The role of non-spore-forming actinobacteria in cleaning up sites contaminated by persistent pollutants and the ability of these microorganisms to survive under unfavourable conditions
Inna P Solyanikova , Natalia E Suzina and Ludmila A GolovlevaG.K. Skryabin Institute of Biochemistry and Physiology of Microorganisms, Russian Academy of Sciences, Pushchino, Moscow Region, Russia. Tel: +7 495 625 74 48, Email: Innas@ibpm.pushchino.ru
Microbiology Australia 39(3) 141-144 https://doi.org/10.1071/MA18043
Published: 7 August 2018
Years of research has shown that actinobacteria, including Rhodococcus, Gordonia, Arthrobacter, Microbacteria, play an important role in cleaning up sites contaminated by persistent organic pollutants. Under special conditions, actinobacteria of different genera are able to form specific forms, cyst-like resting cells (CLC), which maintain the viability during long-term storage (for at least 5–6 years, our unpublished results). These cells quickly germinate when conditions become favourable for growth. As a result, actinobacteria can be used as a basis for creating highly efficient biological preparations for cleaning up the soil with high levels of toxic contaminants such as (chloro)phenols, (chloro)biphenyls, polycyclic hydrocarbons, oil1.
Among the most important environmental concerns today is contamination with toxic and persistent pollutants. Oceans, soils, groundwater, air, living organisms are increasingly polluted due to human activities2. The most serious problem is created by the presence of halogenated aromatic compounds, polycyclic aromatic hydrocarbons and various oil components3–5. A great majority of these compounds are by-products derived from manufacturing processes and found in waste of by-product-coking and petrochemical industries6,7. In some industrial regions, technogenic load on the environment is caused by emissions and waste dumping from large enterprises of the chemical industry, potassium industry, and power plants. One of the largest environmental polluters in Chapaevsk (Samara Region, Russia) is the Middle Volga chemical plant8. A number of large enterprises for the processing of potassium-magnesium and sodium salts are located in Perm Krai, Russia9. Vast quantities of halides in wastewater and the brine sludge are formed as a result of the activities of such enterprises. These components, released by potassium salt mining and processing plants, pollute the environment with compounds of sodium, potassium, magnesium, and other elements, which leads to soil salinisation. Hence, wastes generated during the production process of fluorine- and bromine-containing organic and inorganic compounds are major contributors to the pollution of soils and water bodies5. These compounds are characterised by low water solubility and can be strongly adsorbed by biological tissues2. A strong technogenic impact on the environment is often aggravated by ecological factors that are unfavourable for microbial destruction of pollutants, such as low temperature, high contents of mineral substances, and high or low pH of the substrate. Nevertheless, the unique soil microflora developing in such areas can both survive and decompose different classes of xenobiotics under extreme environmental conditions10,11.
Actinobacteria are able to degrade a great variety of persistent compounds, for instance, phenol, chlorophenols, (chloro)benzoates, chlorinated biphenyls, oil. Biopreparations for soil and water detoxification contain bacteria of one or few strains. Generally, these biopreparations include representatives of actinobacteria. A combination of microorganisms with different viability and metabolic strategy (e.g. Rhodococci and Pseudomonads) increases the efficacy of biopreparations.
To date, there is limited research in microbiology on survival of the species of non-spore-forming microorganisms in nature under conditions of carbon, nitrogen and phosphorus deficiency, species-specific growth factors necessary for growth and development, and under the influence of aggressive physical factors of the environment that may be temporary or seasonal (fluctuations in temperature and humidity, pH of the medium, the presence of aggressive chemical compounds-contaminants of anthropogenic origin, etc.). Cyst-like resting cells (CLC) of non-spore-forming actinobacteria, representatives of the genera: Rhodococcus12, Gordonia, Arthrobacter13 and Microbacterium14 were obtained in experimental laboratory conditions. These actinobacteria are widely distributed in various natural habitats (including extreme ones) and, apparently, have the ability to rapidly and effectively adapt to structural rearrangements remaining in a viable state.
