Cryptic diversity down under: defining species in the subterranean amphipod genus Nedsia Barnard & Williams, 1995 (Hadzioidea: Eriopisidae) from the Pilbara, Western Australia
Rachael A. King A B * , Erinn P. Fagan-Jeffries A B , Tessa M. Bradford
A B * , Erinn P. Fagan-Jeffries A B , Tessa M. Bradford  A B , Danielle N. Stringer A B , Terrie L. Finston C , Stuart A. Halse D , Stefan M. Eberhard E , Garth Humphreys C F G , Bill F. Humphreys C G , Andrew D. Austin A B and Steven J. B. Cooper A B
A B , Danielle N. Stringer A B , Terrie L. Finston C , Stuart A. Halse D , Stefan M. Eberhard E , Garth Humphreys C F G , Bill F. Humphreys C G , Andrew D. Austin A B and Steven J. B. Cooper A B
A South Australian Museum, North Terrace, Adelaide, SA 5000, Australia.
B Australian Centre for Evolutionary Biology and Biodiversity and School of Biological Sciences, University of Adelaide, SA 5005, Australia.
C School of Animal Biology, The University of Western Australia, Crawley, WA 6009, Australia.
D Bennelongia Environmental Consultants, PO Box 384, Wembley, WA 6913, Australia.
E Subterranean Ecology Pty Ltd, 227 Coningham Road, Coningham, Tas. 7054, Australia.
F Biota Environmental Sciences Pty Ltd, PO Box 155, Leederville, WA 6903, Australia.
G Western Australian Museum, Locked Bag 40, Welshpool DC, WA 6986, Australia.
Invertebrate Systematics 36(2) 113-159 https://doi.org/10.1071/IS21041
Submitted: 25 May 2021 Accepted: 17 September 2021 Published: 18 February 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Amphipod crustaceans comprise a significant and enigmatic component of Australian groundwater ecosystems, particularly in the Pilbara region of Western Australia. Many amphipod species in the Pilbara, including species in the genus Nedsia Barnard & Williams, 1995, are considered short range endemics, poorly or contentiously defined by taxonomic treatments based on morphology alone and have uncertain distributions as a consequence of this taxonomy. A modern systematic revision of Nedsia is presented here, utilising both molecular and morphological analyses alongside distributional data to delineate species. We describe 13 new species of Nedsia, confirm three existing species and synonymise eight previously described species. Nedsia species are confirmed to be functionally morphologically cryptic, with COI divergences at the 5–20% level. We present comparatively reduced taxonomic descriptions for these cryptic amphipod species in an effort to provide an accelerated pathway for future taxonomic work. The research provides the basis for future environmental impact assessments involving Nedsia species and ongoing monitoring of the groundwater communities these form part of in the resource-rich Pilbara region.
Keywords: Amphipoda, classification, cryptic species, Eriopisidae, groundwater, phylogenetics, stygofauna, taxonomy.
Introduction
The Pilbara region in north-western Australia is an ancient biogeographic landscape with extraordinarily high levels of biological diversity and endemism (Pepper et al. 2013). This is particularly the case for invertebrates and most significantly, subterranean fauna including groundwater-associated crustaceans (Eberhard et al. 2005; Guzik et al. 2010; Halse 2018). Amphipods comprise a significant component of these stygofaunal (i.e. animals living within the groundwater) crustacean communities. Four diverse stygobiotic families and 35 species have been recorded from the Pilbara, the adjacent North West Cape peninsula and Barrow Island, including Bogidiellidae Hertzog, 1936; Eriopisidae Lowry & Myers, 2013; Hadziidae S. Karaman, 1943 and Paramelitidae Bousfield, 1977. However, more than 80% of the stygobiotic fauna of Western Australian is estimated to be undiscovered and accordingly hundreds of amphipod species potentially remain to be discovered and described (Eberhard et al. 2005; Finston et al. 2008; Guzik et al. 2010). Freshwater amphipods are not common in northern Australian tropical and subtropical surface waters, however a diverse stygobiotic fauna is known that is likely limited by climatic (Bradbury and Williams 1997), biological and habitat-driven dispersal opportunities. Across the Pilbara, there are few surface records except for occasional records of a few species in small springs (Pinder et al. 2010) and hyporheic habitats. Stygobiotic amphipods brood young with specialised ventral pouches, are considered to not swim long distances and subject to the confinement of subterranean habitats (i.e. aquifers, springs, hyporheic habitats or catchment boundaries that may contain physical barriers inhibiting dispersal). As such, many are likely to be short range endemic (SRE) species (Harvey 2002; Eberhard et al. 2009) and are significant in terms of biodiversity conservation (Harvey et al. 2011) and management of groundwater, including as potential ecosystem providers (Boulton et al. 2008).
The amphipod genus Nedsia Barnard & Williams, 1995 (Hadzioidea: Eriopisidae) currently exists as an enigmatic complex of 11 described species distributed on Barrow Island and the North West Cape peninsula (Horton et al. 2020), and many undescribed taxa from the Pilbara (Halse et al. 2014). First described from Exmouth, the North West Cape peninsula (N. douglasi Barnard & Williams, 1995), 10 additional species were subsequently described from Barrow Island using limited morphological characters (largely setation and spination) (Bradbury and Williams 1996; Bradbury 2002). Seven of the Barrow Island species (N. fragilis Bradbury & Williams, 1996; N. humphreysi Bradbury & Williams, 1996; N. hurlberti Bradbury & Williams, 1996; N. macrosculptilis Bradbury & Williams, 1996; N. sculptilis Bradbury & Williams, 1996; N. straskraba Bradbury & Williams, 1996; and N. urifimbriata Bradbury & Williams, 1996) are identified as vulnerable, i.e. ‘facing a high risk of extinction in the wild in the medium-term future’ under the Western Australian Biodiversity Conservation Act 2016. An additional species, N. chevronia Bradbury, 2002 is identified as a ‘Priority 2, poorly known species in urgent need of further survey’ by the Department of Biodiversity, Conservation and Attractions. However, the described species of Nedsia are poorly defined and difficult, if not impossible, to identify to species level. Examination (by R. A. King) of the material used for previous studies on Barrow Island has shown that most species were described from single or few individuals, from single sites and from small (potentially juvenile) or damaged specimens. The nature of stygobiotic sampling often leads to small numbers of individuals per catch and can result in damage to fragile specimens, therefore descriptions based on few specimens are not unusual. However, broader examination of Pilbara Nedsia specimens indicated that previously considered species-level characters were likely to represent population-level morphological variations.
The amphipod genus Nedsia Barnard & Williams, 1995 (Hadzioidea: Eriopisidae) currently exists as an enigmatic complex of 11 described species distributed on Barrow Island and the North West Cape peninsula (Horton et al. 2020), and many undescribed taxa from the Pilbara (Halse et al. 2014). First described from Exmouth, the North West Cape peninsula (N. douglasi Barnard & Williams, 1995), 10 additional species were subsequently described from Barrow Island using limited morphological characters (largely setation and spination) (Bradbury and Williams 1996; Bradbury 2002). Seven of the Barrow Island species (N. fragilis Bradbury & Williams, 1996; N. humphreysi Bradbury & Williams, 1996; N. hurlberti Bradbury & Williams, 1996; N. macrosculptilis Bradbury & Williams, 1996; N. sculptilis Bradbury & Williams, 1996; N. straskraba Bradbury & Williams, 1996; and N. urifimbriata Bradbury & Williams, 1996) are identified as vulnerable, i.e. ‘facing a high risk of extinction in the wild in the medium-term future’ under the Western Australian Biodiversity Conservation Act 2016. An additional species, N. chevronia Bradbury, 2002 is identified as a ‘Priority 2, poorly known species in urgent need of further survey’ by the Department of Biodiversity, Conservation and Attractions. However, the described species of Nedsia are poorly defined and difficult, if not impossible, to identify to species level. Examination (by R. A. King) of the material used for previous studies on Barrow Island has shown that most species were described from single or few individuals, from single sites and from small (potentially juvenile) or damaged specimens. The nature of stygobiotic sampling often leads to small numbers of individuals per catch and can result in damage to fragile specimens, therefore descriptions based on few specimens are not unusual. However, broader examination of Pilbara Nedsia specimens indicated that previously considered species-level characters were likely to represent population-level morphological variations.
Many stygobiotic amphipod genera described from Western Australia are monotypic or contain few species, but molecular analyses have indicated that some described genera contain highly divergent lineages (=putative species) across and within catchments. The paramelitid genera Chydaekata Bradbury, 2000 and Pilbarus Bradbury & Williams, 1997 that are distributed across the Pilbara, are known to have high levels of genetic divergence among populations from different tributaries and in some cases, with little congruence with existing morphological delimitation, thereby suggesting the presence of cryptic species (Finston et al. 2004, 2007). Similar patterns of cryptic species, where species are clearly morphologically indistinguishable, have been seen in chiltoniid amphipods from groundwater-associated mound springs of South Australia (Murphy et al. 2013). Sampling of Nedsia on Barrow Island and in the Pilbara by environmental consultants as part of various surveys, with associated molecular and morphological analyses, has revealed problems with the diagnoses for described species and has also suggested the presence of undescribed species in the Pilbara. Similarly to paramelitid groups in the region, Nedsia likely exists as a complex across Barrow Island and the Pilbara with species that are unable to be adequately defined using morphological characters alone.
The aim of this taxonomic study was to investigate species diversity in the genus Nedsia across the Pilbara, Barrow Island and the North West Cape peninsula. Specifically, to (1) bring data and specimens from disparate regional collecting and environmental impact assessment surveys together; (2) compile and expand existing Cytochrome Oxidase subunit I gene (COI) data and provide additional 28S rRNA gene sequence data to develop a phylogenetic framework; and (3) define species using a combination of morphological and molecular genetic analyses, and examine hydrographic and biogeographic data to inform and support species hypotheses. We adopted this approach acknowledging that many morphological traits in amphipods are considered homoplasious (Hurt et al. 2013; Lowry and Myers 2013), and that multiple lines of evidence are likely required to provide more accurate species delimitation (Padial et al. 2010). This also reflects use of the Generalised Species Concept (GSC) (de Queiroz 1998, 2005, 2007), where species are considered to be ‘separately evolving metapopulation lineages’, and molecular and morphological data provide the ‘operational criteria’ for species delimitation.
Materials and methods
Specimens
More than 400 specimens were sourced from previous surveys of the Pilbara region by the Western Australian Museum, and from three environmental consultancy companies: Bennelongia, Biota Environmental Sciences and Subterranean Ecology. Almost all specimens were collected from bore holes that intercepted groundwater using plankton nets (mesh size range: 50–200 µm, net diameter range: 37–250 mm), that were hauled through the water column, concentrating the samples into a collection tube at the bottom of the net. One sample site in Weelumurra Creek comprised a groundwater and hyporheic upwelling zone that was sampled by excavating a hole in the riparian sediments and allowing the hole to fill with upwelling water that was then filtered through a plankton net. An initial morphological examination of samples was carried out to confirm that the amphipods belonged to the correct family (formerly Melitidae, now Eriopisidae (Lowry & Myers, 2013)), based on the presence of a biramous third uropod, one or multiple robust basofacial seta(e) on the peduncle of the first uropod and the absence of sternal gills. A great deal of this material had not been preserved with the consideration of long-term DNA viability (stored at room temperature, low percentage alcohol preservation) and was not amenable for molecular analyses but was used for morphological assessment (although we note that this material may be suitable for targeted exon capture or genome skimming methods in future; Weitemier et al. 2014).
Published and unpublished COI sequences were obtained from The University of Western Australia (UWA) (n = 269) but DNA voucher material was unavailable or not viable for these samples and additional nuclear gene data could not be obtained from this material (Supplementary Table S1). Using all available museum and consultancy material and some fresh opportunistic collections, a concerted attempt was made to generate additional COI sequences from the main lineages found in the initial COI analysis of the UWA material (using the same bore holes), and to produce corresponding data from the rRNA gene (henceforth 28S; Supplementary Table S1). Additional specimens that were not successfully sequenced were used in the morphological analysis.
Specimens from two genera, Norcapensis (n = 4; including GenBank #JQ608487.1) and a newly detected undescribed genus ‘Eriopisidae gen. undet.’ (n = 18, to be described separately) were used as outgroups for phylogenetic analyses. Confirmation that these genera represented suitable outgroups was obtained using more distantly related taxa from the Hadzioidea: Nurina poulteri Bradbury & Eberhard, 2000; Brachina invasa Barnard & Williams, 1995; Exitomelita sigynae Tandberg, Rapp, Schander, Vader, Sweetman, & Berge, 2011 (COI: GenBank # JN831765.1; Melita plumulosa Zeidler, 1989 (COI: GenBank # JN790072.1); and Eriopisa elongata (Bruzelius, 1859) (28S: GenBank # EU693289.1) in an analysis of both COI and 28S data (results not shown). These distantly related taxa were subsequently excluded from further phylogenetic analyses, due to saturation problems for the COI data and difficulties aligning the 28S sequences, with the exception of highly conserved stem regions that showed limited phylogenetic signal for resolving relationships among species of Nedsia.
DNA extraction, PCR and sequencing
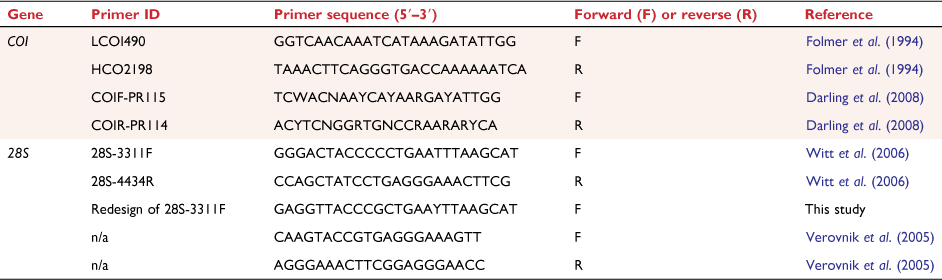
DNA was extracted from two or three pereopods or pleopods removed from the right side of the animal whenever possible. The entire body was used when specimens were very small or badly damaged. Extractions were performed using the Gentra Pure-Gene DNA Purification Kit (Gentra Systems, Minneapolis, MN, USA). Partial sequences of COI and 28S were amplified by Polymerase Chain Reaction (PCR) using existing forward and reverse primer pairs or newly designed ones (Table 1).
|
PCR-amplification was carried out in 25-µL reaction volumes containing 1× Immobuffer (Bioline), 1.5 mM of MgCl2, 0.2 mM of each dNTP, 0.24 µM of forward and reverse primers (0.58 µM for COIF-PR115 and COIR-PR114), 0.5 Units IMMOLASE DNA Polymerase, 0.25 µg µL−1 of bovine serum albumin) and 1 µL (~50 ng) of DNA. Thermal cycling conditions for COI involved an initial hold at 94°C for 10 min followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 47–48°C for 30#160;s and extension at 72°C for 90 s. Following the 35 cycles, a final extension step at 72°C for 6 min completed the reaction. PCR conditions for 28S differed from COI only in the annealing temperature, 55–65°C, varying slightly with the primer combination used and different specimens.
PCR products were purified using Ultraclean PCR cleanup plates (MoBio Laboratories) and sequenced in the forward and reverse direction with the same primers used for PCR amplification. Sequencing was carried out in 20-µL reaction volumes containing 1 µL of ABI Prism BigDye Terminator (Applied Biosytems), 3.5 µL of Big Dye sequencing buffer, 1 µL of 5 µmol of primer and 1–2.5 µL of purified PCR product. Sequencing reactions involved 24 cycles of 96°C for 30 s, 50°C for 15#160;s and 60°C for 4 min. Products were purified using UItraclean sequencing cleanup plates (MoBio Laboratories) and capillary separation was conducted on an ABI 3730xl sequencing platform by the Australian Genome Research Facility Ltd.
Phylogenetic analysis
Forward and reverse sequences were trimmed, edited and assembled into a consensus sequence using Geneious (ver. 9.1.2, see https://www.geneious.com). COI sequences were unambiguously aligned using Clustal W (ver. 1.81, see http://www.clustal.org/clustal2/; Larkin et al. 2007), with default parameters as implemented in Geneious. 28S sequences were initially aligned using Clustal W, as implemented in Geneious, and sections containing many indels (matrix containing distantly related outgroup taxa only) in the alignment were subsequently re-aligned using MAFFT (ver. 7.222, see https://mafft.cbrc.jp/alignment/software/; Katoh et al. 2002) using a gap open penalty of 1.0, a scoring matrix of 200PAM/k = 2 and an offset value of 0.123.
Identical COI haplotypes were removed from the COI alignment using Geneious (Remove Duplicate reads option: Dedupe from BBTools), leaving one representative of each COI haplotype in the final dataset that comprised 90 sequences. Geneious was subsequently used to create a Phylip format input file and PartitionFinder2 (ver. 2.1.0, see https://www.robertlanfear.com/partitionfinder/; Lanfear et al. 2017), as implemented within the CIPRES gateway (ver. 3.3, see https://www.phylo.org/; Miller et al. 2010), was used to assess the most appropriate model of evolution and partitioning scheme for each of the codon positions in COI. The analysis was conducted using the greedy search option and the Bayesian Information Criterion (BIC) for distinguishing the different models and partitioning schemes. The best scheme was as follows: first codon position: GTR+I+G; second codon position: F81+I; and third codon position: GTR+G. These analyses were repeated for the combined COI and 28S dataset, using the greedy and BIC options as before, with the best scheme represented by four partitions as follows: 28S: HKY+G; COI first position: GTR+G; COI second position: F81; and COI third position: GTR.
Bayesian Inference (BI) analyses were conducted independently for each gene and for COI+28S using the Markov chain Monte Carlo (MCMC) approach in MrBayes (ver. 3.2.6, see http://nbisweden.github.io/MrBayes/; Ronquist and Huelsenbeck 2003) with the CIPRES gateway (Miller et al. 2010). For the COI analysis, codon positions were partitioned independently and modelled according to the PartitionFinder results given above. All parameters were unlinked and four chains were run simultaneously for 10 million generations with two independent runs, sampling every 1000th generation. Convergence was established by consideration of the average standard deviation of split frequencies and PSRF values for parameters as calculated by MrBayes (ver. 3.2.6), and using the program Tracer (ver. 1.5, see http://tree.bio.ed.ac.uk/software/tracer/, accessed 1 October 2020; Rambaut et al. 2018) to determine whether estimated sample size (ESS) values were above 200. A burn-in of 25% was chosen and a maximum clade credibility tree was constructed using MrBayes (ver. 3.2.6), and viewed in FigTree (ver. 1.3.1, A. Rambaut, see http://tree.bio.ed.ac.uk/software/figtree/, accessed 1 October 2020). Trees were rooted in FigTree using the outgroup genera Norcapensis and ‘Eriopisidae gen. undet.’. The 28S and COI+28S analyses using MrBayes (ver. 3.2.6) were conducted in a similar way to the above, using the PartitionFinder model, with 10 million generations run and trees sampled every 1000 generations.
Maximum likelihood (ML) trees for each individual gene and COI+28S datasets were generated using RAxML (ver. 7.0.4, see https://cme.h-its.org/exelixis/web/software/raxml/; Stamatakis et al. 2008) and PhyML (ver. 3.0, see http://www.atgc-montpellier.fr/phyml/; Guindon et al. 2010), as implemented in the program Geneious (ver. 9.1.8) or using the CIPRES gateway (Miller et al. 2010). A GTR model with a gamma model of rate heterogeneity was used for each partition (COI codon positions and 28S) and tree robustness was assessed using >500 fast bootstrap pseudo-replicates for the RAxML analyses, using the no-limit option in CIPRES.
Species delimitation analyses
Under the GSC (de Queiroz 1998, 2005, 2007), the operational criteria that we used to delimit species were the presence of monophyletic groups of individuals that were genetically divergent from other groups and showing concordance of the independent genetic markers and morphological data.
The COI data were also analysed using the program Automated Barcode Gap Discovery (ABGD; https://bioinfo.mnhn.fr/abi/public/abgd/; Puillandre et al. 2012) to further assess species boundaries. The parameters used in the analysis were: Pmin = 0.001, Pmax = 0.1, steps = 10, X (relative gap width) = 1 and the Kimura 2 parameter distance model (TS/TV = 2.0). In addition, a PhyML tree of COI data (n = 90) was analysed using the online (http://species.h-its.org/ptp/) version of the program bPTP (Zhang et al. 2013) to generate species hypotheses for comparison. We also utilised the Species Delimitation plugin in Geneious (ver. 9.1.8) that implements a method that calculates the probability of reciprocal monophyly under a null model of random coalescence (Rosenberg 2007). Using the PhyML tree (single partition analysis) and a cut-off probability P(AB) of 0.05, we tested whether different sister groups or species defined using ABGD or bPTP may have arisen through random coalescence. We also used the Species Delimitation plugin to calculate pairwise average distances between these groups or lineages in the PhyML tree and used arbitrary DNA divergence (ML distance) cut-offs of 5, 10 and 15% to assess the divergence of monophyletic groups of individuals for comparison with results from ABGD and bPTP analyses.
