Occurrence of Nocardia in near shore marine environments and its public and environmental implications
Luke Wright A , Mohammad Katouli A and İpek Kurtböke A *A

Dr Luke Wright graduated from the University of the Sunshine Coast, where he earned a BSc, Honours, and a PhD under the supervisions of Assoc. Prof. İpek Kurtböke and Assoc. Prof. Mohammad Katouli investigating the occurrence of Nocardia species in near shore marine environments of the Sunshine Coast Region. His study revealed the presence of novel Nocardia species in the foaming waters, and he described two new species in a collaborative investigation with the DSMZ in Germany. Dr Wright’s study increased awareness in the region and contributed toward international studies related to mapping of nocardiae in four corners of the world. |

Assoc. Prof. Mohammad Katouli is a member of the Centre for Bioinnovation (CBI) and the School of Science, Technology and Engineering at the University of the Sunshine Coast. He has been working for many years on pathogenic mechanisms of bacteria isolated from clinical and environmental sources. His current focus is on how probiotic strains can mitigate the pathogenicity of translocating (TEC) and adherent and invasive Escherichia coli (AIEC) strains in intestinal epithelial and modulates host cell gene expression. Since 1998, he has been a member of the ASM and the chair of the Queensland branch committee between 2014 and 2016. |

Assoc. Prof. İpek Kurtböke has been working in the field of biodiscovery and has been an active member of the international actinomycete research community since 1982. She currently conducts research and teaches in the field of applied microbiology and biotechnology at the University of the Sunshine Coast, Queensland. She has also been an active member of the World Federation of Culture Collections (WFCC) and currently is the President of the Federation. She was also an Editorial Board member of Microbiology Australia for 20 years (2004–2024). |
Abstract
Since 2003, large foaming events resembling ‘chocolate mousse-like foam’ have been reported along several popular beaches on the Sunshine Coast, Queensland, Australia, following storms and turbulent sea water events. Earlier studies at the University of the Sunshine Coast (UniSC) first highlighted that these foaming events contained an abundance of filamentous actinomycete species, specifically from the genus Nocardia. Species of this genus are frequently detected in the foams of sewage treatment plants during the activated sludge process. Nocardiae can form an extensive mycelial network and trap fats, oil and grease, which exacerbates foam formation. In addition, Nocardia species are known to be pathogenic and cause various diseases including nocardiosis in human and animal hosts. This review focusses on the occurrence, diversity and virulence properties of Nocardia species with examples of isolations of Nocardiae from foaming coastal marine waters of the region.
Introduction
Nocardiae were first isolated in cattle in 1888 as the causative agent of fatal bovine farcy by the veterinarian Edmond Nocard.1 In 1889, Trevisan proposed a new genus (Nocardia) and named this strain Nocardia farcinica.2 At present, there are currently more than 119 validly and non-validly published names of species belonging to the Nocardia genus (https://bacterio.net/genus/nocardia).3 Nocardiae are Gram-positive filamentous bacteria and although they are aerobic or facultatively anaerobic many species also display a level of acid fastness.4 Typically, Nocardia species produce branching hyphae which form an extensive network of substrate and aerial mycelia which bears the spores.5
The first Nocardia isolated from a human host was reported in a patient suffering from pneumonia and brain abscess in 1890.6 Since then nocardiae are considered to be opportunistic pathogens and are known to cause a variety of human and animal infections.7,8 Examples of species that are involved in pathogenesis of human disease include Nocardia asteroides and N. nova that are well-documented pathogenic species of the genus Nocardia responsible for causing nocardiosis in human and animal hosts.7,8 Whereas N. seriolae is well-known for its role in causing nocardiosis in marine life, particularly in fish species.9
Ecology of Nocardia
Natural environments
Nocardiae are saprophytic bacteria and are ubiquitous in the environment, commonly found in soil, water bodies, and decaying vegetation.10 Nocardia properties include their ability to produce spores, an extensive substrate and aerial mycelia network5 and genes encoding for osmolytes (compounds which aid cells in maintaining a high intracellular osmotic concentration) which appear to facilitate their existence in harsh environments. This characteristic might be the reason of their occurrence in coastal marine waters and sandy beaches11 where the estimated salt concentration in sea water is approximately 3.5% (w/w).11
Man-made environments
The man-made environments where nocardiae are most predominant are sewage treatment plants (STPs) where they are known to cause stable foams12–14 (Fig. 1). Generally, excess foam formation in STPs is controlled by the addition of chlorine, mechanical removal and/or proprietary chemicals15 or using nocardiae specific bacteriophages.16,17
The vicious foam cycle. Figure is copyright Aquafix and sourced from: http://www.teamaquafix.com/nocardia.aspx (last acccessed 8 November 2020). Reproduced with Permission of Aquafix (https://teamaquafix.com).
