‘The awesome power of yeast’
Ian Macreadie A * and Sudip Dhakal AA School of Science, RMIT University, Bundoora, Vic. 3083, Australia.

Ian Macreadie has a 45-year history of yeast genetics. He was a research scientist at CSIRO for 24 years and then taught at RMIT University until 2020. He is currently an Honorary Professor of RMIT University. He is Editor-in-Chief of Microbiology Australia. |

Sudip Dhakal is a PhD researcher and a tutor/instructor at the School of Science, RMIT University, whose research focuses on investigating therapeutic strategies against Alzheimer’s disease using yeast models. |
Microbiology Australia 43(1) 19-21 https://doi.org/10.1071/MA22007
Submitted: 15 January 2022 Accepted: 25 February 2022 Published: 11 April 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of the ASM. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Yeast is one of the most useful microorganisms in society. Aside from the well known traditional uses in beer, wine and bread making, yeast is currently providing new opportunities for our society. This article examines some of those new opportunities which include using yeast as a model organism, yeast as a cell factory for valuable proteins, including vaccines and new therapeutics, and yeast as a very convenient tool for teaching.
Keywords: ageing research, cancer research, drug discovery, gene engineering, mitochondrial function, Nobel prizes, teaching, yeast-derived vaccines.
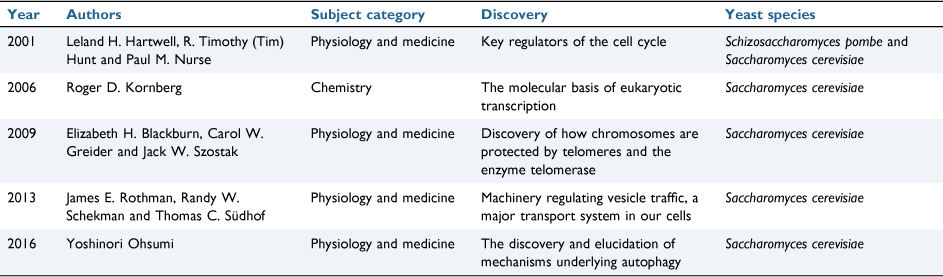
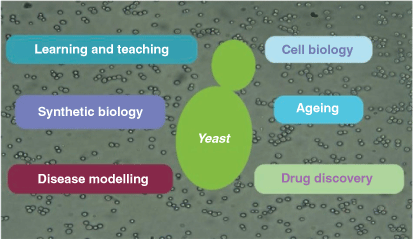
‘The awesome power of yeast genetics’ was a term generally believed to have been first used by Ira Herskowitz (1946–2003).1 With thousands who have experienced the power of yeast genetics the phrase thrived and continues in new contexts today, not just yeast genetics. To date five Nobel prizes have been awarded to yeast researchers for their discoveries of important cellular mechanisms (Table 1). In this article we describe that awesome power that is available today (Fig. 1).
