Phylogenomics and biogeography of leptonetid spiders (Araneae : Leptonetidae)
Joel Ledford A G , Shahan Derkarabetian
A G , Shahan Derkarabetian  B , Carles Ribera C , James Starrett D , Jason E. Bond D , Charles Griswold E and Marshal Hedin F
B , Carles Ribera C , James Starrett D , Jason E. Bond D , Charles Griswold E and Marshal Hedin F
A Department of Plant Biology, University of California—Davis, Davis, CA 95616-5270, USA.
B Department of Organismic and Evolutionary Biology, Museum of Comparative Zoology, Harvard University, Cambridge, MA 02138, USA.
C Departament de Biologia Evolutiva, Ecologia i Ciències Ambientals, Universitat de Barcelona, Barcelona, Spain.
D Department of Entomology and Nematology, University of California—Davis, Davis, CA 95616-5270, USA.
E Department of Entomology, California Academy of Sciences, San Francisco, CA 94118, USA.
F Department of Biology, San Diego State University, San Diego, CA 92182-4614, USA.
G Corresponding author. Email: jmledford@ucdavis.edu
Invertebrate Systematics 35(3) 332-349 https://doi.org/10.1071/IS20065
Submitted: 26 August 2020 Accepted: 27 October 2020 Published: 24 March 2021
Journal Compilation © CSIRO 2021 Open Access CC BY-NC-ND
Abstract
Leptonetidae are rarely encountered spiders, usually associated with caves and mesic habitats, and are disjunctly distributed across the Holarctic. Data from ultraconserved elements (UCEs) were used in concatenated and coalescent-based analyses to estimate the phylogenetic history of the family. Our taxon sample included close outgroups, and 90% of described leptonetid genera, with denser sampling in North America and Mediterranean Europe. Two data matrices were assembled and analysed; the first ‘relaxed’ matrix includes the maximum number of loci and the second ‘strict’ matrix is limited to the same set of core orthologs but with flanking introns mostly removed. A molecular dating analysis incorporating fossil and geological calibration points was used to estimate divergence times, and dispersal–extinction–cladogenesis analysis (DEC) was used to infer ancestral distributions. Analysis of both data matrices using maximum likelihood and coalescent-based methods supports the monophyly of Archoleptonetinae and Leptonetinae. However, relationships among Archoleptonetinae, Leptonetinae, and Austrochiloidea are poorly supported and remain unresolved. Archoleptonetinae is elevated to family rank Archoleptonetidae (new rank) and Leptonetidae (new status) is restricted to include only members of the subfamily Leptonetinae; a taxonomic review with morphological diagnoses is provided for both families. Four well supported lineages within Leptonetidae (new status) are recovered: (1) the Calileptoneta group, (2) the Leptoneta group, (3) the Paraleptoneta group, and (4) the Protoleptoneta group. Most genera within Leptonetidae are monophyletic, although Barusia, Cataleptoneta, and Leptoneta include misplaced species and require taxonomic revision. The origin of Archoleptonetidae (new rank), Leptonetidae, and the four main lineages within Leptonetidae date to the Cretaceous. DEC analysis infers the Leptoneta and Paraleptoneta groups to have ancestral distributions restricted to Mediterranean Europe, whereas the Calileptoneta and Protoleptoneta groups include genera with ancestral distributions spanning eastern and western North America, Mediterranean Europe, and east Asia. Based on a combination of biology, estimated divergence times, and inferred ancestral distributions we hypothesise that Leptonetidae was once widespread across the Holarctic and their present distributions are largely the result of vicariance. Given the wide disjunctions between taxa, we broadly interpret the family as a Holarctic relict fauna and hypothesise that they were once part of the Boreotropical forest ecosystem.
Introduction
Biology of Leptonetidae
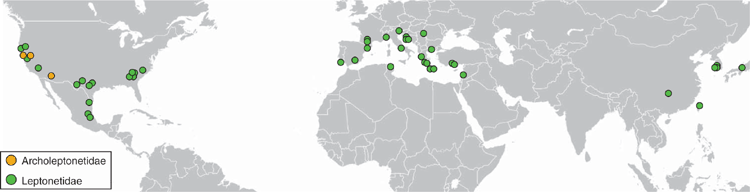
Leptonetids are a lineage of small, rarely encountered spiders that live in moist habitats such as leaf litter, under rocks, and especially in caves. The family includes 21 genera and 355 species placed into two subfamilies, Archoleptonetinae and Leptonetinae (World Spider Catalog, ver. 19.5, see http://wsc.nmbe.ch, accessed 19 August 2020). The archoleptonetines (Fig. 1A) include eight species in two genera and are known from the western USA, southern Mexico, Guatemala, and Panama. Leptonetines (Fig. 1B) are more diverse (21 genera, 355 species) and have a Holarctic distribution with centres of diversity in North America, Mediterranean Europe, and east Asia. Although the archoleptonetines have few features that make them readily diagnosable by non-specialists, all leptonetines share a unique eye arrangement where the posterior median eyes are displaced from the main eye group (Ledford and Griswold 2010, fig. 24, 27, 28).
Among spiders, leptonetids are best known for their association with caves. Over 50% of described species are known only from caves and many species show a range of troglomorphic morphologies including eye reduction, depigmentation, and appendage elongation (Mammola and Isaia 2017). Most species are small (2–5 mm) and reside in delicate sheet webs from which they hang (Fig. 1C). Leptonetids are microhabitat specialists, preferring environments where moisture, temperature and humidity remain stable. Ideal habitat includes breakdown debris in caves and layered rock piles in heavily shaded areas. Observations of reproductive biology have been reported (Cokendolpher 2004; Ledford 2004; Ledford and Griswold 2010) but most aspects of their life history are unknown.
Given their habitat preferences, most species have distributions that are highly localised. Sympatry is rare and known only in a few surface-dwelling populations (Ledford 2004). Even in localities where leptonetids are known to occur, they are rarely encountered and in some regions are recognised as threatened species (US Fish and Wildlife Service 2020). Although gaps in distributional range may be partly explained by inadequate sampling, we propose that the combination of specific habitat preferences and the repeated pattern of narrow endemism for most species worldwide supports a hypothesis of dispersal-limitation for the family. The biological characteristics of limited dispersal ability and high microhabitat preference often lead to biogeographic histories that are dominated by vicariance, with sometimes rare dispersal events, as seen in many other arachnid lineages (e.g. Harrison et al. 2016; Hedin and McCormack 2017; Baker et al. 2020).
Taxonomic history
Most research on leptonetids has focused on improving understanding of α-level diversity. The western European fauna is arguably the best known due to its long history of study (Simon 1872) and the detailed descriptive efforts of Brignoli (1967a, 1967b, 1968, 1971, 1974a, 1974c, 1978, 1979a, 1979b, 1979c), Fage (1913, 1931, 1943), Kratochvíl (1935, 1938, 1978), Machado and Ribera (1986), Ribera (1978, 1988), and Ribera and Lopez (1982). Although most of these works are regionally focused, Fage (1913) treated the European fauna comprehensively and Brignoli (1970, 1979d) provided a global interpretation of leptonetid relationships and biogeography. The North American fauna has a history of monographic study starting with Gertsch (1971, 1974) who described the majority of species and Platnick (1986) who delineated three North American genera. Brignoli (1974b, 1977, 1979e) also worked on the North American fauna, providing a global perspective that sharply contrasted with Gertsch (1971, 1974). Gertsch (1971, 1974) argued that all of the North American leptonetids should be placed in the European genus Leptoneta but, unlike Brignoli, had limited understanding of other European leptonetid genera. By contrast, based on his experience working with European leptonetids, Brignoli hypothesised that the North American fauna likely included multiple genera (Brignoli 1972, 1977). Two studies (Ledford and Griswold 2010; Ledford et al. 2011) assessed the phylogeny of North American leptonetids using nucleotide sequence data and better defined the genera. During the past 10 years, the most striking advances in leptonetid taxonomy have been in Asia where over 100 new species have been described from China and South Korea (Chen et al. 2010; Lin and Li 2010; Wang and Li 2010, 2011; Seo 2015a, 2015b, 2016a, 2016b; Guo et al. 2016; He et al. 2019; Xu et al. 2019). Although most of these studies do not include phylogenetic or biogeographic analyses (but see Wang et al. 2017), they have greatly improved our understanding of the Asian fauna.
Leptonetidae have a long history of controversy surrounding their relationships to other spiders. Fage (1913) was the first to recognise that leptonetids share convergent morphology with other subterranean-adapted spiders, and Brignoli (1979d) suggested that the hypothesised relationships of leptonetids to other spiders were largely based on these convergent features. One example is that leptonetines, Telemidae, and some Ochyroceratidae have independently evolved a peculiar arrangement of the posterior spinnerets where there is a single row of aciniform gland spigots used to build sheet webs (Ledford and Griswold 2010, fig. 65–72). For this reason, many workers have placed leptonetids among the Synspermiata (formerly Haplogynae), usually as sister to Telemidae, despite the fact that much of their morphology conflicts with this position. The discovery of a cribellum in Archoleptoneta Gertsch, 1974 further compounded these conflicts and, given the traditional placement of leptonetids within Synspermiata, had implications for the interpretation of spinning organs as a whole (Ledford and Griswold 2010). Brignoli (1979d) was the first to propose that leptonetids might be more closely related to entelegynes based on the absence of a cheliceral lamina and the presence of expandable male genitalia. Ledford and Griswold (2010) reviewed leptonetid morphology and were the first to suggest the possibility of leptonetid paraphyly, proposing that at least the archoleptonetines are not part of Synspermiata, but instead are more closely related to entelegynes.
Agnarsson et al. (2013) used a supertree approach and was the first to suggest a relationship between Archoleptoneta and austrochiloids, but thought that the result was caused by long-branch attraction. Using transcriptomes, Garrison et al. (2016) recovered leptonetids outside of Synspermiata, placing Calileptoneta Platnick, 1986 as sister to Entelegynae. Several studies built upon the foundation of Garrison et al. (2016), including Shao and Li (2018) who recovered leptonetines as sister to entelegynes but did not include austrochiloids as part of their study. Fernández et al. (2018) added transcriptomes for both leptonetid subfamilies (Archoleptoneta and Calileptoneta) and in their preferred topology recovered a monophyletic Leptonetidae sister to austrochiloids (Fig. 2). Based on a combination of multigene nucleotide data and morphology, Wheeler et al. (2017) recovered a polyphyletic Leptonetidae, but did support a relationship between Archoleptoneta and austrochiloids. As part of a study on basal araneomorphs, Ramírez et al. (2021) included better representation across Leptonetidae and used ultraconserved elements (UCEs) to hypothesise a sister-group relationship between Leptonetinae and austrochiloids, rendering Leptonetidae paraphyletic.

|
Systematics and biogeography of Leptonetidae
In this paper, we present a phylogenomic analysis of Leptonetidae including representatives from across the geographic range of the family. Both genera of archoleptonetines and 90% of described leptonetine genera are sampled. Where possible, multiple exemplars for each genus are used, including several from type localities. We include a broad sample of austrochiloids and use Telemidae as the outgroup. Because this study is an extension of a parallel study on basal araneomorphs (Ramírez et al. 2021), we do not include a broad sampling of entelegynes in order to assess the relationships of leptonetids to other spiders. Instead, we focus on testing the monophyly of leptonetid subfamilies and explore relationships among leptonetid genera. We use our results to review the current taxonomy of Leptonetidae and identify areas in need of taxonomic revision. As the first study to produce a global phylogeny of Leptonetidae, we also gain insight into the historical biogeography of the group. Using a combination of fossils and dates for continental events, we present a chronogram and assess the biogeography of the family using dispersal–vicariance analysis. Our results provide a robust phylogenetic framework for the family that can be used as a scaffold for forthcoming taxonomic works.
Methods
Taxon sampling
Representatives for 19 of the 21 described genera in Leptonetidae were sampled from localities worldwide (Fig. 3). Our sample does not include the genera Masirana Kishida, 1942 and Rhyssoleptoneta Tong & Li, 2007. We gathered original UCE data for 28 specimens, combined with data for 17 specimens taken from previous studies (Wood et al. 2018; Ramírez et al. 2021) (Table S1 of the Supplementary material). Depending on availability, multiple species within a genus were used. We included a broad sample of Austrochiloidea and used Usofila pacifica Banks, 1894 (Telemidae) as the outgroup.
DNA extraction
Most specimens were preserved for DNA studies (preserved in high percentage ethyl alcohol at –80°C), and genomic DNA was extracted from leg tissue using the Qiagen DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA, USA). For a handful of tissues preserved in 70–80% ethyl alcohol we used standard phenol chloroform extractions with 24-h incubation for lysis. Extraction type for each specimen is indicated in Table S1. Extractions were quantified using a Qubit Fluorometer (Life Technologies, Inc.) and quality was assessed by agarose gel electrophoresis. Between 11 and 500 ng of total DNA was used for UCE library preparation.
