Strongyloides fuelleborni kellyi in New Guinea: neglected, ignored and unexplored
Richard S Bradbury A BA School of Health and Life Sciences, Federation University, Berwick Campus, Building 901, level 2, 100 Clyde Road, Berwick, Vic. 3806, Australia
B Tel.: +61 3 5327 6584; Email: r.bradbury@federation.edu.au
Microbiology Australia 42(4) 169-172 https://doi.org/10.1071/MA21048
Submitted: 29 July 2021 Accepted: 31 August 2021 Published: 3 November 2021
Journal Compilation © The Authors 2021 Open Access CC BY-NC-ND, published (by CSIRO Publishing) on behalf of the ASM
Abstract
Strongyloidiasis remains endemic throughout the Island of New Guinea. While many infections are caused by Strongyloides stercoralis, a second human-infecting Strongyloides species, Strongyloides fuelleborni kellyi, is also present. S. f. kellyi infections are most common in infants and young children, and those with high-intensity infections might develop a potentially fatal protein-losing enteropathy, swollen belly syndrome. Surprisingly little work has been performed on S. f. kellyi. Unlike S. stercoralis, S. f. kellyi is passed in faeces as eggs rather than rhabditiform larvae. There is no specific diagnostic test. This review summarises what is currently known about the biology, epidemiology, and clinical impact of S. f. kellyi infections. Features that might be used to differentiate S. f. kellyi from hookworm and S. stercoralis are also discussed. S. f. kellyi remains a neglected, ignored, and unexplored human helminth infection, worthy of further research.
The Western Pacific region has among the highest burden of strongyloidiasis in the world1. While Strongyloides stercoralis is endemic in Papua New Guinea (PNG), the island also harbours a second human-infecting Strongyloides species, Strongyloides fuelleborni kellyi.
The presence of a previously undescribed Strongyloides was first identified by Allan Kelly of the PNG Institute of Medical Research in 19712. Due to close morphological resemblance to S. fuelleborni fuelleborni, a parasite of humans and non-human primates in Africa and Asia, the species was originally described as a Strongyloidesfuelleborni-like helminth, later changed to S. f. kellyi. Sequencing of the 18S ribosomal RNA gene revealed clustering at this target within a distinct clade including S. cebus, S. papillosus and S. venezuelensis but separate from S. f. fuelleborni3.
Infection is primarily found in infants and young children, with egg loads peaking at 20 months of age4–8. High-intensity infection in infants <2 years of age can cause a rapidly fatal disease known as swollen belly syndrome (SBS), characterised by gross abdominal distension and respiratory distress8. SBS is most commonly seen in infants around 2 months of age8. The characteristic swollen belly observed in this condition is due to ascites following a protein-losing enteropathy. The signs and symptoms of SBS include a very low serum protein, ascites, peripheral oedema, mild diarrhoea with very high numbers of S. f. kellyi eggs in faeces, occasional vomiting, a high-pitched cry, fever (in some cases) and respiratory distress8. Haemoglobin levels remain normal8. Heavy infections in older children may be associated with failure to thrive, but many older children appear asymptomatic8.
Strongyloides fuelleborni kellyi has been found in multiple provinces of PNG7 and has also been reported from Irian Jaya in Indonesian Western New Guinea5. No work has been performed on the distribution or prevalence of this parasite since 19979. Historical surveys conducted between 1981 and 1997 reported prevalence in children from endemic areas ranging between 20% and 93%, with most studies finding a prevalence of over 60% in children aged <10 years4,6–13. Prevalence peaked between 20 and 36 months of age4,6. Prevalence in children from high-intensity infection communities may reach 100%4,8 and it was not unusual in such communities for children to pass more than 100 000 eggs per mL of faeces8. Prevalence rose steeply from 1 month to 4 months of age6, but by 10 years of age prevalence fell and the intensity of infection rarely exceeded 500 eggs per mL of faeces8. Prevalence in adults from affected communities was only 5–10%. The parasite was found infecting people in both rural and remote communities, and larger towns and cities6, although with a much lower prevalence in urban areas4,12. Environmental factors such as altitude, slope of ground, landform, rainfall and population densities do not influence distribution of the parasite, although it is uncommon in areas with limestone and polygonal karst7. It is not known if an animal reservoir of infection exists8. Pigs were investigated extensively as a potential zoonotic source but no S. f. kellyi infections were found14.
