Growing soil organic carbon in dryland agricultural systems
Mark Farrell A B C * and Gupta Vadakattu A BA CSIRO Agriculture & Food, Kaurna Country, Locked Bag 2, Glen Osmond, SA 5064, Australia.
B CSIRO Microbiomes for One Systems Health Future Science Platform, Kaurna Country, Locked Bag 2, Glen Osmond, SA 5064, Australia.
C UWA School of Agriculture & Environment, The University of Western Australia, Whadjuk Noongar Country, 35 Stirling Highway, Perth, WA 6000, Australia.

Dr Mark Farrell is a biogeochemist, with primary interests in carbon and nitrogen cycling in soils, and the impacts of management and environmental change on these processes. Principally working alongside microbiologists, he utilises a range of cutting-edge analytical techniques including nuclear magnetic resonance and mid infrared spectroscopy, as well as stable- and radio-isotopes to understand the composition and flows of carbon and nitrogen through the environment, and how these are moderated by the microbial community. |

Dr Gupta Vadakattu investigates on aspects of genetic and functional diversity, functional capability and resilience of soil biota in agricultural soils. Special interests include: genetic, functional and environmental regulators of biological disease suppression in soils, phenotypic and functional diversity of microbiota in the rhizosphere systems, diversity and functional capacity of diazotrophs in annual and perennial crops and turnover to carbon, nutrients and biological health of soils. |
Microbiology Australia 44(1) 18-21 https://doi.org/10.1071/MA23005
Submitted: 17 January 2023 Accepted: 2 February 2023 Published: 20 February 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of the ASM. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Soil organic carbon (SOC) plays a crucial role in dryland agricultural systems, improving resilience, productivity and delivering a range of ecosystem services including carbon (C) sequestration and broader ecosystem health. Although the net primary production (NPP) is the principal source of C inputs to soil, plant–microbe interactions can help increase NPP and stimulate plant C inputs to the soil through a variety of mechanisms. Additionally, the soil microbial community plays a crucial role in the loss (CO2 respiration) and stabilisation of SOC. With improved understanding of soil microbiomes and plant–microbe interactions, there are new emerging strategies in which microorganisms may be harnessed either directly or indirectly to increase the amount of C added and stabilised in dryland soils.
Sources of carbon, the role of microorganisms and the impacts of management practices
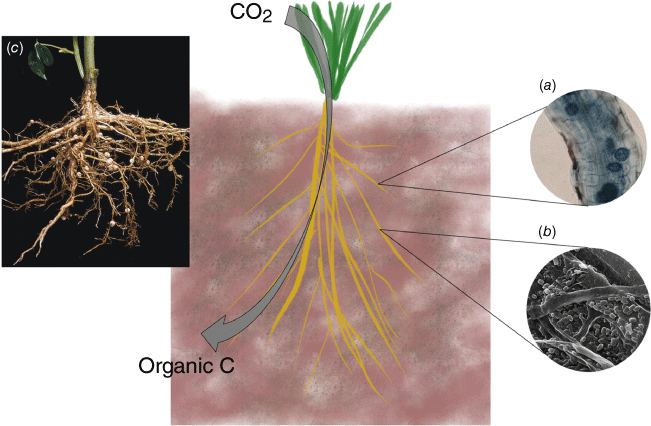
The primary source of carbon (C) inputs to soil is net primary production (NPP) by photosynthetic fixation of atmospheric CO2 to plant biomass and exudates1 (Fig. 1). Although exogenous inputs in the form of organic amendments, such as manures, composts and biochars, may be locally relevant they are not typically available in regionally or nationally significant quantities, and typically involve the diversion of C that has already been removed from the atmosphere; thus they are not the concern of this paper. In dryland systems that account for ~45% of global land area2 and typically receive <500 mm of annual rainfall, water availability is the governing factor that limits NPP and thus flows of C to the soil organic C (SOC) pool. Soil microorganisms process C inputs from plants – famously described by David Jenkinson as ‘The eye of the needle’3 – with a proportion of the C lost through microbial respiration to CO2, and the remainder retained in microbial biomass. This partitioning is termed carbon-use efficiency (CUE).4 In turn, microbial biomass is itself subsequently further cycled upon cell death.
