Bovine spongiform encephalopathy and food safety
Rosalind Dalefield and Scott CrerarFood Standards Australia New Zealand
PO Box 7186, Canberra BC
ACT 2610, Australia
Tel: +61 2 6271 2222
Fax: +61 2 6271 2278
Email: scott.crerar@foodstandards.gov.au
Microbiology Australia 34(2) 86-89 https://doi.org/10.1071/MA13030
Published: 13 May 2013
Bovine spongiform encephalopathy (BSE) is a fatal disease of cattle, caused by infective proteins known as prions. A prion (PrPSc) is a mis-folded isoform of the glycoprotein PrPC, which is highly expressed in the nervous system. Prions replicate by coercing PrPC to refold into PrPSc. The BSE epidemic was propagated by rendering dead cattle to produce meal which was then included in cattle feed. Consumption of BSE PrPSc from contaminated beef resulted in over 200 human cases of variant Creutzfeldt-Jakob (vCJD) disease, which is invariably fatal. There were rare cases of person-to-person vCJD transmission by blood transfusion. Variant CJD is now very rare, due to adoption of measures that prevent the feeding of ruminant protein to ruminants and the contamination of beef with the tissues that harbour PrPSc. Beef from countries with these control systems are safe for human consumption.
The infectious agent
Bovine Spongiform Encephalopathy (BSE) is one of a number of diseases known collectively as Transmissible Spongiform Encephalopathies (TSEs) of which scrapie of sheep, chronic wasting disease (CWD) of deer and elk and Creutzfeldt-Jakob disease (CJD) in humans are also members. TSEs are caused by a mis-folded isoform of the prion glycoprotein (PrP). The mis-folded pathogenic isoform is known as a ‘prion’, a contraction of the words ‘proteinaceous’ and ‘infectious’1. By convention, normal PrP is represented as PrPC, while the prion is represented as PrPSc. Prions replicate themselves by binding to PrPC and acting as a template that coerces PrPC to refold into PrPSc2,3.
In mammals, PrPC is present in a wide variety of tissues but is highly expressed in the nervous system2,4,5. The physiological function of PrPC remains obscure and mice modified to express no PrPC show only subtle, non-lethal differences to wild-type mice3,6.
Three strains of BSE exist, which exhibit differences in prion distribution, histopathology, incubation time and clinical signs7 as well as the appearance of the prions on western blots. Only one strain, classical BSE, was responsible for the BSE epidemic and the associated epidemic of vCJD 8. The atypical H-type and L-type strains typically occur in cattle over eight years of age, and appear to arise spontaneously9,10.
Diseases
Cattle
The incubation period of BSE is estimated to be from 30 months to 8 years and clinical disease usually occurs in cattle of four to five years of age. The course of clinical disease is generally less than 6 months11. Clinical signs in cattle include abnormal posture, incoordination, and changes in temperament9,11.
Humans
Consumption of BSE prions in contaminated beef resulted in over 200 human cases of variant CJD (vCJD)7. The great majority of patients were residents in the United Kingdom (UK) during the period 1985-199612. Patients ranged in age from 17–42 years5. Variant CJD is distinct from the most common human prion disease, sporadic CJD (sCJD), which occurs spontaneously in people, including lifelong vegetarians8, between 55 and 70 years old12. Both forms of CJD are fatal.
Pathogenesis
The first tissues in which PrPSc can be detected in BSE are those of the nervous system supplying the intestine13 although it is not clear how infection reaches the nerves from the intestinal lumen. Infection ascends to the brain via the autonomic nerves2. The routes of spread of prions from cell to cell within the nervous system are not fully understood14,15, and the mechanisms of cerebral damage are unknown. Depletion of PrPC does not appear to be a factor. On the contrary, depletion of PrPC in mice has been shown to reverse early degeneration and prevent progression to clinical disease7.
Transmission and incidence of disease
Animals
The epidemic of BSE was first recognized in 1986 in the UK and was propagated by the rendering of dead cattle to produce meat-and-bone meal (MBM) which was then included in feed for cattle8. The infection was spread elsewhere in the world by exports of cattle and contaminated MBM9. There is no evidence that BSE can be transmitted between living cattle. This is in marked contrast to the horizontal infectivity of scrapie in sheep and CWD in deer16–18.
