1923; Muchmore 1974, 1979, 1980; Harvey 1988; Mahnert 1991; Dashdamirov 1996; Harvey and Šťáhlavský 2010; Gardini and Gavalas 2021). As discussed by Harvey and Šťáhlavský (2010), dorsal anterior glands are present in several garypinid genera, including Aldabrinus, Amblyolpium, Galapagodinus, Neominniza, Neoamblyolpium, Protogarypinus, Pseudogarypinus, Serianus, Solinellus, Solinus and Thaumatolpium but are absent in both species of Oreolpium. Species of Nobilipinus also have a prominent pair of dorsal anterior glands and an enlarged ventral anterior gland (Fig. 47). The sister family of the Garypinidae, the Larcidae, also has prominent dorsal anterior glands (Harvey and Šťáhlavský 2010), providing morphological support for this relationship, in addition to the phylogenomic data that first revealed this relationship (Benavides et al. 2019). The dorsal and ventral anterior glands of Nobilipinus have a noticeably different morphology from the lateral and median genital sacs, and appear dark grey in specimens cleared in lactic acid, whereas the sacs are beige, the same colour as the remainder of the genitalia.
Another feature of potential phylogenetic significance in Garypinidae is the placement of cheliceral setae ls and is. In most taxa, including Amblyolpium, Garypinidius capensis (Ellingsen, 1912) (M. S. Harvey, pers. obs.), Neoamblyolpium, Neominniza divisa Beier, 1930 (M. S. Harvey, pers. obs.), Oreolpium, Protogarypinus, Thaumatolpium silvestrii (Beier, 1930) (M. S. Harvey, pers. obs), Solinus spp. (M. S. Harvey, pers. obs, including species from Australia and USA) and Serianus (Mahnert 2014, fig. 44; M. S. Harvey, pers. obs. including specimens from USA and Chile), these are widely spaced (e.g. Hoff 1956; Heurtault 1970; Mahnert 1976; Benedict and Malcolm 1978; Harvey 1992; Harvey and Šťáhlavský 2010). This configuration is found in other pseudoscorpions, including species of Larcidae (the sister group of the Garypinidae) (Gardini 1983; Harvey 1992; Zaragoza 2005). However, these setae are closely spaced in Caecogarypinus (Dashdamirov 2007, fig. 3H), Garypinus dimidiatus (Hadži 1933, fig. 29a), G. afghanicus (M. S. Harvey, pers. obs.) and Nobilipinus spp. (With 1906, plate 2, fig. 8b) (Fig. 34) and therefore likely to define a monophyletic clade based on a unique synapomorphy. Unfortunately, this feature has not been mentioned or illustrated for any species of the genera Haplogarypinus, Hemisolinus, Nelsoninus, Solinellus or Teratolpium and cannot be determined for garypinids that are lacking seta ls, i.e. the genera Aldabrinus and Paraldabrinus, and the species of Serianus and Solinus with only four setae on the cheliceral hand.
| 1. | Intermediate setal row of carapace straight (Fig. 63); galea of male with very long rami (Fig. 64)...Nobilipinus galeatus sp. nov. Intermediate setal row of carapace recurved (Fig. 49, 57, 70, 72, 84); galea of male with short rami (Fig. 50, 58, 71, 75, 85)...2 |
| 2. | Posterior setal row of carapace situated at same level as lateral setae of the intermediate setal row (Fig. 70, 74)...3 Posterior setal row of carapace situated posterior to level of lateral setae of the intermediate setal row (Fig. 49, 57, 63, 84)...4 |
| 3. | Trichobothrium est situated closer to esb than et (Fig. 72, 73)...Nobilipinus karenae sp. nov. Trichobothrium est situated midway between esb and et (Fig. 78–80)...Nobilipinus kohi sp. nov. |
| 4. | Trichobothrium est situated midway between esb and et (Fig. 53–55, 60–62)...5 Trichobothrium est closer to esb than et (Fig. 87–89)...Nobilipinus tricosus sp. nov. |
| 5. | Intermediate setal row of carapace strongly recurved (Fig. 49)...Nobilipinus nobilis With Intermediate setal row of carapace slightly recurved (Fig. 57)...Nobilipinus affinis sp. nov. |
Nobilipinus nobilis (With, 1906), comb. nov.
ZooBank: urn:lsid:zoobank.org:act:8A37D0C9-1FCC-4F59-95C3-725BC4D71626
Garypinus nobilis With, 1906, pp. 29, 112–115, fig. 7a–b, plate 1 fig. 7a–c, plate 2 fig. 8a–g. Ellingsen (1910), p. 389; Beier (1932b), p. 210, fig. 242; Roewer (1936), fig. 15b, 39a–b, 42a–b, 51; Schawaller (1994), pp. 733–734, fig. 12–13 (in part); Judson (1997), p. 30.
Not Garypinus nobilis With: Tullgren (1907), p. 67 (misidentification, see N. galeatus); Beier (1930), pp. 289–290 (misidentification, see N. tricosus).
THAILAND: Trat Province: 49 ♂, 49 ♀, 5 tritonymphs, Ko Chang, west side, 12°03′N, 102°18′E, 50–200 m, 3–23 December 1999, Winckler extraction, secondary forest with primary spots, A. Schulz (MHNG); 5 ♂, 5 ♀, 1 tritonymph, same data (WAM T152653–152656, T152662); 3 ♂, 1 ♀, 1 tritonymph, Ko Chang National Park, Khlong Phrao Waterfall and hill near White Sand Beach, 12°07′N, 102°16′E, 100 m, 23–25 August 1992, P. Schwendinger (MHNG).
Nobilipinus nobilis differs from all other species of the genus as follows: from N. karenae and N. kohi in the posterior setal row of the carapace being situated basally to the level of the lateral setae of the intermediate setal row (Fig. 49); from N. galeatus in the strongly recurved intermediate setal row of the carapace (Fig. 49) and the male galea possessing short rami (Fig. 50); from N. tricosus in trichobothrium est being situated midway between esb and et (Fig. 54, 55); from N. affinis in the intermediate setal row of carapace being strongly recurved (Fig. 49); and from N. vachoni in the shape of the male chelal hand that is ovoid in N. nobilis (Fig. 53) and more elongated in N. vachoni.
Setae long, mostly straight and acicular; most cuticular surfaces smooth and glossy.
Surface smooth; cheliceral hand with 5 setae, ls and is adjacent to each other; movable finger with 1 subdistal seta; all setae acuminate; galea of ♂ short with 3 small distal rami (Fig. 50), of ♀ with 3 very long rami (Fig. 51); fixed finger with 5 (♂, ♀) small teeth, each approximately same size; movable finger with 1 subdistal tooth; serrula interior with 20 (♂, ♀) blades; lamina exterior present; rallum with 4 blades, anterior blade with anterior spinules, remainder smooth (Fig. 36).
All segments completely smooth; setae acicular, straight or nearly so; trochanter 1.39–1.70× (♂), 1.62–1.77× (♀), femur with 1 subbasal tactile seta on dorsal surface 2.53–2.86× (♂), 2.26–2.50× (♀), patella with several small lyrifissures situated basally on dorsal surface, 2.23–2.37× (♂), 2.06–2.23× (♀), chelal hand ovoid, chela (with pedicel) 3.20–3.50× (♂), 3.03–3.24× (♀), chela (without pedicel) 3.02–3.32× (♂), 2.79–3.05× (♀), hand (without pedicel) 1.58–1.70× (♂), 1.41–1.58× (♀) all longer than broad, movable finger 0.89–1.00× (♂), 0.95–1.08× (♀) longer than hand. Fixed finger with 8 trichobothria: eb and esb situated at base of fixed finger on retrolateral margin, est situated slightly closer to esb than et, ib, isb ist and it situated prolaterally at base of fixed finger, et subdistally; movable finger with 4 trichobothria, st situated dorsal to sb, t situated submedially (Fig. 54, 55). Fixed finger with 31 (♂), 37 (♀) triangular, retrorse chelal teeth; movable finger with 34 (♂), 37 (♀) triangular, retrorse chelal teeth.
Carapace (Fig. 49) 1.23–1.45× (♂), 1.15–1.34× (♀) longer than broad; ♂ and ♀ with 20 setae arranged with 4 in anterior row, 6 in ocular row, 4 in median row, 4 in intermediate row and 2 in posterior row; intermediate row recurved; posterior row situated near posterior margin of carapace; all setae subequal in length; with 1 shallow median furrow; with two pairs of large, corneate eyes. Manducatory process distally triangular; with 1 seta situated distally, another situated medially, and 1 suboral seta, plus 8 (♂), 11 (♀) additional setae. Coxae, setal formula: ♂, 6: 6: 6: 5; ♀, 8: 7: 5: 4.
Femur + patella IV 2.41× (♂), 2.54× (♀) longer than deep; metatarsus IV with long basal tactile seta, TS = 0.20 (♂), 0.21 (♀).
Tergites straight, divided; setal formula ♂, 6: 6: 8: 8: 8: 10: 8: 10: 10: 10 (including 4 tactile setae): 12 (including 2 tactile setae): 2; ♀, 6: 6: 8: 8: 8: 9: 9: 8: 8: 10 (including 4 tactile setae): 8 (including 4 tactile setae): 2; setae arranged in single rows; sternites, setal formula ♂, 6: (1) 6 [1 + 1] (1): (1) 4 (1): 9: 8 + 2 gls: 8 + 2 gls: 7 + 2 gls: 8: 10 (including 4 tactile setae): 10 (including 4 tactile setae): 2; ♀, 4: (1) 4 (1): (1) 4 (1): 8: 6 + 2 gls: 6 + 2 gls: 7 + 2 gls: 7: 10 (including 4 tactile setae): 10 (including 4 tactile setae): 2; posterior tergites and sternites with several tactile setae; 1 pair of glandular setae (gls) situated on sternites VI, VII and VIII, situated near mid-line, slightly in advance of regular setae, each seta slightly longer than other sternal setae; most sternites with broad median suture line.
With prominent pair of dorsal anterior glands, a single ventral anterior gland, paired lateral genital sacs and small, ovoid median genital sac; with 1 pair (sometimes duplicated on one side) of setae within genital atrium (Fig. 47).
With a single median cribriform plate and a pair of lateral cribriform plates (Fig. 48).
Followed by 11 other males in parentheses (when measured): body length (excluding chelicerae) 2.08 (1.61–2.10). Pedipalp: trochanter 0.285/0.180 (0.270–0.300/0.165–0.180), femur 0.500/0.175 (0.425–0.465/0.160–0.180), patella 0.485/0.205 (0.435–0.475/0.195–0.205), chela (with pedicel) 0.875/0.250 (0.800–0.850/0.245–0.265), chela (without pedicel) length 0.830 (0.760–0.800), chelal hand (without pedicel) length 0.420 (0.395–0.425), movable finger length 0.395 (0.375–0.410). Carapace 0.630/0.505 (0.570–0.655/0.440–0.475), anterior eye diameter 0.065, posterior eye diameter 0.060. Leg I: femur 0.175/0.095, patella 0.220/0.105, tibia 0.220/0.070, metatarsus 0.085/0.050, tarsus 0.120/0.050. Leg IV: femur + patella 0.495/0.205, tibia 0.335/0.110, metatarsus 0.125/0.065, tarsus 0.180/0.060, TS = 0.025.
Followed by 11 other females in parentheses (when measured): Body length (excluding chelicerae) 3.05 (1.94–2.88). Pedipalp: trochanter 0.355/0.220 (0.280–0.345/0.165–0.200), femur 0.575/0.240 (0.435–0.510/0.180–0.210), patella 0.575/0.280 (0.450–0.545/0.205–0.250), chela (with pedicel) 1.055/0.345 (0.845–1.005/0.265–0.310), chela (without pedicel) length 1.005 (0.780–0.945), chelal hand (without pedicel) length 0.500 (0.400–0.465), movable finger length 0.510 (0.395–0.500). Carapace 0.775/0.615 (0.610–0.745/0.485–0.565), anterior eye diameter 0.070, posterior eye diameter 0.055. Leg I: femur 0.150/0.115, patella 0.250/0.130, tibia 0.260/0.085, metatarsus 0.095/0.060, tarsus 0.140/0.050. Leg IV: femur + patella 0.610/0.240, tibia 0.410/0.130, metatarsus 0.145/0.075, tarsus 0.180/0.070, TS = 0.030.
Cheliceral hand with 5 setae, ls and is adjacent to each other; movable finger with 1 seta; galea with 3 very long rami (Fig. 52).
Trochanter 1.72×, femur with 1 very long submedial tactile seta on dorsal surface, 2.30×, patella 2.03×, chela (with pedicel) 3.02×, chela (without pedicel) 2.85×, hand (without pedicel) 1.43× all longer than broad, movable finger 1.03× longer than hand. Fixed finger with 7 trichobothria: eb and esb situated at base of fixed finger on retrolateral margin, ib, ist and it situated prolaterally at base of fixed finger, et subdistally, isb absent; movable finger with 3 trichobothria, b and st situated basally, t situated submedially, sb absent (Fig. 56). Fixed finger with 28 teeth; movable finger with 26 teeth.
Carapace 1.31× longer than broad; with 20 setae including 4 in anterior row, and 2 in posterior row; with two pairs of corneate eyes. Coxae, setal formula: 6: 5: 4: 4.
Tergites, setal formula: 6: 8: 8: 8: 8: 8: 8: 8: 8: 8 (including 4 tactile setae): 8 (including 4 tactile setae): 2; sternites, setal formula 2: (1) 4 (1): (1) 4 (1): 6: 2 + 2 gls: 4 + 2 gls: 4 + 2 gls: 8: 6 (including 4 tactile setae): 8 (including 2 tactile setae): 2; 1 pair of glandular setae (gls) situated on sternites VI, VII and VIII, situated near mid-line, slightly in advance of regular setae, each seta slightly longer than other sternal setae.
Body length (excluding chelicerae) 2.27. Pedipalp: trochanter 0.250/0.145, femur 0.380/0.165, patella 0.375/0.185, chela (with pedicel) 0.710/0.235, chela (without pedicel) length 0.670, chelal hand (without pedicel) length 0.335, movable finger length 0.345. Carapace 0.570/0.435, anterior eye diameter 0.050, posterior eye diameter 0.050.
Garypinus nobilis With, 1906 was originally described from six adult specimens (one male and five females) collected from under stones on the island of Ko Chang, Thailand (With 1906). Most of these specimens are lodged in the Zoological Museum, Copenhagen with one female syntype deposited in BMNH (Judson 1997). The species has subsequently been recorded from other locations in Thailand (Tullgren 1907; Schawaller 1994), the Bismarck Archipelago, Papua New Guinea (Ellingsen 1910) and Java (Beier 1930). The specimens from Java were examined for this study and found to represent the new species N. tricosus. The specimen from Bangkok recorded by Tullgren (1907) is a specimen of N. galeatus. The other records of G. nobilis from Thailand and the Bismarck Archipelago may represent misidentifications and will require verification. At least some of the specimens reported from Thailand by Schawaller (1994) are likely to have beeen misidentified, as these were collected close to the known distribution of N. galeatus (Fig. 91).
Garypinus vachoni was synonymised with G. nobilis by Schawaller (1994). As discussed under N. vachoni, this synonymy is provisionally overturned, pending examination of the type specimens or of additional material from Vietnam and Cambodia.
Nobilipinus affinis, sp. nov.