UCE data collection
UCE data were collected in multiple library preparation and sequencing experiments. Up to 500 ng of genomic DNA was used in sonication, using a Covaris M220 Focused-ultrasonicator. Library preparation followed methods previously used for arachnids, as in Starrett et al. (2017), Derkarabetian et al. (2018, 2019), and Hedin et al. (2018a, 2018b). Target enrichment was performed using the MYbaits Arachnida 1.1K kit (ver. 1, Arbor Biosciences; Faircloth 2017) following the Target Enrichment of Illumina Libraries protocol (ver. 1.5, see http://ultraconserved.org/#protocols). Libraries were sequenced with an Illumina HiSeq 2500 with 125 bp paired-end reads (Brigham Young University DNA Sequencing Center).
Matrix filtering and assembly
Raw demultiplexed reads were processed with the PHYLUCE pipeline (ver. 1.6, see https://phyluce.readthedocs.io/en/latest/; Faircloth 2016). Quality control and adaptor removal were conducted with the Illumiprocessor wrapper (B. C. Faircloth, see https://github.com/faircloth-lab/illumiprocessor). Assemblies were created with Velvet (ver. 1.2.10, see https://www.ebi.ac.uk/~zerbino/velvet/; Zerbino and Birney 2008) and Trinity (ver. 2.11.0, see https://github.com/trinityrnaseq/trinityrnaseq/wiki; Grabherr et al. 2011), both at default settings. Assemblies were combined for probe matching, retrieving assembly-specific UCEs and overall increasing the number of UCEs per sample relative to using only a single assembly method. Contigs were matched to probes using minimum coverage and minimum identity at liberal values of 65. UCE loci were aligned with MAFFT (ver. 7.471, see https://mafft.cbrc.jp/alignment/software/ Katoh and Standley 2013) at default settings and trimmed with Gblocks (ver. 0.91b, see http://molevol.cmima.csic.es/castresana/Gblocks/Gblocks_documentation.html; Castresana 2000; Talavera and Castresana 2007), with settings –b1 0.5 –b2 0.5 –b3 6 –b4 6 in the Phyluce pipeline.
We expected some paralogy at the low minimum coverage values used, but considered this as a tradeoff given the ancient divergences considered. In addition to internal checks for paralogy in Phyluce, we checked for paralogy by conducting RAxML analyses on all individual Phyluce alignments. We excluded individual loci that failed to recover the well supported clade Austrochiloidea. We did not exclude duplicate UCE loci, as found in Hedin et al. (2019) – these are distinct UCE alignments that match the same protein, but are expected to be well separated by long introns. Two data matrices were assembled for phylogenomic analyses: (1) 65% (36 of 55 terminals) occupancy matrix, exon + intron, no ‘paralogs’ matrix, and (2) the same matrix as above using very strict Gblocks settings (–b1 0.5 –b2 0.85 –b3 4 –b4 8) to further trim alignments. We visually checked to confirm that these trimmed alignments comprised mostly exon data. These two matrices were developed to test the sensitivity of results to different alignment parameters (e.g. see Portik and Wiens 2020), and are referred to as ‘relaxed’ and ‘strict’ throughout the paper.
Phylogenomic analyses
Partitioned concatenation and coalescent-based analyses were conducted. Maximum likelihood analysis of the concatenated datasets was performed using RAxML (ver. 8, see https://cme.h-its.org/exelixis/web/software/raxml/; Stamatakis 2014) with the data partitioned by locus, the GTR+Γ substitution model, and support estimated by 500 bootstrap pseudoreplicates. Maximum likelihood analysis of the concatenated datasets was also performed using IQ-TREE (ver. 1.7-beta9, see http://www.iqtree.org/; Nguyen et al. 2015; Minh et al. 2020a) in order to calculate gene (gCF) and site (sCF) concordance factors for the relaxed and strict data matrices (Minh et al. 2020b). These measures were used as alternative measures of support (Ane et al. 2006), especially for nodes where topology conflicted between analysis type. An SVDQuartets analysis (ver. 1.0, see https://www.asc.ohio-state.edu/kubatko.2/software/SVDquartets/; Chifman and Kubatko 2014, 2015) was conducted on both matrices using PAUP* (ver. 4.0a, see https://paup.phylosolutions.com/), implementing a multispecies coalescent tree model with exhaustive quartets sampling and 100 bootstrap replicates.
Molecular dating analysis
A molecular clock analysis was conducted in a Bayesian framework with the MCMCtree module in the PAML package (ver. 4.9i, see http://abacus.gene.ucl.ac.uk/software/paml.html; Yang 2007). For the clock analysis we used the relaxed matrix RAxML topology, and treated the concatenated data as unpartitioned. The outgroup was removed from the tree and dataset, and a maximum boundary of 240 Ma was applied to the root node, based on the upper boundary of the 95% highest posterior density credible interval of the Leptonetidae + Austrochiloidea node from Fernández et al. (2018). The analysis was run with a model that assumes independent rates among branches (Yang and Rannala 2006; Rannala and Yang 2007). Estimation of the parameters (shape and scale) of the gamma distribution for the substitution rate prior (μ) was done with Baseml based on four fossil and biogeographic-based calibration points treated as fixed (shape = 1, scale = 5). We used shape = 1 and scale = 4.5 for the gamma distributed prior for σ2, or the variability in substitution rate among branches. The HKY sequence model was used and the analysis was run with birth rate, death rate, and species sampling priors of 2, 2 and 0.1 respectively. Gamma priors for κ (the transition/transversion ratio) and α (shape parameter for among site rate variation) were left as default (Yang 2007). Calibrations (see below) were treated as soft boundaries (i.e. 0.025 probability date falls beyond boundary; Yang and Rannala 2006; Inoue et al. 2010). The first 40 000 iterations were discarded as burnin, followed by 40 000 iterations sampled with 100 iterations (4 million generations). The analysis was run twice to ensure MCMC convergence, with negligible differences in posterior date estimates and 95% highest posterior density credible intervals occurring between the two runs.
Our clock analysis was calibrated based on fossils in Burmese Amber (Wunderlich 2008, 2012) and two continental events in Mediterranean Europe (Lymberakis and Poulakakis 2010; Garcia-Castellanos and Villaseñor 2011). Leptonetinae was assigned a minimum age of 98.19 Ma based on the fossil Palaeoleptoneta calcar Wunderlich, 2008 from Burmese Amber. Burmese Amber has been radiometrically dated to 98.79 ± 0.6 Ma (Selden and Ren 2017). Although this fossil cannot be confidently placed among extant genera (Magalhaes et al. 2020), it appears to share the ocular arrangement of leptonetines and we interpret it as part of the Leptonetinae (Wunderlich 2012). The Messinian Salinity Crisis, MSC (5.96–5.33 Ma) refers to the large-scale desiccation of the Mediterranean basin leading to reconnection of Mediterranean islands and part of north Africa to mainland Europe (Garcia-Castellanos and Villaseñor 2011). We set the nodes uniting Sulcia violacea (Greece) + S. cretica (Crete) and Paraleptoneta spinimana (Italy) + S. bellesi (Tunisia) to a minimum age of 5.33 Ma because these areas were most recently connected during the MSC. Greece and Turkey were formerly connected in a contiguous landmass called Ägäis. The breakup of Ägäis resulted in the formation of Aegean Islands 12–9 Ma (Dermitzakis and Papanikolaou 1981; Papadopoulou et al. 2010; Lymberakis and Poulakakis 2010). The node uniting Cataleptoneta sengleti (Greece) + C. aesculpii (Turkey) was set to a minimum age of 12 Ma based on their present distributions. We acknowledge that using biogeographic events as calibrations for divergence dating to assess biogeographic history is potentially circular in its logic. However, given the scarcity of fossils for Leptonetidae, we feel that using these recent and localised events, and the benefit they provide for estimating the age of leptonetid lineages across the Holarctic, outweighs the negative aspects.
Biogeographic reconstructions
Biogeographic analysis was performed with the Reconstruct Ancestral State in Phylogenies package (ver. 4.2, see http://mnh.scu.edu.cn/soft/blog/RASP/; Yu et al. 2015) using the dispersal–extinction–cladogenesis model (DEC) (Ree and Smith 2008) implemented in the C++ version of Lagrange (S. A. Smith, see http://mnh.scu.edu.cn/soft/blog/RASP/). We use the APE package (Paradis et al. 2004) in R (ver. 4.0, R Foundation for Statistical Computing, Vienna, Austria, see https://www.R-project.org/) to convert the relaxed RAxML tree topology into a relative-rate scaled ultrametric tree (‘chronopl’ using an assigned lambda value of 0.1). Analysis settings allowed for two unit areas in ancestral distributions and equal probabilities of dispersal events between all areas. Terminal taxa were assigned to six distribution ranges: (A) eastern North America, (B) western North America, (C) South America, (D) Mediterranean Europe, (E) Australia or New Zealand, and (F) East Asia. Four of the assigned regions (eastern North America, western North America, East Asia, Mediterranean Europe) correspond to infraregions identified in meta-analyses of Holarctic biogeography (Sanmartín et al. 2001; Donoghue and Smith 2004).
Results
Data
Voucher data, input DNA values, assembled contig numbers, and UCE locus numbers are provided in Table S1. In total, 408 loci were included in the final matrices. The relaxed data matrix included 97 094 basepairs (mean locus length of 238) and 39 114 parsimony informative sites; the strict data matrix included 49 656 basepairs (mean locus length of 122) and 17 638 parsimony informative sites. Raw reads from our 30 original samples have been submitted to the SRA (PRJNA694694); aligned matrices and .TRE files are available at Dryad (doi:10.25338/B8SS5F).
Phylogenomic analyses
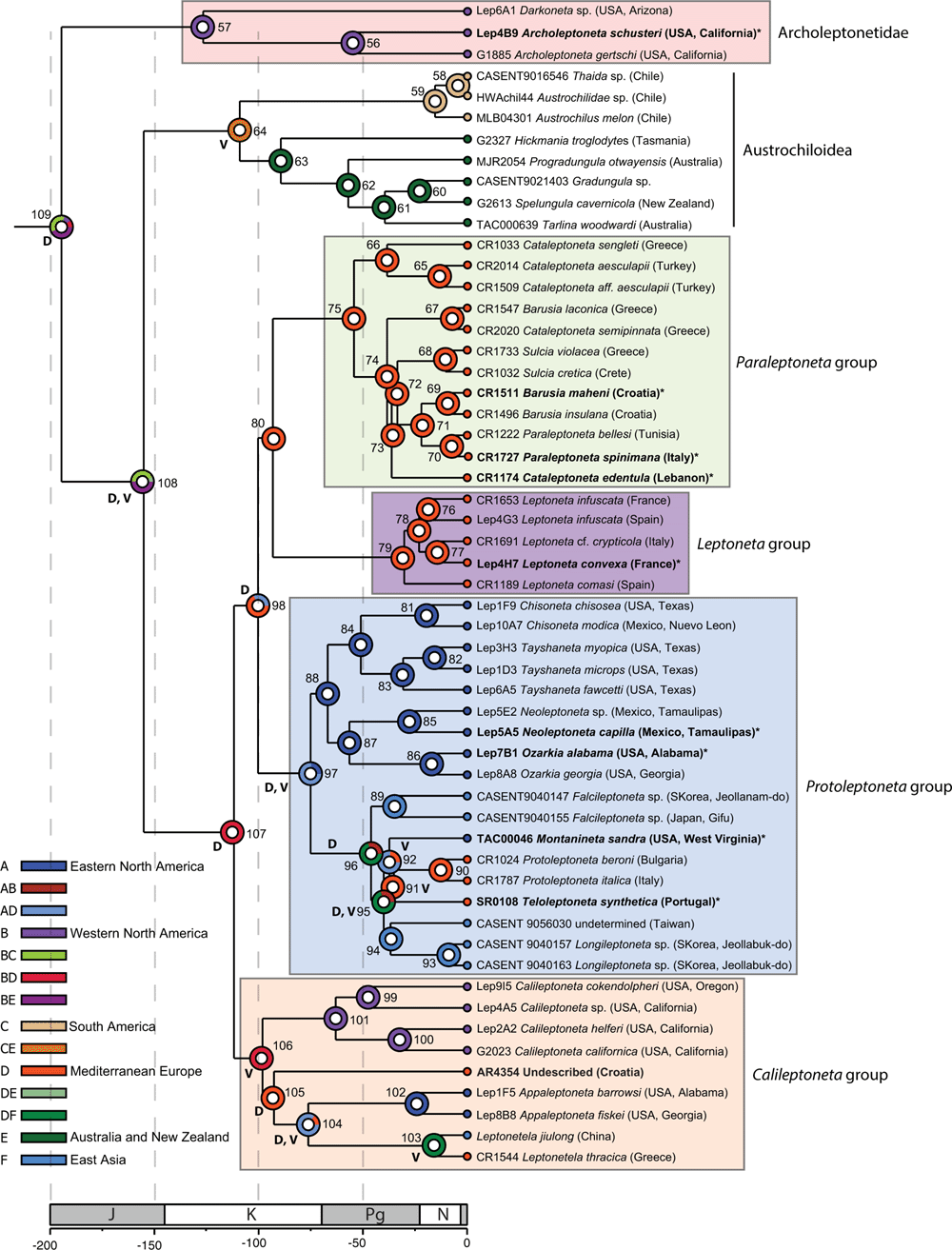
Maximum likelihood analysis using RAxML and IQ-TREE for both data matrices resulted in identical topologies with the exception of the RAxML strict matrix (Fig. 4 and S1–S3 of the Supplementary material). All ML analyses recovered a sister-group relationship between Leptonetinae and Austrochiloidea that is highly supported with a bootstrap value of 100%. However, genealogical and site concordance factor analysis for this node resulted in relatively low values of gCF 33% and sCF 40.5% for the relaxed data matrix (Fig. S2) and gCF 21.5% and sCF 40.3% for the strict data matrix (Fig. S3). We present these values as contrasting measures of support, noting that for both data matrices a majority of gene trees (over 60%) support alternative resolutions of this node despite the 100% bootstrap support.
