Sample design in biodiversity studies matters: a fine-scale study of Lawrence’s velvet worm, Peripatopsis lawrencei (Onychophora: Peripatopsidae), reveals hidden diversity
Julian A. Nieto Lawrence A and Savel R. Daniels
A and Savel R. Daniels  A *
A *
A
Abstract
A fine-scale phylogenetic and phylogeographic analysis of Peripatopsis lawrencei s.l. was conducted with both mitochondrial and nuclear DNA sequence data, using both external morphology and scanning electron microscopy of taxonomically important characters. A total of 119 sequences were used for the mitochondrial cytochrome c oxidase subunit I (COI) whereas a single representative specimen from each locality was sequenced for the nuclear 18S rRNA locus. Phylogenetic analyses were conducted on the total COI data set and the combined COI + 18S rRNA data set using a Bayesian analysis and maximum likelihood analyses. For the combined DNA sequence data set, a divergence time estimation was further undertaken in BEAST and specimens placed in a phylogenetic framework including all the described Peripatopsis species from South Africa. In addition, a phylogeographic study was conducted exclusively on P. lawrencei s.s. (clade A) using an analysis of molecular variance and haplotype network. Phylogenetic results indicated that, at the Oubos sample locality, two highly distinct genetic lineages were present (clades A and B), whereas a divergence time estimation suggests a Miocene cladogenesis of the novel Oubos lineage. Marked phylogeographic structure was observed for P. lawrencei s.s. (restricted to clade A) across the distribution range with limited maternal dispersal. Morphologically, the two sympatric lineages at Oubos A and B differed in leg pair number, ventral colour and dorsal scale rank counts, as evident from scanning electron microscopy. Our results support the recognition of a distinct species that occurs in sympatry with P. lawrencei s.s. The new species, P. aereus sp. nov. (clade B) is described and the implication for fine-scale taxonomic studies on saproxylic taxa is discussed.
ZooBank: urn:lsid:zoobank.org:pub:AB6E0BDA-7B5F-4FD3-A863-BA7C814E278C
Keywords: cladogenesis, phylogenetics, phylogeography, saproxylic invertebrates, SEM, sympatry, systematics.
Introduction
Velvet worms are soft-bodied invertebrates, typically occurring in saproxylic environments within forested regions, living in leaf litter, inside bark or under stones or moss (Hamer et al. 1997). The vulnerability to desiccation encourages an affinity to these sheltered terrestrial habitats from warm tropical to cold temperate climatic regimes (Ruhberg 1985; Daniels pers. obs; Giribet pers. obs). The phylum Onychophora, sister group to Arthropoda (Edgecombe 2009), consists of two families: Peripatopsidae, Bouvier, 1905 and Peripatidae, Aoudouin & Milne-Edwards, 1832. The latter is circumtropically distributed and present in South-east Asia, India, the Neotropics into Mesoamerica and Gabon (Ruhberg 1985). Members of Peripatopsidae have a Gondwanan circumpolar distribution and occur in Australasia, Chile, New Zealand, South Africa and Eswatini (formerly Swaziland) (Ruhberg 1985; Hamer et al. 1997). Ancient climatic oscillations, coupled with tectonic uplift followed by habitat fragmentation are considered to be the main cladogenetic drivers (McDonald and Daniels 2012; Murienne et al. 2014; Giribet et al. 2018; Sato et al. 2018; Myburgh and Daniels 2022).
Two genera within Peripatopsidae, Opisthopatus Purcell, 1899 and Peripatopsis Pocock, 1894 occur in South Africa (Hamer et al. 1997). Towards the end of the 20th century, Opisthopatus and Peripatopsis contained eight and three species respectively, delineated by poorly defined morphological characters (Hamer et al. 1997; Sherbon and Walker 2004; Ruhberg and Hamer 2005). However, the recent application of DNA sequence-based systematic research and scanning electron microscopy on Opisthopatus and Peripatopsis resulted in the description of 7 and 15 species respectively (McDonald et al. 2012; Daniels et al. 2013, 2016; Ruhberg and Daniels 2013; Barnes et al. 2020; Barnes and Daniels 2022; Grobler et al. 2023). Although we note the publication by Oliveira (2023), we critique observations regarding velvet worm taxonomic diversity in South Africa. Oliveira (2023) makes several spurious, scientifically incorrect observations, for example, restricting all species to type localities. Our DNA based evidence derived from widespread sampling demonstrates the monophyly of species, suggesting that, although some species are regionally widespread, other taxa are narrowly endemic, negating the perception that all species names should be restricted exclusively to type localities (McDonald et al. 2012; Daniels et al. 2013, 2016; Ruhberg and Daniels 2013; Barnes et al. 2020; Barnes and Daniels 2022; Grobler et al. 2023). The delineation of South African species is further corroborated by SEM and to a varying degree, gross morphological data. Furthermore, Oliveira’s (2023) global list lacks understanding of biogeographic context and potential barriers or the absence of barriers to gene flow, and no formal species definition is applied. Consequently, we do not adopt the alpha-taxonomic diversity proposed by Oliveira (2023). Clearly additional research into the alpha taxonomy of the South African velvet worm is required.
The greatest diversity of Peripatopsis occurs in the Afrotemperate forests and fynbos regions of the Western Cape province along the south-eastern Cape coast and adjacent interior, extending into the Eastern Cape and KwaZulu–Natal provinces (Daniels et al. 2009). Velvet worms are habitat specialists (Barnes and Daniels 2019), requiring very specific microclimatic regimes for survival, i.e. saproxylic environments and leaf litter. The Afrotemperate forests are highly fragmented and distributed along the Cape Fold Mountain belt, confined to areas of high altitude with high rainfall, often nested in deep gorges and valleys. Large areas of Afrotemperate forest remain unsampled for velvet worms. Considering the limited dispersal capability of velvet worms, several undescribed species are likely to be present in poorly sampled forested areas and fynbos regions. In South Africa, few phylogeographic studies have been conducted on velvet worms. One study assessed the P. capensis species complex in the western and south-western Cape regions of the Western Cape Province (McDonald and Daniels 2012), resulting in the description of two novel species, P. overbergiensis McDonald, Ruhberg & Daniels, 2012 and P. lawrencei McDonald, Ruhberg & Daniels, 2012. Daniels et al. (2013) examined species boundaries in the P. balfouri (Sedgwick, 1855) species complex and described three novel lineages within the species complex. Similarly, a fine-scale study of the P. moseleyi (Wood-Mason, 1979) species complex resulted in the description of five novel species (Ruhberg and Daniels 2013). Furthermore a recent study of the P. birgeri Ruhberg & Daniels, 2013 species complex from KwaZulu–Natal resulted in the description of a novel lineage, P. polychroma Grobler, Myburgh, Barnes & Daniels, 2023 (Ruhberg and Daniels 2013; Grobler et al. 2023). A phylogeographic study of Opisthopatus amaxhosa Daniels, Dambire, Klaus & Sharma, 2016, a narrowly endemic velvet worm in the Eastern Cape by Barnes and Daniels (2019) also resulted in the discovery of a novel species, O. baziya, Barnes & Daniels, 2022 (Barnes and Daniels 2022). These results reiterate the need for fine-scale sampling to document the alpha-taxonomic diversity of velvet worms and other saproxylic taxa including, for example, earthworms, snails, spring tails, centipedes, harvestmen and millipedes.
The first DNA sequence-based phylogeny of Peripatopsis by Daniels et al. (2009) demonstrated that at Riviersonderend, two highly divergent sympatric lineages (A & B) were present in the P. capensis (Grube 1866) species complex. The study by Daniels et al. (2009) revealed three geographically discrete, morphologically diagnosable clades in the latter species complex that were subsequently described as P. capensis sensu stricto (s.s.), sister group to P. lawrencei and P. overbergiensis (McDonald et al. 2012). The main lineage (A), present along the Riviersonderend Mountains, now known as P. lawrencei, also occurs in Caledon, Fernkloof, Gaansbaai, Greyton and Oubos. However, the identity of the second Riviersonderend lineage (B) is unknown, likely signifying a narrowly distributed endemic species awaiting formal description. This novel species forms the basis for the present study.
Underestimated or cryptic diversity often arises from the application of outdated taxonomic methods such as using gross morphology as the only diagnostic tool. This has been frequently demonstrated for velvet worms (Daniels et al. 2009; Sato et al. 2018; Grobler et al. 2023). Even when crypticity has been identified, effective species diagnosis can become problematic because of incongruence between taxonomic methods (Daniels et al. 2016). The most effective means of revising taxonomic lineages and cryptic species complexes involves utilising a combination of gross morphology, scanning electron microscopy (SEM) and genetic characteristics (Dayrat 2005). The fragmented nature of saproxylic environments has led to similar microclimatic pressures, promoting cryptic speciation and convergent evolution (Daniels et al. 2016; Barnes and Daniels 2019). Saproxylic groups show high cryptic differentiation but the alpha taxonomy is poor, further highlighting the importance of molecular systematic research to document diversity (Barnes and Daniels 2019).
During our study, we undertook fine-scale sampling along the Riviersonderend Mountains to identify the novel species and better understand the evolutionary placement in the wider Peripatopsis phylogeny. The cryptic nature of these velvet worms indicates that fine-scale sampling is necessary to understand population genetic structure. The first objective was to identify and describe the novel lineage. We hypothesised that the novel lineage (B) would be morphologically very similar to P. lawrencei s.s. (A) but could be distinguished based on gross morphological characteristics. Additionally, SEM characteristics of the dorsal and ventral papillae would also be diagnostic. For identification, the specimens of the novel lineage were subjected to DNA sequencing, and placed into a dated phylogeny to understand the evolutionary placement and divergence relative to the wider Peripatopsis. In the second part of this study, we focused exclusively on Peripatopsis lawrencei s.s., with the objective being to examine the population genetic structure and colonisation history. Using a fine-scale phylogeographic study, we hypothesised that we would observe marked phylogeographic differentiation across the distribution range of the species that is characterised by limited dispersal based on DNA sequence data.
Materials and methods
Sample collection
A total of 69 new Peripatopsis lawrencei sensu lato (s.l.). specimens was collected from the known distribution range of the species in the Western Cape province, South Africa (Fig. 1). These specimens were combined with data collected from two previous studies (Daniels et al. 2009; McDonald and Daniels 2012), yielding a total of 119 specimens (Table 1). Peripatopsis lawrencei s.s. comprised 116 of the samples, whereas 3 samples were suspected to be the unknown sympatric lineage at Oubos (Riviersonderend Mountains). A handheld GPS was used to record the locality information and a permit (CN44-87-22079) was obtained from CapeNature for velvet worm sampling. Specimens were collected in Afromontane forest patches under decaying logs, leaf litter and stones (McDonald et al. 2012). These specimens were preserved directly in absolute ethanol and stored in a refrigerator at 4°C once returned to the laboratory.
Map showing all sampling localities for Peripatopsis lawrencei s.l., Western Cape Province, South Africa. The red markers indicate sampled localities with numbers corresponding to Table 1.
