Hospitals as amplifiers of infectious diseases
Lyn GilbertCentre for Infectious Disease and Microbiology
Westmead Hospital
Marie Bashir Institute for Emerging Infections and Biosecurity
University of Sydney
Tel: +61 2 9845 6252
Email: lyn.gilbert@sydney.edu.au
Microbiology Australia 35(1) 5-9 https://doi.org/10.1071/MA14003
Published: 17 February 2014
Institutions for the care of the sick and relief of the poor date back at least to ancient Egypt and Greece. In Europe they were generally run by religious communities (Fig. 1), until the Reformation, in the 16–17th Century, when many passed from Church, to secular control. ‘Modern’ hospitals, which would become centres of medical innovation, research and training, were first established during the Enlightenment, in the 18th Century, funded by wealthy benefactors, specifically for care of the sick.

|
These 18th Century hospitals were a mixed blessing for patients. The life-threatening illnesses for which they were admitted were often infectious diseases – of which smallpox was the most prominent and feared – and hospital outbreaks were common. Specialised ‘smallpox’ – or, later, ‘fever’ – hospitals partly relieved the pressure, but outbreaks of infectious diseases still occurred in the overcrowded, dirty, verminous wards where patients often shared beds. The lice that infested patients’ beds spread louse-borne typhus (‘hospital’ fever) and outbreaks of dysentery and respiratory infections were common. As barber surgeons became more ambitious, surgical sepsis and gangrene became more common, with mortality rates, after amputations, up to 40%1. Women hoping for assistance during childbirth were much more likely to die from childbed fever in hospital than if they delivered at home2. Surgical sepsis and childbed fever were recognised, by many practitioners, as epidemiologically linked and much more likely to occur in hospitals; some had begun to suspect their own roles in transmission, despite the prevailing belief in miasmas as the vectors of disease.
In 1789, the Scottish obstetrician Alexander Gordon, in A Treatise on the Epidemic Puerperal Fever of Aberdeen, linked clusters of childbed fever cases to certain doctors’ or midwives’ practices. His assertion that these practitioners were spreading disease was largely ignored for many years3, until, in 1843, the young Boston physician, Oliver Wendall Holmes, presented a review of the literature, including Gordon’s treatise, and results of his extensive correspondence with colleagues, entitled The Contagiousness of Childbed Fever. His research revealed the widespread, often guilty, suspicion among some doctors that they, themselves, were responsible for the spread of childbed fever, between patients or from bodies of dead patients, which they had recently dissected. Many described their attempts to prevent this by strict ablutions and changing clothes between patients. But Holmes stated publicly that: ‘occurrence of three or more closely connected cases, in the practice of one individual … is primâ facie evidence that he is the vehicle of contagion’ and when his paper was republished, in 18554, it provoked abuse and scorn from several influential academics, who could not countenance the possibility that a physician could be responsible for transmission of disease.
Meanwhile, in 1847, another controversial physician/obstetrician entered the fray. Ignaz Semmelweis was assistant lecturer at the first obstetrical division of the Vienna General Hospital. He became greatly concerned by the persistently much higher (up to 10-fold) maternal mortality from childbed fever in the first, compared with the second obstetrical division, although the only obvious difference between them was that the latter was staffed by midwives and the former, by doctors and medical students. Semmelweis spent months systematically investigating every conceivable explanation, without success, until the death of his mentor – the pathologist, Jacob Kolletschka – provided a breakthrough. Kolletschka had died from sepsis, following a scalpel wound sustained during an autopsy; the pathological findings at his autopsy were essentially similar to those of childbed fever victims. Semmelweis realised that Kolletschka had died from ‘cadaverous material’ being introduced into his wound. He reasoned that similar material was the cause of childbed fever and ‘that the transmitting source of the cadaver particles was … the hands of the students and attending physicians’5, each of whom who performed several autopsies a day and moved freely between the mortuary and wards where they examined women in labour. Hand washing was perfunctory and failed to remove the cadaverous smell6.
