The microbiology of microbial electrolysis cells
Lucie Semenec and Ashley E FranksDepartment of Microbiology – Environmental Microbiology
Faculty of Science, Technology and Engineering
La Trobe University
Melbourne, Vic. 3086, Australia
Tel: +61 3 9479 2206
Email: a.franks@latrobe.edu.au
Microbiology Australia 35(4) 201-206 https://doi.org/10.1071/MA14065
Published: 31 October 2014
Electromicrobiology is a new discipline that investigates the ability of microbial species to interact with insoluble external electron acceptors and donors. This ability has most commonly been studied through microbial communities found in association with electrodes as part of a microbial electrolysis cell (MEC). MECs are devices that employ bacteria capable of utilising either an anode as an electron acceptor or a cathode as an electron donor to carry out biologically driven processes. In effect, these devices make use of microbes that are eating and breathing electricity. Potential applications for MECs are ever expanding and currently include bioremediation, biosensing, biofuel production and power generation. MECs that produce overall net power are referred to as microbial fuel cells (MFCs) and have helped to generate much of our initial knowledge regarding electroactive bacteria. Energy consuming MECs have more recently expanded our knowledge on microbial electrosynthesis pathways, whereby microbes reduce CO2 using electrons provided by an electrode. Furthering of our knowledge on electrode-associated microbes has in turn led us to an increased understanding of how microbes in the environment have been developing, powering and utilising their own electricity grids all along. These electrical interactions, between microbes and components of their living and non-living environment, are potentially very important but have been overlooked until very recently.
nota notae est nota rei ipsius – in as much as chemical change being a sign of life, and electrical change a sign of chemical change, it follows that electrical change is a sign of life. Waller1
Electron transfer from microbes to electrodes
The electrical nature of living organisms was eloquently summarised in lectures by Augustus Waller in 19031 and the ability to use an anode to detect an electrical current in a microbial culture during the decomposition of organic compounds was demonstrated by Potter in 19112. However, it was not until half a century later that this knowledge was implemented into the first reported studies using a MFC. In the past 10 years research into electric bacteria has exponentially expanded.
Defining microbial fuel cells
A MFC is typically a two-chambered system containing an anaerobic anode chamber and an oxic cathode chamber, separated by an ion permeable membrane, and is capable of utilising electrons from microbial central metabolism for a net energy gain. Electric microbes in the anode chamber utilise the anode as a final electron acceptor for the anaerobic respiration of organic electron donors such as acetate. Electrons donated to the anode flow to the cathode through electrical wires, where they are reunited with the protons generated in the anode chamber and combine with oxygen or other electron acceptors to form reduced products. The reported anodic power density has increased from initial power outputs of 0.1 W/m2 to more recent reports of 6.9 W/m2 of anode surface area. Despite the many improvements made to MFC electrical current production, these systems do not yet produce enough power for commercially viable large-scale power production applications, but are able to reduce the energy demands of wastewater treatment as well as provide small scale power outputs to power remote sensing devices. Many physical, chemical and biological discoveries remain to be unveiled in order to make the power generation application of MFCs more feasible.
Electricigens: anode respiring bacteria
The anode-associated microbial communities are dominated by electricigens, microbes capable of completely oxidising organic carbon while utilising the anode as the final electron acceptor. While research had been initially built on findings from studies based on dissimilatory metal reduction pathways of metals like Fe(III) and Mn(IV), it was found that dissimilatory metal reduction was not always indicative of anode reduction and vice versa. Mixed communities often form in association with an anode when complex organic matter is used as an energy source, microbial fermentation first reduces the compounds to simple carbohydrates such as acetate and then the fermentation end products serve as electron donors for the anode respiring microbial community members, typically found in an anode-associated biofilm.
The dominance of the Geobacteraceae in anode-associated communities was first reported in studies of sediment MFCs. Sediment MFCs utilise aquatic sediments for inocula, carbon sources and as a proton exchange medium. Through 16S ribosomal DNA (rDNA) analysis, it was found that bacteria from the family Geobacteraceae were enriched at the anode, as compared to a control, along with several other Delta-proteobacteria. It has been shown that Geobacteraceae dominate anode-associated biofilms from a wide range of environmental inocula such as sewage waste and rice paddy soil3.