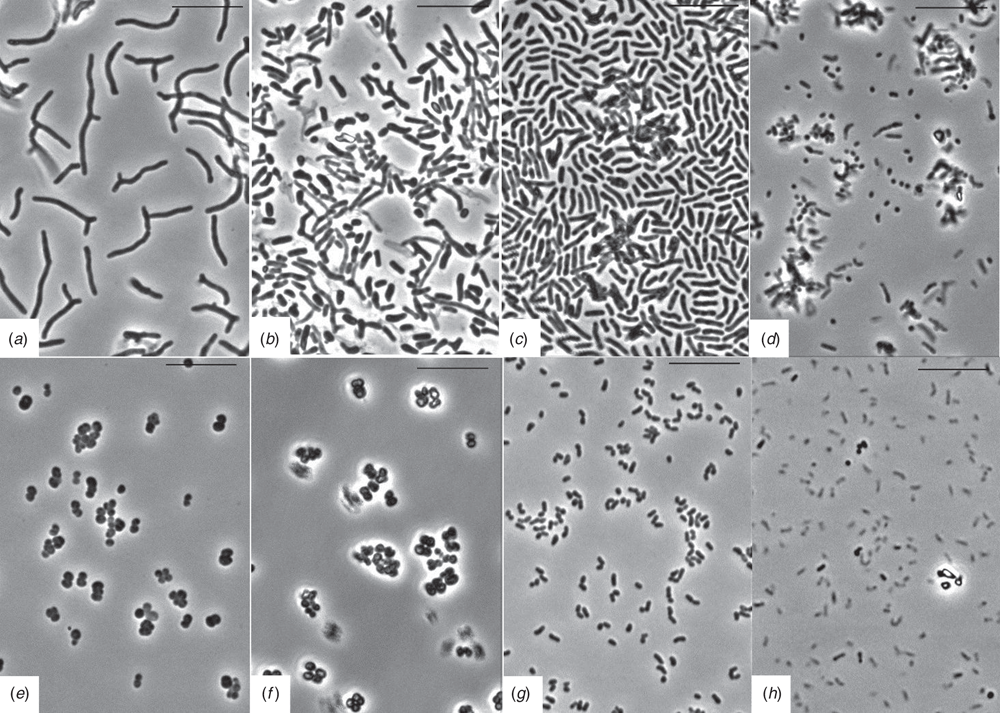
It is shown that the studied non-spore-forming actinobacteria, under unfavourable growth conditions, for example, depletion of the growth substrate, are capable of entering into a resting state (Figure 1). Model strains of bacteria of two genera: Arthrobacter and Microbacterium, have the ability to form, under experimental conditions, small (volume ≤0.1 μm3) and ultra-small (from 0.01 to 0.02 μm3) viable cyst-like resting cells (US CLC). Experimentally obtained CLC are characterised by a high density of the cytoplasm, a compacted nucleoid and a dense cell wall with an external additional cover in the form of a fibrillar capsule (single CLCs of M. foliorum BN52), or represented by conglomerates of such ultra-small CLCs (A. agilis Lush13).
Under favourable conditions, the process of germination of resting cells of non-spore-forming bacteria occurs. As a rule, the process of activation of growth is already discernible at the ultrastructural cellular level during the first 0.5–1 h after cell seeding procedure. Intrapopulation variability of R. opacus was shown to be coupled with germination after dormancy stage, and diversity of the emerged colonial variants depended upon the physiological age (storage time) of CLC and additional stress impacts (thermal treatment)12.
The transition to the vegetative growth of conglomerates of ultra-small CLC A. agilis Lush13 was accompanied by disintegration of conglomerates into single US CLCs followed by their active growth and division. A distinct feature of the process of ‘germination’ of the CLC of this type is simultaneous formation of a dense extracellular and intercellular fibrillar matrix. The transition from a resting state to vegetative growth of the A. agilis Lush13 CLC resulted in the formation of microcolonies of vegetative cells enclosed within a dense fibrillar matrix, the ‘cocoon’. It can be assumed that in this case, the bacterium realises the protective mechanism of rapid multiplication within the fibrillar matrix, which protects the population of young vegetative cells against sudden fluctuations in external factors and unexpectedly unfavourable conditions. In common laboratory practice A. agilis Lush13 cultivated cells do not form a fibrillar matrix13.
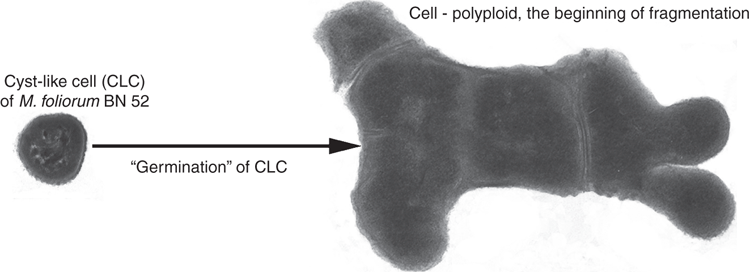
The transition to vegetative growth of the CLC strain M. foliorum BN52 was followed at the first stages by the formation of large cellular forms, polyploids, where the cytoplasm and nucleoplasmic volume was ~40 times greater than the volume of vegetative cells under laboratory cultivation conditions (Fig. 2)14. In the subsequent stages of the ‘germination’ of the CLC of strain BN52, the nucleoplasm was split into discrete nucleoids and multiple fragmentation of large cells into ultra-small ‘single nucleoid’ cell forms (with a volume of 0.01–0.02 μm3). The process of ‘germination’ of US CLC was complete when 15 to 20 ‘daughter’ vegetative cells were formed. At the same time, under laboratory conditions of cultivation binary fission is the most common form of reproduction in bacteria; such fission is complete when two ‘daughter’ cells are formed.