ZooBank: urn:lsid:zoobank.org:act:05A79785-A408-4660-983E-E6C5B5345D30
Holotype. INDONESIA: Kepulauan Riau: ♂, Linnga Island, at the foot of Mount Daik, ~5 km NW of Daik, 0°12′35.9″S, 104°36′58.8″E, 12 June 2001, rainforest along stream, P. Schwendinger (MHNG).
Paratypes. INDONESIA: Kepulauan Riau: 2 ♀, collected with holotype (MHNG).
The specific epithet is an adjective referring to similarity of this species to others in the genus (affinis, Latin, neighbouring, bordering, related) (Brown 1956).
Nobilipinus affinis differs from all other species of the genus as follows: from N. karenae and N. kohi in the posterior setal row of the carapace being situated basally to the level of the lateral setae of the intermediate setal row (Fig. 57); from N. galeatus in the strongly recurved intermediate setal row of the carapace (Fig. 57) and the male galea possessing short rami (Fig. 58); from N. nobilis and N. tricosus in trichobothrium est being situated midway between esb and et (Fig. 59, 62); and from N. vachoni in the shape of the male chelal hand that is ovoid in N. nobilis (Fig. 60) and more elongated in N. vachoni.
Setae long, mostly straight and acicular; most cuticular surfaces smooth and glossy.
Surface smooth; cheliceral hand with 5 setae, ls and is adjacent to each other; movable finger with 1 subdistal seta; all setae acuminate; galea of ♂ short with 3 small distal rami (Fig. 58), of ♀ with 3 very long rami (Fig. 59); fixed finger with 5 (♂, ♀) small teeth, each approximately same size; movable finger with 1 subdistal tooth; serrula interior with 19 (♂), 20 (♀) blades; lamina exterior present; rallum with 4 blades, anterior blade with anterior spinules, others smooth.
All segments completely smooth; setae acicular, straight or nearly so; trochanter 1.75× (♂), 1.79–1.85× (♀), femur with 1 subbasal tactile seta on dorsal surface, 2.56× (♂), 2.52–2.72× (♀), patella with several small lyrifissures situated basally on dorsal surface, 2.21× (♂), 2.11–2.16× (♀), chelal hand ovoid, chela (with pedicel) 3.12× (♂), 3.03–3.06× (♀), chela (without pedicel) 2.91× (♂), 2.82–2.84× (♀), hand (without pedicel) 1.50× (♂), 1.42–1.49× (♀) all longer than broad, movable finger 0.99× (♂), 0.89–0.99× (♀) longer than hand. Fixed finger with 8 trichobothria: eb and esb situated at base of fixed finger on retrolateral margin, est situated slightly closer to esb than et in ♂, and midway between esb and et in ♀, ib, isb ist and it situated prolaterally at base of fixed finger, et subdistally; movable finger with 4 trichobothria, st situated dorsally and basally to sb in ♂, and dorsally to sb in ♀, t situated submedially (Fig. 61, 62). Fixed finger with 36 (♂), 38 (♀) triangular, retrorse chelal teeth; movable finger with 38 (♂), 40 (♀) triangular, retrorse chelal teeth.
Carapace (Fig. 57) 1.21× (♂), 1.12–1.14× (♀) longer than broad; 1 ♀ with 20 setae arranged with 4 in anterior row, 6 in ocular row, 4 in median row, 4 in intermediate row and 2 in posterior row; ♂ with 19 setae arranged same as ♀ except 3 in intermediate row; intermediate row recurved; posterior row situated near posterior margin of carapace; all setae subequal in length; with 1 shallow median furrow; with two pairs of large, corneate eyes. Manducatory process distally triangular; with 1 seta situated distally, another situated medially and 1 suboral seta, plus 8 (♂), 9 (♀) additional setae. Coxae, setal formula: ♂, 5: 5: 4: 4; ♀, 5: 4: 4: 4.
Femur + patella IV 2.67× (♂), 2.71× (♀) longer than deep; metatarsus IV with long basal tactile seta, TS = 0.22 (♂), 0.26 (♀).
Tergites, setal formula ♂: 6: 6: 8: 7: 8: 8: 8: 8: 8: 10 (including 4 tactile setae): 8 (including 4 tactile setae): 2; ♀, 6: 7: 8: 8: 8: 8: 8: 8: 10: 10 (including 2 tactile setae): 10 (including 4 tactile setae): 2; setae arranged in single rows; most tergites with broad median suture line; sternites, setal formula ♂, 4: (1) 6 [1 + 1] (1): (1) 4 (1): 6: 6 + 2 gls: 6 + 2 gls: 6 + 2 gls: 8: 8 (including 4 tactile setae): 8 (including 4 tactile setae): 2; ♀, 4: (1) 4 (1): (1) 4 (1): 6: 6 + 2 gls: 6 + 2 gls: 6 + 2 gls: 8: 12 (including 4 tactile setae): 8 (including 2 tactile setae): 2; posterior tergites and sternites with several tactile setae; 1 pair of glandular setae (gls) situated on sternites VI, VII and VIII, situated near mid-line, slightly in advance of regular setae, each seta slightly longer than other sternal setae; most sternites with broad median suture line.
Male with prominent pair of dorsal anterior glands, a single ventral anterior gland, paired lateral genital sacs and small, ovoid median genital sac; with 1 pair of setae within genital atrium. Female: with a single median cribriform plate and a pair of lateral cribriform plates.
Holotype: Body length (excluding chelicerae) 1.79. Pedipalp: trochanter 0.315/0.180, femur 0.460/0.180, patella 0.475/0.215, chela (with pedicel) 0.875/0.280, chela (without pedicel) length 0.815, chelal hand (without pedicel) length 0.420, movable finger length 0.415. Carapace 0.615/0.510, anterior eye diameter 0.065, posterior eye diameter 0.060. Leg I: femur 0.105/0.110, patella 0.200/0.110, tibia 0.200/0.075, metatarsus 0.090/0.055, tarsus 0.125/0.050. Leg IV: femur + patella 0.480/0.180, tibia 0.320/0.110, metatarsus 0.115/0.070, tarsus 0.165/0.060, TS = 0.025.
Paratype, with other paratype in parentheses (when measured): Body length (excluding chelicerae) 1.76 (but slightly contracted (2.18)). Pedipalp: trochanter 0.370/0.200 (0.350/0.195), femur 0.555/0.220 (0.585/0.215), patella 0.560/0.265 (0.530/0.245), chela (with pedicel) 1.055/0.345 (0.985/0.325); chela (without pedicel) length 0.980 (0.915), chelal hand (without pedicel) length 0.490 (0.485), movable finger length 0.485 (0.430). Carapace 0.685/0.600 (0.640/0.570), anterior eye diameter 0.065, posterior eye diameter 0.065. Leg I: femur 0.135/0.115, patella 0.255/0.130, tibia 0.245/0.090, metatarsus 0.115/0.065, tarsus 0.145/0.060. Leg IV: femur + patella 0.610/0.225, tibia 0.380/0.120, metatarsus 0.155/0.075, tarsus 0.200/0.070, TS = 0.040.
Nobilipinus affinis has been collected from rainforest habitats on Lingga Island situated off the coast of Sumatra (Fig. 91).
Nobilipinus galeatus, sp. nov.
ZooBank: urn:lsid:zoobank.org:act:9FBE7F00-216D-407A-8280-3AD75563D707
Garypinus nobilis With: Tullgren (1907), p. 67 (misidentification).
Holotype. THAILAND: Chumphon Province: ♂, Khao Kai Jae Waterfall, 9°55′04.6″N, 98°56′33.7″E, 5–8 May 2003, semi-evergreen rainforest, P. Schwendinger (MHNG).
Paratypes. THAILAND: Phang Nga Province: 1 ♀, 1 tritonymph, Khao Sok National Park, 30 km E. of Takua Pa, 8°55′N, 98°36′E, 50 m, 21–26 December 1997, in secondary moist forest with primary spots, Winckler, A. Schulz (MHNG); Satun Province: 3 ♂, 1 tritonymph, Thaleban National Park, 6°42′N, 100°07′E, 5 January 1998, in primary moist forest, A. Schulz, K. Vock (MHNG).
Other material. THAILAND: Bangkok: 1 ♀, Bangkok (intercepted at Hamburg), 13°45′N, 100°30′E, from orchids, 24 October 1902, Dr Reh (ZMH A0003820).
The specific epithet is an adjective referring to the presence of three long rami in the galea of males (galeatus, Latin, cover with a helmet).
Nobilipinus galeatus differs from the other species of the genus in the galea of both males and females having long rami (Fig. 64, 65) whereas only females have long rami and males have short distal rami in other species. This species also differs from others in the straight intermediate setal row of the carapace (Fig. 63) that is recurved in other species.
Setae long, mostly straight and acicular; most cuticular surfaces smooth and glossy.
Surface smooth; cheliceral hand with 5 setae, ls and is adjacent to each other; movable finger with 1 subdistal seta; all setae acuminate; galea of ♂ and ♀ with 3 very long rami (Fig. 64, 65); fixed finger with 5 (♂, ♀) small teeth, each approximately same size; movable finger with 1 subdistal tooth; serrula interior with 19 (♂), 20 (♀) blades; lamina exterior present; rallum with 4 blades, anterior blade with anterior spinules, remainder smooth.
All segments completely smooth; setae acicular, straight or nearly so; trochanter 1.86–1.91× (♂), 1.66–1.89× (♀), femur with 1 subbasal tactile seta on dorsal surface, 2.51–3.00× (♂), 2.30–2.51× (♀), patella with several small lyrifissures situated basally on dorsal surface, 2.02–2.27× (♂), 1.94–2.08× (♀), chelal hand ovoid, chela (with pedicel) 3.08–3.38× (♂), 3.00–3.05× (♀), chela (without pedicel) 2.82–3.13 (♂), 2.84–2.85× (♀), hand (without pedicel) 1.43–1.70× (♂), 1.47–1.48× (♀) all longer than broad, movable finger 0.86–1.04× (♂), 0.93–0.94× (♀) longer than hand. Fixed finger with 8 trichobothria: eb and esb situated at base of fixed finger on retrolateral margin, est situated slightly closer to esb than et; ib, isb ist and it situated prolaterally at base of fixed finger, and et subdistally; movable finger with 4 trichobothria, st situated dorsal to sb, t situated submedially (Fig. 67, 68). Fixed finger with 36 (♂), 31 (♀) triangular, retrorse chelal teeth; movable finger with 38 (♂), 33 (♀) triangular, retrorse chelal teeth.
Carapace (Fig. 63) 1.20–1.31× (♂), 1.10–1.17× (♀) longer than broad; ♂ and ♀ with 20 setae arranged with 4 in anterior row, 6 in ocular row, 4 in median row, 4 in intermediate row and 2 in posterior row; median row straight; posterior row situated near posterior margin of carapace; all setae subequal in length; with 1 barely discernible median furrow; with two pairs of large, corneate eyes. Manducatory process distally triangular; with 1 seta situated distally, another situated medially and 1 suboral seta, plus 8 (♂), 9 (♀) additional setae. Coxae, setal formula: ♂, 8: 6: 5: 7; ♀, 10: 8: 5: 5.
Femur + patella IV 2.53× (♂), 2.83× (♀) longer than deep; metatarsus IV with long basal tactile seta, TS = 0.21 (♂, ♀).
Tergites straight, divided; setal formula ♂, 6: 6: 6: 7: 8: 8: 8: 8: 7: 11 (including 4 tactile setae): 8 (including 4 tactile setae): 2; ♀, 6: 6: 7: 7: 8: 8: 8: 8: 8: 10 (including 4 tactile setae): 10 (including 4 tactile setae): 2; setae arranged in single rows; sternites, setal formula ♂, 6: (1) 4 [1 + 1] (1): (1) 4 (1): 8: 6 + 2 gls: 6 + 2 gls: 6 + 2 gls: 8: 12 (including 4 tactile setae): 11 (including 4 tactile setae): 2, ♀, 6: (1) 4 (1): (2) 4 (2): 9: 8 + 2 gls: 8 + 2 gls: 7 + 2 gls: 10: 10 (including 4 tactile setae): 8 (including 4 tactile setae): 2; posterior tergites and sternites with several tactile setae; 1 pair of glandular setae (gls) situated on sternites VI, VII and VIII, situated near mid-line, slightly in advance of regular setae, each seta slightly longer than other sternal setae; most sternites with broad median suture line.
Holotype, followed by 3 paratypes in parentheses (when measured): Body length (excluding chelicerae) 2.26 (1.97–2.11). Pedipalp: trochanter 0.325/0.170 (0.270–0.280/0.145–0.150), femur 0.540/0.185 (0.440–0.450/0.150–0.175), patella 0.500/0.220 (0.415–0.425/0.200–0.210), chela (with pedicel) 0.895/0.265 (0.785–0.800/0.240–0.255), chela (without pedicel) length 0.830 (0.720–0.750), chelal hand (without pedicel) length 0.450 (0.365–0.385), movable finger length 0.385 (0.370–0.385). Carapace 0.635/0.485 (0.540–0.565/0.420–0.470), anterior eye diameter 0.065, posterior eye diameter 0.050. Leg I: femur 0.110/0.110, patella 0.220/0.125, tibia 0.240/0.080, metatarsus 0.095/0.060, tarsus 0.135/0.055. Leg IV: femur + patella 0.545/0.215, tibia 0.370/0.115, metatarsus 0.120/0.075, tarsus 0.180/0.065, TS = 0.025.
Paratype, followed by other female (when measured): Body length (excluding chelicerae) 2.62 (2.59). Pedipalp: trochanter 0.340/0.180 (0.315/0.190), femur 0.540/0.215 (0.495/0.215), patella 0.510/0.245 (0.475/0.245), chela (with pedicel) 0.930/0.305 (0.900/0.300), chela (without pedicel) length 0.865 (0.855), chelal hand (without pedicel) length 0.450 (0.440), movable finger length 0.420 (0.415). Carapace 0.595/0.540 (0.645/0.550), anterior eye diameter 0.060, posterior eye diameter 0.050. Leg I: femur 0.110/0.125, patella 0.250/0.130, tibia 0.250/0.080, metatarsus 0.085/0.065, tarsus 0.130/0.055. Leg IV: femur + patella 0.565/0.200, tibia 0.385/0.120, metatarsus 0.120/0.075, tarsus 0.175/0.070, TS = 0.025.
Cheliceral hand with 5 setae, ls and is adjacent to each other; movable finger with 1 seta; galea with 3 very long rami.
Trochanter 1.75×, femur with 1 very long subbasal tactile seta on dorsal surface, 2.21×, patella 1.89×, chela (with pedicel) 2.73×, chela (without pedicel) 2.55×, hand (without pedicel) 1.31× all longer than broad, movable finger 1.03× longer than hand. Fixed finger with 7 trichobothria: eb and esb situated at base of fixed finger on retrolateral margin, ib, ist and it situated prolaterally at base of fixed finger, et subdistally, isb absent; movable finger with 3 trichobothria, b and st situated basally, t situated submedially, sb absent (Fig. 69). Fixed finger with 28 teeth; movable finger with 27 teeth.
Carapace 1.17× longer than broad; with 20 setae including 4 in anterior row, and 2 in posterior row; with two pairs of corneate eyes. Coxae, setal formula: 4: 6: 4: 3.