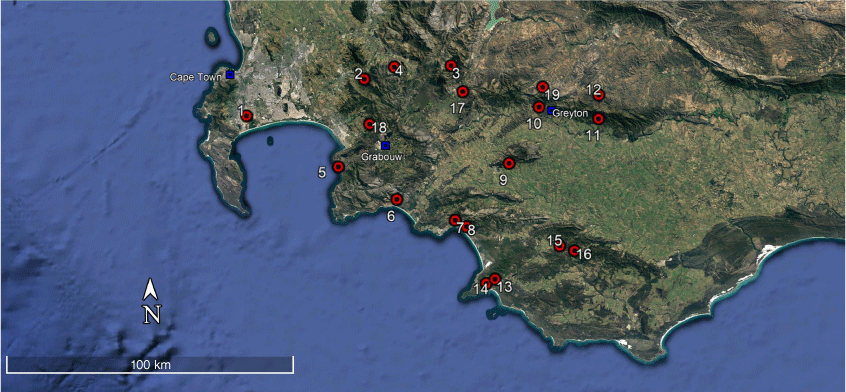
| Number | Locality | Latitude (S) | Longitude (E) | Daniels et al. (2009) | McDonald and Daniels (2012) | Present study | Total | |
|---|---|---|---|---|---|---|---|---|
| 1 | Rondevlei Nature Reserve (NR) | 34°03.375′S | 18°29.597′E | 1 | 1 | |||
| 2 | Jonkershoek NR | 33°58.957′S | 18°56.246′E | 4 | 4 | |||
| 3 | Franschhoek | 33°54.292′S | 19°16.560′E | 4 | 4 | |||
| 4 | High Noon | 33°54.251′S | 19°03.044′E | 4 | 4 | |||
| 5 | Dappat se Gat | 34°13.258′S | 18°50.241′E | 5 | 5 | |||
| 6 | Kogelberg Biosphere NR | 34°05.764′S | 18°50.493′E | 1 | 1 | 3 | 5 | |
| 7 | Fernkloof NR | 34°23.613′S | 19°16.564′E | 1 | 4 | 6 | 11 | |
| 8 | Vogelgat NR | 34°24.021′S | 19°19.330′E | 10 | 10 | |||
| 9 | Caledon | 34°13.442′S | 19°25.739′E | 1 | 11 | 12 | ||
| 10 | Greyton | 34°02.015′S | 19°36.573′E | 3 | 3 | |||
| 11 | Oubos A and B | 34°04.194′S | 19°49.628′E | 4 | 15/2 | 19/2 | ||
| 12 | Riviersonderend A and B | 34°00.480′S | 19°49.480′E | 1/1 | 1/1 | |||
| 13 | Grootbos NR | 34°34.270′S | 19°25.369′E | 5 | 4 | 2 | 11 | |
| 14 | Linden Hill | 34°35.109′S | 19°23.871′E | 5 | 5 | |||
| 15 | Sandies Glen | 34°28.233′S | 19°40.111′E | 1 | 1 | |||
| 16 | Napier | 34°29.237′S | 19°43.202′E | 6 | 4 | 10 | ||
| 17 | Villiersdorp | 33°59.007′S | 19°17.180′E | 1 | 1 | |||
| 18 | Grabouw | 34°05.470′S | 18°57.550′E | 8 | 8 | |||
| 19 | Jonaskop | 33°58.170′S | 19°30.230′E | 1 | 1 |
The numbers (1–19) correspond to those in Fig. 1. A forward slash (/) implies the presence of the novel lineage (clade B). Data generated during the present study were combined with COI sequence data from two previous studies (Daniels et al. 2009; McDonald and Daniels 2012).
DNA sequencing
A tissue biopsy was taken from each specimen (2–3 mm) and a Macherey–Nagel DNA extraction kit was used, following the manufacturer’s protocol. DNA extractions were kept at 4°C until required for use in polymerase chain reaction (PCR). Two loci were targeted for amplification, namely mitochondrial (mtDNA) cytochrome c oxidase subunit I (COI) and nuclear 18S rRNA subunit (18S). These two loci have been extensively used in velvet worm systematics studies of Peripatopsis, allowing us to combine our data with DNA sequences generated during earlier studies (Daniels et al. 2009, 2013, 2016; McDonald and Daniels 2012; Barnes et al. 2020; Grobler et al. 2023). The COI and 18S rRNA primer pairs (specifically 5F and 7R for the latter locus) used in the amplification of the two fragments were obtained from Folmer et al. (1994) and Giribet et al. (1996) respectively. All newly collected specimens were sequenced for COI. Only a single representative of each locality was sequenced for the 18S locus, except for the Oubos locality at which the two lineages (A and B) occurred in sympatry. A total of 14 new 18S rRNA sequences was generated during our study and combined with three 18S rRNA sequences generated by McDonald and Daniels (2012).
PCRs were conducted for a 25-μL reaction and the samples held at 15°C afterwards. The reactions both contained 14.9 μL of deionised water, 2.5 μL of 10× Mg2+ free buffer, 0.5 μL of a 10-mM dNTP solution and 0.5 μL of the primer sets at 10 mM, 0.1 units of JMR SuperTherm Taq polymerase and 1 μL of extracted DNA. The PCR was conducted on a geneAmp PCR System Thermocycler. PCR conditions for the COI locus were as follows: 94°C for 4 min, 94°C for 30 s, 42°C for 35 s and 72°C for 40 s for 36 cycles, with an extension of 72°C for 10 min. For the 18S locus, PCR conditions were as follows: 94°C for 4 min, 94°C for 30 s, 48°C for 40 s and 72°C for 40 s for 34 cycles, with an extension of 72°C for 10 min. Following successful PCR amplification, PCR products were electrophoresed in a 1% agarose gel for 180 min, at 100 V and gel-purified using a Bio flux gel purification kit, following the manufacturer's instruction. An ABI 3730 machine was used to sequence the products at the DNA sequence facility of the University of Stellenbosch.
Phylogenetic analyses
Data collected from the sequences were utilised with the DNA sequences for P. lawrencei s.l. from Daniels et al. (2009) and McDonald and Daniels (2012). Forward and reverse strands were used to compute a consensus sequence and check for base pair ambiguity, whereas sequence alignment for both the COI and 18S rRNA data was computed using ClustalX (ver. 2.0, see https://www.genome.jp/tools-bin/clustalw;Thompson et al. 1997). For 18S rRNA, highly variable indel regions that could not be aligned with accuracy were excluded from the phylogenetic analyses using Gblocks (ver. 0.91b, see http://phylogeny.lirmm.fr/phylo_cgi/one_task.cgi?task_type=gblocks; Talavera and Castresana 2007). Using jModelTest2 (ver. 2.2.10, see https://github.com/ddarriba/jmodeltest2; Posada 2008), the Akaike information criterion (AIC) (Akaike 1973) was utilised to determine the best-fit DNA substitution model on XSEDE (ver. 2.0, see https://www.xsede.org/) in CIPRES (see http://www.phylo.org/; Miller et al. 2010). Bayesian inference (BI) and maximum likelihood (ML) approaches were used to construct phylogenies. Analyses were conducted on CIPRES Science Gateway (Miller et al. 2010), with the Bayesian analysis taking place in MrBayes (ver. 3.2.6, see https://github.com/NBISweden/MrBayes/; Ronquist et al. 2012) and the phylogeny for ML in RAxML (ver. 7.2.8 alpha, see https://github.com/stamatak/standard-RAxML; Stamatakis et al. 2008). For the part of the study focusing on P. lawrencei s.s., the ML analyses were conducted on the IQ-TREE web server (ver. 2.2.2.6, see http://iqtree.cibiv.univie.ac.at/; Trifinopoulos et al. 2016) and BI analyses through CIPRES, to create the tree topology for the COI data set. For the phylogenetic placement of the new lineage, a combined tree topology for COI and 18S rRNA was reconstructed using the aforementioned procedures, and DNA sequences for both loci for all described Peripatopsis species were included to determine the phylogenetic placement of the Riviersonderend specimens (Daniels et al. 2009, 2013; Ruhberg and Daniels 2013; Barnes et al. 2020; Grobler et al. 2023). Uncorrected COI and 18S rRNA p-distances were calculated in PAUP* (ver. 4.10, see http://phylosolutions.com/paup-test/; Swofford 2002) and compared to interspecific sequence values recorded among velvet worms.
Branch support values were calculated through an ultrafast Bootstrap analysis with 10 000 pseudo replicates and bootstrap (BS) values >75% were taken as support for the nodes using IQ-TREE. For Bayesian inference, burn-in was set to 20% and a posterior probability (PP) >0.95 was considered statistically well supported, estimated through percentage of time spent on node recovery (Barnes and Daniels 2022). With a Bayesian framework, a divergence time estimation was conducted for the COI and 18S rRNA loci, using a probability model to describe molecular sequences of divergent lineages. The ages of the clades were subsequently estimated using the Markov Chain Monte Carlo that was run for 50 million generations, sampling the chain every 1000th generation. In total, 20% of generations were discarded as burn-in. Convergence was assessed visually by examining the performance of the chain, as in Zhang et al. (2013). A relaxed molecular clock was implemented, running the analysis through BEAST (ver. 2.7.6, see https://www.beast2.org/; Drummond and Rambaut 2007) and a multiple coalescent model was used (Heled and Drummond 2010). Mutation rates of 1.5–2.3% per million years were used for the COI locus and the mutation rate for the 18S rRNA locus was estimated through the use of a non-informative (1/x) prior. The aforementioned use of mutation rates has been applied in several Peripatopsis divergence time estimations (Barnes et al. 2020; Myburgh and Daniels 2022; Grobler et al. 2023). Finally, for further support we conducted a Shimodaira–Hasegawa test (Shimodaira and Hasegawa 1999) to evaluate the likelihood of a constrained tree topology, where clades A and B are combined into a single clade, rather than being represented as two separate entities.
Phylogeographic analysis of P. lawrencei s.s.
A haplotype network was constructed through the use of TCS (ver. 1.21, see https://bioresearch.byu.edu/tcs/), with a 95% confidence interval (Clement et al. 2000) and population genetic structure was computed in ARLEQUIN (ver. 3.5.2.2, see http://cmpg.unibe.ch/software/arlequin35; Excoffier and Lischer 2010) using the COI data for P. lawrencei s.s. The sample localities were subjected to a hierarchical Analysis of Molecular Variance (AMOVA), and FST values were calculated, along with standard diversity indices including nucleotide diversity, number of haplotypes and haplotype diversity at each locality.
