The relevance of probiotics in Caesarean-born neonates
Hanna E Sidjabat A E , Alaa Mohammed Ali Alsaggaf B , Akshatha Gopalakrishna B , Evelyn Nadar B , Adam Irwin B C and Pieter Koorts DA Menzies Health Institute Queensland, Griffith University, Gold Coast, Qld 4222, Australia
B The University of Queensland, UQ Centre for Clinical Research, Herston, Qld 4029, Australia
C Infection Management and Prevention Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Qld 4101, Australia
D Grantley Stable Neonatal Unit and Queensland Milk Bank, Royal Brisbane and Women’s Hospital, Brisbane, Qld 4029, Australia
E Email: h.sidjabat@griffith.edu.au
Microbiology Australia 41(2) 75-81 https://doi.org/10.1071/MA20020
Published: 27 May 2020
Abstract
There is growing interest in the use of probiotics in neonates. In particular, Lactobacillus rhamnosus, L. acidophilus, Bifidobacterium breve and B. longum have been well studied. Caesarean-section (CS)-born infants often lack Lactobacillus spp. and Bifidobacterium spp., which showed increasing evidence in establishing the neonatal immune system. Furthermore, CS increases the difficulties for mothers in initiating and sustaining breastfeeding. Increasing evidence shows CS-born infants are more susceptible to allergy, infections and chronic inflammatory diseases later in life. The number of CS births has increased continuously, now accounting for 35% of all deliveries Australia wide. In this context, probiotics may have a role in establishing a healthy neonatal gut microbiome.
Introduction
‘An ounce of prevention is worth a pound of cure’ is an axiom by Benjamin Franklin, one that is relevant especially in the current COVID-19 pandemic. In Australia, rates of delivery by Caesarean-section (CS) have increased and reached 35% in 20171. Antibiotics are used regularly for both prophylaxis and treatment of infections in mothers who deliver babies through CS2. This excess use is important for its potential role in driving antimicrobial resistance worldwide3 and also has an impact on the establishment of the neonatal gut microbiome.
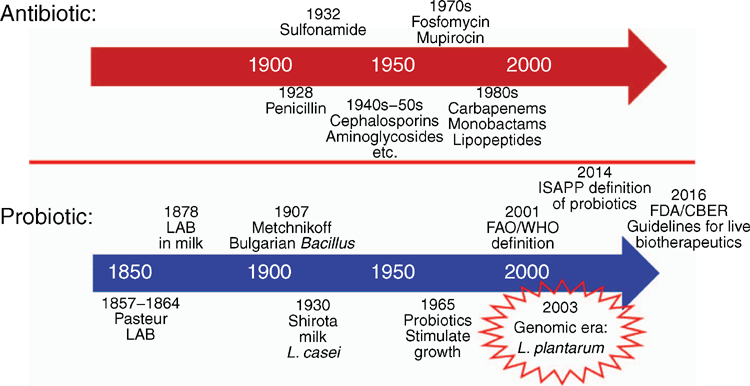
CS is associated with significant difficulties in initiating breastfeeding when compared with vaginal birth4. The microbiome of breast milk contains bacteria, including lactic-acid bacteria (LAB), and is important in establishing the gut microbiome of neonates5. Breastfeeding helps to establish healthy gut microbiome. LAB were first described by Pasteur as part of fermentation to prevent spoilage approximately 70 years before the discovery of penicillin in 19286 (Figure 1).
CS-born infants generally lack LAB, i.e. Lactobacillus spp. and Bifidobacterium spp., which appear important in establishing the neonatal immune system7. Recent data support the theory that probiotic administration to CS-born infants may prevent allergy in children and young people8. Certain species of Bifidobacterium spp. may only be isolated from human breast milk within a few days after birth9. Early intervention through probiotic administration in neonates, especially in neonates born via CS may improve general health, given their susceptibility to various chronic diseases7 as well as potential prevention of chronic inflammatory diseases, such as inflammatory bowel disease, rheumatoid arthritis, coeliac disease and diabetes mellitus later in life10.
Probiotics, in particular Lactobacillus spp. and Bifidobacterium spp., are considered normal flora and part of human gut microbiota7. Lactobacillus spp. and Bifidobacterium spp. are considered generally regarded as safe, especially for oral administration11. In international guidelines such as the FAO/WHO guideline, probiotics are recognised as having a role in maintaining gut health and may modulate host immunity11. In this article, the genomes of Lactobacillus spp. and Bifidobacterium spp. are described along with the mechanisms of action of LAB in interfering against pathogenic bacteria.