Over the past three decades, our understanding of the processes involved in SOC stabilisation have evolved greatly. The traditional view of chemical recalcitrance driving persistence has been largely replaced by multiple lines of evidence that point towards physical and chemical protection of kinetically unstable compounds within aggregates and upon clay particles.5 Although emerging research suggests that direct stabilisation of plant C inputs may have been underestimated,6 it is understood that the soil microbial community plays a pivotal role in both stabilisation and loss of C in soils. Indeed, in low CUE situations, over 90% of C from fresh inputs may be respired7 (Fig. 1).
Soil organic C does not exist in isolation. It is a component of soil organic matter (SOM), and has been shown to broadly conform to Redfield’s stoichiometric ratio, having fairly consistent proportions of C:N:P:S.8 Plant C inputs typically contain far greater C:nutrient ratios than microbial biomass or stabilised SOM. Thus, in order to build SOC, nutrients are also required, and the availability of nutrients in ratios broadly representative of SOM typically increases microbial CUE,4 although clay content and the size of the extant microbial biomass can be more important drivers.9
Modern management practices in dryland systems often seek to maximise water availability for the target crop through the adoption of zero tillage, stubble retention as a mulch and the control of summer weeds. Each of these practices can have a direct or indirect effect on the inputs to and losses of SOC. Additionally, in dryland cropping systems such as those predominantly found across the Australian wheatbelt, nitrogen (N) is frequently a limiting factor for crop production because of conservative application rates that target individual season profitability and may ‘mine’ mineralised N from SOM,10 resulting in increased losses of SOC by mineralisation.
Against this background, a number of practices are emerging that seek to deliver more C to the soil through increasing the proportion of time a living plant is present in the system (e.g. by using cover crops in otherwise fallow periods11), harnessing plant traits that increase C delivery below ground,12 or manipulating the soil, rhizosphere or root microbiome to increase the stabilisation of plant C inputs.
Progress to date
The most widespread type of biological amendment in regular use in dryland agriculture are rhizobia inocula – N2 fixing symbionts of legume crops. Tightly optimised for legume crop type and often soil properties, rhizobia can fix 20–25 kg N Mg dry matter−1 year−1 in dryland systems.13 Because of the lower C:N ratio of legume-derived organic matter inputs, higher CUE and thus greater retention of legume-derived C may be expected.
However, rhizobia are, by and large, the exception. Although there are a multitude of biological amendments available, including both active inocula and biostimulant products, consistency of results remains poor.14 Unlike the highly specific and well-understood legume–Rhizobium symbiosis that is harnessed to perform an exclusive function, many commercially available microbial inocula have more generalist target outcomes. In a lot of cases, including those with reported aims of increasing SOC, mechanisms and modes of action have often remained unclear or poorly verified. Additionally, there is a need to improve mechanisms of application that protect microbial inoculants from the harsh soil environment prior to their colonisation of the root and rhizosphere. It is necessary to develop strategies for effective inoculation methods, so that single species or consortia of microorganisms of interest can gain an advantage in colonisation efficiency over native microbiomes in the field environment. This may present a significant challenge given that native microbial communities are highly adapted to their environment.
An important approach to potentially improve the efficacy of microbial inocula and biostimulants in dryland agricultural systems is to develop a clearer understanding of the limits to crop productivity and thus C inputs. Aligned with this, a better understanding of interactive effects of multiple management interventions across several seasons and rotations on plant C inputs by NPP and microbial stabilisation of C inputs is required. Hallama et al.11 concluded that cover crops increased mycorrhizal abundance, leading to improved colonisation of the main crop and subsequent provision of nutrients. However, although appropriate application of fungicides may control crop disease and increase crop yield and C inputs of an existing crop, fungicides may also negatively affect non-target fungal functions,15 including potentially beneficial mycorrhizae, and this is particularly the case for anti-fungal seed coatings.
A promising future?
Recent plant root research has improved our understanding of the diversity of root traits and their contribution to plant and ecosystem functioning.16 Coupled with this is the progress in understanding root–microbiome interactions both in terms of the drivers of microbiome function and the potential consequences of manipulating microbiomes to stimulate root growth, health and biogeochemical processes – including C turnover and stabilisation.17 It is suggested that root architectural traits known to increase below-ground plant-derived C inputs are important drivers of microbial community structure and biomass, which in turn contribute to turnover and stabilisation of freshly added C.