The number of known electricigens has been increasing and includes species of Geobacter, Shewanella, Rhodoferax, Pseudomonas, Geothrix, Ochrobactrum, Clostridium, Desulfuromonas, Aeromonas, Desulfobulbus, Geopsychrobacter, Escherichia, Rhodopseudomonas, Desulfovibrio, Acidiphilium, Klebsiella, Thermincola, and Pichia. Of these microbes, Geobacter sulfurreducens and Shewanella oneidensis are the most extensively studied in terms of their mechanisms of extracellular electron transfer (EET) to insoluble electron acceptors.
Electrotrophs: cathode-associated microorganisms
Microorganisms can also utilise the cathode as an electron donor in a MEC, in effect consuming electrical current as an energy source. This process requires an input of current, as the electrode often needs to be held electronically at a specific potential to make the redox reactions favourable4. Microorganisms that receive electrons directly from electrodes are referred to as electrotrophs and, if carbon dioxide is fixed for organic synthesis, the process is known as electrosynthesis; named due to similarities to photosynthesis5. Cathodes were initially demonstrated to act as electron donors for microbial metabolism through pure culture studies with Geobacter spp. Only a limited number of known electrotrophs that are capable of utilising a cathode in pure culture have been described thus far, including Sporomusa ovata, S. sphaeroides, Morella thermoacetica, Clostridium ljungdahlii, C. aceticum, G. metallireducens and G. sulfurreducens5. In pure cultures, the cathode-associated biofilms have been found to be only sparse or single layer biofilms. However, cathodes in the environment will often attract a mixed community of electrotrophic bacteria with the capability of improving bioremediation, biosensing and biosynthesis. It has also recently been demonstrated that community population dynamics, and hence electrosynthetic outcomes, can change in response to current supply fluctuations to the cathode. These results highlight the importance of understanding interspecies microbial interactions within MECs to better predict and control products of electrosynthesis under varying environmental conditions especially when the production of specific organic compounds is desired.
Electrotrophic bacteria such as Geobacter play an important role in cathode-assisted bioremediation. Energy from the cathode enables the reduction of nitrates, chlorinated solvents and soluble U(VI) to insoluble U(IV) in the subsurface. When compared with common biomass strategies, the use of renewable energy sources like sunlight for the production of valuable commercial synthetic compounds and transportation fuels such as acetone and butanol has large potential efficient gains without consuming land available for food production.
Unlike the thick biofilms that form on anode surfaces, only sparse or single layer biofilms are usually observed on cathode surfaces in pure cultures. Investigations into relatively thick naturally occurring cathode-associated mixed species biofilms may lead to new insights and improvements. Several avenues of research are now investigating mechanisms by which the cathode-associated biomass and production rates can be increased.