Very recently the taxonomy within genus Lactobacillus spp. was re-classified into 25 genera12. As the changes were very recent, and these new genera have not been adopted to the WHO/FAO guideline for probiotics, genus Lactobacillus will be used for this article. It is proposed that genus Lactobacillus of L. casei, L. paracasei and L. rhamnosus as genus Lacticaseibacillus12. L. salivarius and L. fermentum have been named as Ligilactobacillus salivarius and Limosilactobacillus fermentum, respectively12. Genus Lactobacillus of L. acidophilus and L. gasseri have not changed12.
Probiotic use in neonates
There have been extensive studies of the use of probiotics in neonates including preterm infants13–16. In particular, these studies have focussed on the role of probiotics in reducing the incidence of necrotising enterocolitis (NEC) and sepsis. Most significantly, a randomised controlled trial of a symbiotic preparation including L. plantarum in 4500 term neonates in the community resulted in a 42% reduction in neonatal sepsis17.
In addition to its impact on neonatal sepsis, probiotics may reduce gastrointestinal complications in neonates though the evidence is mixed18. The large Probiotics in Preterm Infants Study (PiPS) Trial randomised 1310 pre-term babies to treatment with Bifidobacterium breve BBG-001 or placebo and showed no reduction in rates of sepsis, NEC or death16. In contrast, the ProPrems trial, a randomised-controlled trial that included 1099 preterm infants from Australia and New Zealand demonstrated a reduction of NEC of approximately 50%19. The strains being used in the ProPrems trial were B. infantis, S. thermophilus and B. lactis. A metagenomic approach to characterise the gut microbiota was also used in a sub-study of ProPrems trial, which showed abundance of Bifidobacterium spp. in the infants administered with probiotic15. In neonates, while considered generally safe, cases of Lactobacillus bacteraemia have been reported including in a <1000 g weight pre-term infant following a laparotomy20.
L. plantarum, L. gasseri and L. salivarius have been isolated from infants’ oral and faecal samples21–23. Therefore, these three genera are considered normal infants’ microbiota and warrant further research. Thus far, there is no published research study of using L. gasseri and L. salivarius in neonates. Further, these strains are not commercially available for infants or neonates yet. Research is required to include species not typically in the current formulation of probiotics for neonates. As L. plantarum may reduce atopic dermatitis24, and L. gasseri and L. salivarius may have immunomodulatory effects21,22, these should be considered for inclusion in probiotic formula for neonates. In the potential formulation of the probiotics, Bifidobacterium spp. have been reported as predominant genus in breastmilk microbiome7. Therefore, to add another strain to the formula would need to consider the species proportion in the breastmilk, i.e. with lower CFU than Bifidobacterium spp. Of note, L. plantarum, L. gasseri and L. salivarius are in the commercial formula available for adults.
Probiotic administration has also significantly reduced the length of stay in pre-term infants23. A cost-saving analysis in pre-term infants supplemented with probiotics showed a saving of €2000 per infant25. The clinical impact and cost effectiveness of probiotic administration require further well designed laboratory, clinical and cost analysis research.
Mechanisms of probiotics in interfering with pathogens and immune modulation
Probiotics interfere with pathogens through acid production, hydrogen peroxide production and bacteriocin activity26. Production of bacteriocins, small peptides with anti-bacterial activity of Lactobacillus spp. has been reported to inhibit pathogen growth26. Specific short-chain fatty acids (SCFA) have been studied to understand their beneficial properties, such as butyrate for the antagonistic activity against cancer cells and anti-inflammatory property27. SCFA production by Bifidobacterium spp. in the gastrointestinal tract results in a lower pH and inhibition of potentially pathogenic bacteria28.
Bacterial exopolysaccharide has been known to possess immunostimulatory properties29,30. Extracellular vesicles (EV) in Gram-negative bacteria have been studied for their pathogenicity, host-pathogen interaction and potential targets in vaccine development31. Very recently, studies on EV were performed in probiotic strains and revealed potential delivery of bacteriocins and other beneficial properties through the EV32,33. Advancing research on EV of probiotics is highly recommended as it will provide further understanding on molecular mechanisms of probiotic bactericidal properties against pathogens and immune modulation. Evidence of Bifidobacterium spp. in boosting immune systems has been demonstrated mostly in the mouse model, marked by the stimulation of IL-6 and IL-10 in the ileal Peyer’s patches and in weaned pig model, marked by the increase of IgA against the parasite and IgG34,35.
Genomes of LAB
Genome data provides comprehensive data that might also help to determine the beneficial properties and the potential virulence determinants in the strains. Genome data enable the comparison of the strains with publicly available genome data. We limit the discussion of the genomes to the strains being used commercially in humans. The recent genus Lactobacillus name changes have not impacted the species and genomes, as we abbreviate the genera.