In high prevalence communities, patent infection may occur in infants only 14–18 days old. The exposure of infants to filariform (infective) larvae in soiled bedding used to line string bags in which they were carried might have led to rapid external autoinfection, causing the very high-intensity infections seen in this age group8. Internal autoinfection, which is seen in S. stercoralis infections, is thought not to occur. This is because S. f. kellyi are passed as eggs in faeces, unlike S. stercoralis, which are passed as larvae8,15. Also, prevalence and egg counts are observed to decline with age4,6,7,9,11,13, indicating that maintenance of infection via an autoinfective process is likely not occurring.
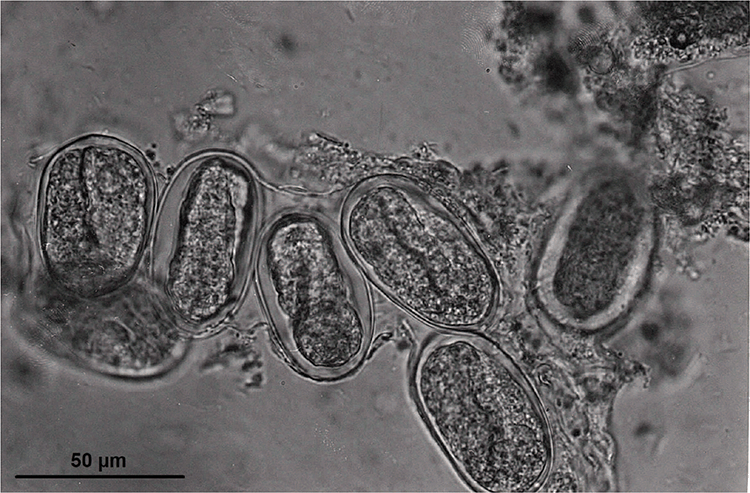
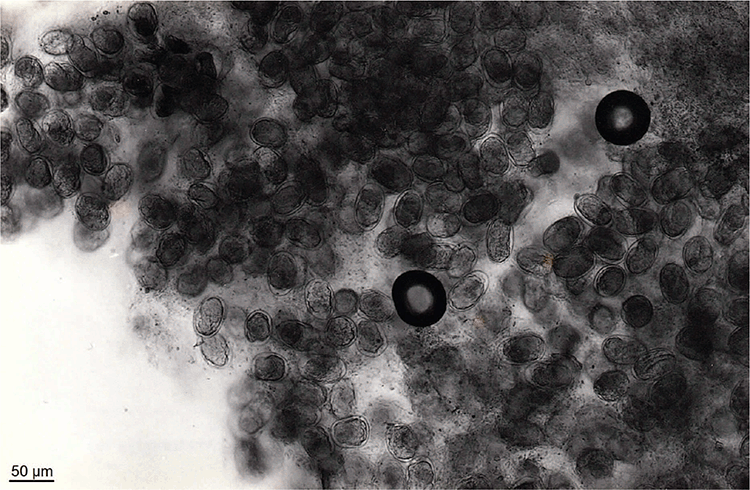
S. f. kellyi eggs average 51.4 (47–55.8) µm in length by 32.1 (31.1–33.1) µm in width15 and contain well developed larvae8 that hatch within hours of passage8. In asymptomatic adults, these are normally single eggs free in the faeces (JM Shield, personal communication). However, in children with heavy infections, strings of eggs encased within a fine membrane (Figure 1)8,15 or masses of eggs (Figure 2) may be seen8. The hatched larvae are morphologically indistinguishable from those of S. stercoralis15.
|
|
Diagnosis of infection in very young infants may often be missed or delayed as it is generally assumed by clinicians that children of this age cannot yet have acquired intestinal helminths8. Misdiagnosis as hookworm is common due to the similar morphology of eggs8. Diagnosis from faeces is reliant on the presence of proficient microscopists who are aware of the existence and morphology of S. f. kellyi eggs, which are smaller and more developed upon passage than those of hookworm.