Tergites, setal formula 6: 6: 6: 6: 6: 8: 8: 8: 8: 10 (including 4 tactile setae): 10 (including 4 tactile setae): 2; sternites, setal formula 3: (2) 4 (1): (2) 2 (1): 8: 6 + 2 gls: 6 + 2 gls: 4 + 2 gls: 7: 10 (including 4 tactile setae): 10 (including 4 tactile setae): 2; 1 pair of glandular setae (gls) situated on sternites VI, VII and VIII, situated near mid-line, slightly in advance of regular setae, each seta slightly longer than other sternal setae.
Body length (excluding chelicerae) 2.13. Pedipalp: trochanter 0.245/0.140, femur 0.365/0.165, patella 0.340/0.180, chela (with pedicel) 0.670/0.245, chela (without pedicel) length 0.625, chelal hand (without pedicel) length 0.320, movable finger length 0.330. Carapace 0.510/0.435, anterior eye diameter 0.050, posterior eye diameter 0.045.
Nobilipinus galeatus has been found at four localities in Thailand, within Chumphon Province, Phang Nga Province, Satun Province and the Bangkok Special Administrative Area (Fig. 91). A single specimen collected from orchids exported from Thailand to Germany was identified as G. nobilis by Tullgren (1907). This specimen was revealed to be an adult female of N. galeatus on examination.
Schawaller (1994) recorded six specimens identified as G. nobilis from Ko Samui, Surat Thani Province that is located close to the other localities, strongly suggesting that these belong to N. galeatus rather than N. nobilis that occurs further to the north-east (Fig. 91).
Nobilipinus karenae, sp. nov.
ZooBank: urn:lsid:zoobank.org:act:78C555B6-F5ED-4E86-970F-85B43156F2FD
Holotype. BRUNEI DARUSSALAM: ♂, Mengkubau, off Jalan Penghubang mentiri, 4°56′40″N, 114°59′24″E, 2 July 2009, sieved rainforest litter, M.S. Harvey, K.L. Edward, J.K.H. Koh (BMKB).
The specific epithet honours Karen Cullen (nee Edward), who assisted with collection of the holotype.
Nobilipinus karenae differs from all other species of the genus except N. kohi in the posterior setal row of the carapace being situated at the same level as the lateral setae of the intermediate setal row (Fig. 70). This species differs from N. kohi in trichobothrium est being situated closer to esb than et (Fig. 72, 73) and from N. vachoni in the shape of the male chelal hand that is ovoid in N. karenae (Fig. 72) and more elongated in N. vachoni.
Setae long, mostly straight and acicular; most cuticular surfaces smooth and glossy.
Surface smooth; cheliceral hand with 5 setae, ls and is adjacent to each other; movable finger with 1 subdistal seta; all setae acuminate; galea of ♂ short with 3 small distal rami (Fig. 71); fixed finger with 6 (♂) small teeth, each approximately same size; movable finger with 1 subdistal tooth; serrula interior with 20 (♂), blades; lamina exterior present; rallum with 4 blades, anterior blade with anterior spinules, remainder smooth.
All segments completely smooth; setae acicular, straight or nearly so; trochanter 1.62× (♂), femur with 1 subbasal tactile seta on dorsal surface, 2.77× (♂), patella with several small lyrifissures situated basally on dorsal surface, 2.12× (♂), chelal hand ovoid, chela (with pedicel) 3.34× (♂), chela (without pedicel) 3.08× (♂), hand (without pedicel) 1.66× (♂) all longer than broad, movable finger 0.93× (♂) longer than hand. Fixed finger with 8 trichobothria: eb and esb situated at base of fixed finger on retrolateral margin, est situated midway between esb and et, ib, isb ist and it situated prolaterally at base of fixed finger, et subdistally; movable finger with 4 trichobothria, st situated dorsal and slightly basal to sb in ♂ in ♀, t situated submedially (Fig. 73). Fixed finger with 30 (♂) triangular, retrorse chelal teeth; movable finger with 32 (♂) triangular, retrorse chelal teeth.
Carapace (Fig. 70) 1.20× (♂) longer than broad; with 22 setae arranged with 4 in anterior row, 8 in ocular row, 4 in median row, 4 in intermediate row and 2 in posterior row; intermediate row recurved; posterior row situated near median row; all setae subequal in length; with 1 shallow median furrow; with two pairs of large, corneate eyes. Manducatory process distally triangular; with 1 seta situated distally, another situated medially and 1 suboral seta, plus 7 (♂) additional setae. Coxae, setal formula: ♂, 6: 6: 5: 5.
Femur + patella IV 2.57× (♂) longer than deep; metatarsus IV with long basal tactile seta, TS = 0.25 (♂).
Tergites straight, undivided; setal formula ♂, 6: 8: 8: 8: 8: 8: 8: 8: 8: 10 (including 4 tactile setae): 10 (including 4 tactile setae): 2; setae arranged in single rows; sternites, setal formula ♂, 6: (1) 5 [1 + 1] (1): (1) 4 (1): 9: 7 + 2 gls: 8 + 2 gls: 6 + 2 gls: 9: 8 (including 4 tactile setae): 8 (including 2 tactile setae): 2; posterior tergites and sternites with several tactile setae; 1 pair of glandular setae (gls) situated on sternites VI, VII and VIII, situated near mid-line, slightly in advance of regular setae, each seta slightly longer than other sternal setae; sternites undivided.
Holotype: 1.71. Pedipalp: trochanter 0.275/0.170, femur 0.485/0.175, patella 0.435/0.205, chela (with pedicel) 0.835/0.250, chela (without pedicel) length 0.770, chelal hand (without pedicel) length 0.415, movable finger length 0.385. Carapace 0.550/0.460, anterior eye diameter 0.065, posterior eye diameter 0.055. Leg I: femur 0.105/0.095, patella 0.215/0.110, tibia 0.220/0.070, metatarsus 0.105/0.060, tarsus 0.135/0.065. Leg IV: femur + patella 0.475/0.185, tibia 0.320/0.100, metatarsus 0.120/0.065, tarsus 0.165/0.065, TS = 0.030.
Nobilipinus kohi, sp. nov.
ZooBank: urn:lsid:zoobank.org:act:93CC2530-CF51-424F-870C-E92901195BA8
Holotype. MALAYSIA: Sarawak: ♂, Mulu National Park, 100 km ESE. of Miri, 4°00′N, 114°49′E, 200 m, 19–24 August 2003, Winckler extraction, A. Schulz (MHNG).
Paratypes. MALAYSIA: Sarawak: 6 ♂, 4 ♀, 1 tritonymph, 1 protonymph, collected with holotype (MHNG); 1 ♂, collected with holotype (WAM T156912). BRUNEI DARUSSALAM: 2 ♀, 1 deutonymph, Ulu Temberong National Park, near Kuala Belalong Field Studies Centre, 4°33′17″N, 115°09′35″E, 5 July 2009, sieved rainforest litter, M.S. Harvey, K.L. Edward, J.K.H. Koh (BMKB).
The specific epithet honours Joseph Koh for friendship, arachnological prowess and support in organising the field trip in Brunei.
Nobilipinus kohi differs from all other species of the genus except N. karenae in the posterior setal row of the carapace being situated at the same level as the lateral setae of the intermediate setal row (Fig. 74). This species differs from N. karenae in trichobothrium est being situated midway between esb and et (Fig. 78–80) and from N. vachoni in the shape of the male chelal hand that is ovoid in N. kohi (Fig. 78) and more elongated in N. vachoni.
Setae long, mostly straight and acicular; most cuticular surfaces smooth and glossy.
Surface smooth; cheliceral hand with 5 setae, ls and is adjacent to each other; movable finger with 1 subdistal seta; all setae acuminate; galea of ♂ short with 3 small distal rami (Fig. 75), of ♀ with 3 very long rami (Fig. 76); fixed finger with 5 (♂, ♀) small teeth, each approximately same size; movable finger with 1 subdistal tooth; serrula interior with 16 (♂), 16–20 (♀) blades; lamina exterior present; rallum with 4 blades, anterior blade with anterior spinules, remainder smooth.
All segments completely smooth; setae acicular, straight or nearly so; trochanter 1.64× (♀), femur with 1 subbasal tactile seta on dorsal surface, 2.41–2.53× (♀), patella with several small lyrifissures situated basally on dorsal surface, 2.14–2.20× (♀), chelal hand ovoid, chela (with pedicel) 2.96–3.15× (♀), chela (without pedicel) 2.81–3.00× (♀), hand (without pedicel) 1.49–1.58× (♀) all longer than broad, movable finger 0.92–0.93× (♀) longer than hand. Fixed finger with 8 trichobothria: eb and esb situated at base of fixed finger on retrolateral margin, est situated midway between esb and et, ib, isb ist and it situated prolaterally at base of fixed finger, et subdistally; movable finger with 4 trichobothria, st situated dorsally and slightly basally to sb, t situated submedially (Fig. 79, 80). Fixed finger with 33 (♀) triangular, retrorse chelal teeth; movable finger with 36 (♀) triangular, retrorse chelal teeth.
Carapace (Fig. 74) 1.20–1.22× (♀) longer than broad; holotype with with 20 (occasionally 21 or 22) setae arranged with 4 in anterior row, 6 (occasionally 7) in ocular row, 4 in median row, 4 in intermediate row and 2 (occasionally 3) in posterior row; intermediate row recurved; posterior row situated near median row; all setae subequal in length; with 1 shallow median furrow; with two pairs of large, corneate eyes. Manducatory process distally triangular; with 1 seta situated distally, another situated medially and 1 suboral seta, plus 7 (♂), 9 (♀) additional setae. Coxae, setal formula: ♂, 6: 6: 5: 5; ♀, 6: 6: 5: 6.
Femur + patella IV 2.86× (♀) longer than deep; metatarsus IV with long basal tactile seta, TS = 0.19 (♀).
Tergites straight, undivided; setal formula ♂, 6: 8: 7: 8: 8: 9: 9: 9: 9: 10 (including 4 tactile setae): 8 (including 2 tactile setae): 2; ♀, 6: 8: 8: 8: 9: 10: 10: 5 (tergite VIII only formed on left side; right tergite IX enlarged): 9: 9: 10 (including 4 tactile setae): 2; setae arranged in single rows; sternites, setal formula ♂, 4: (1) 4 [1 + 1] (1): (1) 8 (1): 8: 6 + 2 gls: 6 + 2 gls: 6 + 2 gls: 8: 8 (including 2 tactile setae): 8 (including 2 tactile setae): 2; ♀, 8: (1) 4 (1): (1) 8 (1): 5: 6 + 2 gls: 6 + 2 gls: 8 + 2 gls: 7: 9 (including 4 tactile setae): 8 (including 4 tactile setae): 2; posterior tergites and sternites with several tactile setae; 1 pair of glandular setae (gls) situated on sternites VI, VII and VIII, near mid-line, slightly in advance of regular setae, each seta slightly longer than other sternal setae; sternites undivided.
Holotype, followed by 7 paratypes in parentheses (when measured): Body length (excluding chelicerae) 1.81 (1.60–2.12). Pedipalp: trochanter 0.275/0.165 (0.245–0.315/0.155–0.195), femur 0.425/0.165 (0.395–0.510/0.155–0.200), patella 0.435/0.200 (0.395–0.510/0.180–0.235), chela (with pedicel) 0.800/0.255 (0.735–0.930/0.235–0.300), chela (without pedicel) length 0.750 (0.700–0.885), chelal hand (without pedicel) length 0.385 (0.360–0.455), movable finger length 0.370 (0.345–0.435). Carapace 0.575/0.475 (0.530–0.655/0.440–0.565), anterior eye diameter 0.065, posterior eye diameter 0.055. Leg I: femur 0.115/0.090, patella 0.200/0.100, tibia 0.215/0.070, metatarsus 0.095/0.055, tarsus 0.125/0.050. Leg IV: femur + patella 0.460/0.185, tibia 0.320/0.095, metatarsus 0.135/0.065, tarsus 0.160/0.060, TS = 0.025.
Paratype from Mulu National Park, followed by 5 other paratypes in parentheses (when measured): Body length (excluding chelicerae) 2.21 (2.05–2.42). Pedipalp: trochanter 0.335/0.205 (0.295–0.345/0.185–0.210), femur 0.555/0.225 (0.500–0.545/0.195–0.270), patella 0.545/0.255 (0.465–0.550/0.225–0.255), chela (with pedicel) 1.005/0.340 (0.890–1.025/0.290–0.345), chela (without pedicel) length 0.950 (0.845–0.975), chelal hand (without pedicel) length 0.505 (0.435–0.515), movable finger length 0.480 (0.405–0.480). Carapace 0.640/0.575 (0.610–0.710/0.530–0.620), anterior eye diameter 0.070, posterior eye diameter 0.055. Leg I: femur 0.130/0.115, patella 0.255/0.125, tibia 0.265/0.085, metatarsus 0.115/0.065, tarsus 0.075/0.065. Leg IV: femur + patella 0.610/0.230, tibia 0.420/0.120, metatarsus 0.160/0.080, tarsus 0.185/0.075, TS = 0.030.
Cheliceral hand with 5 setae, ls and is adjacent to each other; movable finger with 1 subdistal seta; galea with 3 very long rami (Fig. 77).
Trochanter 1.66×, femur with 1 very long subbasal tactile seta on dorsal surface, 2.37×, patella 2.00×, chela (with pedicel) 3.00×, chela (without pedicel) 2.81×, hand (without pedicel) 1.37× all longer than broad, movable finger 1.10× longer than hand. Fixed finger with 7 trichobothria: eb and esb situated at base of fixed finger on retrolateral margin, ib, ist and it situated prolaterally at base of fixed finger, et subdistally, isb absent; movable finger with 3 trichobothria, b and st situated basally, t situated submedially, sb absent (Fig. 81). Fixed finger with 30 teeth; movable finger with 28 teeth.
Carapace 1.13× longer than broad; with 20 setae including 4 in anterior row, and 2 in posterior row; with two pairs of corneate eyes. Coxae, setal formula: 4: 4: 4: 3.
Tergites, setal formula 6: 6: 8: 8: 8: 8: 8: 8: 8: 8 (including 2 tactile setae): 8 (including 2 tactile setae): 2; sternites, setal formula 2: (1) 2 (1): (1) 3 (1): 6: 6 + 2 gls: 6 + 2 gls: 4 + 2 gls: 8: 6 (including 2 tactile setae): 6 (including 2 tactile setae): 2; 1 pair of glandular setae (gls) situated on sternites VI, VII and VIII, situated near mid-line, slightly in advance of regular setae, each seta slightly longer than other sternal setae.
Body length (excluding chelicerae) 1.76. Pedipalp: trochanter 0.265/0.160, femur 0.415/0.175, patella 0.390/0.195, chela (with pedicel) 0.780/0.260, chela (without pedicel) length 0.730, chelal hand (without pedicel) length 0.355, movable finger length 0.390. Carapace 0.575/0.510, anterior eye diameter 0.060, posterior eye diameter 0.055.
Cheliceral hand with 5 setae, ls and is adjacent to each other; movable finger with 1 seta; galea with 3 very long rami.
Trochanter 1.52×, femur with 1 very long subbasal tactile seta on dorsal surface, 2.16×, patella 2.07×, chela (with pedicel) 3.14×, chela (without pedicel) 2.78×, hand (without pedicel) 1.53× all longer than broad, movable finger 0.98× longer than hand. Fixed finger with 6 trichobothria: eb situated at base of fixed finger on retrolateral margin, ib, ist and it situated prolaterally at base of fixed finger, et subdistally, esb and isb absent; movable finger with 2 trichobothria, b situated basally, t situated submedially, sb and st absent (Fig. 82). Fixed finger with 22 teeth; movable finger with 22 teeth.