L. rhamnosus GG (LGG) has been the most commercially popular probiotic strain. More than 1100 studies on L. rhamnosus GG were found in NCBI (accessed 22 April 2020). L. plantarum 299v has shown beneficial properties such as effectiveness to treat irritable bowel syndrome36; regardless, only 112 studies on L. plantarum 299v versus 204 studies on L. plantarum WCFS1 were in NCBI. Very few studies were on L. salivarius with 39 studies of L. salivarius UCC118 found from NCBI. As previously described, the L. plantarum WCFS1 genome was first sequenced in the early 2000s and has been well described with its genome of 3 308 273 bp (GenBank accession number NC_004567.2) and a total of nearly 1200 identified proteins. The beneficial properties of L. plantarum WCFS1 include the ability of this strain to survive in a wide range of environments with temperature and pH changes37. The parental strain of L. plantarum WCFS1 is L. plantarum NCIMB 8826, which was isolated from human saliva38. L. plantarum NCIMB 8826 colonises the oral cavity well but not the human intestine, although it has been demonstrated to survive in the gastrointestinal tract, including faeces39. L. gasseri ATCC33323 (Accession Number of NC_008530.1) was the complete reference L. gasseri genome in the NCBI database with its genome of 1 894 360 bp. L. gasseri ATCC33323 is an autochthonous microbe in the gastrointestinal system40. Therefore, oral application of the L. gasseri ATCC33323 for intestinal colonisation may be well tolerated. For a comprehensive genome description of L. salivarius UCC118, the reference strain being used here is available through the study by Claesson and colleagues41. The size of the chromosomal genome of L. salivarius UCC118 was 1 827 111 bp (GenBank accession number: NC_007929.1). General probiotic properties of L. salivarius were the ability to eliminate pathogens and the adaptation to the gastrointestinal niche42. L. salivarius UCC118 has broad spectrum activity versus Gram-positive bacteria43. Therefore, L. salivarius UCC118 has very strong probiotic properties and is autochthonous to the gastrointestinal tract.
Genomes of Bifidobacterium spp. have also been described, i.e. B. longum (n = 349), B. breve (n = 109), B. bifidum (n = 104) and B. animalis (n = 83) (from NCBI, accessed 2 March 2020). B. longum NCC2705, B. breve DSM 20213, B. bifidum PRL2010 and B. animalis subsp. lactis DSM 10140 are the reference genomes in NCBI with genome sizes of 2.257, 2.257, 2.215 and 1.938 Mb, respectively (GenBank Accession Numbers: NC_004307.2, NZ_JDUD000000000.1, NC_014638.1 and CP001606.1, respectively).
Current evidence
Probiotic supplementation in neonates has been frequently studied. In an era of interventional birth leading to high rates of CS, probiotics may have a role in establishing a healthy gut microbiome. The impact of probiotics in this setting may include a reduction in important acute complications such as neonatal sepsis, and NEC and longer-term impacts relating to the development of mucosal immunity and atopy. The heterogeneity of trial results may relate to the differing strains used. Genomic and metagenomics approaches to analysing the gut microbiome may improve understanding of gut dysbiosis and its role in these complications.
L. rhamnosus, L. casei, L. acidophilus, L. plantarum, L. gasseri and L. salivarius are listed in the three main regulatory bodies in European Food Safety Authority (EFSA), Canada and China as strains can be added in food44, which may broaden the use of these strains as human food supplement in countries outside Europe, e.g. China and Canada. Europe has been the epicentre for probiotic development and generation so far. EFSA allowed 37 different Lactobacillus spp. for consumption through food44. Therefore, supplementation of Lactobacillus spp. and Bifidobacterium spp. to infants and neonates can be categorised as natural administration of beneficial microbes or probiotics to maintain gut microbiota and immune systems45.