|
The obtained experimental data are consistent with the literature data on the detection in a variety of natural biotopes of a large number of ultra-small bacteria and bacterial cell forms (70–90% according to various data)15–17. It is obvious that small and ultra-small viable cell forms have the advantage of spreading in the environment, since they can penetrate into habitats that are inaccessible to large single-celled organisms18. Thus, our studies have shown that the transition of the CLC to active growth differs significantly from conventional binary fission under cultivation conditions on laboratory nutrient media. One can assume that in natural conditions, bacterial cells implement the following mechanisms: (1) the mechanism of ‘protection of offspring’, realised in the natural environment by forming a protective outer matrix-cocoon around young dividing cells; (2) the mechanism of directed reduction in the size / volume of daughter cells to provide an advantage in colonising hard-to-reach ecological niche; and (3) cell numbers increasing by the division of giant polyploid cells, boosting the growth of the cell population in a short time.
The following properties of CLC are important for survival in nature: (1) the ability to quickly reset active metabolism and cell division under favourable conditions; (2) stress resistance; and (3) increased phenotypic variability, which is expressed after CLC germination as a spectrum of phenotypes, with one of them being probably most adapted to the new environment12. Using the strain R. opacus 1CP as an example, it was shown that the renewal of growth after resting stage in actinobacteria can be accompanied by an expansion of the range of utilisable substrates12. The most likely explanation for this phenomenon is a change in the rate of induction/repression of genes responsible for different metabolic networks of the whole cell and facilitating the reorganisation of their regulation.
Conclusion
Actinobacteria can degrade a wide range of compounds under varied environmental conditions due to high metabolic rates, the presence of highly specific enzymes, and the ability to transfer biodegradation genes. Under adverse conditions these bacteria are able to form specific surviving forms, the so-called cyst-like resting cells (CLC). Thanks to the formation of the CLC, the actinobacteria retain their viability for many years in starvation, drier, and higher temperature conditions. Under favourable conditions, the CLC can germinate quickly. The mechanism, which is based on the formation of polyploid cells, can be accompanied by a structural and functional reorganisation of the genomes of forming ‘daughter’ cells, which in turn can lead to the appearance of new properties in forming cells, which can increase the adaptability of cells to new environmental conditions.
Acknowledgements
This work was supported by the grant of the Russian Science Foundation, project no. 14-14-00368.
References
[1] Puntus, I.F. et al. (2018) Contribution of soil bacteria isolated from different regions into crude oil and oil product degradation. J. Soils Sedim. , .| Contribution of soil bacteria isolated from different regions into crude oil and oil product degradation.Crossref | GoogleScholarGoogle Scholar |
[2] Wang, L. et al. (2015) Accumulation of 19 environmental phenolic and xenobiotic heterocyclic aromatic compounds in human adipose tissue. Environ. Int. 78, 45–50.
| Accumulation of 19 environmental phenolic and xenobiotic heterocyclic aromatic compounds in human adipose tissue.Crossref | GoogleScholarGoogle Scholar |
[3] Singh, A. and Ward, O.P. (2004) Biodegradation and Bioremediation. In Soil Biology 2 (Singh, A., and Ward, O.P., eds). Springer-Verlag, Berlin, Heidelberg, XVII. https://www.springer.com/gp/book/9783540211013
[4] Patel, B.P. and Kumar, A. (2016) Multi-substrate biodegradation of chlorophenols by defined microbial consortium. 3 Biotech 6, 191.
| Multi-substrate biodegradation of chlorophenols by defined microbial consortium.Crossref | GoogleScholarGoogle Scholar |
[5] Jin, R. et al. (2017) Congener-specific determination of ultratrace levels of chlorinated and brominated polycyclic aromatic hydrocarbons in atmosphere and industrial stack gas by isotopic dilution gas chromatography/high resolution mass spectrometry method. J. Chromatogr. A 1509, 114–122.