Carapace 1.11× longer than broad; with 18 setae including 4 in anterior row, and 2 in posterior row; with two pairs of corneate eyes. Coxae, setal formula: 4: 4: 2: 2.
Tergites, setal formula 4: 4: 6: 6: 6: 6: 6: 6: 6: 8: 8 (including 2 tactile setae): 2; sternites, setal formula 0: (1) 2 (1): (1) 2 (1): 6: 4 + 2 gls: 4 + 2 gls: 6 + 2 gls: 6: 8 (including 4 tactile setae): 10 (including 4 tactile setae): 2; 1 pair of glandular setae (gls) situated on sternites VI, VII and VIII, situated near mid-line, difficult to discern from other sternal setae.
Body length (excluding chelicerae) 1.36. Pedipalp: trochanter 0.190/0.125, femur 0.270/0.125, patella 0.290/0.140, chela (with pedicel) 0.565/0.180, chela (without pedicel) length 0.500, chelal hand (without pedicel) length 0.275, movable finger length 0.270. Carapace 0.445/0.400, anterior eye diameter 0.040, posterior eye diameter 0.040.
Cheliceral hand with 4 setae, ls and is adjacent to each other; movable finger without seta; galea with 3 very long rami.
Trochanter 1.68×, femur with 1 very long subbasal tactile seta on dorsal surface, 2.16×, patella 1.86×, chela (with pedicel) 3.00×, chela (without pedicel) 2.86×, hand (without pedicel) 1.39× all longer than broad, movable finger 1.08× longer than hand. Fixed finger with 3 trichobothria: eb situated at base of fixed finger on retrolateral margin, ist situated prolaterally at base of fixed finger, et subdistally; movable finger with 1 trichobothrium, t, situated subbasally (Fig. 83). Fixed finger with 16 teeth; movable finger with 16 teeth.
Carapace 1.06× longer than broad; with 16 setae including 4 in anterior row and 2 in posterior row; with two pairs of corneate eyes. Coxae, setal formula: 1: 1: 1: 1.
Tergites, setal formula 2: 4: 4: 4: 4: 4: 4: 4: 4: 4 (including 2 tactile setae): 4 (including 2 tactile setae): 2; sternites, setal formula, 0: (1) 0 (1): (1) 2 (1): 4: 2: 2: 2: 2: 2: 2: 2; glandular setae (gls) absent on sternites VI, VII and VIII.
Body length (excluding chelicerae) 0.98. Pedipalp: trochanter 0.160/0.195, femur 0.205/0.095, patella 0.205/0.110, chela (with pedicel) 0.420/0.140, chela (without pedicel) length 0.040, chelal hand (without pedicel) length 0.195, movable finger length 0.210. Carapace 0.370/0.350, anterior eye diameter 0.030, posterior eye diameter 0.025.
Nobilipinus tricosus, sp. nov.
ZooBank: urn:lsid:zoobank.org:act:D05AEC7A-385A-4007-91AB-DDD03128D785
Garypinus nobilis, With: Beier (1930), pp. 289–290 (misidentification).
Holotype. INDONESIA: Daerah Khusus Ibukota Jakarta: ♂, ‘Java’ but possibly Batavia (currently known as Jakarta) [6°12′S, 106°49′E] (SMF 9901952 – RII/1952–70).
Paratypes. INDONESIA: Daerah Khusus Ibukota Jakarta: 3 ♀, 1 deutonymph, same data (SMF 9901952 – RII/1952–70); 2 ♂, same data (SMF 9901950 – RII/1950–70).
The specific epithet is an adjective referring to the previous misidentification of these specimens as Garypinus nobilis (tricosus, Latin, full of tricks or wiles) (Brown 1956).
Nobilipinus tricosus differs from all other species of the genus as follows: from N. karenae and N. kohi in the posterior setal row of the carapace being situated basally to the level of the lateral setae of the intermediate setal row (Fig. 84); from N. galeatus in the recurved intermediate setal row of the carapace (Fig. 84) and the male galea possessing short rami (Fig. 85); from N. nobilis in trichobothrium est being situated closer to esb than et (Fig. 87–89); from N. affinis in the intermediate setal row of carapace being strongly recurved (Fig. 84); and from N. vachoni in the shape of the male chelal hand that is ovoid in N. tricosus (Fig. 87) and more elongated in N. vachoni.
Setae long, mostly straight and acicular; most cuticular surfaces smooth and glossy.
Surface smooth; cheliceral hand with 5 setae, ls and is adjacent to each other; movable finger with 1 subdistal seta; all setae acuminate; galea of ♂ short with 3 small distal rami (Fig. 85), of ♀ with 3 very long rami (Fig. 87); fixed finger with 5 (♂, ♀) small teeth, each approximately same size; movable finger with 1 subdistal tooth; serrula interior with 17 (♂), 20 (♀) blades; lamina exterior present; rallum with 4 blades, anterior blade with anterior spinules, others smooth.
All segments completely smooth; setae acicular, straight or nearly so; trochanter 1.66–1.88× (♂), 1.68–1.82× (♀), femur with 1 subbasal tactile seta on dorsal surface, 2.71–2.88× (♂), 2.56–2.71× (♀), patella with several small lyrifissures situated basally on dorsal surface, 2.28–2.35× (♂), 2.16–2.26× (♀), chelal hand ovoid, chela (with pedicel) 3.26–3.56× (♂), 3.15–3.22× (♀), chela (without pedicel) 3.08–3.38× (♂), 2.97–3.06× (♀), hand (without pedicel) 1.57–1.69× (♂), 1.48–1.52× (♀) all longer than broad, movable finger 1.02–1.10× (♂), 0.95–1.01× (♀) longer than hand. Fixed finger with 8 trichobothria: eb and esb situated at base of fixed finger on retrolateral margin, est situated closer to esb than et, ib, isb and ist, and it situated prolaterally at base of fixed finger, et subdistally; movable finger with 4 trichobothria, st situated dorsal to sb, t situated submedially (Fig. 88, 89). Fixed finger with 33 (♂), 35 (♀) triangular, retrorse chelal teeth; movable finger with 35 (♂), 38 (♀) triangular, retrorse chelal teeth.
Carapace (Fig. 84) 1.28–1.36× (♂), 1.15–1.28× (♀) longer than broad; with 20 setae arranged with 4 in anterior row, 6 in ocular row, 4 in median row, 4 in intermediate row and 2 in posterior row; posterior row situated near posterior margin of carapace; all setae subequal in length; without discernible furrow; with two pairs of large, corneate eyes. Manducatory process distally triangular; with 1 seta situated distally, another situated medially and 1 suboral seta, plus 7 (♂, ♀) additional setae. Coxae, setal formula: ♂, 7: 7: 4: 5; ♀, 7: 6: 5: 4–5.
Femur + patella IV 2.54× (♂), 2.57× (♀) longer than deep; metatarsus IV with long basal tactile seta, TS = 0.12 (♂), 0.23 (♀).
Tergites, setal formula ♂, 6: 6: 6: 8: 8: 8: 8: 8: 8: 10 (including 4 tactile setae): 10 (including 4 tactile setae): 2; ♀, 6: 6: 8: 8: 7: 7: 7: 7: 7: 8 (including 4 tactile setae): 8 (including 4 tactile setae): 2; setae arranged in single rows; tergites without median suture line; sternites, setal formula ♂, 6: (1) 5 [1 + 1] (1): (1) 4 (1): 8: 6 + 2 gls: 6 + 2 gls: 6 + 2 gls: 8: 10 (including 4 tactile setae): 10 (including 4 tactile setae): 2; ♀, 6: (1) 4 (1): (1) 4 (1): 6: 6 + 2 gls: 6 + 2 gls: 6 + 2 gls: 8: 11 (including 4 tactile setae): 10 (including 2 tactile setae): 2; posterior tergites and sternites with several tactile setae; 1 pair of glandular setae (gls) situated on sternites VI, VII and VIII, situated near mid-line, slightly in advance of regular setae, each seta slightly longer than other sternal setae; sternites without median suture line.
Holotype, with paratypes in parentheses (when measured): Body length (excluding chelicerae) 1.92 (1.98–2.03). Pedipalp: trochanter 0.280/0.165 (0.290–0.300/0.160–0.175), femur 0.475/0.165 (0.470–0.475/0.170–0.175), patella 0.455/0.200 (0.470/0.200-0.205), chela (with pedicel) 0.885/0.240 (0.865–0.875/0.250–0.265), chela (without pedicel) length 0.810 (0.815–0.820), chelal hand (without pedicel) length 0.405 (0.410–0.415), movable finger length 0.445 (0.420–0.430). Carapace 0.610/0.450 (0.615–0.635/0.475–0.480), anterior eye diameter 0.065, posterior eye diameter 0.060. Leg I: femur 0.090/0.090, patella 0.175/0.100, tibia 0.210/0.070, metatarsus 0.095/0.055, tarsus 0.140/0.050. Leg IV: femur + patella 0.470/0.185, tibia 0.305/0.100, metatarsus 0.115/0.060, tarsus 0.165/0.060, TS = 0.020.
Paratype, with other paratypes in parentheses (when measured): Body length (excluding chelicerae) 2.52 (2.20–2.56). Pedipalp: trochanter 0.320/0.190 (0.315–0.355/0.175–0.195), femur 0.515/0.190 (0.500–0.540/0.195–0.210), patella 0.520/0.230 (0.485–0.545/0.225–0.245), chela (with pedicel) 0.960/0.305 (0.945–1.105/0.300–0.315), chela (without pedicel) length 0.910 (0.890–0.965), chelal hand (without pedicel) length 0.465 (0.445–0.500), movable finger length 0.455 (0.450–0.475). Carapace 0.660/0.575 (0.655–0.705/0.510–0.550), anterior eye diameter 0.065, posterior eye diameter 0.060. Leg I: femur 0.135/0.105, patella 0.255/0.120, tibia 0.255/0.080, metatarsus 0.105/0.065, tarsus 0.130/0.055. Leg IV: femur + patella 0.540/0.210, tibia 0.355/0.115, metatarsus 0.130/0.075, tarsus 0.175/0.065, TS = 0.030.
Cheliceral hand with 4 setae, ls and is adjacent to each other, sbs absent; movable finger with 1 seta; galea with 3 very long rami.
Trochanter 1.73×, femur with 1 very long subbasal tactile seta on dorsal surface, 2.45×, patella 1.96×, chela (with pedicel) 3.21×, chela (without pedicel) 3.00×, hand (without pedicel) 1.52× all longer than broad, movable finger 1.02× longer than hand. Fixed finger with 6 trichobothria: eb situated at base of fixed finger on retrolateral margin, ib, ist and it situated prolaterally at base of fixed finger, et subdistally, esb and isb absent; movable finger with 2 trichobothria, b situated basally, t situated submedially, sb and st absent (Fig. 90). Fixed finger with 20 teeth; movable finger with 21 teeth.
Carapace 1.29× longer than broad; with 18 setae including 4 in anterior row and 2 in posterior row; with two pairs of corneate eyes. Coxae, setal formula: 4: 4: 2: 2.
Tergites, setal formula 4: 4: 6: 6: 6: 6: 6: 6: 6: 8 (including 2 tactile setae): 8 (including 2 tactile setae): 2; sternites, setal formula 0: (1) 2 (1): (1) 2 (1): 6: 4 + 2 gls: 4 + 2 gls: 4 + 2 gls: 6: 8 (including 4 tactile setae): 10 (including 4 tactile setae): 2; 1 pair of glandular setae (gls) situated on sternites VI, VII and VIII, situated near mid-line, difficult to discern from other sternal setae.
Body length (excluding chelicerae) 1.46. Pedipalp: trochanter 0.190/0.110, femur 0.270/0.110, patella 0.265/0.135, chela (with pedicel) 0.530/0.165, chela (without pedicel) length 0.495, chelal hand (without pedicel) length 0.250, movable finger length 0.255. Carapace 0.440/0.340, anterior eye diameter 0.020, posterior eye diameter 0.015.
The only known specimens of N. tricosus were previously identified as Garypinus nobilis by Beier (1930) from which these differ in several ways, most notably in the position of trichobothrium est that is situated closer to esb than et (Fig. 87–89). Although Beier (1930) stated that the specimens were collected from Batavia (Fig. 91), currently known as Jakarta, the locality label simply states ‘Java’. Furthermore, Beier (1930) noted that vial ‘1950/32’ contained 1 male, and that vial ‘1952/34’ contained 1 male and 1 female. These vials contain 2 males, and 1 male, 2 females and 1 deutonymph respectively and either Beier was seemingly in error or additional specimens were added to the vials at a later date.
Nobilipinus vachoni (Redikorzev, 1938), comb. nov.
(Fig. 91.)
Garypinus vachoni Redikorzev, 1938, pp. 86–87, fig. 15. Beier (1951), pp. 69–70, fig. 14.
Garypinus nobilis With (in part): Schawaller (1994), p. 733.
The original description of G. vachoni was based on four specimens collected from Ba Ngoi in southern Vietnam but lacks sufficient detail to be useful for discriminating between other species from the region. Beier (1951) attributed additional specimens from Cambodia and southern Vietnam to G. vachoni and provided an illustration of the pedipalp of a male that was evidently collected from either Ha Tien or Plateau von Langbian (now known as Núi Lang Bian), the only localities from which males were collected. Whether these specimens are conspecific with the type specimens of G. vachoni will require further research into the garypinid fauna of the region. However, the shape of the male chelal hand as illustrated by Beier (1951) is notably narrower than that in any other species included in this study and this is likely to be significant at the species level.
The decision by Schawaller (1994) to synonymise G. vachoni with G. nobilis did not contain any compelling arguments and is reversed here. The new data provided in this study show that species diversity in this genus is much higher than previously suspected and until further Vietnamese and Cambodian specimens are examined, considering G. vachoni as a distinct species, albeit poorly defined, would be prudent. The transfer of G. vachoni from Garypinus to Nobilipinus is predicated on the illustration of a male purported to be this species that depicts trichobothrium st situated dorsally to sb (Beier 1951, fig. 14).
Genus Garypinus Daday, 1889
Garypinus Daday, 1889, pp. 124, 179–180.
As discussed elsewhere in this paper, Garypinus nobilis and G. vachoni showed sufficient morphological and molecular differences to warrant the creation of the new genus Nobilipinus, thereby leaving the genus Garypinus with only five species: G. dimidiatus (L. Koch, 1873) from the Mediterranean region (e.g. Koch 1873; Beier 1932b, 1963; Hadži 1933; Gardini and Gavalas 2021); G. asper Beier, 1955 from the Middle East (Beier 1955b, 1967c; Mahnert 1974); G. afghanicus Beier, 1959 from Afghanistan and Iran, with the subspecies G. afghanicus afghanicus and G. afghanicus minor Beier, 1959; G. nicolaii Mahnert, 1988 from South Africa (Mahnert 1988); G. mirabilis With, 1907 from Hawaii (With 1907); and G. electri Beier, 1937 from Eocene amber from the Baltic region (Beier 1937; Judson 2005). The genus is relatively poorly circumscribed and requires a comprehensive review to test whether all species are congeneric with the type species, G. dimidiatus, that has recently been characterised and illustrated (Gardini and Gavalas 2021). The presence of setae within the pleural membrane, reported for G. dimidiatus by Callaini (1982) and Gardini and Gavalas (2021), seems to be a highly useful character state that may be diagnostic at the genus level and will help to define the genus.