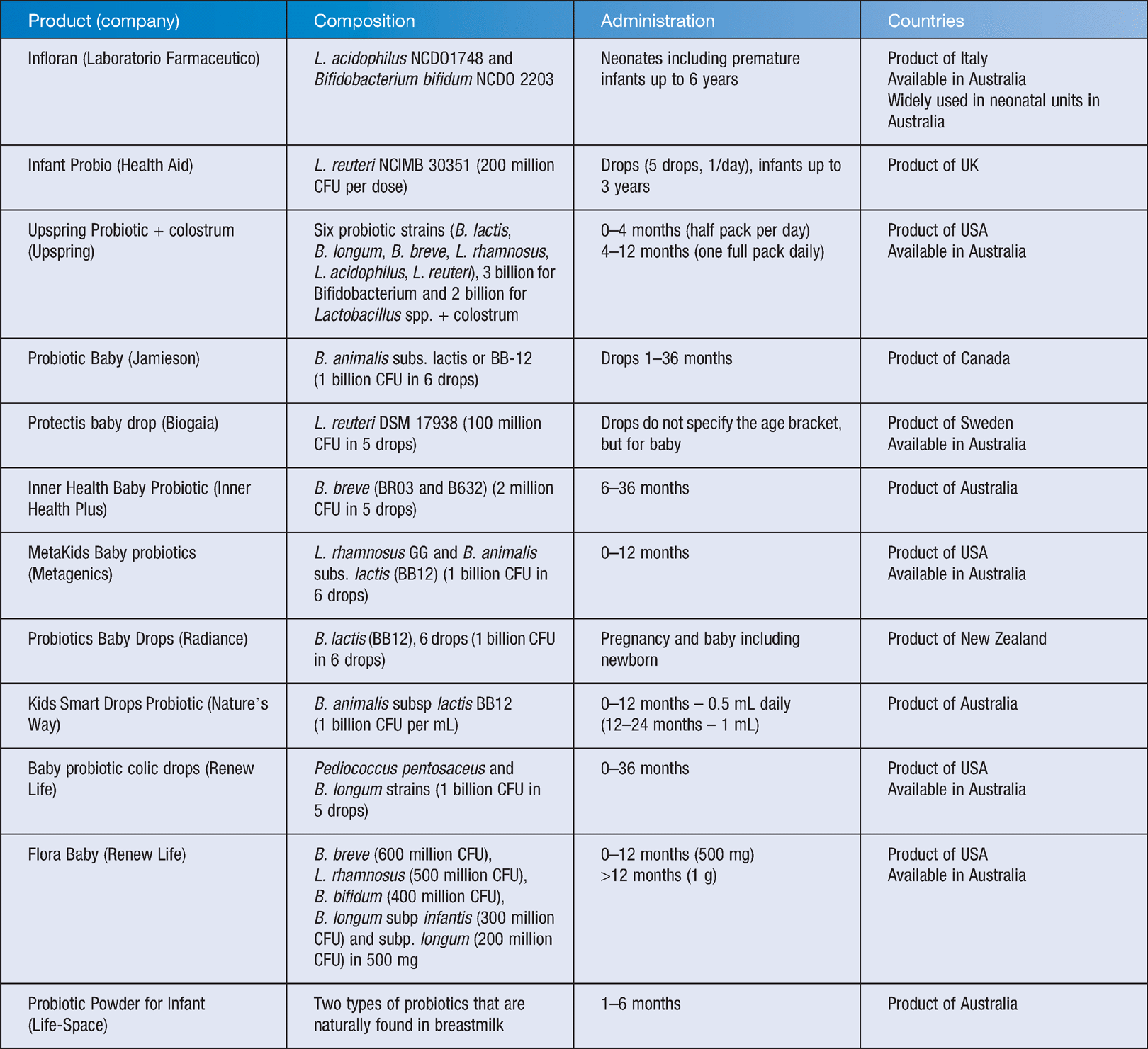
Infloran containing Bifidobacterium bifidum and Lactobacillus acidophilus is a commercial probiotic widely used in neonatal units in Australia. Other probiotics available in pharmacies are listed in Table 1. Many commercial preparations are not included in the table due to a lack of published data on the strain identity and CFU counts. Guidelines in choosing the right probiotics are available from International Scientific Association for Probiotics and Prebiotics website (https://isappscience.org/). Industry-related probiotic information can be found from the International Probiotic Association website (http://internationalprobiotics.org/). As probiotic administration is now becoming broader than oral administration, the use of the food-medicine interface guidance tool within Therapeutic Goods Australia (https://www.tga.gov.au/) is highly recommended in translating probiotic research to industry.
|
In summary, with the increasing evidence of CS births in Australia and worldwide, and antibiotic prophylaxis administration in CS births, as well as the potential delay of the breastfeeding initiation, it would be highly recommended to provide probiotics those commonly isolated from breastmilk, to CS born neonates. Probiotic administration mimicking the LAB of breastmilk will be likely a better option than inoculation of swabs originated from vagina, often called seeding. Future studies that include microbiome analysis, neurocognitive development as well as economic analysis of probiotic administrations are highly recommended.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This research did not receive any specific funding.
References
[1] Australian Institute of Health and Welfare (2019) Australia’s mothers and babies 2017 – in brief. Perinatal statistics series no. 35. Canberra: AIHW.[2] Pinto-Lopes, R. et al. (2017) Single dose versus multiple dose of antibiotic prophylaxis in caesarean section: a systematic review and meta-analysis. BJOG 124, 595–605.
| Single dose versus multiple dose of antibiotic prophylaxis in caesarean section: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar | 27885778PubMed |
[3] O’Neill J. (2014) The Review on Antimicrobial Resistance, Chaired by Jim O’Neill. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. 1–20.