Semmelweis ordered his students and colleagues to wash their hands in chloride of lime between the autopsy room and examining patients. The next year (1848), the maternal mortality in the first obstetrical division fell from more than 10% to an unprecedented low of 1.2% – almost identical with that (1.3%) of the second division6. The critical difference between divisions, which Semmelweis had overlooked before, was that midwives did not perform autopsies! Until many years after his death, Semmelweis’ evidence-based hand hygiene practice was misunderstood or angrily rejected by the medical establishment, even after he belatedly published his findings, in 1861, in Die Aetiologie, der Bergriff und die Prophylaxis des Kindbettfiebers(The Etiology, the Concept and the Prevention of Puerperal Fever). But Semmelweis’ strategy for implementing practice change was far from ideal:
Insult yourenemies, accuse your superiors of causing the deaths of mothers... refuse to publish, but when you do so write incomprehensibly, use public humiliation and haranguing to change behaviour, and be arrogant and angry. This will not work every time…7!
Fortunately, prevention of hospital-acquired infections did not rest only with Semmelweis. Florence Nightingale reduced hospital cross-infection by enforcing strict hygiene – clean linen (boiled to destroy lice) for each patient; disposal of sewage; fresh-air ventilation (Fig. 2)8. Joseph Lister, influenced by the work of Pasteur and Koch on the germ theory of disease, introduced antiseptic principles of surgery: use of carbolic acid spray in the operating theatre and carbolic acid wound dressings to kill bacteria, with dramatic reduction in surgical sepsis9.
Despite these improvements, infectious diseases remained the most common cause of premature death, until the first half of the 20th Century, when safe and effective vaccines and antibiotics were developed. Together, they changed the course of medical (and arguably global and military) history to such an extent that, in 1967, the US Surgeon General, William Stewart, is reputed to have said: ‘It is time to close the book on infectious disease’. This widely held view10 remained largely unchallenged until the 1980s when the emergence of AIDS and other ‘new’ infectious diseases proved how misguided it had been.
By then, hospital infections were no longer taken very seriously. When outbreaks of virulent penicillin-resistant Staphylococcus aureus infection occurred in newborn nurseries in the 1950s, shortly after penicillin was introduced, methicillin and its derivatives were developed; when methicillin-resistant S. aureus first began to spread in hospitals, in the 1970s, vancomycin was used11. Ampicillin, then successive generations of cephalosporins, aminoglycosides and carbapenems were introduced to manage infections due to E. coli and other Gram-negative bacteria, as they became progressively more resistant to one antibiotic after another or, more commonly, to several different antibiotic classes simultaneously. The emergence of multiresistant bacteria has been insidious, like climate change, and warnings of ‘the end of the antibiotic era’ were often dismissed as alarmist – at least until recently, when the flow of new antibiotics has largely dried up.
Viral infections also are amplified and spread in the hospital environment. The importance of blood-borne viruses (BBV) only became apparent in the 1970s, when hepatitis B virus (HBV) was identified and outbreaks of HBV infection occurred among patients, in which healthcare workers (HCW) were the source12. Serological surveys showed that HCW, especially those frequently exposed to blood, were several times more likely to have been infected with HBV than the general population13. The HBV vaccine has virtually eliminated the risk, but hepatitis C, HIV and other BBV remain potential risks for HCWs and patients exposed to blood or blood products in healthcare settings.
Meanwhile, hospital outbreaks of other vaccine preventable diseases still occur, even in countries where immunisation uptake is high. Measles, pertussis and varicella, introduced by an infected HCW or patient, can cause serious, potentially fatal illness in highly vulnerable patients; even a single mild case can cause major disruption, while contacts are traced and quarantined. The risk can be reduced by mandatory HCW immunisation with routine childhood vaccines, as introduced, with moderate success, in New South Wales, in 200714. Also, regular hospital outbreaks of gastrointestinal and respiratory viral infections, of which influenza is the most serious, occur each year. Mandatory annual HCW influenza immunisation remains controversial and logistically difficult, but voluntary uptake is often poor15.
