Engineering biodegradable coatings for sustainable fertilisers
Zahra F. Islam A B * , Pavel V. Cherepanov B C and Hang-Wei Hu A BA School of Agriculture and Food, Faculty of Science, The University of Melbourne, Parkville, Vic. 3010, Australia.
B ARC Research Hub for Smart Fertilisers, The University of Melbourne, Parkville, Vic. 3010, Australia.
C School of Chemical Engineering, Faculty of Engineering and IT, The University of Melbourne, Parkville, Vic. 3010, Australia.

Dr Zahra F. Islam is a research fellow in plant–soil microbiomes within Theme 3 of the ARC Research Hub for Smart Fertilisers at The University of Melbourne. Her research centres on understanding the complexities of plant–soil microbiome interactions, with a focus on isolating plant-beneficial microorganisms for use as probiotics in the agriculture industry. |

Dr Pavel V. Cherepanov is a research fellow in fertiliser coating engineering within Theme 1 of the ARC Research Hub for Smart Fertilisers at The University of Melbourne. His research focuses on engineering fertiliser coatings for controlled nutrient release in soils with a particular focus on biodegradable materials. |

Dr Hang-Wei Hu is a senior lecturer in the School of Agriculture and Food, and leader of Theme 3 of the ARC Research Hub for Smart Fertilisers at The University of Melbourne. His research focuses on connecting multifaceted components among soil organisms (e.g. bacteria, viruses, fungi, archaea, protists and fauna) with ecosystem functions, and translating fundamental microbiological knowledge into agricultural biotechnologies. |
Microbiology Australia 44(1) 9-12 https://doi.org/10.1071/MA23003
Submitted: 11 January 2023 Accepted: 3 February 2023 Published: 20 February 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of the ASM. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
With the pressures of a changing global climate and ever-growing population, the need for sustainable agricultural practices that increase crop yields while decreasing greenhouse gas emissions are critical. Currently used practices to increase yields can often be problematic due to low nitrogen use efficiency or a potential overreliance on agrichemicals that can alter the community composition of a given ecosystem, although this is typically system and situation dependent. As such, the next generation of enhanced efficiency fertilisers that combine chemical, materials engineering and biological components are likely to be a game changer. Integral to their success is a better understanding of how plant–soil microbiomes interact with the new enhanced efficiency fertilisers, and how we can best tailor the fertilisers to suit different plant–soil combinations. In particular, the biodegradation properties of new fertiliser coatings must be given careful consideration so as to not further burden agricultural soils with microplastics or cause ecotoxicity problems. This perspective proposes novel, interdisciplinary strategies to generate highly efficient, biodegradable fertiliser coatings for use in the agricultural sector.
Keywords: agriculture, biodegradation, biotechnology, fertilisers, plant–microbiome interactions, polymers, soil microbiology, sustainability.
The challenges of feeding the world on finite agricultural land
With global populations set to reach 10 billion by 2050,1 there is increasing pressure to match food production within existing agricultural lands in the face of a changing global climate. Integral to global food security is an increasing reliance on synthetic fertilisers to improve crop yields,2 while simultaneously reducing their negative environmental impacts.3 Though there have been recent shifts towards designing fertilisers with enhanced efficiency1,4,5 including those that have been coated with a polymeric substance such as metal–phenolic networks,6,7 these have not been widely adopted by the global agricultural industry. In addition, strategies to further increase crop yields, such as the deployment of pesticides, herbicides and enzymatic inhibitors,8,9 may also lead to disruptions in the balanced plant holobiont (i.e. the collection of microorganisms such as bacteria, fungi, archaea and protists, that form close associations with the plant host).10,11 Thus, the design of new generation fertilisers must take into consideration sustained and tailorable release profiles, the degradation of coatings and ecotoxicity potential, as well as the potential benefits of incorporating probiotic microorganisms into engineered coatings to enhance plant performance.
Among the major design challenges for the development of new fertilisers is the composition of coatings that cannot only slow the release of the internal nitrogenous compound, but also can be completely biodegraded by indigenous soil microorganisms. This remains an understudied challenge within both the fertiliser and agricultural industry, as the microorganisms that comprise plant holobionts are often host or soil specific,12 and may not be shared among different crop types.13,14 As such, innovative microbial solutions are required to ensure that newly developed biopolymeric fertiliser coatings can be degraded by a wide range of microorganisms native to different soil types and plant species. In addition, ensuring that the polymers are completely degraded and do not generate microplastics15–17 is paramount to ensure that ecosystems are not further burdened. We thus outline a cross-disciplinary strategy combining materials engineering and plant–soil microbiology approaches to generate innovative hybrid chemical–biological fertilisers for use in Australian agricultural systems.