[4] Wu, Y. et al. (2018) The association between caesarean delivery and the initiation and duration of breastfeeding: a prospective cohort study in China. Eur. J. Clin. Nutr. 72, 1644–1654.
| The association between caesarean delivery and the initiation and duration of breastfeeding: a prospective cohort study in China.Crossref | GoogleScholarGoogle Scholar | 29670258PubMed |
[5] Biagi, E. et al. (2017) The bacterial ecosystem of mother’s milk and infant’s mouth and gut. Front. Microbiol. 8, 1214.
| The bacterial ecosystem of mother’s milk and infant’s mouth and gut.Crossref | GoogleScholarGoogle Scholar | 28713343PubMed |
[6] O’Toole, P.W. et al. (2017) Next-generation probiotics: the spectrum from probiotics to live biotherapeutics. Nat. Microbiol. 2, 17057.
| Next-generation probiotics: the spectrum from probiotics to live biotherapeutics.Crossref | GoogleScholarGoogle Scholar | 28440276PubMed |
[7] Milani, C. et al. (2017) The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiol. Mol. Biol. Rev. 81, e00036-17.
| The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota.Crossref | GoogleScholarGoogle Scholar | 29118049PubMed |
[8] Kallio, S. et al. (2019) Perinatal probiotic intervention prevented allergic disease in a Caesarean-delivered subgroup at 13-year follow-up. Clin. Exp. Allergy 49, 506–515.
| Perinatal probiotic intervention prevented allergic disease in a Caesarean-delivered subgroup at 13-year follow-up.Crossref | GoogleScholarGoogle Scholar | 30472801PubMed |
[9] Moossavi, S et al. (2019) Composition and variation of the human milk microbiota are influenced by maternal and early-life factors. Cell Host Microbe. 25, 324–335.e4.
| Composition and variation of the human milk microbiota are influenced by maternal and early-life factors.Crossref | GoogleScholarGoogle Scholar | 30763539PubMed |
[10] Andersen, V. et al. (2020) Caesarean delivery and risk of chronic inflammatory diseases (inflammatory bowel disease, rheumatoid arthritis, coeliac disease, and diabetes mellitus): a population based registry study of 2,699,479 births in Denmark during 1973–2016. Clin. Epidemiol. 12, 287–293.
| Caesarean delivery and risk of chronic inflammatory diseases (inflammatory bowel disease, rheumatoid arthritis, coeliac disease, and diabetes mellitus): a population based registry study of 2,699,479 births in Denmark during 1973–2016.Crossref | GoogleScholarGoogle Scholar | 32210632PubMed |
[11] Food and Agriculture Organization of the United Nations, World Health Organization (2006) Probiotics in food: health and nutritional properties and guidelines for evaluation. Rome: Food and Agriculture Organization of the United Nations, World Health Organization.
[12] Zheng, J. et al. (2020) A taxonomic note on the genus Lactobacillus: description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 70, 2782–2858.
| A taxonomic note on the genus Lactobacillus: description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae.Crossref | GoogleScholarGoogle Scholar |
[13] Bi, L.W. et al. (2019) Probiotic strategies to prevent necrotizing enterocolitis in preterm infants: a meta-analysis. Pediatr. Surg. Int. 35, 1143–1162.
| Probiotic strategies to prevent necrotizing enterocolitis in preterm infants: a meta-analysis.Crossref | GoogleScholarGoogle Scholar | 31420743PubMed |
[14] Athalye-Jape, G. and Patole, S. (2019) Probiotics for preterm infants – time to end all controversies. Microb. Biotechnol. 12, 249–253.
| Probiotics for preterm infants – time to end all controversies.Crossref | GoogleScholarGoogle Scholar | 30637944PubMed |
[15] Plummer, E.L. et al. (2018) Gut microbiota of preterm infants supplemented with probiotics: sub-study of the ProPrems trial. BMC Microbiol. 18, 184.
| Gut microbiota of preterm infants supplemented with probiotics: sub-study of the ProPrems trial.Crossref | GoogleScholarGoogle Scholar | 30424728PubMed |
[16] Costeloe, K. et al. (2016) A randomised controlled trial of the probiotic Bifidobacterium breve BBG-001 in preterm babies to prevent sepsis, necrotising enterocolitis and death: the Probiotics in Preterm infantS (PiPS) trial. Health Technol. Assess. 20, 1–194.
| A randomised controlled trial of the probiotic Bifidobacterium breve BBG-001 in preterm babies to prevent sepsis, necrotising enterocolitis and death: the Probiotics in Preterm infantS (PiPS) trial.Crossref | GoogleScholarGoogle Scholar | 27594381PubMed |
[17] Panigrahi, P. et al. (2017) A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature 548, 407–412.
