Faecal microbiota transplantation: is it the future for pig production?
Tanya L Nowland A B and Roy N Kirkwood AA School of Animal and Veterinary Sciences, The University of Adelaide, Roseworthy, SA 5400, Australia
B Tel.: +61 8 8313 7664, Email: tanya.nowland@adelaide.edu.au
Microbiology Australia 41(2) 91-94 https://doi.org/10.1071/MA20023
Published: 5 May 2020
Abstract
Piglet mortality is a major issue for the pork industry globally and until recently, the main method for improving growth performance and reducing disease in commercial practice is centred on anti-microbial use. Antibiotic resistance is a global concern and, as such, animal production industries are seeking alternatives to antibiotics. Different approaches under investigation include but are not limited to management of the intestinal microbial environment. The gastrointestinal microbiota is involved in a myriad of processes that impact host health and well-being. Recently, interest in maintaining a healthy microbiome in order to improve herd health is increasing. In this article, we focus on faecal microbiota transplantation as a method for manipulating and improving the gastrointestinal microbiota in pigs in order to improve health and performance.
Currently, 11–15% of all piglets born alive die prior to weaning within the pork industry globally1–3. This represents a major welfare concern and economic loss to industry. To date, much research has gone into reducing this loss but with varied success. The current management methods for reducing piglet mortality caused by sickness, such as diarrhoea, and improving growth performance in weaned pigs, is the administration of antibiotics, with their use often being both therapeutic and prophylactic. Organisations such as the World Health Organization, the US Centres for Disease Control and Prevention, and the European Centre for Disease Prevention and Control have identified antibiotic resistance as a global concern, as what were once common treatable infections are now becoming life threatening4. As such, alternatives to antibiotics need to be explored.
The intestinal tract houses a community of microorganisms that has a mutualistic relationship with the host, known as the enteric microbiome5. These microorganisms include bacteria, fungi, archaea, protozoa and viruses6–8. The enteric microbiome is involved in a myriad of processes, some of which include immune system maintenance and development, intestinal barrier function, nutrient metabolism and competitive exclusion of pathogens8–10. While antibiotics are effective in pathogen removal, they also impact the commensal microbiome11. If a healthy microbiome is maintained, the need for therapeutic interventions such as antibiotic administration will be reduced as the animal will be better equipped to cope with external stressors. This is where the interest surrounding methods for influencing the microbiome, through management such as pre- and pro-biotics and faecal microbiota transfers, has expanded.
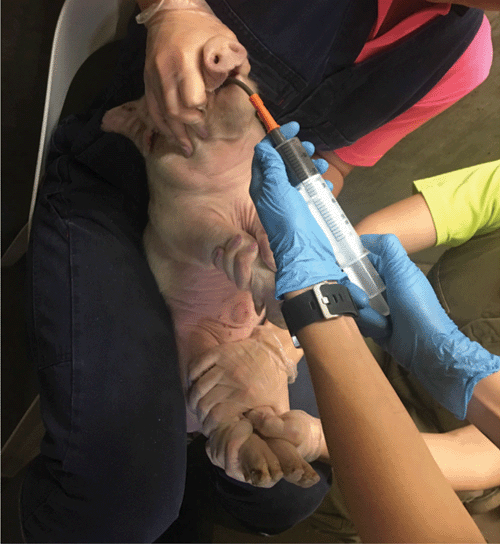
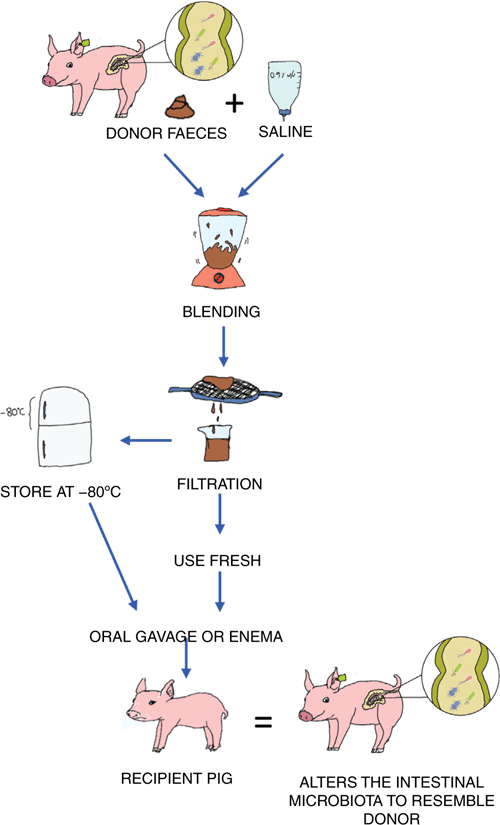
In particular, one such method that has demonstrated efficacy in treating Clostridium difficile infections in humans is faecal microbiota transplantation (FMT). FMT involves the transfer of faeces from a healthy donor into the gastrointestinal tract of a recipient. This can be done either orally (Figure 1) or rectally via an enema12. The objective being that the beneficial bacteria within the healthy donors’ faeces will competitively exclude the pathogenic bacteria within the unhealthy or sick recipient, therefore altering the microbiota and in the case of C. difficile infections, treating the disease12 (Figure 2). This method can also be used for altering the microbiota of the recipient to resemble that of the donor for the objective of creating a phenotypic change13. FMT was first described by Ge Hong in 4th century China for the treatment of food poisoning and severe diarrhoea12. Today, FMT is commonly known for its efficacy for the treatment of C. difficile infections in humans. FMT has demonstrated a success rate of >90% in patients with reoccurring C. difficile where antibiotic use has been unsuccessful due to the formation of spores14. The use of FMT in other areas of human health and disease prevention are becoming increasingly popular; however, its efficacy in treating other diseases in humans to date is not as high. Although this is the case, investigation into its use within production animals such as pigs is increasing.