Extracellular electron transfer mechanisms
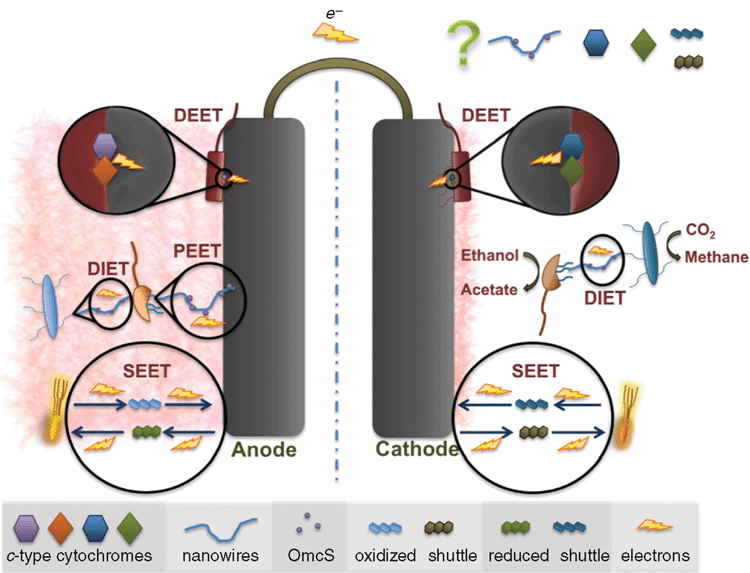
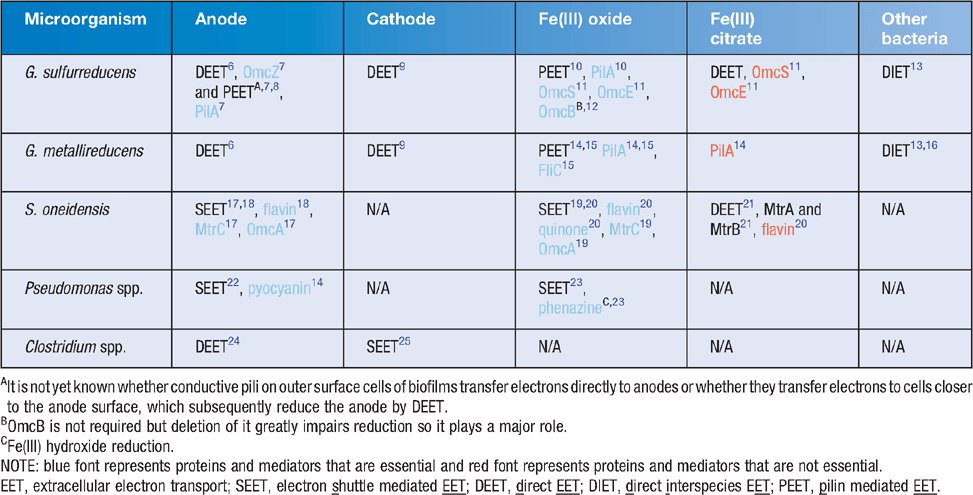
Electrogens are a more general term for electricigens and encompass all microbes that can interact in a community via extracellular electron transport using any suitable extracellular electron acceptor; whereas electricigens are microbes that specifically use an electrode as the final electron acceptor for extracellular electron transport. Initial studies of electrogenic bacteria focused mainly on Geobacter and Shewanella species. These species had been extensively studied in relation to iron reduction and were known to have different EET mechanisms (Table 1). Currently there are three known mechanisms of EET that were recently reviewed in great detail26: (1) electron shuttle mediated EET (SEET); (2) direct EET (DEET); and (3) pilin mediated EET (PEET). These mechanisms are discussed below in relation to anode reduction (Figure 1).
|
(1) Electron shuttle mediated EET
SEET utilises extracellular electron shuttles to act as a carrier between the cell and the electrode surface. Shuttles may be biotic or abiotic compounds. If available in the environment, many microbial species are capable of utilising exogenous electron shuttles but not all microbes produce them. S. oneidensis has been studied extensively due to flavin production that cycles electrons from the outer membrane cytochrome MtrC19. Likewise, pyocyanin production by Pseudomonas aeruginosa enables electrode interaction22. Abiotic shuttles include humic substances and sulfur compounds amongst others. The use of solid-phase humic substances as electron shuttles to reduce Fe(III) oxide has been demonstrated to occur in G. sulfurreducens and Shewanella putrefaciens despite the ability of these organisms to reduce Fe(III) oxide via other EET mechanisms (Table 1).