The parthenogenic adult parasitic female of S. f. kellyi appears to be morphologically indistinguishable from S. f. fuelleborni15. It may be differentiated from S. stercoralis by the presence of spiral rather than straight ovaries15. Due to the difficulty in obtaining adult parasitic females this approach is not viable for routine diagnosis. S. f. kellyi may be differentiated from S. f. fuelleborni and S. stercoralis in its cultured free-living adult stage. Like S. f. fuelleborni, the free-living female of S. f. kellyi has a prominent post-vulval constriction, which is not seen in S. stercoralis15. There is also slight variation in the morphology of a portion of the peri-vulval cuticle of the S. f. kellyi free-living female when compared to that seen in S. f. fuelleborni14. Differences in the morphology of free-living males involve a more anterior position of the phasmidal pore relative to the sub-ventral, sub-dorsal post-cloacal papillae14. Detection and differentiation of these very subtle morphologic differences requires a very advanced level of parasitological expertise.
A treatment intervention study using thiabendazole (25 mg/kg twice daily for 3 days) was conducted between 1983 and 1984. This study found that thiabendazole eliminated egg passage in 86 of 88 infected children, with a very significant reduction in egg passage in the remaining two participants16. Reinfection after treatment was common6. Fifty-eight percent of treated children were found to be reinfected 18 months after treatment6. The modal time for reinfection was 9 months6. The standard treatment in PNG for Strongyloides in adults in PNG is albendazole 400 mg daily for 3 days17. Standard treatment for children with anaemia and oedema is albendazole 200 mg for those 5–9.9 kg and 400 mg for those ≥10kg daily for 3 days. Oedema in this context may include SBS due to S. f. kellyi17. The efficacy of ivermectin, moxidectin or combination anthelmintic therapies against S. f. kellyi has not been explored.
Very little work has been performed on S. f. kellyi in the past 30 years. Important questions, such as what is the current prevalence and distribution, and if it might be a zoonosis with an animal reservoir, have not been answered. This human helminth remains neglected, ignored, and unexplored, despite being present and likely widespread in Australia’s nearest neighbour.
Conflicts of interest
The author declares no conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
Acknowledgements
The author thanks Dr Jennifer Shield of La Trobe University, Bendigo Campus, and formerly the parasitologist at PNG Institute of Medical Research, for her advice and critical review of this manuscript.
References
[1] Buonfrate, D. et al. (2020) The global prevalence of Strongyloides stercoralis infection. Pathogens 9, 468.| The global prevalence of Strongyloides stercoralis infection.Crossref | GoogleScholarGoogle Scholar |
[2] Kelly, A. and Voge, M. (1973) Report of a nematode found in humans at Kiunga, Western District. P. N. G. Med. J. 16, 59.
[3] Dorris, M. et al. (2002) Molecular phylogenetic analysis of the genus Strongyloides and related nematodes. Int. J. Parasitol. 32, 1507–1517.
| Molecular phylogenetic analysis of the genus Strongyloides and related nematodes.Crossref | GoogleScholarGoogle Scholar | 12392916PubMed |
[4] Shield, J.M. et al. (1987) Hookworm (Necator americanus) and Strongyloides fuelleborni-like prevalence and egg count with age in highlands fringe people of Papua New Guinea. P. N. G. Med. J. 30, 21–26.
| 3475865PubMed |
[5] Muller, R. et al. (1987) Human cysticercosis and intestinal parasitism amongst the Ekari people of Irian Jaya. J. Trop. Med. Hyg. 90, 291–296.
| 3430662PubMed |
[6] Barnish, G. and Ashford, R.W. (1989) Strongyloides cf. fuelleborni in Papua New Guinea: epidemiology in an isolated community, and results of an intervention study. Ann. Trop. Med. Parasitol. 83, 499–506.