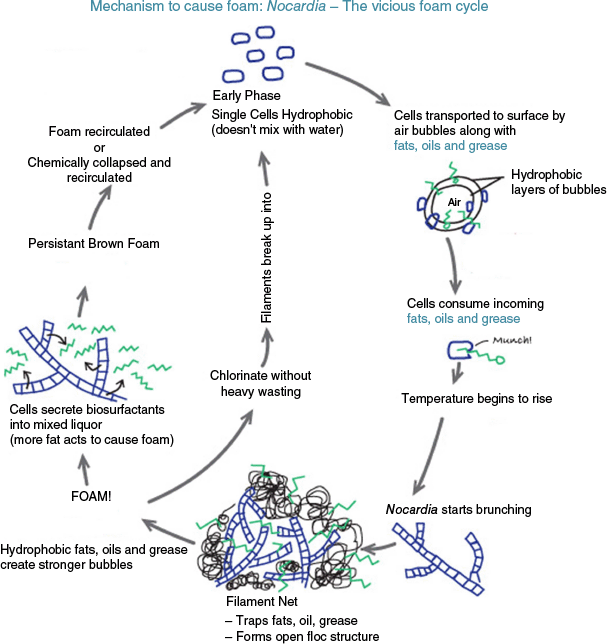
High amounts of lipids (e.g. greases and fatty acids) in the sewage is the primarily cause of excess Nocardia growth, while warmer temperatures (e.g. 28–35°C) further contribute to its proliferation.18
Aetiology and epidemiology of nocardiae
The most common site of nocardiosis is generally the pulmonary system (lung/s; alveoli), cutaneous (skin) or the subcutaneous tissue.19 A primary site of infection has potential to spread (disseminate) to other parts/organs of the body producing a secondary infection site, e.g. brain/cerebral. Persons most at risk of developing nocardiosis are those with compromised immune systems, but on rarer occasions, nocardiosis may also affect immunocompetent persons.20
Pulmonary nocardiosis typically arises from the inhalation of nocardia cells and or spores. In contrast, cutaneous/sub-cutaneous nocardiosis is usually caused via direct skin contact (usually through an open wound).19 Repeated exposure to pathogenic species of Nocardia through these routes of transmission may increase the risk of developing disease. Different climatic zones and geographic locations have been shown to play a role in the distribution of pathogenic bacterial species; most notably in tropical and subtropical regions.21 This is evident with actinomycetoma which is considered endemic, and a major health concern in many countries/areas situated in tropical and subtropical regions, e.g. Sudan.21
According to the Centres for Disease Control and Prevention (CDC; https://www.cdc.gov/nocardiosis/hcp/clinical-overview/) in the United States, approximately 500–1000 new cases of nocardiosis occur every year. However globally, little information is available of the incidence of nocardiosis. For example, the incidence of nocardiosis in Australia is relatively unknown as this information has not been made available by The Australian Institute of Health and Welfare (AIHW; https://www.aihw.gov.au/). The institute is an independent statutory Australian Government agency responsible for the collection and dissemination of health data gathered from Australian Government agencies, e.g. states and territories.