(2) Direct EET
EET is the direct microbial transfer of electrons to an electrode without the use of an electron shuttle and commonly utilises outer membrane cytochromes. This typically occurs over short distances and requires cells to be in close proximity to the electrode. G. sulfurreducens is able to reduce an electrode in pure culture even though it does not produce an electron shuttle but instead utilises outer membrane c-type cytochromes for DEET. The genome of G. sulfurreducens encodes for more than 100 c-type cytochromes, which may help explain this microorganism’s versatility in MECs. During electrode reduction OmcZ has been shown to be essential for thick biofilm formation and it is likely that OmcS plays some role as well7. Studies of insoluble Fe(III) oxide reduction found differences in the electron pathways as compared to those associated with the anode, with OmcS, which is localised on pili, to be essential and OmcE to be also important for DEET to insoluble Fe(III) oxide11. The Mtr cytochrome system of Shewanella likewise transfers electrons from central metabolism to the electrode surface. Interestingly the Mtr system is reversible and capable of accepting electrons from the electrode surface where the systems in Geobacter are not. OmcS and OmcZ do not affect electrode oxidation whereas GSU3274, a heme containing cytochrome does27. Furthermore, direct electron transfer differs when the extracellular electron acceptor is soluble. For instance, OmcS and pili are not required for reduction of Fe(III) citrate by Geobacter whereas OmcZ is11,28.
(3) Pilin mediated EET
G. sulfurreducens is capable of producing a multicellular thick biofilm (>50 μm) on anode surfaces that is dependent on the expression of pili7,8,29. The entire biofilm is metabolically active and contributes to power production and, unusual for a biofilm, is transcriptionally homologous throughout8,30. The PilA mutant of G. sulfurreducens, defective in pilin production, is incapable of insoluble Fe(III) oxide reduction but is still able to reduce soluble Fe(III) citrate10. This mutant also does not produce the characteristically thick anode-associated biofilms. The results of recent studies on Geobacter strongly suggest that electrons do not travel along the pili by the conventional mechanism of electron hopping between redox proteins. Instead, the movement of electrons along the pili appears to be occurring via metallic-like conduction through the pili outer surface via specific amino acid side chains31. In contrast to Geobacter nanowires, it has been recently demonstrated that the molecular composition of S. oneidensis nanowires are actually protrusions of outer membrane and periplasm. They are thus comprised of membrane material, including cytochromes involved in EET rather than being pilin based like those found in Geobacter. These differences in long-range EET further highlight the importance of understanding the different EET mechanisms between various electrogens and the electrode. With knowledge gained in this field, more tools will become apparent on how to increase the efficiency of EET and hence increase the bioremediative and energy production capacity of MFCs.
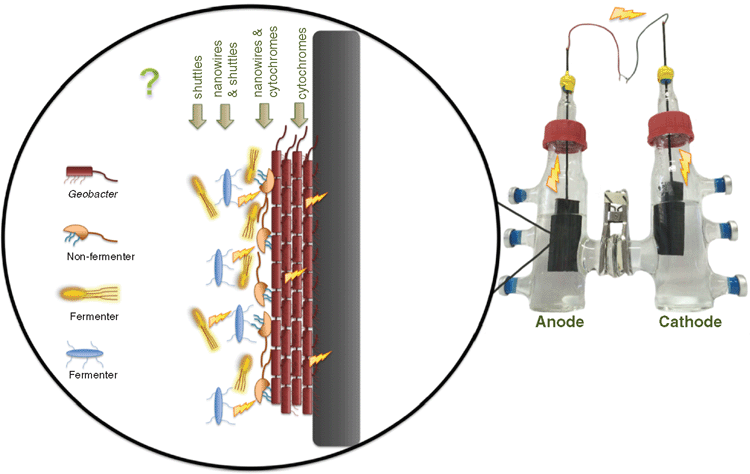
Community dynamics of electrogenic biofilms
It is now becoming apparent the microbes in the environment can directly transfer electrons to each other within an electrically conductive biofilm. Most practical applications of MECs involve a mixed microbial community from an environmental inoculum that is capable of interactions via extracellular electron transport. Although various studies on mixed species biofilms have been performed, the structural organisation and electron transfer mechanisms of mixed species electrogenic biofilms still needs further study. Some predictions however can be made based on the existing studies (Figure 2).
Depending on the environmental conditions, the interactions between electrogens may be either syntrophic or competitive. Syntrophic aggregates of G. metallireducens and G. sulfurreducens, utilising ethanol as an electron donor and fumarate as an electron acceptor, have been shown to exchange electrons through an electrically conductive network comprised of pilin and OmcS13. This interaction is known as direct interspecies electron transfer (DIET). Conversely, in environments where both microorganisms can oxidise the same electron donors for respiration, competition for this electron source ensues.