A number of microbial genera and species generally referred to as plant-growth promoting rhizobacteria (PGPR; e.g. Pseudomonas, Azospirillum, Bacillus spp.) have been shown to produce phytohormones (e.g. auxins, gibberellins and cytokinin) and other quorum sensing molecules18 that promote root growth (lateral root formation, root hair development), thus expanding rhizosphere and rhizodeposit C addition. PGPR may also benefit root growth and functioning through establishment of rhizobial and mycorrhizal symbioses, protection from soil-borne root pathogens and by eliciting plant defences including induced systemic resistance. Beneficial effects from such interactions, in particular from introduced organisms, depend upon the correct microbe–plant combination and the expression of the required functional traits (e.g. producing optimal concentrations of hormones at the appropriate time). Therefore, harnessing this type of root–microbe interaction requires a deeper understanding of the complex feedback mechanisms associated with the abundances of specific microbial species, the types and concentrations of chemical signals, and genetic and environmental controls for functional expression.18
Despite extensive research demonstrating potential benefits from the symbiotic associations between arbuscular mycorrhizae and agricultural crops, practical applications of these associations are yet to be utilised, largely because the complex influences of edaphic and environmental factors in field environments are not well understood. In Australian dryland agricultural soils with low SOM concentrations, arbuscular mycorrhizae can make a significant contribution to improving plant C inputs and turnover processes through their extensive hyphal networks combined with enzymatic, metabolic and nutrient acquisition capabilities and effects on C translocation below-ground.16
Plant genotype may play a significant role in the recruitment, assembly and activities of the rhizosphere microbiome. The composition of highly diverse rhizosphere microbiomes is largely selected by the host plant but primarily modulated by soil type.19 For example, a core root microbiome dominated by a restricted group of bacterial taxa has been found in multiple phyla growing in close proximity. This suggests shared functionality relating to traits in the core root bacterial communities and opportunities for a targeted approach to manipulate root growth across a broad spectrum of plant types.20 Crop-based variations in rhizosphere microbiomes have been well documented, with diverse rotations being shown to increase disease suppression and avoid negative legacies.21 Recent evidence has shown that structural or taxonomic diversification of rhizosphere-associated microbial communities exists within crop varieties and between wild and domesticated accessions of many crops (barley, wheat, maize, pearl millet and arabidopsis) – that is, root-associated microbiomes have greater heritable variation.22 Such differences in root traits and microbiomes could contribute to variability among cultivars for soil C cycling and SOC sequestration potential. A strong relationship between phylogenetic distance and rhizosphere microbiome dissimilarity is reported for different species in both monocotyledons and dicotyledons.23
As mentioned earlier, N availability is the greatest constraint to Australian dryland wheat production. Engineering crops and synthetic plant–microbe symbioses to introduce N2 fixation machinery to cereals and other non-legume crops is decades away. Therefore, designer plant–diazotrophic combinations are an attractive option to remove N constraints to production and to increase C inputs in agricultural systems.24 The ecological significance of free-living or associative diazotrophic N2-fixing bacteria in agricultural soils is becoming more appreciated. A diverse diazotrophic community exists in soils and in below- and above-ground plant parts, and a growing body of evidence suggests that they could be a significant contributor to cereal crop N budgets and thus C inputs.25 This presents an opportunity to harness their capacity in cereal dominated cropping systems.
Although improvements to plant production have been facilitated through breeding and targeted gene manipulation of agricultural crops, there is growing evidence that microorganisms associated with crops can impart positive outcomes on biomass production and biogeochemical processes including C turnover in the vicinity of plant roots. Thus, by understanding the relationship between plants and microbes, more-efficient agricultural systems, particularly in dryland or rainfed cropping regions, could be developed through selection of improved plant genotype–microbiome combinations. Another recent approach is the direct crop application of microbe-to-plant signal molecules, such as isoflavonoids to improve crop tolerance to stresses and enhance plant growth, thus avoiding the constraints related to the successful introduction of microbial inoculants.26
In conclusion, clear potential pathways are emerging by which microorganisms may be harnessed either directly or indirectly to increase the amount of C added and stabilised in dryland soils. These will come from both wider rotation and system-based changes, such as appropriate adoption of cover crops and their feedback effects, and also by targeted plant–microbe interactions and introduction of PGPRs, etc. However, all are underpinned by sustaining or enhancing C inputs from the cash crop, and thus there will be occasions when management to maximise this may involve the targeted use of agrochemicals as part of a sustainable agricultural system.