More than 184,000 cases of BSE have been diagnosed in cattle. At the peak of the epidemic 1,000 cases were being diagnosed each week in the UK18. The feeding of MBM to cattle was banned in the UK in 1988, but because of the long incubation period and initially ineffective implementation of the feed ban, clinical incidence continued to rise, peaking in 1992. The incidence has steadily declined since, and the disease is now very rare19.
A number of zoo animals, including Bovidae, Felidae and non-human primates, developed TSEs at the same time as the BSE epidemic20. Cases of TSE were also diagnosed in two domestic goats20 and a number of domestic cats8,18. All these cases were attributed to ingestion of BSE prions in beef or processed feed.
The epidemic is believed to have been amplified from a single common source7, which remains unknown. Sporadic cases of BSE occur in cattle, although to date only the atypical L- and H-type strains have been found. It is possible that classical BSE may also occur spontaneously. Although it has sometimes been suggested that BSE arose from rendering of scrapie-infected sheep, encephalopathy induced in cattle by intracerebral inoculation with scrapie prions does not resemble BSE, and experimental BSE in sheep does not resemble scrapie. Furthermore, cattle are resistant to oral infection with scrapie or CWD8.
Humans
Since the first ten cases were reported in April 1996, over 200 vCJD cases have been identified7,21. The epidemic of vCJD is attributed to consumption of beef contaminated with central nervous system tissue containing BSE PrPSc. BSE PrPSc and vCJD PrPSc have identical biochemical properties and cause identical lesions in mice, and on a country-by-country basis the incidence of vCJD in humans generally correlates with the prevalence of BSE in cattle7. The infective dose of bovine PrPSc to human beings is unknown12.
Four cases of person-to-person vCJD transmission by blood transfusion have been reported in the UK13. Iatrogenic transmission of vCJD remains a concern because retrospective analysis of tonsil and appendix specimens suggests that up to 1 in 4000 persons exposed during the UK epidemic may be a sub-clinical carrier8,22. Internationally, blood donations are generally not accepted from people who lived in the UK between 1980 and 1996, or who received a blood transfusion in the UK since 1980. These precautions are in place in Australia23 and in New Zealand24.
A polymorphism at position 129 of the PrPC amino acid sequence has been identified in humans, which appears to affect susceptibility to TSEs. Approximately 40% of Caucasians are homozygous for methionine (Met) at position 129, 10% are homozygous for valine (Val) and 50% are Met/Val heterozygotes. To date, all confirmed clinical cases of vCJD have been Met/Met homozygotes12. However, PrPSc was found in the spleen of a Met/Val heterozygote who died of unrelated causes five years after receiving a blood transfusion from a person incubating vCJD2,12, and PrPSc was also found in anonymous postsurgical samples of appendices from two Val/Val homozygotes. Thus, lymphoid tissue of all three genotypes may become infected8,12,25. It is not yet clear whether the Met/Val and Val/Val genotypes prevent or only delay neurological infection with vCJD12. Besides vCJD, the only other orally acquired TSE known in humans is kuru, a historical disease of some communities in Papua New Guinea who practiced funerary cannibalism. The mean incubation period of kuru is 12 years, but the incubation period has exceeded 50 years in some individuals13. Retrospective analysis of samples has shown that the majority of those with unusually long incubation periods were Met/Val heterozygotes12. Some authors have predicted a late peak of vCJD cases affecting Met/Val heterozygotes7,25.
Food safety and controls
A key component of prevention of both BSE in cattle and vCJD in humans is the prohibition on feeding mammalian-derived protein to food animals. Feeding of mammalian-derived proteins, other than dairy proteins, to livestock has been prohibited in the UK since 1996 and throughout the European Union since 200126,27. Numerous other countries worldwide have enacted similar legislation. Enforcement of this ban includes27:
-
Registration and regular auditing of feed producers
-
Mandatory physical separation of ruminant feed production, storage and transport from production, storage and transport of non-ruminant feed that may contain animal proteins
-
Testing of raw materials and finished feeds for presence of mammalian proteins
-
Warning labels on feed bags
-
Education of livestock keepers.
