The threat of zoonotic coronaviruses
Matthew J Gartner A and Kanta Subbarao A B CA Department of Microbiology and Immunology, The University of Melbourne at the Peter Doherty Institute for Infection and Immunity, Melbourne, Vic., Australia
B WHO Collaborating Centre for Reference and Research on Influenza, Melbourne, Vic., Australia
C Tel.: +61 3 9342 9310; Email: kanta.subbarao@influenzacentre.org
Microbiology Australia 42(1) 4-9 https://doi.org/10.1071/MA21003
Submitted: 13 February 2021 Accepted: 1 March 2021 Published: 12 April 2021
Journal Compilation © The Authors 2021 Open Access CC BY, published (by CSIRO Publishing) on behalf of the ASM
Abstract
Since 2002, three zoonotic coronaviruses (CoV), SARS-CoV, MERS-CoV and SARS-CoV-2 have emerged in humans, establishing that emergence of coronaviruses from animal reservoirs represents a significant pandemic threat. SARS-CoV and MERS-CoV led to smaller epidemics with very high case fatality rates while SARS-CoV-2 resulted in a global pandemic. These zoonotic coronaviruses have their likely origins in bat species and they transmit to humans through intermediate hosts. Coronaviruses can occasionally jump between host species due to their high rate of recombination. Pandemic preparedness requires surveillance in animals and occupationally exposed humans and prevention and treatment strategies that have broad activity against coronaviruses.
Introduction
Zoonotic transmission of viruses represents a significant threat to human health and viruses that spread via the respiratory route increase their pandemic potential. To date, seven CoVs have been identified in humans, four are endemic in the human (h) population (hCoV-NL63, hCoV-229E, hCoV-OC43 and hCoV-HKU1) and generally cause mild respiratory illness1. Since 2002, zoonotic CoVs severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) emerged and led to epidemics associated with severe disease2,3. The potential pandemic risk of zoonotic CoVs was cemented when SARS-CoV-2 emerged in 2019, leading to the coronavirus disease 2019 (COVID-19) pandemic.
Coronavirus epidemics and pandemics
The SARS epidemic, caused by SARS-CoV, occurred in 2002-2003 and led to 8098 infections and 774 deaths (9.6% case fatality rate (CFR))3–5. The virus emerged in a live-animal market in Guangdong, China6 and, SARS-CoV spread to 29 countries over eight months5. Fortunately, public health measures halted the epidemic in July 20037. With the exception of a small cluster of cases the following year, SARS-CoV has not been detected in humans since8.
MERS-CoV was first isolated from a patient who died of multiorgan failure following severe pneumonia in Saudi Arabia in April 20129. Since then, 2519 cases and 866 deaths have been reported (34.3% CFR)10 from 27 countries2 due to sporadic zoonotic transmission from dromedary camels to humans, with person-to-person transmission limited to healthcare or household settings2,10.
In December 2019, seven individuals in Wuhan, China were hospitalised with fever, cough, chest discomfort and bilateral lung infiltration11. The etiological agent of this new respiratory illness was a novel coronavirus, initially called 2019-nCoV and subsequently formally named SARS-CoV-2 by the International Committee on Taxonomy of Viruses12. On 11 March 2020, the World Health Organization (WHO) declared COVID-19 a public health emergency of international concern. Since January 2020, SARS-CoV-2 has spread to every continent with 106 008 375 cases and 2 313 677 deaths (2.2% CFR, Johns Hopkins University Coronavirus Resource Centre) as of 7 February 2021.