|
|
Recent studies investigating its use in pig production have shown promising but inconsistent results. Several research groups have demonstrated that the administration of multiple oral FMT to piglets from birth can increase average daily gain, reduce the incidence of diarrhoea and improve intestinal barrier and immune system function15–18. However, in contrast to this, others demonstrated a negative effect on intestinal integrity and growth when piglets received FMT directly or were reared on sows receiving FMT19,20. When examining the human literature, where additional phenotypic traits were transferred with FMT that mimicked the donor, it is evident that the donor used can significantly impact the results observed21. As such, particular care needs to be taken when selecting the appropriate donor as the risk of transferring undesirable traits is high. Further, Niederwerder et al.22 found that FMT was an effective preventative effect against porcine circovirus associated disease in pigs co-infected with porcine circovirus type-2 and porcine reproductive and respiratory syndrome virus. The pigs that received one dose of FMT daily for seven days following weaning from healthy donor sows had a significant reduction in morbidity and mortality and increased antibody levels.
Studies where FMT in pigs was employed as a research model for humans have also found promising results that not only provide evidence for its effects on enteric microbiota modulation but also host metabolism. Wan et al.23 demonstrated that oral FMT from 1 to 6 days of age reduced fatty acid oxidative catabolism and amino acid biosynthesis of piglets. Additionally, Brunse et al.24 observed that rectal FMT from 10-day-old donor pigs to caesarean-derived pre-term piglets changed their colonic carbohydrate metabolism from lactate to propionate production, increasing colonic pH. Rectal FMT also preserved goblet cell mucin stores and reduced the incidence of necrotizing enterocolitis. When comparing routes for FMT, it has been noted that when combining oral and rectal transplantation, piglet mortality increased. Conversely, those that received only rectal administration did not suffer the same problems24. Further supporting the findings of the previous studies, Geng et al.25 demonstrated that FMT reduced susceptibility to epithelial injury and modulated tryptophan metabolism in a piglet inflammatory bowel disease model. When taken collectively, it is evident that FMT in pigs not only alters microbial membership but also has effects on host metabolism, intestinal barrier function and the immune system.
Although FMT is a promising prospect it is not commercially applicable in its current form, with most studies administering multiple doses for 1–2 weeks in order to demonstrate an effect and fasting or stomach acid reduction protocols in place to improve post-gastric bacterial survival. Recently, our research group identified that the administration of a single FMT dose at weaning resulted in durable changes to 35 days of age (14 days post FMT) (T L Nowland et al., unpubl. data). To our knowledge, this is the first study to demonstrate changes to the microbiome of piglets after a single dose of FMT. However, whether this is possible in a younger pig and whether it lasts long term is yet to be determined. Additionally, some scepticism surrounds the use of FMT commercially due to the biosecurity risk that it entails as rigorous testing is needed in order to prevent the transfer of diseases13. If FMT is being considered in pigs for the treatment of a disease, then it is likely that the recipients are sick and probably relatively immunocompromised. Thus, the risk from possible transfer of pathogens will be increased. However, a possible refinement to FMT to minimise this risk is suggested by the work of Hu et al.18. These authors used a native Chinese pig breed with increased resistance to stress-induced diarrhoea to determine the identity of specific bacteria involved in this resistance. Such a targeted approach to disease control would have a major advantage over the ‘shot gun’ approach of conventional FMT. It is evident that research surrounding the use of FMT within pig production is still in its infancy. Although, an increasing number of studies are investigating the use of FMT as a tool for increasing growth, feed efficiency and treating enteric diseases in pigs, there is still a long way to go before it will be applicable to industry.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors acknowledge the contributions to the project by Professor Mary Barton and Sophia Ward and the University of Adelaide and Australian Pork Limited for their support of this research.