Morphology and scanning electron microscopy (SEM)
A Canon EOS camera was used to photograph the specimens, and gross morphological traits were examined using a Leica MZ 7.5 stereomicroscope, noting dorsal and ventral integument colour, number of leg pairs and the presence of a claw on the posterior leg pair. A digital calliper was used to measure total length of the ethanol-preserved specimen from the anterior-most point to the posterior side, and additional comparisons between P. lawrencei s.s. and the new lineage were undertaken using a Leica S9i digital stereo microscope at the South African Museum of Cape Town. The rarity of the novel lineage (clade B) poses limitations on the methods of examination. Only two individuals of the new lineage were available for examination and due to the destructive nature of the process, traditional scanning electron microscopy (SEM) was not possible. However, the South African Museum of Cape Town has an environmental SEM, Hitachi TM4000Plus that allows for the analysis of specimens without requiring critical point drying or sputter coating. This allowed us to preserve our limited specimens for later use as holotype and paratype. Through the use of this SEM technique, dorsal and ventral scale ranks for representatives of each of the two main clades were examined. Specimens were accessioned into the entomology collection of the South African Museum of Cape Town (SAM-ENW-C013473–SAM-ENW-C013487). The new species name was deposited in ZooBank (urn:lsid:zoobank.org:act:71276915-79C6-4759-9244-20BEF6303F58).
Results
Phylogenetic analysis of COI for P. lawrencei s.l.
We amplified and sequenced a 644 base-pair (bp) fragment of the COI locus from 69 P. lawrencei s.l. specimens, two of which were the suspected novel lineage. Novel sequences were deposited in GenBank (Accession numbers OR423143–OR423211). We combined COI data generated during our study with 50 P. lawrencei s.l. sequences, of which 1 is known to be the novel species, from two previous studies to yield a total of 119 sequences, 116 of P. lawrencei s.s. and 3 of the novel lineage (clade B). Using corrected AIC, we selected TIM1 + G as our DNA substitution model (–lnL = 2224.64). The base frequencies for the COI locus were as follows: A = 25.00%, C = 12.56%, G = 16.29% and T = 46.14%, whereas the rate matrix included R(a) [A–C] = 1.00, R(b) [A–G] = 19.17, R(c) [A–T] = 2.72, R(d) [C–G] = 2.72, R(e) [C–T] = 8.76 and R(f) [G–T] = 1.00. Gamma (G) shape distribution was 0.15. Both Bayesian inference and maximum likelihood analyses yielded near-identical tree topologies, hence only the Bayesian inference topology is shown (Fig. 2). The tree topology shows two highly divergent statistically well-supported monophyletic clades (A & B) (>75% bootstrap support, BS/>0.95 posterior probability, PP). Within P. lawrencei s.s. (clade A) (84% BS/<0.95 PP), the most basal lineage occurs at Rondevlei NR and Jonkershoek NR (77% BS/0.95 PP). A clade comprising Villiersdorp, Jonaskop, Greyton, Riviersonderend and Oubos A (haplocluster A2; Fig. 2, 3) is sister group to a clade found exclusively at Caledon (92% BS/<0.95 PP), likely reflecting a recent colonisation history between the Riviersonderend Mountains and Klein Swartberg at Caledon (Fig. 2). A coastal clade exists at Vogelgat and Fernkloof NRs (haplocluster A1; Fig. 2, 3) that is sister group to the groups found further south-east at Grootbos, Linden Hill, Sandies Glen and Napier (haplocluster A3; Fig. 2, 3). These groups are found to be sister group to a clade comprising Grabouw, Dappat se Gat, Franschhoek, High Noon and Kogelberg Biosphere NR (haplocluster A3; Fig. 2, 3). Clade B, the novel lineage, was exclusive to the Riviersonderend Mountains and sympatric with P. lawrencei s.s. at Oubos B.
Bayesian phylogenetic tree topology derived from the COI locus, showing the evolutionary relationship across the P. lawrencei s.s. population and separation from the sympatric novel lineage. Statistical support for the nodes is shown as posterior probability (>0.95 PP) above the nodes and bootstrap values (>75% BS) below the node. Posterior probability and bootstrap values <0.95 PP and <75% BS respectively are indicated with a hash (‘#’). Clades A and B have been indicated, showing Peripatopsis lawrencei s.s. and the novel lineage respectively. Clade A (P. lawrencei s.s.) is divided into three haploclusters (A1–A3) and corresponds to the haplotype network in Fig. 3.

The haplotype network based on the COI sequence data showing the genetic distribution of Peripatopsis lawrencei s.s. across 19 localities in the Western Cape, South Africa. A total of 57 haplotypes was identified, with circle size corresponding to the frequency of the haplotypes. The codes A1–A3 represent the clades (haploclusters) present on Fig. 2. Missing or unsampled haplotypes are indicated with a solid black circle. Haplotype numbers correspond to those in Appendix A1.

The uncorrected p-distance for the COI locus between P. lawrencei s.s. (clade A) and clade B ranged from 9.13 to 10.62%. The uncorrected p-distance between P. capensis and P. overbergiensis was 6.58% (our two outgroups), and between P. overbergiensis and P. lawrencei s.s., this ranged from 5.93 to 7.49%. The uncorrected p-distance ranged from 10.53 to 10.73% between clade B and P. overbergiensis. The marked p-distance between the two clades (A and B) of P. lawrencei s.l. further corroborates the genetic distinction of clade B.
Phylogeographic analysis of P. lawrencei s.s. (clade A)
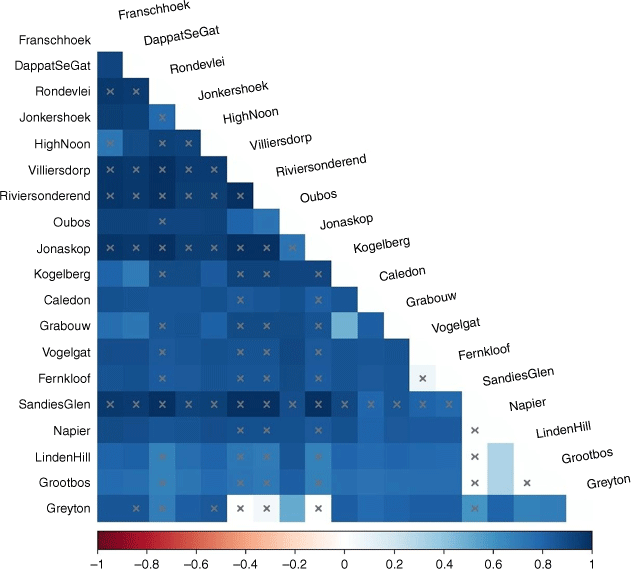
The TCS analyses of 116 COI sequences revealed 57 haplotypes, comprising 3 large unconnected haploclusters that were genetically and geographically discrete (Fig. 3). Haplocluster A1 was exclusive to a narrow coastal strip at Hermanus (Fernkloof and Vogelgat Nature Reserves), whereas haplocluster A2 was restricted to the Riviersonderend Mountains (Oubos A, Greyton, Villiersdorp, Jonaskop and Riviersonderend). Finally, haplocluster A3 was geographically more widespread from Rondevlei to Napier. Within the aforementioned three haploclusters, we observed evidence for maternal dispersal exclusively between Fernkloof NR and Vogelgat, separated by 18 km because these two localities shared one haplotype (Appendix A1). Overall no evidence of shared haplotypes existed among the remaining sample localities, indicating a lack of maternal dispersal based on the COI sequence data (Appendix A1). The lack of maternal dispersal is validated by the AMOVA results. The AMOVA demonstrated that 83.06% of the genetic variation was present among populations (Va = 6.32, d.f. = 18, s.s. = 693.91, P < 0.01), whereas 16.94% of the genetic variation was present within populations (Vb = 1.29, d.f. = 97, s.s. = 125.05, P < 0.01). Furthermore, pairwise and statistically significant FST values showed marked to moderate levels of genetic differentiation among conspecific populations (Table 2, Fig. 4). Non-significant results in Sandies Glen, Riviersonderend, Jonaskop, Villiersdorp and Rondevlei can likely be attributed to small sample sizes (n = 1) at these localities.
| Locality | Vogelgat | Fernkloof | Villiersdorp | Jonaskop | Greyton | Rivierson-derend A | Oubos A | Grabouw | Kogelberg | Franschh-oek | High Noon | Dappat se Gat | Jonkersh-oek | Rondevlei | Caledon | Napier | Grootbos | Sandies Glen | Linden Hill | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vogelgat | 0.00000 | |||||||||||||||||||
| Fernkloof | 0.07913 | 0.00000 | ||||||||||||||||||
| Villiersdorp | 0.86029 | 0.85113 | 0.00000 | |||||||||||||||||
| Jonaskop | 0.84004 | 0.83004 | 1.00000 | 0.00000 | ||||||||||||||||
| Greyton | 0.82717 | 0.82186 | 0.00000 | −0.63636 | 0.00000 | |||||||||||||||
| Riviersonderend A | 0.86029 | 0.85113 | 1.00000 | 1.00000 | 0.05263 | 0.00000 | ||||||||||||||
| Oubos A | 0.90202 | 0.89771 | 0.80117 | 0.73946 | 0.50408 | 0.73016 | 0.00000 | |||||||||||||
| Grabouw | 0.86493 | 0.85880 | 0.89260 | 0.86832 | 0.80653 | 0.89106 | 0.89454 | 0.00000 | ||||||||||||
| Kogelberg | 0.86886 | 0.86066 | 0.92929 | 0.91463 | 0.82668 | 0.92135 | 0.91433 | 0.47225 | 0.00000 | |||||||||||
| Franschhoek | 0.88103 | 0.86861 | 0.9759 | 0.97183 | 0.85117 | 0.97333 | 0.92541 | 0.76382 | 0.80067 | 0.00000 | ||||||||||
| High Noon | 0.87900 | 0.86734 | 0.95349 | 0.94595 | 0.84141 | 0.94872 | 0.92416 | 0.81674 | 0.83210 | 0.72727 | 0.00000 | |||||||||
| Dappat se Gat | 0.88020 | 0.87092 | 0.96939 | 0.96386 | 0.85703 | 0.96591 | 0.92231 | 0.72762 | 0.70588 | 0.91872 | 0.89109 | 0.00000 | ||||||||
| Jonkershoek | 0.85682 | 0.84740 | 0.95000 | 0.94118 | 0.82775 | 0.94444 | 0.91024 | 0.86012 | 0.89603 | 0.94118 | 0.92593 | 0.93340 | 0.00000 | |||||||
| Rondevlei | 0.84004 | 0.83004 | 1.00000 | 1.00000 | 0.69492 | 1.00000 | 0.91463 | 0.85979 | 0.89855 | 0.96610 | 0.93548 | 0.95588 | 0.77778 | 0.00000 | ||||||
| Caledon | 0.85168 | 0.84670 | 0.84284 | 0.82504 | 0.77275 | 0.85734 | 0.87055 | 0.82117 | 0.85406 | 0.87442 | 0.87284 | 0.86850 | 0.85969 | 0.85734 | 0.00000 | |||||
| Napier | 0.83916 | 0.83248 | 0.87266 | 0.84685 | 0.80036 | 0.87266 | 0.87332 | 0.84500 | 0.87277 | 0.89168 | 0.88866 | 0.88780 | 0.86085 | 0.85654 | 0.80905 | 0.00000 | ||||
| Grootbos | 0.76375 | 0.76381 | 0.73274 | 0.68632 | 0.69054 | 0.71483 | 0.82002 | 0.75380 | 0.75496 | 0.77037 | 0.77222 | 0.76707 | 0.72019 | 0.67609 | 0.73679 | 0.31294 | 0.00000 | |||
| Sandies Glen | 0.80173 | 0.78936 | 1.00000 | 1.00000 | 0.59091 | 1.00000 | 0.88331 | 0.85154 | 0.90541 | 0.96825 | 0.93939 | 0.95890 | 0.92308 | 1.00000 | 0.79394 | −0.74359 | −0.12453 | 0.00000 | ||
| Linden Hill | 0.78157 | 0.77787 | 0.72277 | 0.67442 | 0.67846 | 0.70526 | 0.85379 | 0.79018 | 0.79167 | 0.80999 | 0.80593 | 0.81325 | 0.76123 | 0.67059 | 0.77007 | 0.30519 | −0.07664 | −0.27273 | 0.00000 |
Significant values are shown in bold.