Synapomorphic sites for COI and 28S associated with putative species were identified using PAUP* (ver. 3.99.167.0, see http://phylosolutions.com/paup-test/). Parsimony uninformative sites were initially excluded and the RECONSTRUCT command was subsequently used to map informative sites to the phylogeny. Only sites that were unique to and distinguished among Nedsia species were recorded (Supplementary Table S2).
Results
Phylogenetic analyses: COI data
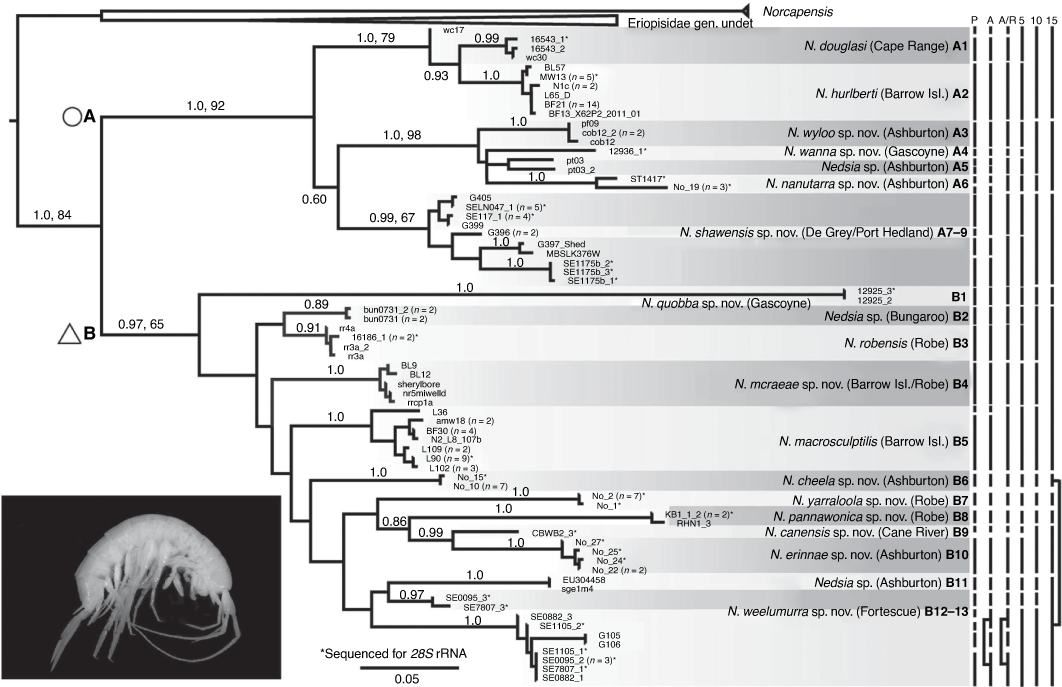
The final COI dataset comprised 157 sequences, including four Norcapensis, 18 ‘Eriopisidae gen. undet.’, and 134 Nedsia sequences. Prior to phylogenetic analyses, the COI sequences were further trimmed to 90 unique sequences by the removal of identical COI haplotypes. ML and BI analyses using a single gene partition or separate partitions for each codon position generally produced congruent trees, with the exception of the placement of a lineage comprising two individuals (12925_2 and 12925_3; from the Gascoyne region, located slightly south of the Pilbara). This lineage was positioned, with a very long branch, within a monophyletic group (here labelled group B) in all the partitioned analyses (i.e. codon positions treated as separate partitions (Supplementary Fig. S1), but was placed as a sister lineage to group B in non-partitioned analyses (i.e. using a single partition for COI, Fig. 1). Given that independent analyses of combined COI+28S rRNA data also placed the 12 925 individuals outside a group comprising all other lineage B taxa (see below), we consider the COI tree based on the non-partitioned analyses to represent a more likely hypothesis of relationships among the taxa, but note that there is considerable uncertainty in the position of the Gascoyne lineage. Overall, Nedsia was resolved into two distinct monophyletic groups (A and B), each receiving high posterior probability support (>0.97) and, for group A, high bootstrap support of 92% (Fig. 1).
Phylogenetic analyses: 28S rRNA data
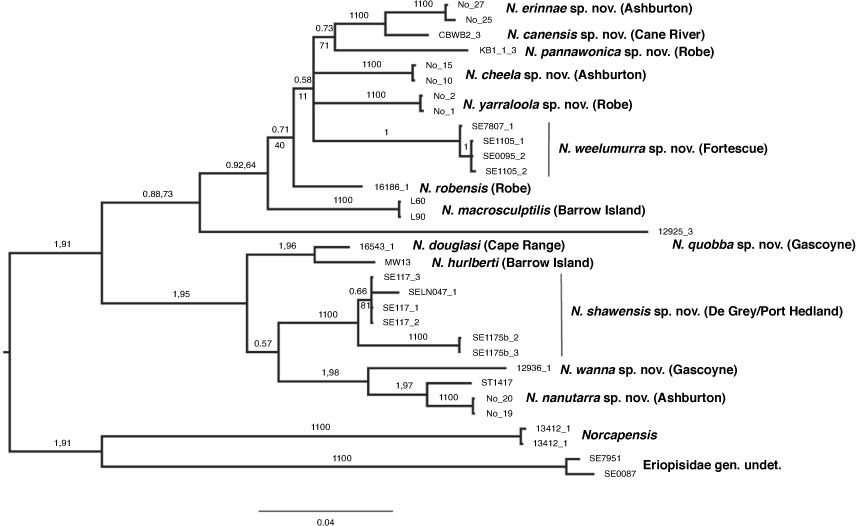
28S sequences were obtained from 38 specimens, 34 of which were Nedsia, with the final alignment matrix being 790 bp long. Owing to technical problems, only partial 28S rRNA data (344 bp in the matrix) were obtained from sample 16543.1 (lineage A1) but inclusion in analyses did not change the overall topology of the trees obtained. Phylogenetic analyses using ML and BI produced relatively poorly resolved trees, with low bootstrap support for most internal branches. However, 17 distinct lineages identified in the COI gene tree were supported in the 28S tree (Supplementary Fig. S2), with the exception of a COI lineage comprising SE7807_3 and SE0095_3 from the Fortescue catchment. In the 28S tree, the SE7807_3 sequence was identical to SE7807_1 that were both from the same bore, and SE0095_3 was identical to SE0095_2 that were from the same bore. Re-sequencing of COI revealed the presence of multiple copies of the gene for SE7807_1 and SE0095_3 and, therefore, the presence of a separate evolutionary lineage or species comprising these samples is likely to be erroneous (Fig. 1, Supplementary Fig. S2). Also, in contrast to the COI phenogram, MW13 did not form a sister lineage to 16543.1, although overall the relationships among the lineages of group A taxa were poorly resolved (Supplementary Fig. S2).
Phylogenetic analyses: combined COI and 28S data
Phylogenetic analyses (ML and BI) of the combined COI+28S data gave stronger resolution of relationships among taxa than that provided from the analyses of single genes, with monophyly of Nedsia relative to Norcapensis and the new genus supported by high posterior probability (=1) and bootstrap support (=91%) (Fig. 2). These analyses also provided further support for the presence of two main monophyletic groups of Nedsia in the Pilbara region. These also placed the Gascoyne specimen 12925_3 as sister to the remaining group B lineages, although this arrangement was only moderately supported (bootstrap value = 73%; posterior probability = 0.88; see Fig. 1, 2).
Species delimitation using bPTP, ABGD and random coalescence analyses
Using bPTP analyses of a COI ML tree, 29 Nedsia lineages, excluding the erroneous COI lineage comprising SE7807_3 and SE0095_3 were identified as putative species with Bayesian support levels ranging from 0.31 to 1.0 (Fig. 1). A similar result was obtained for the ABGD analyses, with the number of groups v. prior intra-specific divergence showing the recursive partitions levelling off at 25 groups of Nedsia (excluding SE7807_3 and SE0095_3) (Fig. 1). Most of these ABGD groups correspond to distinct monophyletic groups of COI haplotypes or divergent COI lineages containing a single individual (Fig. 1). When analysing whether each of the ABGD groups could lead to rejection of a null hypothesis of random coalescence acting on a single panmictic population of constant size (Rosenberg 2007), the hypothesis could not be rejected for four groups defined using ABGD analyses, resulting in 21 distinct groups that could be considered as putative species (Fig. 1). This latter partitioning of monophyletic groups into discrete species was nearly identical to a partitioning scheme based on a 5% (20 taxa) divergence level. Higher threshold levels of 10 and 15% yielded much lower estimates of the number of putative species, with 14 and 9 taxa estimated respectively (Fig. 1).
Morphological analyses
Extensive evaluation of morphological variation between and within populations directly linked to COI lineages confirmed that species within Nedsia are highly morphologically conserved. Only a small number of derived characters corresponding with a few specific lineages occur, largely aligned with a 5–10% divergence level. Despite this, our morphological results broadly corroborate parts of the phylogeny including the two main well supported groups (A and B). Group A consists of several morphologically cryptic lineages (Fig. 1, lineages A1–A9), with all lineages united by possession of one basofacial seta on the peduncle of uropod 1 and smooth dorsal pleonal and urosomal margins (as opposed to any sculpturing). No additional morphological characters were found to reliably distinguish any of the lineages within group A. In slight contrast, group B possesses morphological characters that unite most lineages: broadly, the possession of 2–3 basofacial setae on the peduncle of uropod 1 (except for lineages B3, B5 and B9, Fig. 1) and, more specifically, the presence of sculpturing on the dorsal pleonal and urosomal margins and strong ventral and lateral setation of the epimera in lineages B2–B5 (Fig. 1).
We infer that many of the characters used to delimit the previously described species of Nedsia likely represent population-level morphological variation (and sometimes at the individual level e.g. asymmetry of appendages), as a result of inadequate material for comparative purposes. Five of the previously described Nedsia species were described from a single specimen (N. fragilis, N. humphreysi, N. hurlberti, N. macrosculptis and N. sculptis), and others were described from small (possibly juvenile) specimens of less than 4 mm. Indeed, Bradbury (2002) noted that additional Barrow Island material could not always be definitively identified to a described species.
Given that previous morphological delimitation of species was likely flawed, we here review the main morphological characters used to differentiate the 11 previously described species of Nedsia (Barnard and Williams 1995; Bradbury and Williams 1996; Bradbury 2002) from Barrow Island and the North West Cape peninsula.
Maxilla 2 marginal setation on the inner plate was recorded by Bradbury and Williams (1996) as sparse for N. straskraba, N. fragilis and N. humphreysi that were all described from single small male specimens (3 mm or less). We have subsequently found this character to be size class dependent with fewer setae in larger specimens (>4 mm).
Male pleopod 1 rami with three to four articles v. six or more articles (latter seen in N. macrosculptilis, N. sculptilis and N. hurlberti) was also recorded by Bradbury and Williams (1996); however, all the males examined with six or more articles were larger than 4.5 mm (v. 4 mm or less) and again, we found this character to be variable between size classes with smaller individuals possessing fewer pleopodal rami articles.
Pleonites with sculpturing on the dorsal and dorsolateral margins was recorded by Bradbury and Williams (1996) for N. macrosculptilis (pleonites 1–3, described from a 5.5-mm female) and N. sculptilis (pleonites 2–3 only, described from a 4-mm male), yet we found these differences equated to size and sex, with larger females from species with this sculpturing always possessing it on pleonites 1–3 and males (generally smaller than females) with the sculpturing on pleonites 2–3 only. In the current study, sculpturing occurred within four separate lineages (B2–B5; Fig. 1): one on Barrow Island and three across the Pilbara mainland.
Epimera facial ‘spines’ (described herein as robust setae distributed across the lateral face of the epimera) were recorded as present in N. macrosculptilis and N. sculptilis by Bradbury and Williams (1996). We found this setation on the epimera in all species with dorsal pleonal sculpturing.
Pleonites with or without dorsal ‘spines’ (robust setae) was used by Bradbury and Williams (1996) to separate N. straskraba from N. fragilis and N. humphreysi, whereas we found this setation character to be variable within populations and not directly related to size or sex.
Epimera distolateral ‘spines’ (a row of robust setae that may be present on the distal margin of the epimera 1–3) was used by Bradbury and Williams (1996) to separate N. fragilis from N. humphreysi, and by Bradbury (2002) to distinguish N. stefania from N. chevronia and N. hallettii. However, we found this to be variable within populations and not directly related to size or sex.
Gnathopod 1 propodus shape (subquadrate or rounded) was used by Bradbury and Williams (1996) to separate N. fragilis from N. humphreysi, and by Bradbury (2002) to distinguish N. stefania, whereas we found that propodus shape and setation varied within populations, possibly related to size or sex.
The shape of the maxillipedal outer plate (1.6 to more than 2 times width and rugose or not rugose) was used by Bradbury and Williams (1996) to distinguish N. douglasi from N. urifimbriata and N. macrosculptilis from N. sculptilis, respectively and by Bradbury (2002) to distinguish N. halletti and N. chevronia from N. stefania; however, we found these characters to be variable within populations possibly related to size, with larger individuals exhibiting a broader (and seemingly more rugose) outer plate.
Urosomite 1 with a spine-like extension at the base of uropod 1 was recorded for N. hurlberti by Bradbury and Williams (1996) but this appears to be a size-related character with larger individuals possessing a more prominent extension at the base of uropod 1.
Coxa 1 with anterior ‘spines’ (robust setae) was recorded by Bradbury and Williams (1996) for N. urifimbriata; however, we found this setation character to be variable within populations, possibly related to size with larger individuals exhibiting more setae.
Telson with or without dorsal setae was used by Bradbury and Williams (1996) to distinguish N. douglasi from N. urifimbriata, yet we found this to be variable within populations and not directly related to size or sex.
Hydrographic information
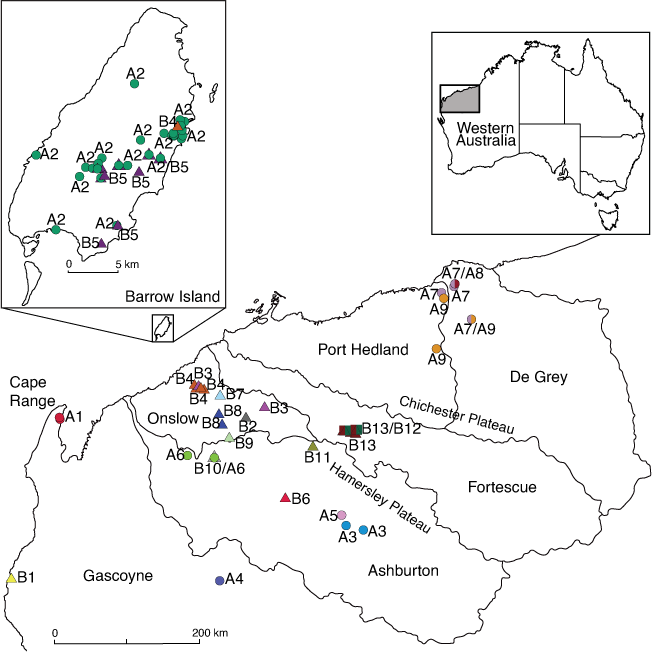
Hydrographic distributional data provide additional support for the molecular lineages found here, with lineages largely corresponding to specific drainages within catchments and furthermore that groups of related lineages occur together within single or adjacent drainage basins (Fig. 3).
Group A lineage distributions arc around the Hamersley and Chichester Plateaus in the De Grey, Port Hedland and Ashburton hydrographic basins in the Pilbara, and Lyon catchment (Gascoyne), Barrow Island and the North West Cape peninsula. Lineage A1 includes individuals from the Exmouth aquifer (Water Corporation bore field) at the North West Cape peninsula, while A2 (5% COI divergence from A1) contains individuals from adjacent Barrow Island. Lineages A3, A5–A6 are distributed along the Ashburton River catchment: Metawandy Creek–Hardey River (A3), Bellary Creek (A5) and the Nanutarra area (A6), along with a lineage (A4) from the adjacent Lyons River catchment, Gascoyne. Lineages A7–A9 include individuals distributed across the adjacent De Grey and Port Hedland basins in the northern Pilbara.
Group B lineages are distributed adjacent to and west of the Hamersley Plateau in the Ashburton, Fortescue and Onslow Coast hydrographic basins, Barrow Island and Gascoyne region. Lineage B1 is from Quobba Station, north of Carnarvon in the Gascoyne region and is the most geographically isolated species from the main Pilbara group and the most genetically divergent species (at 39.9% for COI). Lineages B2–B3 are from Bungaroo Creek and the lower Robe River catchment (both Onslow Coastal basin). Lineage B4 individuals come from Barrow Island and the Robe River catchment (Onslow Coastal basin). Lineage B5 is endemic to Barrow Island. Lineage B6 individuals are from Calgara station in the Ashburton River catchment and basin (Calgara station bore). Lineage B7 is from Yarraloola in the Robe River catchment, west of Pannawonica and lineage B8 is from Red Hill Creek, also within the Robe River catchment (Onslow Coastal basin). Lineage B9 is from the upper Cane River (Onslow Coastal catchment) and lineage B10 is from near Nanutarra in the Ashburton River catchment and basin. Lineages B11–B13 are distributed within the Hamersley Range alluvial valleys: Caves Creek (B11) and Weelumurra Creek (B12–B13); areas mapped as adjacent to Fortescue River and Ashburton River drainages, but unpublished survey data indicate that groundwater species distributions do not strictly follow drainage patterns in these areas (S. A. Halse, unpubl. data).
Most Nedsia specimens were collected in palaeovalleys and channels in quaternary alluvial aquifers suggesting that this is a preferred habitat for members of the genus (Halse et al. 2014).
Defining species
Nedsia is clearly a speciose and diverse genus with numerous divergent lineages distributed across the Pilbara, the North West Cape peninsula, Barrow Island and Gascoyne region of Western Australia. Species delimitation methods used on the more extensive COI phylogeny indicated that 14–29 species of Nedsia exist (Fig. 1). However, the bPTP and ABGD methods also detected phylogeographic structure within species and hence this necessitates taking an integrative approach and also utilising other lines of evidence, including independent genetic markers, morphology and hydrographic data. Scrutiny of the COI lineages using morphological and hydrographic data, and analyses of the 28S data, have led us to hypothesise that 17 species of Nedsia exist across Barrow Island, the North West Cape peninsula and the Pilbara with two additional divergent Nedsia species in the Gascoyne.
Across group A, we propose seven putative morphologically cryptic Nedsia species (with smooth (not sculptured) dorsal pleonites, one uropodal basofacial seta) from nine distinct lineages. Lineages from Exmouth on the North West Cape peninsula (A1) and neighbouring Barrow Island (A2) are identified as separate species that correspond to the described Nedsia douglasi and N. hurlberti respectively. Three lineages (A3, A5 and A6) within the Ashburton River basin form a natural group with an adjacent Gascoyne lineage (A4) that we recognise as four separate species with more than 5% divergence levels. Lastly, lineages found across the De Grey and Port Hedland basins (A7–A9) potentially form two separate species: A9 and the lineages A7+A8 united into a single species with less than 3.5% divergence. However, sequences from A7 and A9 (LN047, SE0117 and MBSLK376) are from bores within a 40-m radius of each other and there may be multiple copies of COI confounding the phylogenetic signal. Within A9, sequences forming the SE1175b cluster are more geographically isolated from this group (~60 km south of LN047) and may represent a unique species for lineage A9, but we have not defined this as such as we were unable to examine adult specimens and could not provide a full description of the species.
Across group B, we recognise B1 from Quobba Station in the Gascoyne region as a distinct species that, based largely on COI data, is sister to the remaining group B lineages but morphologically similar to group A species (smooth dorsal pleonites, one uropodal basofacial seta). Interestingly, 28S data place this Gascoyne lineage basal to both groups A and B in contrast to the COI data so placement within group B is treated cautiously in terms of inferring relationships. We recognise 11 putative species from the 12 remaining lineages. Lineages B2–B5 are strongly supported and distinct lineages forming a close group, with unequivocal morphological characters uniting these (sculptured dorsal pleonites, setose epimera, 1–2 uropodal basofacial setae). Hydrographic data show that these lineages are closely located within the Robe River catchment (Onslow coastal basin) and the adjacent Barrow Island. Our conclusions are that each lineage represents a unique species. (B5) is isolated on Barrow Island, while another species (B4) exists with individuals distributed across Barrow Island and the adjacent Robe coastal plain, and lastly two unique species (B2, B3) exist at discrete sites in the upper Robe River and coastal plains. The eight remaining lineages are recognised as seven morphologically similar species (smooth dorsal pleonites, 2–3 uropodal basofacial setae). One distinct species is recognised from lineage B6 at Cheela Plains, in the Ashburton River catchment. Two distinct species are recognised from lineages B7 and B8 in the Upper Robe River catchment. A unique species from lineage B9 is identified from the upper Cane River area. One species is identified from lineage B10 in the Nanutarra area. Lastly, three lineages from around the Hamersley Ranges, Caves Creek, Ashburton River (B11) and the Fortescue River area (B12–B13) are recognised as two distinct species. The latter two lineages (B12 and B13) are combined into a single species despite divergence levels of >6.5% in COI, as re-evaluation of the molecular data indicated that there were multiple copies of COI for SE0095 and SE7807 that grouped closely based on 28S data.