Microbial culturing studies from aquatic sediments have revealed that electrodes selectively enrich for bacteria from the Geobacteraceae. Despite the competitive advantage that Geobacter hold in these conditions, it has been demonstrated that having a mixed species biofilm on the anode also produces high electrical current and efficient oxidation of the electron donor. It is important to understand the community dynamics that occur in these mixed species biofilms in order to optimise their functionality for future applications.
Therefore, by introducing other species that can interact directly with the electric Geobacter biofilm, it should be possible to increase biofilm thickness and thus electrical current production. However, increasing biofilm thickness does not come without its limitations such as accumulation of protons leading to lower pH, build up of metabolic waste products and reduced penetration of substrate into the biofilm.
An understanding of the electric community also has benefits in other systems. Direct interspecies electron transfer in anaerobic granules used in anaerobic digesters has been improved through the addition of electron conductive material such as biochar or granulated carbon32. An understanding for the ability of bacteria to utilise electrons in syntrophic interactions will have many potential applications in the future32.
Conclusion
Although MEC technology has seen the proposal of a wide range of technological applications, real world applications have only begun to appear recently. The MudWatt™ is a small sediment MFC that powers an LED and has increased interest in electric microbiology through its educational applications. The US navy has developed benthic unattended generators (BUGs) to power remote sensing devices using MEC technology and the efficiency of upflow anaerobic sludge blankets (UASBs) has also been improved through the application of lessons learnt from MECs. The first industrial application of MECs has been the EcoVolt®, developed by Cambrian Innovation to improve treatment of large-scale wastewater treatment while producing methane ‘biogas’. Various applications of MECs are also being actively pursued by NASA for the powering of space robots, wastewater treatment in space and air revitalisation systems. Australia has seen a large-scale pilot MEC at Foster’s brewery in Queensland to determine if brewery wastewater could be more sustainably treated. Further long term plans are emerging for low power applications such as using saliva powered micro-MFCs for portable point-of-care diagnostics or Lab-on-a-Chip devices.
Many refinements have been made to improve the physical engineering of MFCs by experimenting with different electrode materials and mechanical and structural orientations. However, learning more about the biological phenomena of MECs and electrogens deserves special attention to maximally optimise the capabilities of these systems in various applications. Insights gained through the application and study in these systems is also providing information on the microbial ecology of electrogenic bacteria and their processes in the environment.
Acknowledgements
The Franks laboratory receives support from the Defence Science Institute and the Defence Science and Technology Organisation (Synthetic Biology Initiative), Office of Naval Research Global Award No. N626909-13-1-N259 and the ARC LP140100459.
References
[1] Waller, A.D. (1903) University of Liverpool. The Signs of Life from their Electrical Aspect. New York, E.P. Dutton.[2] Potter, M.C. (1911) Electrical effects accompanying the decomposition of organic compounds. Proc. R. Soc. Lond., B 84, 260–276.
| Electrical effects accompanying the decomposition of organic compounds.Crossref | GoogleScholarGoogle Scholar |
[3] Rosenbaum, M.A. and Franks, A.E. (2014) Microbial catalysis in bioelectrochemical technologies: status quo, challenges and perspectives. Appl. Microbiol. Biotechnol. 98, 509–518.
| Microbial catalysis in bioelectrochemical technologies: status quo, challenges and perspectives.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhvVGru7bL&md5=ac9619757008c8fda5226621bb800b9eCAS | 24270896PubMed |
[4] Nevin, K.P. et al. (2010) Microbial electrosynthesis: feeding microbes electricity to convert carbon dioxide and water to multicarbon extracellular organic compounds. mBio 1, e00103-10.