| A randomized synbiotic trial to prevent sepsis among infants in rural India.Crossref | GoogleScholarGoogle Scholar | 28813414PubMed |
[18] Indrio, F. et al. (2008) The effects of probiotics on feeding tolerance, bowel habits, and gastrointestinal motility in preterm newborns. J. Pediatr. 152, 801–806.
| The effects of probiotics on feeding tolerance, bowel habits, and gastrointestinal motility in preterm newborns.Crossref | GoogleScholarGoogle Scholar | 18492520PubMed |
[19] Jacobs, S.E. et al. (2013) Probiotic effects on late-onset sepsis in very preterm infants: a randomized controlled trial. Pediatrics 132, 1055–1062.
| Probiotic effects on late-onset sepsis in very preterm infants: a randomized controlled trial.Crossref | GoogleScholarGoogle Scholar | 24249817PubMed |
[20] Brecht, M. et al. (2016) Lactobacillus sepsis following a laparotomy in a preterm infant: a note of caution. Neonatology 109, 186–189.
| Lactobacillus sepsis following a laparotomy in a preterm infant: a note of caution.Crossref | GoogleScholarGoogle Scholar | 26780534PubMed |
[21] Holowacz, S. et al. (2018) Lactobacillus salivarius LA307 and Lactobacillus rhamnosus LA305 attenuate skin inflammation in mice. Benef. Microbes 9, 299–309.
| Lactobacillus salivarius LA307 and Lactobacillus rhamnosus LA305 attenuate skin inflammation in mice.Crossref | GoogleScholarGoogle Scholar | 29409331PubMed |
[22] Hsieh, M.H. et al. (2018) Lactobacillus gasseri attenuates allergic airway inflammation through PPARγ activation in dendritic cells. J. Mol. Med. 96, 39–51.
| Lactobacillus gasseri attenuates allergic airway inflammation through PPARγ activation in dendritic cells.Crossref | GoogleScholarGoogle Scholar | 29032406PubMed |
[23] Rao, S.C. et al. (2016) Probiotic supplementation and late-onset sepsis in preterm infants: a meta-analysis. Pediatrics 137, e20153684.
| Probiotic supplementation and late-onset sepsis in preterm infants: a meta-analysis.Crossref | GoogleScholarGoogle Scholar | 26908700PubMed |
[24] Prakoeswa, C.R.S. et al. (2017) Lactobacillus plantarum IS-10506 supplementation reduced SCORAD in children with atopic dermatitis. Benef. Microbes 8, 833–840.
| Lactobacillus plantarum IS-10506 supplementation reduced SCORAD in children with atopic dermatitis.Crossref | GoogleScholarGoogle Scholar |
[25] Indrio, F. et al. (2017) Probiotic supplementation in preterm: feeding intolerance and hospital cost. Nutrients 9, 965.
| Probiotic supplementation in preterm: feeding intolerance and hospital cost.Crossref | GoogleScholarGoogle Scholar |
[26] Plaza-Diaz, J, Ruiz-Ojeda, FJ, Gil-Campos, M and Gil, A (2019) Mechanisms of action of probiotics. Adv. Nutr. 10, S49–S66.
| Mechanisms of action of probiotics.Crossref | GoogleScholarGoogle Scholar | 30721959PubMed |
[27] Park, S. et al. (2018) Cholesterol-lowering effect of Lactobacillus rhamnosus BFE5264 and its influence on the gut microbiome and propionate level in a murine model. PLoS One 13, e0203150.
| Cholesterol-lowering effect of Lactobacillus rhamnosus BFE5264 and its influence on the gut microbiome and propionate level in a murine model.Crossref | GoogleScholarGoogle Scholar | 30586360PubMed |
[28] O’Callaghan, A. and van Sinderen, D. (2016) Bifidobacteria and their role as members of the human gut microbiota. Front. Microbiol. 7, 925.
| Bifidobacteria and their role as members of the human gut microbiota.Crossref | GoogleScholarGoogle Scholar | 27379055PubMed |
[29] Xu, Y. et al. (2019) Purification, characterization and bioactivity of exopolysaccharides produced by Lactobacillus plantarum KX041. Int. J. Biol. Macromol. 128, 480–492.
| Purification, characterization and bioactivity of exopolysaccharides produced by Lactobacillus plantarum KX041.Crossref | GoogleScholarGoogle Scholar | 30682478PubMed |
[30] Castro-Bravo, N. et al. (2018) Interactions of surface exopolysaccharides from Bifidobacterium and Lactobacillus within the intestinal environment. Front. Microbiol. 9, 2426.