Throughout the EU, cattle are individually and permanently identified, and each country has an electronic database recording the details, locations, movements and fate of all cattle. Cattle that die or require emergency slaughter on farms are rendered and then incinerated to ensure that infective material is destroyed27 and cannot be used in animal feed.
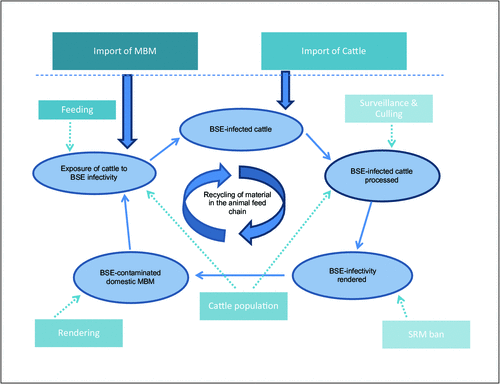
As a result of these measures (also see Figure 1 for a summary of the critical BSE control points) the BSE epidemic was controlled, and classical BSE is now very rare 19. Surveillance programs for BSE are in place in many countries throughout the world, and occasionally detect isolated cases of atypical BSE28.
|
The risk of oral infection of humans with vCJD can be eliminated by preventing contamination of the food supply with the animal tissues know to harbour infectivity, which are known as specific risk materials (SRM). SRM include the brain, spinal cord, eyes, palatine tonsils and gastrointestinal tract. Slaughter and processing procedures have been implemented throughout Europe and other countries to prevent contamination of beef with SRM. Following removal, SRM are transported to rendering plants under strict controls, and are rendered and incinerated under conditions known to destroy infectivity27. Beef and beef products from countries with these control systems are safe for human consumption.
Australia and New Zealand are among eleven countries internationally recognized as being at ‘negligible risk’ for BSE. Live cattle may not be imported into Australia from BSE-affected countries. A ban on the importation of MBM from countries other than New Zealand was in place almost two decades before the UK BSE outbreak, as a measure to prevent importation of anthrax spores. Should infected material enter Australia, propagation to the national cattle herd would not occur, because the feeding of ruminants with MBM is prohibited. Information on Australia’s approach to BSE may be found on the website of the Department of Agriculture, Fisheries and Forestry29.
With respect to the importation of beef products, Australia implemented a revised policy in 2010 whereby any country wishing to export beef to Australia must undergo a rigorous risk assessment of their BSE-related control measures30. The risk assessment, consistent with the principles outlined by the World Organisation for Animal Health, is undertaken by Food Standards Australia New Zealand who also visits each country to verify the effectiveness of the control measures. Countries are given a BSE category that establishes the conditions under which they may import beef products into Australia31.
References
[1] Prusiner, S.B. (1982) Novel proteinaceous infectious particles cause scrapie. Science 216, 136–144.| Novel proteinaceous infectious particles cause scrapie.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL38XhsFyqtro%3D&md5=c70f12036beba07d509d7833f031fe3dCAS | 6801762PubMed |
[2] Gains, M.J. and LeBlanc, A.C. (2007) Prion protein and prion disease: the good and the bad. Can. J. Neurol. Sci. 34, 126–145.
| 17598589PubMed |
[3] Cobb, N.J. and Surewicz, W.K. (2009) Prion diseases and their biochemical mechanisms. Biochemistry 48, 2574–2585.
| Prion diseases and their biochemical mechanisms.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXivFSnsrk%3D&md5=49d5d5c5f8e968f616438b82284b4a48CAS | 19239250PubMed |
[4] Linden, R. et al. (2008) Physiology of the prion protein. Physiol. Rev. 88, 673–728.
| Physiology of the prion protein.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXls1KhsLo%3D&md5=488932bb7bb94acb2d63f6c177394a8dCAS | 18391177PubMed |
[5] Brown, K. and Mastrianni, J.A. (2010) The prion diseases. J. Geriatr. Psychiatry 23, 277–298.