Virology
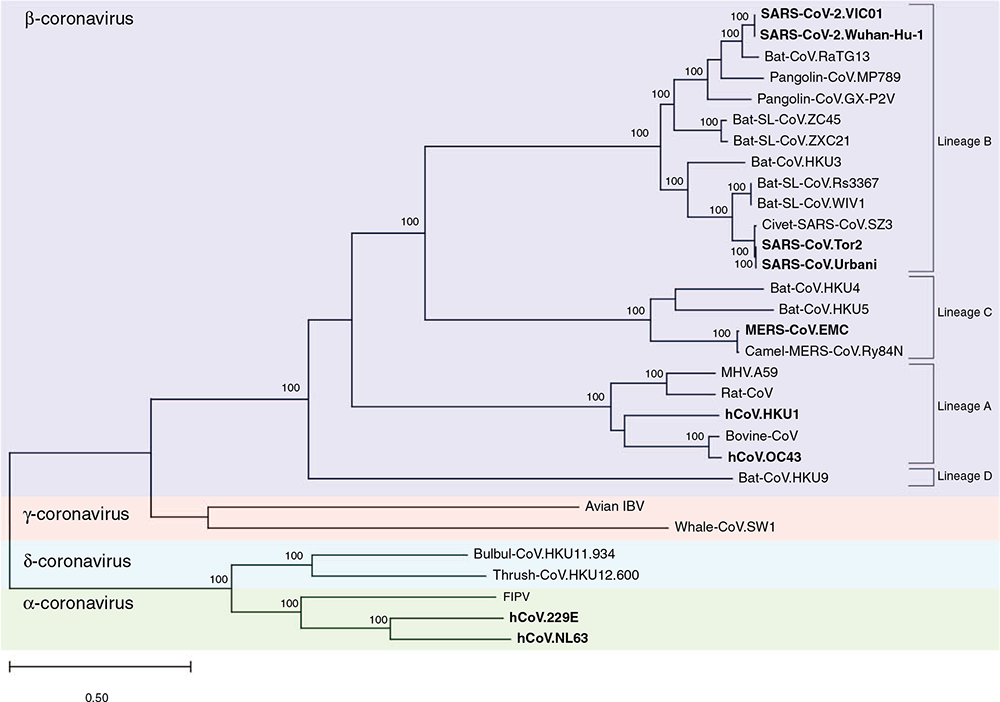
The Coronaviridae family are classified into four genera: alpha-, beta-, gamma- and deltacoronaviruses. Betacoronaviruses are further split into four lineages (A, B, C and D). HCoVs NL63 and 229E are alphacoronaviruses and HKU-1 and OC43 are lineage A betacoronaviruses. SARS-CoV, MERS-CoV and SARS-CoV-2 are lineage B betacoronaviruses (Figure 1). CoVs encode a ~30 kb strand of single-stranded positive-sense RNA coated in nucleocapsid (N) protein and enclosed within a lipid bilayer with spike (S), membrane (M) and envelope (E) proteins.
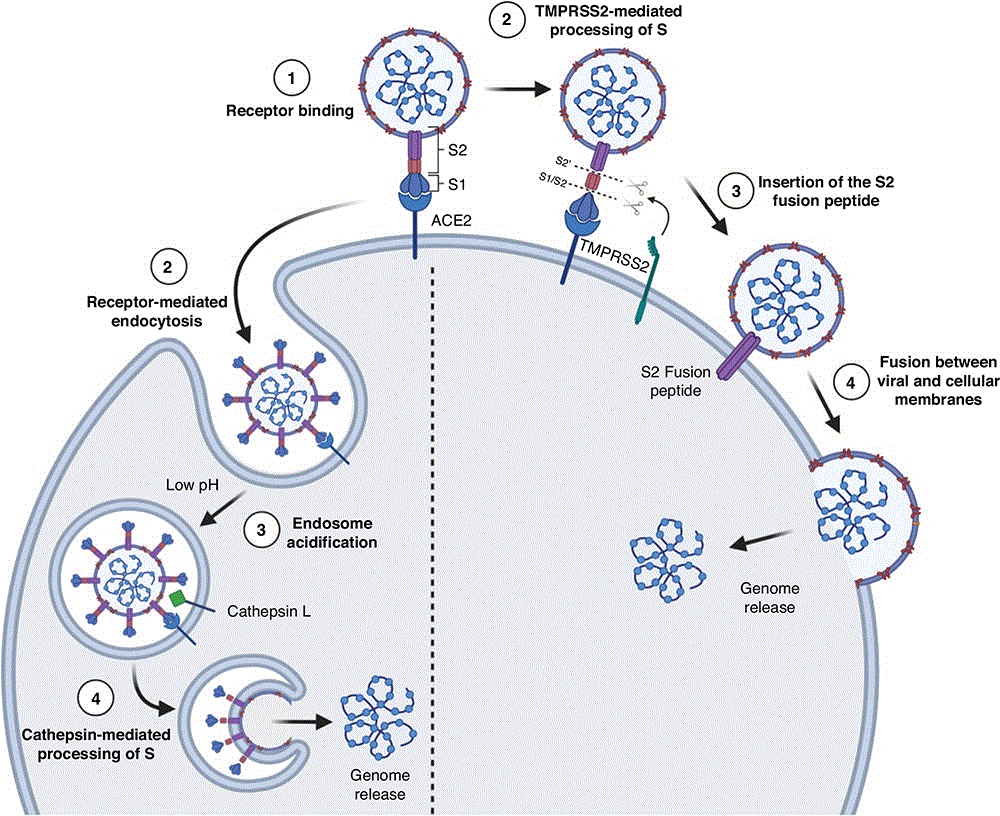
The S protein is comprised of two subunits; S1 is the surface-exposed subunit that includes the receptor binding domain (RBD) that attaches to the host receptor, and S2 domain that mediates fusion of viral and host membranes13. The receptor for SARS-CoV-2, SARS-CoV and hCoV-NL63 is angiotensin converting enzyme 2 (ACE2) and for MERS-CoV is dipeptidyl peptidase 4 (DPP4). Following receptor attachment, the S protein is proteolytically cleaved at two sites to mediate fusion (Figure 2)13,14. SARS-CoV, SARS-CoV-2 and MERS-CoV have all been shown to mediate entry through direct membrane fusion or through endosomal entry, depending on the cell type14.