Heatmap diagram with the colour showing the strength of the FST value for each respective pairwise FST combination. Darker colours show higher levels of FST between different localities and are therefore more distinct. The stronger the signal, the more easily the different populations can be distinguished from one another by looking at the individual's DNA. The lighter the colour, the less distinct. Blocks marked by a superimposed X indicate non-significance.
The number of samples, haplotypes, polymorphic sites and amounts of haplotype (h) and nucleotide diversity (π) are reported in Table 3 for all 19 localities. The number of haplotypes ranged from one to seven, with most occurring between one and three. The highest haplotype and nucleotide diversities were found in Greyton (h = 1.0000 ± 0.2722; π = 0.009081 ± 0.007451) despite the comparatively low sample size (n = 3) and the second highest was found in Linden Hill (h = 0.9000 ± 0.1610; π = 0.008696 ± 0.005872). The lowest haplotype diversity (with nh > 1) was found in Grabouw (h = 0.4643 ± 0.2000) and the lowest nucleotide diversity (with nh > 1) in Franschhoek (π = 0.000776 ± 0.000963). Rondevlei NR, Riviersonderend, Sandies Glen, Villiersdorp and Jonaskop all had only one haplotype. The highest diversity was present in Greyton and Linden Hill.
| Locality | n | nh | np | Haplotype diversity (H) | Nucleotide diversity (π) | |
|---|---|---|---|---|---|---|
| Rondevlei NR | 1 | 1 | 0 | 1.0000 ± 0.0000 | 0.000000 ± 0.000000 | |
| Jonkershoek NR | 4 | 3 | 2 | 0.8333 ± 0.2224 | 0.001553 ± 0.001538 | |
| Franschhoek | 4 | 2 | 1 | 0.5000 ± 0.2652 | 0.000776 ± 0.000963 | |
| High Noon | 4 | 2 | 2 | 0.5000 ± 0.2652 | 0.001553 ± 0.001538 | |
| Dappat se Gat | 5 | 2 | 1 | 0.6000 ± 0.1753 | 0.000932 ± 0.001021 | |
| Kogelberg Biosphere NR | 5 | 3 | 3 | 0.8000 ± 0.1640 | 0.002174 ± 0.001849 | |
| Fernkloof NR | 11 | 7 | 11 | 0.8909 ± 0.0740 | 0.005590 ± 0.003463 | |
| Vogelgat NR | 10 | 5 | 8 | 0.8444 ± 0.0796 | 0.005141 ± 0.003260 | |
| Caledon | 12 | 5 | 6 | 0.8333 ± 0.0691 | 0.003600 ± 0.002381 | |
| Greyton | 3 | 3 | 8 | 1.0000 ± 0.2722 | 0.009081 ± 0.007451 | |
| Oubos A | 19 | 5 | 10 | 0.5263 ± 0.1266 | 0.002470 ± 0.001714 | |
| Riviersonderend | 1 | 1 | 0 | 1.0000 ± 0.0000 | 0.000000 ± 0.000000 | |
| Grootbos NR | 11 | 7 | 16 | 0.8727 ± 0.0891 | 0.008669 ± 0.005103 | |
| Linden Hill | 5 | 4 | 10 | 0.9000 ± 0.1610 | 0.008696 ± 0.005872 | |
| Sandies Glen | 1 | 1 | 0 | 1.0000 ± 0.0000 | 0.000000 ± 0.000000 | |
| Napier | 10 | 6 | 8 | 0.8444 ± 0.1029 | 0.003520 ± 0.002384 | |
| Villiersdorp | 1 | 1 | 0 | 1.0000 ± 0.0000 | 0.000000 ± 0.000000 | |
| Grabouw | 8 | 3 | 5 | 0.4643 ± 0.2000 | 0.002006 ± 0.001606 | |
| Jonaskop | 1 | 1 | 0 | 1.0000 ± 0.0000 | 0.000000 ± 0.000000 |
n represents the number of samples per locality, nh is the number of haplotypes and np the number of polymorphic sites.
Combined DNA sequence (COI and 18S rRNA) phylogeny and divergence time estimation
We amplified a 519-bp fragment of the 18S rRNA locus for 14 P. lawrencei s.l. specimens, one of which was the suspected novel lineage (clade B). The novel 18S rRNA data were deposited in GenBank (Accession numbers: OR421492–OR421505). The uncorrected p-distance between the two sympatric Oubos lineages (A and B) was 5.58%. To determine the phylogenetic placement of the novel lineage (clade B), we combined all 18S rRNA sequences with COI sequence data from published sequence data for Peripatopsis.
The DNA substitution model for 18S rRNA, selected using AIC, was TIM1 + I + G (–lnL = 1242.71). The base frequencies for the 18S rRNA locus were as follows: A = 19.37%, C = 27.27%, G = 30.28% and T = 23.09%, whereas the rate matrix included R(a) [A–C] = 1.00, R(b) [A–G] = 0.94, R(c) [A–T] = 2.08, R(d) [C–G] = 2.08, R(e) [C–T] = 4.02 and R(f) [G–T] = 1.00. The gamma (G) shape distribution was 1.00. For COI of Peripatopsis, the selected DNA substitution model was GTR + I + G (–lnL = 6422.14) and base frequencies were A = 34.69%, C = 3.71%, G = 9.53% and T = 52.07%, with the rate matrix being R(a) [A–C] = 8.47, R(b) [A–G] = 52.93, R(c) [A–T] = 2.01, R(d) [C–G] = 24.23, R(e) [C–T] = 75.49 and R(f) [G–T] = 1.00. The gamma (G) shape distribution was 0.35. The combined COI and 18S rRNA data yielded a 1163-bp fragment, and the topologies produced from maximum likelihood and Bayesian inference were nearly identical, therefore only the maximum likelihood tree topology is shown (Fig. 5).
Divergence time estimation based on COI and 18S rRNA sequence data for Peripatopsis. The 95% HPD interval bars are shown at each node, with a timescale bar representing the relevant epochs. Clade A shows the monophyletic P. lawrencei s.s. whereas clade B indicates the ancestral novel lineage, denoted as P. aereus sp. nov. Blue bars indicate HPD ranges. Posterior probability values >0.95 PP are shown above each node and bootstrap values >75% BS are shown below the node. Posterior probability and bootstrap values <0.95 PP and <75% BS respectively are indicated with a hash (‘#’).

Peripatopsis was retrieved as monophyletic (>75% BS/>0.95 PP) (Fig. 5). The most basal lineage was P. alba, found in the Wynberg Caves on Table Mountain. The latter species was sister group to a clade of P. balfouri exclusively from the Kogelberg and Cape Peninsula. Peripatopsis balfouri was in turn sister group to a clade where P. bolandi Daniels, McDonald & Picker, 2013 was sister to P. purpureus Daniels, McDonald & Picker, 2013. The next clade comprised P. ferrox Barnes, Reiss & Daniels, 2020 sister to P. edenensis Barnes, Reiss & Daniels, 2020 and these two species were sister group to a clade comprising P. tulbaghensis Barnes, Reiss & Daniels, 2020, sister to P. clavigera Purcell, 1899 and the latter species was sister to P. cederbergiensis Daniels, McDonald & Picker, 2013. The novel lineage, P. aereus sp. nov. (clade B) was basal to the remaining Peripatopsis species, affirming the evolutionary distinction from the sympatric P. lawrencei s.s. specimens at Oubos A. In the next clade, we observed a monophyletic P. lawrencei s.s. (clade A) as sister group to P. capensis. The latter species is in turn sister group to P. overbergiensis. The latter clade, containing the three aforementioned species P. lawrencei s.s., P. capensis and P. overbergiensis was sister group to a larger clade of species distributed from the southern Cape into the Eastern Cape and KwaZulu–Natal. Within the last clade, P. sedgwicki Purcell, 1899 was basal to two other clades. Within these, the first clade comprised P. janni Ruhberg & Daniels, 2013 as sister group to three species P. birgeri Ruhberg & Daniels, 2013, sister group to P. polychroma with these two species being sister group to P. hamerae Ruhberg & Daniels, 2013. The second sister clade comprised P. storchi Ruhberg & Daniels, 2013 as sister group to P. moseleyi. Results of the Shimodaira-Hasegawa test indicated that the difference between the unconstrained topologies and constrained topology enforcing the monophyly of the Oubos clades (A sister to B) is highly significant (Δ –lnL = 185.11, P < 0.01), further validating the distinction of the two clades.
Divergence time estimates (Fig. 5) based on the combined COI and 18S data set suggested that Peripatopsis originated 28.83 Ma (95% highest posterior density, HPD 21.27–37.49 Ma) during the Oligocene–Eocene. Peripatopsis alba originated 13.62 Ma (95% HPD: 10.37–17.42 Ma), whereas P. balfouri separated shortly afterwards, 13.38 Ma (95% HPD: 9.53–15.73 Ma). Sister clades P. purpureus and P. bolandi diverged 11.21 Ma (95% HPD: 8.62–14.44 Ma), whereas P. ferox and P. edenensis diverged 10.23 Ma (95% HPD: 6.62–14.35 Ma). Peripatopsis tulbaghensis originated 11.07 Ma (95% HPD: 7.90–14.70 Ma) and P. clavigera diverged from P. cederbergiensis 8.72 Ma (95% HPD: 5.94–11.88 Ma). Furthermore, the novel lineage (clade B) diverged from the remaining Peripatopsis lineages 13.68 Ma (95% HPD: 10.46–17.73 Ma) during the Miocene. Within P. lawrencei s.s., sister clades P. overbergiensis and P. capensis diverged 6.02 Ma (95% HPD: 4.34–8.11 Ma), whereas P. lawrencei s.s. originated 5.23 Ma (95% HPD: 3.75–7.02 Ma) during the late Miocene to the early Pliocene. In a similar time period, within the more derived members of Peripatopsis, P. sedgwicki diverged 8.69 Ma (95% HPD: 6.59–11.11 Ma), whereas P. moseleyi diverged 5.17 Ma (95% HPD: 3.79–6.82 Ma). During the Pliocene, we observed the origin of P. janni 5.26 Ma (95% HPD: 3.97–6.74 Ma), P. storchi 3.77 Ma (95% HPD: 2.59–5.18 Ma), P. hamerae 4.60 Ma (95% HPD: 3.35–6.96 Ma) and the split between P. polychroma and P. birgeri 3.41 Ma (95% HPD: 2.32–4.81 Ma).