It is easy to dismiss the gradual increase in rates of healthcare-associated infection (HAIs) due to multiresistant bacteria or regular seasonal outbreaks of viral infection, as unavoidable collateral damage inherent in modern hospital practice. But the estimated burden of HAIs and the associated morbidity, mortality and excess healthcare costs is huge: an estimated 1.7 million HAIs and 99,000 deaths at a cost of US$10–25 billion, in the USA, annually. Approximately 50% or more of these HAIs are preventable16, using simple measures, of which hand hygiene – as advocated with relatively little success by Semmelweis, but recently embraced by healthcare authorities, worldwide17 – is the most important. Despite the simplicity and logic of hand hygiene and massive international efforts to facilitate and promote it, compliance by HCW remains patchy and often inadequate. HAI prevention is an urgent ethical issue, but it remains a relatively low priority in many countries, including Australia, with increasingly expensive, sophisticated healthcare systems.
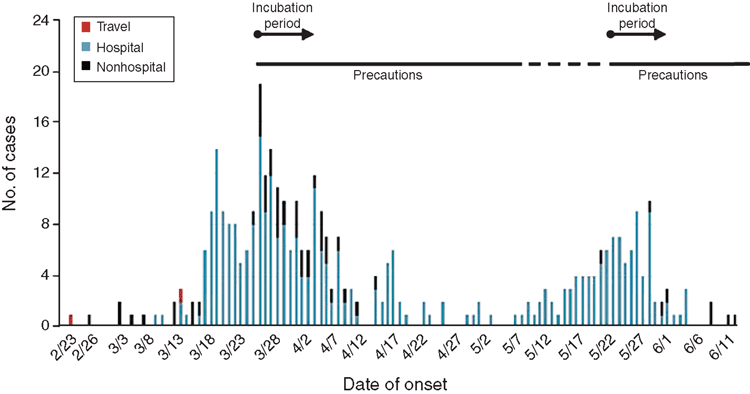
Occasionally, unexpected events seriously challenge our complacency. In February 2003, a Chinese doctor with a mysterious respiratory illness checked into the Metropole Hotel, Hong Kong, where he infected 12 people, who subsequently travelled to several countries where they, in turn, infected another 350 people with a disease that became known as severe acute respiratory syndrome (SARS), due to a novel coronavirus. More than 8000 people (one fifth of whom were HCW) were infected and nearly 800 died from SARS before the outbreak was controlled. Two of the people infected at the Metropole Hotel were Canadians. One, an elderly woman from Toronto, died at home; her son, who became ill while caring for her, was admitted to a Toronto hospital where, directly or indirectly, he infected 84 people, including 37 HCW of whom three died. After strict precautions were implemented18 the outbreaks appeared to have been controlled and the Ontario government eased restrictions, under pressure because of serious disruption to medical services, businesses and tourism. However, new cases had been occurring, unrecognised, in another hospital and a second epidemic wave affected 118 people (including 64 HCWs), causing 17 deaths. Overall, in Ontario, there were 375 cases (72% in healthcare settings), including 44 deaths (Fig. 3). This was in stark contrast to what occurred in British Columbia where there were only four proven SARS cases and no deaths after the other Canadian, who had been infected at the Metropole Hotel, was admitted to hospital in Vancouver.
|
The SARS Commission, established in 2006 to investigate these tragic events, revealed that in Toronto the index patient waited in the busy Emergency Department for 16 hours and was not placed in respiratory isolation until 21 hours after his arrival at the hospital. The Commission described a poor worker safety culture, dissociated from infection control, and described the Ontario public health system as ‘…broken, neglected, inadequate and dysfunctional’. In contrast, British Columbia had a well-rehearsed pandemic plan, awareness among HCW of infection control, including appropriate use of N95 masks, and a proactive workplace regulator. However, the Commission conceded:
No one foresaw the sudden emergence of an invisible unknown disease with no diagnostic test, no diagnostic criteria, uncertain symptoms, an unknown clinical course, … incubation period, … duration of infectivity, … method of transmission…[etc.] … no known treatment and no known vaccine19.