Current state of agricultural practices and potential innovation strategies
Current practices within the agricultural industry are heavily weighted towards the usage of conventional fertilisers that are typically applied in liquid form or as uncoated granules.4 As Australia possesses one of the highest nitrogen footprints in the world (~47 kg of nitrogen per capita per year), with food production comprising the largest component,18 it is of great importance to develop new products to reduce the environmental and socioeconomic impacts of fertiliser use.1 It is well established that intensive and improper use of nitrogen-based fertilisers can lead to numerous undesirable effects including mining of soil nitrogen in low-rainfall cropping areas,18 nitrate leaching into waterways causing eutrophication,19 and nitrous oxide and ammonia emissions into the atmosphere, contributing to global warming.20 With the current cost of developing, producing and deploying enhanced efficiency fertilisers up to 10 times higher than that of commercial fertilisers within the agricultural sector,5,21 the use of these commercial fertilisers will continue to be widespread and are unlikely to decrease unless next-generation fertilisers are comparably priced and are higher efficiency.
Within the agricultural sector, three major approaches are currently used to mitigate excess nitrogen loss within cropping lands as well as increase crop nitrogen use efficiencies: (1) addition of chemical urease and nitrification inhibitors4,10,11; (2) utilisation of the 4R Nutrient Stewardship concept (right source of nutrients, at the right rate, at the right time and in the right place)22; and (3) use of physical barriers to slow the release of fertilisers.7,23 Although the benefits of urease and nitrification inhibitors have been well documented,5,11 the use of polymer-coated fertilisers has been comparatively less studied. The effectiveness of polymer-coated fertilisers specifically was demonstrated to have negative to negligible positive effects on drylands and grasslands, and was highly influenced by soil pH.5 This is a concerning phenomenon for translation into the Australian agricultural sector, which is predominantly grasslands and drylands.24,25 Additionally, the type of polymer used in the coating needs to be carefully selected to ensure that it is capable of natural degradation, meets national biodegradability standards and by-products do not have ecotoxicological effects. It is important to note that currently there are no global standards governing the biodegradation parameters of fertiliser coatings. More work is required to understand the effects of additives on polymer degradation by microorganisms, as numerous reports have highlighted deleterious effects of microplastics on soil organisms and functions.26 Subsequently, the question remains, can enhanced-efficiency polymer-coated fertilisers be generated for use within the Australian agricultural industry, taking into consideration the unique properties of Australian soils?
Upcoming multidisciplinary approaches to engineering biodegradable fertiliser coatings
The effectiveness of controlled release fertilisers could potentially be improved by the incorporation of biological additives, such as plant-growth promoting bacteria (PGPB) as well as polymer-degrading microorganisms (PDMs). Biofertilisers, or microbial inoculants, can be split into two major classes, rhizobia-based inoculants that are primarily applied to legumes, and non-rhizobia based inoculants to non-legumous crops. Non-rhizobia biofertilisers in the form of peat or liquid supplements have been demonstrated to increase the yield of numerous crops including soybeans, maize and rice, though positive effects can vary greatly across different applications.27,28 Although biofertilisers have been implemented for decades,28 they are scarcely used within the Australian agricultural sector aside from in forage legumes.27 In particular, the uptake of these biofertilisers has been sporadic in wheat-producing nations and has had inconsistent results between countries, indicating that species-specific interactions between plant subtypes and microbial inoculants might be critical to consider.27
Similarly, the discovery and characterisation of PDMs is rapidly growing in response to the overuse of plastics worldwide, though their efficiencies in different ecosystems remains understudied.16 A recent review by Gambarini et al. determined that, although the degradation capacity for microorganisms is taxonomically widespread, experimental evidence of this has been minimal so far.29 Some of the better-characterised PDMs include Ideonella sakaiensis, which has been shown to degrade polyethylene terephthalate, numerous species within the order Bacillales, which are capable of polypropylene and polystyrene degradation, and species from the Amycolatopsis genus, which have been shown to degrade polylactic acid polymers.29 In particular, the conditions within which polymers are partially or completely degraded can differ extensively between different polymer types, with synthetic polymers derived from fossil fuels (e.g. polyethylene terephthalate, polypropylene and polystyrene) typically only degraded by microorganisms under specific conditions.15,29 Conversely, coatings developed from biopolymers, polymers that are made from renewable resources (e.g. polyhydroxybutyrate) would likely be better candidates as they have a greater biodegradation potential than synthetic polymers.29 Thus, careful consideration of polymer type as well as the in situ degradation capacity of the agricultural soil tested must be at the forefront of the generation of fertiliser coatings. Soil properties, such as pH and organic carbon content, in conjunction with the plant species grown must also be considered as these can drastically alter the composition of microorganisms within the rhizosphere.12 As such, the testing of multiple soil types and incubation conditions on the same polymer type must be carried out to assess the generalisability of degradation across different agricultural systems. It is likely that multiple polymer and microbial combinations need to be generated for each plant–soil combination due to specific nature of plant–soil–microbiome interactions.13 Thus, an ongoing challenge is finding microbial combinations that will promote the growth of crops, remain in the soil long term and are able to be incorporated into existing fertiliser delivery strategies such as coatings.