Morphology of P. lawrencei s.l.
Diagnostic gross morphological differences were observed between clades A and B, including the number of leg pairs and ventral colour (Table 4). In both clades, dorsal colour ranged from navy blue to slate black, to black with tints of orange and various shades of orange. The ventral colour for P. lawrencei s.s. was predominantly cream white and sometimes pale orange or yellow (Fig. 6a, c). For the new lineage (clade B) the ventral colour was a bronze-like golden brown (Fig. 6b, d). The presence of two claws was noted on the back legs of all individuals across both clades. The number of leg pairs differed between the two clades, as P. lawrencei s.s. had 17 leg pairs and clade B had 18, with the last leg pair being reduced in size. The SEM micrographs revealed marked differences in the number of scale rank counts on the primary papillae between the two lineages, with clade B having more than double the number of scale rank counts for both the dorsal and ventral papillae. P. lawrencei s.s. had seven scale rank counts on the dorsal papilla and four on the ventral papilla (Fig. 7a, c), whereas clade B had fifteen scale ranks for the dorsal papilla and nine on the ventral papilla (Fig. 7b, d).
| Locality | Sample size (n) | Clade | Dorsal colour | Ventral colour | Leg pairs | Length (mm) | Width | |
|---|---|---|---|---|---|---|---|---|
| Rondevlei NR | 1 | P. lawrencei s.s. | ? | Cream white | 17 | ? | ? | |
| Jonkershoek NR | 4 | P. lawrencei s.s. | Dark brown–orange | Cream white | 17 | ? | ? | |
| Franschhoek | 4 | P. lawrencei s.s. | Slate black | Cream white | 17 | ? | ? | |
| High Noon | 4 | P. lawrencei s.s. | Dark orange–orange | Cream white | 17 | ? | ? | |
| Dappat se Gat | 5 | P. lawrencei s.s. | Slate black–dark orange | Cream white | 17 | ? | ? | |
| Kogelberg Biosphere NR | 5 | P. lawrencei s.s. | Dark orange–orange | Cream white | 17 | ? | ? | |
| Fernkloof NR | 11 | P. lawrencei s.s. | Navy blue–orange | Cream white | 17 | 18–24.5 | 5.5 | |
| Vogelgat NR | 10 | P. lawrencei s.s. | Dark orange–orange | Cream white | 17 | 13–20.5 | 1.5–4 | |
| Caledon | 12 | P. lawrencei s.s. | Slate black–orange | Cream white–pale orange | 17 | 15–29 | 2–5.5 | |
| Greyton | 3 | P. lawrencei s.s. | Dark orange | Cream white | 17 | ? | ? | |
| Oubos A | 19 | P. lawrencei s.s. | Dark orange–orange | Cream white | 17 | 18–40.5 | 2–4.5 | |
| Oubos B | 2 | P. aereus sp. nov. | Dark brown | Golden brown | 18 | 34–38 | 4–4.5 | |
| Riviersonderend A | 1 | P. lawrencei s.s. | Orange | Cream white | 17 | 13.5–43 | 1.5–6 | |
| Riviersonderend B | 1 | P. aereus sp. nov. | Dark brown | Golden brown | 18 | ? | ? | |
| Grootbos NR | 11 | P. lawrencei s.s. | Navy blue–dark orange | Cream white–pale orange | 17 | 18–40.5 | 2–6.5 | |
| Linden Hill | 5 | P. lawrencei s.s. | Dark orange | Cream white | 17 | ? | ? | |
| Sandies Glen | 1 | P. lawrencei s.s. | Dark orange | Cream white–olive green | 17 | 29.5 | 5 | |
| Napier | 10 | P. lawrencei s.s. | Navy blue–orange | Cream white | 17 | 13–33 | 1.5–3.5 | |
| Villiersdorp | 1 | P. lawrencei s.s. | Dark orange | Orange | 17 | 21 | 4 | |
| Grabouw | 8 | P. lawrencei s.s. | Dark orange–orange | Cream white–pale orange | 17 | 7.5–21 | 1.5–4.5 | |
| Jonaskop | 1 | P. lawrencei s.s. | Dark orange | Cream white | 17 | ? | ? |
Missing data are indicated by a question mark (?). Morphological data generated during our study were combined with data from Daniels et al. (2009) and McDonald and Daniels (2012).
Photographs of recently collected velvet worm specimens suspended in ethanol, taken with a Leica light microscope. (a) Ventral view of P. lawrencei s.s. (b) Ventral view of the novel lineage, P. aereus sp. nov. (clade B). Enhanced ventral views showing the ventral organs in (c) P. lawrencei s.s. and (d) the novel lineage, P. aereus sp. nov. Scale bars: 2 mm.
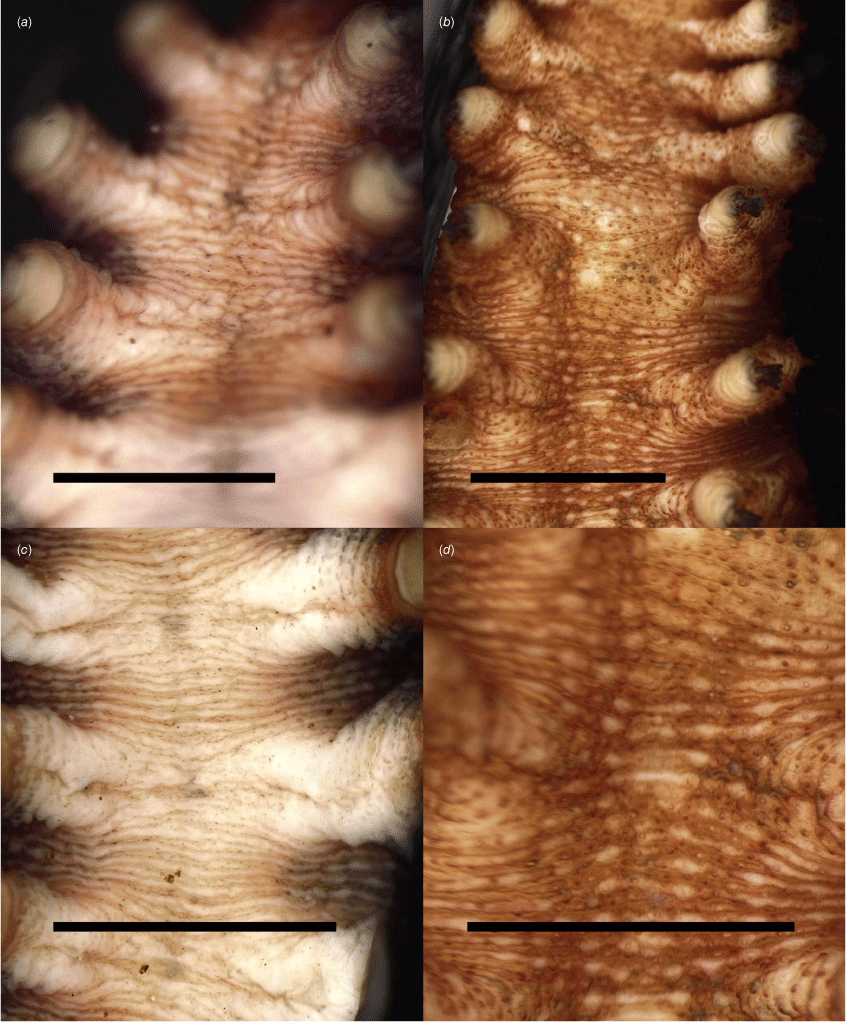
Scanning electron micrographs of dermal papillae on dorsal and ventral surfaces of P. lawrencei s.s. and P. aereus sp. nov. Black dots represent a single scale rank. (a) Dorsal papilla for P. lawrencei s.s. with seven scale ranks. (b) Dorsal papilla for the novel lineage (clade B, P. aereus sp. nov.) with 15 scale ranks. (c) Ventral papilla for P. lawrencei s.s. with four scale ranks. (d) Ventral papilla for the novel lineage (clade B, P. aereus sp. nov.) with nine scale ranks. Scale bars: 100 μm.

Discussion
We observed marked genetic differentiation and detected the presence of two genetically highly divergent clades (clades A and B; Fig. 2 and 5) within Periptatopsis lawrencei s.l. Clade A corresponds to P. lawrencei s.s. whereas clade B represents a novel species that is described in the current manuscript. Marked phylogeographic structure was observed within P. lawrencei s.s. that was characterised by the absence of maternal dispersal evident from the COI haplotype network and FST heatmap (Fig. 3, 4, Table 2). Similar levels of marked genetic differentiation have been observed in several velvet worm species (Myburgh and Daniels 2015; Barnes et al. 2020). Clade B, the narrowly endemic lineage present exclusively in the Riviersonderend Mountains at Oubos, was not monophyletic with P. lawrencei s.s. (clade A) in our phylogeny (Fig. 5). Enforcing the monophyly of clades A and B retrieved a statistically less satisfactory tree topology, corroborating the evolutionary divergence. Furthermore, the two clades were characterised by marked differentiation based on the COI p-distances that fall within the interspecific range and a marked sequence p-distance value for the conserved 18S rRNA locus. The two clades can be differentiated based on leg pair number, ventral colour patterns and fixed scale rank differences. Collectively these results provide supportive evidence for the recognition of a novel species for specimens in clade B. We note that the deeper nodal relationship in our COI + 18S rRNA phylogeny was poorly supported and recommend the use of additional loci that represents a broader suite of evolutionary tempos to potentially resolve this problem.
Novel species delineation
Using the ‘unified species concept’ advocated by de Queiroz (2007), we find P. lawrencei s.s. (clade A) and the sympatric lineage at the Riviersonderend Mountains (clade B) to be separately evolving metapopulations, with multiple lines of evidence substantiating the classification of clade B as a divergent species. Following conventional taxonomic criteria, this can be identified as a unique species based on discernible morphological differences (Fig. 6a–d and 7a–d, Table 4). Using both gross morphology and scanning electron microscopy the two clades within P. lawrencei s.l. could be delineated. In clade A (P. lawrencei s.s.), the ventral colour was highly variable (Table 4) however in clade B, based on two samples, the ventral surface colour was bronze. In velvet worm species, the dorsal colour pattern can notably be highly variable. For example, in Peripatopsis polychroma the dorsal colour surface ranged from olive green, through slate black to rusty brown (Grobler et al. 2023). Similarly, in P. overbergiensis, the dorsal surface colour can range from rusty brown to bright red (Daniels pers. obs). Although leg pair count is a variable indicator of species boundaries (Daniels et al. 2009, 2013, 2016; Barnes et al. 2020), the number of leg pairs differed between the species in our study, as 17 were present in P. lawrencei s.s. and 18 in clade B. McDonald et al. (2012) reported that in P. capensis and P. overbergiensis the number of leg pairs ranged from 17 to 18 respectively, with limited overlap in this character. By contrast, Grobler et al. (2023) reported that between sister species P. birgeri and P. polychroma the number of leg pairs always overlapped and ranged from 20 to 23. Leg pair numbers, together with the colour of the dorsal surface should be interpreted cautiously and preferably within a phylogenetic context to limit artificial splitting or lumping of species. The arrangement of dermal papillae has more often been used as a diagnostic feature in earlier velvet worm studies (Oliveira et al. 2011; McDonald and Daniels 2012; Daniels et al. 2013, 2016; Sato et al. 2018; Grobler et al. 2023). The novel lineage (Oubos B, clade B, P. aereus sp. nov.) was shown to have a reduced number of scale ranks on the papillae for both the dorsal and ventral surfaces.