The other species that are currently attributed to Garypinus differ from G. dimidiatus as described here. Garypinus asper differs in the position of trichobothrium st that is situated dorsally to sb (Beier 1955a, fig. 3). Garypinus afghanicus and G. afghanicus minor differ in the lack of setae in the pleural membrane (M. S. Harvey, pers. obs.) and the median position of the chelal pedicel (Beier 1959c) that is prolaterally displaced in G. dimidiatus (Hadži 1933; Beier 1963; Gardini and Gavalas 2021). Garypinus nicolaii differs in the position of trichobothrium est that is situated much closer to esb than et (Mahnert 1988, fig. 2, 3) but is situated approximately midway between esb and et in G. dimidiatus (e.g. Hadži 1933, fig. 30; Beier 1963, fig. 240; Gardini and Gavalas 2021, fig. 108, 109). Judson (2005) noted that Garypinus electri was most likely misplaced in the genus due to the presence of fields of glandular setae on sternites VI–VIII, as reported for the holotype by Beier (1937). This feature is characteristic of the genus Serianus and G. electri may be better placed in Serianus than Garypinus. The final species, G. mirabilis, is redescribed from the holotype here and shown to not belong to the genus Garypinus, although a definitive placement is lacking due to the paucity of adult specimens.
Garypinus mirabilis With, 1907
ZooBank: urn:lsid:zoobank.org:act:03D9E3AA-2A77-466B-BE90-C7060133CF31
Amblyolpium longiventer (L. Koch & Keyserling): Simon (1900), p. 519 (misidentification).
Garypinus, sp. nov.: With (1905), p. 98; With (1906), p. 89, text-fig. 2.
Garypinus mirabilis With, 1907, pp. 79–80, fig. 48–53.
Holotype. USA: Hawaii: tritonymph, Kau, Hawaii Island [19°06′N, 155°36′W], 1895, R.C.L. Perkins (BMNH 1904.10.24.451).
With 5 setae on hand and 1 subdistal seta on movable finger; all setae acuminate; galea stout with 2 distal and 1 subdistal rami (Fig. 93); rallum of 4 blades, anterior blades I–III with serrations on anterior margin, fourth blade slender and smooth; serrula exterior with 18 blades, basalmost blade enlarged; lamina exterior present.
All surfaces smooth; patella with 1 long and 4 short subbasal lyrifissures; all segments robust, trochanter 1.85, femur 2.91, patella 2.24, chela (with pedicel) 3.38, chela (without pedicel) 3.18, hand (without pedicel) 1.63× longer than broad, movable finger 1.00× longer than hand (without pedicel). Fixed chelal finger with 7 trichobothria, movable chelal finger with 3 trichobothria (Fig. 92): isb and sb absent; eb and esb situated basally; est situated much closer to esb than et; ib, ist and it situated subbasally; t situated submedially; st situated slightly closer to sb than t. Venom apparatus present in both chelal fingers, venom duct short. Chelal teeth juxtadentate; fixed finger with ~23 teeth; movable finger with ~22 teeth.
Carapace (Fig. 94) smooth; 1.26× longer than broad; without eyes or eye-spots; with 18 setae, including 4 in anterior row, 4 in ocular zone, 8 in median zone and 2 in posterior zone; without furrows. Manducatory process with 3 apical acuminate setae and 5 additional setae; median maxillary lyrifissure rounded and situated submedially; posterior maxillary lyrifissure rounded. Chaetotaxy of coxae I–IV: 5: 4: 3: 3.
Femora I and II shorter than patellae I and II (Fig. 95); junction between anterior femora and patellae perpendicular (Fig. 96); junction between femora and patellae III and IV highly angular (Fig. 96); femora III and IV much smaller than patellae III and IV; leg segments rather stocky; femur + patella of leg IV 2.41× longer than broad; metatarsi III and IV with long tactile seta, situated basally; subterminal tarsal setae arcuate and acute; claws not modified; arolium deeply divided, much longer than claws.
Tergites II–X with median suture line. Tergal chaetotaxy: 4: 2: 4: 6: 6: 6: 6: 6: 6: 9: 8: 1; setae acuminate. Sternal chaetotaxy: 3: (2) 4 (2): (2) 4 (2): 7: 6 [1 + 1 gls]: 6 [1 + 1 gls]: 6 [1 + 1 gls]: 6: 8: 8: 2. Spiracles with helix. Pleural membrane longitudinally striate; without setae.
Body length 2.47. Pedipalps: trochanter 0.315/0.170, femur 0.510/0.175, patella 0.470/0.210, chela (with pedicel) 0.945/0.280, chela (without pedicel) 0.890, hand (without pedicel) length 0.455, movable finger length 0.455. Chelicera 0.240/0.135, movable finger length 0.150. Carapace 0.655/0.520; anterior eye 0.050; posterior eye 0.045. Leg I: femur 0.130/0.140, patella 0.185/0.145, tibia 0.225/0.105, metatarsus 0.065/0.080, tarsus 0.115/0.070. Leg IV: femur + patella 0.495/0.205, tibia 0.335/0.135, metatarsus 0.095/0.095, tarsus 0.145/0.080.
The holotype of G. mirabilis is, unfortunately, a tritonymph and therefore lacks some significant morphological features that would help determine the generic status. However, there are some characters that can be used to test the affinities. The pleural membrane lacks setae that are present in the type species of Garypinus, G. dimidiatus (Callaini 1982; Gardini and Gavalas 2021) and likely to be highly diagnostic at the generic level. Trichobothrium est is situated much closer to esb than et, a character state that is also found in the genera Haplogarypinus from the Democratic Republic of the Congo (Beier 1959b), Hemisolinus from Saint Helena (Beier 1977), Garypinus nicolaii Mahnert, 1988 from South Africa (Mahnert 1988) and the widespread genera Solinus (e.g. Morikawa 1953a; Beier 1966c, 1967b; Dashdamirov 1996) and Serianus (e.g. Chamberlin 1930; Hoff 1950, 1956, 1964; Beier 1964a; Muchmore 1968). This is easily excluded from Solinus as all Solinus species lack trichobothrium st (e.g. Chamberlin 1930; Murthy and Ananthakrishnan 1977; Dashdamirov 1996) that is present in G. mirabilis. The species differs from G. nicolaii in the morphological characteristics of the galea that has 2 distal rami and a medial or subbasal ramus in G. nicolaii (Mahnert 1988) but three distal rami in G. mirabilis. Incidentally, G. nicolaii is most likely misplaced in the genus Garypinus due to the position of est that is midway between esb and et in the type species G. mirabilis (Gardini and Gavalas 2021) but much closer to esb in G. nicolaii. Garypinus mirabilis differs from most species of Serianus in the morphological character of the galea that has three distal rami in G. mirabilis but a subbasal ramus in most Serianus species (e.g. Hoff 1950, fig. 10, 1956, 1964; Muchmore 1968, fig. 1, 1981b, fig. 1; Mahnert 1988, 1991; Piedra-Jiménez et al. 2019, fig. 30). Known exceptions to this morphological pattern are S. birabeni from Argentina (Feio 1945, fig. 10, 11), S. elongatus from the Galapagos Islands (Mahnert 2014) and S. salomonensis from the Solomon Islands (Beier 1966b), both of which might be misplaced in Serianus as these lack the characteristic trichobothrial pattern of st situated dorsally to sb.
A definitive assessment of the generic status of G. mirabilis must await the collection of adult specimens. No specimens collected from Hawaii have been identified as Garypinidae or Olpiidae in the Bernice P. Bishop Museum collection (James Boone, in litt.) and I have examined numerous collections of Pacific Ocean pseudoscorpions without finding any garypinids. Until adults are available for study, G. mirabilis will be provisionally retained in Garypinus.
Although Simon (1900) noted that the holotype was collected from Kau taht is located on Hawaii Island, With (1907) erroneously gave the type locality as the island of Kauai. This error was corrected by Judson (1997).
Genus Solinus Chamberlin, 1930
Solinus Chamberlin, 1930, p. 596.
Indogarypinus Murthy & Ananthakrishnan, 1977, p. 98. New synonymy.
Solinus: Garypinus corticolus Chamberlin, 1930, by original designation.
Indogarypinus: Indogarypinus minutus Murthy & Ananthakrishnan, 1977, by original designation.
The genus Solinus Chamberlin, 1930 is sporadically distributed around the world and includes specimens that are generally small, pale and flattened, and live under tree bark. The genus currently includes eight species from Mexico and Colombia (S. corticola (Chamberlin, 1923)), the Mediterranean region and Central Asia (S. cyrenaicus Beier, 1929, S. hispanus Beier, 1939 and S. rhodius Beier, 1966), east Africa (S. africanus Beier, 1967), Japan (S. japonicus Morikawa, 1953), Australia (S. australiensis Chamberlin, 1930) and Papua New Guinea (S. pusillus Beier, 1971). Dashdamirov (1996) suggested that the three Mediterranean species might eventually be shown to be synonyms but refrained from treating these as a single species until all of the type specimens had been examined. In addition to S. australiensis, the Australian fauna comprises several species that can be best distinguished by the number of setae on the male genital sternites. Unfortunately, the holotype of S. Australiensis is female (Chamberlin 1930) that precludes knowledge of the male genital region. Until males are collected from near the type locality (Barringun, New South Wales, 29°01′S, 145°42′E), the identity of this species will remain uncertain. In addition to the genital sternites, Australian species of Solinus can be distinguished using molecular sequence data (Fig. 1). One of these species is described here to provide further data on the Australian Solinus fauna and document the unusual setal patterns found in some Australian species.
Chamberlin (1930) provided conflicting information regarding the number of setae on the cheliceral hand when establishing the genus Solinus. In the key, S. corticola was stated to possess four setae and S. australiensis five setae but in the description on the following page, S. australiensis was stated to only have four setae and S. corticola implied to have five. Correct numbers were provided by Beier (1932b) and my examination of the type specimens of both species in the California Academy of Sciences revealed that Beier’s (1932b) interpretation is correct. Since that time, additional species have been described from different parts of the world that have either four or five setae, with species from central America (S. corticola), the Palaerctic region (S. cyrenaicus, S. hispanus, S. japonicus and S. rhodius) possessing five setae (Chamberlin 1930; Morikawa 1953a; Beier 1966c; Dashdamirov 1996) and those from Africa (S. africanus), India (S. minutus), New Guinea (S. pusillus) and Australia (S. australiensis) having four setae (Beier 1967b, 1971a; Murthy and Ananthakrishnan 1977). The new species described from south-western Australia also has only four setae.
The genus Indogarypinus was described by Murthy and Ananthakrishnan (1977) for the type species I. minutus Murthy and Ananthakrishnan, 1977 from Tamil Nadu, southern India. The description is relatively comprehensive and the genus was considered to be ‘related to the Solinus–Aldabrinus genus group’ due to the absence of trichobothria st and t, and the ‘vertical, immovable’ articulation between the femur and patella of leg I. These comparisons were evidently based on the groupings and terminology used by Chamberlin (1930). Murthy and Ananthakrishnan (1977) noted several differences between Indogarypinus, Solinus and Aldabrinus and used the basal position of the prolateral trichobothria on the fixed chelal finger, a deep posterior carapaceal furrow and the presence of ‘short spine like setae set in areola in the sternites VI to VIII’ to justify the recognition of Indogarypinus. Comparison of the figures of I. minutus supplied by Murthy and Ananthakrishnan (1977) demonstrate, however, that the trichobothria are located in positions that are extremely similar to those that occur in species of Solinus (e.g. Chamberlin 1931a; Morikawa 1953a; Beier 1963, 1966c, 1967b; Dashdamirov 1996). Murthy and Ananthakrishnan (1977) were possibly influenced by the illustration of a pedipalp of S. corticola by Beier (1932b, fig. 243) in which trichobothrium it is situated submedially on the fixed chelal finger. However, this illustration does not agree with that of the same species provided by Chamberlin (1931a, fig. 37C) in which it is situated subbasally adjacent to the other trichobothria of the prolateral series. This dilemma can only be resolved by comparison of the type specimens of S. corticola and the specimen illustrated by Beier (1932b). Nevertheless, the placement of trichobothrium it cannot be used as a character state to distinguish Indogarypinus from Solinus. The second character used by Murthy and Ananthakrishnan (1977), the presence of a deep posterior carapaceal furrow, seems to be a misinterpretation of the carapace morphology of Solinus species in which the posterior portion is desclerotised in a semi-circular arc, as portrayed for S. corticola by Chamberlin (1931a, fig. 61). The third character, the presence of short spine-like setae on sternites VI–VIII, is a pair of glandular setae that is present in most garypinids (e.g. Harvey and Šťáhlavský 2010).
Therefore, all the morphological features used by Murthy and Ananthakrishnan (1977) to establish Indogarypinus are identical to those occurring in species of Solinus and these are here synonymised with each other. Indogarypinus minutus is transferred to the genus Solinus, forming the new combination, Solinus minutus (Murthy and Ananthakrishnan, 1977).
Solinus pingrup, sp. nov.
ZooBank: urn:lsid:zoobank.org:act:001FF09A-6A6E-4063-B7C6-1A6B38745197
Holotype. AUSTRALIA: Western Australia: ♂, Pingrup Primary School, 33°31ʹ55"S, 118°30ʹ40"E, 11–18 March 2022, Malaise trap, Pingrup Primary School students (Insect Investigators BIOUG85132-F10) (WAM T160525).
Other material. AUSTRALIA: Western Australia: 1 ♂, ~2 km E. of North Bannister, 32°34ʹ54.0"S, 116°30ʹ06.1"E, 25 April 2019, pyrethrum knockdown, Eucalyptus wandoo bark, A. Postle (WAM T147127); 1 tritonymph, ~9 km NNE. of North Bannister, 32°30ʹ03.3"S, 116°28ʹ05.0"E, 25 April 2019, pyrethrum knockdown, Eucalyptus marginata bark, A. Postle (WAM T150840).
The specific epithet is a noun in apposition that was selected by collectors of the holotype, the students from Pingrup Primary School.
The presence of only four setae on the cheliceral hand (Fig. 100) separates Solinus pingrup from S. corticola, S. cyrenaicus, S. hispanus, S. japonicus and S. rhodius that have five setae. This species differs from the species with four setae on the hand as follows: from S. africanus in the lack of galeal rami (Fig. 100) (3 small terminal rami in S. africanus), the larger size (e.g. male pedipalpal femur 0.285–0.315 v. 0.25 mm in the female of S. africanus) and the position of trichobothrium t that is situated further away from b than in S. africanus; from S. australiensis in the relative size of the chelal fingers that are described as ‘distinctly shorter than hand’ in S. australiensis (Chamberlin 1930) but are approximately the same length as the hand in S. pingrup (Fig. 102); from S. minutus in the larger size (e.g. male pedipalpal femur 0.285–0.315 v. 0.24 mm in the female of S. minutus); and from S. pusillus in the presence of only 4 setae on the tergites (6 setae in S. pusillus) and the lack of any galeal rami (Fig. 100) (a delicate ramus in S. pusillus).