|
SVDQuartets results are presented for both data matrices in Fig. S4–S7 of the Supplementary material. As suggested by the low gCF and sCF values, the SVD trees and associated 50% majority rule consensus trees show two conflicting resolutions for relationships within Leptonetidae. For both data matrices, SVD optimal trees recover leptonetid monophyly; archoleptonetines are sister to leptonetines and each subfamily is monophyletic. By contrast, the SVD 50% majority rule consensus trees show leptonetid paraphyly identical to our ML results, although support values are low. Despite the ambiguous resolution between the leptonetid subfamilies and Austrochiloidea, all three major lineages (Archoleptonetinae, Leptonetinae, Austrochiloidea) are strongly supported as monophyletic in all analyses. We therefore elevate the subfamily Archoleptonetinae to Archoleptonetidae (new rank) and restrict Leptonetidae to include only members of the subfamily Leptonetinae. This taxonomic structure is used throughout the remainder of the paper.
Relationships among the three species representing Archoleptonetidae are consistent and well supported across all analyses. We recover four main lineages within Leptonetidae which we refer to as the Paraleptoneta, Leptoneta, Protoleptoneta, and Calileptoneta groups. Although relationships among these groups vary by analysis, the Calileptoneta group is consistently sister to the Paraleptoneta + Leptoneta + Protoleptoneta groups. RAxML analyses of the relaxed data matrix results in a sister group relationship between the Paraleptoneta and Leptoneta groups (Fig. 4). However, RAxML analysis of our strict data matrix results in a sister group relationship between the Protoleptoneta and Leptoneta groups (Fig. S1). This node has low bootstrap support in both cases, with 91% support for the relaxed data matrix and 58% for the strict data matrix. Further, gCF and sCF values show that only 6–11% of the gene trees support a relationship between the Paraleptoneta and Leptoneta groups which we interpret as additional evidence of topological uncertainty (Fig. S2–S3). SVDQuartets analysis for both data matrices result in a sister group relationship between the Protoleptoneta and Leptoneta groups (Fig. S4–S7); however, 50% majority rule consensus trees show lower support for this node.
The European genera Leptoneta Simon, 1872, Leptonetela Kratochvíl, 1978, Paraleptoneta Fage, 1913, Protoleptoneta Deltshev, 1972, and Sulcia Kratochvíl, 1938 are all supported as monophyletic. However, the type species Cataleptoneta edentula Denis, 1955 does not group with C. aesculapii (Brignoli, 1968) or C. sengleti (Brignoli, 1974). The type species Barusia maheni (Kratochvíl & Miller, 1939) is sister to B. insulana (Kratochvíl & Miller, 1939) but B. laconica (Brignoli, 1974) is sister to Cataleptoneta semipinnata Wang & Li, 2010 and does not group with the types of Barusia or Cataleptoneta. Teloleptoneta Ribera, 1988 is weakly supported as sister to Protoleptoneta. One undetermined specimen from Croatia (AR4354) is sister to Appaleptoneta Platnick, 1986 + Leptonetela Kratochvíl, 1978. Leptonetela thracia Gasparo, 2005 is supported as sister to L. jiulong Lin & Li, 2010. All North American genera are monophyletic, including Appaleptoneta Platnick, 1986, Calileptoneta Platnick, 1986, Chisoneta Ledford & Griswold, 2011, Neoleptoneta Brignoli, 1972, Ozarkia Ledford & Griswold, 2011, and Tayshaneta Ledford & Griswold, 2011. Montanineta Ledford & Griswold, 2011 is weakly supported as sister to Protoleptoneta + Teloleptoneta. The Asian genera Falcileptoneta Komatsu, 1970 and Longileptoneta Seo, 2015 are both monophyletic. A single female specimen from Taiwan (CASENT9056030) is sister to Longileptoneta but is a juvenile and unable to be confidently assigned to this genus.
Molecular dating
Results from our dating analysis are presented in Fig. 5. We summarise posterior mean divergence times for major splits below and provide a list of key divergence times in Table S2 of the Supplementary material. The age of the root node is estimated as early Jurassic (189 Ma). Both Archoleptonetidae (112 Ma) and Leptonetidae (126 Ma) are of Cretaceous origin and the most recent common ancestor of Austrochiloidea + Leptonetidae dates to the mid-Jurassic (163 Ma). Most of the major divergences within Leptonetidae occur in the middle to late Cretaceous. The Paraleptoneta, Leptoneta, Protoleptoneta, and Calileptoneta groups arose 87–110 Ma and the majority of more recent divergence occurred in the mid-Paleogene.

|
Biogeography
Results for our biogeographic analysis are presented in Fig. 6. Multiple dispersal and vicariance events are inferred and, with few exceptions, their probabilities are 1.0 (Table S3 of the Supplementary material). No extinction events are reconstructed on the phylogeny. Archoleptonetidae is reconstructed to have an ancestral distribution in western North America. Leptonetidae is predicted to have originated in a region spanning western North America and Mediterranean Europe. The ancestor of the Paraleptoneta, Leptoneta, and Protoleptoneta groups most likely originated in Mediterranean Europe with a low probability of dispersal (0.52) to eastern North America. The Paraleptoneta and Leptoneta groups both have reconstructed origins in Mediterranean Europe. The Protoleptoneta group most likely had an ancestral distribution spanning eastern North America and Mediterranean Europe with a low probability of dispersal (0.54) to east Asia. The Calileptoneta group is reconstructed to have originated in a region spanning western North America and Mediterranean Europe.
Discussion
Systematics
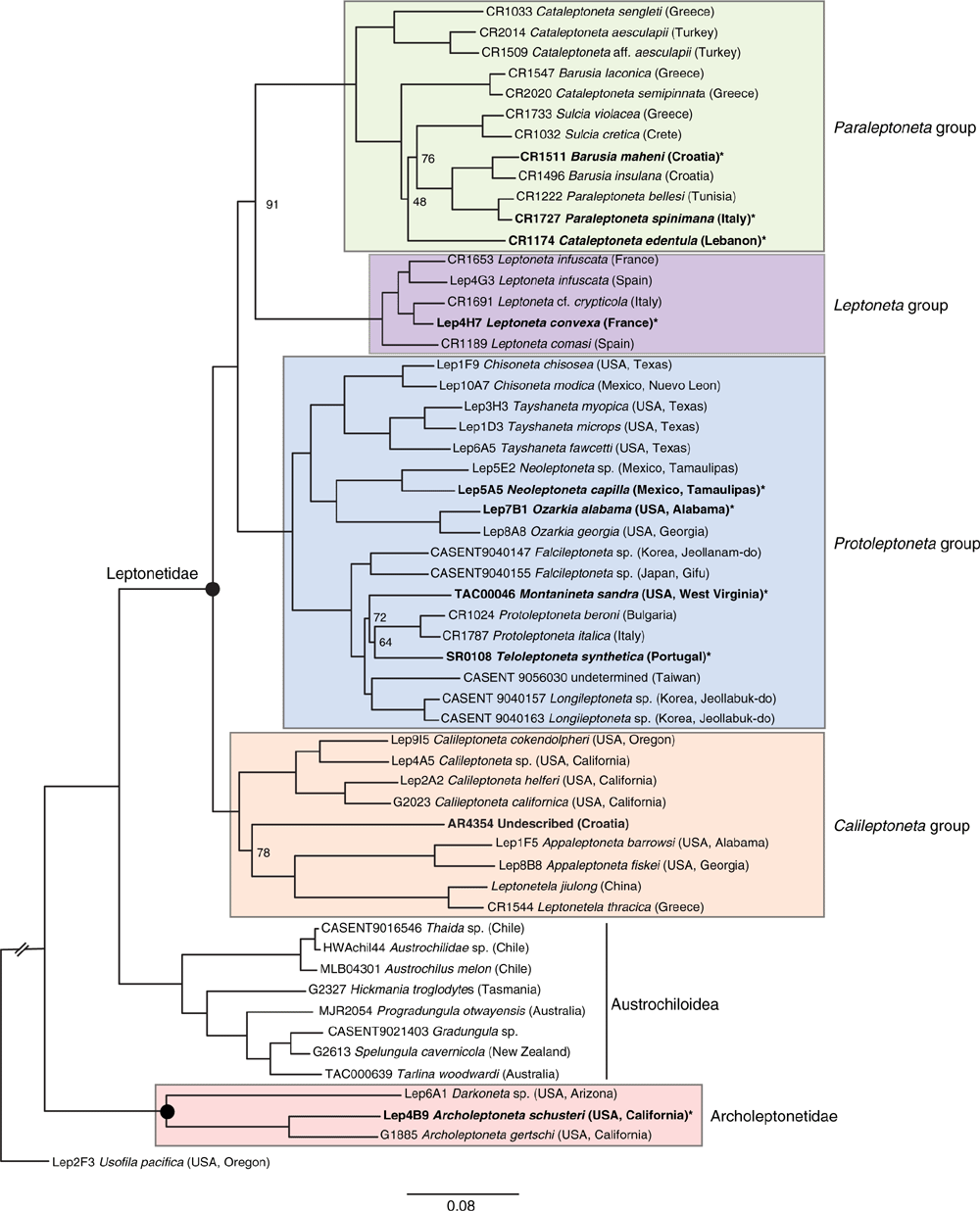
Recent studies of spider phylogeny have recovered conflicting results for the relationships of Archoleptonetidae and Leptonetidae, ranging from hypotheses of monophyly (Fernández et al. 2018), to paraphyly (Ramírez et al. 2021), and polyphyly (Wheeler et al. 2017). Given the conflict observed between these studies (Fig. 2), and the results of our analyses, it is not unreasonable to question the elevation of Archoleptonetidae. Further, what are the possible causes for the discordance between these results and how does our study address their limitations?
Among the primary strengths of our study is taxon sampling. We include specimens collected over the past 16 years, many species of which are monotypic, rare, or live in difficult to access habitats such as caves. Admittedly, our sampling is limited in Asia, but efforts to access additional material from this region have been challenging. By contrast, most recent studies have relatively sparse sampling of archoleptonetids and leptonetids. Fernández et al. (2018) includes two exemplars (Archoleptoneta and Calileptoneta) and provides insight into their relationships to other spiders, but lacks sufficient depth to test the monophyly of Archoleptonetidae and Leptonetidae. Wheeler et al. (2017) has broader representation with Archoleptoneta, Calileptoneta, Leptoneta, and Neoleptoneta but does not include the ecribellate Darkoneta in order to test the monophyly of Archoleptonetidae. Ramírez et al. (2021) includes denser sampling within Archoleptonetidae and Leptonetidae but is mostly focused on the evolution of tracheael systems in basal araneomorphs. Our study expands on Ramírez et al. (2021) by adding representatives of most leptonetid genera, thereby providing a robust test of the monophyly of Archoleptonetidae and Leptonetidae while gaining insight into relationships among the genera.
In addition to differences in sampling, each of these studies used a different type of data. Wheeler et al. (2017) used nucleotide data from six genes routinely used in spider systematics. Their results are perplexing as Leptonetidae is polyphyletic with Leptoneta sister to Austrochilidae and Calileptoneta + Neoleptoneta sister to Entelegynae (Fig. 3). Analytical instability was recognised as a problem by the authors, who also argued that a polyphyletic Leptonetidae was unlikely to be based on morphological synapomorphies. Given the problems of the Wheeler et al. (2017) analysis, the question becomes focused on whether or not Archoleptonetidae and Leptonetidae are sister groups.