Incorporating DNA sequence data into our argument for the recognition of a new species, we observed several lines of evidence that aid in the delineation. Marked uncorrected sequence p-distance values, using the COI locus between the two clades falls into a similar range as reported among velvet worm species. For example, the uncorrected p-distances between P. capensis, P. overbergiensis and P. lawrencei s.s. ranged between 5.93 and 7.49%, however, in our study the uncorrected p-distance COI distance value between P. lawrencei s.s. and the novel lineage (clade B) was between 9.13 and 10.62%. Similarly, between clade B and P. overbergiensis, the uncorrected p-distance was between 10.57 and 10.73%. The values found between clade B and P. lawrencrei s.s. are higher than what has been reported for sister species of velvet worms in prior studies (McDonald and Daniels 2012), suggesting a more distant relationship; a result corroborated by our phylogeny for Peripatopsis (Fig. 5). Barnes et al. (2020) used similar p-distance values ranging from 8.46 to 10.64% to delineate velvet worm species. Sequence divergence values as low as 3% have been used to delineate novel velvet worm species (Rockman et al. 2001). In addition, the 5.58% uncorrected p-distance difference between the two sympatric phylogenetically distinct Oubos lineages (A and B) observed for the highly conserved 18S rRNA locus further corroborates the genetic distinction of the two velvet worm species. Barnes et al. (2020) also used uncorrected p-distance differences in the 18S rRNA locus to validate the recognition of species in the P. clavigera species complex. For the 18S locus the latter authors noted p-distance values ranging from 0.68 to 3.40% to aid the recognition of novel species. The 18S rRNA p-distance value we report is higher than those from earlier velvet worm studies, providing corroborative nuclear differences for the recognition of a novel lineage. To further strengthen our argument for distinct taxonomic status, a multi-locus tree topology (incorporating both mt and nuDNA sequence data) of the South African Peripatopsis suggests a Miocene divergence between the two Oubos clades (A and B) (Fig. 5). Given the multifaceted evidence for the distinction of this novel lineage in clade B, we are confident in the delineation and recognition of the new species.
The divergence time estimation indicates an Oligocene origin of Peripatopsis, 28.83 Ma (95% HPD: 21.27–37.49 Ma), followed by rapid diversification in the mid-Miocene to the Pleistocene. The Miocene divergence of the stem lineage, 13.68 Ma (95% HPD: 10.46–17.73 Ma) suggests a more ancient origin of this velvet worm and a wider distribution than previously considered (Daniels et al. 2009). This can be attributed to the different analytical approaches employed by the two studies. The Miocene epoch was characterised by humid subtropical forests in the Cape region of southern Africa (Steinthorsdottir et al. 2021) but was succeeded by major climatic ameliorations such as the development of the proto Benguela current along the west coast. This resulted in increased aridification, possibly contributing to cladogenesis (Siesser 1980). Cladogenesis was also observed at a comparable time period, 13.63 Ma (95% HPD: 9.53–15.73 Ma), between the sister taxa P. bolandi and P. balfouri (Daniels et al. 2013). Notably, these have a similar distribution, encompassing the Western Cape region and would have experienced similar climatic pressures. The dry conditions of the late Miocene persisted throughout the Plio–Pleistocene (Mucina and Rutherford 2006; Sepulchre et al. 2006). Contemporary populations of Peripatopsis generally reside at high altitudes, suggesting biome contraction in lowlands. This could have driven many lineages upward to forest refugia, further separating populations. As velvet worms are extremely mesic-adapted animals, the long-distance gene flow is highly restricted. In such cases, local adaptation or genetic drift is expected to play an important role in species divergence and genetic differentiation.
Phylogeography of P. lawrencei s.s.
The phylogeographic analysis of P. lawrencei s.s. using the COI dataset yielded results congruent with the tree topologies observed thus far (Fig. 2 and 5). Velvet worms are known to be highly fragmented and generally restricted to high elevation forest patches (Daniels et al. 2009, 2016; Barnes and Daniels 2019; Barnes et al. 2020; Grobler et al. 2023). The observed distribution of P. lawrencei s.s. is consistent with such habitat restriction. Haplocluster A3 had a wide distribution, A2 was confined to the Riviersonderend Mountains and A1 showed exclusivity to a narrow coastal region at Hermanus (Fig. 2 and 3). The geographically isolated haploclusters underscore the presence of strong barriers to gene flow and limited maternal dispersal among populations, as observed in prior velvet worm studies (Daniels and Ruhberg 2010; Barnes et al. 2020; Barnes and Daniels 2019, 2022). There is a clear interplay of geographical factors, such as physical barriers or habitat fragmentation and genetic differentiation across these populations. This distribution may have been influenced by localised extinctions in neighbouring or connecting populations, resulting in the observed genetic drift and allelic fixation. The FST values further support this, showing considerable genetic differentiation among the conspecific populations, indicating high genetic structuring and low rates of dispersal (Table 2). The lack of connectedness promotes genetic divergence, as supported by the AMOVA results, highlighting that a substantial portion of genetic variation occurred among populations. The higher diversity values in certain localities such as Greyton and Linden Hill could reflect the presence of refugia, allowing for accumulation of genetic diversity (Table 3). These findings suggest the need for conservation units within Peripatopsis lawrencei s.s., as the Afromontane habitats are highly reduced and highly threatened. The scarcity of samples for the novel lineage further supports the need for habitat maintenance.
Fine-scale sampling and future directions
Fine-scale sampling plays a crucial role in uncovering hidden alpha-taxonomic diversity, especially in poorly studied saproxylic taxa. These organisms are often overlooked or understudied due to habitat complexity or crypticity. Saproxylic zones have been observed to harbour a wealth of taxonomic diversity, as these comprise numerous microhabitats allowing for highly specific niche divergence among taxa that may live in proximity (Daniels et al. 2009; Oliveira et al. 2011; Sato et al. 2018). Furthermore, weak taxonomic classifications can arise through poor sampling, leading to divergent sympatric species being lumped together into a single clade (Barnes and Daniels 2019). Many saproxylic taxa are rare and potentially endangered due to the nature of the habitats that are prone to habitat degradation and fragmentation. Fine-scale sampling allows researchers to detect these rare species and assess the conservation status precisely. A study by Barnes and Daniels (2019) demonstrated the difference between large spatial scale- and fine-scale sampling in detecting novel lineages. Fine-scale sampling, as undertaken here, enables refinement of taxonomic classifications where diversity has previously been underestimated. In our case, fine-scale sampling revealed an ancient novel lineage (clade B) living in sympatry with a well-documented species, P. lawrencei s.s. in the absence of these detailed studies, such species might be unnoticed, impeding conservation efforts. A fine-scale approach is highly recommended when investigating species of limited dispersal capacity and those that inhabit saproxylic zones. Future studies could intensify field surveys and assess the conservation status of this novel lineage. Population size estimates and ongoing monitoring of this lineage and the populations of P. lawrencei s.s. can aid in evaluating the vulnerability and informing targeted conservation strategies, including the identification of potential conservation units. Genome-wide analyses could be conducted to pinpoint specific genes responsible for morphological divergence, while also providing insights into broader evolutionary and developmental processes in velvet worms within these rapidly changing landscapes.
Conclusion
This study offers comprehensive evidence supporting the classification of a novel lineage of velvet worms found in the Riviersonderend Mountains as a distinct species from the sympatric counterpart, P. lawrencei s.s. Multiple lines of independent evidence are congruent and reinforce the credibility of this new species designation. Morphological differences such as ventral coloration, papillae structure and leg pair variations were observed, and ancestral tree construction demonstrated the separation of clade B from the monophyletic clade of P. lawrencei s.s. The genetic distinctiveness of clade B was strengthened by substantial sequence divergence, dating back to the Miocene, an epoch characterised by rapid aridification. Phylogeographic analyses revealed the role of geographical barriers and habitat fragmentation in shaping the genetic structure of P. lawrencei s.s. Furthermore, distinct haploclusters, characterised by limited gene flow, underscore the importance of habitat preservation and the establishment of conservation units to safeguard the unique genetic diversity. Moreover, the study’s findings highlight the necessity of fine-scale sampling to uncover concealed alpha-taxonomic diversity, particularly among saproxylic organisms. The novel lineage identified in this study is described as Peripatopsis aereus sp. nov.
Taxonomy
Genus Peripatopsis Pocock, 1894
(Fig. 2, 5, 6a, b, 7a, b, Table 4.)
Peripatopsis lawrencei s.s. McDonald, Ruhberg & Daniels, 2012.
Male (SAM-ENW-C6468a), Oubos, Riviersonderend, Western Cape Province, South Africa, collected by D. McDonald and A. Abels, 20 October 2010.
Four males (SAM-ENW-C6468b), Oubos, Riviersonderend, Western Cape Province, South Africa, collected by D. McDonald and A. Abels, 20 September 2010.