Interaction of nocardiae with the host cells
The adhesion, invasion and/or translocation capabilities of pathogenic Nocardia species have not been well defined using human cell culture models in vitro (e.g. lung, skin and brain/cerebral cell lines), especially for those species associated with foaming coastal marine waters. Adhesion, invasion, and translocation assays in vitro using human cell lines are typically used to assess and quantify the protective capability of such cells against a foreign invader/organism, and the ability of the invader/organism to adhere and navigate the host’s immune defence. Several studies to date have used cell culture models to investigate interaction of pathogenic bacteria such as Mycobacterium tuberculosis with human cells.22 However, few studies have been conducted using cell culture models (particularly human lung cells) for analysis of the adhesion and translocation properties of pathogenic Nocardia species.23 Those studies have typically used cultured strains isolated from diseased human tissue, but there is a paucity of information about the adhesion and invasion capability of pathogenic Nocardia species isolated from environmental samples, particularly from foaming coastal marine waters and sandy beaches that are popular with beachgoers.
Antibiotic susceptibility of major pathogenic nocardiae
Due to the variation in drug susceptibility and the increasing number of clinically significant Nocardia species identified in the literature, six drug-susceptibility profiles (resistance categories) have been constructed based on Nocardia susceptibility patterns24,25 (Table 1), serving as a reference for clinicians to accurately determine the appropriate antibiotic(s) for treating nocardiosis.
| Species | Corresponding type drug pattern | Major drug pattern characteristics | |
|---|---|---|---|
| N. abscessus | I | Susceptible to ampicillin, amoxicillin-clavulanic acid, ceftriaxone, linezolid, and amikacin; most have resistant MICs for imipenem; resistant to ciprofloxacin and clarithromycin | |
| N. brevicatena/paucivorans complex, unnamed group | II | Same as type I but kanamycin MICs low (<1 µg/mL) and susceptible to ciprofloxacin; usually resistant to gentamicin; resistant to clarithromycin | |
| N. nova complex (N. nova, N. veterana, N. africana, N. kruczakiae) | III | Susceptible to ampicillin but resistant to amoxicillin-clavulanic acid; susceptible to erythromycin, clarithromycin, linezolid, and ceftriaxone; very low MICs to imipenem and amikacin | |
| N. transvalensis complex | IV | Resistant to all aminoglycosides, including amikacin; susceptible to ciprofloxacin, ceftriaxone, linezolid, and imipenem; resistant to erythromycin and clarithromycin | |
| N. farcinica | V | Resistant to ampicillin, broad-spectrum cephalosporins, and clarithromycin; resistant to aminoglycosides except amikacin; susceptible to ciprofloxacin, linezolid, and imipenem | |
| N. asteroides complex | VI | Resistant to ampicillin, amoxicillin-clavulanic acid, clarithromycin, and ciprofloxacin; susceptible to ceftriaxone, amikacin, linezolid, and imipenem | |
| N. cyriacigeorgica | Removed from VI | N. cyriacigeorgica (formerly considered part of the N. asteroides complex, specifically type VI) was removed from the complex due to 3 main reasons: (1) shows a different drug susceptibility profile (notably ampicillin sensitivity), (2) genetically unique and (3) has distinct phenotype features The ATCC type strain is susceptible to ampicillin, other drug susceptibilities are the same as for pattern VI | |
| N. brasiliensis | NA A | Susceptible to minocycline, amoxicillin-clavulanic acid, carbenicillin, and sulfamethoxazole; resistant to kanamycin, cefamandole, ampicillin, ciprofloxacin, and clarithromycin | |
| N. pseudobrasiliensis | NA A | Susceptible to carbenicillin, ciprofloxacin, clarithromycin, and sulfamethoxazole; resistant to kanamycin, cefamandole, ampicillin, minocycline, and amoxicillin-clavulanic acid | |
| N. otitidiscaviarum | NA A | Susceptible to kanamycin, gentamicin, amikacin, sulfamethoxazole, and ciprofloxacin; resistant to ceftriaxone, ampicillin, amoxicillin-clavulanic acid, carbenicillin, and imipenem (often resistant to all β-lactam antibiotics) |
Linezolid and amikacin were found to be the most effective antibiotic/s for the treatment of nocardiosis.25 A study by Tan et al. (2020) confirmed that amikacin was a highly effective antibiotic against clinical Nocardia isolates from a leading tertiary laboratory in Australia.27
Nocardiae associated with foaming coastal waters of Sunshine Coast Region of Australia
Beach foam is generally caused by the agitation of sea water (e.g. by storms and adverse weather conditions) and can be exacerbated with the addition (or higher concentrations) of organic compounds such as lipids and proteins, which may act as surfactants (Fig. 2). These surfactants may further trap air bubbles, in turn increasing foam viscosity. Surfactants originating from bacteria may also be a major contributor to beach foam formation.28 Moreover, the world’s tropical zone has been identified (as or to be) expanding poleward at an increasing rate.29,30 This phenomenon may lead to a shift in a disease-causing agent’s ecological niche, in turn, producing endemicity of the disease in new locations. Australia is a large continent containing three main climatic zones; equatorial, tropical and sub-tropical.31 However, information on the occurrence, distribution and diversity of possible foam-associated bacteria, particularly nocardiae, is incomplete at the Australian and global level.