While we were able to confidently translate lineages to species, we were unable to describe all the species identified due to lack of additional material to designate as type specimens. Therefore species associated with A5, A9 (for SE1175b), B2 and B11 are not formally described herein. In addition, we were able to morphologically assess a single juvenile specimen for B7 that may not exhibit all the characters necessary for an unambiguous morphological description. In this case, we have described the species based on sequence data with a limited morphological diagnosis and illustrations.
Most of the 11 previously described Nedsia species (Bradbury and Williams 1996; Bradbury 2000) need to be synonymised. Only three species, N. douglasi, N. hurlberti and N. macrosculptilis remain valid. Nedsia douglasi (lineage A1) is confirmed to be restricted to the North West Cape peninsula, but closely related to N. hurlberti (A2) from Barrow Island. Nedsia hurlberti has been selected as the replacement name for one of the two widespread Barrow Island species. In our study, no sequences were obtained from the exact bores from which the type material of the eight synonymised names was collected (see under Systematics, below). Despite this, we have comprehensively explored genetic diversity within Nedsia across Barrow Island (Fig. 3) and are confident in our taxonomic decisions. Nedsia hurlberti was selected to represent lineage A2 because the holotype (a female, 4.5 mm), that matched the morphology of this sequenced lineage, was the largest and presumably most mature of all the previously described species from Bradbury and Williams (1996). The second widespread Barrow Island species (lineage B5) (Fig. 1, 3) has distinct dorsal sculpturing on pereonites 1–3 and corresponds to N. macrosculptilis and N. sculptilis, both described in Bradbury and Williams (1996). We have chosen N. macrosculptilis as the valid name and synonymise N. sculptilis, as N. macrosculptilis was originally described from a large mature female (5.5 mm) and should represent the species more appropriately.