One female (SAM-ENW-C6457), Fernkloof Nature Reserve, Hermanus, Western Cape province, South Africa, collected 2006 by S. R. Daniels and H. van den Worm. Three males and three females (SAM-ENW-C6458a and b), collected 2010 by D. McDonald and A. Abels from the same locality. Six males and three females (SAM-ENW-C6455), Dappat se Gat, Gordon’s Bay, Western Cape province, South Africa, collected 2011 by F. Van Zyl. One male and three females (SAM-ENW-C6456a and b), Kogelberg Biosphere Nature Reserve, Kleinmond, Western Cape province, South Africa, collected 2011 by D. McDonald and A. Abels. One female and three n/a (SAMENW-C6462), Grootbos Private Reserve, Gansbaai, Western Cape province, South Africa, collected 2006 by S. R. Daniels. Five males and six females (SAM-ENW-C6463a and b), collected 2010 by D. McDonald and A. Abels from the same locality. Five females (SAM-ENW-C6464), Napier, Western Cape province, South Africa, collected 2010 by F. Van Zyl. Two males and ten females (SAM-ENW-C6465), collected 2006 by S. R. Daniels and M. Picker from the same locality. Nine juveniles (SAM-ENW-C6454), High Noon, Villiersdorp, Western Cape province, South Africa, collected 2006 by M. Picker, R. Cowlin, and Merl. Five n/a (SAM-ENW-C6453) collected 2006 by M. Picker and R. Cowlin from the same locality. Three n/a (SAM-ENW-C6466), Greyton, Western Cape province, South Africa, (date and collector not specified). One male and three females (SAM-ENW-C6448), Jonkershoek Nature Reserve, Stellenbosch, Western Cape province, South Africa, collected 2011 by D. McDonald and A. Abels. Two males and two females (SAM-ENW-C6449) collected 2011 by D. McDonald and A. Abels from the same locality. One n/a (SAM-ENWX7290) unspecified locality, Stellenbosch, Western Cape province, South Africa, collected 1905 by Brown. One n/a (SAM-ENW-C6488), Rondevlei, Cape Flats, Western Cape province, South Africa, collected by Picker (date not specified). Five juveniles (SAM-ENW-C6467), Oubos, Riviersonderend, Western Cape province, South Africa, collected 2010 by D. McDonald and A. Abels. One female (SAMENW-C6461) Klein Swartberg, Caledon, Western Cape province, South Africa, collected 2011 by D. McDonald and A. Abels. Three males and four females (SAM-ENW-C6460), collected 2011 by G. Diedericks and C. Broeckhoven from the same locality; 15 n/a (SAM-ENW-X6390) collected by Watermeyer and Purcell (date unknown) from the same locality. Four males and two females (SAM-ENWC6450, C6451, and C6452), Berg River Dam, Franschhoek, Western Cape province, South Africa, collected 2010 by F. Van Zyl. Five n/a (SAM-ENW-X6388), Houw Hoek, Western Cape province, South Africa, collected 1900 by Purcell. One n/a (SAM-ENW-X4024), Sir Lowry’s Pass, Western Cape province, South Africa, collected 1899 by Purcell; four females and two n/a (SAM-ENW-C013476), Fernkloof Nature Reserve, Hermanus, Western Cape province, South Africa, collected by S. R. Daniels and K. Gunkel, 10 June 2023. Four males, three females, and four n/a (SAM-ENW-C013486), Vogelgat Nature Reserve, Hermanus, Western Cape province, South Africa, collected by S. R. Daniels, 25 November 2022. Four males and one female (SAM-ENW-C013475), Caledon, Western Cape province, South Africa, collected by R. Basson and S.R. Daniels, 4 September 2022; three males and three females (SAM-ENW-C013480), collected by H. Barnard and R. Barnard, 24 September 2022, from the same locality. Four males, five females, and one n/a (SAM-ENW-C013487), Oubos, Riviersonderend, Western Cape province, South Africa, collected by R. Basson, S. R. Daniels and F. Gordon, 5 September 2022. Two males, two females, one n/a, and two juveniles (SAM-ENW-C013477), collected by S. R. Daniels and J. A Nieto Lawrence, 22 April 2023, from the same locality. One male, six females, and one n/a (SAM-ENW-C013481), Grootbos, Gansbaai, Western Cape province, South Africa, collected by P. Strauss, January 2022. One n/a (SAM-ENW-C013473), Sandies Glen, Western Cape province, South Africa, collected by R. Basson, 9 September 2022. One male, one female, and two n/a (SAM-ENW-C013478), Napier, Western Cape province, South Africa, collected by F. van Zyl, 8 September 2022. One male (SAM-ENW-C013483), Villiersdorp, Western Cape province, South Africa, collected by J. Durie, 29 April 2023. Four females, four n/a, and two juveniles (SAM-ENW-C013485), Grabouw, Western Cape province, South Africa, collected by J. Durie, 15 May 2023. One n/a (SAM-ENW-C013484), Jonaskop, Western Cape province, South Africa, collected by J. Durie, 2 February 2023.
High Noon, Villiersdorp: EU855288–EU855291, Grootbos Private Nature Reserve: EU855344–EU85548, Greyton: EU855340–EU855342, Fernkloof Nature Reserve: EU855336, JN798123–JN798126, Dappat se Gat: JN798096–JN798100, Caledon: JN798101, Jonkershoek: JN798102–JN79805, Kogelberg: JN798106–JN79807, Oubos: JN798108–JN798111, Rondevlei: JN798112, Napier: JN798113–JN798118. Sequence divergence values between P. lawrencei and (P. capensis and P. overbergiensis) are 8.1 and 7.8% respectively (McDonald et al. 2012). GenBank 18S rRNA data: High Noon, Villiersdorp: EU855531, JN798146, Greyton: EU855528, JN798153, Fernkloof Nature Reserve: EU855526, JN798145, Dappat se Gat: JN798151, Caledon: JN798142, Jonkershoek: JN798161, Kogelberg: JN798162, Oubos: JN798157, Rondevlei: JN798159, Napier: JN798160, Grootbos Private Nature Reserve: EU 855532, JN798154. In the combined DNA sequence topology, P. lawrencei s.l. formed a genetically distinct and statistically well supported monophyletic clade (McDonald and Daniels 2012). GenBank COI Data: OR423143–OR423209. GenBank 18S rRNA Data: OR421492–OR421504 from the present study. Plus, all GenBank numbers for COI and 18S included by McDonald et al. (2012). The uncorrected p-distance between P. capensis and P. overbergiensis was 6.58%; between P. overbergiensis and P. lawrencei s.s., 7.49%; and between P. capensis and P. lawrencei s.s., 8.1% (McDonald et al. 2012). The combined COI + 18S rRNA tree topology (Fig. 5) shows P. lawrencei s.s. to be a statistically well supported monophyletic clade (clade A).
Large variation in dorsal colour surface, ranging from navy blues, to shades of brown and orange. Ventral surfaces are generally lighter, cream or orange, but some specimens have darker shades of red and orange. Dermal papillae are moderately spaced and there are four scale ranks on the papillae structures of the ventral surface, whereas there are seven on the dorsal papillae. There are always 17 leg pairs with two claws on each foot (Table 4).
Length: 20 mm, Paratypes (four male adults): 12–19 mm (McDonald et al. 2012). Specimens from our study were between 7 and 43 mm long and 1.5–6.5 mm wide (Table 4).
Navy blue, slate black, dark brown, rust orange to lighter oranges. A dark dorsal midline is present with light lateral band above legs along the entire body. Similarly, a darker midline can be seen on the ventral surface of some individuals (Fig. 6a, b).
Moderately spaced primary dermal papillae with 2–3 intermittent accessory papillae. SEM: conical or semicircular dermal papillae with seven scale ranks on the dorsal papillae and four scale ranks on the ventral papillae (Fig. 7a, b).
17 leg pairs (Table 4). Dorsal foot surface with ridges. Three complete spinous pads with the fourth being completely to partially fragmented (McDonald et al. 2012). Two claws are present on each posterior leg.
Restricted to Afromontane forest patches but widely distributed in the south-western part of the Western Cape province, from the Cape flats at Rondevlei, to Grabouw, Oubos, Greyton and Napier. This species is regionally widespread, based on genetic data (Fig. 2, 5). This result negates the observation by Oliveira (2023) that the species name be restricted to the type locality.
Peripatopsis aereus Daniels & Nieto Lawrence, sp. nov.
(Fig. 2, 5, 6c, d, 7c, d, Table 4.)
ZooBank: urn:lsid:zoobank.org:act:71276915-79C6-4759-9244-20BEF6303F58
(SAM-ENW-C013474), Oubos B, Riviersonderend, Western Cape Province, South Africa, collected by R. Basson, S. R. Daniels and F. Gordon, 5 September 2022.
GenBank accession numbers: COI Data: OR423210 and OR423211. GenBank 18S rRNA Data: OR421505. The uncorrected p-distance between P. lawrencei s.s. and P. aereus sp. nov. was 10.62%, and between P. overbergiensis and P. aereus sp. nov., 10.73%. In the combined DNA sequence topology (Fig. 5), P. aereus sp. nov. formed a genetically distinct, statistically well-supported monophyletic clade ancestral to P. lawrencei s.s., P. capensis and P. overbergiensis, among other more derived members of Peripatopsis. The p-distance between P. lawrencei s.s. and P. aereus sp. nov. was 5.58% using the 18S rRNA locus.
Dark-brown colouration with a distinct bronze-like colour on the ventral surface. Specimens with 18 leg pairs, with the posterior pair being reduced in size (Table 4). Two claws are present on each foot, including the reduced posterior legs. Deep ridges present between papillae structures and dermal papillae tightly packed. Ventrally with nine scale ranks on the primary papillae structures, whereas there are fifteen on the dorsal primary papillae (Fig. 7c, d).
Holotype (n/a) length: 38 mm, width: 4 mm; paratype (n/a): length 34 mm, width: 4.5 mm. No additional specimens were available for measurement (Table 4).
Both specimens were dark brown, with a bronze-like ventral surface. No discernible lines could be seen on either ventral or dorsal surfaces and no other distinct colour variations were visible (Fig. 6c, d).
Densely spaced primary dermal papillae, with deeper ridges between papillae structures. Dermal papillae conical with nine scale ranks on the ventral papillae and 15 scale ranks on the dorsal papillae (Fig. 7c, d).
18 leg pairs, with the posterior leg pairs being reduced in size. Two claws are present on each leg, including the posterior leg pairs (Table 4).
The species is endemic to the Oubos site B on the Riviersonderend Mountains, Western Cape province, South Africa.
Acknowledgements
The following individuals are thanked for contributing to sampling: Miss F. Gordon, Mr K. Gunkel, Mr F. van Zyl, Mr R. J. Basson, Mr J. Durie, Miss P. Strauss, and brothers Mr R. and Mr H. Barnard. Mr G. Lombardi of Vogelgat Nature Reserve is thanked for allowing us to sample in the reserve and providing accommodation. We also extend thanks to CapeNature for providing a permit (CN44-87-22079) for velvet worm collection. The South African Museum is thanked for allowing us to use the environmental SEM, and Dr T. Samaai and Mr D. Clark are thanked for helping with SEM and light microscope use. Mr A. Barnes is thanked for helping with the analysis of the data. Dr M. Picker is thanked for taking the image of P. lawrencei from Grootbos Nature Reserve. The Department of Botany and Zoology is thanked for providing a bursary to the first author and logistical support. We are grateful for access to the DNA sequence facility of Stellenbosch University. Two anonymous reviewers and the editor, Prof G. Giribet are thanked for constructive comments that improved the quality of the manuscript.