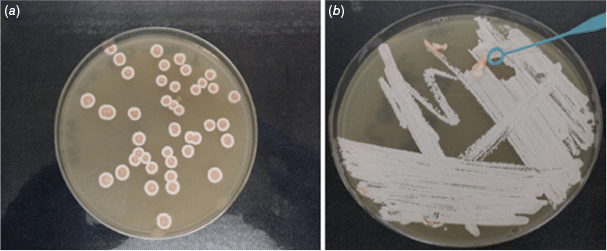
Ongoing studies at the University of the Sunshine Coast since 2003 revealed the presence of nocardiae within the foaming coastal marine waters including novel species32–36 (Fig. 3). The Sunshine Coast is situated in the sub-tropical region of Australia. The mean (average) maximum summer temperature on the Sunshine Coast has risen by 2°C from 2007 to 2017. The optimal growth temperature of most actinomycetes, including most Nocardia species, is between 28 and 30°C. Nocardia-mediated foam formation generally occurs in warmer climates within this temperature range.18 Such an increase may lead to higher numbers of nocardiae present in these environments.
Nocardia isolation plate (a) and a pure isolate (b) from Sunshine Coast foaming marine waters.
Public risk of nocardiae
Aerosols generated by the formation of beach foam which carry microorganisms may have the potential to be transmitted further inland through the beach and coastal zone.37,38 This could thus be a public health hazard if aerosols are transmitting pathogenic Nocardia species as well. However, there is little to no data in the scientific literature in recent years on the potential of foam creation resulting in the aerosolisation of pathogenic bacteria. An early study by Filipkowska et al. (2000)39 assessed the volume of bacteria aerosolised from a wastewater treatment plant with activated sludge tanks. The highest number of heterotrophic bacteria was greater than 1000/m3 of air detected at 100 m outside the wastewater plant’s fence line (boundary). The authors surmise that the main source of microbial air pollution was due to the aeration tanks which are exposed to the atmosphere. Although the Sunshine Coast Region of Australia has experienced several foaming events (particularly in the summer seasons) along its coastal shoreline since the early 2000s, no studies to date have correlated a link between beach foaming events and an increase in human pulmonary and or cutaneous nocardiosis cases.
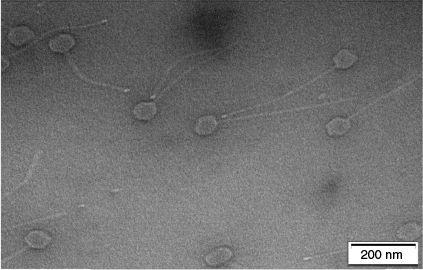
Moreover, at present, there is little data available on the ecology of pathogenic Nocardia species inhabiting sandy beaches, although a study by Seo and Lee (2006)11 isolated Nocardia harenae in the Jeju Island of Korea. Subsequently in 2012, Nocardia harenae was identified as the causative agent of mycetoma in a human patient.40 Another study by Bai et al. (2016)41 isolated another novel actinomycete strain (KLBMP S0027) from coastal soil of the coast of Lianyungang, Jiangsu Province, east China, which was found to be a new species of the genus Nocardia and named as Nocardia jiangsuensis. The closest Nocardia relative was shown to be N. harenae, with 98.5% 16S rRNA gene sequence similarity. University of the Sunshine Coast studies have also identified large numbers of Nocardia-specific phages in the nearshore marine environments which indicates presence of the members of this genus as hosts including human pathogenic species (Tables 2 and 3, Fig. 4).