Taxonomy
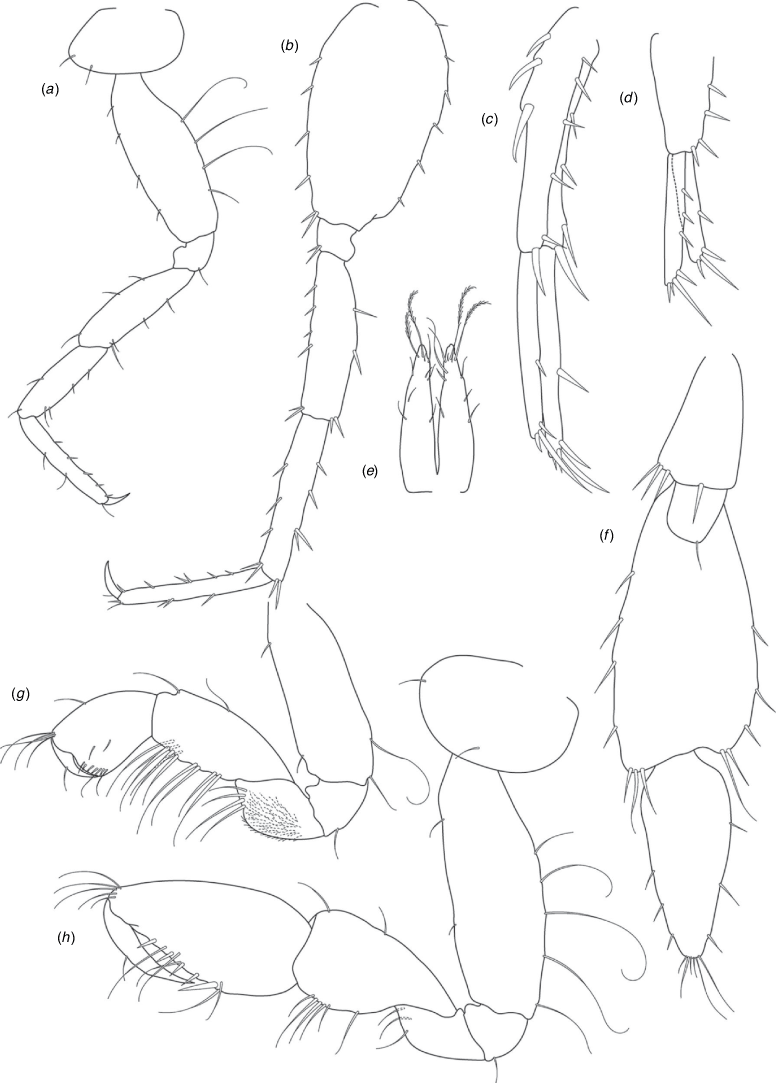
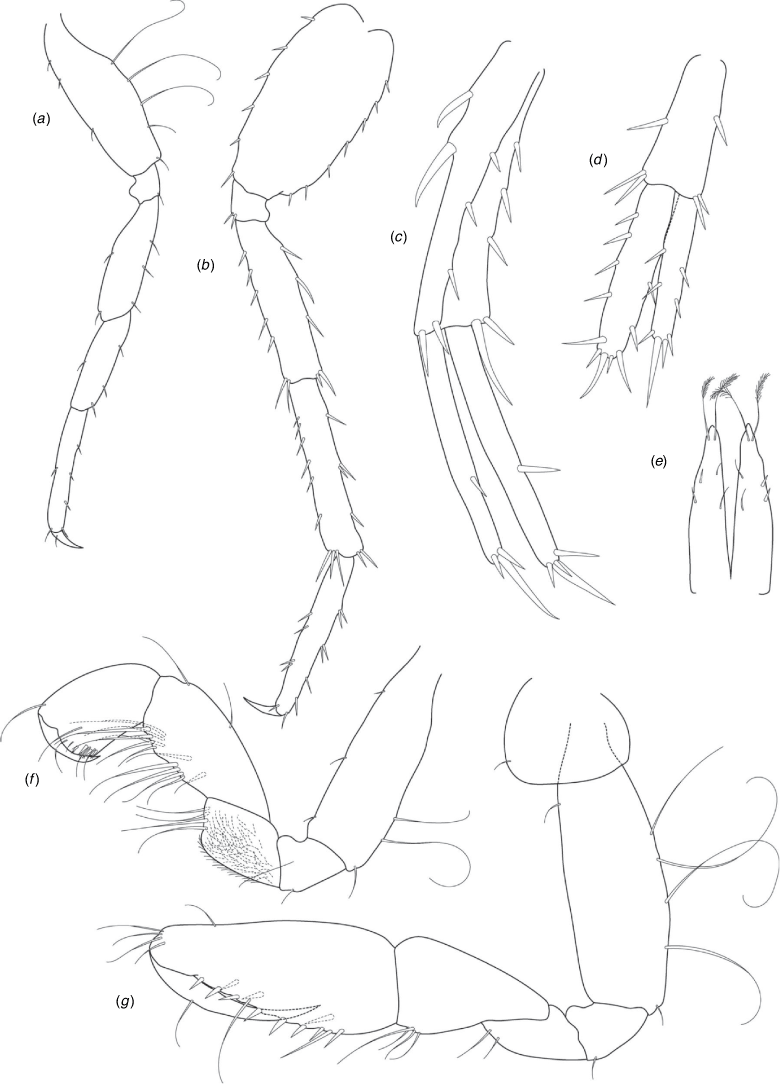
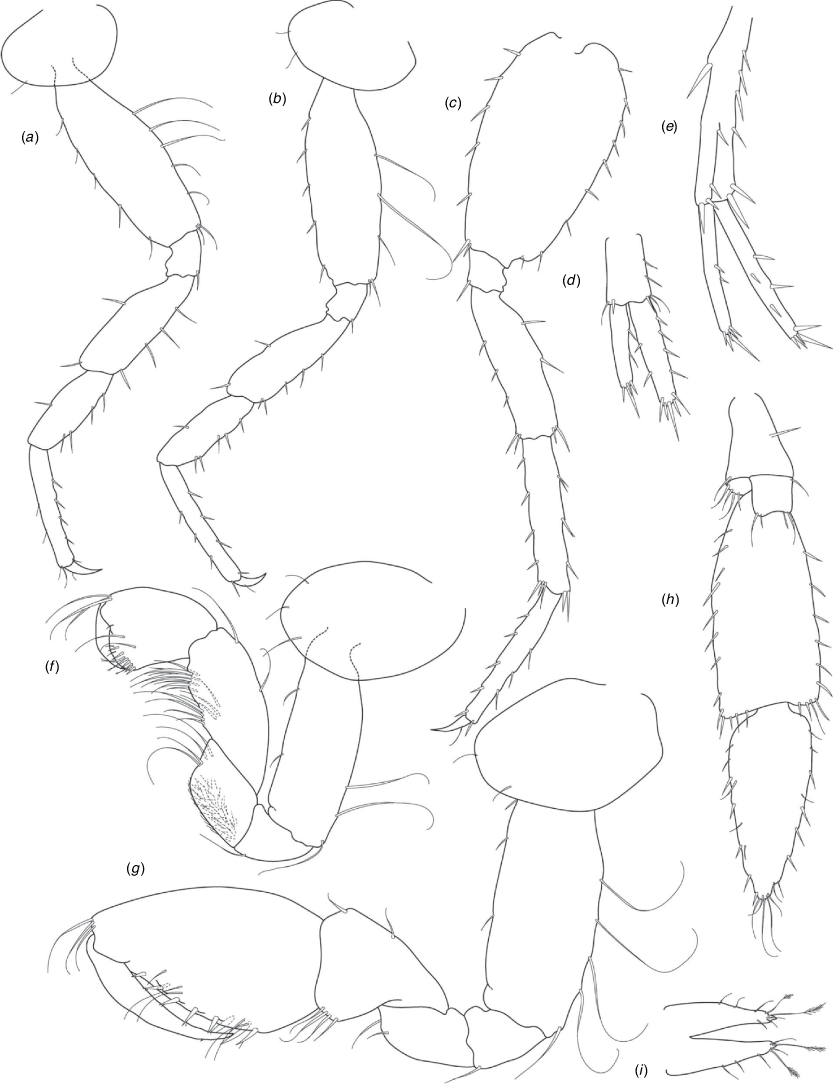
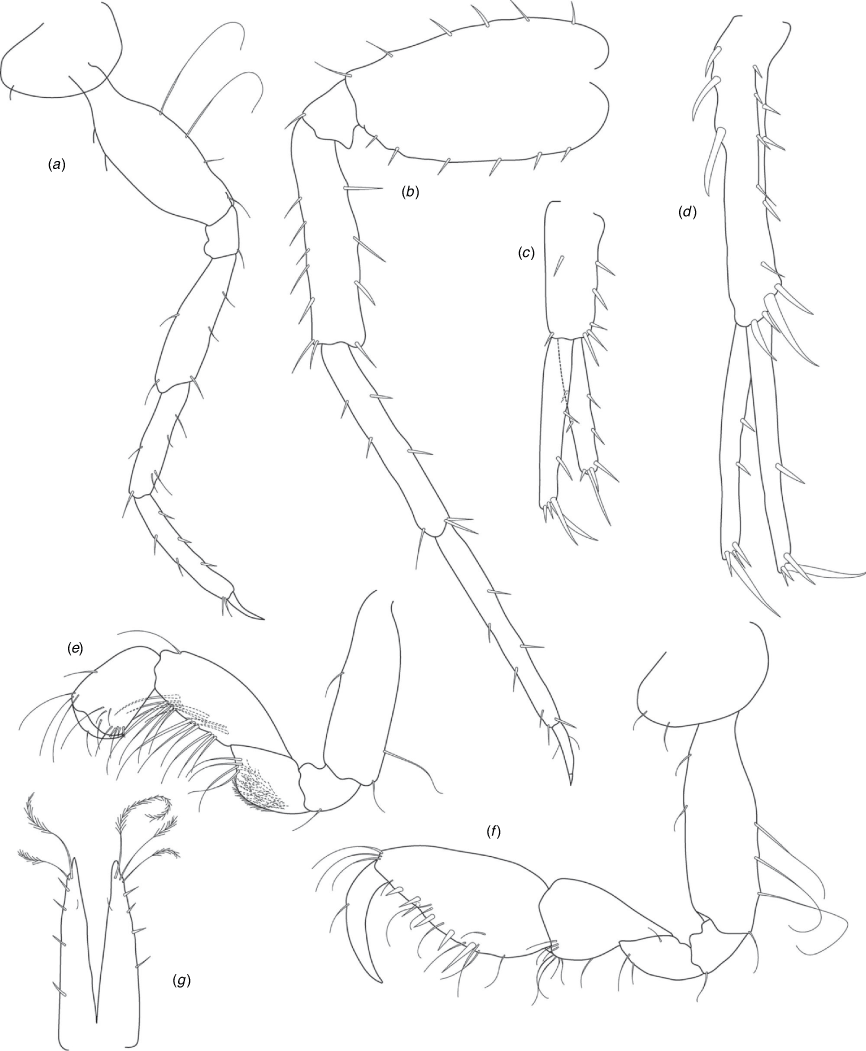
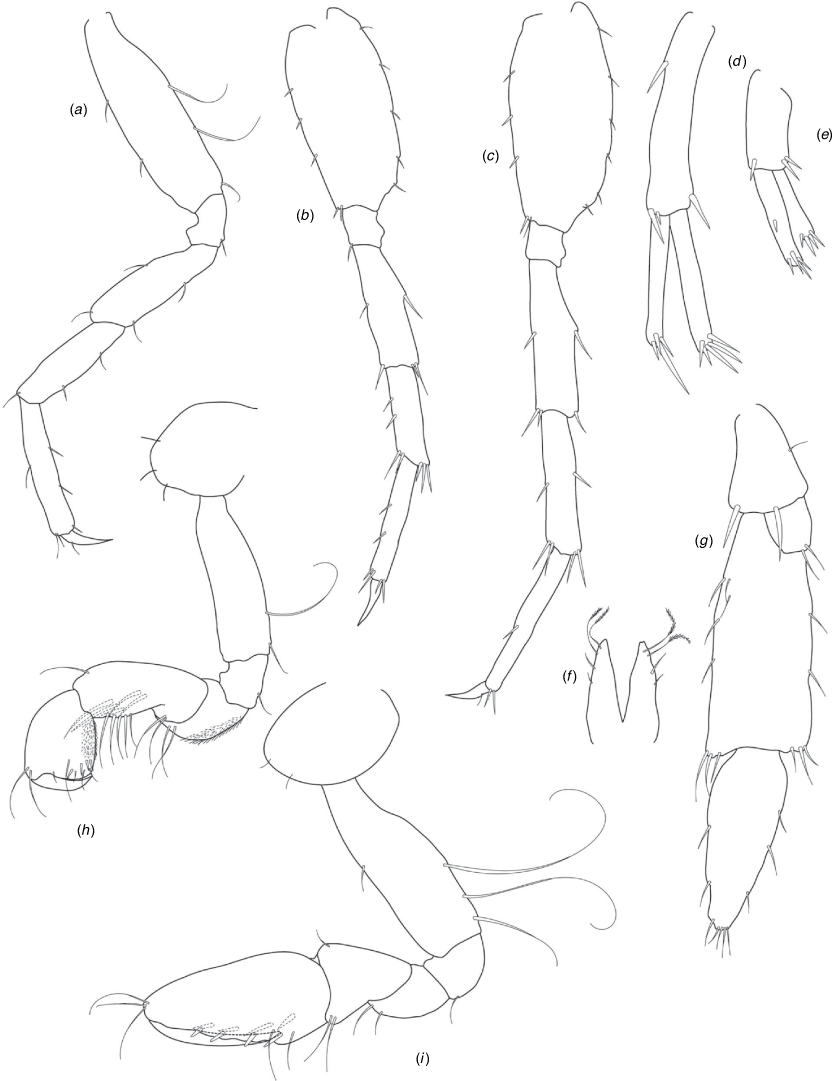
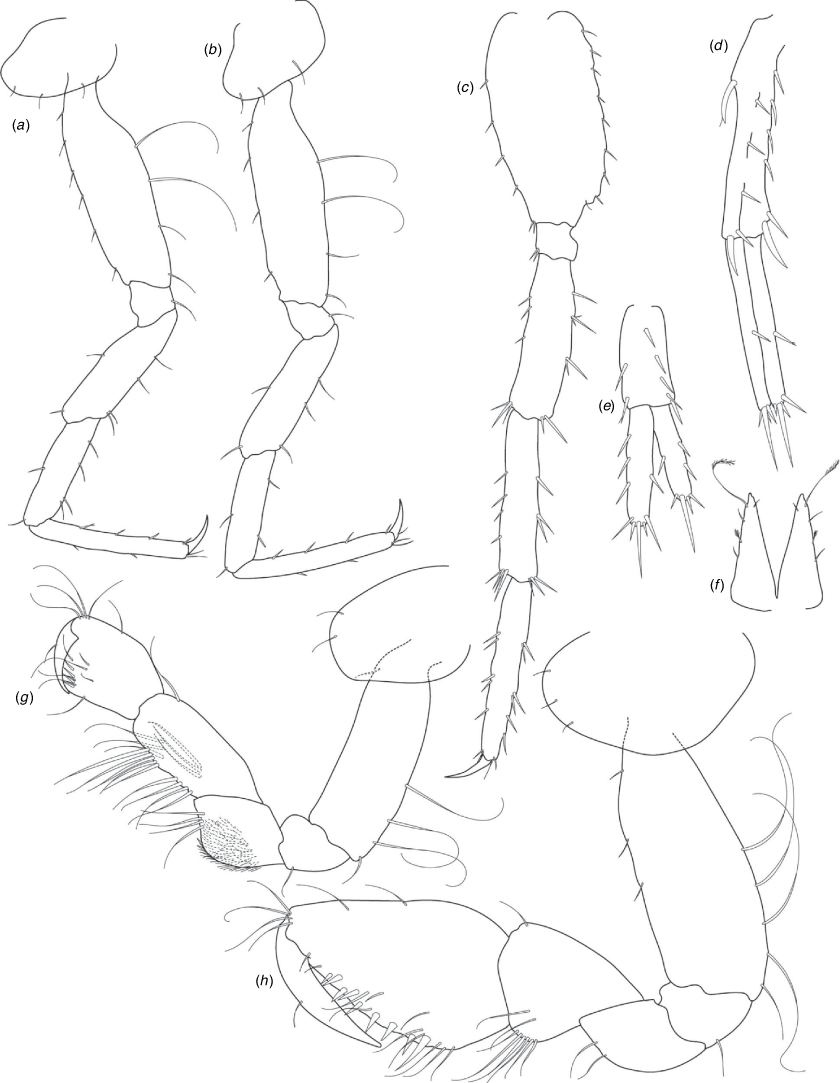
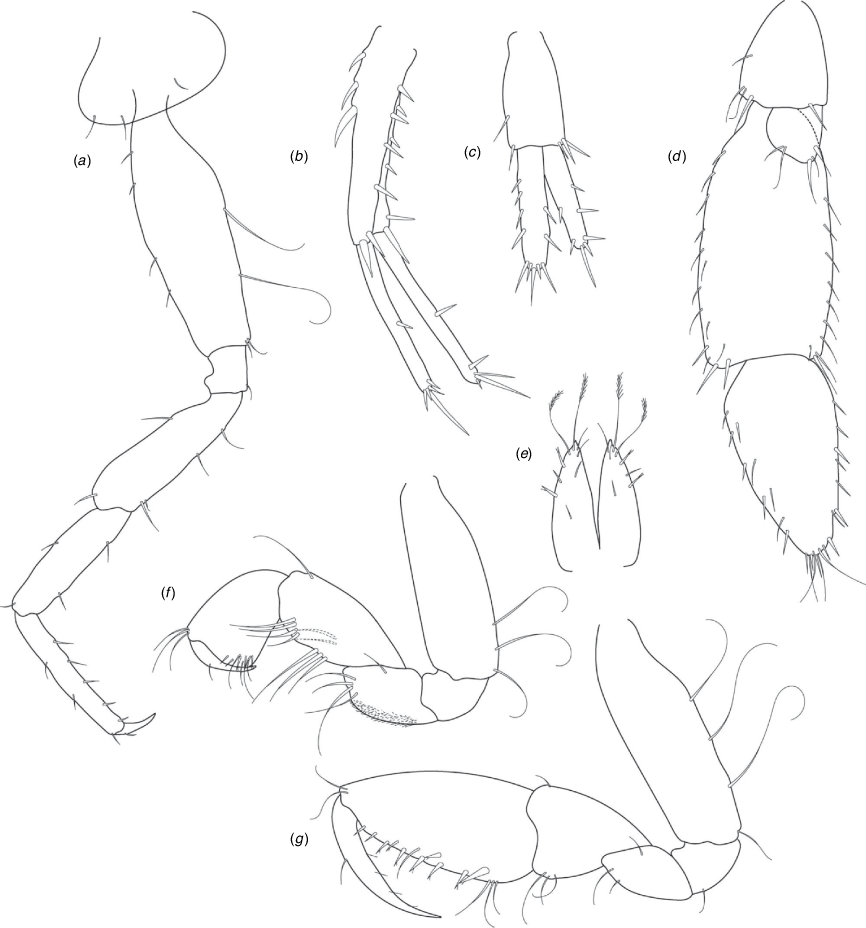
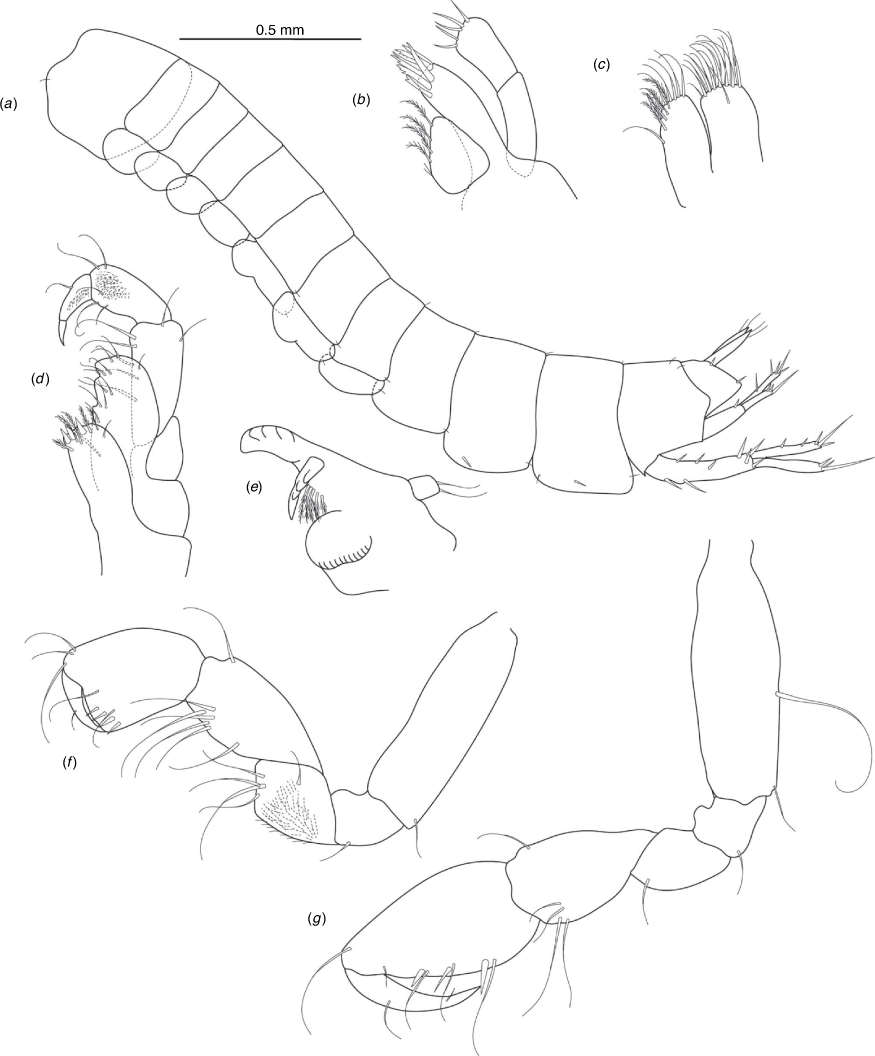
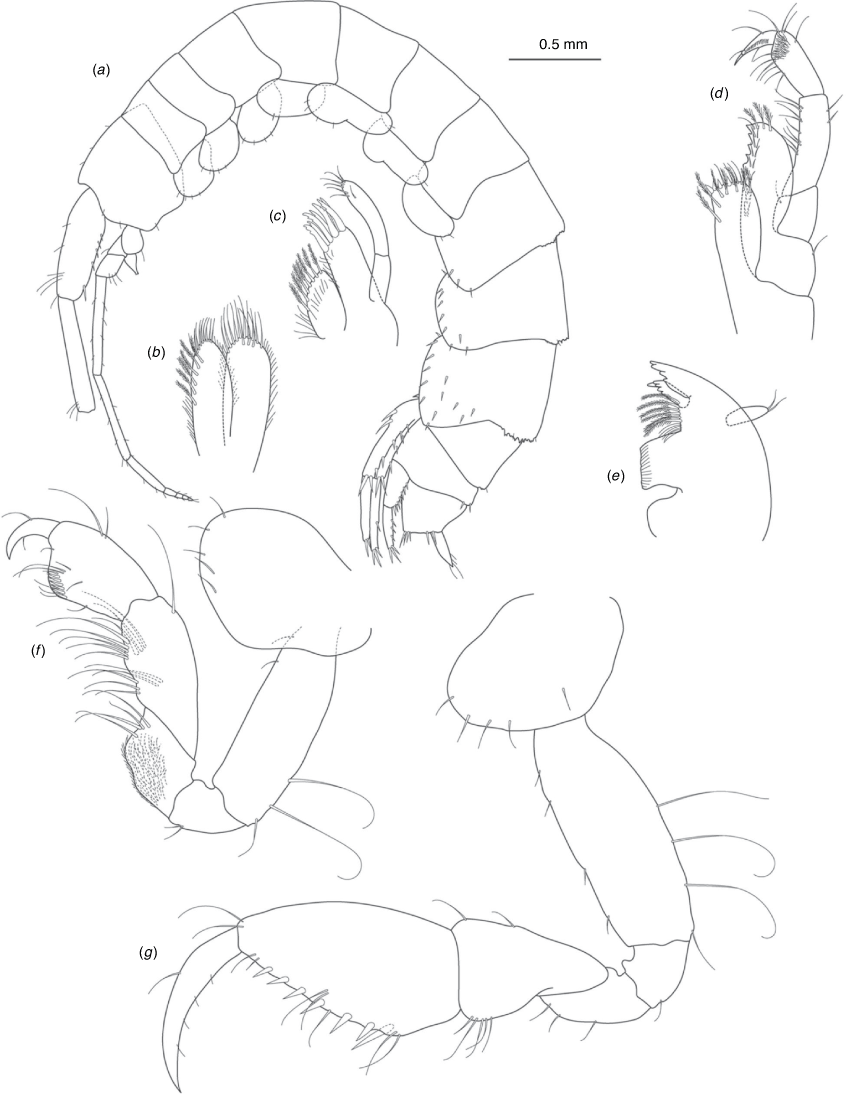
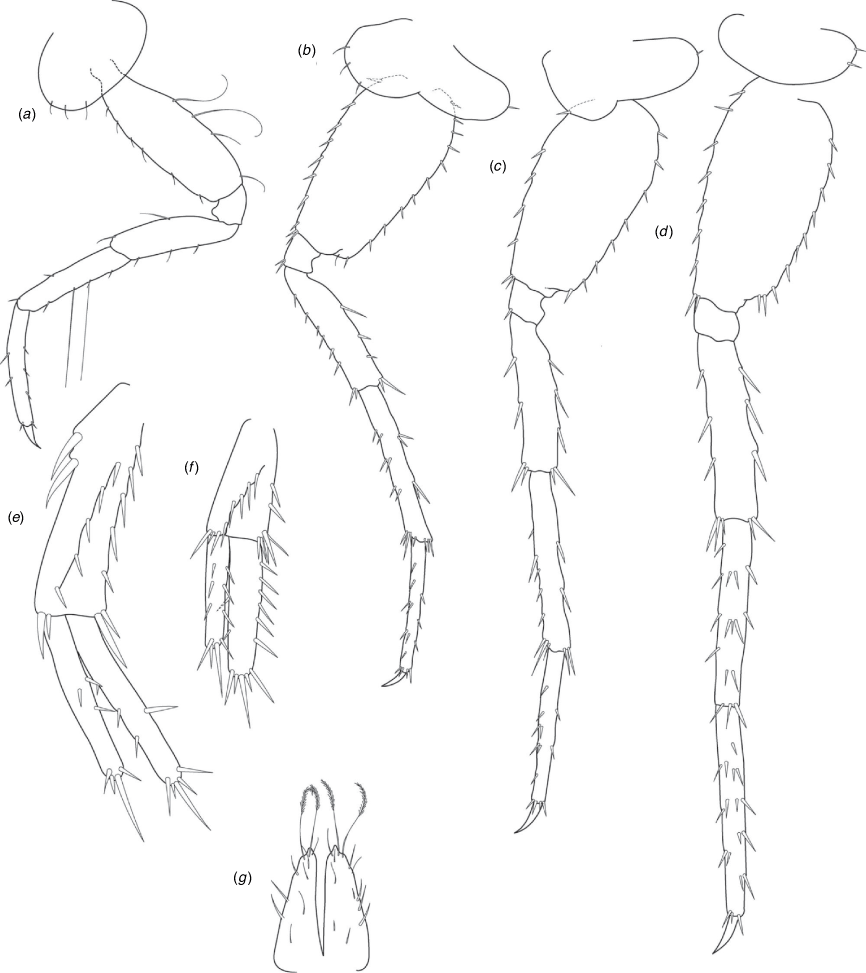
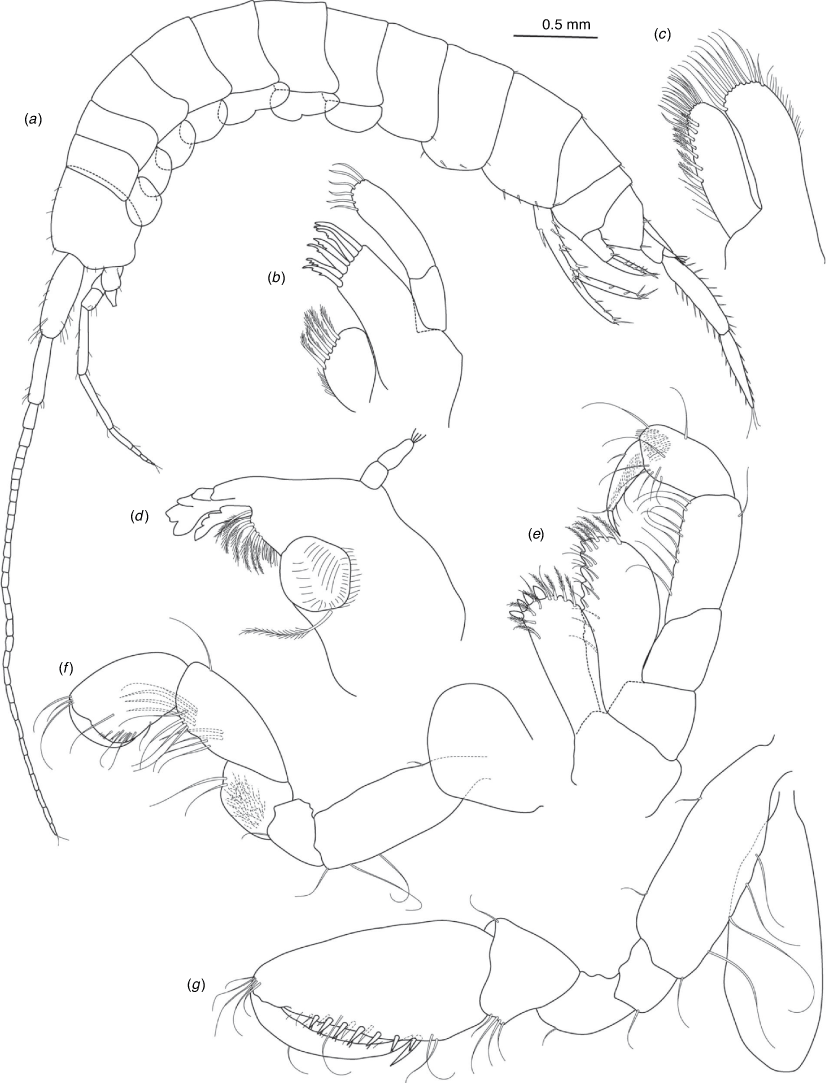
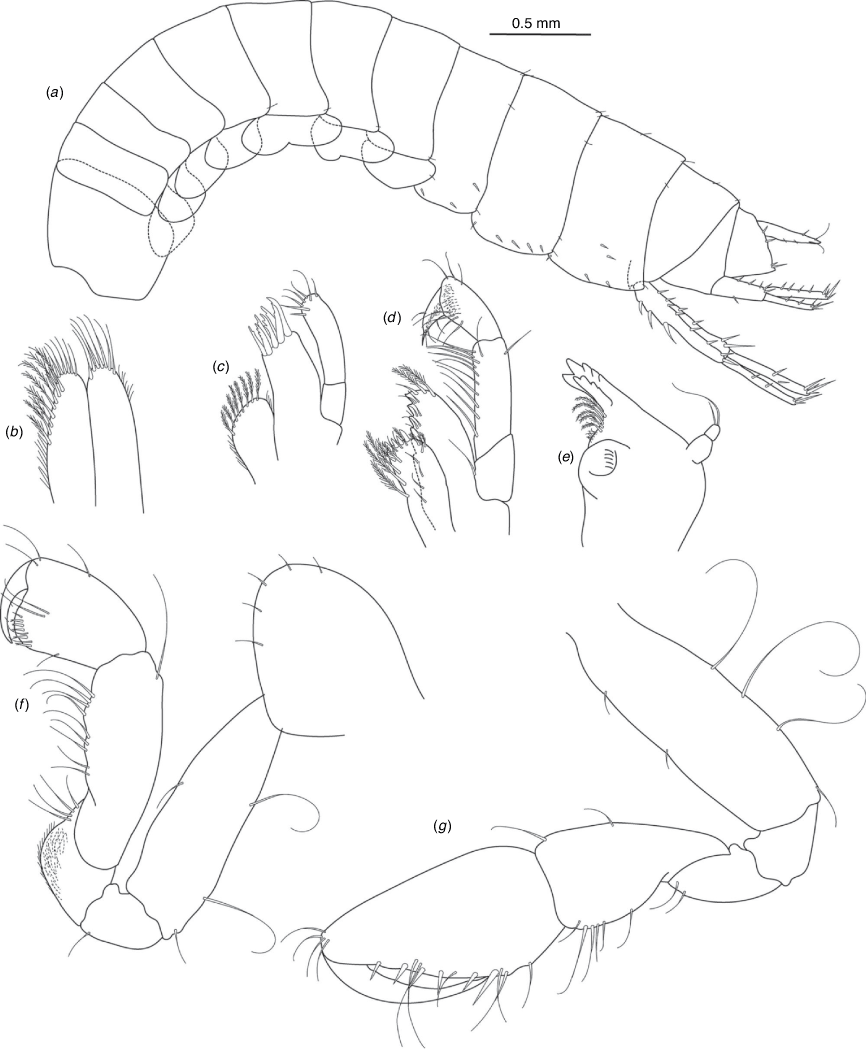
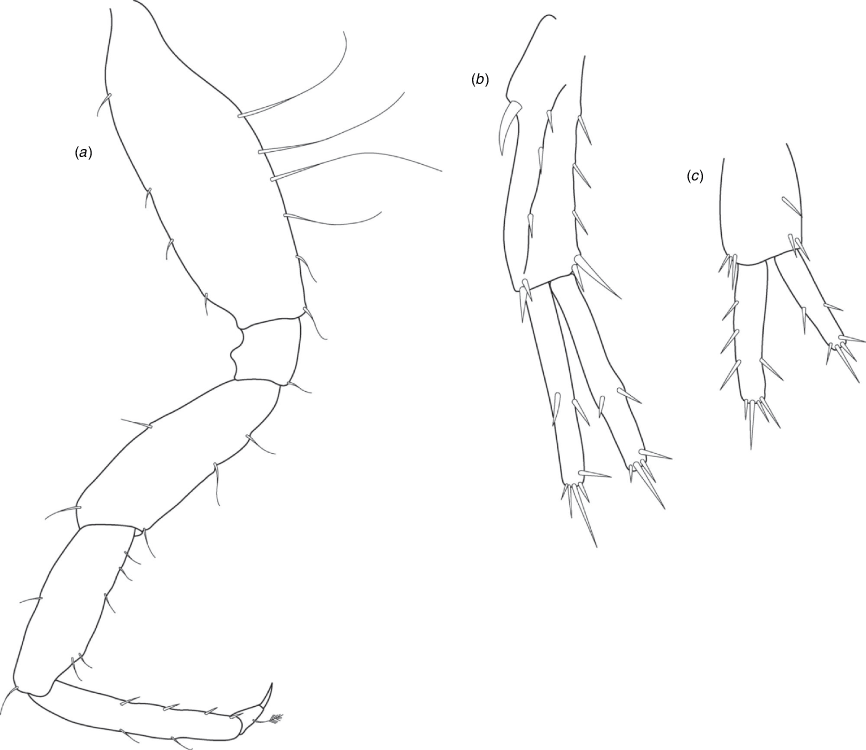
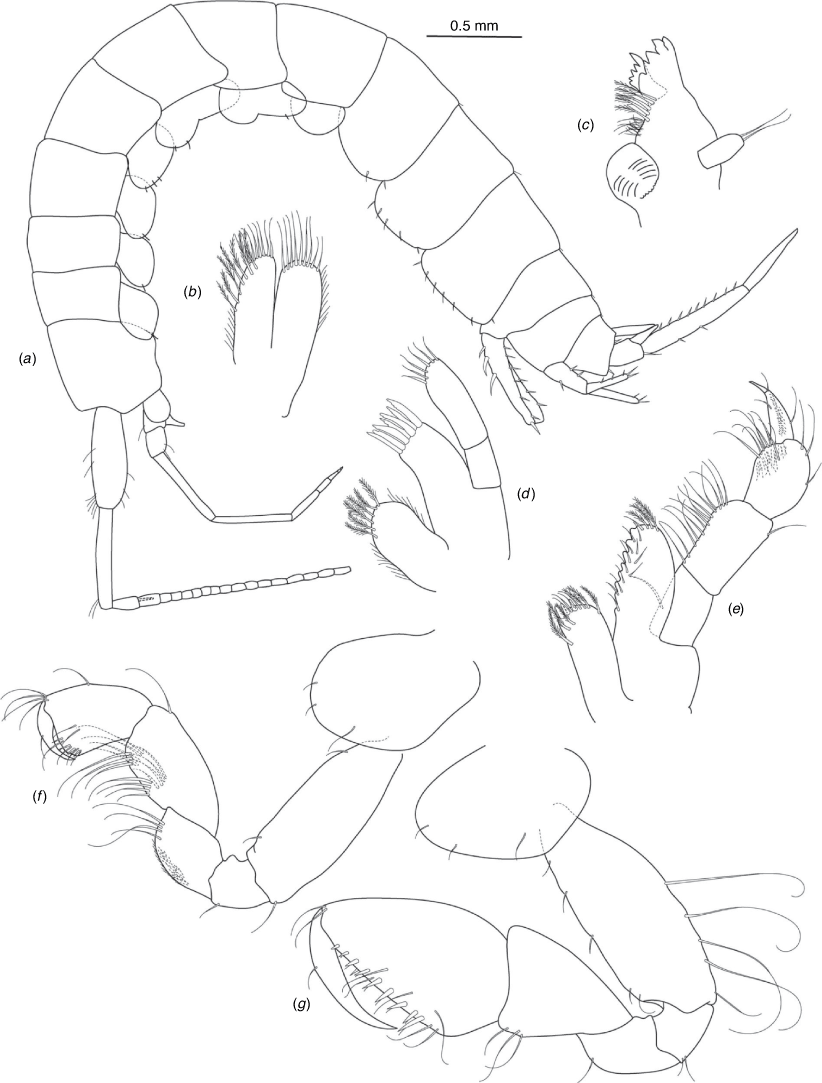
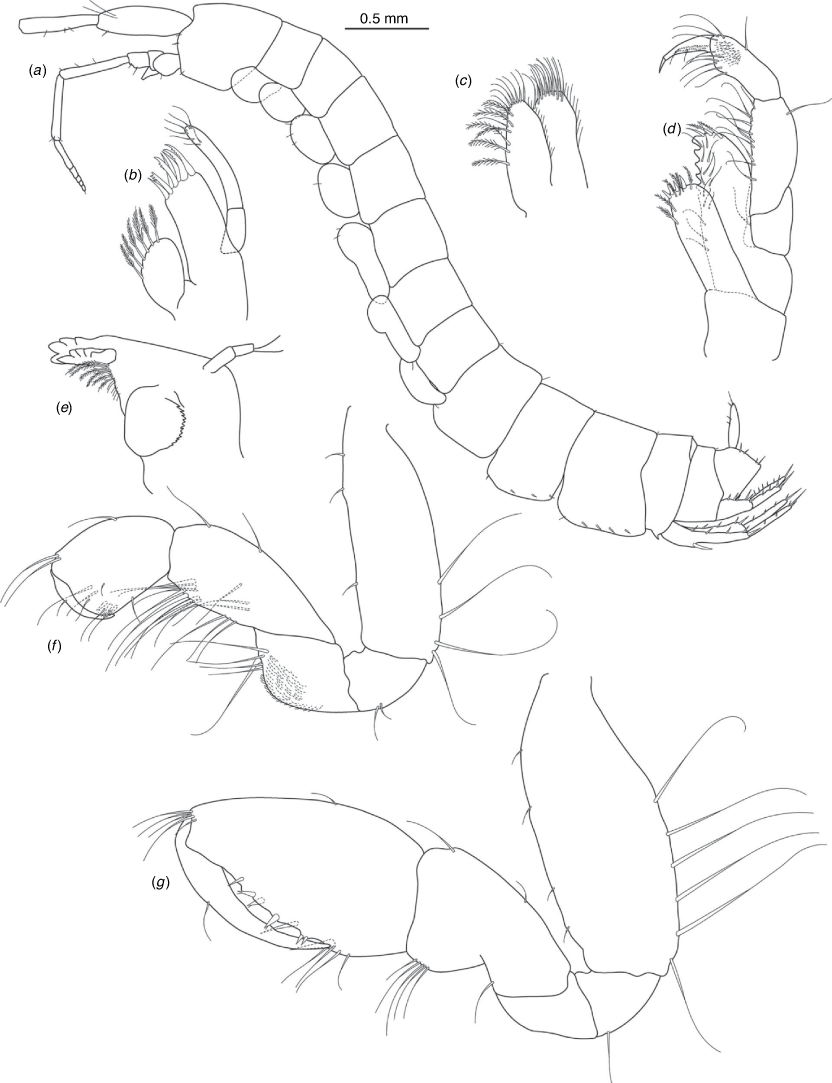
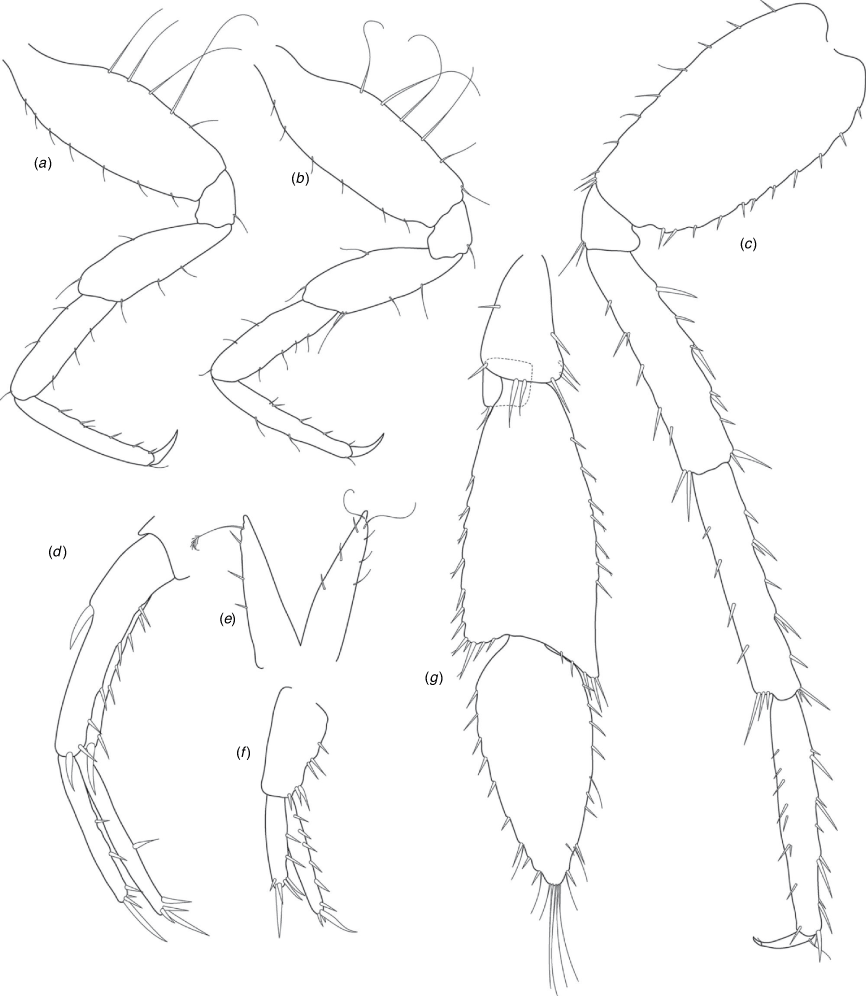
A series of scientific engagement forums was conducted in April 2020 under the auspices of Taxonomy Australia, a program of the Australian Academy of Science (https://www.taxonomyaustralia.org.au/). These forums exist to advocate for the exploration and documentation of Australian biodiversity, with the recurring theme being a perceived need for more rapid species descriptions that maintained a robust scientific method. Following this lead, species descriptions in this paper are reduced compared with more traditional amphipod taxonomy publications. Here, we have found very little evidence of consistent species-level morphological variation in Nedsia despite significantly divergent COI lineages. Given this, the new species here are functionally morphologically cryptic and best described by the (generic-level) Nedsia characters provided, plus an additional small diagnosis for each species that includes a set of additional molecular and any available morphological characters. Illustrations of type material (limited to two figures per species) that include traditionally dissected articles are included for clarity and comparative purposes as we recognise that type specimens cannot always be easily accessed for future study.