Surface smooth; cheliceral hand with 4 setae, ls absent; movable finger with 1 subdistal seta; all setae acuminate; galea slender, ♂ without rami (Fig. 100); fixed finger with 4 small teeth, each approximately same size; movable finger with 1 subdistal tooth; with 2 dorsal and 1 ventral lyrifissures; serrula interior modified to form velum; serrula interior with 14 (♂) blades; lamina exterior absent; rallum with 4 blades, anterior blade with anterior spinules, remainder smooth.
Solinus pingrup sp. nov., holotype male (WAM T160525), unless stated otherwise: 99, carapace; 100, left chelicera, dorsal; 101, right pedipalp, dorsal; 102, left chela, retrolateral; 103, detail of chelal finger tips; 104, left chela, retrolateral, paratype tritonymph (WAM T150840); 105, left leg IV, prolateral. Scale lines: 0.2 mm (99, 101, 102, 104, 105), 0.1 mm (100, 103).

Slender (Fig. 101); all segments completely smooth; setae acicular, straight or nearly so; trochanter 2.05–2.13× (♂); femur cylindrical, with 1 tactile seta on dorsal surface, situated ~1/3 length of femur, 3.35–3.50× (♂); patella with several small lyrifissures situated basally on dorsal surface, 2.41–2.59× (♂); chelal hand slender, retrolateral chelal condyle small and rounded, chela (with pedicel) 3.55–3.93× (♂), chela (without pedicel) 3.37–3.70× (♂), hand (without pedicel) 1.81–1.89× (♂) all longer than broad, movable finger 0.90–0.98× (♂) longer than hand. Fixed finger with 8 trichobothria, movable finger with 2 trichobothria, sb and st absent, eb and esb situated at base of fixed finger on retrolateral margin, est situated much closer to esb than et, ib, isb ist and it situated prolaterally at base of fixed finger, et subdistal (Fig. 102); chelal fingers straight in lateral view (Fig. 102). Chelal teeth juxtadentate; fixed finger with 18 (♂) flattened chelal teeth; movable finger with 13 (♂) retrorse chelal teeth, the 6 most distal teeth pointed and the remainder rounded; accessory teeth absent; chelal fingers without spine-like setae on prolateral margins. Venom teeth broad; venom apparatus present in both chelal fingers, duct very short, terminating in nodus ramosus near tip of fingers (Fig. 103), nodus ramosus not inflated.
Carapace subrectangular (Fig. 99); anterior margin slightly convex; epistome absent; completely smooth; 1.37–1.50× (♂) longer than broad, with 18 setae arranged 4: 2: 2: 4: 4: 2; all setae subequal in length; without furrows; with two pairs of large, corneate eyes, situated near anterior margin of carapace, not on tubercle. Manducatory process distally triangular; with 1 seta situated distally, another situated medially; with 4 (♂) additional setae; suboral seta present, not reduced to a small nubbin. Maxillary shoulder absent; median maxillary lyrifissure situated medially. Coxa I approximately same width as coxa IV; coxae setal formula: ♂, 5: 5: 3: 3.
Femora I and II shorter than patellae I and II respectively; femora I and II with primary slit sensillum directed transversely; junction between anterior femora and patellae perpendicular; junction between posterior femora and patellae strongly oblique; femora III and IV much smaller than patellae III and IV respectively; femur + patella IV 2.33× (♂) longer than deep; metatarsi IV with long basal tactile seta (Fig. 105); metatarsi shorter than tarsi; claws smooth; arolium much longer than claws, deeply divided (Fig. 105).
Tergites straight, II–X divided (Fig. 97); setal formula ♂, 4: 2: 4: 4: 4: 4: 4: 4: 5: 11 (including 4 tactile setae): 8 (including 4 tactile setae): 2; setae arranged in single rows; sternites, setal formula ♂, 16: (1) 4 [2 + 2] (1): (2) 4 (2): 4: 4 + 2 gls: 4 + 2 gls: 4 + 2 gls: 4: 9 (including 4 tactile setae): 7 (including 4 tactile setae): 2; posterior tergites and sternites with several tactile setae; 1 pair of enlarged glandular setae (gls) situated on sternites VI, VII and VIII (Fig. 106) near mid-line, well in advance of regular setae, each seta thicker and longer than other sternal setae; most sternites with broad median suture line; pleural membrane uniformly longitudinally striate, without setae; stigmatic helix present; anus (tergite XII and sternite XII) without raised rim; situated between tergite XI and sternite XI.
Dorsal anterior glands greatly enlarged; lateral genital sacs and median genital sacs absent; with 2 pairs of setae within genital atrium (Fig. 108).
Holotype male (WAM T160525) (followed by 1 other male, when measured, in parentheses): Body length (excluding chelicerae) 1.58 (1.96). Pedipalp: trochanter 0.175/0.085 (0.170/0.080), femur 0.315/0.090 (0.285/0.085), patella 0.285/0.110 (0.265/0.110), chela (with pedicel) 0.530/0.135 (0.480/0.135), chela (without pedicel) length 0.500 (0.455), chelal hand (without pedicel) length 0.255 (0.245), movable finger length 0.250 (0.220). Carapace 0.450/0.300 (0.465/0.340); anterior eye diameter 0.040, posterior eye diameter 0.035. Leg IV: femur + patella 0.280/0.120, tibia 0.175/0.060, metatarsus 0.060/0.040, tarsus 0.075/0.040.
Most cuticular surfaces yellow–brown, pedipalps red–brown, posterior portion of carapace paler.
Trochanter 2.06×, femur 3.24×, patella 2.43×, chela (with pedicel) 3.65×, chela (without pedicel) 3.42×, hand (without pedicel) 1.88× all longer than broad, movable finger 0.86× longer than hand (without pedicel). Fixed chelal finger with 7 trichobothria, eb, esb, est, et, ib, ist and it present; movable finger with 2 trichobothria, b and t present (Fig. 104). Fixed chelal finger with 13 pointed teeth; movable chelal finger ~9 pointed teeth.
1.61× longer than broad; with 18 setae; arranged 4: 2: 2: 4: 4: 2; smooth, without furrows; 2 pair of eyes.
The male specimen from North Bannister shares an identical setal pattern of sternite II to that of the holotype from Pingrup and is therefore easily attributed to Solinus pingrup. Other males from south-western Australia have differing setal patterns and are considered to represent different undescribed species.
Sequence data are available for two other Australian species of Solinus in the molecular phylogeny, designated as S. ‘PSE214’ from the Nullarbor Plain and S. ‘PSE222’ from the Pilbara region of Western Australia, all collected from tree bark. Neither is conspecific with S. pingrup, as these have divergent COI barcodes and sufficient morphological differences to be regarded as distinct species.
Subfamily PROTOGARYPININAE, subfam. nov.
ZooBank: urn:lsid:zoobank.org:act:EEBC79AB-274B-4B86-A19A-E50DAE5F6FA8
Members of the subfamily Protogarypininae differ from other garypinids in the undivided arolium that is distinctly bipartite in Garypininae and Amblyolpiinae.
As for family, except as follows.
Neominniza Beier, 1930, Oreolpium Benedict & Malcolm, 1978, Protogarypinus Beier, 1954, Teratolpium Beier, 1959 and Thaumatolpium Beier, 1931.
Currently there is no compelling evidence that Protogarypininae is a monophyletic taxon, even though this are easily distinguished from the other garypinids in the undivided arolium. The only other group of pseudoscorpions that have divided arolia are some genera of Ideoroncidae including Albiorix Chamberlin, 1930 from the Americas, Proalbiorix Geißler, Kotthoff, Hammel, Harvey and Harms, 2022 from Cretaceous amber and to a lesser extent, Xorilbia Harvey & Mahnert (Harvey and Muchmore 2013; Geißler et al. 2022). The remaining ideoroncid genera have undivided arolia as found in the vast majority of pseudoscorpions but there are no explicit tests to determine whether the divided condition is monophyletic for these three ideoroncid genera.
Most protogarypinines occur in the southern hemisphere, with Protogarypinus in Australia (Beier 1954a, 1975), Neominniza and Thaumatolpium in Chile (Beier 1964a), and Teratolpium in Peru (Beier 1959a). The genus Oreolpium has a highly disjunct distribution with species in north-western USA and Tasmania (Benedict and Malcolm 1978; Harvey and Šťáhlavský 2010).
| 1. | Pedicel of pedipalpal chela offset prolaterally...2 Pedicel of pedipalpal chela situated medially...4 |
| 2. | Trichobothrium st situated dorsally to sb...3 Trichobothrium st distal to sb...Oreolpium Benedict and Malcolm (USA and Tasmania) |
| 3. | Four eyes present; trichobothria sb and st situated closer to b than t...Protogarypinus Beier (Australia) Two eyes present; trichobothria sb and st situated closer to t than b...Teratolpium Beier (Peru) |
| 4. | Four eyes present...Neominniza Beier (Chile) Two eyes present or eyes absent...Thaumatolpium Beier (Chile) |
Data availability
All data generated or analysed during this study are included in this article. Sequence information generated in this study is available in GenBank as outlined in Table 1.
Conflicts of interest
M. S. Harvey is an editor for Invertebrate Systematics but did not at any stage have editor-level access to this manuscript while in peer review, as is the standard practice when handling manuscripts submitted by an editor to this journal. Invertebrate Systematics encourages its editors to publish in the journal and they are kept totally separate from the decision-making processes for their manuscripts. The author has no further conflicts of interest to declare.
Acknowledgements
The author is very grateful to Janet Beccaloni (BMNH), Cristoph Hörweg (NHMW), Peter Jäger and Julia Altmann (SMF), Peter Schwendinger (MHNG), Nadine Dupérré and Danilo Harms (ZMH) for access to the specimens used in this study, and the late James Boone (Bernice P. Bishop Museum, Honolulu) for checking the BPBM collection for garypinid specimens. Field work in Brunei was made possible through the kind auspices of Joseph Koh. A specimen of Protogarypinus giganteus was collected during a Walpole Wilderness Bioblitz organised by the Walpole Nornalup National Parks Association. The specimen of Serianus was kindly made available by Dr Elizabeth Arias (Essig Museum of Entomology, University of California Berkeley) and Dr Charles Griswold (California Academy of Sciences, San Francisco) and the specimen of Larca granulata was made available by Julia Cosgrove (Harvard University). This project forms part of the Insect Investigators Citizen Science Project (https://insectinvestigators.com.au) that received grant funding from the Australian Government and is supported in Western Australia by the Western Australian Gould League and the Western Australian Museum. The author is also very grateful to Dr Erinn Fagan-Jeffries for support and encouragement during this project. I thank the students from Pingrup Primary School, the teacher Karl Moll and Principal Katrina Carnicell, and the students from Kwoorabup Nature School and the teacher Olly Watkins for participation and collection of specimens of Solinus pingrup and Aldabrinus rixi respectively. I thank Sheila Murray for permission to use her image of a living Aldabrinus rixi (Fig. 7), Karen Cullen for the line drawing depicted in Fig. 2 and the Western Australian Museum’s Molecular Systematics Unit for supplying some the new sequence data.
References
Arabi J, Judson MLI, Deharveng L, Lourenço WR, Cruaud C, Hassanin A (2012) Nucleotide composition of CO1 sequences in Chelicerata (Arthropoda): detecting new mitogenomic rearrangements. Journal of Molecular Evolution 74, 81-95.
| Crossref | Google Scholar |
Bedoya-Roqueme E, Pérez-Agudelo M, Zaragoza JA, Quirós JA (2017) Nuevos reportes de Pseudoescorpiones (Arachnida) de Bosques de Manglar en Córdoba, Caribe colombiano. Revista Ibérica de Aracnología 30, 25-36 [In Spanish].
| Google Scholar |
Beier M (1930) Die Pseudoskorpione der Sammlung Roewer. Zoologischer Anzeiger 91, 284-300 [In German].
| Google Scholar |
Beier M (1932a) Pseudoscorpionidea II. Subord. C. Cheliferinea. Tierreich 58, 1-297 [In German].
| Google Scholar |
Beier M (1932b) Pseudoscorpionidea I. Subord. Chthoniinea et Neobisiinea. Tierreich 57, 1-258 [In German].
| Google Scholar |
Beier M (1936) Zoologische Ergebnisse einer Reise nach Bonaire, Curaçao und Aruba im Jahre 1930. No. 21. Einige neue neotropische Pseudoscorpione. Zoologische Jahrbücher, Abteilung für Systematik, Ökologie und Geographie der Tiere 67, 443-447 [In German].
| Google Scholar |
Beier M (1944) Über Pseudoscorpioniden aus Ostafrika. Eos, Madrid 20, 173-212 [In German].
| Google Scholar |
Beier M (1947) Zur Kenntnis der Pseudoscorpionidenfauna des südlichen Afrika, insbesondere der südwest- und südafrikanischen Trockengebiete. Eos, Madrid 23, 285-339 [In German].
| Google Scholar |
Beier M (1951) Die Pseudoscorpione Indochinas. Mémoires du Muséum National d’Histoire Naturelle, Paris, Nouvelle Série 1, 47-123 [In German].
| Google Scholar |
Beier M (1954a) Report from Prof. T. Gislén’s expedition to Australia in 1951–1952. 7. Pseudoscorpionidea. Acta Universitatis Lundensis, Nova Series (2) 50, 1-26.
| Google Scholar |
Beier M (1954b) Zwei neue Pseudoscorpione aus Ostafrika. Zoologischer Anzeiger 152, 84-88 [In German].
| Google Scholar |
Beier M (1955b) Über Pseudoscorpione aus Syrien und Palästina. Annalen des Naturhistorischen Museums in Wien 60, 212-219 [In German].
| Google Scholar |
Beier M (1958) The Pseudoscorpionidea (false-scorpions) of Natal and Zululand. Annals of the Natal Museum 14, 155-187.
| Google Scholar |
Beier M (1959a) Zur Kenntnis der Pseudoscorpioniden-Fauna des Andengebietes. Beiträge zur Neotropischen Fauna 1, 185-228 [In German].
| Google Scholar |
Beier M (1959b) Pseudoscorpione aus dem Belgischen Congo gesammelt von Herrn N. Leleup. Annales du Musée du Congo Belge, Sciences Zoologiques 72, 5-69 [In German].
| Google Scholar |
Beier M (1959c) Zur Kenntnis der Pseudoscorpioniden‐Fauna Afghanistans. Zoologische Jahrbücher, Abteilung für Systematik, Ökologie und Geographie der Tiere 87, 257-282 [In German].
| Google Scholar |
Beier M (1962) Pseudoscorpioniden aus der Namib-Wüste. Annals of the Transvaal Museum 24, 223-230 [In German].
| Google Scholar |
Beier M (1964a) Die Pseudoscorpioniden-Fauna Chiles. Annalen des Naturhistorischen Museums in Wien 67, 307-375 [In German].
| Google Scholar |
Beier M (1964b) Weiteres zur Kenntnis der Pseudoscorpioniden-Fauna des südlichen Afrika. Annals of the Natal Museum 16, 30-90 [In German].
| Google Scholar |
Beier M (1964c) Some further nidicolous Chelonethi (Pseudoscorpionidea) from Malaya. Pacific Insects 6, 312-313.
| Google Scholar |
Beier M (1964d) Pseudoscorpione von der Insel San Ambrosio. Annalen des Naturhistorischen Museums in Wien 67, 303-306 [In German].
| Google Scholar |
Beier M (1966a) Ergebnisse der österreichischen Neukaledonien-Expedition 1965. Pseudoscorpionidea. Annalen des Naturhistorischen Museums in Wien 69, 363-371 [In German].