|
|
Yeast as a model organism
Yeasts are the best studied eukaryotes. Our advances in genetics, molecular and cell biology have been greatly aided by yeast, in particular Saccharomyces cerevisiae, whose genome was the first eukaryotic genome to be sequenced. Much of the information on the contributions of yeast to biology is readily available on the Saccharomyces Genome Database.2 This information has aided the characterisation of many genes and proteins in humans. Functional complementation of yeast gene deletants with human genes is often observed in yeast, further allowing insights into human proteins in health and disease. To a lesser extent, several Candida species have been similarly exploited and the information compiled on the Candida Genome Database.3
Due to the conservation of important fundamental processes of eukaryotes from yeasts to humans, yeast have played crucial roles in the study of several human diseases.4 The similarities such as age-associated loss of proteostasis makes them valuable models for diseases involving age-related proteinopathies. Through advanced synthetic biology approaches it is also possible to reconstruct yeast for the study of diseases like Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, prion disease, and cancers.5–7
Yeast also offers unique opportunities to study diseases involving mitochondrial defects since it can grow with defective mitochondria.8 In addition, the conservation of cellular signalling and pathways from yeast to higher eukaryotes make it more highly useful in studying diseases involving a multitude of processes. For example, these attributes have been used in recent studies that have been outstanding in identifying compounds that can modify mitochondrial health.9,10
Yeast as a host for vaccines
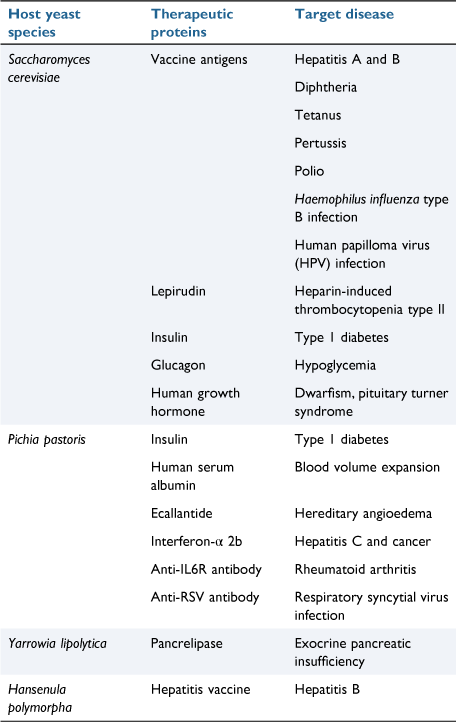
The first viral subunit (for HBV and HPV) vaccines were made in yeast and they have been used safely for more than three decades. Since then, several important therapeutic proteins including vaccines have already been synthesised using yeast as a cell factory (Table 2).11 In the 1980s, Macreadie and colleagues at CSIRO were involved in the development of the 2nd viral subunit vaccine for a poultry virus, infectious bursal disease virus.12
|
Can yeast be used to produce a COVID-19 vaccine? So far progress to the development of a yeast-derived COVID-19 vaccine has been slow compared to the many other COVID-19 vaccines. However, the efficacy of a yeast-derived receptor binding domain (RBD)-based COVID-19 vaccine looks to be promising.13 A challenge with producing human proteins in yeast is the type of carbohydrate added to proteins (glycosylation).11 Human glycosylation is complex, while yeast adds high mannose. This challenge has now been met, by synthetic biology approaches. Pichia pastoris was re-engineered with genes involved in human glycosylation to produce up to 16 different types of mammalian glycosylation patterns. This has revolutionised the pharmaceutical industry and the use of P. pastoris for vaccine production.14 Corbevax is an example of a P. pastoris expressed subunit vaccine, for COVID-19. It is patent free and is regarded as an effective, low cost vaccine candidate.15
Two hybrid technology and drug screening
Protein interactions are immensely important for assembly of protein complexes and for cell signalling. The yeast two hybrid system, pioneered by Fields and Song (1989) has provided technologies to discover human protein–protein interactions, including their precise molecular interaction interfaces.16 Further, it is possible to use yeast displaying human protein interactions to find small molecules that disrupt these interactions.17 This is a powerful approach for drug screening since molecules found in such an approach must already have a degree of bioavailability.18
Ageing
Yeast is also a useful model to study aging. Throughout their life most yeast species produce progeny by budding. With each bud that is released a bud scar is left at the surface and these scars can readily be stained with calcofluor white.8 In every generation, 50% of the population are newborn yeast, while the remainder have one or more bud scars. Flow cytometry is a very convenient means to be analyse or isolate yeast populations, which provides a convenient means of observing the ageing of yeast and can facilitate study of the expression of ageing-related genes.19
As noted by Dhakal (2021) proteostasis declines with aging and can readily be observed in yeast having the Alzheimer’s disease protein, amyloid beta.8 Anti-ageing drugs can readily be tested in such yeast models. For example, a study to screen several bioactive compounds found two excellent candidates (baicalein and trans-chalcone) that have potential to treat and prevent AD. Additionally, the synergistic activity of these two compounds in improving ageing health in the yeast that expressed amyloid beta was identified using such yeast models.20
Yeast as a tool in teaching
Yeast is GRAS (generally regarded as safe), fast growing and can be used as a convenient organism for teaching aseptic technique, metabolism, synthetic biology and cell biology.11 Yeast are usually grown in rich media and the total absence of antibiotics, even for selection of transformants. Therefore, proper technique is required.