Replication of CoVs occurs within double membrane vesicles, where positive-sense RNA acts as a template for synthesis of full-length negative-sense RNA, which in turn serve as templates for production of full-length positive-sense RNA to be packaged into virions14. Unlike other RNA viruses, coronavirus Nsp14 encodes an exoribonuclease with proof-reading function limiting their error-rate during RNA synthesis14 in substitutions/nucleotide/replication cycle from 9 × 10–7 for SARS-CoV versus 10–3 to 10–6 for other RNA viruses15.
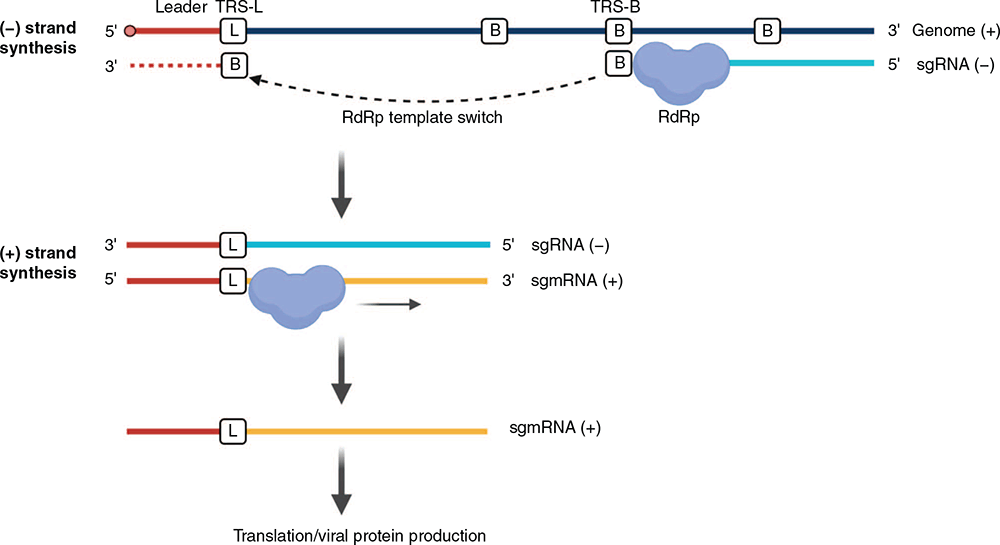
Transcription of CoVs follows a unique discontinuous process that leads to high rates of recombination among CoV genomes during co-infection (Figure 3). CoV genomes contain a transcription-regulatory ‘leader’ sequence (TRS-L) at the 5′ end16 and each open reading frame (ORF) contains a ‘body’ transcription-regulatory sequence (TRS-B) immediately 5′ of the ORF14. During negative-strand synthesis, the RNA-dependent RNA polymerase (RdRp) pauses when it reaches the TRS-B and switches template to the TRS-L, fusing the ‘leader’ sequence to the 5′ end of the gene being copied. Positive strand subgenomic messenger RNAs (mRNAs) are transcribed and translated from these fused negative-strand intermediates14. Assembly and release of CoVs requires the nucleocapsid-bound RNA and structural proteins M, S and E to combine near the cell membrane and bud from the cell surface14.