| Google Scholar |
Beier M (1966b) Die Pseudoscorpioniden der Salomon-Inseln. Annalen des Naturhistorischen Museums in Wien 69, 133-159 [In German].
| Google Scholar |
Beier M (1966c) Über Pseudoskorpione der Insel Rhodos. Annalen des Naturhistorischen Museums in Wien 69, 161-167 [In German].
| Google Scholar |
Beier M (1967a) Contributions to the knowledge of the Pseudoscorpionidea from New Zealand. Records of the Dominion Museum 5, 277-303.
| Google Scholar |
Beier M (1967b) Pseudoskorpione aus dem tropischen Ostafrika (Kenya, Tansania, Uganda, etc.). Annalen des Naturhistorischen Museums in Wien 70, 73-93 [In German].
| Google Scholar |
Beier M (1967c) Ergebnisse zoologischer Sammelreisen in der Türkei. Annalen des Naturhistorischen Museums in Wien 70, 301-323 [In German].
| Google Scholar |
Beier M (1970a) Die Pseudoscorpione der Royal Society Expedition 1965 zu den Salomon-Inseln. Journal of Natural History 4, 315-328 [In German].
| Google Scholar |
Beier M (1970b) Ergänzungen zur Pseudoskorpionidenfauna der Kanaren. Annalen des Naturhistorischen Museums in Wien 74, 45-49 [In German].
| Google Scholar |
Beier M (1971a) Pseudoskorpione unter Araucarien-Rinde in Neu-Guinea. Annalen des Naturhistorischen Museums in Wien 75, 367-373 [In German].
| Google Scholar |
Beier M (1971b) Pseudoskorpione aus dem Iran. Annalen des Naturhistorischen Museums in Wien 75, 357-366 [In German].
| Google Scholar |
Beier M (1973) Weiteres zur Kenntnis der Pseudoscorpioniden Südwestafrikas. Cimbebasia, A 2, 97-101 [In German].
| Google Scholar |
Beier M (1975) Neue Pseudoskorpione aus Australien und Neu-Guinea. Annalen des Naturhistorischen Museums in Wien 78, 203-213 [In German].
| Google Scholar |
Beier M (1976) The pseudoscorpions of New Zealand, Norfolk and Lord Howe. New Zealand Journal of Zoology 3, 199-246.
| Crossref | Google Scholar |
Beier M (1977) Pseudoscorpiones. In: La faune terrestre de l’île de Sainte-Hélène IV. Annales du Musée Royal de l’Afrique Centrale, Zoologie (8) 220, 2-11 [In French].
| Google Scholar |
Beier M (1978) Pseudoskorpione von den Galapagos-Inseln. Annalen des Naturhistorischen Museums in Wien 81, 533-547 [In German].
| Google Scholar |
Benavides LR, Cosgrove JG, Harvey MS, Giribet G (2019) Phylogenomic interrogation resolves the backbone of the Pseudoscorpiones Tree of Life. Molecular Phylogenetics and Evolution 139, 106509.
| Crossref | Google Scholar |
Benedict EM (1978) False scorpions of the genus Apocheiridium Chamberlin from western North America (Pseudoscorpionida, Cheiridiidae). Journal of Arachnology 5, 231-241.
| Google Scholar |
Benedict EM, Malcolm DR (1978) Some garypoid false scorpions from western North America (Pseudoscorpionida: Garypidae and Olpiidae). Journal of Arachnology 5, 113-132.
| Google Scholar |
Callaini G (1982) Étude comparative de la membrane pleurale des pseudoscorpions au microscope électronique à balayage. Memorie, Atti della Società Toscana di Scienze Naturali Residente in Pisa B 88(supplemento), 16-26 [In French].
| Google Scholar |
Chamberlin JC (1923) New and little known pseudoscorpions, principally from the islands and adjacent shores of the Gulf of California. Proceedings of the California Academy of Science, 4th Series 12, 353-387.
| Google Scholar |
Chamberlin JC (1924) The Cheiridiinae of North America (Arachnida - Pseudoscorpionida). Pan-Pacific Entomologist 1, 32-40.
| Google Scholar |
Chamberlin JC (1930) LXI.—A synoptic classification of the false scorpions or chela-spinners, with a report on a cosmopolitan collection of the same.—Part II. The Diplosphyronida (Arachnida-Chelonethida).5. Annals and Magazine of Natural History 5(30), 585-620.
| Crossref | Google Scholar |
Chamberlin JC (1931a) The arachnid order Chelonethida. Stanford University Publications, Biological Sciences 7(1), 1-284.
| Google Scholar |
Chamberlin JC (1931b) Parachernes ronnaii, a new genus and species of false scorpion from Brazil (Arachnida – Chelonethida). Entomological News 42, 192-195.
| Google Scholar |
Chamberlin JC (1932) On some false scorpions of the superfamily Cheiridioidea (Arachnida – Chelonethida). Pan-Pacific Entomologist 8, 137-144.
| Google Scholar |
Chamberlin JC (1943) The taxonomy of the false scorpion genus Synsphyronus with remarks on the sporadic loss of stability in generally constant morphological characters (Arachnida: Chelonethida). Annals of the Entomological Society of America 36, 486-500.
| Crossref | Google Scholar |
Cockerell TDA (1920) XXXVI.—Fossil arthropods in the British Museum.—I. Annals and Magazine of Natural History 5(27), 273-279.
| Crossref | Google Scholar |
Daday E (1889) A Magyar Nemzeti Muzeum álskorpióinak áttekintése. Természetrajzi Füzetek 11, 111–136, 165-192 [In Hungarian].
| Google Scholar |
Dashdamirov S (1996) Notes on the false scorpion genus Solinus in the Caucasus (Pseudoscorpionida: Olpiidae). Revue Suisse de Zoologie, Hors Série 1, 117-123.
| Google Scholar |
Dashdamirov S (2007) A new genus and species of false scorpion from Vietnam showing remarkable chelal modifications (Arachnida: Chelonethida). Acta Biologica Benrodis 13, 219-229.
| Google Scholar |
Dashdamirov S, Schawaller W (1993) Pseudoscorpions from Middle Asia, Part 2 (Arachnida: Pseudoscorpiones). Stuttgarter Beiträge zur Naturkunde (A) 496, 1-14.
| Google Scholar |
Ellingsen E (1910) Die Pseudoskorpione des Berliner Museums. Mitteilung aus dem Zoologischen Museum in Berlin 4, 357-423 [In German].
| Google Scholar |
Ellingsen E (1914) On the pseudoscorpions of the Indian Museum, Calcutta. Records of the Indian Museum 10, 1-14.
| Google Scholar |
Feio JLA (1945) Novos pseudoscorpiões de região neotropical (com a descriçao de uma subfamilia, dois géneros e sete espécies). Boletim do Museu Nacional Rio de Janeiro, n.s. Zoologia 44, 1-47 [In Portuguese].
| Google Scholar |
Gardini G (1983) Larca italica n. sp. cavernicola dell’Appennino Abruzzese (Pseudoscorpionida, Garypidae) (Pseudoscorpioni d’Italia XV). Bollettino della Società Entomologica Italiana 115, 63-69 [In Italian].
| Google Scholar |
Gardini G, Gavalas I (2021) The pseudoscorpions of the island of Iraklia (Cyclades, Greece). Parnassiana Archives 9, 43-78.
| Google Scholar |
Gardini G, Galli L, Zinni M (2017) Redescription of Geogarypus minor, type species of the genus Geogarypus, and description of a new species from Italy (Pseudoscorpiones: Geogarypidae). Journal of Arachnology 45, 424-443.
| Crossref | Google Scholar |
Geißler C, Kotthoff U, Hammel J, Harvey MS, Harms D (2022) The first fossil of the pseudoscorpion family Ideoroncidae (Arachnida: Pseudoscorpiones): a new taxon from the mid-Cretaceous of norarabithern Myanmar. Cretaceous Research 130, 105030.
| Crossref | Google Scholar |
Hadži J (1933) Beitrag zur Kenntnis der Pseudoskorpionen-Fauna des Küstenlandes. Bulletin International de l’Académie Yugoslave des Sciences et des Beaux-arts 27, 173-199 [In French].
| Google Scholar |
Harms D, Roberts JD, Harvey MS (2019) Climate variability impacts on diversification processes in a biodiversity hotspot: a phylogeography of ancient pseudoscorpions in southwestern Australia. Zoological Journal of the Linnean Society 186, 934-949.
| Crossref | Google Scholar |
Harvey MS (1985) The systematics of the family Sternophoridae (Pseudoscorpionida). Journal of Arachnology 13, 141-209.
| Google Scholar |
Harvey MS (1986) The Australian Geogarypidae, new status, with a review of the generic classification (Arachnida: Pseudoscorpionida). Australian Journal of Zoology 34, 753-778.
| Crossref | Google Scholar |
Harvey MS (1987a) A revision of the genus Synsphyronus Chamberlin (Garypidae: Pseudoscorpionida: Arachnida). Australian Journal of Zoology, Supplementary Series 126, 1-99.
| Crossref | Google Scholar |
Harvey MS (1987b) Redescriptions of Geogarypus bucculentus Beier and G. pustulatus Beier (Geogarypidae: Pseudoscorpionida). Bulletin of the British Arachnological Society 7, 137-141.
| Google Scholar |
Harvey MS (1988) Pseudoscorpions from Krakatau Islands and adjacent regions, Indonesia (Chelicerata: Pseudoscorpionida). Memoirs of the Museum of Victoria 49, 309-353.
| Crossref | Google Scholar |
Harvey MS (1990) New pseudoscorpions of the genera Americhernes Muchmore and Cordylochernes Beier from Australia (Pseudoscorpionida: Chernetidae). Memoirs of the Museum of Victoria 50, 325-336.
| Crossref | Google Scholar |
Harvey MS (1992) The phylogeny and classification of the Pseudoscorpionida (Chelicerata: Arachnida). Invertebrate Systematics 6, 1373-1435.
| Crossref | Google Scholar |
Harvey MS (2000) From Siam to Rapa Nui – the identity and distribution of Geogarypus longidigitatus (Rainbow) (Pseudoscorpiones: Geogarypidae). Bulletin of the British Arachnological Society 11, 377-384.
| Google Scholar |
Harvey MS (2010) Two new species of Synsphyronus (Pseudoscorpiones: Garypidae) from southern Western Australian granite landforms. Records of the Western Australian Museum 26, 11-22.
| Crossref | Google Scholar |
Harvey MS (2016) The systematics of the pseudoscorpion family Ideoroncidae (Pseudoscorpiones, Neobisioidea) in the Asian region. Journal of Arachnology 44, 272-329.
| Crossref | Google Scholar |
Harvey MS, Du Preez G (2014) A new troglobitic ideoroncid pseudoscorpion (Pseudoscorpiones: Ideoroncidae) from southern Africa. Journal of Arachnology 42, 105-110.
| Crossref | Google Scholar |
Harvey MS, Muchmore WB (1989) The systematics of the family Menthidae (Pseudoscorpionida). Invertebrate Taxonomy 3, 941-964.
| Crossref | Google Scholar |
Harvey MS, Muchmore WB (2013) The systematics of the pseudoscorpion family Ideoroncidae (Pseudoscorpiones: Neobisioidea) in the New World. Journal of Arachnology 41, 229-290.
| Crossref | Google Scholar |
Harvey MS, Šťáhlavský F (2010) A review of the pseudoscorpion genus Oreolpium (Pseudoscorpiones: Garypinidae), with remarks on the composition of the Garypinidae and on pseudoscorpions with bipolar distributions. Journal of Arachnology 38, 294-308.
| Crossref | Google Scholar |
Harvey MS, Wynne JJ (2014) Troglomorphic pseudoscorpions (Arachnida: Pseudoscorpiones) of northern Arizona, with the description of two new short-range endemic species. Journal of Arachnology 42, 205-219.
| Crossref | Google Scholar |
Harvey MS, Ratnaweera PB, Udagama PV, Wijesinghe MR (2012) A new species of the pseudoscorpion genus Megachernes (Pseudoscorpiones: Chernetidae) associated with a threatened Sri Lankan rainforest rodent, with a review of host associations of Megachernes. Journal of Natural History 46, 2519-2535.
| Crossref | Google Scholar |
Harvey MS, Lopes PC, Goldsmith GR, Halajian A, Hillyer MJ, Huey JA (2015) A novel symbiotic relationship between sociable weaver birds (Philetairus socius) and a new cheliferid pseudoscorpion (Pseudoscorpiones: Cheliferidae) in southern Africa. Invertebrate Systematics 29, 444-456.
| Crossref | Google Scholar |
Harvey MS, Abrams KM, Beavis AS, Hillyer MJ, Huey JA (2016a) Pseudoscorpions of the family Feaellidae (Pseudoscorpiones: Feaelloidea) from the Pilbara region of Western Australia show extreme short-range endemism. Invertebrate Systematics 30, 491-508.
| Crossref | Google Scholar |
Harvey MS, Huey JA, Hillyer MJ, McIntyre E, Giribet G (2016b) The first troglobitic species of Gymnobisiidae (Pseudoscorpiones, Neobisioidea), from Table Mountain (Western Cape Province, South Africa) and its phylogenetic position. Invertebrate Systematics 30, 75-85.
| Crossref | Google Scholar |
Harvey MS, Hillyer MJ, Carvajal JI, Huey JA (2020) Supralittoral pseudoscorpions of the genus Garypus (Pseudoscorpiones: Garypidae) from the Indo-West Pacific region, with a review of the subfamily classification of Garypidae. Invertebrate Systematics 34, 34-87.
| Crossref | Google Scholar |
Henderickx H, Vets V (2002) A new Larca (Arachnida: Pseudoscorpiones: Larcidae) from Crete. Bulletin of the British Arachnological Society 12, 280-282.
| Google Scholar |
Hernández-Corral J, Zaragoza JA, Micó E (2018) New species of Pseudoscorpiones (Arachnida) from tree hollows in a Mediterranean oak forest in Spain. Zootaxa 4497, 201-225.
| Crossref | Google Scholar |
Heurtault J (1970) Pseudoscorpions du Tibesti (Tchad). I. Olpiidae. Bulletin du Muséum National d’Histoire Naturelle, Paris, 2e Série 41(5), 1164-1174 [In French].
| Google Scholar |
Heurtault J (1982) Le développement postembryonnaire chez deux espèces nouvelles de Pseudoscorpions Olpiinae du Venezuela. Revue de Nordest Biologie 3, 57-85 [In French].
| Google Scholar |
Hlebec D, Podnar M, Kučinić M, Harms D (2023) Molecular analyses of pseudoscorpions in a subterranean biodiversity hotspot reveal cryptic diversity and microendemism. Scientific Reports 13, 430.
| Crossref | Google Scholar |
Hoff CC (1950) Pseudoescorpionidos nuevos o poco conocidos de la Argentina (Arachnida, Pseudoscorpionida). Arthropoda, Buenos Aires 1, 225-237 [In Spanish].
| Google Scholar |
Hoff CC (1952) Two new species of pseudoscorpions from Illinois. Transactions of the Illinois State Academy of Sciences 45, 188-195.
| Google Scholar |
Hoff CC (1956) Diplosphyronid pseudoscorpions from New Mexico. American Museum Novitates 1780, 1-49.