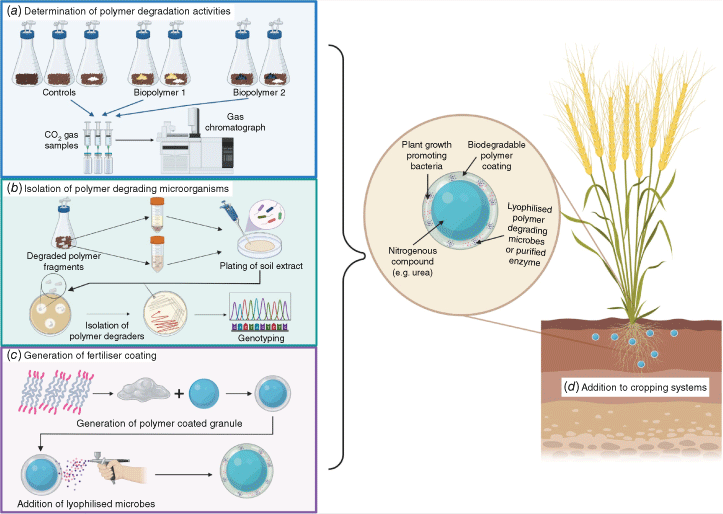
Our strategy for developing next-generation smart fertilisers is to use a multidisciplinary approach, combining complimentary microbiological, chemical and materials engineering strategies. As demonstrated by the schematic in Fig. 1, we aim to combine culture-independent and culture-dependent microbiological techniques with materials engineering to develop economically viable smart fertilisers capable of increasing yields and reducing nitrogen losses. Determination of soil physicochemical properties as well as overall microbial community structure could potentially enable genome-informed cultivation strategies to target both PGPB and PDMs specific to each major Australian crop species–soil combination (Fig. 1b).30 In situ biodegradation studies, assayed by gas chromatography (GC) of CO2 production and scanning electron microscopy (SEM), using biodegradable polymer candidates will also inform downstream coating design, with candidates able to be degraded by multiple soil types and in multiple conditions prioritised (Fig. 1a). The direct measurement of degradation by GC and SEM will be accompanied by additional, complementary analytical techniques, such as complete soil physiochemical analysis and Fourier-transform infrared spectroscopy. Partially degraded polymers will then be used as the starting inoculum for PDM isolation using minimal media with fresh polymer as the sole carbon source (Fig. 1b). Isolates will then be phenotyped to determine the mechanism by which polymer degradation was occurring, with the potential to purify degradation enzymes. Within a coating, it is theoretically possible to include urease and nitrification inhibitors1 as well as a microbial cocktail of lyophilised PGPB and PDM or purified enzyme (Fig. 1c). This would allow for controlled release of the encapsulated chemical fertiliser (e.g. urea) because of the degradation effects of the PDM, the inhibition of major enzymatic pathways leading to nitrogen losses and the delivery of PGPB directly to the rhizosphere (Fig. 1d).
Concluding remarks
With an increasing global population and a changing global climate, addressing food scarcity through innovative microorganism-forward agriculture is paramount. Only through a deep understanding of plant–soil–microbiome interactions and using multi-disciplinary approaches can new biodegradable polymer coatings for chemical fertilisers be generated. This new generation of enhanced efficiency fertilisers should be tailored to specific plant–soil combinations to obtain the best yields and nitrogen use efficiencies while also being a viable economic alternative to currently used chemical fertilisers.
