Antimicrobial resistance a threat to public health
John MerlinoA Department of Infectious Diseases and Immunology, Sydney Medical School, University of Sydney, Sydney, NSW, Australia
B Department of Microbiology and Infectious Diseases, Concord Hospital, NSW Health Pathology, Sydney, NSW, Australia
C Tel: +61 2 9767 6658
Fax: +61 2 9767 7868
Email: JMerlino@med.usyd.edu.au; John.Merlino@health.nsw.gov.au
Microbiology Australia 38(4) 165-167 https://doi.org/10.1071/MA17059
Published: 3 November 2017
Antimicrobial resistance is a complex issue and is a threat to public health globally. While emerging and re-emerging diseases have captured news headlines with outbreaks such SARS, bird flu, Ebola and Zika virus around the world, leading health experts tells us that there is a more serious threat to public health – antimicrobial resistance (AMR)1.
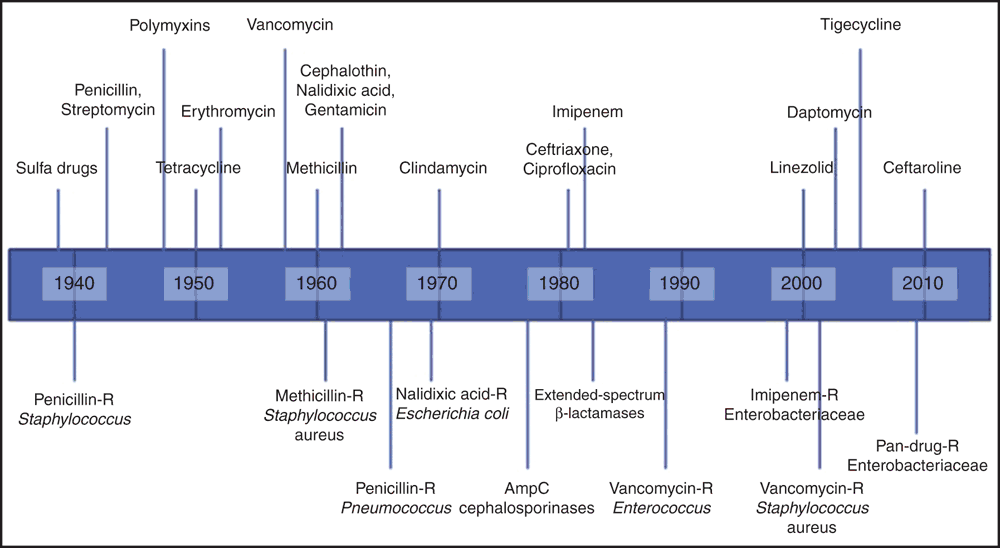
The World Health Organization (WHO) defines AMR as microorganisms, which include bacteria, fungi, viruses, and parasites, that change when exposed to antimicrobial drugs such as antibiotics, antifungals, antivirals, antimalarials, and antihelminthics formulations making them ineffective in the treatment of many infectious diseases2. This is not a new phenomenon. We have been aware of AMR since the beginning of antibiotic usage in therapeutics medicine (Figure 1)3. When AMR organisms known as ‘superbugs’ emerge and spread in society they become a major public health issue. AMR is an increasingly serious threat to global public health that requires action across all government sectors and society4.
|
In many cases the cost of healthcare for patients with resistant infections is higher due to longer duration of illness, additional medical/laboratory testing, and the use of more expensive drugs. As reported by Gilbert et al. in a previous issue of Microbiology Australia in ‘Healthcare infection prevention and control really is everyone’s business’5 it is estimated 200 000 health associated infections (HAI), some of which are multi-drug resistant, occur in Australia annually; contribute to 7000 deaths and are responsible for A$2–3 billion in health costs5. The WHO estimates globally 480 000 people develop multi-drug resistant tuberculosis each year, and drug resistance is complicating the fight against malaria and HIV2.
Key public health issues relating to antimicrobial resistance have been summarised below by Balsalobre et al. in ‘An overview of antimicrobial resistance and its public health significance’4 and Jindal et al. in ‘Antimicrobial resistance: a public health challenge’6.
AMR and costs of controlling infections
AMR delays appropriate treatment, making patients infectious for longer periods of time. Ineffective treatment increases the chance of transmission of AMR organisms putting at risk more members of the community and healthcare workers. The family of the individual and society bears the burden of the associated economic health-care costs. While empirical antibiotic therapy is effective for antimicrobial susceptible organisms, the costs for treating AMR organisms are much higher. Additional costs are associated with controlling outbreaks of resistance.