| Interactions of surface exopolysaccharides from Bifidobacterium and Lactobacillus within the intestinal environment.Crossref | GoogleScholarGoogle Scholar | 30364185PubMed |
[31] Turner, L. et al. (2018) Helicobacter pylori outer membrane vesicle size determines their mechanisms of host cell entry and protein content. Front. Immunol. 9, 1466.
| Helicobacter pylori outer membrane vesicle size determines their mechanisms of host cell entry and protein content.Crossref | GoogleScholarGoogle Scholar | 30013553PubMed |
[32] Liu, Y. et al. (2018) Gram-positive bacterial extracellular vesicles and their impact on health and disease. Front. Microbiol. 9, 1502.
| Gram-positive bacterial extracellular vesicles and their impact on health and disease.Crossref | GoogleScholarGoogle Scholar | 30038605PubMed |
[33] Liu, Y. et al. (2019) Delivery of genome editing tools by bacterial extracellular vesicles. Microb. Biotechnol. 12, 71–73.
| 30549228PubMed |
[34] Solano-Aguilar, G. et al. (2018) Bifidobacterium animalis subspecies lactis modulates the local immune response and glucose uptake in the small intestine of juvenile pigs infected with the parasitic nematode Ascaris suum. Gut Microbes 9, 422–436.
| Bifidobacterium animalis subspecies lactis modulates the local immune response and glucose uptake in the small intestine of juvenile pigs infected with the parasitic nematode Ascaris suum.Crossref | GoogleScholarGoogle Scholar | 30024817PubMed |
[35] Makioka, Y. et al. (2018) Oral supplementation of Bifidobacterium longum strain BR-108 alters cecal microbiota by stimulating gut immune system in mice irrespectively of viability. Biosci. Biotechnol. Biochem. 82, 1180–1187.
| Oral supplementation of Bifidobacterium longum strain BR-108 alters cecal microbiota by stimulating gut immune system in mice irrespectively of viability.Crossref | GoogleScholarGoogle Scholar | 29557273PubMed |
[36] Ducrotté, P. et al. (2012) Clinical trial: Lactobacillus plantarum 299v (DSM 9843) improves symptoms of irritable bowel syndrome. World J. Gastroenterol. 18, 4012–4018.
| Clinical trial: Lactobacillus plantarum 299v (DSM 9843) improves symptoms of irritable bowel syndrome.Crossref | GoogleScholarGoogle Scholar | 22912552PubMed |
[37] Huang, Y. and Tanaka, T. (2015) Characterization of two putative prolinases (PepR1 and PepR2) from Lactobacillus plantarum WCFS1: occurrence of two isozymes with structural similarity and different catalytic properties. Biochim. Biophys. Acta 1854, 91–100.
| Characterization of two putative prolinases (PepR1 and PepR2) from Lactobacillus plantarum WCFS1: occurrence of two isozymes with structural similarity and different catalytic properties.Crossref | GoogleScholarGoogle Scholar | 25463046PubMed |
[38] van den Nieuwboer, M. et al. (2016) Lactobacillus plantarum WCFS1 and its host interaction: a dozen years after the genome. Microb. Biotechnol. 9, 452–465.
| Lactobacillus plantarum WCFS1 and its host interaction: a dozen years after the genome.Crossref | GoogleScholarGoogle Scholar | 27231133PubMed |
[39] van Bokhorst-van de Veen, H. et al. (2012) Modulation of Lactobacillus plantarum gastrointestinal robustness by fermentation conditions enables identification of bacterial robustness markers. PLoS One 7, e39053.
| Modulation of Lactobacillus plantarum gastrointestinal robustness by fermentation conditions enables identification of bacterial robustness markers.Crossref | GoogleScholarGoogle Scholar | 22970257PubMed |
[40] Azcarate-Peril, M.A. et al. (2008) Analysis of the genome sequence of Lactobacillus gasseri ATCC 33323 reveals the molecular basis of an autochthonous intestinal organism. Appl. Environ. Microbiol. 74, 4610–4625.
| Analysis of the genome sequence of Lactobacillus gasseri ATCC 33323 reveals the molecular basis of an autochthonous intestinal organism.Crossref | GoogleScholarGoogle Scholar | 18539810PubMed |
[41] Claesson, M.J. et al. (2006) Multireplicon genome architecture of Lactobacillus salivarius. Proc. Natl. Acad. Sci. USA 103, 6718–6723.
| Multireplicon genome architecture of Lactobacillus salivarius.Crossref | GoogleScholarGoogle Scholar | 16617113PubMed |
[42] Neville, B.A. and O’Toole, P.W. (2010) Probiotic properties of Lactobacillus salivarius and closely related Lactobacillus species. Future Microbiol. 5, 759–774.