| Microbial electrosynthesis: feeding microbes electricity to convert carbon dioxide and water to multicarbon extracellular organic compounds.Crossref | GoogleScholarGoogle Scholar | 20714445PubMed |
[5] Lovley, D.R. and Nevin, K.P. (2013) Electrobiocommodities: powering microbial production of fuels and commodity chemicals from carbon dioxide with electricity. Curr. Opin. Biotechnol. 24, 385–390.
| Electrobiocommodities: powering microbial production of fuels and commodity chemicals from carbon dioxide with electricity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXjsFCisr0%3D&md5=996cccd03183b9ae020399f7261f654dCAS | 23465755PubMed |
[6] Bond, D.R. et al. (2002) Electrode-reducing microorganisms that harvest energy from marine sediments. Science 295, 483–485.
| Electrode-reducing microorganisms that harvest energy from marine sediments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XnvFWgtg%3D%3D&md5=837c0d19aa466920ccdd72eb61d6b3e7CAS | 11799240PubMed |
[7] Nevin, K.P. et al. (2009) Anode biofilm transcriptomics reveals outer surface components essential for high density current production in Geobacter sulfurreducens fuel cells. PLoS ONE 4, e5628.
| Anode biofilm transcriptomics reveals outer surface components essential for high density current production in Geobacter sulfurreducens fuel cells.Crossref | GoogleScholarGoogle Scholar | 19461962PubMed |
[8] Reguera, G. et al. (2006) Biofilm and nanowire production leads to increased current in Geobacter sulfurreducens fuel cells. Appl. Environ. Microbiol. 72, 7345–7348.
| Biofilm and nanowire production leads to increased current in Geobacter sulfurreducens fuel cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1GksrrM&md5=66d4cf7b49a96c28f4eb6195d58eb307CAS | 16936064PubMed |
[9] Gregory, K.B. et al. (2004) Graphite electrodes as electron donors for anaerobic respiration. Environ. Microbiol. 6, 596–604.
| Graphite electrodes as electron donors for anaerobic respiration.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXlsVSmsbY%3D&md5=3af8180037eb0670abd790a11368d565CAS | 15142248PubMed |
[10] Reguera, G. et al. (2005) Extracellular electron transfer via microbial nanowires. Nature 435, 1098–1101.
| Extracellular electron transfer via microbial nanowires.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXltl2ntrc%3D&md5=429f9eafb692e9819dd1e032f02c2d30CAS | 15973408PubMed |
[11] Mehta, T. et al. (2005) Outer membrane c-type cytochromes required for Fe(III) and Mn(IV) oxide reduction in Geobacter sulfurreducens. Appl. Environ. Microbiol. 71, 8634–8641.
| Outer membrane c-type cytochromes required for Fe(III) and Mn(IV) oxide reduction in Geobacter sulfurreducens.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtlehtbjM&md5=3c244225a0fc4e3e0aa18146328ab0b0CAS | 16332857PubMed |
[12] Leang, C. et al. (2003) OmcB, a c-type polyheme cytochrome, involved in Fe(III) reduction in Geobacter sulfurreducens. J. Bacteriol. 185, 2096–2103.
| OmcB, a c-type polyheme cytochrome, involved in Fe(III) reduction in Geobacter sulfurreducens.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXisVCms7o%3D&md5=4b419343abed4611848e198839c81530CAS | 12644478PubMed |
[13] Summers, Z.M. et al. (2010) Direct exchange of electrons within aggregates of an evolved syntrophic coculture of anaerobic bacteria. Science 330, 1413–1415.
| Direct exchange of electrons within aggregates of an evolved syntrophic coculture of anaerobic bacteria.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsVyrsbbJ&md5=8d436b99db6af41210888331d4fa98cbCAS | 21127257PubMed |
[14] Childers, S.E. et al. (2002) Geobacter metallireducens accesses insoluble Fe(III) oxide by chemotaxis. Nature 416, 767–769.
| Geobacter metallireducens accesses insoluble Fe(III) oxide by chemotaxis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XjtVKksLY%3D&md5=972716f49e6ab0e6167235ba13ae294aCAS | 11961561PubMed |
[15] Tremblay, P-L. et al. (2012) A genetic system for Geobacter metallireducens: role of the flagellin and pilin in the reduction of Fe(III) oxide. Environ. Microbiol. Rep. 4, 82–88.