AMR and no new antimicrobials in the pipeline
Microorganisms are becoming increasingly resistant to existing antibiotics. In the previous decades changes in susceptibility patterns of many microorganisms (in bacteria Gram-positive but mainly in Gram-negative bacteria – especially plasmid encoded carbapenemase producing organisms) has had a dramatic impact in the therapeutic options in treating many community-acquired and hospital-acquired infections. In the immunocompromised host, following a viral infection many AMR fungal or bacterial infections frequently occur. Two dramatic challenges arise: first, resistance may not be predictable and combined with virulence increases the challenge in treating such infections. Secondly there are no new antibiotics in the pipeline in treating AMR infections. For many clinicians, the threat of AMR is becoming a reality both in the community and in the hospital setting, a worrisome situation experienced in the pre-antibiotic era.
AMR and no new antimicrobial discoveries
Many antibiotics used today are the result of scientific discoveries from many years ago3. While research has been done in modifying pre-existent antibiotics such as tigecycline, a tetracycline derivative, or Ceftolazane/Tazobactam, a combined 5th generation cephalosporin, many clinicians are returning to previously discovered antibiotics such as colistin. Colistin, a polymixin antibiotic that was discovered in the late 1940s for the treatment of Gram-negative infections, has resurfaced as a last line of treatment option for multi-drug resistant organisms such as Pseudomonas aeruginosa, Acinetobacter baumannii, Klebsiella pneumoniae and carbapenemase resistant Gram-negative bacteria. One of the reasons for why there is a lack of new antibiotics being discovered is that microorganisms will rapidly modify and adapt to many new antibiotics making them ineffective in the short and long term. Consequently, many pharmaceutical companies have directed their research in chronic illness medication, such as hypertension and diabetes, ensuring a more profitable business proposition6.
In 2017 the WHO published its first ever list of antibiotic-resistant ‘priority pathogens’ – a catalogue of 12 families of bacteria that pose the greatest threat to human health.
WHO priority pathogens list for research and development of new antibiotics7
Priority 1: CRITICAL
-
Acinetobacter baumannii, carbapenem-resistant
-
Pseudomonas aeruginosa, carbapenem-resistant
-
Enterobacteriaceae, carbapenem-resistant, ESBL-producing
Priority 2: HIGH
-
Enterococcus faecium, vancomycin-resistant
-
Staphylococcus aureus, methicillin-resistant, vancomycin-intermediate and resistant
-
Helicobacter pylori, clarithromycin-resistant
-
Campylobacter spp., fluoroquinolone-resistant
-
Salmonellae, fluoroquinolone-resistant
-
Neisseria gonorrhoeae, cephalosporin-resistant, fluoroquinolone-resistant
Priority 3: MEDIUM
-
Streptococcus pneumoniae, penicillin-non-susceptible
-
Haemophilus influenzae, ampicillin-resistant
-
Shigella spp., fluoroquinolone-resistant
The list was drawn up in a bid to guide and promote research and development of new antibiotics, as part of the WHO’s efforts to address growing global resistance to antimicrobial medicines7.
AMR and primary health care in hospitals and community
Antimicrobial use and AMR is one of the most pressing problems faced in healthcare services. Antimicrobial use and overuse is also a concern in the community and is rising steadily globally8. In response, initiatives at the local, national and international levels promote ‘antibiotic stewardship’, with the aim to improve the appropriateness of antimicrobial use. Such initiatives rely on success on the continuing education of prescribers and patients in the community. In the healthcare setting, success relies on programs administered by multidisciplinary teams composed of infectious diseases physicians, clinical pharmacists, clinical microbiologists, and infection control practitioners and hospital administrators. Antibiotic rotation strategies based on population epidemiology dynamically control the prescribing process by schedule changes of antimicrobial classes used for empirical therapy9.
As a public health strategy, Australia has a range of existing initiatives in both human and animal health to address aspects of AMR. These include regulatory restrictions on the prescription and use of antimicrobials, AMR surveillance activities, hand hygiene and antimicrobial stewardship (AMS) programs, strict requirements to manage pathogen levels along the food production and processing chain, education for prescribers on the judicious use of antibiotics, and research into new products and approaches to prevent and respond to AMR. While there has been national coordination of some AMR-related activities, there are opportunities to improve coordination across all elements to better integrate efforts to address gaps and ensure a more comprehensive response to AMR in Australia9.