SARS should have been a wakeup call to healthcare organisations everywhere. The events in Toronto could have occurred in any hospital system with inadequate infection control emergency plans, including many in Australia. It could happen again. In September 2012, the discovery was announced of a new coronavirus that caused a SARS-like illness in humans – the Middle Eastern respiratory syndrome (MERS) coronavirus. By November 2013, 175 confirmed or probable cases had been reported, of which 97 resulted from human-to-human transmission, including at least 60 in healthcare settings20. Despite the lessons of SARS, healthcare settings will continue to harbour and amplify established and, potentially, new pathogens and the resistance genes by which they resist our dwindling therapeutic arsenal, unless the whole healthcare community accepts the moral responsibility of protecting the patients in our care.
References
[1] Smith, P.W. et al. (2012) Infection control through the ages. Am. J. Infect. Control 40, 35–42.| Infection control through the ages.Crossref | GoogleScholarGoogle Scholar | 21783278PubMed |
[2] Hallett, C. (2005) The attempt to understand puerperal fever in the eighteenth and early nineteenth centuries: the influence of inflammation theory. Med. Hist. 49, 1–28.
| The attempt to understand puerperal fever in the eighteenth and early nineteenth centuries: the influence of inflammation theory.Crossref | GoogleScholarGoogle Scholar | 15730128PubMed |
[3] Gould, I.M. (2010) Alexander Gordon, puerperal sepsis, and modern theories of infection control- Semmelweis in perspective. Lancet Infect. Dis. 10, 275–278.
| Alexander Gordon, puerperal sepsis, and modern theories of infection control- Semmelweis in perspective.Crossref | GoogleScholarGoogle Scholar | 20334850PubMed |
[4] Holmes, O.W. (2001) The Contagiousness of Puerperal Fever. Vol. XXXVIII, Part 5. The Harvard Classics. P.F. Collier & Son, New York.1909–14. www.bartleby.com/38/5/
[5] Semmelweis, I. (2008) Classics in social medicine. The etiology, concept and prophylaxis of childbed fever (excerpts). Social Medicine 3, 4–12.
[6] Nuland, S.B. (1979) The enigma of Semmelweis--an interpretation. J. Hist. Med. Allied Sci. 34, 255–272.
| The enigma of Semmelweis--an interpretation.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL3c%2Fgslequw%3D%3D&md5=afad69f09ad13783c170c4761dac9235CAS | 383779PubMed |
[7] Best, M. and Neuhauser, D. (2004) Ignaz Semmelweis and the birth of infection control. Qual. Saf. Health Care 13, 233–234.
| Ignaz Semmelweis and the birth of infection control.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2c3ns12msw%3D%3D&md5=9099fb1875a11815eed5dcd3a5d4bdb1CAS | 15175497PubMed |
[8] Gill, C.J. and Gill, G.C. (2005) Nightingale in Scutari. Her legacy re-examined. Clin. Infect. Dis. 40, 1799–1805.
| Nightingale in Scutari. Her legacy re-examined.Crossref | GoogleScholarGoogle Scholar | 15909269PubMed |
[9] Lister, J. (2010) On the antiseptic principle in the practice of surgery. Brit. Med. J. (1867) vol. ii, p. 24. Reprinted in Clin Orthop Relat Res 468, 2012–2016.
| On the antiseptic principle in the practice of surgery. Brit. Med. J. (1867) vol. ii, p. 24. Reprinted inCrossref | GoogleScholarGoogle Scholar |
[10] Sassetti, C.M. and Rubin, E. (2007) The open book of infectious diseases. Nat. Med. 13, 279–280.