References
[1] Daigle, C. (2018) Parallels between postpartum disorders in humans and preweaning piglet mortality in sows. Animals (Basel) 8, 22.| Parallels between postpartum disorders in humans and preweaning piglet mortality in sows.Crossref | GoogleScholarGoogle Scholar |
[2] Mota-Rojas, D. et al. (2012) Animal welfare in the newborn piglet: a review. Vet. Med. 57, 338–349.
| Animal welfare in the newborn piglet: a review.Crossref | GoogleScholarGoogle Scholar |
[3] Nuntapaitoon, M. et al. (2018) Newborn traits associated with pre-weaning growth and survival in piglets. Asian-Australas. J. Anim. Sci. 31, 237–244.
| Newborn traits associated with pre-weaning growth and survival in piglets.Crossref | GoogleScholarGoogle Scholar | 28728403PubMed |
[4] Roca, I. et al. (2015) The global threat of antimicrobial resistance: science for intervention. New Microbes New Infect. 6, 22–29.
| The global threat of antimicrobial resistance: science for intervention.Crossref | GoogleScholarGoogle Scholar | 26029375PubMed |
[5] Young, V.B. (2017) The role of the microbiome in human health and disease: an introduction for clinicians. BMJ 356, j831.
| The role of the microbiome in human health and disease: an introduction for clinicians.Crossref | GoogleScholarGoogle Scholar | 28298355PubMed |
[6] Abeles, S.R. and Pride, D.T. (2014) Molecular bases and role of viruses in the human microbiome. J. Mol. Biol. 426, 3892–3906.
| Molecular bases and role of viruses in the human microbiome.Crossref | GoogleScholarGoogle Scholar | 25020228PubMed |
[7] Hallen-Adams, H.E. and Suhr, M.J. (2017) Fungi in the healthy human gastrointestinal tract. Virulence 8, 352–358.
| Fungi in the healthy human gastrointestinal tract.Crossref | GoogleScholarGoogle Scholar | 27736307PubMed |
[8] Cahenzli, J. et al. (2013) Intestinal microbial diversity during early-life colonization shapes long-term IgE levels. Cell Host Microbe 14, 559–570.
| Intestinal microbial diversity during early-life colonization shapes long-term IgE levels.Crossref | GoogleScholarGoogle Scholar | 24237701PubMed |
[9] Gensollen, T. et al. (2016) How colonization by microbiota in early life shapes the immune system. Science 352, 539–544.
| How colonization by microbiota in early life shapes the immune system.Crossref | GoogleScholarGoogle Scholar | 27126036PubMed |
[10] Wang, M. et al. (eds) (2016) Impact of early gut microbiota on immune and metabolic development and function. Seminars in Fetal and Neonatal Medicine.
[11] Looft, T. et al. (2014) Bacteria, phages and pigs: the effects of in-feed antibiotics on the microbiome at different gut locations. ISME J. 8, 1566–1576.
| Bacteria, phages and pigs: the effects of in-feed antibiotics on the microbiome at different gut locations.Crossref | GoogleScholarGoogle Scholar | 24522263PubMed |
[12] Brandt, L.J. and Aroniadis, O.C. (2013) An overview of fecal microbiota transplantation: techniques, indications, and outcomes. Gastrointest. Endosc. 78, 240–249.
| An overview of fecal microbiota transplantation: techniques, indications, and outcomes.Crossref | GoogleScholarGoogle Scholar | 23642791PubMed |
[13] Canibe, N. et al. (2019) Potential relevance of pig gut content transplantation for production and research. J. Anim. Sci. Biotechnol. 10, 55.
| Potential relevance of pig gut content transplantation for production and research.Crossref | GoogleScholarGoogle Scholar | 31304012PubMed |
[14] Bakken, J.S. et al. (2011) Treating clostridium difficile infection with fecal microbiota transplantation. Clin. Gastroenterol. Hepatol. 9, 1044–1049.
| Treating clostridium difficile infection with fecal microbiota transplantation.Crossref | GoogleScholarGoogle Scholar | 21871249PubMed |
[15] Cheng, C.S. et al. (2019) Early intervention with faecal microbiota transplantation: an effective means to improve growth performance and the intestinal development of suckling piglets. Animal 13, 533–541.
