Bugs in floods
Mark A. T. Blaskovich A * and Patrick N. A. Harris
A * and Patrick N. A. Harris  B C
B C
A
B
C

Prof. Mark Blaskovich is an ‘antibiotic hunter’ and Director of Translation for the Institute for Molecular Bioscience at The University of Queensland, as well as Director of the ARC Industrial Transformation Training Centre for Environmental and Agricultural Solutions to Antimicrobial Resistance. A medicinal chemist with 15 years of industrial drug development experience that produced a clinical candidate, since 2010 he has been developing new antibiotics, non-antibiotic therapies and diagnostics to detect and treat resistant bacterial and fungal infections. He cofounded the Community for Open Antimicrobial Drug Discovery (CO-ADD), a global ‘crowdsourcing’ antibiotic discovery initiative that has collaborated with over 300 research groups, and has led multiple industry collaborations focused on antibiotic development. |

Dr Patrick Harris is the Acting Statewide Director of Microbiology at Pathology Queensland and UQ Amplify Fellow at The University of Queensland Centre for Clinical Research (UQCCR) in Brisbane, Australia. He holds specialist accreditation in both infectious disease and clinical microbiology, and has worked in the UK, Malawi, Singapore and Australia. His research has a focus on antibiotic resistant bacteria and the use of randomised trials to define optimal management for ESBL- and AmpC-producing Enterobacterales, and was lead author for the influential MERINO trial published in JAMA in 2018. He has also been the clinical lead for the Queensland Genomics infection program, aiming to introduce the routine application of microbial genomics to infection control practice. |
Abstract
Floods are natural disasters that affect millions of people every year, with escalating impact due to a combination of factors that include increasing urbanisation of previously uninhabited land, deforestation, and climate change. Floods do not discriminate between lower–middle income countries (LMICs) and high-income countries, though the types of damage can differ. As a ‘fire or flood’ country, Australia is no exception. Apart from the obvious physical damage to infrastructure and direct impact on human health due to injury and drowning, there is a more insidious danger lurking in floodwaters – a range of microbial pathogens that can opportunistically cause additional morbidity and mortality. These health effects can be both acute, and longer term. This review focuses on bacterial infections that can be attributed to floods, divided into sections that summarise opportunistic infections by commonly seen human pathogens, versus infections caused by more unusual microbes that are normally not encountered until they are released by floods.
Keywords: Burkholderia, disaster management climate, flood pathogens, infectious disease, Leptospirosis, Vibrio.
Australia has a long history of both fire and floods (Fig. 1) – and the two are not unrelated, as wildfires strip the land of water-retaining vegetation and create a water-repellant soil surface, increasing run-off and the likelihood of floods. A 2022 report1 by the UN Environment Programme (UNEP) and GRID-Arendal predicts climate change will lead to a global increase of extreme fires of up to 50% by the end of the century, and this will be accompanied by increased flood events. An analysis of data captured between 1900 and 2019 by the Institute for Economics and Peace found flooding is the most common natural disaster since 1990, with floods accounting for 42% of 9924 natural disasters recorded from 1990 to 2019.2
Urban floodwater in Brisbane in February 2021: local park in Bardon, during and after floods (note person walking through floodwater, circled in yellow); Brisbane River inundating Southbank walkway (images: M. A. T. Blaskovich).