Infraorder HADZIIDA S. Karaman, 1932 (Lowry & Myers, 2013)
Superfamily HADZIOIDEA S. Karaman, 1943 (Bousfield, 1983)
Family ERIOPISIDAE Lowry & Myers, 2013
Genus Nedsia Barnard & Williams, 1995
Included species
Type species
Diagnosis
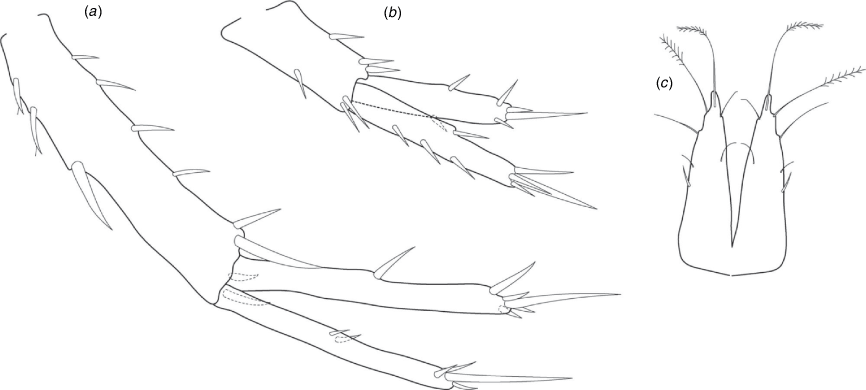
Head with shallow or indistinct antennal sinus. Antenna 1 half body length. Coxae reduced (coxae 1–4 lengths (depths) distinctly shorter than pereonite depths), coxae 1–4 simple and ovoid shaped, coxa 4 without posterior excavation, coxae 5–6 with small anterior lobe. Coxal gills present on coxae 2–6, sternal gills absent. Gnathopod 1 propodus with transverse palm. Gnathopod 2 propodus 1.5 times length of carpus, palm oblique with robust setae covering ~0.75 times propodus length. Uropod 1 peduncle with 1–3 basofacial robust setae. Uropod 3 distinctly larger than uropod 1, outer ramus larger than inner ramus, flattened and leaf shaped, with two articles; inner ramus short and scale-like.
Description
Pleonites with few scattered dorsal setae. Head with rostrum weak to obsolete; lateral cephalic lobes moderately to strongly projecting, broad, no antennal sinus present; eyes absent. Antenna 1 elongate, reaching to at least half body length; longer than antenna 2; accessory flagellum of two articles. Antenna 2 flagellum shorter than peduncle; calceoli absent. Mandible palp with 1–3 short articles; terminal article linear or tapered and setose. Labium with inner lobes. Maxilla 1 inner plate ovate with simple and plumose setae along medial margin; outer plate with denticulate robust setae; palp with two articles. Maxilla 2 inner plate with row of medial plumose setae extending onto face apically.
Coxae 1–7 short, broader than long with few or no posterior setae; coxa 4 without posterior excavation; coxae 2–6 with simple ovate gills; coxae 2–5 in females with thin poorly setose oostegites. Thoracic segments lacking sternal gills. Gnathopods 1–2 subchelate, not sexually dimorphic. Gnathopod 1 smaller than gnathopod 2; merus without hyaline lobe; carpus longer than or similar length to propodus; propodus with palm transverse, with robust setae at palm corner and along palm margin. Gnathopod 2 carpus shorter than propodus, not produced along posterior margin of propodus; propodus with palm oblique, with robust setae at palm corner and along palm margin. Pereopods 3–4 similar, basis not expanded posteriorly. Pereopods 5–7 moderately elongate; basis with moderate posterior expansion but remaining longer than broad; pereopod 7 longer than pereopods 5–6.
Epimera 1–2 with rounded posterodistal margins, epimeron 3 posterodistal margin shaped into a small spine or squared. Epimera 1–3 with robust setae scattered along posterolateral margins and lateral face. Uropod 1 peduncle with 1–3 basofacial robust setae. Uropod 2 smaller than uropod 1. Uropod 3 not sexually dimorphic, larger than uropod 1, parviramous (inner ramus reduced); outer ramus flattened and leaf shaped, with two articles; inner ramus short and scale-like. Telson longer than broad, deeply cleft into two lobes with apical and sub-apical setae.
Remarks
Nedsia is morphologically most similar to Norcapensis that is known from four caves in Cape Range on the North West Cape peninsula, Western Australia, but can be easily distinguished: gnathopod 2 propodus in Norcapensis is distinctly two times longer than the carpus and the palm straight edged and extending almost across the length of the propodus, and uropod 3 outer ramus article 1 in Norcapensis is elongate (five times as long as broad).
The original generic diagnosis and description of Nedsia were based on female specimens of N. douglasi (Barnard & Williams, 1995) and later updated to include the N. douglasi male and additional Nedsia species (Bradbury and Williams 1996). Lowry and Myers (2013) removed Nedsia from the Melitidae and placed this within the Eriopisidae noting that eriopisids could be largely distinguished from melitids and maerids by the lack of a sexually dimorphic gnathopod 2 (i.e. male and female gnathopod 2 largely indistinguishable).
Nedsia canensis King & Cooper, sp. nov.
Type material
Other material examined
Diagnosis
Morphological: mandible palp of one article; pleonites 1–3 posterior margins smooth (no sculpturing); urosomites 1–3 posterior margins smooth (no sculpturing); uropod 1 peduncle with two to three basofacial robust setae. Molecular: lineage B9 differs from most closely related lineage B10 by 9.6% for COI and also has a site synapomorphy at position 234 (C) of the 473-bp COI sequence matrix relative to all other Nedsia taxa that were sequenced.
Distribution
Upper Cane River catchment area, ~60–70 km south of Pannawonica, Western Australia.
Etymology
This species was named for the Cane River, where the holotype and paratype material was collected.
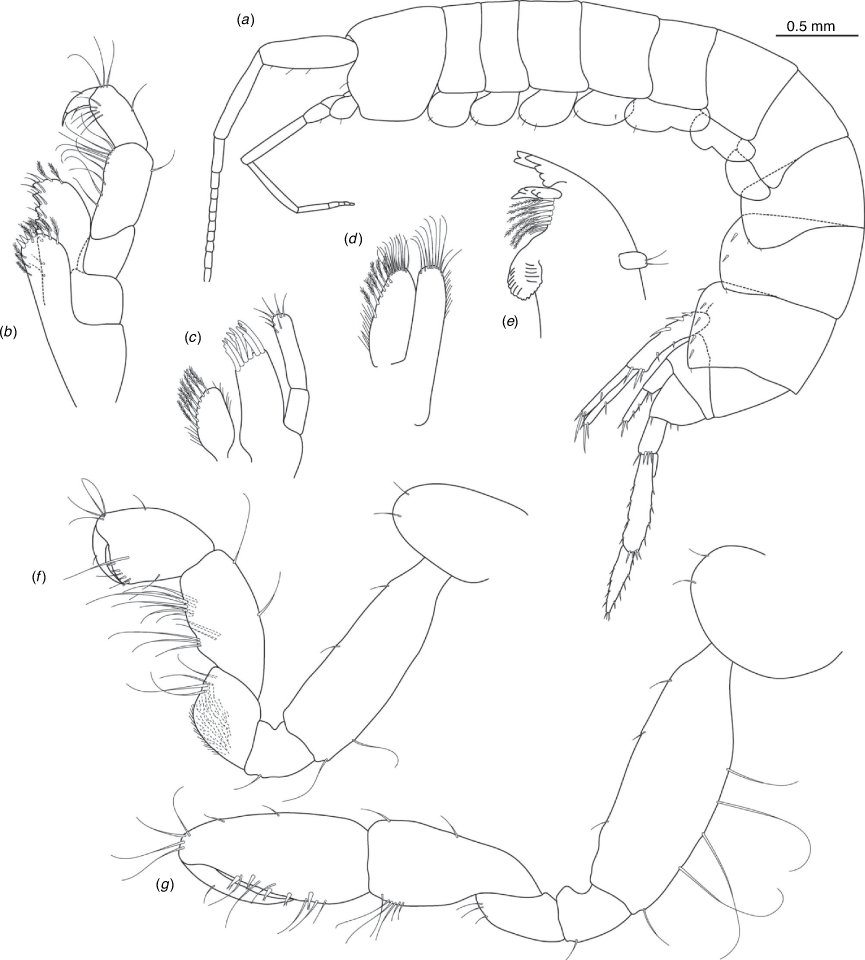
|
Remarks
The relatively large male holotype illustrated has three robust basofacial setae on the peduncle of uropod 1. However, females and smaller males from the same sample were observed with two robust basofacial setae and juveniles were recorded from the sample with none so these character states are seemingly variable with size (smaller individuals with fewer setae). Most similar to N. erinnae sp. nov., the two species remain functionally morphologically cryptic.
Nedsia cheela King & Cooper, sp. nov.
Type material
Diagnosis
Morphological: mandible palp of two articles; pleonites 1–3 posterior margins smooth (no sculpturing); urosomites 1–3 posterior margins smooth (no sculpturing); uropod 1 peduncle with three robust basofacial setae. Molecular: lineage B6 differs from most closely related lineage (lineage B3) by 13.3% for COI and also has a site synapomorphy at position 432 (T) of the 473-bp COI sequence matrix relative to all other Nedsia taxa that were sequenced.
Distribution
Calgara bore, bore CP4, Ashburton River catchment, 60–70 km north-west of Paraburdoo, Western Australia.
Etymology
Named for the Cheela Plains Station where the type specimens and sequenced specimens for lineage B6 were collected.
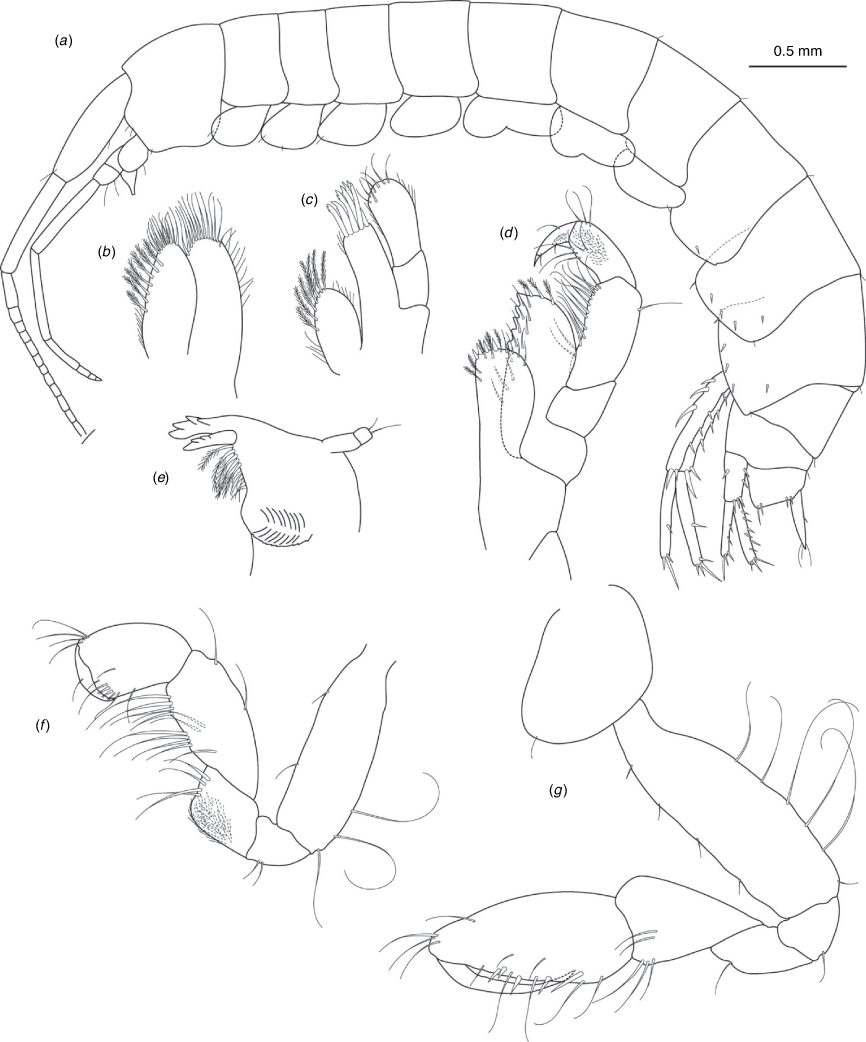
|
Remarks
Clearly a divergent lineage, with 13.3% COI divergence from the most closely related N. robensis sp. nov. from the Robe River. The sequenced material for N. cheela sp. nov. (lineage B6) (Calgara bore on Cheela Plains Station) was not available for morphological study. Specimens from bore CP4 (~5 km from Calgara bore and on the same drainage channel) on Cheela Plains Station were available for examination and considered representative of this species.
Nedsia douglasi Barnard & Williams, 1995
Type material
Other material examined
Diagnosis
Morphological: mandible palp of two articles; pleonites 1–3 posterior margins smooth (no sculpturing); urosomites 1–3 posterior margins smooth (no sculpturing), uropod 1 peduncle with one robust basofacial seta. Molecular: lineage A1: this lineage differs from most closely related species lineage A2 (N. hurlberti) by 5.3% for COI.
Distribution
Exmouth bore field and Yardie Creek Station, North West Cape peninsula, Western Australia.
Remarks
This species, lineage A1 in our analyses, is confirmed as being restricted to the North West Cape peninsula and closely related to N. hurlberti from Barrow Island (lineage A2), with 5.3% COI divergence levels. No additional illustrations are included here as specimens examined did not significantly differ from the published descriptions of N. douglasi (Barnard and Williams 1995; Bradbury and Williams 1996).
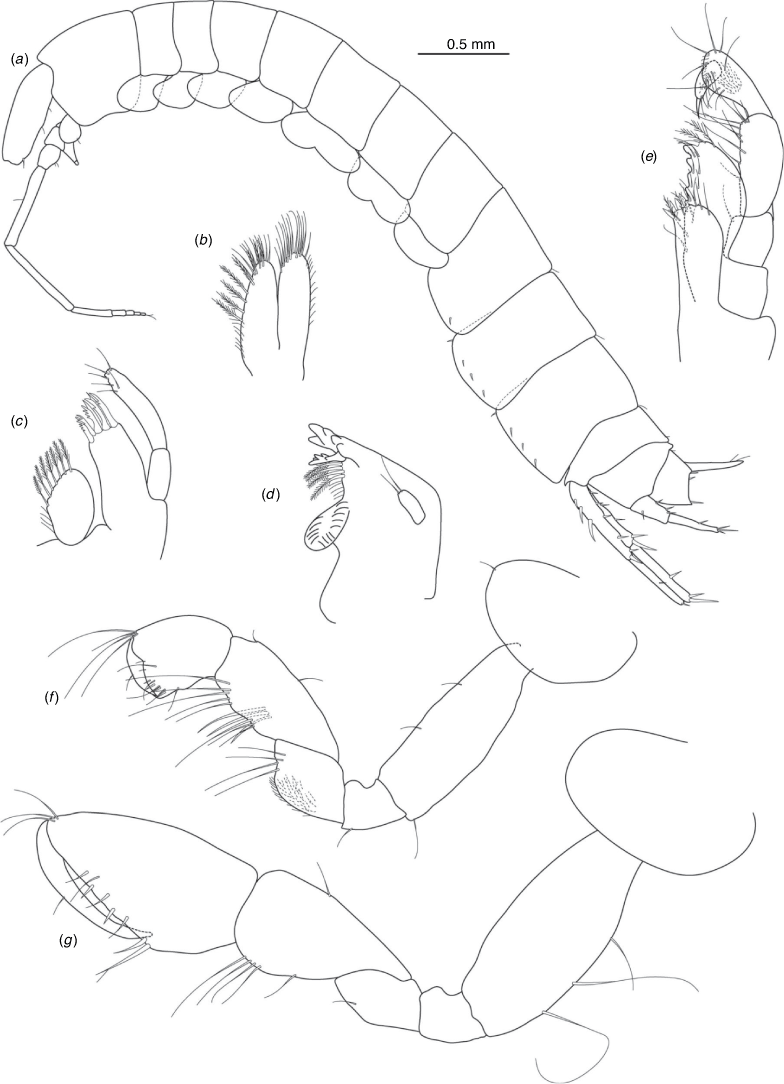
Nedsia erinnae King & Cooper, sp. nov.
Type material
Diagnosis
Morphological: mandible palp of one article; pleonite 1–3 posterior margins smooth (no sculpturing); urosomites 1–3 posterior margins smooth (no sculpturing); uropod 1 peduncle with 2–3 robust basofacial setae. Molecular: lineage B10 differs from most closely related lineage (lineage B9) by 9.6% for COI and also has a site synapomorphy at position 234 (T) of the 473-bp COI sequence matrix relative to all other Nedsia taxa that were sequenced.
Distribution
House Creek, Ashburton River catchment and basin, Pilbara, Western Australia.