| Congener-specific determination of ultratrace levels of chlorinated and brominated polycyclic aromatic hydrocarbons in atmosphere and industrial stack gas by isotopic dilution gas chromatography/high resolution mass spectrometry method.Crossref | GoogleScholarGoogle Scholar |
[6] Deblonde, T. et al. (2011) Emerging pollutants in wastewater: a review of the literature. Int. J. Hyg. Environ. Health 214, 442–448.
| Emerging pollutants in wastewater: a review of the literature.Crossref | GoogleScholarGoogle Scholar |
[7] Gavrilescu, M. et al. (2015) Emerging pollutants in the environment: present and future challenges in biomonitoring, ecological risks and bioremediation. N. Biotechnol. 32, 147–156.
| Emerging pollutants in the environment: present and future challenges in biomonitoring, ecological risks and bioremediation.Crossref | GoogleScholarGoogle Scholar |
[8] Revich, B. et al. (2001) Dioxin exposure and public health in Chapaevsk, Russia. Chemosphere 43, 951–966.
| Dioxin exposure and public health in Chapaevsk, Russia.Crossref | GoogleScholarGoogle Scholar |
[9] Korsakova, E.S. et al. (2013) Diversity in the bacterial genus Halomonadaceae in the Upper Kama salts mining region. Mikrobiologiia 82, 247–250.
[10] Diels, L. et al. (2009) From industrial sites to environmental applications with Cupriavidus metallidurans. Antonie van Leeuwenhoek 96, 247–258.
| From industrial sites to environmental applications with Cupriavidus metallidurans.Crossref | GoogleScholarGoogle Scholar |
[11] Bello-Akinosho, M. et al. (2016) Potential of polycyclic aromatic hydrocarbon-degrading bacterial isolates to contribute to soil fertility. Biomed. Res. Int. , 5798593.
| Potential of polycyclic aromatic hydrocarbon-degrading bacterial isolates to contribute to soil fertility.Crossref | GoogleScholarGoogle Scholar |
[12] Solyanikova, I.P. et al. (2011) The effect of dormant stage on degradative activity of the strain Rhodococcus opacus 1CP. J. Environ. Sci. Health B 46, 638–647.
| The effect of dormant stage on degradative activity of the strain Rhodococcus opacus 1CP.Crossref | GoogleScholarGoogle Scholar |
[13] Solyanikova, I.P. et al. (2017) The response of soil-dwelling Arthrobacter agilis Lush13 to stress impact: transition between vegetative growth and dormancy state. J. Environ. Sci. Health B 52, 745–751.
| The response of soil-dwelling Arthrobacter agilis Lush13 to stress impact: transition between vegetative growth and dormancy state.Crossref | GoogleScholarGoogle Scholar |
[14] Solyanikova, I.P. et al. (2017) Structural and functional rearrangements in the cells of actinobacteria Microbacterium foliorum BN52 during transition from vegetative growth to a dormant state and during germination of dormant forms. Mikrobiologiia 86, 476–486.
| Structural and functional rearrangements in the cells of actinobacteria Microbacterium foliorum BN52 during transition from vegetative growth to a dormant state and during germination of dormant forms.Crossref | GoogleScholarGoogle Scholar |
[15] Bae, H.C. et al. (1972) Microflora of soil as viewed by transmission electron microscopy. Appl. Microbiol. 23, 637–648.
[16] Panikov, N.S. (2005) Contribution of nanosized bacteria to the total biomass and activity of a soil microbial community. Adv. Appl. Microbiol. 57, 245–296.
| Contribution of nanosized bacteria to the total biomass and activity of a soil microbial community.Crossref | GoogleScholarGoogle Scholar |
[17] Dmitriev, V.V. et al. (2008) Electron microscopic detection and in situ characterization of bacterial nanoforms in extreme biotopes. Microbiology 77, 39–46.
| Electron microscopic detection and in situ characterization of bacterial nanoforms in extreme biotopes.Crossref | GoogleScholarGoogle Scholar |
[18] Duda, V.I. et al. (2012) Ultramicrobacteria: formation of the concept and contribution of ultramicrobacteria to biology. Mikrobiologiia 81, 415–427.
Biographies
Inna Solyanikova, DSc, is the leading researcher in the laboratory of enzymatic degradation of organic compounds. Her major research interest is the investigation of microorganisms with the ability to degrade various persistent pollutants.
Natalia Suzina, PhD, works in the Laboratory of Cytology of Microorganisms. Her main area of interest belongs to investigation of small and ultra-small bacteria and intra-microbial parasitism.
Professor Ludmila Golovleva, DSc, is the Head of the laboratory of enzymatic degradation of organic compounds. For many years she is the supervisor of research on bacterial and fungal metabolism in respect to their ability to degrade persistent compounds.