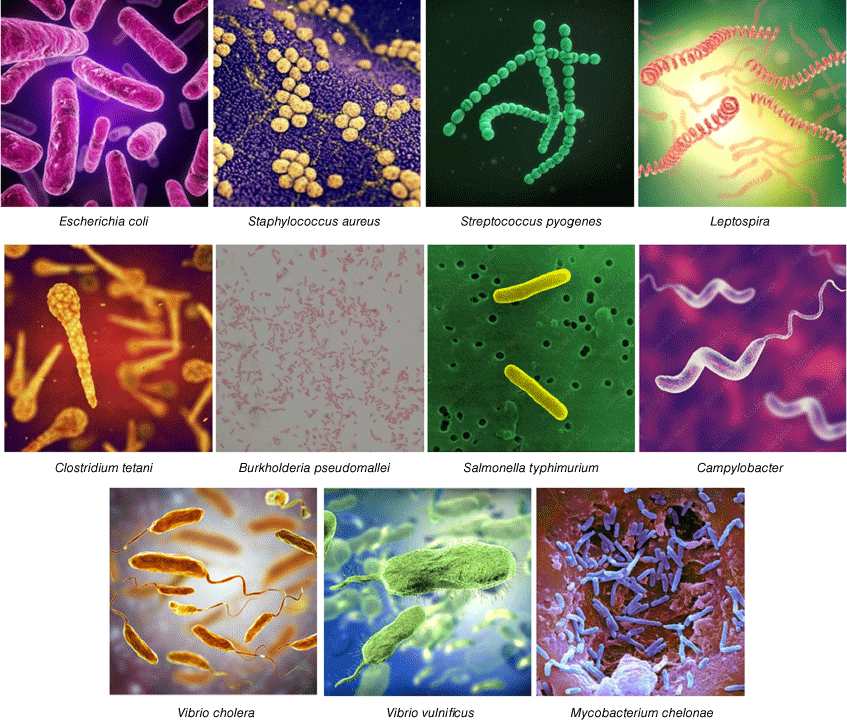
The immediate physical threat of floods to both human health and infrastructure is obvious, but floods also carry a more insidious hidden threat: a range of disease-causing pathogens that lead to infection and illness after the waters have subsided and clean-up begins.3,4 Their impact is exacerbated by cuts and injuries and accidental ingestion sustained during the flood event or during clean-up activities,5 potentially further aggravated by compromised immune systems resulting from the stress of dealing with injury or displacement.6 Although viruses, parasites and fungi also play an important role in flood-related infections, this article focuses on bacterial threats (Fig. 2). Other infecting species and potential treatments for both bacterial and non-bacterial infections are nicely summarised in a 2015 ‘field manual’ developed for responding providers who are engaged in care after a flooding event.7
One obvious effect of floods is environmental contamination due to the spread of animal and human waste containing reasonably common bacteria that are normally of little concern as they are properly contained and controlled. Sewage lines and plants are inundated releasing large quantities of raw sewage, while animal waste from farms and feedlots is swept up by the floodwaters and widely distributed. The main culprit, particularly in resource-rich areas, is Escherichia coli. Accidental ingestion of floodwaters or contaminated drinking water supplies can lead to gastrointestinal illnesses, including vomiting, diarrhoea, stomach pains and fever, with dehydration in young children and the elderly a particular associated risk. These effects can be persistent, with elevated levels of E. coli (and, concerningly, associated antibiotic resistance genes) found in river water samples 6 months after Hurricane Harvey flooding in Houston, Texas.8 Another gastrointestinal concern are Salmonella enterica serotypes, which also spread by water contaminated with faeces of infected humans and animals. Flood events in rural settings have consistently led to the detection of antibiotic-resistant strains of Salmonella, such as in water samples collected in North Carolina after Hurricane Florence.9 Campylobacter spp. and Arcobacter are additional animal-associated pathogens causing gastrointestinal infections that have been found in floodwaters, such as in North Carolina (a region highly dense in facilities that produce food animals, particularly swine and poultry) after Hurricane Florence in 2018.10
Other common human pathogens that lead to increased infections as a result of floods are Staphylococcus aureus and Streptococcus pyogenes. These bacteria can lead to skin infections, including cellulitis and impetigo. S. pyogenes can also lead to respiratory infections by colonisation of the throat (strep throat). The most severe manifestation of S. pyogenes infection includes necrotising fasciitis and toxic shock syndrome, which are associated with high risk of mortality and severe morbidity (e.g. need for limb amputation). When flood victims have aspirated water during near-drowning, pneumonia is highly likely, with a range of pathogens possible.11 These cases are often polymicrobial and complicated by abscess formation or empyema. Although some reports have identified acute respiratory infections (cough, sore throat, fatigue, aches and chest or nasal congestion) as the most common infectious disease after flooding, the vast majority are likely due to viral infections.12
Another zoonotic infection spread by floods is leptospirosis, caused by the corkscrew-shaped obligate aerobic spirochaete Gram-negative bacteria Leptospira spp. (including L. interrogans and L. borgpetersenii) with symptoms including muscle pain, fever and headaches. These bacteria are spread through the urine of infected animals, including rodents, livestock and domestic pets.13 A meta-analysis of observational studies suggests that flooding is associated with the risk of occurrence of leptospirosis in endemic countries.14 North Queensland has one of the highest incidences of leptospirosis in the world.15 There was a 65% upswing in cases in South-East Queensland in the weeks after the 2011 floods16; in Central Queensland nine cases were identified in the 3 months following the floods, compared to nine confirmed cases in the 7 years prior to the flood.17