Etymology
Named for Erinn Fagan-Jeffries who completed an Honours Project in 2012 that included the first phylogenetic analyses of Nedsia on which the current project was based.
|
|
Remarks
Nedsia erinnae sp. nov. is one of two species co-occurring at House Creek bore (NWSLK58), with the other species being N. nanutarra sp. nov. (lineage A6). We were unable to examine the lineage B10 specimens sequenced as the whole animals were used in the extraction process. Additional specimens from this bore were subsequently obtained but we were not able to sequence these. Examination of this material indicated two morphospecies, with larger (adult) individuals easily distinguishable based primarily on the number of robust basofacial setae on the peduncle of uropod 1. We are confident in these morphological distinctions defining the two lineages as species. Nedsia erinnae sp. nov. with 2–3 robust basofacial setae v. N. nanutarra sp. nov. with only 1 robust basofacial seta on the peduncle of uropod 1. Note should be made that all small juvenile specimens from the bore had 1–2 robust basofacial setae and juveniles of N. erinnae sp. nov. cannot be ruled out to possess a single robust basofacial seta on the peduncle of uropod 1. According to our molecular data N. erinnae sp. nov. is most closely related to N. canensis sp. nov. from the upper Cane River catchment, with 9.6% COI divergence; the two species are morphologically cryptic.
Nedsia hurlberti Bradbury & Williams, 1996
Type material
Other material examined
Diagnosis
Morphological: mandible palp of two articles; pleonites 1–3 posterior margins smooth (no sculpturing); urosomites 1–3 posterior margins smooth (no sculpturing); uropod 1 peduncle with one robust basofacial seta. Molecular: lineage A2 differs from most closely related species, N. douglasi (lineage A1), by 5.3% for COI and also has a site synapomorphy at position 210 (G) of the 473-bp COI sequence matrix relative to all other Nedsia taxa that were sequenced.
Distribution
Barrow Island, Western Australia.
Remarks
Analyses of the molecular data (lineage A2) and examination of all available material indicated that seven previously described species of Nedsia were synonymous. Nedsia hurlberti was subsequently selected as the valid name because the type material (female, WAM C22383) represented the best mature specimen compared with the types of the synonymised species (Bradbury and Williams 1996). No figures are included here as specimens examined did not significantly differ from the published description of N. hulberti in Bradbury and Williams (1996). With 5.3% COI divergence, this species is closely related to N. douglasi (lineage A1) but is clearly isolated on Barrow Island while N. douglasi is found on the adjacent mainland at the North West Cape peninsula.
Nedsia macrosculptilis Bradbury & Williams, 1996
Type material
Other material examined
Diagnosis
Morphological: mandible palp of two articles; pleonites 1–3 posterior margins with sculpturing; urosomites 1–3 posterior margins with sculpturing; uropod 1 peduncle with one robust basofacial seta. Molecular: lineage B5 differs from most closely related lineage (lineage B3) by 13.3% for COI and also has site synapomorphies at positions 233 (T), 281 (AT insertion), 299 (A), 341 (A) and 718 (T) of the 790-bp 28S rRNA sequence alignment from 38 Nedsia samples.
Distribution
Barrow Island, Western Australia.
Remarks
Isolation on Barrow Island, >13.3% COI divergence levels from related species (N. robensis sp. nov. and N. mcraeae sp. nov.), and morphological characters including pleonite and urosomite sculpturing and epimera setation, render this species hypothesis robust. Nedsia macrosculptilis and N. robensis sp. nov. are the only Pilbara species in group B with distinctly mature or large specimens that possess one robust basofacial seta on the peduncle of uropod 1 (excepting N. quobba sp. nov. from the Gascoyne).
Nedsia sculptilis is herein synonymised with N. macrosculptilis as there is no evidence of further molecular lineages on Barrow Island and morphological examination indicates that the description of N. sculptilis is based on a small male specimen of N. macrosculptilis.
Nedsia mcraeae King & Cooper, sp. nov.
Type material
Diagnosis
Morphological: mandible palp of one article; pleonites 1–3 posterior margins with sculpturing; urosomites 1–3 posterior margins with sculpturing; uropod 1 peduncle with two robust basofacial setae. Molecular: lineage B4 differs from most closely related lineage (lineage B3) by 10.7% for COI.
Distribution
Barrow Island and Robe River (coastal) catchment, Onslow Coastal basin, Pilbara, Western Australia.
Etymology
Named for Jane McRae who has spent many hours over many years sorting and identifying amphipods and other stygofauna at the environmental consultancy company Bennelongia.
|
|
Remarks
Nedsia mcraeae sp. nov. is a rare species on Barrow Island (known from one bore locality) and only a few sequences exist from Robe River coastal bores. Specimens from bore G70730107, ~10 km from the bores with sequenced material, were available for morphological study but were not able to be sequenced. The presence of two robust basofacial setae indicates that the material fits within clade B and we accept this as B4. The presence of two robust basofacial setae might be diagnostic of the species but may also indicate that the type material is an immature or smaller female and that a more mature female might have three robust setae (similarly to N. erinnae sp. nov. and N. canensis sp. nov.).
Nedsia nanutarra King & Cooper, sp. nov.
Type material
Diagnosis
Morphological: mandible palp of two articles; pleonites 1–3 posterior margins smooth (no sculpturing); urosomites 1–3 posterior margins smooth (no sculpturing); uropod 1 peduncle with one robust basofacial seta. Molecular: lineage A6 differs from most closely related lineage (lineage A5) by 11.3% for COI and also has site synapomorphies at positions 471 (T) and 229 (T) of the 473-bp COI sequence matrix relative to all other Nedsia taxa that were sequenced.
Distribution
Nanutarra region, Ashburton River catchment, Pilbara, Western Australia.
Etymology
Named for the Nanutarra region, where the type material was collected.
|
Remarks
Nedsia nanutarra sp. nov. is a distinct species (lineage A6) with 11.3% COI divergence from the closest lineage (Nedsia sp. A5 from the Upper Ashburton catchment) and distinct molecular synapomorphies for the COI fragment analysed. Nedsia nanutarra sp. nov. and Nedsia sp. A5 are morphologically cryptic, with the former having been collected from the same bore as N. erinnae sp. nov. (see remarks about N. erinnae sp. nov. for more information).
Nedsia pannawonica King & Cooper, sp. nov.
Type material
Other material examined
Diagnosis
Morphological: mandible palp of two articles; pleonites 1–3 posterior margins smooth (no sculpturing); urosomites 1–3 posterior margins smooth (no sculpturing); uropod 1 peduncle with three robust basofacial setae. Molecular: lineage B8 differs from most closely related lineage (lineage B9) by 18.2% for COI and also has site synapomorphies at position 255 (G), 351 (C), 352 (C), 360 (G), 367 (A) and 447 (C) of the 473-bp COI sequence matrix relative to all other Nedsia taxa that were sequenced. This species also shows a site synapomorphy at position 171 (T) of the 790-bp 28S alignment from 38 samples.
Distribution
At ~47 km south-west of Pannawonica, Robe River catchment, Onslow Coastal basin, Pilbara, Western Australia.
|
Etymology
This species is named for the area where the type material was collected (Pannawonica).
Remarks
The molecular COI divergence of 18.2% from the closest species, N. canensis sp. nov. (lineage B9) provides robustness to the recognition of this species. The two species are morphologically cryptic.
The holotype male possesses a mandible with a two articulate palp on one side and a palp of one article on the other (single palp article with a seta apically and not simply damaged). All other specimens have mandibles with two articulate palps on both sides.
Nedsia quobba King & Cooper, sp. nov.
Type material
Other material examined
Diagnosis
Morphological: mandible palp of one article; pleonites 1–3 posterior margin smooth (no sculpturing); urosomites 1–3 posterior margins smooth (no sculpturing); uropod 1 peduncle with one robust basofacial seta. Molecular: lineage B1 differs from most closely related lineage (lineage B3) by 39.9% for COI and has site synapomorphies at positions 159 (T), 182 (C), 256 (T), 360 (T), 395 (C) and 426 (T) of the 473-bp COI sequence matrix relative to all other Nedsia taxa that were sequenced. This species also has site synapomorphies at positions 610 (C) and 634 (C) in a 790-bp alignment of 28S rRNA from 38 samples.
Distribution
Quobba Station, ~100 km north of Carnarvon, Gascoyne region, Western Australia.
Etymology
The species is named for the area where the type material was collected (Quobba Station).

|
Remarks
This is not a Pilbara species but is clearly distinct from other Nedsia species, with 39.9% COI divergence from N. robensis sp. nov. and distinct molecular synapomorphies in the COI fragment analysed. This species clearly belongs to Nedsia, although placement within the molecular phylogeny is not certain and the plain morphological characters indicate alignment closer to group A than group B.
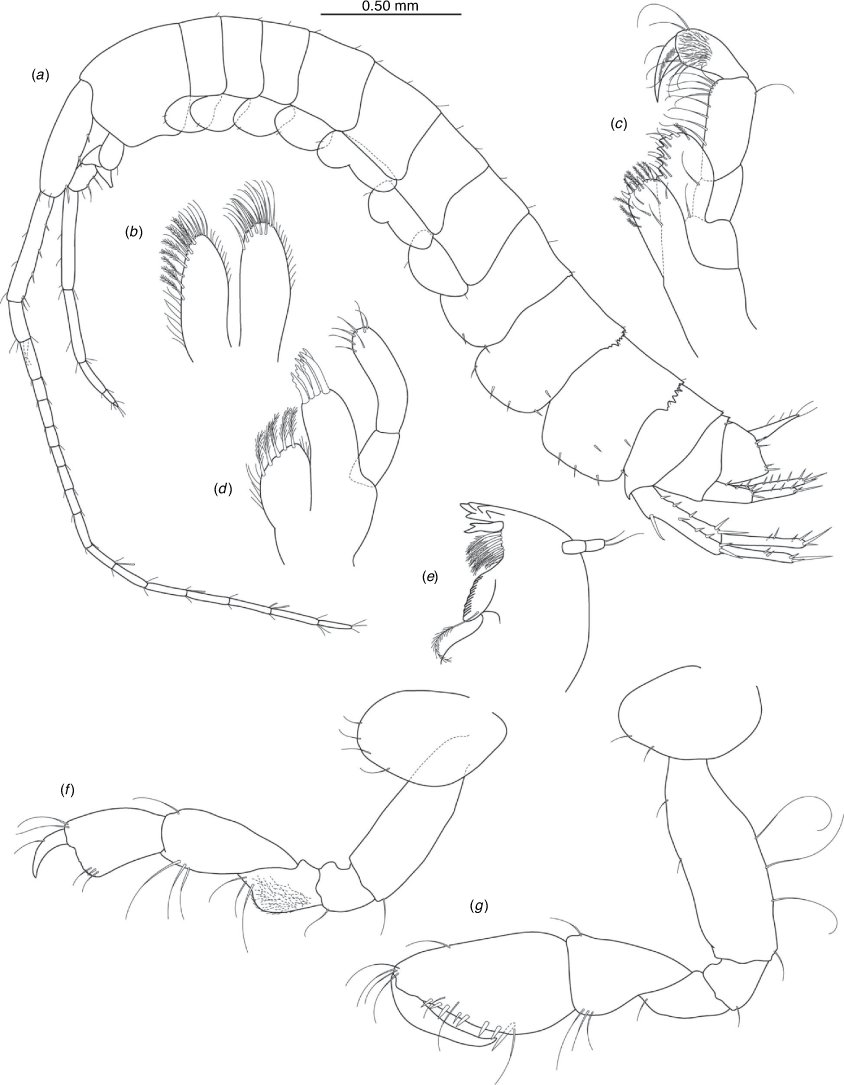
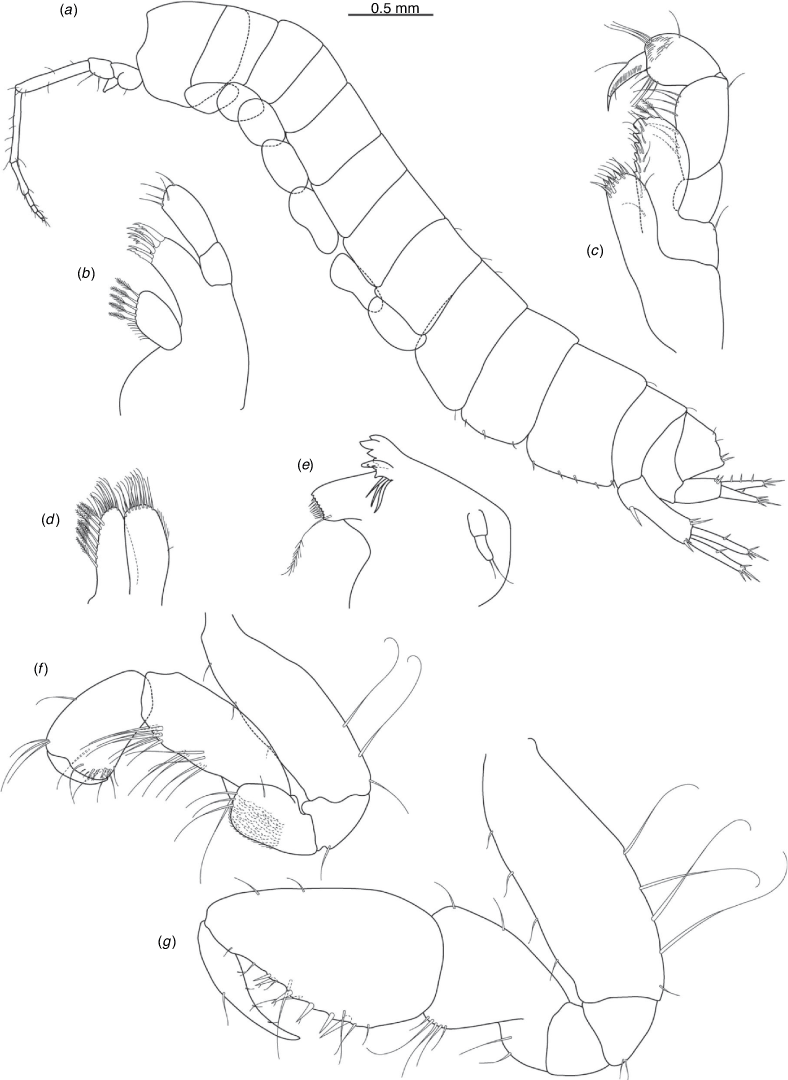
Nedsia robensis King & Cooper, sp. nov.
Type material
Other material examined
Diagnosis
Morphological: mandible palp of two articles; pleonites 1–3 posterior margins with sculpturing; urosomites 1–3 posterior margins with sculpturing; uropod 1 peduncle with one robust basofacial seta. Molecular: lineage B3 differs from most closely related lineage (lineage B4) by 11% for COI.
Distribution
Robe River catchment (Upper River and coastal bores), Onslow Coastal basin, Pilbara, Western Australia.
Etymology
Named for the Robe River catchment from which the type material was collected.
|
|
Remarks
Nedsia robensis sp. nov. is one of the four identified lineages from group B that share sculpturing on the posterior margins of the pleonites and urosomites, the others being Nedsia sp. B2, N. mcraeae sp. nov. (B4) and N. macrosculptilis (B5). All four are from the Onslow Coastal basin. Nedsia robensis sp. nov. is geographically isolated but morphologically cryptic in relation to N. macrosculptilis, with both possessing one robust basofacial seta on the peduncle of uropod 1 and a mandibular palp of two articles; and differs from N. mcraeae sp. nov. as that species has two robust basofacial setae on the peduncle of uropod 1 and mandibular palp with one article.
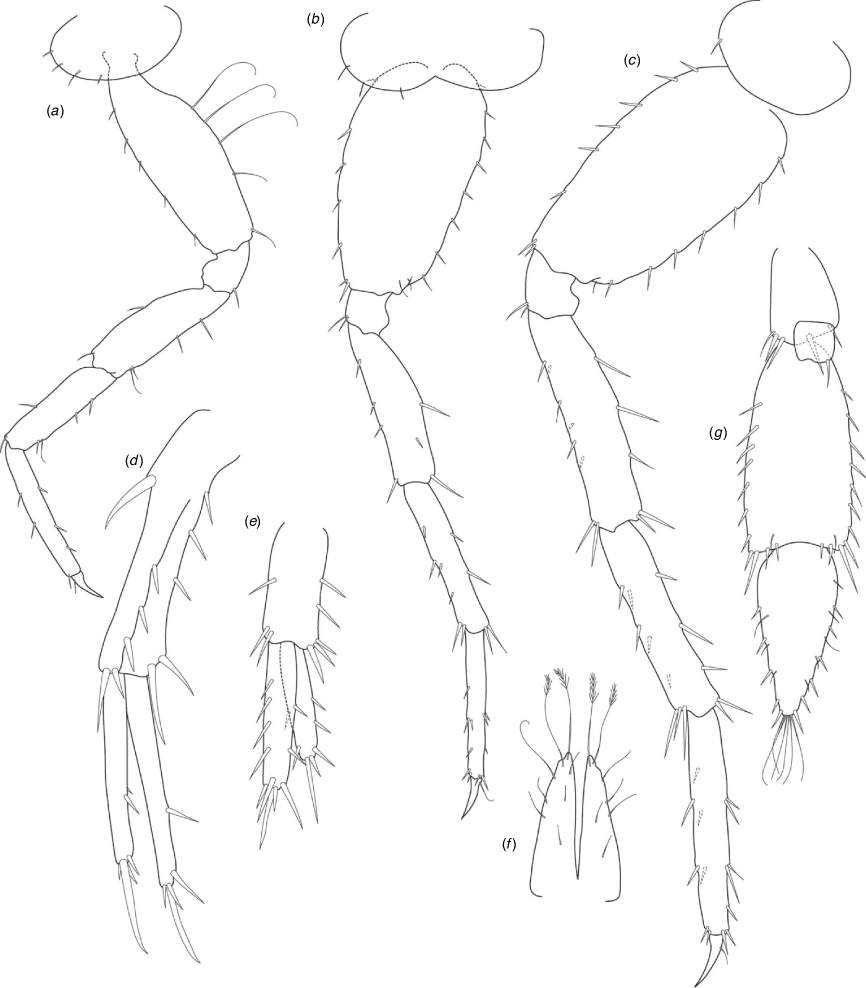
Nedsia shawensis King & Cooper, sp. nov.
Type material
Other material examined
Diagnosis
Morphological: mandible palp of three articles; pleonite 1–3 posterior margin smooth (no sculpturing); urosomites 1–3 posterior margins smooth (no sculpturing); uropod 1 peduncle with one robust basofacial seta. Molecular: lineages A7–A9 differ from most closely related lineage (lineage A5) by 19.2% for COI and also has a site synapomorphy at position 18 (T) of the 473-bp COI sequence matrix relative to all other Nedsia taxa that were sequenced.
Distribution
Approximately 70 km north-west of Marble Bar, Shaw River catchment, De Grey basin, Pilbara, Western Australia. Additional material: Coppings bore samples from Turner River catchment, Port Hedland basin, Pilbara, Western Australia.
Etymology
Named for the Shaw River, where the holotype and paratype specimens were collected.
|
Remarks
Nedsia shawensis sp. nov. is the only species recorded thus far with a mandibular palp of three articles. There are potentially two species from three lineages (A7–A9) present in this material that are currently conservatively treated as a single species. Several of the samples from lineages A7 and A9 were located from bores that were close to each other (for example MBSLK376E, MBSLK376W and MBSLK376A and B are bores within a 40-m radius of each other) in the Shaw River catchment. Coppings Bore specimens appear to be a more isolated group from the Turner River catchment and may represent a separate species (~60 km south of MBSLK376E) but we were unable to find adult specimens for morphological description and to select as types for the species.
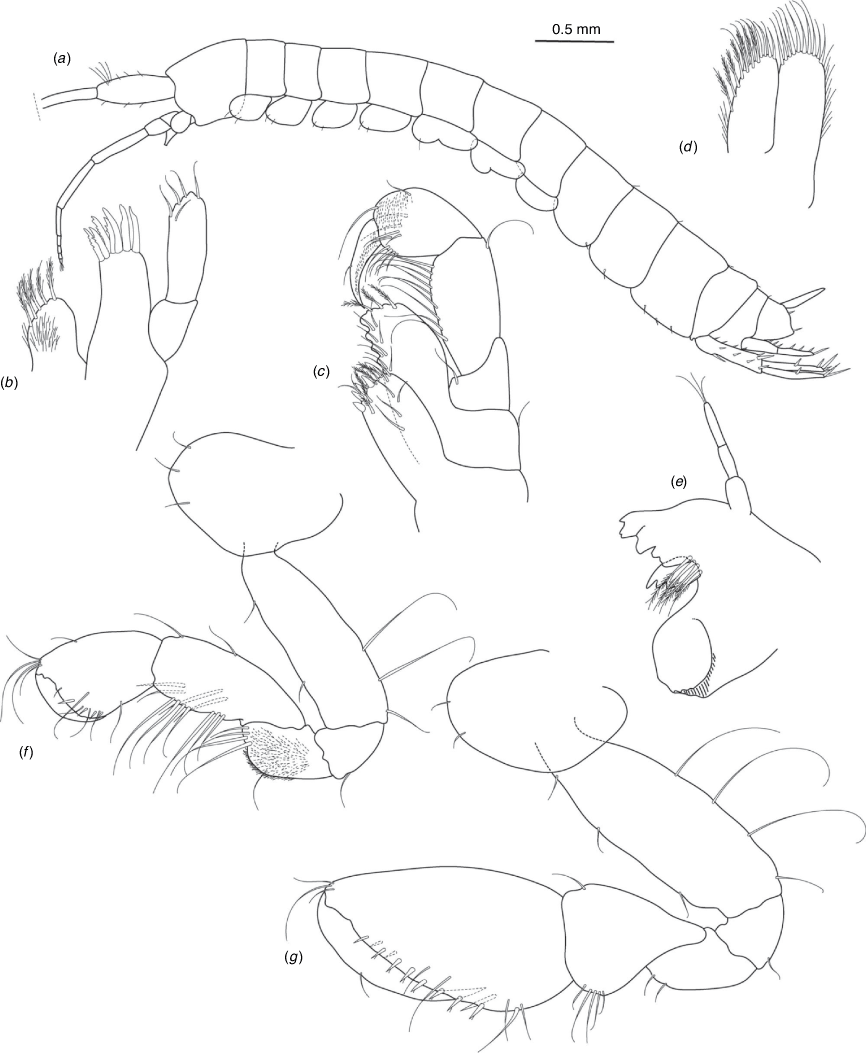
Nedsia wanna King & Cooper, sp. nov.