Yeast are eukaryotes and have the organelles of eukaryotes. There is beauty in the sight of mitochondria in live yeast, being able to look at them as structures resembling roots in a plant pot, rather the textbook image: an oval shape showing their cross section. Furthermore, the effect of mitochondria on growth can be readily demonstrated on media with a non-fermentable carbon source, and yeast lacking the ability to grow on such media can be observed. This lack of growth can happen when a mitochondrial inhibitor is added to the media (e.g. antimycin, oligomycin, erythromycin, chloramphenicol) or when the yeast contains a defect in its mitochondrial genome. This defect can include a point mutation or a deletion of a portion or all of the mitochondrial genome. Such mutants are known as mit− or petite mutants.
Conclusion
So far, yeasts have proved to be an awesome research tool for studying human diseases, a very convenient biological factory for production of several important therapeutic proteins and a platform for screening drugs against human diseases. Important discoveries made in yeast have always provided solutions to big problems in simpler ways.
Data availability
Data sharing is not applicable as no new data were generated or analysed during this study.
Conflicts of interest
Ian Macreadie is the Editor-in-Chief of Microbiology Australia but was blinded from the peer-review process for this paper.
Declaration of funding
This study did not receive any specific funding.
References
[1] Botstein, D (2004) Ira Herskowitz: 1946–2003. Genetics 166, 653–660.| Ira Herskowitz: 1946–2003.Crossref | GoogleScholarGoogle Scholar | 15020456PubMed |
[2] Cherry, JM et al.. (1998) SGD: Saccharomyces Genome Database. Nucleic Acids Res 26, 73–79.
| SGD: Saccharomyces Genome Database.Crossref | GoogleScholarGoogle Scholar | 9399804PubMed |
[3] Skrzypek, MS et al.. (2017) The Candida Genome Database (CGD): incorporation of Assembly 22, systematic identifiers and visualization of high throughput sequencing data. Nucleic Acids Res 45, D592–D596.
| The Candida Genome Database (CGD): incorporation of Assembly 22, systematic identifiers and visualization of high throughput sequencing data.Crossref | GoogleScholarGoogle Scholar | 27738138PubMed |
[4] Dhakal, S and Macreadie, I (2020) Protein homeostasis networks and the use of yeast to guide interventions in Alzheimer’s disease. Int J Mol Sci 21, 8014.
| Protein homeostasis networks and the use of yeast to guide interventions in Alzheimer’s disease.Crossref | GoogleScholarGoogle Scholar |
[5] Pereira, C et al.. (2012) Contribution of yeast models to neurodegeneration research. J Biomed Biotechnol 2012, 941232.
| Contribution of yeast models to neurodegeneration research.Crossref | GoogleScholarGoogle Scholar | 22910375PubMed |
[6] Guaragnella, N et al.. (2014) The expanding role of yeast in cancer research and diagnosis: insights into the function of the oncosuppressors p53 and BRCA1/2. FEMS Yeast Res 14, 2–16.
| The expanding role of yeast in cancer research and diagnosis: insights into the function of the oncosuppressors p53 and BRCA1/2.Crossref | GoogleScholarGoogle Scholar | 24103154PubMed |
[7] Mcdonald, JB et al.. (2020) Yeast contributions to Alzheimer’s disease. J Hum Clin Genet 2, 1–19.
| Yeast contributions to Alzheimer’s disease.Crossref | GoogleScholarGoogle Scholar |
[8] Dhakal, S (2021) The awesome power of yeast’ in Alzheimer’s disease research. Microbiol Aust 42, 130–133.