The second broad category of flood-associated infections result from microorganisms that live in the environment. One of the best known is Clostridium tetani, a common spore-forming Gram-positive motile, anaerobic soil bacterium that is the cause of tetanus. Tetanus immunisation programs normally provide protection, but in areas where immunisation is low, the combination of C. tetani released by flooding and skin abrasion or cuts due to physical damage can lead to a significant number of cases. The 2004 tsunami in Indonesia led to 106 cases of tetanus in Aceh province, of which 18.9% were fatal.18 Of particular concern to Australia is Burkholderia pseudomallei, another soil-dwelling bacterium that is endemic in tropical regions such as northern Australia and Southeast Asia, though it has also been reported along the Gulf Coast of Mississippi in the United States (US Centers for Disease Control and Prevention, see www.cdc.gov/melioidosis/index.html, accessed 20 July 2023). Infections by this Gram-negative, bipolar, aerobic, non-spore-forming, motile, rod-shaped bacterium cause the clinical syndrome of melioidosis, often presenting with severe pneumonia, sepsis and bloodstream infection, visceral abscesses or with involvement of the central nervous system. The disease can affect multiple organs, or cause localised infections in the skin. Chronic respiratory, cutaneous or visceral disease may also present less acutely and mimic other conditions such as tuberculosis or malignancy (Queensland Government, see http://conditions.health.qld.gov.au/HealthCondition/condition/14/33/455/melioidosis, accessed 20 July 2023). Treatment requires extended antibiotic therapy for over 3 months. Case clusters of melioidosis in the Northern Territory of Australia are associated with extreme weather events.19 A study of melioidosis in the remote Katherine region of northern Australia identified 128 patients from 1989 to 2021, with nine attributed to the flooding of the Katherine River in 1998.20 Flooding in Townsville, Qld, in 2019 affected more than 3000 properties and led to more than 10 cases of melioidosis, with one death.21 Melioidosis can often be deadly – a cluster of 10 cases in eastern Sri Lanka identified in the 2 months following an extreme weather event in 2015 had a mortality rate of 40%.22
Other environmental bacteria live in the water. Many natural disasters, including floods, lead to outbreaks of potentially lethal cholera, particularly in poorly resourced countries. Cholera is caused by highly virulent Vibrio cholerae (e.g. serogroups O1 or O139), a comma-shaped Gram-negative, facultative anaerobe which colonises the small intestine. It then releases large quantities of a toxin (cholera toxin, CT or CTX), a protein complex formed from six subunits that activates the CFTR ion channel, releasing chloride ions that draws water into the intestine (World Health Organization, see www.who.int/news-room/fact-sheets/detail/cholera, accessed 20 July 2023). Cholera can be easily treated through oral rehydration solution or intravenous fluids. Antibiotics are not recommended to stop outbreaks, but vaccination is an effective preventative strategy (see www.who.int/news-room/fact-sheets/detail/cholera). Floods in Nigeria in 2022 led to more than 6000 suspected cases and a 4–5% case fatality ratio,23 while monsoon floods in Pakistan in 2022 led to nearly 2000 confirmed cases of cholera across all four provinces.24 V. cholerae is halotolerant and is naturally found in brackish and estuarine environments.25 Other Vibrio spp. infections are associated with saltwater exposure resulting from storm surges: Hurricane Katrina in 2005 led to 22 identified cases of Vibrio illness and five deaths, mainly due to wound infections caused by V. vulnificus or V. parahaemolyticus, with five deaths reported.26
Aeromonas species are Gram-negative, rod-shaped facultatively anaerobic bacteria found in fresh or brackish water, and have been shown to form biofilms on various biotic and abiotic surfaces.27 The species considered to be potential human pathogens include A. hydrophila, A. caviae and A. veronii biovar sobria.28 They predominantly cause gastrointestinal tract disorders, but are also associated with infections in wounds, soft tissues, and other sites. A study of a subset of hospitalised patients from the 2004 tsunami in Thailand found 515 of 777 had skin and soft-tissue infections: 22% of cultured isolates were Aeromonas spp.29 An Australian study of bacterial pathogens in 5 consecutive years (2015–2019) from 375 842 stool samples of patients with gastroenteritis found Aeromonas species were the third most common bacterial pathogens, following Campylobacter and Salmonella, though no flood association was noted.30