Data availability
Data sharing is not applicable as no new data were generated or analysed during this study.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
The authors received support from the CSIRO Future Science Platform ‘Microbiomes for One‐System Health’.
Acknowledgements
Support from the CSIRO Future Science Platform ‘Microbiomes for One-System Health’ is gratefully acknowledged.
References
[1] Minasny, B et al. (2022) Current NPP cannot predict future soil organic carbon sequestration potential. Comment on “Photosynthetic limits on carbon sequestration in croplands”. Geoderma 424, 115975.| Current NPP cannot predict future soil organic carbon sequestration potential. Comment on “Photosynthetic limits on carbon sequestration in croplands”.Crossref | GoogleScholarGoogle Scholar |
[2] FAO, ITPS (2021) Recarbonizing global soils: a technical manual of recommended management practices. Vol. 2. Food and Agriculture Organization of the United Nations, Rome, Italy.
[3] Jenkinson, DS (1977) The soil microbial biomass. New Zealand Soil News 25, 213–218.
[4] Sinsabaugh, RL et al. (2013) Carbon use efficiency of microbial communities: stoichiometry, methodology and modelling. Ecol Lett 16, 930–939.
| Carbon use efficiency of microbial communities: stoichiometry, methodology and modelling.Crossref | GoogleScholarGoogle Scholar |
[5] Schmidt, MWI et al. (2011) Persistence of soil organic matter as an ecosystem property. Nature 478, 49–56.
| Persistence of soil organic matter as an ecosystem property.Crossref | GoogleScholarGoogle Scholar |
[6] Whalen, ED et al. (2022) Clarifying the evidence for microbial- and plant-derived soil organic matter, and the path toward a more quantitative understanding. Glob Chang Biol 28, 7167–7185.
| Clarifying the evidence for microbial- and plant-derived soil organic matter, and the path toward a more quantitative understanding.Crossref | GoogleScholarGoogle Scholar |
[7] Angers, D et al. (2022) A well-established fact: rapid mineralization of organic inputs is an important factor for soil carbon sequestration. Eur J Soil Sci 73, e13242.
| A well-established fact: rapid mineralization of organic inputs is an important factor for soil carbon sequestration.Crossref | GoogleScholarGoogle Scholar |
[8] Kirkby, CA et al. (2011) Stable soil organic matter: a comparison of C:N:P:S ratios in Australian and other world soils. Geoderma 163, 197–208.
| Stable soil organic matter: a comparison of C:N:P:S ratios in Australian and other world soils.Crossref | GoogleScholarGoogle Scholar |
[9] Creamer, CA et al. (2016) Is the fate of glucose-derived carbon more strongly driven by nutrient availability, soil texture, or microbial biomass size? Soil Biol Biochem 103, 201–212.
| Is the fate of glucose-derived carbon more strongly driven by nutrient availability, soil texture, or microbial biomass size?Crossref | GoogleScholarGoogle Scholar |
[10] Meier, EA et al. (2021) Evaluation of nitrogen bank, a soil nitrogen management strategy for sustainably closing wheat yield gaps. Field Crops Res 261, 108017.
| Evaluation of nitrogen bank, a soil nitrogen management strategy for sustainably closing wheat yield gaps.Crossref | GoogleScholarGoogle Scholar |
[11] Hallama, M et al. (2019) Hidden miners – the roles of cover crops and soil microorganisms in phosphorus cycling through agroecosystems. Plant Soil 434, 7–45.
| Hidden miners – the roles of cover crops and soil microorganisms in phosphorus cycling through agroecosystems.Crossref | GoogleScholarGoogle Scholar |
[12] Jansson, C et al. (2021) Crops for carbon farming. Front Plant Sci 12, 636709.