| The awesome power of yeast’ in Alzheimer’s disease research.Crossref | GoogleScholarGoogle Scholar |
[9] Mcdonald, JB et al.. (2021) A Toxic synergy between aluminium and amyloid beta in yeast. Int J Mol Sci 22, 1835.
| A Toxic synergy between aluminium and amyloid beta in yeast.Crossref | GoogleScholarGoogle Scholar | 33673244PubMed |
[10] Dhakal, S and Macreadie, I (2020) Tyramine and amyloid beta 42: a toxic synergy. Biomedicines 8, 145.
| Tyramine and amyloid beta 42: a toxic synergy.Crossref | GoogleScholarGoogle Scholar |
[11] Kim, H et al.. (2015) Yeast synthetic biology for the production of recombinant therapeutic proteins. FEMS Yeast Res 15, 1–16.
| Yeast synthetic biology for the production of recombinant therapeutic proteins.Crossref | GoogleScholarGoogle Scholar | 25130199PubMed |
[12] Macreadie, IG et al.. (1990) Passive protection against infectious bursal disease virus by viral VP2 expressed in yeast. Vaccine 8, 549–552.
| Passive protection against infectious bursal disease virus by viral VP2 expressed in yeast.Crossref | GoogleScholarGoogle Scholar | 1965076PubMed |
[13] Zang, J et al.. (2021) Yeast-produced RBD-based recombinant protein vaccines elicit broadly neutralizing antibodies and durable protective immunity against SARS-CoV-2 infection. Cell Discov 7, 71.
| Yeast-produced RBD-based recombinant protein vaccines elicit broadly neutralizing antibodies and durable protective immunity against SARS-CoV-2 infection.Crossref | GoogleScholarGoogle Scholar | 34408130PubMed |
[14] Karbalaei, M et al.. (2020) Pichia pastoris: a highly successful expression system for optimal synthesis of heterologous proteins. J Cell Physiol 235, 5867–5881.
| Pichia pastoris: a highly successful expression system for optimal synthesis of heterologous proteins.Crossref | GoogleScholarGoogle Scholar | 32057111PubMed |
[15] Chen, WH et al.. (2021) Genetic modification to design a stable yeast-expressed recombinant SARS-CoV-2 receptor binding domain as a COVID-19 vaccine candidate. Biochim Biophys Acta Gen Subj 1865, 129893.
| Genetic modification to design a stable yeast-expressed recombinant SARS-CoV-2 receptor binding domain as a COVID-19 vaccine candidate.Crossref | GoogleScholarGoogle Scholar | 33731300PubMed |
[16] Fields, S and Song, O-k (1989) A novel genetic system to detect protein–protein interactions. Nature 340, 245–246.
| A novel genetic system to detect protein–protein interactions.Crossref | GoogleScholarGoogle Scholar | 2547163PubMed |
[17] Brückner, A et al.. (2009) Yeast two-hybrid, a powerful tool for systems biology. Int J Mol Sci 10, 2763–2788.
| Yeast two-hybrid, a powerful tool for systems biology.Crossref | GoogleScholarGoogle Scholar | 19582228PubMed |
[18] Lentze, N and Auerbach, D (2008) The yeast two-hybrid system and its role in drug discovery. Expert Opin Ther Targets 12, 505–515.
| The yeast two-hybrid system and its role in drug discovery.Crossref | GoogleScholarGoogle Scholar | 18348685PubMed |
[19] Sutphin, GL et al.. (2012) Genome-wide analysis of yeast aging. Subcell Biochem 57, 251–289.
| 22094426PubMed |
[20] Dhakal, S et al.. (2021) Trans-Chalcone plus baicalein synergistically reduce intracellular amyloid beta (Aβ42) and protect from Aβ42 induced oxidative damage in yeast models of Alzheimer’s disease. Int J Mol Sci 22, 9456.
| Trans-Chalcone plus baicalein synergistically reduce intracellular amyloid beta (Aβ42) and protect from Aβ42 induced oxidative damage in yeast models of Alzheimer’s disease.Crossref | GoogleScholarGoogle Scholar | 34502362PubMed |