A range of other Gram-negative bacteria that may lead to soft-tissue infections after water exposure include facultatively anaerobic motile Shewanella spp., a normal component of the surface flora of fish. Shewanella putrefaciens, S. algae, S. haliotis and S. xiamenensis all cause rare cases of human infection31,32; though most literature cases do not appear to be specifically associated with flood exposure, a news report describes a North Carolina case of S. putrefaciens after Hurricane Florence in 2018.33 Leclercia adecarboxylata is a facultative–anaerobic motile bacillus of the Enterobacteriaceae family recognised as an emerging opportunistic pathogen, with rare infections generally found in immunocompromised patients.34 A polymicrobial infection including L. adecarboxylata was identified in a man in his 80s who had cleaned up floodwater in his basement after Hurricane Irene in the US in 2011.35 Chromobacterium violaceum is a facultatively anaerobic coccobacillus that usually inhabits soil and stagnant water; it produces a unique violacein pigment when cultured. Skin infections can rapidly progress to sepsis, with mortality rates of 48–64%.36
Non-tuberculous mycobacteria (NTM) are ubiquitous in the environment and may also cause infections after exposure to floodwater, particularly rapid growing Mycobacterium species such as M. fortuitum, M. chelonae and M. abscessus.37 In an analysis of NTM infections in Missouri (USA), risk of geographical case clustering was significantly higher in counties with >5 flooding events per year v. no flooding (rate ratio 2.82, 95% CI 1.90–4.19).38 Typically, NTM can cause indolent skin and soft tissue infections in healthy individuals, which may fail to respond to first-line antibiotics, but can also cause more aggressive or disseminated disease in immunocompromised hosts. Specific laboratory techniques may be required to isolate and identify mycobacteria from clinical samples, so close liaison with the laboratory is essential in suspected cases.
In summary, bacterial infections resulting from exposure to contaminated water during floods present a significant health risk. Physicians should be aware of the potential for unusually rare cases in the months following severe weather events, and patients with infection symptoms should remember to inform their doctors if they have been exposed to floodwaters. Simple precautions by the general population, such as avoiding entry into floodwaters and wearing protective equipment during cleanup, can substantially alleviate the threat.
References
1 United Nations Environment Programme (2022) Spreading like wildfire – the rising threat of extraordinary landscape fires. A UNEP Rapid Response Assessment. UNEP, Nairobi, Kenya. www.unep.org/resources/report/spreading-wildfire-rising-threat-extraordinary-landscape-fires(accessed 20 July 2023)
2 Vision of Humanity (2020) Climate: increase in natural disasters on a global scale by ten times. Institute for Economics & Peace. www.visionofhumanity.org/global-number-of-natural-disasters-increases-ten-times/(accessed 20 July 2023)
3 Paterson DL et al. (2018) Health risks of flood disasters. Clin Infect Dis 67, 1450-1454.
| Crossref | Google Scholar | PubMed |
4 Talbot CJ et al. (2018) The impact of flooding on aquatic ecosystem services. Biogeochemistry 141, 439-461.
| Crossref | Google Scholar | PubMed |
5 Fewtrell L et al. (2011) The microbiology of urban UK floodwaters and a quantitative microbial risk assessment of flooding and gastrointestinal illness. J Flood Risk Manag 4, 77-87.
| Crossref | Google Scholar |
6 Segerstrom SC, Miller GE (2004) Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull 130, 601-630.
| Crossref | Google Scholar | PubMed |
7 Bandino JP et al. (2015) The infectious and noninfectious dermatological consequences of flooding: a field manual for the responding provider. Am J Clin Dermatol 16, 399-424.
| Crossref | Google Scholar | PubMed |
8 Yu P et al. (2018) Elevated levels of pathogenic indicator bacteria and antibiotic resistance genes after Hurricane Harvey’s flooding in Houston. Environ Sci Technol Lett 5, 481-486.