The Australian Government Department of Health funds the Australian Commission on Safety and Quality in Health Care to develop and implement a national surveillance system for AMR and antibiotic usage. This initiative includes building on existing passive and targeted AMR surveillance systems, addressing antibiotic usage in humans across hospital, community and aged care settings and establishing a national alert system to inform clinicians and policy makers about emerging AMR trends that may impact on public health9,10.
Australia is also engaging with other countries through the WHO, to ensure alignment with key international policies and strategies and to support developing countries in our region in their efforts to prevent and contain AMR. Australia also contributes to the development of a WHO Global Action Plan (GAP) for AMR9.
As part of the Australian Government’s ‘First National Antimicrobial Resistance Strategy 2015–2019’11 to prevent and contain AMR (Figure 2), the Australian Commission on Safety and Quality in Health Care was funded by the Department of Health to establish and coordinate a national surveillance system for Antimicrobial Use and Resistance in Australia (AURA). This system is now in place and the commission is expanding the data collected and analysed to provide reports that will inform policy and practice. In addition, Australia’s response recognises AMR is a One Health issue that requires a coordinated response in all sectors where antimicrobials are used, including in human health, animal health, and in the food and agriculture sectors11,12.
Slowing the rate of AMR, preparing for and responding to new and emerging threats, and ensuring that antimicrobials are used appropriately are key components of the Commission’s work with the states and territories, and the private sector, to ensure safety and quality of health care in Australia12.
References
[1] Boyce, M. (2017) A new threat to public health: antibiotic-resistant bacteria. http://globalhealth.duke.edu/media/blogs/mscgh/new-threat-public-health-antibiotic-resistant-bacteria[2] WHO (2016) Antimicrobial resistance. Fact sheet updated September 2016. http://www.ho.int/mediacentre/factsheets/fs194/en/
[3] Tang, S.S. et al. (2014) Mechanisms of β-lactam antimicrobial resistance and epidemiology of major community- and healthcare-associated multidrug-resistant bacteria. Adv. Drug Deliv. Rev. 78, 3–13.
| Mechanisms of β-lactam antimicrobial resistance and epidemiology of major community- and healthcare-associated multidrug-resistant bacteria.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhsVSqtrzM&md5=ea495036437ad12b738dd61323e3d85dCAS |
[4] Balsalobre, L.C. et al. (2014) An overview of antimicrobial resistance and its public health significance. Braz. J. Microbiol. 45, 1–5.
| An overview of antimicrobial resistance and its public health significance.Crossref | GoogleScholarGoogle Scholar |
[5] Gilbert, L. et al. (2014) Healthcare infection prevention and control really is everyone’s business. Microbiol. Aust. 35, 3–4.
| Healthcare infection prevention and control really is everyone’s business.Crossref | GoogleScholarGoogle Scholar |
[6] Jindal, A.K. et al. (2015) Antimicrobial resistance: a public health challenge. Med. J. Armed Forces India 71, 178–181.
| Antimicrobial resistance: a public health challenge.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC2MjivVagsA%3D%3D&md5=ba4ea55b2b328afd7bb3291bc58621b6CAS |
[7] WHO Media Centre (2017) WHO publishes list of bacteria for which new antibiotics are urgently needed. http://www.who.int/mediacentre/news/releases/2017/bacteria-antibiotics-needed/en/
[8] O’Neill, J. (2014) Review on antimicrobial resistance. http://amr-review.org/
[9] Costelloe, C. et al. (2010) Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ 340, c2096.
| Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar |
[10] Australian Government, Department of Health (2016) Antimicrobial resistance (AMR). http://www.health.gov.au/internet/main/publishing.nsf/Content/ohp-amr.htm
[11] Australian Government, Department of Health, Department of Agriculture (2015) Responding to the threat of antimicrobial resistance. Australia’s First National Antimicrobial Resistance Strategy 2015–2019. http://www.health.gov.au/internet/main/publishing.nsf/Content/1803C433C71415CACA257C8400121B1F/%24File/amr-strategy-2015-2019.pdf
[12] Australian Commission on Safety and Quality in Health Care (2017) Antibiotic Use and Resistance in Australia (AURA). https://www.safetyandquality.gov.au/antimicrobial-use-and-resistance-in-australia/2017-report/
Biography
Dr John Merlino is a Senior Scientist; he completed his MSc with honours and a PhD in Medicine on antimicrobial resistance and lectures at the University of Sydney. He is a Fellow of the Australian Society for Microbiology and a Founding Fellow of the Faculty of Science of the Royal College of Pathology Australasia. For the past 15 years he has been the National Convenor of the Antimicrobial Special Interest Group (ASIG) of the Australian Society for Microbiology.