Data availability
Data sharing is not applicable as no new data were generated or analysed during this study.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
This work was supported by the Australian Research Council through the Industrial Transformation Research Hub Scheme (grant number IH200100023) awarded to H-W. Hu.
References
[1] Lam, SK et al. (2022) Next-generation enhanced-efficiency fertilizers for sustained food security. Nat Food 3, 575–580.| Next-generation enhanced-efficiency fertilizers for sustained food security.Crossref | GoogleScholarGoogle Scholar |
[2] Zhang, X et al. (2015) Managing nitrogen for sustainable development. Nature 528, 51–59.
| Managing nitrogen for sustainable development.Crossref | GoogleScholarGoogle Scholar |
[3] Foley, JA et al. (2011) Solutions for a cultivated planet. Nature 478, 337–342.
| Solutions for a cultivated planet.Crossref | GoogleScholarGoogle Scholar |
[4] Dimkpa, CO et al. (2020) Development of fertilizers for enhanced nitrogen use efficiency – trends and perspectives. Sci Total Environ 731, 139113.
| Development of fertilizers for enhanced nitrogen use efficiency – trends and perspectives.Crossref | GoogleScholarGoogle Scholar |
[5] Li, T et al. (2018) Enhanced-efficiency fertilizers are not a panacea for resolving the nitrogen problem. Glob Chang Biol 24, e511–e521.
| Enhanced-efficiency fertilizers are not a panacea for resolving the nitrogen problem.Crossref | GoogleScholarGoogle Scholar |
[6] Mazaheri, O et al. (2022) Assembly of metal–phenolic networks on water-soluble substrates in nonaqueous media. Adv Funct Mater 32, 2111942.
| Assembly of metal–phenolic networks on water-soluble substrates in nonaqueous media.Crossref | GoogleScholarGoogle Scholar |
[7] Azeem, B et al. (2014) Review on materials & methods to produce controlled release coated urea fertilizer. J Control Release 181, 11–21.
| Review on materials & methods to produce controlled release coated urea fertilizer.Crossref | GoogleScholarGoogle Scholar |
[8] MacLeod, A et al. (2010) Evolution of the international regulation of plant pests and challenges for future plant health. Food Secur 2, 49–70.
| Evolution of the international regulation of plant pests and challenges for future plant health.Crossref | GoogleScholarGoogle Scholar |
[9] Riah, W et al. (2014) Effects of pesticides on soil enzymes: a review. Environ Chem Lett 12, 257–273.
| Effects of pesticides on soil enzymes: a review.Crossref | GoogleScholarGoogle Scholar |
[10] Duff, AM et al. (2022) Assessing the long-term impact of urease and nitrification inhibitor use on microbial community composition, diversity and function in grassland soil. Soil Biol Biochem 170, 108709.
| Assessing the long-term impact of urease and nitrification inhibitor use on microbial community composition, diversity and function in grassland soil.Crossref | GoogleScholarGoogle Scholar |
[11] Wang, X et al. (2021) Effects of biological nitrification inhibitors on nitrogen use efficiency and greenhouse gas emissions in agricultural soils: a review. Ecotoxicol Environ Saf 220, 112338.
| Effects of biological nitrification inhibitors on nitrogen use efficiency and greenhouse gas emissions in agricultural soils: a review.Crossref | GoogleScholarGoogle Scholar |
[12] Trivedi, P et al. (2020) Plant–microbiome interactions: from community assembly to plant health. Nat Rev Microbiol 18, 607–621.
| Plant–microbiome interactions: from community assembly to plant health.Crossref | GoogleScholarGoogle Scholar |
[13] Hartmann, A et al. (2009) Plant-driven selection of microbes. Plant Soil 321, 235–257.
| Plant-driven selection of microbes.Crossref | GoogleScholarGoogle Scholar |
[14] Bulgarelli, D et al. (2013) Structure and functions of the bacterial microbiota of plants. Annu Rev Plant Biol 64, 807–838.
| Structure and functions of the bacterial microbiota of plants.Crossref | GoogleScholarGoogle Scholar |
[15] Chamas, A et al. (2020) Degradation rates of plastics in the environment. ACS Sustain Chem Eng 8, 3494–3511.
| Degradation rates of plastics in the environment.Crossref | GoogleScholarGoogle Scholar |
[16] Mohanan, N et al. (2020) Microbial and enzymatic degradation of synthetic plastics. Front Microbiol 11, 580709.