| Culture collection strain IDs | Phage susceptibility | |
|---|---|---|
| N. shimofusensis (DSMZ-44733) | − | |
| N. takedensis (DSMZ-44802) | ± | |
| N. cumidelens/soli (DSMZ-44488) | ++ | |
| N. soli (DSMZ-44490) | +++ | |
| N. uniformis (DSMZ-43136) | ++ | |
| N. salmonicida (DSMZ-40472) | ++ | |
| N. pseudovaccinii (DSMZ-43406) | ++ | |
| N. veterana (DSMZ-44445) | ++ | |
| N. fluminea (DSMZ-44489) | − | |
| N. flavorosea (DSMZ-44480) | +++ | |
| N. asteroides (ACM-131) | ++ | |
| N. asteroides (ACM-2963) | ++ |
+++, highly susceptible (complete lysis); ++, susceptible (complete partial lysis); +, moderately susceptible (lysis and single plaques); ±, low susceptibility (lysis but regrowth of the host); −, not susceptible.
Nocardiae phages isolated from Sunshine Coast near shore marine environments displaying icosahedral heads and non-contractile tails (magnification: 80,000×, high voltage: 100 kV).
In conclusion, further research is necessary, including compiling data from various global regions affected by extreme climate events to gain a better understanding of the microbial ecology of sandy beaches and nearshore marine environments, especially focusing on microorganisms that could pose health risks to swimmers and beachgoers. Two contemporary methods for detecting pathogenic bacteria in sandy beaches and nearshore marine environments are metagenomics, which can analyse DNA directly from those environments to identify a broad range of microorganisms, and quantitative PCR (qPCR), which can quantify specific pathogens based on genetic markers with a high degree of sensitivity and specificity. Advanced molecular techniques combined with conventional methods such as determination of host-phage interactions in such environments will facilitate obtaining information on changing ecology of nocardiae in relation to changing climatic conditions. As this issue is jointly produced by Malaysian Society for Microbiology, authors invite South to South Cooperation and beyond (https://unsouthsouth.org/) to generate further information on nocardiae in the Southeast Asia Pacific Region.
References
1 Nocard E (1888) Note sur la maladie des boeufs de la Gouadeloupe connue sous le nom de farcin. Ann Inst Pasteur (Paris) 2, 293-302 [In French].
| Google Scholar |
3 List of Prokaryotic names with Standing in Nomenclature (LPSN). Nocardia. Available at https://bacterio.net/genus/nocardia
4 Rawat V, Umesh U, Thapliyal N, Punera DC (2012) Primary pulmonary infection caused by 20% acid fast Nocardia brasiliensis. Indian J Med Microbiol 29, 446-447.
| Google Scholar |
6 Eppinger HL (1890) Über eine neue pathogene Cladothrix und eine durch sie hervorgerufene Pseudotuberkulosis (Cladothrichia). Beitr Pathol Anat Allg Pathol 9, 287-328 [In German].
| Google Scholar |
7 CDC. Nocardiosis—About Nocardiosis; 2024. https://www.cdc.gov/nocardiosis/about/index.html
8 MSD Veterinary Manual. Nocardiosis in animals; 2024. https://www.msdvetmanual.com/infectious-diseases/nocardiosis/nocardiosis-in-animals#Clinical-Findings_v3273859
9 Lee D-C, Cho M-Y, Park S-I (2007) A review of nocardial infection in fishes. J Fish Pathol 20, 1-23.
| Google Scholar |
11 Seo JP, Lee SD (2006) Nocardia harenae sp. nov., an actinomycete isolated from beach sand. Int J Syst Evol Microbiol 56, 2203-2207.
| Crossref | Google Scholar | PubMed |
12 Heard J, Harvey E, Johnson BB, Wells JD, Angove MJ (2008) The effect of filamentous bacteria on foam production and stability. Colloids Surf B Biointerfaces 63, 21-26.
| Crossref | Google Scholar | PubMed |
13 Jiang C, McIlroy SJ, Qi R, Petriglieri F, Yashiro E, Kondrotaite Z, Nielsen PH (2021) Identification of microorganisms responsible for foam formation in mesophilic anaerobic digesters treating surplus activated sludge. Water Res 191, 116779.