| Probiotic properties of Lactobacillus salivarius and closely related Lactobacillus species.Crossref | GoogleScholarGoogle Scholar | 20441548PubMed |
[43] Flynn, S. et al. (2002) Characterization of the genetic locus responsible for the production of ABP-118, a novel bacteriocin produced by the probiotic bacterium Lactobacillus salivarius subsp. salivarius UCC118. Microbiology 148, 973–984.
| Characterization of the genetic locus responsible for the production of ABP-118, a novel bacteriocin produced by the probiotic bacterium Lactobacillus salivarius subsp. salivarius UCC118.Crossref | GoogleScholarGoogle Scholar | 11932444PubMed |
[44] Zhang, W. and Zhang, H. (2014) Genomics of lactic acid bacteria. In: Zhang H., Cai Y., eds. Lactic Acid Bacteria: Fundamentals and Practice. Dordrecht: Springer Netherlands. pp. 205-247.
[45] Simpson, M.R. et al. (2018) Breastfeeding-associated microbiota in human milk following supplementation with Lactobacillus rhamnosus GG, Lactobacillus acidophilus La-5, and Bifidobacterium animalis ssp. lactis Bb-12. J. Dairy Sci. 101, 889–899.
| Breastfeeding-associated microbiota in human milk following supplementation with Lactobacillus rhamnosus GG, Lactobacillus acidophilus La-5, and Bifidobacterium animalis ssp. lactis Bb-12.Crossref | GoogleScholarGoogle Scholar | 29248229PubMed |
Biographies
Dr Hanna E Sidjabat is a molecular microbiologist with a strong industry link in translating her probiotic research to manufacturing. In addition to her probiotic expertise, she has a solid background in antibiotic resistance mechanisms including genome and proteome due to 15 years of research experience. She has strong research focus in the bacterial genome, proteome of pathogens and probiotics. To date, Dr Sidjabat has published 87 peer-reviewed articles in international journals. Dr Sidjabat has supervised and mentored 35 PhD students, Postdoctoral Research Fellows, Master and Honours students, Microbiology Registrars, local and international Infectious Diseases Visiting Academics following the completion of her PhD in 2007.
Alaa Mohammed Ali Alsaggaf is a Master graduate in molecular microbiology from the University of Queensland, School of Chemistry and Molecular Biosciences. Alaa has worked extensively on the genome of Lactobacillus spp. within Sidjabat’s team at the University of Queensland Centre for Clinical Research (UQCCR). She has clinical microbiology role in Saudi Arabia within Ministry of Health. She received the UQ SCMB Dean’s award for her project in the second semester of 2018.
Akshatha Gopalakrishna has a Masters degree in molecular biology research and is equipped with extensive microbiology and biochemistry laboratory skills from the University of Queensland, School of Chemistry and Molecular Biosciences. She has worked extensively on Lactobacillus spp. for screening of probiotic strains within Sidjabat’s team at the UQCCR. She has further extended her skills on histology, immunohistochemistry staining, various microscopic skills and sample preparation for MRI at the UQ Centre for Advanced Imaging.
Evelyn Nadar is a graduate of Master’s in molecular biology research extensive with extensive laboratory skills from the University of Queensland, School of Chemistry and Molecular Biosciences. She has worked extensively on Lactobacillus spp. proteomic analysis of probiotic research within Sidjabat’s team at the UQCCR. She has also expanded her animal handling skills and histology including microscopy skills.
Dr Adam Irwin is a conjoint Senior Lecturer and academic lead for Paediatric Infectious Disease at Children’s Health Queensland and the University of Queensland. His research focuses on diagnostic evaluations to optimise the use of antimicrobials in children. Specifically, he is interested in healthcare-associated infections and infections resistant to antimicrobial therapy. He is also interested in the clinical and molecular epidemiology of invasive Gram-negative infections. Dr Irwin studied at the University of Birmingham Medical School and completed training in Paediatric Infectious Disease and Immunology in London. He was awarded his PhD by the University of Liverpool Institute of Infection and Global Health in 2016.
Dr Pieter Koorts is the Director of Neonatology Royal Brisbane and Women’s Hospital since 2016, Acting Director of Neonatology RBWH (2015–2016), Deputy Director of Neonatology RBWH (2009–2015), Staff Specialist Neonatologist RBWH (2007–2009). Dr Koorts has affiliation as a Senior Lecturer with School of Medicine, University of Queensland since 2007. Dr Koorts started his career in paediatrics in 1998 and into neonatology as a Senior Lecturer and Senior Staff Neonatologist (2005–2006) at Pretoria University, South Africa. Dr Koorts was a neonatal fellow at Mercy Hospital for Women, Melbourne, in 2002–2005.