| The prion diseases.Crossref | GoogleScholarGoogle Scholar |
[6] Chakrabarti, O. et al. (2009) Prion protein biosynthesis and its emerging role in neurodegeneration. Trends Biochem. Sci. 34, 287–295.
| Prion protein biosynthesis and its emerging role in neurodegeneration.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXnt1Kisrs%3D&md5=b9b7139baae1456c45c66542d751c64cCAS | 19447626PubMed |
[7] Aguzzi, A. and Calella, A.M. (2009) Prions: protein aggregation and infectious diseases. Physiol. Rev. 89, 1105–1152.
| Prions: protein aggregation and infectious diseases.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtlyqs73L&md5=d1e5e4b274a01bbee6d99c463f6deebdCAS | 19789378PubMed |
[8] Harman, J.L. and Silva, C.J. (2009) Bovine spongiform encephalopathy. J. Am. Vet. Med. Assoc. 234, 59–72.
| Bovine spongiform encephalopathy.Crossref | GoogleScholarGoogle Scholar | 19119967PubMed |
[9] Seuberlich, T. et al. (2010) Atypical transmissible spongiform encephalopathies in ruminants: a challenge for disease surveillance and control. J. Vet. Diagn. Invest. 22, 823–842.
| Atypical transmissible spongiform encephalopathies in ruminants: a challenge for disease surveillance and control.Crossref | GoogleScholarGoogle Scholar | 21088166PubMed |
[10] Konold, T. et al. (2012) Experimental H-type and L-type bovine spongiform encephalopathy in cattle: observation of two clinical syndromes and diagnostic challenges. BMC Vet. Res. 8, 22.
| Experimental H-type and L-type bovine spongiform encephalopathy in cattle: observation of two clinical syndromes and diagnostic challenges.Crossref | GoogleScholarGoogle Scholar | 22401036PubMed |
[11] USDA FSIS (2005) Bovine spongiform encephalopathy – "Mad cow disease". http://www.fsis.usda.gov/Factsheets/Bovine_Spongiform_Encephalopathy_Mad_Cow_Disease/index.asp#10 (accessed 9 August 2012).
[12] Mackay, G.A. et al. (2011) The molecular epidemiology of variant CJD. International Journal of Molecular Epidemiology and Genetics 2, 217–227.
| 21915360PubMed |
[13] van Keulen, L.J.M. et al. (2008) TSE pathogenesis in cattle and sheep. Vet. Res. 39, 24.
| TSE pathogenesis in cattle and sheep.Crossref | GoogleScholarGoogle Scholar |
[14] Caughey, B. et al. (2009) Getting a grip on prions: oligomers, amyloids and pathological membrane interactions. Annu. Rev. Biochem. 78, 177–204.
| Getting a grip on prions: oligomers, amyloids and pathological membrane interactions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXos1Ghtb0%3D&md5=1ab7041af8b606e2e6a697fe0bb78f8cCAS | 19231987PubMed |
[15] Kovacs, G.G. and Budka, H. (2008) Prion diseases: from protein to cell pathology. Am. J. Pathol. 172, 555–565.
| Prion diseases: from protein to cell pathology.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXktVKhtLc%3D&md5=6bd30b61ab370d79680f79871c99814aCAS | 18245809PubMed |
[16] Solomon, I.H. et al. (2009) Prion neurotoxicity: insights from prion protein mutants. Curr. Issues Mol. Biol. 12, 51–62.
| 19767650PubMed |
[17] Haley, N.J. et al. (2011) Detection of chronic wasting disease prions in salivary, urinary, and intestinal tissues of deer: potential mechanisms of prion shedding and transmission. J. Virol. 85, 6309–6318.
| Detection of chronic wasting disease prions in salivary, urinary, and intestinal tissues of deer: potential mechanisms of prion shedding and transmission.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXotFOntbw%3D&md5=e1da58fd21e5dae6ffd0eb63948fcaaaCAS | 21525361PubMed |
[18] Imran, M. and Mahmood, S. (2011)b An overview of animal prion diseases. Virol. J. 8, 493.
| An overview of animal prion diseases.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsFSrur3F&md5=1305c609c4fa528f1e5521453972cbfeCAS | 22044871PubMed |
[19] OIE (2012) Bovine spongiform encephalopathy (BSE) – Geographical distribution of countries that reported BSE confirmed cases since 1989. http://www.oie.int/en/animal-health-in-the-world/bse-specific-data/ accessed (12 February 2013).