| Crossref | Google Scholar |
9 Mao Y et al. (2021) Distribution and antibiotic resistance profiles of Salmonella enterica in rural areas of North Carolina after Hurricane Florence in 2018. GeoHealth 5, e2020GH000294.
| Crossref | Google Scholar | PubMed |
10 Niedermeyer JA et al. (2020) Search for Campylobacter spp. reveals high prevalence and pronounced genetic diversity of Arcobacter butzleri in floodwater samples associated with Hurricane Florence in North Carolina, USA. Appl Environ Microbiol 86, e01118-20.
| Crossref | Google Scholar | PubMed |
11 Maegele M et al. (2006) One year ago not business as usual: wound management, infection and psychoemotional control during tertiary medical care following the 2004 tsunami disaster in Southeast Asia. Critical Care 10, R50.
| Crossref | Google Scholar | PubMed |
12 Carroll B et al. (2010) Health and social impacts of a flood disaster: responding to needs and implications for practice. Disasters 34, 1045-1063.
| Crossref | Google Scholar | PubMed |
13 Lau CL et al. (2010) Climate change, flooding, urbanisation and leptospirosis: fuelling the fire? Trans R Soc Trop Med Hyg 104, 631-638.
| Crossref | Google Scholar | PubMed |
14 Naing C et al. (2019) Risk factors for human leptospirosis following flooding: a meta-analysis of observational studies. PLoS One 14, e0217643.
| Crossref | Google Scholar | PubMed |
15 Pappas G et al. (2008) The globalization of leptospirosis: worldwide incidence trends. Int J Infect Dis 12, 351-357.
| Crossref | Google Scholar | PubMed |
16 NNDSS Annual Report Writing Group (2012) Australia’s notifiable disease status, 2012: annual report of the National Notifiable Diseases Surveillance System. www1.health.gov.au/internet/main/publishing.nsf/Content/cda-cdi3901-pdf-cnt.htm/$FILE/cdi3901g.pdf (accessed 20 July 2023)
17 Smith JKG et al. (2013) Leptospirosis following a major flood in Central Queensland, Australia. Epidemiol Infect 141, 585-590.
| Crossref | Google Scholar | PubMed |
18 Aceh Epidemiology Group (2006) Outbreak of tetanus cases following the tsunami in Aceh province, Indonesia. Glob Public Health 1, 173-177.
| Crossref | Google Scholar | PubMed |
19 Cheng AC et al. (2006) Extreme weather events and environmental contamination are associated with case-clusters of melioidosis in the Northern Territory of Australia. Int J Epidemiol 35, 323-329.
| Crossref | Google Scholar | PubMed |
20 Hodgetts K et al. (2022) Melioidosis in the remote Katherine region of northern Australia. PLoS Negl Trop Dis 16, e0010486.
| Crossref | Google Scholar | PubMed |
21 Layt S (2019) Deadly bacteria highlights dangers of Townsville flood clean-up. In Brisbane Times, 13 February 2019. www.brisbanetimes.com.au/national/queensland/deadly-bacteria-highlights-dangers-of-townsville-flood-clean-up-20190213-p50xkq.html (accessed 20 July 2023)
22 Jayasinghearachchi HS et al. (2021) Nonclonal Burkholderia pseudomallei population in melioidosis case cluster, Sri Lanka. Emerging Infect Dis 27, 2955-2957.
| Crossref | Google Scholar | PubMed |
23 International Rescue Committee (2022) Deadly flooding in Nigeria leads to major cholera outbreak; IRC scaling up flood and health response. Press Release 21 October 2022. www.rescue.org/press-release/deadly-flooding-nigeria-leads-major-cholera-outbreak-irc-scaling-flood-and-health (accessed 20 July 2023)
24 Malikzai A et al. (2023) Cholera spike following monsoon floods in Pakistan: challenges, efforts and recommendations (short communication). Int J Surg Open 57, 100652.
| Crossref | Google Scholar |
25 Vezzulli L et al. (2010) Environmental reservoirs of Vibrio cholerae and their role in cholera. Environ Microbiol Rep 2, 27-33.
| Crossref | Google Scholar | PubMed |
26 Centers for Disease Control and Prevention (2005) Vibrio illnesses after Hurricane Katrina – multiple states, August–September 2005. MMWR Morb Mortal Wkly Rep 54, 928-931.