| Microbial and enzymatic degradation of synthetic plastics.Crossref | GoogleScholarGoogle Scholar |
[17] Ng, E-L et al. (2018) An overview of microplastic and nanoplastic pollution in agroecosystems. Sci Total Environ 627, 1377–1388.
| An overview of microplastic and nanoplastic pollution in agroecosystems.Crossref | GoogleScholarGoogle Scholar |
[18] Liang, X et al. (2016) Beef and coal are key drivers of Australia’s high nitrogen footprint. Sci Rep 6, 39644.
| Beef and coal are key drivers of Australia’s high nitrogen footprint.Crossref | GoogleScholarGoogle Scholar |
[19] Michael Beman, J et al. (2005) Agricultural runoff fuels large phytoplankton blooms in vulnerable areas of the ocean. Nature 434, 211–214.
| Agricultural runoff fuels large phytoplankton blooms in vulnerable areas of the ocean.Crossref | GoogleScholarGoogle Scholar |
[20] Hu, H-W et al. (2015) Microbial regulation of terrestrial nitrous oxide formation: understanding the biological pathways for prediction of emission rates. FEMS Microbiol Rev 39, 729–749.
| Microbial regulation of terrestrial nitrous oxide formation: understanding the biological pathways for prediction of emission rates.Crossref | GoogleScholarGoogle Scholar |
[21] Chen, D et al. (2008) Prospects of improving efficiency of fertiliser nitrogen in Australian agriculture: a review of enhanced efficiency fertilisers. Soil Res 46, 289–301.
| Prospects of improving efficiency of fertiliser nitrogen in Australian agriculture: a review of enhanced efficiency fertilisers.Crossref | GoogleScholarGoogle Scholar |
[22] Johnston, AM and Bruulsema, TW (2014) 4R Nutrient stewardship for Improved nutrient use efficiency. Procedia Eng 83, 365–370.
| 4R Nutrient stewardship for Improved nutrient use efficiency.Crossref | GoogleScholarGoogle Scholar |
[23] Lawrencia, D et al. (2021) Controlled release fertilizers: a review on coating materials and mechanism of release. Plants 10, 238.
| Controlled release fertilizers: a review on coating materials and mechanism of release.Crossref | GoogleScholarGoogle Scholar |
[24] Bell, LW et al. (2014) Opportunities and challenges in Australian grasslands: pathways to achieve future sustainability and productivity imperatives. Crop Pasture Sci 65, 489–507.
| Opportunities and challenges in Australian grasslands: pathways to achieve future sustainability and productivity imperatives.Crossref | GoogleScholarGoogle Scholar |
[25] Carberry, PS et al. (2011) Innovation and productivity in dryland agriculture: a return–risk analysis for Australia. J Agric Sci 149, 77–89.
| Innovation and productivity in dryland agriculture: a return–risk analysis for Australia.Crossref | GoogleScholarGoogle Scholar |
[26] Diao, T et al. (2023) Microplastics derived from polymer-coated fertilizer altered soil properties and bacterial community in a Cd-contaminated soil. Appl Soil Ecol 183, 104694.
| Microplastics derived from polymer-coated fertilizer altered soil properties and bacterial community in a Cd-contaminated soil.Crossref | GoogleScholarGoogle Scholar |
[27] Santos, MS et al. (2019) Microbial inoculants: reviewing the past, discussing the present and previewing an outstanding future for the use of beneficial bacteria in agriculture. AMB Express 9, 205.
| Microbial inoculants: reviewing the past, discussing the present and previewing an outstanding future for the use of beneficial bacteria in agriculture.Crossref | GoogleScholarGoogle Scholar |
[28] Abbott, LK et al. (2018) Potential roles of biological amendments for profitable grain production – a review. Agric Ecosyst Environ 256, 34–50.
| Potential roles of biological amendments for profitable grain production – a review.Crossref | GoogleScholarGoogle Scholar |
[29] Gambarini, V et al. (2021) Phylogenetic distribution of plastic-degrading microorganisms. mSystems 6, e01112-20.
| Phylogenetic distribution of plastic-degrading microorganisms.Crossref | GoogleScholarGoogle Scholar |
[30] Liu, S et al. (2022) Opportunities and challenges of using metagenomic data to bring uncultured microbes into cultivation. Microbiome 10, 76.
| Opportunities and challenges of using metagenomic data to bring uncultured microbes into cultivation.Crossref | GoogleScholarGoogle Scholar |