| A genetic system for Geobacter metallireducens: role of the flagellin and pilin in the reduction of Fe(III) oxide.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xjs1KlsLc%3D&md5=55a08c67c0fc27da5549d73832acc927CAS | 23757233PubMed |
[16] Rotaru, A-E. et al. (2014) A new model for electron flow during anaerobic digestion: direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane. Energy Environ. Sci. 7, 408–415.
| A new model for electron flow during anaerobic digestion: direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhvFKhtrbL&md5=36525ba47366ed67d9ffe810bb80b228CAS |
[17] Baron, D. et al. (2009) Electrochemical measurement of electron transfer kinetics by Shewanella oneidensis MR-1. J. Biol. Chem. 284, 28865–28873.
| Electrochemical measurement of electron transfer kinetics by Shewanella oneidensis MR-1.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXht1Cmt73P&md5=081a270eccfc3809ddba467c14ce69b1CAS | 19661057PubMed |
[18] Marsili, E. et al. (2008) Shewanella secretes flavins that mediate extracellular electron transfer. Proc. Natl. Acad. Sci. USA 105, 3968–3973.
| Shewanella secretes flavins that mediate extracellular electron transfer.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXjs1Oms7g%3D&md5=e41000aebc9ebb169ceaf668b5ad8e8aCAS | 18316736PubMed |
[19] Ross, D.E. et al. (2009) Kinetic characterization of OmcA and MtrC, terminal reductases involved in respiratory electron transfer for dissimilatory iron reduction in Shewanella oneidensis MR-1. Appl. Environ. Microbiol. 75, 5218–5226.
| Kinetic characterization of OmcA and MtrC, terminal reductases involved in respiratory electron transfer for dissimilatory iron reduction in Shewanella oneidensis MR-1.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtVeltLvJ&md5=7aa8cb853e1558e73bc631af6eaa5577CAS | 19542342PubMed |
[20] von Canstein, H. et al. (2008) Secretion of flavins by Shewanella species and their role in extracellular electron transfer. Appl. Environ. Microbiol. 74, 615–623.
| Secretion of flavins by Shewanella species and their role in extracellular electron transfer.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhvVSqurw%3D&md5=39c455f2cc4a9b718979236af278188dCAS | 18065612PubMed |
[21] Bücking, C. et al. (2012) Outer-membrane cytochrome-independent reduction of extracellular electron acceptors in Shewanella oneidensis. Microbiology 158, 2144–2157.
| Outer-membrane cytochrome-independent reduction of extracellular electron acceptors in Shewanella oneidensis.Crossref | GoogleScholarGoogle Scholar | 22493303PubMed |
[22] Rabaey, K. et al. (2005) Microbial phenazine production enhances electron transfer in biofuel cells. Environ. Sci. Technol. 39, 3401–3408.
| Microbial phenazine production enhances electron transfer in biofuel cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXisFajsbo%3D&md5=13c1ab44343004c8089ab129358aa14eCAS | 15926596PubMed |
[23] Hernandez, M.E. et al. (2004) Phenazines and other redox-active antibiotics promote microbial mineral reduction. Appl. Environ. Microbiol. 70, 921–928.
| Phenazines and other redox-active antibiotics promote microbial mineral reduction.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhs1Chu7Y%3D&md5=9c5caea7348058b74838357d9dea2403CAS | 14766572PubMed |
[24] Park, H.S. et al. (2001) A novel electrochemically active and Fe(III)-reducing bacterium phylogenetically related to Clostridium butyricum isolated from a microbial fuel cell. Anaerobe 7, 297–306.
| A novel electrochemically active and Fe(III)-reducing bacterium phylogenetically related to Clostridium butyricum isolated from a microbial fuel cell.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XhtVajtbg%3D&md5=18e6287ccad3847b5a06151a01fbce92CAS |
[25] Choi, O. et al. (2012) Butyrate production enhancement by Clostridium tyrobutyricum using electron mediators and a cathodic electron donor. Biotechnol. Bioeng. 109, 2494–2502.