References
Barnes A, Daniels SR (2019) On the importance of fine-scale sampling in detecting alpha taxonomic diversity among saproxylic invertebrates: a velvet worm (Onychophora: Opisthopatus amaxhosa) template. Zoologica Scripta 48, 243-262.
| Crossref | Google Scholar |
Barnes A, Daniels SR (2022) Refining species boundaries among velvet worms (Onychophora, Peripatopsidae), with the description of two new species of Opisthopatus from South Africa. Invertebrate Biology 141, e12368.
| Crossref | Google Scholar |
Barnes A, Reiss T, Daniels SR (2020) Systematics of the Peripatopsis clavigera species complex (Onychophora: Peripatopsidae) reveals cryptic cladogenic patterning, with the description of five new species. Invertebrate Systematics 34, 569-590.
| Crossref | Google Scholar |
Clement M, Posada D, Crandall KA (2000) TCS: A computer program to estimate gene genealogies. Molecular Ecology 9, 1657-1659.
| Crossref | Google Scholar | PubMed |
Daniels SR, Ruhberg H (2010) Molecular and morphological variation in a South African velvet worm Peripatopsis moseleyi (Onychophora, Peripatopsidae): evidence for cryptic speciation. Journal of Zoology 282(3), 171-179.
| Crossref | Google Scholar |
Daniels SR, Picker MD, Cowlin RM, Hamer ML (2009) Unravelling evolutionary lineages among South African velvet worms (Onychophora: Peripatopsis) provides evidence for widespread cryptic speciation. Biological Journal of the Linnean Society 97, 200-216.
| Crossref | Google Scholar |
Daniels SR, McDonald DE, Picker MD (2013) Evolutionary insight into the Peripatopsis balfouri sensu lato species complex (Onychophora: Peripatopsidae) reveals novel lineages and zoogeographic patterning. Zoologica Scripta 42, 656-674.
| Crossref | Google Scholar |
Daniels SR, Dambire C, Klaus S, Sharma PP (2016) Unmasking alpha diversity, cladogenesis and biogeographical patterning in an ancient panarthropod lineage (Onychophora: Peripatopsidae: Opisthopatus cinctipes) with the description of five novel species. Cladistics 32, 506-537.
| Crossref | Google Scholar | PubMed |
Dayrat B (2005) Towards integrative taxonomy. Biological Journal of the Linnean Society 85, 407-415.
| Crossref | Google Scholar |
de Queiroz K (2007) Species concepts and species delimitation. Systematic Biology 56, 879-886.
| Crossref | Google Scholar | PubMed |
Drummond AJ, Rambaut A (2007) BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evolutionary Biology 7, 214.
| Crossref | Google Scholar | PubMed |
Edgecombe GD (2009) Palaeontological and molecular evidence linking Arthropods, Onychophorans, and other Ecdysozoa. Evolution: Education and Outreach 2, 178-190.
| Crossref | Google Scholar |
Excoffier L, Lischer HEL (2010) ARLEQUIN suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Molecular Ecology Resources 10, 564-567.
| Crossref | Google Scholar | PubMed |
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3, 294-299.
| Google Scholar | PubMed |
Giribet G, Carranza S, Baguñà J, Riutort M, Ribera C (1996) First molecular evidence for the existence of a Tardigrada + Arthropoda clade. Molecular Biology and Evolution 13, 76-84.
| Crossref | Google Scholar | PubMed |
Giribet G, Buckman-Young RS, Costa CS, Baker CM, Benavides LR, Branstetter MG, Daniels SR, Pinto-da-Rocha R (2018) The ‘Peripatos’ in Eurogondwana? Lack of evidence that south-east Asian onychophorans walked through Europe. Invertebrate Systematics 32, 842-865.
| Crossref | Google Scholar |
Grobler PCJ, Myburgh AM, Barnes A, Daniels SR (2023) Integrative taxonomy provides evidence for a cryptic lineage in the velvet worm Peripatopsis birgeri species complex (Onychophora: Peripatopsidae) in KwaZulu–Natal, South Africa. Systematics and Biodiversity 21, 2207574.
| Crossref | Google Scholar |
Hamer ML, Samways MJ, Ruhherg H (1997) A review of the Onychophora of South Africa, with discussion of their conservation. Annuals of the Natal Museum 38, 283-312.
| Crossref | Google Scholar |
Heled J, Drummond AJ (2010) Bayesian Inference of species trees from multilocus data. Molecular Biology and Evolution 27, 570-580.
| Crossref | Google Scholar | PubMed |
McDonald DE, Daniels SR (2012) Phylogeography of the Cape velvet worm (Onychophora: Peripatopsis capensis) reveals the impact of Pliocene/Pleistocene climatic oscillations on Afromontane forest in the Western Cape, South Africa. Journal of Evolutionary Biology 25, 824-835.
| Crossref | Google Scholar | PubMed |
McDonald DE, Ruhberg H, Daniels SR (2012) Two new Peripatopsis species (Onychophora: Peripatopsidae) from the Western Cape province, South Africa. Zootaxa 3380, 55-68.
| Crossref | Google Scholar |
Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES science gateway for inference of large phylogenetic trees. In ‘2010 Gateway Computing Environments Workshop (GCE)’, 14 November 2010, New Orleans, LA, USA. Accession Number 11705685. (IEEE) 10.1109/GCE.2010.5676129
Murienne J, Daniels SR, Buckley TR, Mayer G, Giribet G (2014) A living fossil tale of Pangaean biogeography. Proceedings of the Royal Society: Biological Sciences 281, 20132648.
| Crossref | Google Scholar | PubMed |
Myburgh AM, Daniels SR (2015) Exploring the impact of habitat size on phylogeographic patterning in the Overberg velvet worm Peripatopsis overbergiensis (Onychophora: Peripatopsidae). Journal of Heredity 106, 296-305.
| Crossref | Google Scholar | PubMed |
Myburgh AM, Daniels SR (2022) Between the Cape Fold Mountains and the deep blue sea: comparative phylogeography of selected codistributed ectotherms reveals asynchronous cladogenesis. Evolutionary Applications 15, 1967-1987.
| Crossref | Google Scholar | PubMed |
Oliveira IS (2023) An updated world checklist of velvet worms (Onychophora) with notes on nomenclature and status of names. ZooKeys 1184, 133-260.
| Crossref | Google Scholar | PubMed |
Oliveira IS, Lacorte GA, Fonseca CG, Wieloch AH, Mayer G (2011) Cryptic Speciation in Brazilian Epiperipatus (Onychophora: Peripatidae) reveals an underestimated diversity among the peripatid velvet worms. PLoS One 6, e19973.
| Crossref | Google Scholar | PubMed |
Posada D (2008) jModelTest: phylogenetic averaging. Molecular Biology and Evolution 25, 1253-1256.
| Crossref | Google Scholar | PubMed |
Rockman MV, Rowell DM, Tait NN (2001) Phylogenetics of Planipapillus, lawn headed onychophorans of the Australian Alps, based on nuclear and mitochondrial gene sequences. Molecular Phylogenetics and Evolution 21, 103-116.
| Crossref | Google Scholar | PubMed |
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61, 539-542.
| Crossref | Google Scholar | PubMed |
Ruhberg H (1985) Die Peripatopsidae (Onychophora). Systematik, Ökologie, Chorologie und phylogenetische Aspekte. Zoologica 137, 1-183.
| Google Scholar |
Ruhberg H, Hamer ML (2005) A new species of Opisthopatus Purcell, 1899 (Onychophora: Peripatopsidae) from KwaZulu–Natal, South Africa. Zootaxa 1039, 27-38.
| Crossref | Google Scholar |
Ruhberg H, Daniels SR (2013) Morphological assessment supports the recognition of four novel species in the widely distributed velvet worm Peripatopsis moseleyi sensu lato (Onychophora: Peripatopsidae). Invertebrate Systematics 27, 131-145.
| Crossref | Google Scholar |
Sato S, Buckman-Young RS, Harvey MS, Giribet G (2018) Cryptic speciation in a biodiversity hotspot: multilocus molecular data reveal new velvet worm species from Western Australia (Onychophora: Peripatopsidae: Kumbadjena). Invertebrate Systematics 32, 1249-1264.
| Crossref | Google Scholar |
Sepulchre P, Ramstein G, Fluteau F, Schuster M, Tiercelin JJ, Brunet M (2006) Tectonic uplift and Eastern Africa aridification. Science 313, 1419-1423.
| Crossref | Google Scholar | PubMed |
Sherbon BJ, Walker MH (2004) A new species of Peripatopsis from South Africa, P. stelliporata, with observations on embryonic development and sperm degradation (Onychophora, Peripatopsidae). Journal of Zoology London 264, 295-305.
| Crossref | Google Scholar |
Shimodaira H, Hasegawa M (1999) Multiple comparisons of log-likelihoods with applications to phylogenetic inference. Molecular Biology and Evolution 16, 1114-1116.
| Crossref | Google Scholar |
Siesser WG (1980) Late Miocene origin of the Benguela upswelling system off northern Namibia. Science 208, 283-285.
| Crossref | Google Scholar | PubMed |
Stamatakis A, Hoover P, Rougemont J (2008) A rapid bootstrap algorithm for the RAxML web servers. Systematic Biology 57, 758-771.
| Crossref | Google Scholar | PubMed |
Steinthorsdottir M, Coxall HK, de Boer AM, Huber M, Barbolini N, Bradshaw CD, Burls NJ, Feakins SJ, Gasson E, Henderiks J, Holbourn AE, Kiel S, Kohn MJ, Knorr G, Kürschner WM, Lear CH, Liebrand D, Lunt DJ, Mörs T, Pearson PN, Pound MJ, Stoll H, Strömberg CAE (2021) The Miocene: The future of the past. Paleoceanography and Paleoclimatology 36, e2020PA004037.
| Crossref | Google Scholar |
Talavera G, Castresana J (2007) Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Systematic Biology 56, 564-577.
| Crossref | Google Scholar | PubMed |
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research 25, 4876-4882.
| Crossref | Google Scholar | PubMed |
Trifinopoulos J, Nguyen L-T, von Haeseler A, Minh BQ (2016) W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Research 44, 232-235.
| Crossref | Google Scholar | PubMed |
Zhang J, Kapli P, Pavlidis P, Stamatakis A (2013) A general species delimitation method with applications to phylogenetic placements. Bioinformatics 29, 2869-2876.
| Crossref | Google Scholar | PubMed |
Appendix A1.COI haplotype numbers and the number of sequences within each sample locality, corresponding to Fig. 2.
| Locality | Haplotype number | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 | 42 | 43 | 44 | 45 | 46 | 47 | 48 | 49 | 50 | 51 | 52 | 53 | 54 | 55 | 56 | 57 | ||
| Vogelgat NR | 3 | 1 | 3 | 1 | 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fernkloof NR | 3 | 1 | 1 | 1 | 1 | 3 | 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Oubos A and B | 13 | 3 | 1 | 1 | 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Greyton | 1 | 1 | 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Riviersonderend A and B | 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Jonaskop | 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Villiersdorp | 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Grabouw | 1 | 6 | 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kogelberg Biosphere NR | 1 | 2 | 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Franschhoek | 3 | 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| High Noon | 3 | 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dappat se Gat | 2 | 3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Jonkershoek NR | 1 | 2 | 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rondevlei NR | 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Caledon | 3 | 1 | 4 | 2 | 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Napier | 1 | 4 | 1 | 1 | 2 | 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sandies Glen | 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Grootbos NR | 1 | 2 | 1 | 1 | 4 | 1 | 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Linden Hill | 1 | 1 | 2 | 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||