Fernández et al. (2018) expanded on the results of Garrison et al. (2016), which used transcriptomes to infer relationships across the spider tree of life. Given that both transcriptomes and the UCEs in our study are exonic (following results of Hedin et al. 2019), the core data should be comparable and we expected similar results. As part of their study, Fernández et al. (2018) developed several data matrices, most of which recovered a monophyletic Leptonetidae (Archoleptonetinae + Leptonetinae). However, their ML analysis of a truncated, strict orthology matrix resulted in a paraphyletic Leptonetidae with Calileptoneta sister to austrochiloids. Our study also shows conflicting results for the resolution of this node with ML analyses supporting leptonetid paraphyly (Austrochiloidea + Leptonetidae) and some SVDQuartets results showing leptonetid monophyly (Archoleptonetidae + Leptonetidae), although weakly supported. Similar to Fernández et al. (2018), our strict matrix was partly intended as a sensitivity test by restricting our data to a core set of orthologs and removing flanking introns where alignment may be uncertain. Even with nearly 50% of the data removed, the strict matrix still does not consistently resolve the node as some SVDQuartets results recover leptonetid monophyly (Fig. S4, S6). Interestingly, despite the fact that our ML analyses show 100% bootstrap support for a sister group relationship between Austrochiloidea and Leptonetidae (Fig. 4, S1–S3), the relatively low gCF and sCF values suggest that most of the gene trees recover alternate resolutions for the node. In summary, our results and those of Fernández et al. (2018) show ambiguity in the resolution of this node as reflected in both analytical sensitivity and gCF and sCF values.
Although we are encouraged by the results in our study, we recognise that conflict in the relationships of Austrochiloidea, Archoleptonetidae and Leptonetidae persists. As a consequence, we argue that the node is best viewed as a trichotomy in need of further study. One key outcome of all recent studies is that Archoleptonetidae and Leptonetidae are not part of Synspermiata, a result predicted by morphology (Brignoli 1979d; Ledford and Griswold 2010) and corroborated by all phylogenomic analyses. Regardless of the eventual placement of Archoleptonetidae and Leptonetidae, the elevation of Archoleptonetidae is supported by their morphology (Ledford and Griswold 2010), facilitates their diagnosis, and serves a grouping function. We therefore view the elevation of Archoleptonetidae as both warranted and timely, especially as our understanding of spider phylogeny improves with increased adoption of phylogenomic methods. As newly defined, Archoleptonetidae is a small family consisting of two genera; however, many areas within their distributional range are undersampled and we expect that more species likely await discovery.
In each of our analyses, we recover four main lineages within Leptonetidae which we refer to as the Paraleptoneta, Leptoneta, Protoleptoneta, and Calileptoneta groups. The Paraleptoneta and Leptoneta groups include species limited to Mediterranean Europe, whereas both the Protoleptoneta and Calileptoneta groups include species distributed on multiple continents. The North American fauna is well represented in our study, and all genera have high statistical support corroborating the hypotheses of Ledford et al. (2011, 2012). Chisoneta, Neoleptoneta, Ozarkia, and Tayshaneta comprise a single lineage that is distributed from the south-western USA to Mexico and is sister to the remainder of the Protoleptoneta group. Representation of the Asian fauna is weaker, but Falcileptoneta and Longileptoneta are monophyletic and well supported as part of the Protoleptoneta group. Although our sample includes only a single Leptonetela species from China, L. jiulong Lin & Li, 2010 is sister to L. thracia Gasparo, 2005, supporting the synonymies of the genera Guineta, Qianleptoneta, and Sinoneta (Lin & Li, 2010). With the exception of Cataleptoneta, the European genera are supported as monophyletic. However, as discussed below, several species are likely misplaced. Although our study does not include Asian representatives of Leptoneta, several Leptoneta species are part of our analyses, including the type L. convexa Simon, 1872. Based on the features of their genitalic morphology (J. Ledford, pers. obs.) and the distribution of Leptoneta in western Mediterranean Europe, we predict that all of the currently described Asian Leptoneta are misplaced.
As the first study to assess leptonetid phylogeny using a sample of most genera, it is not surprising to discover taxonomic problems and learn that some genera need revision. In particular, the generic limits among Barusia, Cataleptoneta, and Paraleptoneta are unclear. The type species Cataleptoneta edentula (Denis 1955) does not group with C. semipinnata (Wang and Li 2010) and C. sengleti (Brignoli 1974a). Barusia has similar problems as B. laconica (Brignoli 1974a) does not group with the type species B. maheni. Taxonomic issues within Barusia and Cataleptoneta date to Kratochvíl (1978), who based his diagnoses on spination differences on the male palpal tibia and not details of palpal bulb morphology as had been done by previous workers. Barusia, Cataleptoneta, Paraleptoneta, and Sulcia are all characterised by complex spination patterns on the male palpal tibia (Le Peru 2011), making the morphological distinction between them unclear. Although recent studies on Cataleptoneta (Wang and Li 2010; Deltshev and Li 2013; Gavish-Regev et al. 2016; Demircan 2020) have contributed to our understanding of its diversity, they have not provided a broad perspective on the diagnosis, distribution, or relationships of the genus as a whole. Work in progress (C. Ribera, unpubl. data) further shows that the genetic diversity within Paraleptoneta is higher than expected despite a history of synonymy (Brignoli 1979c). Based on our results, we hypothesise that at least two additional genera remain undescribed; the first includes B. laconica and C. semipinnata and the second includes C. sengleti and C. aesculapii. However, we believe that taxonomic changes to Barusia and Cataleptoneta need the context of a more comprehensive revision and we defer any changes until the lineage can be more carefully studied.
Within Leptonetidae, there are three described monotypic genera: Montanineta Ledford & Griswold, 2010 from the eastern USA, Teloleptoneta Ribera, 1988 from Portugal, and Rhyssoleptoneta Tong & Li, 2007 from China. Until this study, none of these taxa have been included in a phylogenetic analysis and the justification used in their descriptions was based solely on the degree of difference in their genitalic morphology. Although our study does not include Rhyssoleptoneta, both Montanineta and Teloleptoneta group with Protoleptoneta (Deltshev 1972) although support within this clade is low. Unpublished single-gene analyses also show an ambiguous relationship between Protoleptoneta and Teloleptoneta (C. Ribera, unpubl. data). Close inspection of the genitalic morphology of Montanineta, Protoleptoneta, and Teloleptoneta show some similarities (J. Ledford, pers. obs.) and one solution is that all of these genera could be combined into a single genus. Although this would simplify the nomenclature of the group, it would not change our interpretation of the relationships in the lineage as a whole. Namely, given the wide geographic disjunction between these genera we view them as relictual; the closest relative of Montanineta, for example, lives on a different continent. One specimen from Croatia (AR4354) likely represents yet another monotypic genus given the geographic disjunction with its closest relatives (Appaleptoneta and Leptonetela). Lastly, although Rhyssoleptoneta was not included in our analysis examination of its genitalic morphology shows similarity in palpal structures to Appaleptoneta (J. Ledford, pers. obs.) and we predict that the genus may be part of the Calileptoneta group.
Biogeography
Given that our sampling of Archoleptonetidae is limited to representatives from California and Arizona, it is not surprising that the ancestral distribution for the group is reconstructed as western North America (B). As more species are described, we expect that the biogeography of Archoleptonetidae will become better understood.
Results of our molecular dating analysis estimate the origin of Leptonetidae at 126 Ma and its ancestral distribution is inferred to encompass an area spanning western North America and Mediterranean Europe (BD, node 107, Fig. 6). Although the Paraleptoneta and Leptoneta groups are reconstructed to have ancestral distributions restricted to Mediterranean Europe (D, node 80, Fig. 6), the Protoleptoneta and Calileptoneta groups are relatively older and have ancestral distributions that include combinations of areas including eastern and western North America, Mediterranean Europe, and east Asia (nodes 97 and 106, Fig. 6).
During the mid-Jurassic, Pangaea split into the northern and southern supercontinents Laurasia and Gondwana. The origin of Leptonetidae (mid-Cretaceous) coincides with a time when Laurasia was largely connected, although North America and Asia remained separated. Most of the major divergences within Leptonetidae occurred 100–70 Ma. Within this time, Laurasia split into two palaeocontinents: Euramerica (Europe + eastern North America) and Asiamerica (western North America + Asia). North America was divided by the Mid-Continental Seaway and western North America was connected to east Asia through Beringia. Europe was divided from Asia by the Turgai Strait and eastern North America remained connected to Europe through multiple, periodic land bridges across the Atlantic (reviewed in Sanmartín et al. 2001).
During the early Tertiary a continuous region of vegetation, the Boreotropical forest, was distributed across Laurasia. The boreotropics hypothesis (Wolfe 1975; Tiffney 1985) postulates that the biogeographical disjunctions often seen in plants between eastern North America–Europe and western North America–Asia resulted from the fragmentation of Laurasia and the recession of the Boreotropical forest. Based on the age estimates, ancestral distributions, and the biology of leptonetids, we hypothesise that they were once widespread across Laurasia. One possibility is that they were associated with the Boreotropical forest ecosystem and their phylogeny is largely the product of vicariance as Laurasia fragmented. This scenario not only aligns well with our results, but also explains the repeated pattern of narrow endemism for most leptonetid species worldwide. As Laurasia drifted apart, the climate became drier and leptonetids were restricted to areas where mesic conditions similar to those found in the Boreotropical forest persisted. This may also explain the close association of leptonetids with caves, which, due to their cool and moist environments, may have functioned as refugia. As such, we argue that leptonetids may be regarded as a largely relictual fauna, similar to Holarctic relict floras (Tiffney 1985; Lavin and Luckow 1993; Milne and Abbott 2002).
As a relictual fauna, we predict that extinction has played a significant role in shaping the present distribution of leptonetids. Although our DEC analyses recover no extinction, we view this result as improbable given the age and reconstructed ancestral distributions for most groups in our analyses (Fig. 5, 6). The underestimation of local extinction is also a known issue with DEC analysis (Ree and Smith 2008). Although dispersal is inferred in our DEC results, the probabilities associated with these events are low (Table S3). We also find the pattern of sympatry within Leptonetidae of particular interest; in most cases leptonetid genera do not have overlapping distributions, even among distantly related lineages. In areas where the distributions of genera are close, such as eastern Europe, our phylogeny reveals taxonomic problems. There are few cases of sympatry within genera although sampling error may be a contributing factor given the relative rarity of leptonetids in collections. We interpret these patterns as evidence for niche conservatism in the group through deep geologic time, a pattern predicted by their biology and supported by phylogeny.
Paraleptoneta and Leptoneta groups
The origin of the Paraleptoneta and Leptoneta groups dates to 105 Ma and coincides with the separation of Europe and Asia by the Turgai Strait. The Paraleptoneta group includes Barusia, Cataleptoneta, Paraleptoneta, and Sulcia, which are primarily distributed in the eastern Mediterranean but also have records in the Levant and Tunisia (Ribera and Lopez 1982; Gavish-Regev et al. 2016). At the time of the origin of this group, the eastern Mediterranean formed a continuous plate from Turkey to the Balkans, including the Aegean and Croatian Islands. The present geographical distribution of the Paraleptoneta group includes all of southern Europe (except for the Iberian Peninsula), where Pleistocene glaciations had minimal impact (Clark et al. 2009; Batchelor et al. 2019). It is likely that the group extends further north, although currently there are no known species at higher latitudes. Our analysis suggests an eastern origin (Turkey or Greece) with subsequent colonisation to the Balkan Mountains, from Albania to Croatia and Italy, extending southward to Tunisia during the Messinian Salinity Crisis (Garcia-Castellanos and Villaseñor 2011).
The Leptoneta group includes 30 species distributed throughout the western Mediterranean and 38 species in Asia (World Spider Catalog, see http://wsc.nmbe.ch). Based on the species included in our analysis, and the distributions of all other Leptoneta from Europe, we interpret the genus to be of Iberian origin; all Leptoneta species in Europe occur in regions that were formerly part of or connected to the peninsula. Results from our molecular dating analysis estimate the divergence of Leptoneta at 33 Ma. During this time the Iberian Peninsula was located between Eurasia and North America but separated from the northern mainland of Africa. This timing also precedes the drying of the Turgai Strait and subsequent uplift of the Himalayas. A key consequence of this timing is that Leptoneta is restricted to the western Mediterranean. In conjunction with our observations of its morphology, we view this as evidence that all currently described Asian Leptoneta are misplaced and belong to other genera.