Type material
Diagnosis
Morphological: mandible palp of two articles; pleonites 1–3 posterior margins smooth (no sculpturing); urosomites 1–3 posterior margins smooth (no sculpturing); uropod 1 peduncle with one robust basofacial seta. Molecular: lineage A4 differs from most closely related lineage (lineage A5) by 9.0% for COI and also has site synapomorphies at positions 81 (T) and 153 (G) of the 473-bp COI sequence matrix relative to all other Nedsia taxa that were sequenced.
Distribution
Wanna Station, West Lyons River region, Gascoyne, Western Australia.
|
|
Etymology
This species was named for Wanna Station, where the type material was collected.
Remarks
Nedsia wanna sp. nov. is a distinct lineage with 9% COI divergences and several synapomorphies in the COI fragment sequenced. This species is clearly closely related to N. wyloo sp. nov. (A3), Nedsia sp. (A5) and N. nanutarra sp. nov. (A6) that are found in the adjacent Ashburton River catchment and basin. Nedsia wanna sp. nov. is morphologically cryptic in relation to these species.
Nedsia weelumurra King & Cooper, sp. nov.
Type material
Other material examined
Diagnosis
Morphological: mandible palp of one article; pleonites 1–3 posterior margins smooth (no sculpturing); urosomites 1–3 posterior margins smooth (no sculpturing); uropod 1 peduncle with three robust basofacial setae. Molecular: lineage B12+B13 differs from most closely related lineage (lineage B11) by 17.9% for COI and also has site synapomorphies at position 722 (G) and 736 (C) of the 790-bp 28S alignment from 38 Nedsia samples.
Distribution
Weelumurra Creek, Fortescue River catchment and basin, Pilbara, Western Australia.
Etymology
This species is named for Weelumurra Creek, where the type and additional material were collected.
|
Remarks
Nedsia weelumurra sp. nov. is clearly distinct with 17.9% COI divergence from Nedsia sp. B11 and having several synapomorphies in the COI fragment sequenced. There are two distinct lineages (B12 and B13) that we have conservatively treated as N. weelumurra sp. nov., as our 28S data showed that SE7807–1 and SE7807–3 were not divergent (compared to the COI data that placed these in two distinct lineages).
Nedsia wyloo King & Cooper, sp. nov.
Type material
Other material examined
Diagnosis
Morphological: mandible palp of two articles; pleonites 1–3 posterior margins smooth (no sculpturing); urosomites 1–3 posterior margins smooth (no sculpturing); uropod 1 peduncle with one robust basofacial seta. Molecular: lineage A3 differs from most closely related lineage (lineage A5) by 8.6% for COI and also has a site synapomorphy at position 415 (C) of the 473-bp COI sequence matrix relative to all other Nedsia taxa that were sequenced.
Distribution
Hardey River and Metawandy Creek, Ashburton River catchment and basin, Pilbara, Western Australia.
Etymology
Wyloo comes from the name of the station near Metawandy Creek and the Hardey River where the specimens including the type material were collected.
Remarks
This species has been established for a distinct lineage from Hardey River area, with 8.6% COI divergences and a synapomorphy on the COI fragment sequenced.
|
|
Nedsia yarraloola King & Cooper, sp. nov.
Type material
Diagnosis
Morphological: pleonites 1–3 posterior margins smooth (no sculpturing); urosomites 1–3 posterior margins smooth (no sculpturing); uropod 1 peduncle with 2–3 robust basofacial setae. Molecular: lineage B7 differs from most closely related lineage (lineage B9) by 17.5% for COI and has site synapomorphies at position 261 (G) and 408 (C) of the 473-bp COI sequence matrix relative to all other Nedsia taxa that were sequenced. This species also shows a site synapomorphy at position 194 (G) of the 790-bp 28S alignment from 38 samples.
Distribution
Yarraloola Well, Robe River catchment, Onslow Coastal basin, Pilbara, Western Australia.
Etymology
This species is named for the well and station (Yarraloola) where the type material was collected.
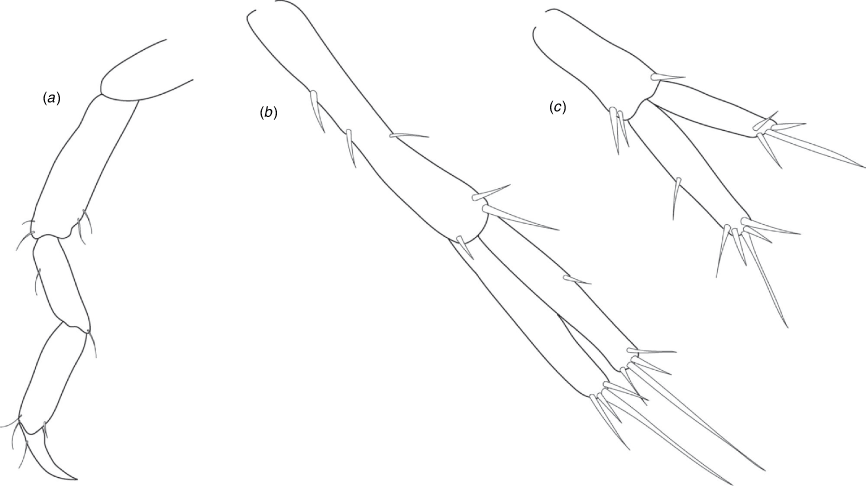
|
Remarks
The only material available for morphological assessment and for lodging as type material was a juvenile. The mandible palp could not be examined and remains unknown. The type specimen has two robust basofacial setae on the peduncle of uropod 1 but the adults likely have 2–3 depending on size.
Discussion
Species determination and cryptic lineages
The highly complex nature of the subterranean habitats in which Nedsia occur are such that we may not fully comprehend the extent of hydrological connections within these, even when the size and scope of each aquifer system has been determined. If a system is particularly complex with multiple barriers, phylogeographic structuring of populations is likely to occur and must be examined appropriately. With this in mind, our approach to defining species has been conservative, sometimes uniting divergent lineages, but largely follows the structure of the phylogeny presented for Nedsia at the 5–10% species divergence level. Recent research suggests that whilst species divergence levels are informative, these can be highly variable (~5–20%) across amphipod families (Delić et al. 2017; Tempestini et al. 2018); thus, thresholds should be used cautiously and preferably include additional corroborative evidence for species delimitation.
An improved understanding of morphological characters as these relate to homoplasy, morphological stasis and cryptic species within Nedsia is of particular importance in the current study. Morphological examination of the lineages within the phylogeny presents clear evidence of significant morphological stasis in Nedsia species across characters traditionally used in amphipod taxonomy (mouthparts, gnathopods and pereopods, and uropods). Also, our analyses showed evidence of possible homoplasy (non-sculptured pleonites appearing in separate lineages across the tree). Evidence of homoplasy is not unusual within the Amphipoda, and has been documented for many groups, especially those existing under extreme selective pressures, such as pelagic species (Browne et al. 2007) and other marine taxa (Knox et al. 2012; Havermans et al. 2013; Verheye et al. 2016) and groundwater-associated taxa (Trontelj et al. 2009; Bauzà-Ribot et al. 2011; Murphy et al. 2015). In the context of Nedsia, the uncoupling of morphological and molecular evolution across a great deal of the phylogeny may be due to the selective pressures of a subterranean lifestyle including the lack of light, low nutrient availability, lack of ecological opportunity and potential niche specialisation (Witt et al. 2003; Macdonald et al. 2005; Bauzà-Ribot et al. 2011). Clearly, we have found very little evidence of morphological variation across Nedsia species and therefore contend that the species be treated as functionally morphologically cryptic.
Systematics and distribution
The Pilbara Eriopisidae comprises Nedsia, Norcapensis and an undescribed genus ‘Eriopisidae gen. undet.’ (delimited by us with molecular and morphological characters but to be described elsewhere) and, due to high levels of divergence, the genera and species are likely to have long been isolated from the other Australian hadzioidean taxa briefly examined here (Brachina, Nurina) (See Materials and methods). Our results correspond well with the current morphology-based classification of Lowry and Myers (2013) that placed Brachina and Nurina within the Melitidae, and Nedsia and Norcapensis firmly within the Eriopisidae. Analysis of the two single locus phylogenies here (COI, 28S) produced largely congruent results and we were able to confirm Nedsia, Norcapensis and Eriopisidae gen. undet. as being reciprocally monophyletic groups. Further analyses are required to rigorously assess the interrelationships of the three genera.
Our molecular results contrast sharply with the existing morphology-only classification for Nedsia, in which 10 species were described from Barrow Island (with seven later classified as vulnerable fauna by the Government of Western Australia) and the North West Cape peninsula. Instead, we redefine Nedsia as a diverse genus of stygobiotic amphipods with at least 16 species of which 13 are described here using molecular data supported by evidence from morphology and hydrography, and distributed widely across the Pilbara mainland, Barrow Island and into the Gascoyne region. Importantly, our results show that only three Nedsia species are found on Barrow Island (Fig 1, 3), as opposed to the 10 previously recognised. Two are endemic to the Island, with N. macrosculptilis distributed across the south-eastern half of the Island, and N. hurlberti clustered in a central and western part of the Island (Fig. 3). The third species, N. mcraeae sp. nov., was identified from a single borehole on Barrow Island near the gas terminal (GW05) along with individuals from Yarraloola (Robe River, Onslow coastal catchment) that had quite low divergence levels (<1%).
Nedsia is far more prevalent across the mainland Pilbara than was previously considered and our data suggest that two separate radiation events have occurred. These are largely overlapping in distribution from the Gascoyne to Pt Hedland and De Grey for group A, and from the Gascoyne to the Fortescue River for group B (Fig. 3). The timing of these radiations requires further analyses, but the deep divergences among lineages in group B in particular (>39% for lineage B1 v. other B lineages), suggest an early divergence in the Miocene, when surface waters and hydrographic connections were likely to be more extensive, before the aridification of Australia in the late Miocene (Byrne et al. 2008). Significant communities of stygobiotic invertebrates have been identified across Western Australia (Humphreys 2019), that, like Nedsia, are likely to have sought refuge within, and subsequently adapted to, groundwater dependent habitats during these aridification events (Byrne et al. 2008). For example, similarly timed divergences for groundwater species in the Pilbara have recently been found among species of Parabathynellidae (Matthews et al. 2020) and Bathynellidae (Perina et al. 2018, 2019a, 2019b), with the latter study finding intra-generic divergences that date to the Miocene using molecular clock analyses.
Limitations of the dataset
Our project sought to compile existing data and specimens (some more than 15 years old) from several environmental consultancy companies, government agencies and museums. A broad limitation in the data collection process relates to the difficulty of sampling groundwater and aquifers across the landscape. This sampling must be done via bores and wells that have all been installed for purposes other than sampling amphipods by a variety of owners and this meant re-sampling bores to obtain better material was frequently not possible. Nedsia samples are therefore relatively rare and we were not always able to generate corresponding COI and 28S sequences from all individuals.
Whilst next generation sequencing and large-scale genomics projects are becoming more prevalent, in the short-term we will likely still need to rely on COI driven approaches, similar to that presented here, for stygobionts. Given the importance and rarity of stygobiotic specimens and the often-slow process of traditional taxonomic methods, the identification of stygobiotic species by recognising significant evolutionary lineages as a means for preserving biodiversity is a valid approach. We argue that our method of using multiple independent molecular markers (COI and 28S), supported by morphology and hydrographic data, is more rigorous than morphology-only species delimitation.
Conservation implications
Mining for iron ore, natural gas and other minerals in the Pilbara produces ~40% of Australia’s export income. Pastoral activities (mainly cattle grazing) are widespread, and mining, pastoralism, small irrigation projects and Pilbara towns all utilise groundwater. The extent of groundwater abstraction ranges from kilolitres per annum at small stock bores to large-scale aquifer dewatering involving tens of gigalitres to enable deep open cut mining below the water table. At the same time, studies are routinely undertaken to model groundwater flow patterns and aquifer connectivity, to predict the extent of groundwater change from such developments, and these provide the basis for environmental impact assessment. Species diversity assessments contribute significant information to these models: specifically, these identify and provide necessary components (species) for conservation and management strategies. Formal description of species is a key component of the documentation of groundwater communities and associated impacts. In addition to improving the clarity of ecological documentation and assessment outputs, formal description also increases the accuracy of relevant studies. Recent research argues that, alongside species identification, genetic diversity must also be assessed to truly comprehend the scale of biodiversity within groundwater systems (Cooper et al. 2007, 2008; Guzik et al. 2008; Juan et al. 2010; Davis et al. 2013; Delić et al. 2017).
Significantly, in terms of conservation, we have synonymised six of the eight species named on the Western Australian Threatened and Priority Fauna list. Nedsia macrosculptilis and N. hurlberti remain as valid species with restricted distributions and endemic to Barrow Island, and we would argue that the other species on Barrow Island (N. mcraeae sp. nov.) that is also found on the adjacent mainland, is also an SRE species of similarly restricted distribution and should consequently receive formal protection. This reduction of threatened species is not, however, a statement on the vulnerability nor the conservation worthiness of Nedsia species. Instead this is an artefact of previous flawed taxonomy from limited morphological characters and specimens. In our study, examination of molecular lineages revealed that individual Nedsia species’ distributions are regularly less than 100 km2 in size. Clearly, most Nedsia species can be considered ultra-short range endemics (uSRE) (Guzik et al. 2019), as the term ‘short range endemic’ was originally devised for species with distributions of 10 000 km2 (Harvey 2002). As confirmed uSREs, Nedsia species are at considerable risk from extreme modification or destruction of the inhabited groundwater systems.
Conclusions
Our study offers an integrated approach combining molecular, morphological, distributional and hydrological data to assess the diversity of Nedsia, an Australian stygobiotic hadzioid amphipod genus that is now identified as prevalent across the Pilbara, Barrow Island and adjacent Gascoyne region. We also confirm the significance of the Pilbara region in Western Australia for groundwater-associated amphipod diversity and stygofauna diversity more broadly. We present a treatment of new Nedsia species, significantly expand the distribution of the genus and provide a framework for identifying Nedsia species in future. This information will be crucial for more appropriately informed groundwater conservation and management practices across the Pilbara.
Data availability
The data that support this study are available in the article and accompanying Supplementary material. Nucleotide sequence data that support this study are available in GenBank at https://www.ncbi.nlm.nih.gov/genbank/. In addition, we present nexus files on the three main phylogenetic analyses presented in the paper and in supplementary figures (COI only, 28S only, COI + 28S combined).
Conflicts of interest
Andy Austin is an Associate Editor of Invertebrate Systematics but did not at any stage have editor-level access to this manuscript while in peer review as is the standard practice when handling manuscripts submitted by an editor to this journal. Invertebrate Systematics encourages editors to publish in the journal and editors are separated from the decision-making processes for manuscripts. The authors have no further conflicts of interest to declare.
Declaration of funding
Funding for this research was obtained from the Australian Biological Resources Study (ABRS) National Taxonomy Research Grant Program (RF 211–20) to R. A. King, S. J. B. Cooper and W. F. Humphreys (with funding support from the South Australian Museum, The University of Adelaide’s School of Biological Sciences and the Australian Centre of Evolutionary Biology and Biodiversity, Subterranean Ecology and Bennelongia Environmental Consultants), and an ABRS Capacity Building Honours Scholarship (CH212–06) to E. Fagan-Jeffries.
Supplementary material
Supplementary material is available online.
Acknowledgements
We thank Dr Remko Leijs for providing sequence data for several outgroup and ingroup taxa and Yvette Hitchen at Helix Molecular solutions for assistance (to T. L. Finston) with some of the sequencing. We thank Dr Michelle Guzik (University of Adelaide) for insightful and supportive phylogenetics discussions. We thank Dr Mark Harvey (Western Australian Museum) for informative nomenclatural discussions. The lead author (R. A. King) gave birth to two children during the research undertaken to produce this paper and acknowledges the support received from the South Australian Museum, co-authors of this research and colleagues, with the best intentions of retaining women (with children) in scientific research positions.
References
Barnard, JL, and Williams, WD (1995). The taxonomy of freshwater Amphipoda (Crustacea) from Australian fresh waters: Part 2. Records of the Australian Museum 47, 161–201.| The taxonomy of freshwater Amphipoda (Crustacea) from Australian fresh waters: Part 2.Crossref | GoogleScholarGoogle Scholar |
Bauzà-Ribot, MM, Jaume, D, Fornós, JJ, Juan, C, and Pons, J (2011). Islands beneath islands: phylogeography of a groundwater amphipod crustacean in the Balearic archipelago. BMC Evolutionary Biology 11, 221.
| Islands beneath islands: phylogeography of a groundwater amphipod crustacean in the Balearic archipelago.Crossref | GoogleScholarGoogle Scholar | 21791038PubMed |
Boulton, AJ, Fenwick, G, Hancock, PJ, and Harvey, MS (2008). Biodiversity, functional roles and ecosystem services of groundwater invertebrates. Invertebrate Systematics 22, 103–116.
| Biodiversity, functional roles and ecosystem services of groundwater invertebrates.Crossref | GoogleScholarGoogle Scholar |
Bousfield, EL (1977). A new look at the systematics of gammaridean amphipods of the world. Crustaceana. Supplements 4, 282–316.
Bousfield EL (1983) An updated phyletic classification and palaeohistory of the Amphipoda. In ‘Crustacean Phylogeny’. (Ed. FR Schram) pp. 257–277. (Museum of Natural History: San Diego, CA, USA)
Bradbury, JH (2000). Western Australian stygobiont amphipods (Crustacea: Paramelitidae) from the Mt Newman and Millstream regions. Records of the Western Australian Museum 60, 102.
Bradbury, JH (2002). Melitid amphipods of Barrow Island, Western Australia. Part II – recent discoveries. Records of the Western Australian Museum 21, 83–103.
| Melitid amphipods of Barrow Island, Western Australia. Part II – recent discoveries.Crossref | GoogleScholarGoogle Scholar |
Bradbury, JH, and Eberhard, S (2000). A new stygobiont melitid amphipod from the Nullarbor Plain. Records of the Western Australian Museum 20, 39–50.