Reservoirs of coronaviruses
CoVs infect a wide range of host species. Interspecies transfer occurs sporadically (reviewed in1). Human CoVs are believed to have originated from bats, bovine and murine hosts1. Bats are the most divergent, widely distributed non-human mammalian species on earth17. They harbor a wide range of viruses that cause disease in humans and animals, including coronaviruses, henipaviruses, filoviruses and lyssaviruses17.
Horseshoe bats were identified as the likely natural reservoir of SARS-CoV based on detection of antibodies to SARS-CoV N protein in bat populations in China18. Furthermore, diverse SARS-like-CoVs have been detected in bat populations in Asia, Africa and Europe1. While no direct progenitor of SARS-CoV has been isolated, related genomes were identified in Chinese horseshoe bats19, that could infect cells expressing ACE2 from humans, civets and Chinese horseshoe bats. Zoonotic transmission of SARS-CoV likely occurred either through direct bat-human contact19, or through an intermediate host like palm civets or racoon dogs in live-animal markets6.
Case reports of MERS patients identified contact with dromedary camels as a risk factor for MERS-CoV infection20. Studies in dromedary camels from the Middle East found high seroprevalence (80–100%) to MERS-CoV21 that may have been circulating in camels since 19831. Furthermore, MERS-like-CoV sequences have been detected in nasal and rectal samples from dromedary camels in the Arabian Peninsula2. Additional studies revealed diverse MERS-like CoVs in bat populations in Africa, Europe, Asia and the Middle East1. Taken together, these findings suggest that bats were the origin of MERS-CoV, while dromedary camels are the intermediate host.
Since the first SARS-CoV-2 sequence was publicly available in January 2020, researchers have sought to understand the origins of the virus. The epidemiological link to a seafood market in Wuhan suggested that zoonotic transmission may have occurred at the market11 and SARS-like CoVs with 91.02% identity to SARS-CoV-2 were reported in lung samples from two dead Malayan pangolins two months earlier22,23. However, all of the earliest reported cases of SARS-CoV-2 infection in humans were not linked to the wet market24. Phylogenetic analysis suggests RaTG13 and RmYN02 from horseshoe bats in Yunnan, China are most closely related to SARS-CoV-2, with 96.2% and 93.3% similarity, respectively11,25. However, these isolates are more than 30 years evolutionarily divergent from SARS-CoV-226. Therefore, additional sampling of CoVs in animals is required to identify the reservoir and possible intermediate hosts for SARS-CoV-2.
Pandemic potential of emerging coronaviruses
Coronaviruses exist in bats in heterogenous, quasispecies pools17. CoVs are highly recombinogenic26; small regions of the genome are derived from independent CoVs by homologous recombination. Studies have shown that the SARS-CoV genome has a mosaic ancestry, with subgenomic regions from multiple origins27,28 including HCoV-229E, mouse hepatitis virus, avian infectious bronchitis virus, bovine coronavirus, transmissible gastroenteritis virus and porcine epidemic diarrhea virus27. Furthermore, substitutions of entire S1 and S2 regions may play an essential role in mediating expansion of CoV host range. Swapping the RBD of a SARS-like bat CoV, with that of SARS-CoV allowed the new hybrid virus to replicate efficiently in human airway epithelial cells29, suggesting that recombination insertion of a different CoV RBD may be a key step in driving cross-species transmission of CoVs. Ultimately, the high frequency of CoV recombination and the propensity to jump host-species mean that emerging CoVs will remain a future pandemic risk in humans.