[20] Spiropoulos, J. et al. (2011) Isolation of prion with BSE properties from farmed goat. Emerg. Infect. Dis. 17, 2253–2261.
| Isolation of prion with BSE properties from farmed goat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xht1Olsr8%3D&md5=39078d379862a70fa72de7aff77feaa6CAS | 22172149PubMed |
[21] Imran, M. and Mahmood, S. (2011)b An overview of human prion diseases. Virol. J. 8, 559.
| An overview of human prion diseases.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xlsl2ltLs%3D&md5=78db2ada5313744535dacb75a60dc8d5CAS | 22196171PubMed |
[22] Collinge, J. (2012) The risk of prion zoonoses. Science 335, 411–413.
| The risk of prion zoonoses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XjtVyqsbs%3D&md5=60b3214a5c569d8fa4defaaec679da24CAS | 22282797PubMed |
[23] Australian Red Cross Blood Service http://www.donateblood.com.au/faq/eligibility/travel-i-have-travelled-outside-australia-can-i-still-donate-blood#mad-cow (accessed 7 February 2013).
[24] New Zealand Blood Service http://www.nzblood.co.nz/content/download/620/3988/file/111I077.pdf (accessed 7 February 2013).
[25] Will, B. (2010) Variant CJD: where has it gone, or has it? Pract. Neurol. 10, 250–251.
| Variant CJD: where has it gone, or has it?Crossref | GoogleScholarGoogle Scholar | 20858625PubMed |
[26] Bradley, R. et al. (2006) Variant CJD (vCJD) and bovine spongiform encephalopathy (BSE): 10 and 20 years on: part 1. Folia Neuropathol. 44, 93–101.
| 16823691PubMed |
[27] Regulation (EC) No 999/2001 of the European Parliament and of the Council of 22 May 2001 laying down rules for the precention, control and eradication of certain transmissible spongiform encephalopathies http://ec.europa.eu/food/fs/afs/marktlab/marktlab14_en.pdf (accessed February 2013).
[28] OIE (2013) Number of reported cases of bovine spongiform encephalopathy (BSE) in farmed cattle worldwide* (excluding the United Kingdom) http://www.oie.int/en/animal-health-in-the-world/bse-specific-data/number-of-reported-cases-worldwide-excluding-the-united-kingdom/ (accessed February 2013).
[29] Department of Agriculture Fisheries and Forestry (2013). Bovine spongiform encephalopathy http://www.daff.gov.au/animal-plant-health/pests-diseases-weeds/animal/bse (accessed 11 February 2013).
[30] Food Standards Australia New Zealand (2013). Bovine spongiform encephalopathy (BSE): requirements for the importation of beef and beef products for human consumption. http://www.foodstandards.gov.au/consumerinformation/bovinespongiformencephalopathybse/requirementsfortheim4751.cfm (accessed February 2013).
[31] Food Standards Australia New Zealand (2013). Status of country BSE food safety risk assessements. http://www.foodstandards.gov.au/consumerinformation/bovinespongiformencephalopathybse/statusofcountrybsefo5388.cfm (accessed February 2013).
Biographies
Dr Scott Crerar manages the implementation of Australia’s BSE risk assessment policy with respect to the safety of imported beef and beef products at Food Standards Australia New Zealand (FSANZ). Dr Crerar has worked in food regulation and safety for 15 years and previously coordinated imported foods and food recall operations at FSANZ. He has also worked in Hong Kong and New Zealand in food regulation.
Dr Rosalind Dalefield participates in the implementation of Australia’s BSE risk assessment policy with respect to the safety of imported beef and beef products at Food Standards Australia New Zealand (FSANZ).