| Strongyloides cf. fuelleborni in Papua New Guinea: epidemiology in an isolated community, and results of an intervention study.Crossref | GoogleScholarGoogle Scholar | 2619364PubMed |
[7] Barnish, G. and Ashford, R.W. (1990) Strongyloides cf. fuelleborni and other intestinal helminths in Papua New Guinea: distribution according to environmental factors. Parassitologia 32, 245–263.
| 2132436PubMed |
[8] Ashford, R.W. et al. (1992) Strongyloides fuelleborni kellyi: infection and disease in Papua New Guinea. Parasitol. Today 8, 314–318.
| Strongyloides fuelleborni kellyi: infection and disease in Papua New Guinea.Crossref | GoogleScholarGoogle Scholar | 15463651PubMed |
[9] King, S.E. et al. (2004) Strongyloides fuelleborni kellyi and other intestinal helminths in children from Papua New Guinea: associations with nutritional status and socioeconomic factors. P. N. G. Med. J. 47, 181–191.
| 16862942PubMed |
[10] Barnish, G. and Barker, J. (1987) An intervention study using thiabendazole suspension against Strongyloides fuelleborni-like infections in Papua New Guinea. Trans. R. Soc. Trop. Med. Hyg. 81, 60–63.
| An intervention study using thiabendazole suspension against Strongyloides fuelleborni-like infections in Papua New Guinea.Crossref | GoogleScholarGoogle Scholar | 3445323PubMed |
[11] Smith, T. et al. (1991) Host genetic factors do not account for variation in parasite loads in Strongyloides fuelleborni kellyi. Ann. Trop. Med. Parasitol. 85, 533–537.
| Host genetic factors do not account for variation in parasite loads in Strongyloides fuelleborni kellyi.Crossref | GoogleScholarGoogle Scholar | 1809247PubMed |
[12] Shield, J.M. and Kow, F. (2013) A comparative study of intestinal helminths in pre-school-age urban and rural children in Morobe Province, Papua New Guinea. P. N. G. Med. J. 56, 14–31.
| 25423854PubMed |
[13] Ashford, R.W. et al. (1979) Strongyloides infection in a mid-mountain Papua New Guinea community: results of an epidemiological survey. P. N. G. Med. J. 22, 128–135.
| 298723PubMed |
[14] Viney, M.E. et al. (1991) A taxonomic study of Strongyloides Grassi, 1879 (Nematoda) with special reference to Strongyloides fuelleborni von Linstow, 1905 in man in Papua New Guinea and the description of a new subspecies. Syst. Parasitol. 18, 95–109.
| A taxonomic study of Strongyloides Grassi, 1879 (Nematoda) with special reference to Strongyloides fuelleborni von Linstow, 1905 in man in Papua New Guinea and the description of a new subspecies.Crossref | GoogleScholarGoogle Scholar |
[15] Speare, R. (1989) Identification of species of Strongyloides. In: Grove D.I. (ed). Strongyloidiasis: a major roundworm infection of man. Taylor and Francis: Basingstoke. pp. 11–84.
[16] Barnish, G. and Ashford, R.W. (1989) Strongyloides cf fuelleborni in Papua New Guinea: epidemiology in an isolated community, and results of an intervention study. Ann. Trop. Med. Parasitol. 83, 499–506.
| Strongyloides cf fuelleborni in Papua New Guinea: epidemiology in an isolated community, and results of an intervention study.Crossref | GoogleScholarGoogle Scholar | 2619364PubMed |
[17] The Paediatric Society of Papua New Guinea (2016) Standard Treatment for Common Illnesses of Children in Papua New Guinea. A Manual for Nurses, Community Health Workers, Health Extension Officers, and Doctors, 10th edition. The Paediatric Society of Papua New Guinea: Port Moresby.
Biography
Richard Bradbury is a Senior Lecturer in Microbiology and Molecular Biology at Federation University Australia. His areas of research interest include diagnostics and epidemiology in medical parasitology and zoonoses.