| Crops for carbon farming.Crossref | GoogleScholarGoogle Scholar |
[13] Herridge, DF et al. (2022) Quantifying country-to-global scale nitrogen fixation for grain legumes II. Coefficients, templates and estimates for soybean, groundnut and pulses. Plant Soil 474, 1–15.
| Quantifying country-to-global scale nitrogen fixation for grain legumes II. Coefficients, templates and estimates for soybean, groundnut and pulses.Crossref | GoogleScholarGoogle Scholar |
[14] Abbott, LK et al. (2018) Potential roles of biological amendments for profitable grain production – a review. Agr Ecosyst Environ 256, 34–50.
| Potential roles of biological amendments for profitable grain production – a review.Crossref | GoogleScholarGoogle Scholar |
[15] Roman, DL et al. (2021) Effects of triazole fungicides on soil microbiota and on the activities of enzymes found in soil: a review. Agriculture 11, 893.
| Effects of triazole fungicides on soil microbiota and on the activities of enzymes found in soil: a review.Crossref | GoogleScholarGoogle Scholar |
[16] Iversen, CM and McCormack, ML (2021) Filling gaps in our understanding of belowground plant traits across the world: an introduction to a virtual issue. New Phytol 231, 2097–2103.
| Filling gaps in our understanding of belowground plant traits across the world: an introduction to a virtual issue.Crossref | GoogleScholarGoogle Scholar |
[17] Freschet, GT et al. (2021) Root traits as drivers of plant and ecosystem functioning: current understanding, pitfalls and future research needs. New Phytol 232, 1123–1158.
| Root traits as drivers of plant and ecosystem functioning: current understanding, pitfalls and future research needs.Crossref | GoogleScholarGoogle Scholar |
[18] Vacheron, J et al. (2013) Plant growth-promoting rhizobacteria and root system functioning. Front Plant Sci 4, 365.
| Plant growth-promoting rhizobacteria and root system functioning.Crossref | GoogleScholarGoogle Scholar |
[19] Gupta VVSR, Sharma AK (eds) (2021) Interactions between microbes and plants. Springer Nature.
[20] Yeoh, YK et al. (2017) Evolutionary conservation of a core root microbiome across plant phyla along a tropical soil chronosequence. Nat Commun 8, 215.
| Evolutionary conservation of a core root microbiome across plant phyla along a tropical soil chronosequence.Crossref | GoogleScholarGoogle Scholar |
[21] Jing, JY et al. (2022) Legacies at work: plant–soil–microbiome interactions underpinning agricultural sustainability. Trends Plant Sci 27, 781–792.
| Legacies at work: plant–soil–microbiome interactions underpinning agricultural sustainability.Crossref | GoogleScholarGoogle Scholar |
[22] Reinhold-Hurek, B et al. (2015) Roots shaping their microbiome: global hotspots for microbial activity. Annu Rev Phytopathol 53, 403–424.
| Roots shaping their microbiome: global hotspots for microbial activity.Crossref | GoogleScholarGoogle Scholar |
[23] Schlaeppi, K et al. (2014) Quantitative divergence of the bacterial root microbiota in Arabidopsis thaliana relatives. Proc Natl Acad Sci USA 111, 585–592.
| Quantitative divergence of the bacterial root microbiota in Arabidopsis thaliana relatives.Crossref | GoogleScholarGoogle Scholar |
[24] Cleveland, CC et al. (2022) Exploring the role of cryptic nitrogen fixers in terrestrial ecosystems: a frontier in nitrogen cycling research. Ecosystems 25, 1653–1669.
| Exploring the role of cryptic nitrogen fixers in terrestrial ecosystems: a frontier in nitrogen cycling research.Crossref | GoogleScholarGoogle Scholar |
[25] Gupta VVSR, Roley S (2023) Nitrogen: non-symbiotic nitrogen fixation in soils. In Encyclopedia of Soils in the Environment (Goss MJ, Oliver MA, eds). Elsevier.
[26] Backer, R et al. (2018) Plant Growth-promoting rhizobacteria: context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front Plant Sci 9, 1473.
| Plant Growth-promoting rhizobacteria: context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture.Crossref | GoogleScholarGoogle Scholar |