| The open book of infectious diseases.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXisVKktbo%3D&md5=9883558bbb19194759762552f47dd207CAS | 17342136PubMed |
[11] Rountree, P.M. (1978) The history of staphyloccal infection Australia. Med. J. Aust. 2, 543–546.
| 1:STN:280:DyaE1M%2Fnt1GitA%3D%3D&md5=9222a01a841e76f888bbf1a2ca6d0660CAS | 152838PubMed |
[12] Heptonstall, J. (1991) Outbreaks of hepatitis B virus infection associated with infected surgical staff. PHLS Commun. Dis. Rep. 1, R81–R85.
| 1:STN:280:DyaK2c7jvVCmuw%3D%3D&md5=d64989a072ba29cfb7d9262d1f954cc1CAS |
[13] West, D.J. (1984) The risk of hepatitis B infection among health professionals in the United States. Am. J. Med. Sci. 287, 26–33.
| The risk of hepatitis B infection among health professionals in the United States.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL2c7ns1SksA%3D%3D&md5=48757551ae20ecd6d51dded882c0e47dCAS | 6369984PubMed |
[14] Helms, C. et al. (2011) Implementation of mandatory immunisation of healthcare workers: Observations from New South Wales, Australia. Vaccine 29, 2895–2901.
| Implementation of mandatory immunisation of healthcare workers: Observations from New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar | 21338677PubMed |
[15] Rakita, R.M. et al. (2010) Mandatory influenza vaccination of healthcare workers: a 5-year study. Infect. Control Hosp. Epidemiol. 31, 881–888.
| Mandatory influenza vaccination of healthcare workers: a 5-year study.Crossref | GoogleScholarGoogle Scholar | 20653445PubMed |
[16] Umscheid, C.A. et al. (2011) Estimating the proportion of healthcare-associated infections that are reasonably preventable and the related mortality and costs. Infect. Control Hosp. Epidemiol. 32, 101–114.
| Estimating the proportion of healthcare-associated infections that are reasonably preventable and the related mortality and costs.Crossref | GoogleScholarGoogle Scholar | 21460463PubMed |
[17] Allegranzi, B. et al. (2013) Global implementation of WHO’s multimodal strategy for improvement of hand hygiene: a quasi-experimental study. Lancet Infect. Dis. 13, 843–851.
| Global implementation of WHO’s multimodal strategy for improvement of hand hygiene: a quasi-experimental study.Crossref | GoogleScholarGoogle Scholar | 23972825PubMed |
[18] Low, D. and McGeer, A. (2003) SARS – one year later. N. Engl. J. Med. 349, 2381–2382.
| SARS – one year later.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXpvFGqsL4%3D&md5=14a8bfb201811f67ff7ea9a7a95caaa8CAS | 14681502PubMed |
[19] Campbell, A. (2006) The SARS Commission. Spring of Fear. Volume 1. Executive Summary. http://www.health.gov.on.ca/en/common/ministry/publications/reports/campbell06/Vol1Chp1.pdf (accessed 23 December 2013).
[20] Assiri, A. et al. (2013) Hospital outbreak of Middle East respiratory syndrome coronavirus. N. Engl. J. Med. 369, 407–416.
| Hospital outbreak of Middle East respiratory syndrome coronavirus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXht1GmsrrL&md5=2c6e03cab46488def6bcecc50fff37abCAS | 23782161PubMed |
Biography
Lyn Gilbert (MD, FRACP, FRCPA, MBioethics, FASM) is a clinical microbiologist and infectious diseases physician who, until recently, was Director of Centre for Infectious Diseases (CIDM) Laboratory Services and CIDM-Public Health, Westmead Hospital. She is currently Clinical Lead in Infection Prevention and Control for Western Sydney Local Health District. Her research interests include clinical and molecular epidemiology, prevention and control and ethics of communicable diseases of public health importance, including healthcare-associated infections.