Protoleptoneta group
The Protoleptoneta group is estimated to have diverged 87.5 Ma and includes genera from eastern North America, Mediterranean Europe, and east Asia. The ancestral distribution of the lineage has a mixed probability of origin, but most likely includes an area spanning eastern North America and Mediterranean Europe (AD, node 97, Fig. 6). Two main lineages occur within the group; the first includes four genera (Chisoneta, Neoleptoneta, Ozarkia, and Tayshaneta) distributed in the southern USA and Mexico. This group is estimated to have arisen 77 Ma in eastern North America after the closure of the Mid-Continental Seaway and the subsequent uplift of the western mountain ranges as part of the Laramide orogeny. The westernmost species in this lineage, Ozarkia apachaea (Gertsch, 1974) is known from the Chiricahua Mountains and we predict that the western mountains presented a barrier to further dispersal of the group in western North America.
The second Protoleptoneta group lineage includes five widely disjunct genera: Falcileptoneta (east Asia), Longileptoneta (east Asia), Montanineta (eastern North America), Protoleptoneta (Mediterranean Europe), and Teloleptoneta (Mediterranean Europe). Given the geographic complexity, the DEC analysis infers a mixed probability of origin for this group (node 96, Fig. 6). Two possibilities are presented: (1) Mediterranean Europe + east Asia (DF, 70.9%), and (2) eastern North America + east Asia (AF, 29%). The first scenario requires dispersal to eastern North America, perhaps by the Thulean or De Greer land bridges, both of which are recognised as important routes between Mediterranean Europe and eastern North America (Sanmartín et al. 2001; Brikiatis 2014). The second scenario requires dispersal to Mediterranean Europe which seems less likely given the persistence of the Turgai Strait until the late Tertiary (30 Ma). One possible explanation for the ambiguity is insufficient sampling in Asia. Falcileptoneta and Longileptoneta, for example, are broadly distributed in Japan, Korea, and Taiwan and together include over 50 species. Our data includes only four representatives of these genera and we predict that increased sampling will greatly inform the inferred ancestral distribution of the group. Based on the number of described species, the centre of diversity for the Protoleptoneta group appears to be east Asia. The genera Montanineta and Teloleptoneta are monotypic and Protoleptoneta includes only four species, mostly known from isolated caves. Given the age of the Protoleptoneta group and its inferred ancestral distribution, we hypothesise that Montanineta, Teloleptoneta, and Protoleptoneta are relictual and perhaps the last extant members of formerly widespread lineages.
Calileptoneta group
The origin of the Calileptoneta group is estimated at 110 Ma and is reconstructed as having an ancestral distribution spanning western North America and Mediterranean Europe (BD, node 106, Fig. 6). This timing is close to the origin of the mid-Continental Seaway, which separated North America into two subcontinents (Laramidia in the west and Appalachia in the east). Given the age of the group, a plausible scenario is that the group was formerly widespread and then divided by the formation of the mid-Continental Seaway.
Within the Calileptoneta group, one lineage corresponds to Calileptoneta, which is known only from western North America (B, node 101, Fig. 6). Although we see no relationships between Calileptoneta and taxa from east Asia, the western North America–east Asia disjunction is well established and we predict that some unsampled taxa in Asia will have close affinity with Calileptoneta. The monotypic genus Rhyssoleptoneta, for example, shares palpal homologies with Calileptoneta (J. Ledford, pers. obs.) and may prove important to understanding relationships and biogeography within this group.
The second lineage includes taxa from Mediterranean Europe, eastern North America, and east Asia. The group is reconstructed to have originated in Mediterranean Europe (D, node 105, Fig. 6) with an estimated age of 103 Ma. Given the reasonably ancient age of the group, we predict that it was formerly widespread and some lineages (AR 4354 and Appaleptoneta) are relictual. Within this group, the genus Leptonetela is the most diverse with over 100 species described from Asia (China and Vietnam) and 12 from the eastern Mediterranean (Turkey, Caucasus, and Greece). Although we only have two Leptonetela species in our dataset, both Mediterranean Europe and east Asia are represented. The estimated age for the split of L. jiulong (Guizhou, China) and L. thracia (Greece) is 19 Ma (the origin of the genus Leptonetela is older, c. 80 Ma) after the closure of the Turgai Strait (Tangelder 1988; Sanmartín et al. 2001). We interpret the occurrence of Leptonetela in Europe as a recent dispersal from South Asia, that occurred before the rise of the Tibetan plateau or the formation, further north, of the Gobi Desert, which prevented connection between Central Asia and the Eastern Mediterranean. Increased sampling will likely improve our understanding of the biogeography of Leptonetela, especially in the region from Turkey to India, which remains undersampled and may hold more species.
Conclusions
Archoleptonetids and leptonetids are ancient lineages of spiders whose relationships have been enigmatic for over 100 years. Brignoli (1979d) was the first to recognise that they were likely not part of Synspermiata (then Haplogynae) although he did not have a clear conception of where they fit on the spider tree of life. He also recognised that the placement of leptonetids with telemids and ochyroceratids was a result of the poor state of knowledge about these families at the time. Since Brignoli, most workers have placed Leptonetidae (including Archoleptonetidae) as sister to Telemidae (Platnick et al. 1991; Ramírez 2000) although it was widely recognised that aspects of their morphology (absence of cheliceral lamina, cylindrical gland spigots, expandable male genitalia, respiratory structures) argued against this arrangement. Ledford and Griswold (2010) reviewed the morphology of both groups and found additional problems, proposing that they were more closely related to entelegynes.
Among the challenges with both families is that they are rarely encountered spiders, even by specialists, and their small size and delicate features make them difficult to study. For example, in the absence of scanning electron microscopy, the intricate features of the male palpal bulb are rarely observed and most species worldwide remain inadequately described and diagnosed (but see Ledford 2004; Ledford and Griswold 2010; Ledford et al. 2011, 2012). The increasingly widespread adoption of phylogenomics has provided insight into relationships among all spiders, although, as demonstrated in this study, this is not a panacea. The deep splits inherent on the spider tree of life are difficult to resolve, especially among early-diverging araneomorph groups such as archoleptonetids and leptonetids. As shown in a recent study on spider fossils (Magalhaes et al. 2020), many of the early-diverging araneomorphs known from fossils are difficult to place and extinction of these lineages is likely a confounding factor in understanding present-day spider phylogeny. However, despite the inability of our study and recent phylogenomic studies to resolve relationships among austrochiloids, archoleptonetids, and leptonetids it is clear that they are not part of Synspermiata, a hypothesis supported by both their morphology and molecular data. Resolving relationships among these groups will require special attention, likely incorporating data from fossils and careful analysis of their morphology.
Although problems with the current taxonomy of leptonetids are identified in our study, most described genera are monophyletic. The problems within the Paraleptoneta group are especially intriguing as they have a long history of study, including detailed illustrations and descriptions of the male and female genitalia. However, none of these groups has undergone comprehensive revision and no European genera have ever been quantitatively analysed. Further, significant sampling gaps remain, especially in eastern Europe, where a wealth of diversity undoubtedly remains undiscovered as evidenced by the undescribed genus from Croatia included in this study. The most substantial challenge in leptonetid systematics is the Asian fauna. Recent efforts focused on Asian leptonetids have provided insight into their diversity, but only one of these studies (Wang et al. 2017) include quantitative analyses of their phylogeny. The result of these descriptive efforts is that most Asian leptonetids remain unplaced, and genera such as Leptoneta continue to be used as placeholders thereby preventing a complete picture of their biogeography. Given the age and distribution of the family, we predict that connections especially to the western North American fauna await discovery. Among the goals of this study is to provide a comprehensive phylogenetic scaffold such that new species in Asia can be more confidently placed.
Taxonomy
Justification
We have decided to raise both subfamilies Archoleptonetinae and Leptonetinae to family status on the grounds that to do so is practical, making each easier to diagnose and that it does no phylogenetic harm, i.e. each is monophyletic. Further, if our preferred tree is correct (Fig. 4), with Leptonetinae sister to the Austrochiloidea to the exclusion of the Archoleptonetinae, Leptonetidae sensu lato becomes paraphyletic and relimiting the family becomes essential.
Austrochiloidea, Archoleptonetidae and Leptonetidae
This analysis and other recent analyses (e.g. Fernández et al. 2018) suggest that Archoleptonetidae and Leptonetidae are closely related to Austrochiloidea (Austrochilidae, including Hickmania, and Gradungulidae). Whereas molecular data unite these taxa, they do not share any obvious morphological synapomorphies. Austrochiloids are readily distinguished from Archoleptonetidae and Leptonetidae. The austrochiloid families share the morphological synapomorphies of a clypeal hood, i.e. a median projection over the middle cheliceral base (Griswold et al. 2005, character 30), a distal notch in the orifice of the trichobothrial base (Griswold et al. 2005, character 9), and a peculiar genitalic morphology in which the gonopore is visible anterior to the epigastric fold (Griswold et al. 2005, character 135; Ramírez 2014 character 363). Austrochiloids are large spiders, and all have austral distributions. Members of Kaiya, smallest of the gradungulids, still have a body length of 1.2 cm and a leg span of more than 3 cm, whereas some austrochiloids are veritable giants. Hickmania troglodytes (Austrochlidae) may have a body length of 2 cm and a leg span of 17 cm, and Macrogradungula moonya (Gradungulidae) may have a body length of 2 cm and a leg span of more than 18 cm! By contrast, temperate Archoleptonetidae and Leptonetidae are small spiders (less than 5 mm body length) with long, slender legs, with a leg spread of less than 2.5 cm. All have only six eyes (or none), three claws with the STC (superior tarsal claws) simple (unlike the asymmetrical claws of Gradungulidae, raptorial claws of Trogloraptoridae or enlarged, bipectinate STC of many Dysderoidea), no cheliceral lamina, unusual iridescence, especially on the legs and carapace (shared with Ochyroceratidae and Psilodercidae, among others), autospasy at the patella–tibia joint (shared with Filistatidae, Austrochilinae (Thaida and Austrochilus) and linyphioids), patella–tibia gland plates (shared with the Telemidae; similar structures occur in various Entelegynae), no external epigynum (unlike Entelegyne and some Palpimanoidea and Synspermiata), a single posterior spiracle, a divided cribellum (unlike the entire cribellum of Hypochilidae and cribellate Austrochiloidea) or a small colulus (unlike the large coluli of Telemidae, Ochyroceratidae and Psilodercidae and some Synspermiata), clypeus with margin evenly concave (unlike the median hood of Austrochiloidea), and no peg teeth (unlike Palpimanoidea).
Ledford and Griswold (2010, p. 4) diagnosed Leptonetidae sensu latu based on a set of homoplastic putative synapomorphies, which assumed placement of Leptonetidae in the Haplogynae, an hypothesis and grouping now discarded. Features cited were unusual iridescence, especially on the legs and carapace, patellar–tibial gland plates, autospasy at the patella–tibia joint, the presence of tartipores on the ALS (shared widely but absent in Synspermiata), male palpi with a fused tegulum and subtegulum but with an expandable basal haematodocha (a peculiar morphology, which requires further study in austrochiloids), and a respiratory system consisting of a pair of short median branches with long laterals that open to a single spiracle anteriad of the ALS (also found in many Entelegynae). This diagnosis overlooks notable differences between Archoleptonetinae and Leptonetinae in eye position, spinning organs and male and female genitalia: recognising Archoleptonetidae and Leptonetidae as families will make each far easier to diagnose.
Family ARCHOLEPTONETIDAE Gertsch, 1974 (new rank)
Diagnosis
Archoleptonetidae may be diagnosed from all other spiders except Leptonetidae by the characters listed above. Archoleptonetids may be diagnosed from leptonetids by having the endites with a pair of conspicuous stout setae, by the simple ocular arrangement with PME and PLE contiguous, and by the form of the tarsal organ, spinning organs and genitalia. All Archoleptonetidae have the tarsal organ with at least one elongate sensillum, multiple MAP (major ampullate gland) spigots on the ALS (anterior lateral spinnerets) and have only a few scattered AC (aciniform gland) spigots on the PMS and PLS (posterior median and lateral spinnerets); at least Archoleptoneta have a divided cribellum. Archoleptonetidae have the male palpal tibia and tarsus simple and cylindrical and the palpal bulb with an elongate embolus and three accessory sclerites, two of which straddle the embolus at the base (PRS, MS) and one that is situated prolaterally (RLS). In addition, the simple female genitalia with two receptacula and oval to elongate patellar–tibial glands may distinguish Archoleptonetidae from Leptonetidae.
Type species: Archoleptoneta schusteri Gertsch, 1974.
Composition
Two genera: Archoleptoneta Gertsch, 1974 (2 species) and Darkoneta Ledford & Griswold, 2010 (6 species).