| Google Scholar |
Hoff CC (1961) Pseudoscorpions from Colorado. Bulletin of the American Museum of Natural History 122, 409-464.
| Google Scholar |
Hoff CC (1964) The pseudoscorpions of Jamaica. Part 3. The suborder Diplosphyronida. Bulletin of the Institute of Jamaica, Science Series 10(3), 1-47.
| Google Scholar |
Johnson J, Loria SF, Joseph MM, Harms D (2022) Biogeographical and diversification analyses of Indian pseudoscorpions reveal the Western Ghats as museums of ancient biodiversity. Molecular Phylogenetics and Evolution 175, 107495.
| Crossref | Google Scholar |
Judson MLI (1997) Catalogue of the pseudoscorpion types (Arachnida: Chelonethi) in the Natural History Museum, London. Occasional Papers on Systematic Entomology 11, 1-54.
| Google Scholar |
Judson MLI (2005) Baltic amber fossil of Garypinus electri Beier provides first evidence of phoresy in the pseudoscorpion family Garypinidae (Arachnida: Chelonethi). In ‘European Arachnology 2003 – Proceedings of the 21st European Colloquium of Arachnology’, 4–9 August 2003, Saint Petersburg, Russian Federation. (Eds DV Logunov, D Penney) pp. 127–131. (KMK Scientific Press Ltd: Moscow, Russian Federation)
Judson MLI (2007) A new and endangered species of the pseudoscorpion genus Lagynochthonius from a cave in Vietnam, with notes on chelal morphology and the composition of the Tyrannochthoniini (Arachnida, Chelonethi, Chthoniidae). Zootaxa 1627, 53-68.
| Crossref | Google Scholar |
Judson MLI, Legg G (1996) Discovery of the pseudoscorpion Larca lata (Garypoidea, Larcidae) in Britain. Bulletin of the British Arachnological Society 10, 205-210.
| Google Scholar |
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30, 772-780.
| Crossref | Google Scholar |
Katoh K, Misawa K, Kuma K, Mityata T (2002) MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research 30, 3059-3066.
| Crossref | Google Scholar |
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A (2012) Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28(12), 1647-1649.
| Crossref | Google Scholar |
Lee VF (1979) The maritime pseudoscorpions of Baja California, México (Arachnida: Pseudoscorpionida). Occasional Papers of the California Academy of Sciences 131, 1-38.
| Google Scholar |
Mahnert V (1974) Einige Pseudoskorpione aus Israel. Revue Suisse de Zoologie 81, 377-386 [In German].
| Crossref | Google Scholar |
Mahnert V (1976) Zwei neue Pseudoskorpion-Arten (Arachnida) aus griechischen Höhlen (Über griechische Pseudoskorpione VII). Berichte des Naturwissenschaftlich-Medizinischen Vereins in Innsbruck 63, 177-183 [In German].
| Google Scholar |
Mahnert V (1981a) Sigles trichobothriaux chez les pseudoscorpions (Arachnida). Memorie, Atti della Società Toscana di Scienze Naturali Residente in Pisa B 88(supplemento), 185-192 [In French].
| Google Scholar |
Mahnert V (1981b) Die Pseudoskorpione (Arachnida) Kenyas. I. Neobisiidae und Ideoroncidae. Revue Suisse de Zoologie 88, 535-559 [In German].
| Crossref | Google Scholar |
Mahnert V (1982a) Die Pseudoskorpione (Arachnida) Kenyas II. Feaellidae; Cheiridiidae. Revue Suisse de Zoologie 89, 115-134 [In German].
| Google Scholar |
Mahnert V (1982b) Die Pseudoskorpion (Arachnida) Kenyas, IV. Garypidae. Annales Historico-Naturales Musei Nationalis Hungarici 74, 307-329 [In German].
| Google Scholar |
Mahnert V (1984a) Forschungen an der Somalilandküste. Am Strand und auf den Dünen bei Sar Uanle. 36. Pseudoscorpiones (Arachnida). Monitore Zoologico Italiano, n.s. 19(supplemento), 43-66 [In German].
| Google Scholar |
Mahnert V (1984b) Beitrag zu einer besseren Kenntnis der Ideoroncidae (Arachnida: Pseudoscorpiones), mit Beschreibung von sechs neuen Arten. Revue Suisse de Zoologie 91, 651-686 [In German].
| Google Scholar |
Mahnert V (1985) Weitere Pseudoskorpione (Arachnida) aus dem zentralen Amazonasgebiet (Brasilien). Amazoniana 9, 215-241 [In German].
| Google Scholar |
Mahnert V (1988) Zwei neue Garypininae-Arten (Pseudoscorpiones: Olpiidae) aus Afrika mit Bemerkungen zu den Gattungen Serianus Chamberlin und Paraserianus Beier. Stuttgarter Beiträge zur Naturkunde (A) 420, 1-11 [In German].
| Google Scholar |
Mahnert V (1991) Pseudoscorpions (Arachnida) from the Arabian Peninsula. Fauna of Saudi Arabia 12, 171-199.
| Google Scholar |
Mahnert V (1993) Pseudoskorpione (Arachnida: Pseudoscorpiones) von Inseln des Mittelmeers und des Atlantiks (Balearen, Kanarische Inseln, Madeira, Ascension), mit vorwiegend subterraner Lebensweise. Revue Suisse de Zoologie 100, 971-992 [In German].
| Crossref | Google Scholar |
Mahnert V (2007) Pseudoscorpions (Arachnida: Pseudoscorpiones) of the Socotra Archipelago, Yemen. Fauna of Arabia 23, 271-307.
| Google Scholar |
Mahnert V (2014) Pseudoscorpions (Arachnida: Pseudoscorpiones) from the Galapagos Islands (Ecuador). Revue Suisse de Zoologie 121, 135-210.
| Google Scholar |
Mahnert V, Schuster R (1981) Pachyolpium atlanticum n. sp., ein Pseudoskorpion aus der Gezeitenzone der Bermudas – Morphologie und Ökologie (Pseudoscorpiones: Olpiidae). Revue Suisse de Zoologie 88, 265-273 [In German].
| Crossref | Google Scholar |
Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, von Haeseler A, Lanfear R (2020) IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Molecular Biology and Evolution 37, 1530-1534.
| Crossref | Google Scholar |
Morikawa K (1953a) [On a new pseudoscorpion of family Olpiidae.] Zoological Magazine, Tokyo 62, 327-328 [In Japanese].
| Google Scholar |
Morikawa K (1953b) Notes on Japanese Pseudoscorpiones. II. Family Cheiridiidae, Atemnidae and Chernetidae. Memoirs of Ehime University (2B) 1, 345-354.
| Google Scholar |
Morikawa K (1960) Systematic studies of Japanese pseudoscorpions. Memoirs of Ehime University (2B) 4, 85-172.
| Google Scholar |
Muchmore WB (1968) A new species of the pseudoscorpion genus Serianus (Arachnida, Chelonethida, Olpiidae) from North Carolina. Entomological News 79, 145-150.
| Google Scholar |
Muchmore WB (1974) Pseudoscorpions from Florida. 1. The genus Aldabrinus (Pseudoscorpionida: Olpiidae). The Florida Entomologist 57, 1-7.
| Crossref | Google Scholar |
Muchmore WB (1976) Pseudoscorpions from Florida and the Caribbean area. 5. Americhernes, a new genus based upon Chelifer oblongus Say (Chernetidae). The Florida Entomologist 59, 151-163.
| Crossref | Google Scholar |
Muchmore WB (1979) Pseudoscorpions from Florida and the Caribbean area. 7. Floridian diplosphyronids. The Florida Entomologist 62, 193-213.
| Crossref | Google Scholar |
Muchmore WB (1980) Three new olpiid pseudoscorpions from California (Pseudoscorpionida, Olpiidae). Pan-Pacific Entomologist 56, 161-169.
| Google Scholar |
Muchmore WB (1981a) Cavernicolous species of Larca, Archeolarca and Pseudogarypus with notes on the genera (Pseudoscorpionida, Garypidae and Pseudogarypidae). Journal of Arachnology 9, 47-60.
| Google Scholar |
Muchmore WB (1981b) The identity of Olpium minutum Banks (Pseudoscorpionida, Olpiidae). Journal of Arachnology 9, 337-338.
| Google Scholar |
Muchmore WB (1982) The genus Anagarypus (Pseudoscorpionida: Garypidae). Pacific Insects 24, 159-163.
| Google Scholar |
Muchmore WB (1992) Cavernicolous pseudoscorpions from Texas and New Mexico (Arachnida: Pseudoscorpionida). Texas Memorial Museum, Speleological Monographs 3, 127-153.
| Google Scholar |
Muchmore WB, Alteri CH (1974) The genus Parachernes (Pseudoscorpionida, Chernetidae) in the United States, with descriptions of new species. Transactions of the American Entomological Society 99, 477-506.
| Google Scholar |
Murienne J, Harvey MS, Giribet G (2008) First molecular phylogeny of the major clades of Pseudoscorpiones (Arthropoda: Chelicerata. Molecular Phylogenetics and Evolution 49, 170-184.
| Crossref | Google Scholar |
Murthy VA, Ananthakrishnan TN (1977) Indian Chelonethi. Oriental Insects Monograph 4, 1-210.
| Google Scholar |
Nassirkhani M, Doustaresharaf MM (2019) Description of a new pseudoscorpion species of the genus Amblyolpium Simon (Pseudoscorpiones: Garypinidae) from north-western Iran. Zoology in the Middle East 65, 268-273.
| Crossref | Google Scholar |
Nassirkhani M, Shoushtari RV, Abadi MR (2016) A new pseudoscorpion species in Amblyolpium (Pseudoscorpiones: Garypinidae) from a house in Kermanshah Province, Iran. Arachnologische Mitteilungen 52, 1-3.
| Crossref | Google Scholar |
Neethling JA, Haddad CR (2016) A systematic revision of the South African pseudoscorpions of the family Geogarypidae (Arachnida: Pseudoscorpiones). Indago 32, 1-80.
| Google Scholar |
Nelson SO Jr (1982) The external morphology and life history of the pseudoscorpion Microbisium confusum Hoff. Journal of Arachnology 10, 261-274.
| Google Scholar |
Novák J (2013) First records of Larca lata (Hansen, 1884) and Neobisium biharicum Beier, 1939 in Hungary. Opuscula Zoologica, Budapest 44, 161-166.
| Google Scholar |
Novák J, Harvey MS, Szabó M, Hammel JU, Harms D, Kotthoff U, Hörweg C, Brazidec M, Ösi A (2023) A new Mesozoic record of the pseudoscorpion family Garypinidae from Upper Cretaceous (Santonian) Ajkaite amber, Ajka area, Hungary. Cretaceous Research, 105709. [Published online 9 September 2023] 10.1016/j.cretres.2023.105709
Novák J, Harvey MS (2018) New species and records of the pseudoscorpion genus Geogarypus (Pseudoscorpiones: Geogarypidae) from India, Sri Lanka and New Guinea. Zootaxa 4394, 417-427.
| Crossref | Google Scholar |
Piedra-Jiménez DF, Alvarez-Padilla F, González Santillán E (2019) Two new species of pseudoscorpions (Arachnida: Pseudoscorpiones) from a Mexican oak forest near Pico de Orizaba National Park. The Journal of Arachnology 47, 95-109.
| Crossref | Google Scholar |
Redikorzev V (1938) Les pseudoscorpions de l’Indochine française recueillis par M. C. Dawydoff. Mémoires du Muséum National d’Histoire Naturelle, Paris 10, 69-116 [In French].
| Google Scholar |
Sakayori H (1989) Postembryonic development of a neotenic pseudoscorpion, Microbisium pygmaeum (Ellingsen, 1907). Acta Arachnologica 38, 55-62.
| Crossref | Google Scholar |
Schawaller W (1994) Pseudoskorpione aus Thailand (Arachnida: Pseudoscorpiones). Revue Suisse de Zoologie 101, 725-759 [In German].
| Crossref | Google Scholar |
Simon E (1898) Étude sur les Arachnides de la région des Maures (Var). Feuille des Jeunes Naturalistes (3)(29), 2-4 [In French].
| Google Scholar |
Stanczak N, Harvey MS, Harms D, Hammel J, Kotthoff U, Loria SF. () Discovery of an extinct Eocene pseudoscorpion genus (Garypinoidea: Garypinidae) supports extinction and range contraction in Europe. PeerJ , in press.
| Google Scholar |
Tömösváry Ö (1884) Adatok az álskorpiók ismeretéhez (Data ad cognitionem Pseudoscorpionum). Természetrajzi Füzetek 8, 16-27 [In Hungarian].
| Google Scholar |
Tooren D (2002a) Pseudoscorpions of the genera Pachyolpium, Novohorus and Amblyolpium (Pseudoscorpiones: Olpiidae) from St Eustatius (Statia), St Martin (Sint Maarten) and Anguilla (Lesser Antilles, Leeward group). Zoologische Mededelingen 76, 451-472.
| Google Scholar |
Tooren D (2002b) Pseudoscorpions (Pseudoscorpiones: Olpiidae) of the genus Apolpium from Venezuela, and the genera Pachyolpium, Leptolpium gen. nov. and Serianus from Curaçao, Aruba and Bonaire. Zoologische Mededelingen 76, 141-192.
| Google Scholar |
Trifinopoulos J, Nguyen L-T, von Haeseler A, Minh BQ (2016) W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Research 44(W1), W232-W235.
| Crossref | Google Scholar |
Tullgren A (1907) Zur Kenntnis aussereuropäischer Chelonethiden des Naturhistorischen Museums in Hamburg. Mitteilungen aus dem Naturhistorischen Museum in Hamburg 24, 21-75 [In German].
| Google Scholar |
Vachon M (1964) Sur l'établissement de formules précisant l’ordre d’apparition des trichobothries au cours du développement post-embryonnaire chez les Pseudoscorpions (Arachnides). Comptes Rendus Hebdomadaires des Séances de l’Académie des Sciences, Paris 258, 4839-4842 [In French].
| Google Scholar |
Vitali-di Castri V (1962) La familia Cheiridiidae (Pseudoscorpionida) en Chile. Investigaciones Zoológicas Chilenas 8, 119-142 [In Spanish].
| Google Scholar |
Vitali-di Castri V (1965) Cheiridium danconai n. sp. (Pseudoscorpionida) con consideraciones sobre su desarrollo postembrionario. Investigaciones Zoológicas Chilenas 12, 67-92 [In Spanish].
| Google Scholar |
With CJ (1905) X.—On Chclonethi, chiefly from the Australian region, in the collection of the British Museum, with observations on the “coxal sac” and on some cases of abnormal segmentation. Annals and Magazine of Natural History 15(85), 94-143.
| Crossref | Google Scholar |
With CJ (1906) The Danish expedition to Siam 1899–1900. III. Chelonethi. An account of the Indian false-scorpions together with studies on the anatomy and classification of the order. Oversigt over det Konigelige Danske Videnskabernes Selskabs Forhandlinger (7) 3, 1-214.
| Google Scholar |
With CJ (1907) On some New Species of Cheliferidæ, Hans., and Garypidæ, Hans., in the British Museum. Journal of the Linnean Society of London, Zoology 30, 49-85.
| Crossref | Google Scholar |
Zaragoza JA (2005) Two new cave-dwelling Larca species from the south-east of Spain (Arachnida, Pseudoscorpiones, Larcidae). Revue Suisse de Zoologie 112, 195-213.
| Crossref | Google Scholar |