| Google Scholar | PubMed |
27 Batra P et al. (2016) Aeromonas spp.: an emerging nosocomial pathogen. J Lab Physicians 8, 001-004.
| Crossref | Google Scholar | PubMed |
28 Pessoa RBG et al. (2022) Aeromonas and human health disorders: clinical approaches. Front Microbiol 13, 868890.
| Crossref | Google Scholar | PubMed |
29 Hiransuthikul N et al. (2005) Skin and soft-tissue infections among tsunami survivors in southern Thailand. Clin Infect Dis 41, e93-e96.
| Crossref | Google Scholar | PubMed |
30 Yuwono C et al. (2021) The isolation of Aeromonas species and other common enteric bacterial pathogens from patients with gastroenteritis in an Australian population. Microorganisms 9, 1440.
| Crossref | Google Scholar | PubMed |
31 Poovorawan K et al. (2013) Shewanella haliotis associated with severe soft tissue infection, Thailand, 2012. Emerg Infect Dis 19, 1019-1021.
| Crossref | Google Scholar | PubMed |
32 Zong Z (2011) Nosocomial peripancreatic infection associated with Shewanella xiamenensis. J Med Microbiol 60(9), 1387-1390.
| Crossref | Google Scholar |
33 Akpan N (2018) After hurricanes, why is it so hard to test for waterborne diseases? In PBS News Hour, 21 November 2018. www.pbs.org/newshour/science/after-hurricanes-why-is-it-so-hard-to-test-for-waterborne-diseases (accessed July 20 2023)
34 Zayet S et al. (2021) Leclercia adecarboxylata as emerging pathogen in human infections: clinical features and antimicrobial susceptibility testing. Pathogens 10, 1399.
| Crossref | Google Scholar | PubMed |
35 Tam V, Nayak S (2012) Isolation of Leclercia adecarboxylata from a wound infection after exposure to hurricane-related floodwater. BMJ Case Rep 2012, bcr-2012-007298.
| Crossref | Google Scholar | PubMed |
36 Ponte R, Jenkins SG (1992) Fatal Chromobacterium violaceum infections associated with exposure to stagnant waters. Pediatr Infect Dis J 11, 583-586.
| Crossref | Google Scholar | PubMed |
37 Appelgren P et al. (2008) Late-onset posttraumatic skin and soft-tissue infections caused by rapid-growing mycobacteria in tsunami survivors. Clin Infect Dis 47, e11-e16.
| Crossref | Google Scholar | PubMed |
38 Mejia-Chew C et al. (2023) Spatial epidemiologic analysis and risk factors for nontuberculous mycobacteria infections, Missouri, USA, 2008–2019. Emerg Infect Dis 29(8), 1540-1546.
| Crossref | Google Scholar | PubMed |
 Prof. Mark Blaskovich is an ‘antibiotic hunter’ and Director of Translation for the Institute for Molecular Bioscience at The University of Queensland, as well as Director of the ARC Industrial Transformation Training Centre for Environmental and Agricultural Solutions to Antimicrobial Resistance. A medicinal chemist with 15 years of industrial drug development experience that produced a clinical candidate, since 2010 he has been developing new antibiotics, non-antibiotic therapies and diagnostics to detect and treat resistant bacterial and fungal infections. He cofounded the Community for Open Antimicrobial Drug Discovery (CO-ADD), a global ‘crowdsourcing’ antibiotic discovery initiative that has collaborated with over 300 research groups, and has led multiple industry collaborations focused on antibiotic development. |
 Dr Patrick Harris is the Acting Statewide Director of Microbiology at Pathology Queensland and UQ Amplify Fellow at The University of Queensland Centre for Clinical Research (UQCCR) in Brisbane, Australia. He holds specialist accreditation in both infectious disease and clinical microbiology, and has worked in the UK, Malawi, Singapore and Australia. His research has a focus on antibiotic resistant bacteria and the use of randomised trials to define optimal management for ESBL- and AmpC-producing Enterobacterales, and was lead author for the influential MERINO trial published in JAMA in 2018. He has also been the clinical lead for the Queensland Genomics infection program, aiming to introduce the routine application of microbial genomics to infection control practice. |