| Butyrate production enhancement by Clostridium tyrobutyricum using electron mediators and a cathodic electron donor.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XmtVKku7w%3D&md5=a080ef71b76f8eb5f3ffb0075ad0e81cCAS | 22511218PubMed |
[26] Lovley, D.R. (2012) Electromicrobiology. Annu. Rev. Microbiol. 66, 391–409.
| Electromicrobiology.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsF2iurrP&md5=4bca6c3772f667dba783ef9b7c56b35bCAS | 22746334PubMed |
[27] Strycharz, S.M. et al. (2011) Gene expression and deletion analysis of mechanisms for electron transfer from electrodes to Geobacter sulfurreducens. Bioelectrochemistry 80, 142–150.
| Gene expression and deletion analysis of mechanisms for electron transfer from electrodes to Geobacter sulfurreducens.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsF2htLbJ&md5=1be2d3622587d5b5019475f9b4d1c6e4CAS | 20696622PubMed |
[28] Inoue, K. et al. (2010) Purification and characterization of OmcZ, an outer-surface, octaheme c-type cytochrome essential for optimal current production by Geobacter sulfurreducens. Appl. Environ. Microbiol. 76, 3999–4007.
| Purification and characterization of OmcZ, an outer-surface, octaheme c-type cytochrome essential for optimal current production by Geobacter sulfurreducens.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXptVems7w%3D&md5=0e7558c95826ebc361839f83efc5f90fCAS | 20400562PubMed |
[29] Franks, A.E. et al. (2009) Novel strategy for three-dimensional real-time imaging of microbial fuel cell communities: monitoring the inhibitory effects of proton accumulation within the anode biofilm. Energ. Environ. Sci. 2, 113.
| Novel strategy for three-dimensional real-time imaging of microbial fuel cell communities: monitoring the inhibitory effects of proton accumulation within the anode biofilm.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXnsVSnsLw%3D&md5=b71ae12e3b02f9ff767dd42be3337fa4CAS |
[30] Franks, A.E. et al. (2010) Microtoming coupled to microarray analysis to evaluate the spatial metabolic status of Geobacter sulfurreducens biofilms. ISME J. 4, 509–519.
| Microtoming coupled to microarray analysis to evaluate the spatial metabolic status of Geobacter sulfurreducens biofilms.Crossref | GoogleScholarGoogle Scholar | 20033069PubMed |
[31] Malvankar, N.S. et al. (2011) Tunable metallic-like conductivity in microbial nanowire networks. Nat. Nanotechnol. 6, 573–579.
| Tunable metallic-like conductivity in microbial nanowire networks.Crossref | GoogleScholarGoogle Scholar | 21822253PubMed |
[32] Chen, S et al. (2014) Promoting interspecies electron transfer with biochar. Scientific Reports 4, 5019.
| 24846283PubMed |
Biographies
Lucie Semenec is a PhD candidate at Dr Ashley Franks laboratory in the Faculty of Science, Technology and Engineering at La Trobe University. Her research focuses on the community dynamics of electrogenic biofilms and the underlying mechanisms of syntrophic and competitive microbial interactions.
Ashley Edwin Franks is a senior lecturer in the Department of Microbiology at La Trobe University and head of the Applied and Environmental Microbiology research laboratory. He conducted his doctorate research as part of the Centre of Marine Biofouling and Bioinnovation at the University of New South Wales by investigating antifungal compounds produced by marine bacteria in biofilms. During his PhD he spent 4 months at the University of Exeter in the UK on an Adrian Lee Fellowship to develop dual bacterial/yeast biofilm systems. On graduating he moved to the Biomerit Research Centre in Cork, Ireland to work on bacterial plant interactions as a Government of Ireland Fellow in Science Technology and Engineering. This research looked at how to use bacteria to help plant growth. He then took a position as a Senior Scientist and Research Professor within the Geobacter Project at the University of Massachusetts Amherst in the USA where he worked on microbes that make electricity.