Family LEPTONETIDAE Simon, 1890 (new status)
Diagnosis
Leptonetidae may be readily diagnosed from all other spiders by the unique ocular arrangement with PME displaced behind the ALE and PLE, AME lost. Another character seemingly unique to the leptonetids is the metatarsus III with an apical preening comb. Leptonetids may be distinguished from archoleptonetids by the endites lacking conspicuous stout setae and by the form of the tarsal organ, spinning organs and genitalia. Leptonetid tarsal organs may have an elongate base but the sensillum(a) are short and inconspicuous. All leptonetids are ecribellate with a small colulus (like the archoleptonetids Darkoneta) but the ALS have only a single MAP (major ampullate gland) spigot and at least the PLS have tightly packed rows of AC (aciniform gland) spigots. Leptonetid male palpi typically have the tibia and tarsus modified: the lateral surfaces of the tibia typically have a variety of spines and twisted setae that in many genera are produced into large spine-like apophyses and the palpal tarsus is dorsally constricted and often modified apically and retrolaterally, usually bearing chemosensory and a variety of other specialised setae. The leptonetid female genitalia that present a vulva with a large, central atrium with a pair of lateral twisted spermathecae bearing numerous flagellate pores, which are connected laterally to the atrium by short, twisted tubes, are unique. In addition, leptonetids have patellar–tibial glands but these differ in shape from those of archoleptonetids and from the family Telemidae.
Type species: Leptoneta convexa Simon, 1872.
Composition
In total, 19 genera and 347 species: Appaleptoneta (Platnick 1986) (7 species), Barusia (Kratochvíl 1978) (5 species), Calileptoneta (Platnick 1986) (9 species), Cataleptoneta (Denis 1955) (8 species), Chisoneta (Ledford et al. 2011) (4 species), Falcileptoneta (Komatsu 1970) (50 species), Leptoneta (Simon 1872) (70 species), Leptonetela (Kratochvíl 1978) (108 species), Longileptoneta (Seo 2015b) (5 species), Masirana (Kishida, 1942, in Komatsu 1942) (26 species), Montanineta (Ledford et al. 2011) (1 species), Neoleptoneta (Brignoli 1972) (8 species), Ozarkia (Ledford et al. 2011) (9 species), Paraleptoneta (Fage 1913) (2 species), Protoleptoneta (Deltshev 1972) (4 species), Rhyssoleptoneta (Tong and Li 2007) (1 species), Sulcia (Kratochvíl 1938) (10 species), Tayshaneta (Ledford et al. 2011) (19 species) and Teloleptoneta Ribera, 1988 (1 species).
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
US National Science Foundation (DEB 1754591 to M. Hedin). The Schlinger Postdoctoral Fellowship at the California Academy of Sciences supported the participation of J. Ledford in the early stages of this research.
Acknowledgments
This study is a culmination of a combined effort between J. Ledford and C. Ribera over the past 10 years based on a shared fascination with leptonetid spiders and caves. We acknowledge the many people who have contributed, especially Enric Planas who helped during the early stages of our research. We thank Jillian Cowles, Christo Deltshev, Mark Harvey, Albert López, Pierre Paquin, Martina Pavlek, Enric Planas, and Darrell Ubick for specimens and discussion of leptonetids and cave biology. We thank Martín Ramírez and an anonymous reviewer for their thoughtful suggestions.
References
Agnarsson, I., Coddington, J. A., and Kuntner, M. (2013). Systematics – progress in the study of spider diversity and evolution. In ‘Spider Research in the 21st Century’. (Ed. D. Penney.) Chapter 2, pp. 58–111. (Siri Scientific Press: Manchester, UK.)Ane, C., Larget, B., Baum, D. A., Smith, S. D., and Rokas, A. (2006). Bayesian estimation of concordance among gene trees. Molecular Biology and Evolution 24, 412–426.
| Bayesian estimation of concordance among gene trees.Crossref | GoogleScholarGoogle Scholar | 17095535PubMed |
Baker, C. M., Sheridan, K., Derkarabetian, S., Pérez-González, A., Vélez, S., and Giribet, G. (2020). Molecular phylogeny and biogeography of the temperate Gondwanan family Triaenonychidae (Opiliones: Laniatores) reveals pre-Gondwanan regionalisation, common vicariance, and rare dispersal. Invertebrate Systematics 34, 637–660.
| Molecular phylogeny and biogeography of the temperate Gondwanan family Triaenonychidae (Opiliones: Laniatores) reveals pre-Gondwanan regionalisation, common vicariance, and rare dispersal.Crossref | GoogleScholarGoogle Scholar |
Batchelor, C. L., Margold, M., Krapp, M., Murton, D. K., Dalton, A. S., Gibbard, P. L., Stokes, C. R., Murton, J. B., and Manica, A. (2019). The configuration of northern hemisphere ice sheets through the Quaternary. Nature Communications 10, 3713.
| The configuration of northern hemisphere ice sheets through the Quaternary.Crossref | GoogleScholarGoogle Scholar | 31420542PubMed |
Brignoli, P. M. (1967a). Considerazioni sul genere Paraleptoneta e descrizione di una nuova specie italiana (Araneae, Leptonetidae). Fragmenta Entomologica 4, 157–169.
Brignoli, P. M. (1967b). Su alcuni Leptonetidae della Sardegna (Araneae). Rendiconti – Istituto Lombardo di Scienze e Lettere 101, 352–359.
Brignoli, P. M. (1968). Due nuove Paraleptoneta cavernicole dell’Asia Minore (Araneae, Leptonetidae). Fragmenta Entomologica 6, 23–37.
Brignoli, P. M. (1970). Considerazioni biogeografiche sulla famiglia Leptonetidae (Araneae). Bulletin du Muséum National d’Histoire Naturelle 41, 189–195.
Brignoli, P. M. (1971). Note su ragni cavernicoli italiani (Araneae). Fragmenta Entomologica 7, 121–229.
Brignoli, P. M. (1972). Some cavernicolous spiders from Mexico (Araneae). Quaderno Accademia Nazionale dei Lincei. Fondazione Leone Caetani 171, 129–155.
Brignoli, P. M. (1974a). Araignées de Grèce VIII. Quelques Leptonetidae de la Laconie et de l’île de Crète (Arachnida, Araneae). Annales de Spéléologie 29, 63–70.
Brignoli, P. M. (1974b). Notes on spiders, mainly cave-dwelling, of southern Mexico and Guatemala (Araneae). Quaderno. Accademia Nazionale dei Lincei. Fondazione Leone Caetani 171, 195–238.
Brignoli, P. M. (1974c). Ragni di Grecia VII. Raccolte in grotte dell’Attica del Dr P. Strinati (Araneae). Revue Suisse de Zoologie 81, 493–499.
| Ragni di Grecia VII. Raccolte in grotte dell’Attica del Dr P. Strinati (Araneae).Crossref | GoogleScholarGoogle Scholar |
Brignoli, P. M. (1977). Spiders of Mexico, III. A new leptonetid from Oaxaca (Araneae, Leptonetidae). Quaderno. Accademia Nazionale dei Lincei. Fondazione Leone Caetani 171, 213–218.
Brignoli, P. M. (1978). Ragni di Turchia IV. Leptonetidae, Dysderidae ed Agelenidae nuovi o interessanti di grotte della Turchia meridionale (Araneae). Quaderni di Speleologia – Circolo Speleologico Romano 3, 37–54.
Brignoli, P. M. (1979a). Spiders from Turkey, VI. Four new species from the coast of the Black Sea (Araneae). Bulletin of the British Arachnological Society 4, 310–313.
Brignoli, P. M. (1979b). Ragni di Grecia XI. Specie nuove o interessanti, cavernicole ed epigee. Revue Suisse de Zoologie 86, 181–202.
| Ragni di Grecia XI. Specie nuove o interessanti, cavernicole ed epigee.Crossref | GoogleScholarGoogle Scholar |
Brignoli, P. M. (1979c). Ragni d’Italia XXXI. Specie cavernicole nuove o interessanti (Araneae). Quaderni del Museo di Speleologia – V. Rivera 5, 1–48.
Brignoli, P. M. (1979d). The morphology and relationships of the Leptonetidae (Arachnida, Araneae). The Journal of Arachnology 7, 231–236.
Brignoli, P. M. (1979e). On some cave spiders from Guatemala and the United States (Araneae). Revue Suisse de Zoologie 86, 435–443.
| On some cave spiders from Guatemala and the United States (Araneae).Crossref | GoogleScholarGoogle Scholar |
Brikiatis, L. (2014). The De Geer, Thulean and Beringia routes: key concepts for understanding early Cenozoic biogeography. Journal of Biogeography 41, 1036–1054.
| The De Geer, Thulean and Beringia routes: key concepts for understanding early Cenozoic biogeography.Crossref | GoogleScholarGoogle Scholar |
Castresana, J. (2000). Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Molecular Biology and Evolution 17, 540–552.
| Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis.Crossref | GoogleScholarGoogle Scholar | 10742046PubMed |
Chen, H. M., Jia, Q., and Wang, S. J. (2010). A revision of the genus Qianleptoneta (Araneae: Leptonetidae). Journal of Natural History 44, 2873–2915.
| A revision of the genus Qianleptoneta (Araneae: Leptonetidae).Crossref | GoogleScholarGoogle Scholar |
Chifman, J., and Kubatko, L. (2014). Quartet inference from SNP data under the coalescent. Bioinformatics 30, 3317–3324.
| Quartet inference from SNP data under the coalescent.Crossref | GoogleScholarGoogle Scholar | 25104814PubMed |
Chifman, J., and Kubatko, L. (2015). Identifiability of the unrooted species tree topology under the coalescent model with time-reversible substitution processes, site-specific rate variation, and invariable sites. Journal of Theoretical Biology 374, 35–47.
| Identifiability of the unrooted species tree topology under the coalescent model with time-reversible substitution processes, site-specific rate variation, and invariable sites.Crossref | GoogleScholarGoogle Scholar | 25791286PubMed |
Clark, P. U., Dyke, A. S., Shakun, J. D., Carlson, A. E., Clark, J., Wohlfarth, B., Mitrovica, J. X., Hostetler, S. W., and McCabe, A. M. (2009). The last glacial maximum. Science 325, 710.
| The last glacial maximum.Crossref | GoogleScholarGoogle Scholar | 19661421PubMed |
Cokendolpher, J. C. (2004). A new Neoleptoneta spider from a cave in Camp Bullis, Bexar County, Texas (Araneae: Leptonetidae). Texas Memorial Museum Speleological Monographs 6, 63–69.
Deltshev, C. (1972). A new genus of Bulgarian cave spiders (Protoleptoneta bulgarica n.g., n. sp.). International Journal of Speleology 4, 275–283.
| A new genus of Bulgarian cave spiders (Protoleptoneta bulgarica n.g., n. sp.).Crossref | GoogleScholarGoogle Scholar |
Deltshev, C., and Li, S. Q. (2013). A new species of the genus Cataleptoneta from Belasitsa Mts, Bulgaria (Araneae, Leptonetidae). Dong Wu Fen Lei Xue Bao 38, 514–519.
Demircan, N. (2020). A new species of the genus Cataleptoneta Denis, 1955 (Araneae: Leptonetidae) from a cave in Turkey. Acta Zoologica Bulgarica 72, 187–191.
Denis, J. (1955). Biospeologica 75. Mission Henri Coiffait au Liban (1951), 7. Araignées. Archives de Zoologie Expérimentale et Générale 91, 437–454.
Derkarabetian, S., Starrett, J., Tsurusaki, N., Ubick, D., Castillo, S., and Hedin, M. (2018). A stable phylogenomic classification of Travunioidea (Arachnida, Opiliones, Laniatores) based on sequence capture of ultraconserved elements. ZooKeys 760, 1–36.
| A stable phylogenomic classification of Travunioidea (Arachnida, Opiliones, Laniatores) based on sequence capture of ultraconserved elements.Crossref | GoogleScholarGoogle Scholar |
Derkarabetian, S., Benavides, L. R., and Giribet, G. (2019). Sequence capture phylogenomics of historical ethanol‐preserved museum specimens: unlocking the rest of the vault. Molecular Ecology Resources 19, 1531–1544.
| Sequence capture phylogenomics of historical ethanol‐preserved museum specimens: unlocking the rest of the vault.Crossref | GoogleScholarGoogle Scholar | 31448547PubMed |
Dermitzakis, M. D., and Papanikolaou, D. J. (1981). Paleogeography and geodynamics of the Aegean region during the Neogene. Annales Géologiques des Pays Helléniques 30, 245–289.
Donoghue, M. J., and Smith, S. A. (2004). Patterns in the assembly of temperate forests around the northern hemisphere. Philosophical Transactions of the Royal Society of London – B. Biological Sciences 359, 1633–1644.
| Patterns in the assembly of temperate forests around the northern hemisphere.Crossref | GoogleScholarGoogle Scholar | 15519978PubMed |
Fage, L. (1913). Biospelogica XXIX. Études sur les araignées cavernicoles II. Revision des Leptonetidae. Archives de Zoologie Expérimentale et Générale 10, 479–576.
Fage, L. (1931). Araneae, 5e série, précédée d’un essai sur l’évolution souterraine et son déterminisme. In: Biospeologica, LV. Archives de Zoologie Expérimentale et Générale 71, 91–291.