Several conditions must be met for a novel CoV to emerge and pose a pandemic threat. It must attach, infect and replicate in human cells often using a host receptor with a human homologue expressed in an accessible anatomical location e.g. the gastrointestinal or respiratory tract. Second, it must be able to transmit efficiently from person-to-person. Third, the human population should lack pre-existing immunity to the virus. Fourth, there must be sufficient exposure to the reservoir species through hunting and consumption of wild-caught animals, trading in live animal markets or occupational exposure in meat and poultry industries. Finally, the virus must cause overt disease in humans.
Pandemic preparedness
The COVID-19 pandemic confirmed the warnings that SARS and MERS epidemics provided of the pandemic potential of betacoronaviruses. Remarkable progress has been made to understand SARS-CoV-2 including global transmission dynamics and shifts in viral variants, development of monoclonal antibodies, antivirals and vaccines, and public health strategies. These lessons must be implemented into preparing for future emerging CoVs. This relies on sampling and evaluation of CoVs circulating in the wild for their potential for human emergence of CoVs. Reverse genetics systems30–32 can be applied to identify which novel CoVs are able to infect human cells. Surveillance in occupationally exposed people may be an effective approach to identifying animal CoVs that can cross the species barrier. The development of pan-CoV therapeutics will be valuable for responding to the emergence of novel CoVs.
Conclusions
The COVID-19 pandemic and recurrent zoonotic transmission of CoVs from animal reservoirs into humans, along with the large diversity of SARS-like CoVs circulating in animals suggests that CoVs will continue to pose a global public health threat and that we must be prepared for the next emerging CoV.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This study did not receive specific funding.
References
[1] Cui, J. et al. (2019) Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 17, 181–192.| Origin and evolution of pathogenic coronaviruses.Crossref | GoogleScholarGoogle Scholar | 30531947PubMed |
[2] Memish, Z.A. et al. (2020) Middle East respiratory syndrome. Lancet 395, 1063–1077.
| Middle East respiratory syndrome.Crossref | GoogleScholarGoogle Scholar | 32145185PubMed |
[3] Drosten, C. et al. (2003) Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 348, 1967–1976.
| Identification of a novel coronavirus in patients with severe acute respiratory syndrome.Crossref | GoogleScholarGoogle Scholar | 12690091PubMed |
[4] Ksiazek, T.G. et al. (2003) A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 348, 1953–1966.
| A novel coronavirus associated with severe acute respiratory syndrome.Crossref | GoogleScholarGoogle Scholar | 12690092PubMed |
[5] Lam, W.K. et al. (2003) Overview on SARS in Asia and the world. Respirology 8, S2–S5.
| Overview on SARS in Asia and the world.Crossref | GoogleScholarGoogle Scholar | 15018125PubMed |
[6] Guan, Y. et al. (2003) Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science 302, 276–278.
| Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China.Crossref | GoogleScholarGoogle Scholar | 12958366PubMed |
[7] Svoboda, T. et al. (2004) Public health measures to control the spread of the severe acute respiratory syndrome during the outbreak in Toronto. N. Engl. J. Med. 350, 2352–2361.
| Public health measures to control the spread of the severe acute respiratory syndrome during the outbreak in Toronto.Crossref | GoogleScholarGoogle Scholar | 15175437PubMed |
[8] Liang, G. et al. (2004) Laboratory diagnosis of four recent sporadic cases of community-acquired SARS, Guangdong Province, China. Emerg. Infect. Dis. 10, 1774–1781.