| Early intervention with faecal microbiota transplantation: an effective means to improve growth performance and the intestinal development of suckling piglets.Crossref | GoogleScholarGoogle Scholar | 29983136PubMed |
[16] Hu, L. et al. (2018) Exogenous fecal microbiota transplantation from local adult pigs to crossbred newborn piglets. Front. Microbiol. 8, 2663.
| Exogenous fecal microbiota transplantation from local adult pigs to crossbred newborn piglets.Crossref | GoogleScholarGoogle Scholar | 29375527PubMed |
[17] Xiao, Y. et al. (2017) Early gut microbiota intervention suppresses DSS-induced inflammatory responses by deactivating TLR/NLR signalling in pigs. Sci. Rep. 7, 3224.
| Early gut microbiota intervention suppresses DSS-induced inflammatory responses by deactivating TLR/NLR signalling in pigs.Crossref | GoogleScholarGoogle Scholar | 28607413PubMed |
[18] Hu, J. et al. (2018) A microbiota-derived bacteriocin targets the host to confer diarrhea resistance in early-weaned piglets. Cell Host Microbe 24, 817–832.e8.
| A microbiota-derived bacteriocin targets the host to confer diarrhea resistance in early-weaned piglets.Crossref | GoogleScholarGoogle Scholar | 30543777PubMed |
[19] McCormack, U.M. et al. (2018) Fecal microbiota transplantation in gestating sows and neonatal offspring alters lifetime intestinal microbiota and growth in offspring. mSystems 3, e00134-17.
| Fecal microbiota transplantation in gestating sows and neonatal offspring alters lifetime intestinal microbiota and growth in offspring.Crossref | GoogleScholarGoogle Scholar | 29577087PubMed |
[20] McCormack, U.M. et al. (2019) Improvement of feed efficiency in pigs through microbial modulation via fecal microbiota transplantation in sows and dietary supplementation of inulin in offspring. Appl. Environ. Microbiol. 85, e01255-19.
| Improvement of feed efficiency in pigs through microbial modulation via fecal microbiota transplantation in sows and dietary supplementation of inulin in offspring.Crossref | GoogleScholarGoogle Scholar | 31519656PubMed |
[21] Maruvada, P. et al. (2017) The human microbiome and obesity: moving beyond associations. Cell Host Microbe 22, 589–599.
| The human microbiome and obesity: moving beyond associations.Crossref | GoogleScholarGoogle Scholar | 29120742PubMed |
[22] Niederwerder, M.C. et al. (2018) Fecal microbiota transplantation is associated with reduced morbidity and mortality in porcine circovirus associated disease. Front. Microbiol. 9, 1631.
| Fecal microbiota transplantation is associated with reduced morbidity and mortality in porcine circovirus associated disease.Crossref | GoogleScholarGoogle Scholar | 30083142PubMed |
[23] Wan, J.J. et al. (2019) Effects of early intervention with maternal fecal bacteria and antibiotics on liver metabolome and transcription in neonatal pigs. Front. Physiol. 10, 171.
| Effects of early intervention with maternal fecal bacteria and antibiotics on liver metabolome and transcription in neonatal pigs.Crossref | GoogleScholarGoogle Scholar | 30890952PubMed |
[24] Brunse, A. et al. (2019) Effect of fecal microbiota transplantation route of administration on gut colonization and host response in preterm pigs. ISME J. 13, 720–733.
| Effect of fecal microbiota transplantation route of administration on gut colonization and host response in preterm pigs.Crossref | GoogleScholarGoogle Scholar | 30367124PubMed |
[25] Geng, S. et al. (2018) Faecal microbiota transplantation reduces susceptibility to epithelial injury and modulates tryptophan metabolism of the microbial community in a piglet model. J. Crohns Colitis 12, 1359–1374.
| Faecal microbiota transplantation reduces susceptibility to epithelial injury and modulates tryptophan metabolism of the microbial community in a piglet model.Crossref | GoogleScholarGoogle Scholar | 30010734PubMed |
Biographies
Tanya Nowland is a PhD student at the University of Adelaide, Australia. Her research focuses on how the intestinal microbiome impacts piglet health and survival. Specifically, focusing on the development of non-antimicrobial industry applicable practices to help support healthy microbiome development and improve preweaning performance.
Roy Kirkwood is an Associate Professor in swine production medicine whose research areas include sow reproductive management and impacts on piglet health and survival. In particular, the involvement of microbiome structure on production outcomes is a current focus area.