Fage, L. (1943). Description d’une leptonète de Corse suivie de remarques sur les araignées cavernicoles du genre Stalita. Bulletin du Muséum National d’Histoire Naturelle 2, 171–174.
Faircloth, B. C. (2016). PHYLUCE is a software package for the analysis of conserved genomic loci. Bioinformatics 32, 786–788.
| PHYLUCE is a software package for the analysis of conserved genomic loci.Crossref | GoogleScholarGoogle Scholar | 26530724PubMed |
Faircloth, B. C. (2017). Identifying conserved genomic elements and designing universal probe sets to enrich them. Methods in Ecology and Evolution 8, 1103–1112.
| Identifying conserved genomic elements and designing universal probe sets to enrich them.Crossref | GoogleScholarGoogle Scholar |
Fernández, R., Kallal, R. J., Dimitrov, D., Ballesteros, J. A., Arnedo, M. A., Giribet, G., and Hormiga, G. (2018). Phylogenomics, diversification dynamics, and comparative transcriptomics across the Spider Tree of Life. Current Biology 28, 1489–1497.
| Phylogenomics, diversification dynamics, and comparative transcriptomics across the Spider Tree of Life.Crossref | GoogleScholarGoogle Scholar | 29706520PubMed |
Garcia-Castellanos, D., and Villaseñor, A. (2011). Messinian Salinity Crisis regulated by competing tectonics and erosion at the Gibraltar Arc. Nature 480, 359–363.
| Messinian Salinity Crisis regulated by competing tectonics and erosion at the Gibraltar Arc.Crossref | GoogleScholarGoogle Scholar | 22170684PubMed |
Garrison, N. L., Rodriguez, J., Agnarsson, I., Coddington, J. A., Griswold, C. E., Hamilton, C. A., Hedin, M., Kocot, K. M., Ledford, J. M., and Bond, J. E. (2016). Spider phylogenomics: untangling the Spider Tree of Life. PeerJ 4, e1719.
| Spider phylogenomics: untangling the Spider Tree of Life.Crossref | GoogleScholarGoogle Scholar | 26925338PubMed |
Gavish-Regev, E., Aharon, S., Armiach, I., and Lubin, Y. D. (2016). Cave survey yields a new spider family record for Israel. Arachnologische Mitteilungen 51, 39–42.
| Cave survey yields a new spider family record for Israel.Crossref | GoogleScholarGoogle Scholar |
Gertsch, W. J. (1971). A report on some Mexican cave spiders. Association for Mexican Cave Studies Bulletin 4, 47–111.
Gertsch, W. J. (1974). The spider family Leptonetidae in North America. The Journal of Arachnology 1, 145–203.
Grabherr, M. G., Haas, B. J., Yassour, M., Levin, J. Z., Thompson, D. A., Amit, I., Adiconis, X., Fan, L., Raychowdhury, R., Zeng, Q., and Chen, Z. (2011). Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nature Biotechnology 29, 644.
| Full-length transcriptome assembly from RNA-Seq data without a reference genome.Crossref | GoogleScholarGoogle Scholar | 21572440PubMed |
Griswold, C. E., Ramírez, M. J., Coddington, J. A., and Platnick, N. I. (2005). Atlas of phylogenetic data for entelegyne spiders (Araneae: Araneomorphae: Entelegynae) with comments on their phylogeny. Proceedings of the California Academy of Sciences 56, 1–324.
Guo, X., Yu, Z. G., and Chen, H. M. (2016). One new spider species of genus Leptonetela (Araneae: Leptonetidae) from cave in Guizhou, China. Sichuan Journal of Zoology 35, 395–399.
Harrison, S. E., Rix, M. G., Harvey, M. S., and Austin, A. D. (2016). An African mygalomorph lineage in temperate Australia: the trapdoor spider genus Moggridgea (Araneae: Migidae) on Kangaroo Island, South Australia. Austral Entomology 55, 208–216.
| An African mygalomorph lineage in temperate Australia: the trapdoor spider genus Moggridgea (Araneae: Migidae) on Kangaroo Island, South Australia.Crossref | GoogleScholarGoogle Scholar |
He, A. L., Liu, J. X., Xu, X. A., Yin, H. Q., and Peng, X. J. (2019). Description of three new species of spider genus Leptonetela Kratochvíl, 1978 from caves of Hunan Province, China (Araneae, Leptonetidae). Zootaxa 4554, 584–600.
| Description of three new species of spider genus Leptonetela Kratochvíl, 1978 from caves of Hunan Province, China (Araneae, Leptonetidae).Crossref | GoogleScholarGoogle Scholar |
Hedin, M., and McCormack, M. (2017). Biogeographical evidence for common vicariance and rare dispersal in a southern Appalachian harvestman (Sabaconidae, Sabacon cavicolens). Journal of Biogeography 44, 1665–1678.
| Biogeographical evidence for common vicariance and rare dispersal in a southern Appalachian harvestman (Sabaconidae, Sabacon cavicolens).Crossref | GoogleScholarGoogle Scholar |
Hedin, M., Derkarabetian, S., Ramírez, M., Vink, C., and Bond, J. (2018a). Phylogenomic reclassification of the world’s most venomous spiders (Mygalomorphae, Atracinae), with implications for venom evolution. Scientific Reports 8, 1636.
| Phylogenomic reclassification of the world’s most venomous spiders (Mygalomorphae, Atracinae), with implications for venom evolution.Crossref | GoogleScholarGoogle Scholar | 29374214PubMed |
Hedin, M., Derkarabetian, S., Blair, J., and Paquin, P. (2018b). Sequence capture phylogenomics of eyeless Cicurina spiders from Texas caves, with emphasis on US federally endangered species from Bexar County (Araneae, Hahniidae). ZooKeys 769, 49–76.
| Sequence capture phylogenomics of eyeless Cicurina spiders from Texas caves, with emphasis on US federally endangered species from Bexar County (Araneae, Hahniidae).Crossref | GoogleScholarGoogle Scholar |
Hedin, M., Derkarabetian, S., Alfaro, A., Ramírez, M. J., and Bond, J. E. (2019). Phylogenomic analysis and revised classification of atypoid mygalomorph spiders (Araneae, Mygalomorphae), with notes on arachnid ultraconserved element loci. PeerJ 7, e6864.
| Phylogenomic analysis and revised classification of atypoid mygalomorph spiders (Araneae, Mygalomorphae), with notes on arachnid ultraconserved element loci.Crossref | GoogleScholarGoogle Scholar | 31110925PubMed |
Inoue, J., Donoghue, P. C. J., and Yang, Z. (2010). The impact of the representation of fossil calibrations on Bayesian estimation of species divergence times. Systematic Biology 59, 74–89.
| The impact of the representation of fossil calibrations on Bayesian estimation of species divergence times.Crossref | GoogleScholarGoogle Scholar | 20525621PubMed |
Katoh, D., and Standley, D. M. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30, 772–780.
| MAFFT multiple sequence alignment software version 7: improvements in performance and usability.Crossref | GoogleScholarGoogle Scholar |
Komatsu, T. (1942). [Spiders from Saisho-do caves]. Acta Arachnologica 7, 54–70.
Komatsu, T. (1970). A new genus and a new species of Japanese spiders (Falcileptoneta n. g. and Sarutana kawasawai n. sp., Leptonetidae). Acta Arachnologica 23, 1–12.
| A new genus and a new species of Japanese spiders (Falcileptoneta n. g. and Sarutana kawasawai n. sp., Leptonetidae).Crossref | GoogleScholarGoogle Scholar |
Kratochvíl, J. (1935). Araignées cavernicoles de Krivošije. Práce Moravské Přírodovědecké Společnosti 9, 1–25.
Kratochvíl, J. (1938). Étude sur les araignées cavernicoles du genre Sulcia nov. gen. Práce Moravské Přírodovědecké Společnosti 11, 1–25.
Kratochvíl, J. (1978). Araignées cavernicoles des îles Dalmates. Přírodovědné práce ústavů Československé Akademie Věd v Brně (N. S.) 12, 1–59.
Lavin, M., and Luckow, M. (1993). Origins and relationships of tropical North America in the context of the boreotropics hypothesis. American Journal of Botany 80, 1–14.
| Origins and relationships of tropical North America in the context of the boreotropics hypothesis.Crossref | GoogleScholarGoogle Scholar |
Le Peru, B. (2011). The spiders of Europe, a synthesis of data: Volume 1 Atypidae to Theridiidae. Mémoires de la Société Linnéenne de Lyon 2, 1–522.
Ledford, J. M. (2004). A revision of the spider genus Calileptoneta Platnick (Araneae, Leptonetidae), with notes on morphology, natural history and biogeography. The Journal of Arachnology 32, 231–269.
| A revision of the spider genus Calileptoneta Platnick (Araneae, Leptonetidae), with notes on morphology, natural history and biogeography.Crossref | GoogleScholarGoogle Scholar |
Ledford, J. M., and Griswold, C. E. (2010). A study of the subfamily Archoleptonetinae (Araneae, Leptonetidae) with a review of the morphology and relationships for the Leptonetidae. Zootaxa 2391, 1–32.
| A study of the subfamily Archoleptonetinae (Araneae, Leptonetidae) with a review of the morphology and relationships for the Leptonetidae.Crossref | GoogleScholarGoogle Scholar |
Ledford, J., Paquin, P., Cokendolpher, J., Campbell, J., and Griswold, C. (2011). Systematics of the spider genus Neoleptoneta Brignoli, 1972 (Araneae: Leptonetidae) with a discussion of the morphology and relationships for the North American Leptonetidae. Invertebrate Systematics 25, 334–388.
| Systematics of the spider genus Neoleptoneta Brignoli, 1972 (Araneae: Leptonetidae) with a discussion of the morphology and relationships for the North American Leptonetidae.Crossref | GoogleScholarGoogle Scholar |
Ledford, J. M., Paquin, P., Cokendolpher, J. C., Campbell, J., and Griswold, C. (2012). Systematics, conservation and morphology of the spider genus Tayshaneta (Araneae, Leptonetidae) in central Texas Caves. ZooKeys 167, 1–102.
| Systematics, conservation and morphology of the spider genus Tayshaneta (Araneae, Leptonetidae) in central Texas Caves.Crossref | GoogleScholarGoogle Scholar |
Lin, Y. C., and Li, S. Q. (2010). Leptonetid spiders from caves of the Yunnan–Guizhou plateau, China (Araneae: Leptonetidae). Zootaxa 2587, 1–93.
| Leptonetid spiders from caves of the Yunnan–Guizhou plateau, China (Araneae: Leptonetidae).Crossref | GoogleScholarGoogle Scholar |
Lymberakis, P., and Poulakakis, N. (2010). Three continents claiming an archipelago: the evolution of Aegean’s herpetofaunal diversity. Diversity 2, 233–255.
| Three continents claiming an archipelago: the evolution of Aegean’s herpetofaunal diversity.Crossref | GoogleScholarGoogle Scholar |
Machado, A. de B., and Ribera, C. (1986). Araneidos cavernícolas de Portugal: Familia Leptonetidae (Araneae). Actas X Congreso Internacional de Aracnologia, Barcelona 1, 355–366.
Magalhaes, I. L., Azevedo, G. H., Michalik, P., and Ramírez, M. J. (2020). The fossil record of spiders revisited: implications for calibrating trees and evidence for a major faunal turnover since the Mesozoic. Biological Reviews of the Cambridge Philosophical Society 95, 184–217.
| The fossil record of spiders revisited: implications for calibrating trees and evidence for a major faunal turnover since the Mesozoic.Crossref | GoogleScholarGoogle Scholar |
Mammola, S., and Isaia, M. (2017). Spiders in caves. Proceedings of the Royal Society of London – B. Biological Sciences 284, 20170193.
| Spiders in caves.Crossref | GoogleScholarGoogle Scholar |
Milne, R. I., and Abbott, R. J. (2002). The origin and evolution of Tertiary relict floras. Advances in Botanical Research 38, 281–314.
| The origin and evolution of Tertiary relict floras.Crossref | GoogleScholarGoogle Scholar |
Minh, B. Q., Schmidt, H. A., Chernomor, O., Schrempf, D., Woodhams, M. D., von Haeseler, A., and Lanfear, R. (2020a). IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Molecular Biology and Evolution 37, 1530–1534.
| IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era.Crossref | GoogleScholarGoogle Scholar | 32011700PubMed |
Minh, B., Hahn, M., and Lanfear, R. (2020b). New methods to calculate concordance factors for phylogenomic datasets. Molecular Biology and Evolution 37, 2727–2733.
| New methods to calculate concordance factors for phylogenomic datasets.Crossref | GoogleScholarGoogle Scholar | 32365179PubMed |
Nguyen, L. T., Schmidt, H. A., von Haeseler, A., and Minh, B. Q. (2015). IQ-TREE: A fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Molecular Biology and Evolution 32, 268–274.
| IQ-TREE: A fast and effective stochastic algorithm for estimating maximum likelihood phylogenies.Crossref | GoogleScholarGoogle Scholar | 25371430PubMed |
Papadopoulou, A., Anastasiou, I., and Vogler, A. P. (2010). Revisiting the insect mitochondrial molecular clock: the mid-Aegean trench calibration. Molecular Biology and Evolution 27, 1659–1672.
| Revisiting the insect mitochondrial molecular clock: the mid-Aegean trench calibration.Crossref | GoogleScholarGoogle Scholar | 20167609PubMed |
Paradis, E., Claude, J., and Strimmer, K. (2004). APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290.
| APE: analyses of phylogenetics and evolution in R language.Crossref | GoogleScholarGoogle Scholar | 14734327PubMed |
Platnick, N. I. (1986). On the tibial and patellar glands, relationships, and American genera of the spider family Leptonetidae (Arachnida, Araneae). American Museum Novitates 2855, 1–16.