| Laboratory diagnosis of four recent sporadic cases of community-acquired SARS, Guangdong Province, China.Crossref | GoogleScholarGoogle Scholar | 15504263PubMed |
[9] Zaki, A.M. et al. (2012) Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 367, 1814–1820.
| Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia.Crossref | GoogleScholarGoogle Scholar | 23075143PubMed |
[10] Xiao, J. et al. (2020) SARS, MERS and COVID-19 among healthcare workers: a narrative review. J. Infect. Public Health 13, 843–848.
| SARS, MERS and COVID-19 among healthcare workers: a narrative review.Crossref | GoogleScholarGoogle Scholar | 32493671PubMed |
[11] Zhou, P. et al. (2020) A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579, 270–273.
| A pneumonia outbreak associated with a new coronavirus of probable bat origin.Crossref | GoogleScholarGoogle Scholar | 32015507PubMed |
[12] Coronaviridae Study Group of the International Committee on Taxonomy of Viruses (2020) The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 5, 536–544.
| The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2.Crossref | GoogleScholarGoogle Scholar | 32123347PubMed |
[13] Walls, A.C. et al. (2017) Tectonic conformational changes of a coronavirus spike glycoprotein promote membrane fusion. Proc. Natl. Acad. Sci. USA 114, 11157–11162.
| Tectonic conformational changes of a coronavirus spike glycoprotein promote membrane fusion.Crossref | GoogleScholarGoogle Scholar | 29073020PubMed |
[14] Hartenian, E. et al. (2020) The molecular virology of coronaviruses. J. Biol. Chem. 295, 12910–12934.
| The molecular virology of coronaviruses.Crossref | GoogleScholarGoogle Scholar | 32661197PubMed |
[15] Eckerle, L.D. et al. (2010) Infidelity of SARS-CoV Nsp14-exonuclease mutant virus replication is revealed by complete genome sequencing. PLoS Pathog. 6, e1000896.
| Infidelity of SARS-CoV Nsp14-exonuclease mutant virus replication is revealed by complete genome sequencing.Crossref | GoogleScholarGoogle Scholar | 20463816PubMed |
[16] Sola, I. et al. (2015) Continuous and discontinuous RNA synthesis in coronaviruses. Annu. Rev. Virol. 2, 265–288.
| Continuous and discontinuous RNA synthesis in coronaviruses.Crossref | GoogleScholarGoogle Scholar | 26958916PubMed |
[17] Wang, L.F. et al. (2019) Viruses in bats and potential spillover to animals and humans. Curr. Opin. Virol. 34, 79–89.
| Viruses in bats and potential spillover to animals and humans.Crossref | GoogleScholarGoogle Scholar | 30665189PubMed |
[18] Li, W. et al. (2005) Bats are natural reservoirs of SARS-like coronaviruses. Science 310, 676–679.
| Bats are natural reservoirs of SARS-like coronaviruses.Crossref | GoogleScholarGoogle Scholar | 16195424PubMed |
[19] Ge, X.Y. et al. (2013) Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature 503, 535–538.
| Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor.Crossref | GoogleScholarGoogle Scholar | 24172901PubMed |
[20] Azhar, E.I. et al. (2014) Evidence for camel-to-human transmission of MERS coronavirus. N. Engl. J. Med. 370, 2499–2505.
| Evidence for camel-to-human transmission of MERS coronavirus.Crossref | GoogleScholarGoogle Scholar | 24896817PubMed |
[21] Dighe, A. et al. (2019) A systematic review of MERS-CoV seroprevalence and RNA prevalence in dromedary camels: implications for animal vaccination. Epidemics 29, 100350.
| A systematic review of MERS-CoV seroprevalence and RNA prevalence in dromedary camels: implications for animal vaccination.Crossref | GoogleScholarGoogle Scholar | 31201040PubMed |
[22] Liu, P. et al. (2019) Viral metagenomics revealed Sendai virus and Coronavirus infection of Malayan pangolins (Manis javanica). Viruses 11, 979.
| Viral metagenomics revealed Sendai virus and Coronavirus infection of Malayan pangolins (Manis javanica).Crossref | GoogleScholarGoogle Scholar |
[23] Zhang, T. et al. (2020) Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Curr. Biol. 30, 1346–1351.e2.
| Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak.Crossref | GoogleScholarGoogle Scholar | 32197085PubMed |
[24] Huang, C. et al. (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506.
| Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China.Crossref | GoogleScholarGoogle Scholar | 31986264PubMed |
[25] Zhou, H. et al. (2020) A novel bat coronavirus closely related to SARS-CoV-2 contains natural insertions at the S1/S2 cleavage site of the spike protein. Curr. Biol. 30, 2196–2203.e3.
| A novel bat coronavirus closely related to SARS-CoV-2 contains natural insertions at the S1/S2 cleavage site of the spike protein.Crossref | GoogleScholarGoogle Scholar | 32416074PubMed |
[26] Boni, M.F. et al. (2020) Evolutionary origins of the SARS-CoV-2 sarbecovirus lineage responsible for the COVID-19 pandemic. Nat. Microbiol. 5, 1408–1417.
| Evolutionary origins of the SARS-CoV-2 sarbecovirus lineage responsible for the COVID-19 pandemic.Crossref | GoogleScholarGoogle Scholar | 32724171PubMed |
[27] Zhang, X.W. et al. (2005) Testing the hypothesis of a recombinant origin of the SARS-associated coronavirus. Arch. Virol. 150, 1–20.
| Testing the hypothesis of a recombinant origin of the SARS-associated coronavirus.Crossref | GoogleScholarGoogle Scholar | 15480857PubMed |
[28] Stavrinides, J. et al. (2004) Mosaic evolution of the severe acute respiratory syndrome coronavirus. J. Virol. 78, 76–82.
| Mosaic evolution of the severe acute respiratory syndrome coronavirus.Crossref | GoogleScholarGoogle Scholar | 14671089PubMed |
[29] Becker, M.M. et al. (2008) Synthetic recombinant bat SARS-like coronavirus is infectious in cultured cells and in mice. Proc. Natl. Acad. Sci. USA 105, 19944–19949.
| Synthetic recombinant bat SARS-like coronavirus is infectious in cultured cells and in mice.Crossref | GoogleScholarGoogle Scholar | 19036930PubMed |
[30] van den Worm, S.H. et al. (2012) Reverse genetics of SARS-related coronavirus using vaccinia virus-based recombination. PLoS One 7, e32857.
| Reverse genetics of SARS-related coronavirus using vaccinia virus-based recombination.Crossref | GoogleScholarGoogle Scholar | 22412934PubMed |
[31] Menachery, V.D. et al. (2015) A SARS-like cluster of circulating bat coronaviruses shows potential for human emergence. Nat. Med. 21, 1508–1513.
| A SARS-like cluster of circulating bat coronaviruses shows potential for human emergence.Crossref | GoogleScholarGoogle Scholar | 26552008PubMed |
[32] Almazán, F. et al. (2006) Construction of a severe acute respiratory syndrome coronavirus infectious cDNA clone and a replicon to study coronavirus RNA synthesis. J. Virol. 80, 10900–10906.
| Construction of a severe acute respiratory syndrome coronavirus infectious cDNA clone and a replicon to study coronavirus RNA synthesis.Crossref | GoogleScholarGoogle Scholar | 16928748PubMed |
Biographies
Matthew Gartner is a Research Officer in Professor Subbarao’s research group at the Peter Doherty Institute for Infection and Immunity. He completed his PhD in 2021 at RMIT University researching HIV cellular tropism and viral reservoirs. His research interests involve understanding key mechanisms of coronavirus pathogenesis, including virus entry, cellular tropism and virus-host interactions.
Professor Kanta Subbarao is the Director of the WHO Collaborating Centre for Reference and Research on Influenza and Professor, Department of Microbiology and Immunology, The University of Melbourne at the Peter Doherty Institute for Infection and Immunity. She is a virologist and a physician with specialty training in paediatrics and paediatric infectious diseases. Her research is focused on the biology and vaccines against influenza viruses and coronaviruses.