| Crossref | Google Scholar | PubMed |
14 Blackall LL, Harbers AE, Greenfield PF, Hayward AC (1991) Foaming in activated sludge plants: a survey in Queensland, Australia and an evaluation of some control strategies. Water Res 25, 313-317.
| Crossref | Google Scholar |
15 Mamais D, Kalaitzi E, Andreadakis A (2011) Foaming control in activated sludge treatment plants by coagulants addition. Glob Nest J 13, 237-245.
| Google Scholar |
16 Thomas JA, Soddell JA, Kurtböke Dİ (2002) Fighting foam with phages? Water Sci Technol 46, 511-518.
| Crossref | Google Scholar | PubMed |
17 Petrovski S, Seviour RJ, Tillett D (2011) Prevention of Gordonia and Nocardia stabilized foam formation by using bacteriophage GTE7. Appl Environ Microbiol 77, 7864-7867.
| Crossref | Google Scholar | PubMed |
18 Soddell JA, Seviour RJ (1995) Relationship between temperature and growth of organisms causing Nocardia foams in activated sludge plants. Water Res 29, 1555-1558.
| Crossref | Google Scholar |
19 Schoen L, Santoro JD, Milla C, Bhargava S (2015) Pulmonary nocardiosis in an immunocompetent patient with cystic fibrosis. Case Rep Pulmonol 2015, 984171.
| Crossref | Google Scholar | PubMed |
20 Das AK, Nandy S, Dudeja M, Tiwari R, Alam S (2013) The incidence of nocardiosis at pulmonary and extra–pulmonary sites. J Clin Diagn Res 7, 1427-1429.
| Crossref | Google Scholar | PubMed |
21 Zijlstra EE, van de Sande W, Welsh O, Mahgoub ES, Goodfellow M, Fahal AH (2016) Mycetoma: a unique neglected tropical disease. Lancet Infect Dis 16, 100-112.
| Crossref | Google Scholar | PubMed |
22 Ashiru OT, Pillay M, Sturm AW (2010) Adhesion to and invasion of pulmonary epithelial cells by the F15/LAM4/KZN and Beijing strains of Mycobacterium tuberculosis. J Med Microbiol 59, 528-533.
| Crossref | Google Scholar | PubMed |
23 Barry DP, Beaman BL (2007) Nocardia asteroides strain GUH-2 induces proteasome inhibition and apoptotic death of cultured cells. Res Microbiol 158, 86-96.
| Crossref | Google Scholar | PubMed |
24 Conville PS, Brown-Elliott BA, Smith T, Zelazny AM (2018) The complexities of Nocardia taxonomy and identification. J Clin Microbiol 56, e01419-17.
| Crossref | Google Scholar | PubMed |
25 Duggal SD, Chugh TD (2020) Nocardiosis: a neglected disease. Med Princ Pract 29, 514-523.
| Crossref | Google Scholar | PubMed |
26 Brown-Elliott BA, Brown JM, Conville PS, Wallace RJ Jr (2006) Clinical and laboratory reatures of the Nocardia spp. based on current molecular taxonomy. Clin Microbiol Rev 19, 259-282.
| Crossref | Google Scholar | PubMed |
27 Tan YE, Chen SC, Halliday CL (2020) Antimicrobial susceptibility profiles and species distribution of medically relevant Nocardia species: results from a large tertiary laboratory in Australia. J Glob Antimicrob Resist 20, 110-117.
| Crossref | Google Scholar | PubMed |
28 Schilling K, Zessner M (2011) Foam in the aquatic environment. Water Res 45, 4355-4366.
| Crossref | Google Scholar | PubMed |
29 Quan X-W, Hoerling MP, Perlwitz J, Diaz HF, Xu T (2014) How fast are the tropics expanding? J Climate 27, 1999-2013.
| Crossref | Google Scholar |
30 Turton SM (2017) Expansion of the tropics: revisiting frontiers of geographical knowledge. Geogr Res 55, 3-12.
| Crossref | Google Scholar |
31 Timbal B, Drosdowsky W (2013) The relationship between the decline of Southeastern Australian rainfall and the strengthening of the subtropical ridge. Int J Climatol 33, 1021-1034.