Platnick, N. I., Coddington, J. A., Forster, R. R., and Griswold, C. E. (1991). Spinneret morphology and the phylogeny of haplogyne spiders (Araneae, Araneomorphae). American Museum Novitates 3016, 1–73.
Portik, D. M., and Wiens, J. J. (2020). Do alignment and trimming methods matter for phylogenomic (UCE) analyses? Systematic Biology , syaa064.
| Do alignment and trimming methods matter for phylogenomic (UCE) analyses?Crossref | GoogleScholarGoogle Scholar | 32797207PubMed |
Ramírez, M. J. (2000). Respiratory system morphology and the phylogeny of haplogyne spiders (Araneae, Araneomorphae). The Journal of Arachnology 28, 149–157.
| Respiratory system morphology and the phylogeny of haplogyne spiders (Araneae, Araneomorphae).Crossref | GoogleScholarGoogle Scholar |
Ramírez, M. J. (2014). The morphology and phylogeny of dionychan spiders (Araneae: Araneomorphae). Bulletin of the American Museum of Natural History 390, 1–374.
| The morphology and phylogeny of dionychan spiders (Araneae: Araneomorphae).Crossref | GoogleScholarGoogle Scholar |
Ramírez, M. J., Magalhaes, I. L. F., Derkarabetian, S., Ledford, J., Griswold, C. E., Wood, H. M., and Hedin, M. (2021). Sequence capture phylogenomics of true spiders reveals convergent evolution of respiratory systems Systematic Biology 70, 14–20.
| Sequence capture phylogenomics of true spiders reveals convergent evolution of respiratory systemsCrossref | GoogleScholarGoogle Scholar | 32497195PubMed |
Rannala, B., and Yang, Z. (2007). Inferring speciation times under an episodic molecular clock. Systematic Biology 56, 453–466.
| Inferring speciation times under an episodic molecular clock.Crossref | GoogleScholarGoogle Scholar | 17558967PubMed |
Ree, R. H., and Smith, S. A. (2008). Maximum likelihood inference of geographic range evolution by dispersal, local extinction, and cladogenesis. Systematic Biology 57, 4–14.
| Maximum likelihood inference of geographic range evolution by dispersal, local extinction, and cladogenesis.Crossref | GoogleScholarGoogle Scholar | 18253896PubMed |
Ribera, C. (1978). Leptoneta comasi n. sp. (Araneae, Leptonetidae) una nueva especie cavernicola del Levante Español. Miscelánea Zoológica 4, 25–29.
Ribera, C. (1988). La familia Leptonetidae (Arachnida, Araneae) in la Península Ibérica. Technische Universität Berlin Dokumentation Kongresse und Tagungen 38, 267–281.
Ribera, C., and Lopez, A. (1982). Résultats d’une campagne biospéologique en Tunisie et description d’une espèce nouvelle de Leptonetidae (Araneae): Paraleptoneta bellesi. Revue Arachnologique 4, 57–64.
Sanmartín, I., Enghoff, H., and Ronquist, F. (2001). Patterns of animal dispersal, vicariance and diversification in the Holarctic. Biological Journal of the Linnean Society. Linnean Society of London 73, 345–390.
| Patterns of animal dispersal, vicariance and diversification in the Holarctic.Crossref | GoogleScholarGoogle Scholar |
Selden, P. A., and Ren, D. (2017). A review of Burmese amber arachnids. The Journal of Arachnology 45, 324–343.
| A review of Burmese amber arachnids.Crossref | GoogleScholarGoogle Scholar |
Seo, B. K. (2015a). Ten new species of the genus Falcileptoneta (Araneae, Leptonetidae) from Korea. Korean Journal of Environmental Biology 33, 290–305.
| Ten new species of the genus Falcileptoneta (Araneae, Leptonetidae) from Korea.Crossref | GoogleScholarGoogle Scholar |
Seo, B. K. (2015b). Four new species of the genera Masirana and Longileptoneta (Araneae, Leptonetidae) from Korea. Korean Journal of Environmental Biology 33, 306–313.
| Four new species of the genera Masirana and Longileptoneta (Araneae, Leptonetidae) from Korea.Crossref | GoogleScholarGoogle Scholar |
Seo, B. K. (2016a). Four new species of the genus Longileptoneta (Araneae, Leptonetidae) from Korea. Journal of Species Research 5, 584–589.
| Four new species of the genus Longileptoneta (Araneae, Leptonetidae) from Korea.Crossref | GoogleScholarGoogle Scholar |
Seo, B. K. (2016b). Four new species of the genus Falcileptoneta (Araneae, Leptonetidae) from Korea. Journal of Species Research 5, 590–595.
| Four new species of the genus Falcileptoneta (Araneae, Leptonetidae) from Korea.Crossref | GoogleScholarGoogle Scholar |
Shao, L. L., and Li, S. Q. (2018). Early Cretaceous greenhouse pumped higher taxa diversification in spiders. Molecular Phylogenetics and Evolution 127, 146–155.
| Early Cretaceous greenhouse pumped higher taxa diversification in spiders.Crossref | GoogleScholarGoogle Scholar |
Simon, E. (1872). Notice complémentaire sur les arachnides cavernicoles et hypogés. Annales de la Société Entomologique de France 5, 473–488.
Stamatakis, A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313.
| RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies.Crossref | GoogleScholarGoogle Scholar | 24451623PubMed |
Starrett, J., Derkarabetian, S., Hedin, M., Bryson, R. W., McCormack, J. E., and Faircloth, B. C. (2017). High phylogenetic utility of an ultraconserved element probe set designed for Arachnida. Molecular Ecology Resources 17, 812–823.
| High phylogenetic utility of an ultraconserved element probe set designed for Arachnida.Crossref | GoogleScholarGoogle Scholar | 27768256PubMed |
Talavera, G., and Castresana, J. (2007). Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Systematic Biology 56, 564–577.
| Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments.Crossref | GoogleScholarGoogle Scholar | 17654362PubMed |
Tangelder, I. R. M. (1988). The biogeography of the Holarctic Nephrotoma dorsalis species-group (Diptera, Tipulidae). Beaufortia 38, 1–35.
Tiffney, B. H. (1985). Perspectives on the origin of the floristic similarity between eastern Asia and eastern North America. Journal of the Arnold Arboretum 66, 73–94.
| Perspectives on the origin of the floristic similarity between eastern Asia and eastern North America.Crossref | GoogleScholarGoogle Scholar |
Tong, Y. F., and Li, S. Q. (2007). Description of Rhyssoleptoneta latitarsa gen. nov. et sp. nov. (Araneae, Leptonetidae) from Hebei Province, China. Dong Wu Fen Lei Xue Bao 32, 35–37.
US Fish and Wildlife Service (2020). US Endangered Species List for Arachnida. Available at https://www.fws.gov/endangered/species/us-species.html [Verified 19 August 2020].
Wang, C. X., and Li, S. Q. (2010). Two new species of the spider genus Cataleptoneta from Balkan Peninsula (Araneae, Leptonetidae). Zootaxa 2730, 57–68.
| Two new species of the spider genus Cataleptoneta from Balkan Peninsula (Araneae, Leptonetidae).Crossref | GoogleScholarGoogle Scholar |
Wang, C. X., and Li, S. Q. (2011). A further study on the species of the spider genus Leptonetela (Araneae: Leptonetidae). Zootaxa 2841, 1–90.
| A further study on the species of the spider genus Leptonetela (Araneae: Leptonetidae).Crossref | GoogleScholarGoogle Scholar |
Wang, C. X., Xu, X., and Li, S. Q. (2017). Integrative taxonomy of Leptonetela spiders (Araneae, Leptonetidae), with descriptions of 46 new species. Zoological Research 38, 321–448.
| 29280363PubMed |
Wheeler, W. C., Coddington, J. A., Crowley, L. M., Dimitrov, D., Goloboff, P. A., Griswold, C. E., Hormiga, G., Prendini, L., Ramírez, M. J., Sierwald, P., Almeida‐Silva, L., Alvarez‐Padilla, F., Arnedo, M. A., Benavides Silva, L. R., Benjamin, S. P., Bond, J. E., Grismado, C. J., Hasan, E., Hedin, M., Izquierdo, M. A., Labarque, F. M., Ledford, J., Lopardo, L., Maddison, W. P., Miller, J. A., Piacentini, L. N., Platnick, N. I., Polotow, D., Silva‐Dávila, D., Scharff, N., Szűts, T., Ubick, D., Vink, C. J., Wood, H. M., and Zhang, J. (2017). The spider tree of life: phylogeny of Araneae based on target‐gene analyses from an extensive taxon sampling. Cladistics 33, 574–616.
| The spider tree of life: phylogeny of Araneae based on target‐gene analyses from an extensive taxon sampling.Crossref | GoogleScholarGoogle Scholar |
Wolfe, J. A. (1975). Some aspects of plant geography of the northern hemisphere during the Late Cretaceous and Tertiary. Annals of the Missouri Botanical Garden 62, 264–279.
| Some aspects of plant geography of the northern hemisphere during the Late Cretaceous and Tertiary.Crossref | GoogleScholarGoogle Scholar |
Wood, H. M., González, V. L., Lloyd, M., Coddington, J., and Scharff, N. (2018). Next-generation museum genomics: phylogenetic relationships among palpimanoid spiders using sequence capture techniques (Araneae: Palpimanoidea). Molecular Phylogenetics and Evolution 127, 907–918.
| Next-generation museum genomics: phylogenetic relationships among palpimanoid spiders using sequence capture techniques (Araneae: Palpimanoidea).Crossref | GoogleScholarGoogle Scholar | 29966686PubMed |
Wunderlich, J. (2008). The dominance of ancient spider families of the Araneae: Haplogyne in the Cretaceous, and the late diversification of advanced ecribellate spiders of the Entelegynae after the Cretaceous – tertiary boundary extinction events, with descriptions of new family. Beiträge zur Araneologie 5, 524–675.
Wunderlich, J. (2012). On the fossil spider (Araneae) fauna in Cretaceous ambers, with descriptions of new taxa from Burmese (Burma) and Jordan, and on the relationships of the superfamily Leptonetoidea. Beiträge zur Araneologie 7, 157–232.
Xu, M. J., Kim, S. T., Yoo, J. S., Nam, E. J., and Li, S. Q. (2019). Three new species of the genus Falcileptoneta Komatsu, 1970 (Araneae, Leptonetidae) from Korea. ZooKeys 872, 1–12.
| Three new species of the genus Falcileptoneta Komatsu, 1970 (Araneae, Leptonetidae) from Korea.Crossref | GoogleScholarGoogle Scholar |
Yang, Z. (2007). PAML 4: phylogenetic analysis by maximum likelihood. Molecular Biology and Evolution 24, 1586–1591.
| PAML 4: phylogenetic analysis by maximum likelihood.Crossref | GoogleScholarGoogle Scholar | 17483113PubMed |
Yang, Z., and Rannala, B. (2006). Bayesian estimation of species divergence times under a molecular clock using multiple fossil calibrations with soft bounds. Molecular Biology and Evolution 23, 212–226.
| Bayesian estimation of species divergence times under a molecular clock using multiple fossil calibrations with soft bounds.Crossref | GoogleScholarGoogle Scholar | 16177230PubMed |
Yu, Y., Harris, A. J., Blair, C., and He, X. (2015). RASP (reconstruct ancestral state in phylogenies): a tool for historical biogeography. Molecular Phylogenetics and Evolution 87, 46–49.
| RASP (reconstruct ancestral state in phylogenies): a tool for historical biogeography.Crossref | GoogleScholarGoogle Scholar | 25819445PubMed |
Zerbino, D. R., and Birney, E. (2008). Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Research 18, 821–829.
| Velvet: algorithms for de novo short read assembly using de Bruijn graphs.Crossref | GoogleScholarGoogle Scholar | 18349386PubMed |