Bradbury, JH, and Williams, WD (1996). Freshwater amphipods from Barrow Island, Western Australia. Records of the Australian Museum 48, 33–74.
| Freshwater amphipods from Barrow Island, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Bradbury, JH, and Williams, WD (1997). Amphipod (Crustacea) diversity in underground waters in Australia: an Aladdin’s Cave. Memoirs of the Museum of Victoria 56, 513–519.
| Amphipod (Crustacea) diversity in underground waters in Australia: an Aladdin’s Cave.Crossref | GoogleScholarGoogle Scholar |
Browne, WE, Haddock, SHD, and Martindale, MQ (2007). Phylogenetic analysis of lineage relationships among hyperiid amphipods as revealed by examination of the mitochondrial gene, cytochrome oxidase I (COI). Integrative and Comparative Biology 47, 815–830.
| Phylogenetic analysis of lineage relationships among hyperiid amphipods as revealed by examination of the mitochondrial gene, cytochrome oxidase I (COI).Crossref | GoogleScholarGoogle Scholar | 21669761PubMed |
Bruzelius, RM (1859). Bidrag till Kännedomen om Skandinaviens Amphipoda gammaridea. Kongliga Svenska Vetenskaps-Akademiens Handlingar, Ny följd 3, 1–104.
| Bidrag till Kännedomen om Skandinaviens Amphipoda gammaridea.Crossref | GoogleScholarGoogle Scholar |
Byrne, M, Yeates, DK, Joseph, L, Kearney, M, Bowler, J, Williams, MA, Cooper, SJB, Donnellan, SC, Keogh, S, Leijs, R, Melville, J, Murphy, D, Porch, N, and Wyrwoll, K-H (2008). Birth of a biome: Synthesizing environmental and molecular studies of the assembly and maintenance of the Australian arid zone biota. Molecular Ecology 17, 4398–4417.
| Birth of a biome: Synthesizing environmental and molecular studies of the assembly and maintenance of the Australian arid zone biota.Crossref | GoogleScholarGoogle Scholar | 18761619PubMed |
Cooper, SJB, Bradbury, JH, Saint, KM, Leys, R, Austin, AD, and Humphreys, WF (2007). Subterranean archipelago in the Australian arid zone: mitochondrial DNA phylogeography of amphipods from central Western Australia. Molecular Ecology 16, 1533–1544.
| Subterranean archipelago in the Australian arid zone: mitochondrial DNA phylogeography of amphipods from central Western Australia.Crossref | GoogleScholarGoogle Scholar |
Cooper, SJB, Saint, KM, Taiti, S, Austin, AD, and Humphreys, WF (2008). Subterranean archipelago: mitochondrial DNA phylogeography of stygobitic isopods (Oniscidea: Haloniscus) from the Yilgarn region of Western Australia. Invertebrate Systematics 22, 195–203.
| Subterranean archipelago: mitochondrial DNA phylogeography of stygobitic isopods (Oniscidea: Haloniscus) from the Yilgarn region of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Darling, JA, Bagley, MJ, Roman, J, Tepolt, CK, and Geller, JB (2008). Genetic patterns across multiple introductions of the globally invasive crab genus Carcinus. Molecular Ecology 17, 4992–5007.
| Genetic patterns across multiple introductions of the globally invasive crab genus Carcinus.Crossref | GoogleScholarGoogle Scholar | 19120987PubMed |
Davis, J, Pavlova, A, Thompson, R, and Sunnucks, P (2013). Evolutionary refugia and ecological refuges: key concepts for conserving Australian arid zone freshwater biodiversity under climate change. Global Change Biology 19, 1970–1984.
| Evolutionary refugia and ecological refuges: key concepts for conserving Australian arid zone freshwater biodiversity under climate change.Crossref | GoogleScholarGoogle Scholar | 23526791PubMed |
de Queiroz K (1998) The general lineage concept of species, species criteria, and the process of speciation. In ‘Endless forms: species and speciation’. (Eds DJ Howard, SH Berlocher) pp. 57–75. (Oxford University Press)
de Queiroz, K (2005). A unified concept of species and its consequences for the future of taxonomy. Proceedings of the California Academy of Sciences 56, 196–215.
de Queiroz, K (2007). Species concepts and species delimitation. Systematic Biology 56, 879–886.
| Species concepts and species delimitation.Crossref | GoogleScholarGoogle Scholar | 18027281PubMed |
Delić, T, Trontelj, P, Rendoš, M, and Fišer, C (2017). The importance of naming cryptic species and the conservation of endemic subterranean amphipods. Scientific Reports 7, 3391.
| The importance of naming cryptic species and the conservation of endemic subterranean amphipods.Crossref | GoogleScholarGoogle Scholar | 28611400PubMed |
Eberhard, SM, Halse, SA, and Humphreys, WF (2005). Stygofauna in the Pilbara region, north-west Western Australia: a review. Journal of the Royal Society of Western Australia 88, 167–176.
Eberhard, SM, Halse, SA, Williams, MR, Scanlon, MD, Cocking, J, and Barron, HJ (2009). Exploring the relationship between sampling efficiency and short range endemism for groundwater fauna in the Pilbara region, Western Australia. Freshwater Biology 54, 885–901.
| Exploring the relationship between sampling efficiency and short range endemism for groundwater fauna in the Pilbara region, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Finston, TL, Bradbury, JH, Johnson, MS, and Knott, B (2004). When morphology and molecular markers conflict: a case history of subterranean amphipods from the Pilbara, Western Australia. Animal Biodiversity and Conservation 27, 83–94.
Finston, TL, Johnson, MS, Humphreys, WF, Eberhard, SM, and Halse, SA (2007). Cryptic speciation in two widespread subterranean amphipod genera reflects historical drainage patterns in an ancient landscape. Molecular Ecology 16, 355–365.
| Cryptic speciation in two widespread subterranean amphipod genera reflects historical drainage patterns in an ancient landscape.Crossref | GoogleScholarGoogle Scholar | 17217350PubMed |
Finston, TL, Johnson, MS, and Knott, B (2008). A new genus and species of stygo bitic paramelitid amphipod from the Pilbara, Western Australia. Records of the Western Australian Museum 24, 395–410.
| A new genus and species of stygo bitic paramelitid amphipod from the Pilbara, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Folmer, O, Black, M, Hoeh, W, Lutz, R, and Vrijenhoek, R (1994). DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3, 294–299.
| 7881515PubMed |
Guindon, S, Dufayard, JF, Lefort, V, Anismova, M, Hordijk, W, and Gascuel, O (2010). New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic Biology 59, 307–321.
| New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0.Crossref | GoogleScholarGoogle Scholar | 20525638PubMed |
Guzik, MT, Abrams, KM, Cooper, SJB, Humphreys, WF, Cho, J-L, and Austin, AD (2008). Phylogeography of the ancient Parabathynellidae (Crustacea: Bathynellacea) from the Yilgarn region of Western Australia. Invertebrate Systematics 22, 205–216.
| Phylogeography of the ancient Parabathynellidae (Crustacea: Bathynellacea) from the Yilgarn region of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Guzik, MT, Austin, AD, Cooper, SJB, Harvey, M, Humphreys, WF, Bradford, T, Eberhard, SM, King, RA, Leys, R, Muirhead, KA, and Tomlinson, M (2010). Is the Australian subterranean fauna uniquely diverse? Invertebrate Systematics 24, 407–418.
| Is the Australian subterranean fauna uniquely diverse?Crossref | GoogleScholarGoogle Scholar |
Guzik, MT, Stringer, DN, Murphy, NP, Cooper, SJB, Taiti, S, King, RA, Humphreys, WF, and Austin, AD (2019). Molecular phylogenetic analysis of Australian arid-zone oniscidean isopods (Crustacea: Haloniscus) reveals strong regional endemicity and new putative species. Invertebrate Systematics 33, 556–574.
Halse SA (2018) Subterranean Fauna of the Arid Zone. In ‘On the Ecology of Australia’s Arid Zone’. (Ed. H Lambers) pp. 215–241. (Springer: Cham, Switzerland)
Halse, SA, Scanlon, MD, Cocking, JS, Barron, HJ, Richardson, JB, and Eberhard, SM (2014). Pilbara stygofauna: deep groundwater of an arid landscape contains globally significant radiation of biodiversity. Records of the Western Australian Museum 78, 443–483.
| Pilbara stygofauna: deep groundwater of an arid landscape contains globally significant radiation of biodiversity.Crossref | GoogleScholarGoogle Scholar |
Harvey, MS (2002). Short-range endemism among the Australian fauna: some examples from non-marine environments. Invertebrate Systematics 16, 555–570.
| Short-range endemism among the Australian fauna: some examples from non-marine environments.Crossref | GoogleScholarGoogle Scholar |
Harvey, MS, Rix, MG, Framenau, VW, Hamilton, ZR, Johnson, MS, Teale, R, Humphreys, G, and Humphreys, WF (2011). Protecting the innocent: studying short-range endemic taxa enhances conservation outcomes. Invertebrate Systematics 25, 1–10.
| Protecting the innocent: studying short-range endemic taxa enhances conservation outcomes.Crossref | GoogleScholarGoogle Scholar |
Havermans, C, Sonet, G, d’Udekem d’Acoz, C, Nagy, ZT, Martin, P, Brix, S, Riehl, T, Agrawal, S, and Held, C (2013). Genetic and morphological divergences in the cosmopolitan deep-sea amphipod Eurythenes gryllus reveal a diverse abyss and a bipolar species. PLoS One 8, e74218.
| Genetic and morphological divergences in the cosmopolitan deep-sea amphipod Eurythenes gryllus reveal a diverse abyss and a bipolar species.Crossref | GoogleScholarGoogle Scholar | 24086322PubMed |
Hertzog, L (1936). Crustacés de biotopes hypogées de la vallée du Rhin d’Alsace. Bulletin de la Société Zoologique de France 61, 356–372.
Horton T, Lowry J, De Broyer C, Bellan-Santini D, Coleman CO, Corbari L, Costello MJ, Daneliya M, Dauvin J-C, Fišer C, Gasca R, Grabowski M, Guerra-García JM, Hendrycks E, Hughes L, Jaume D, Jazdzewski K, Kim Y-H, King RA, Krapp-Schickel T, LeCroy S, Lörz A-N, Mamos T, Senna AR, Serejo C, Sket B, Souza-Filho JF, Tandberg AH, Thomas JD, Thurston M, Vader W, Väinölä R, Vonk R, White K, Zeidler W (2020) Paramelitidae Bousfield, 1977. In ‘World Amphipoda Database’. (World Register of Marine Species). Available at http://www.marinespecies.org/aphia.php?P=taxdetails&id=548188 [Verified 20 April 2021]
Humphreys WF (2019) Diversity patterns in Australia. In ‘Encyclopedia of Caves’, 3rd edn. (Eds WB White, DC Culver, T Pipan) pp. 109–126. (Academic Press: San Diego, CA, USA)
Hurt, C, Haddock, SHD, and Browne, WE (2013). Molecular phylogenetic evidence for the reorganization of the Hyperiid amphipods, a diverse group of pelagic crustaceans. Molecular Phylogenetics and Evolution 67, 28–37.
| Molecular phylogenetic evidence for the reorganization of the Hyperiid amphipods, a diverse group of pelagic crustaceans.Crossref | GoogleScholarGoogle Scholar | 23319084PubMed |
Juan, C, Guzik, MT, Jaume, D, and Cooper, SJB (2010). Evolution in caves: Darwin’s ‘wrecks of ancient life’ in the molecular era. Molecular Ecology 19, 3865–3880.
| Evolution in caves: Darwin’s ‘wrecks of ancient life’ in the molecular era.Crossref | GoogleScholarGoogle Scholar | 20637049PubMed |
Karaman, SL (1932). Beitrag zur Kenntnis der Süsswasser-Amphipoden. Prorodoslovne Razprave 2, 179–232.
Karaman, SL (1943). Die unterirdischen Amphipoden Südserbiens. Srpska Akademya Nauka, Posebna Izdana, 135 (Prirodn’achki i Mathematichki Spici) 34, 161–312.
Katoh, K, Misawa, K, Kuma, K, and Miyata, T (2002). MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research 30, 3059–3066.
| MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform.Crossref | GoogleScholarGoogle Scholar | 12136088PubMed |
Knox, MA, Hogg, ID, Pilditch, CA, Lörz, A-N, Hebert, PDN, and Steinke, D (2012). Mitochondrial DNA (COI) analyses reveal that amphipod diversity is associated with environmental heterogeneity in deep-sea habitats. Molecular Ecology 21, 4885–4897.
| Mitochondrial DNA (COI) analyses reveal that amphipod diversity is associated with environmental heterogeneity in deep-sea habitats.Crossref | GoogleScholarGoogle Scholar | 22924838PubMed |
Lanfear, R, Frandsen, PB, Wright, AM, Senfeld, T, and Calcott, B (2017). PartitionFinder 2: new methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Molecular Biology and Evolution 34, 772–773.
| 28013191PubMed |
Larkin, MA, Blackshields, G, Brown, NP, Chenna, R, McGettigan, PA, McWilliam, H, Valentin, F, Wallace, IM, Wilm, A, Lopez, R, Thompson, JD, Gibson, TJ, and Higgins, DG (2007). Clustal W and Clustal X version 2.0. Bioinformatics 23, 2947–2948.
| Clustal W and Clustal X version 2.0.Crossref | GoogleScholarGoogle Scholar | 17846036PubMed |
Lowry, J, and Myers, AA (2013). A phylogeny and classification of the Senticaudata subord. nov (Crustacea: Amphipoda). Zootaxa 3610, 1–80.
| A phylogeny and classification of the Senticaudata subord. nov (Crustacea: Amphipoda).Crossref | GoogleScholarGoogle Scholar | 24699701PubMed |
Macdonald, KS, Yampolsky, L, and Duffy, JE (2005). Molecular and morphological evolution of the amphipod radiation of Lake Baikal. Molecular Phylogenetics and Evolution 35, 323–343.
| Molecular and morphological evolution of the amphipod radiation of Lake Baikal.Crossref | GoogleScholarGoogle Scholar | 15804407PubMed |
Matthews, EF, Abrams, KM, Cooper, SJB, Huey, JA, Hillyer, MJ, Humphreys, WF, Austin, AD, and Guzik, MT (2020). Scratching the surface of subterranean biodiversity: molecular analysis reveals a diverse and previously unknown fauna of Parabathynellidae (Crustacea: Bathynellacea) from the Pilbara, Western Australia. Molecular Phylogenetics and Evolution 142, 106643.
| Scratching the surface of subterranean biodiversity: molecular analysis reveals a diverse and previously unknown fauna of Parabathynellidae (Crustacea: Bathynellacea) from the Pilbara, Western Australia.Crossref | GoogleScholarGoogle Scholar | 31622741PubMed |
Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In ‘Proceedings of the Gateway Computing Environments Workshop (GCE)’, 14 November 2010, New Orleans, LA, USA. INSPEC Accession Number 11705685. (IEEE)
| Crossref |
Murphy, NP, Adams, M, Guzik, MT, and Austin, AD (2013). Extraordinary micro-endemism in Australian desert spring amphipods. Molecular Phylogenetics and Evolution 66, 645–653.
| Extraordinary micro-endemism in Australian desert spring amphipods.Crossref | GoogleScholarGoogle Scholar | 23142695PubMed |
Murphy, NP, King, RA, and Delean, S (2015). Species, ESUs or populations? Delimiting and describing morphologically cryptic diversity in Australian desert spring amphipods. Invertebrate Systematics 29, 457–467.
| Species, ESUs or populations? Delimiting and describing morphologically cryptic diversity in Australian desert spring amphipods.Crossref | GoogleScholarGoogle Scholar |
Padial, JM, Miralles, A, De la Riva, I, and Vences, M (2010). The integrative future of taxonomy. Frontiers in Zoology 7, 16.
| The integrative future of taxonomy.Crossref | GoogleScholarGoogle Scholar | 20500846PubMed |
Pepper, M, Doughty, P, and Keogh, JS (2013). Geodiversity and endemism in the iconic Australian Pilbara region: a review of landscape evolution and biotic response in an ancient refugium. Journal of Biogeography 40, 1225–1239.
| Geodiversity and endemism in the iconic Australian Pilbara region: a review of landscape evolution and biotic response in an ancient refugium.Crossref | GoogleScholarGoogle Scholar |
Perina, G, Camacho, AI, Huey, J, Horwitz, P, and Koenders, A (2018). Understanding subterranean variability: the first genus of Bathynellidae (Bathynellacea, Crustacea) from Western Australia described through a morphological and multigene approach. Invertebrate Systematics 32, 423–447.
| Understanding subterranean variability: the first genus of Bathynellidae (Bathynellacea, Crustacea) from Western Australia described through a morphological and multigene approach.Crossref | GoogleScholarGoogle Scholar |
Perina, G, Camacho, AI, Huey, J, Horwitz, P, and Koenders, A (2019a). New Bathynellidae (Crustacea) taxa and their relationships in the Fortescue catchment aquifers of the Pilbara region, Western Australia. Systematics and Biodiversity 17, 148–164.
| New Bathynellidae (Crustacea) taxa and their relationships in the Fortescue catchment aquifers of the Pilbara region, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Perina, G, Camacho, AI, Huey, J, Horwitz, P, and Koenders, A (2019b). The role of allopatric speciation and ancient origins of Bathynellidae (Crustacea) in the Pilbara (Western Australia): two new genera from the De Grey River catchment. Contributions to Zoology 88, 452–497.
| The role of allopatric speciation and ancient origins of Bathynellidae (Crustacea) in the Pilbara (Western Australia): two new genera from the De Grey River catchment.Crossref | GoogleScholarGoogle Scholar |
Pinder, AM, Halse, SA, Shiel, RJ, and McRae, JM (2010). An arid zone awash with diversity: patterns in the distribution of aquatic invertebrates in the Pilbara region of Western Australia. Records of the Western Australian Museum 78, 205–246.
| An arid zone awash with diversity: patterns in the distribution of aquatic invertebrates in the Pilbara region of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Puillandre, N, Lambert, A, Brouillet, S, and Achaz, G (2012). ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Molecular Ecology 21, 1864–1877.
| ABGD, Automatic Barcode Gap Discovery for primary species delimitation.Crossref | GoogleScholarGoogle Scholar | 21883587PubMed |
Rambaut, A, Drummond, AJ, Xie, D, Baele, G, and Suchard, MA (2018). Posterior summarisation in Bayesian phylogenetics using Tracer 1.7. Systematic Biology 67, 901–904.
| Posterior summarisation in Bayesian phylogenetics using Tracer 1.7.Crossref | GoogleScholarGoogle Scholar | 29718447PubMed |
Ronquist, F, and Huelsenbeck, JP (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574.
| MrBayes 3: Bayesian phylogenetic inference under mixed models.Crossref | GoogleScholarGoogle Scholar | 12912839PubMed |
Rosenberg, NA (2007). Statistical tests for taxonomic distinctiveness from observations of monophyly. Evolution 61, 317–323.
| Statistical tests for taxonomic distinctiveness from observations of monophyly.Crossref | GoogleScholarGoogle Scholar | 17348942PubMed |
Stamatakis, A, Hoover, P, and Rougemont, J (2008). A rapid algorithm for the RAxML Web servers. Systematic Biology 57, 758–771.
| A rapid algorithm for the RAxML Web servers.Crossref | GoogleScholarGoogle Scholar | 18853362PubMed |
Tandberg, AH, Rapp, HT, Schander, C, Vader, W, Sweetman, AK, and Berge, J (2011). Exitomelita sigynae gen. et sp. nov.: a new amphipod from the Arctic Loki Castle vent field with potential gill ectosymbionts. Polar Biology 35, 705–716.
| Exitomelita sigynae gen. et sp. nov.: a new amphipod from the Arctic Loki Castle vent field with potential gill ectosymbionts.Crossref | GoogleScholarGoogle Scholar |
Tempestini, A, Rysgaard, S, and Dufresne, F (2018). Species identification and connectivity of marine amphipods in Canada’s three oceans. PLoS One 13, e0197174.
| Species identification and connectivity of marine amphipods in Canada’s three oceans.Crossref | GoogleScholarGoogle Scholar | 29791459PubMed |
Trontelj, P, Douady, CJ, Fišer, C, Gibert, J, Špela, G, Lefébure, T, Sket, B, and Zakšek, V (2009). A molecular test for cryptic diversity in ground water: how large are the ranges of macro-stygobionts? Freshwater Biology 54, 727–744.
| A molecular test for cryptic diversity in ground water: how large are the ranges of macro-stygobionts?Crossref | GoogleScholarGoogle Scholar |
Verheye, M, Martin, P, Backeljau, T, and d’Udekem d’Acoz, C (2016). DNA sequence data suggests abundant homoplasy in taxonomically important morphological characters of Eusiroidea (Crustacea, Amphipoda). Zoologica Scripta 45, 300–321.
| DNA sequence data suggests abundant homoplasy in taxonomically important morphological characters of Eusiroidea (Crustacea, Amphipoda).Crossref | GoogleScholarGoogle Scholar |
Verovnik, R, Sket, B, and Trontelj, P (2005). The colonization of Europe by the freshwater crustacean Asellus aquaticus (Crustacea: Isopoda) proceeded from ancient refugia and was directed by habitat connectivity. Molecular Ecology 14, 4355–4369.
| The colonization of Europe by the freshwater crustacean Asellus aquaticus (Crustacea: Isopoda) proceeded from ancient refugia and was directed by habitat connectivity.Crossref | GoogleScholarGoogle Scholar | 16313598PubMed |
Weitemier, K, Straub, SCK, Cronn, RC, Fishbein, M, Schmickl, R, McDonnell, A, and Liston, A (2014). Hyb-Seq: combining target enrichment and genome skimming for plant phylogenomics. Applications in Plant Sciences 2, 1400042.
| Hyb-Seq: combining target enrichment and genome skimming for plant phylogenomics.Crossref | GoogleScholarGoogle Scholar |
Witt, JDS, Blinn, DW, and Hebert, PDN (2003). The recent evolutionary origin of the phenotypically novel amphipod Hyalella montezuma offers an ecological explanation for morphological stasis in a closely allied species complex. Molecular Ecology 12, 405–413.
| The recent evolutionary origin of the phenotypically novel amphipod Hyalella montezuma offers an ecological explanation for morphological stasis in a closely allied species complex.Crossref | GoogleScholarGoogle Scholar |
Witt, JDS, Threloff, DL, and Hebert, PDN (2006). DNA barcoding reveals extraordinary cryptic diversity in an amphipod genus: implications for desert spring conservation. Molecular Ecology 15, 3073–3082.
| DNA barcoding reveals extraordinary cryptic diversity in an amphipod genus: implications for desert spring conservation.Crossref | GoogleScholarGoogle Scholar |
Zeidler, W (1989). A new species of Melita (Crustacea: Amphipoda: Melitidae) from northern New South Wales with a note on the genus Abludomelita Karaman, 1981. Proceedings of the Linnean Society of New South Wales 110, 327–338.
Zhang, J, Kapli, P, Pavlidis, P, and Stamatakis, A (2013). A general species delimitation method with applications to phylogenetic placements. Bioinformatics 29, 2869–2876.
| A general species delimitation method with applications to phylogenetic placements.Crossref | GoogleScholarGoogle Scholar | 23990417PubMed |