| Crossref | Google Scholar |
32 Kurtböke Dİ (2008) ‘Chocolate mousse’ on Sunshine Coast beaches. Microbiol Aust 29, 104-105.
| Google Scholar |
34 Wright L, Katouli M, Kurtböke Dİ (2021) Isolation and characterization of Nocardiae associated with foaming coastal marine waters. Pathogens 10, 579.
| Crossref | Google Scholar | PubMed |
35 Wright L, Nouioui I, Mast Y, Bunk B, Spröer C, Neumann-Schaal M, Wolf J, Katouli M, Kurtböke Dİ (2023) Nocardia australiensis sp. nov. and Nocardia spumae sp. nov., isolated from sea foam in Queensland, Australia. Int J Syst Evol Microbiol 73, 005952.
| Crossref | Google Scholar | PubMed |
37 Velonakis E, Dimitriadi D, Papadogiannakis E, Vatopoulos A (2014) Present status of effect of microorganisms from sand beach on public health. J Coast Life Med 2, 746-756.
| Google Scholar |
38 Whitman R, Harwood VJ, Edge TA, Nevers M, Byappanahalli M, Vijayavel K, Brandão J, Sadowsky MJ, Alm EW, Crowe A, Ferguson D, Ge Z, Halliday E, Kinzelman J, Kleinheinz G, Przybyla-Kelly K, Staley C, Staley Z, Solo-Gabriele HM (2014) Microbes in beach sands: integrating environment, ecology and public health. Rev Environ Sci Biotechnol 13, 329-368.
| Crossref | Google Scholar | PubMed |
39 Filipkowska Z, Janczukowicz W, Krzemieniewski M, Pesta J (2000) Microbiological air pollution in the surroundings of the wastewater treatment plant with activated-sludge tanks aerated by horizontal rotors. Pol J Environ Stud 9, 273-280.
| Google Scholar |
40 Kresch-Tronik NS, Carrillo-Casas EM, Arenas R, Atoche C, Ochoa-Carrera LA, Xicohtencatl-Cortes J, Manjarrez-Hernández ÁH, Hernández-Castro R (2012) Nocardia harenae, an uncommon causative organism of mycetoma: report on two patients. J Med Microbiol 61, 1153-1155.
| Crossref | Google Scholar | PubMed |
41 Bai JL, Wang Y, Qin S, Ding P, Xing K, Yuan B, Cao CL, Huang Y, Zhang YQ, Jiang JH (2016) Nocardia jiangsuensis sp. nov., an actinomycete isolated from coastal soil. Int J Syst Evol Microbiol 66, 4633-4638.
| Crossref | Google Scholar | PubMed |
 Dr Luke Wright graduated from the University of the Sunshine Coast, where he earned a BSc, Honours, and a PhD under the supervisions of Assoc. Prof. İpek Kurtböke and Assoc. Prof. Mohammad Katouli investigating the occurrence of Nocardia species in near shore marine environments of the Sunshine Coast Region. His study revealed the presence of novel Nocardia species in the foaming waters, and he described two new species in a collaborative investigation with the DSMZ in Germany. Dr Wright’s study increased awareness in the region and contributed toward international studies related to mapping of nocardiae in four corners of the world. |
 Assoc. Prof. Mohammad Katouli is a member of the Centre for Bioinnovation (CBI) and the School of Science, Technology and Engineering at the University of the Sunshine Coast. He has been working for many years on pathogenic mechanisms of bacteria isolated from clinical and environmental sources. His current focus is on how probiotic strains can mitigate the pathogenicity of translocating (TEC) and adherent and invasive Escherichia coli (AIEC) strains in intestinal epithelial and modulates host cell gene expression. Since 1998, he has been a member of the ASM and the chair of the Queensland branch committee between 2014 and 2016. |
 Assoc. Prof. İpek Kurtböke has been working in the field of biodiscovery and has been an active member of the international actinomycete research community since 1982. She currently conducts research and teaches in the field of applied microbiology and biotechnology at the University of the Sunshine Coast, Queensland. She has also been an active member of the World Federation of Culture Collections (WFCC) and currently is the President of the Federation. She was also an Editorial Board member of Microbiology Australia for 20 years (2004–2024). |



