The microbiology of acid sulfate soils and sulfidic sediments
Jacqueline L Stroud A and Mike Manefield BA Water Research Centre
School of Civil and Environmental Engineering
University of New South Wales
Sydney, NSW 2052, Australia
Tel: +61 2 9385 5604
Fax: +61 2 9313 8624 469036
Email: jacstroud@gmail.com
B School of Biotechnology and Biomolecular Sciences
University of New South Wales
Sydney, NSW 2052, Australia
Microbiology Australia 35(4) 195-198 https://doi.org/10.1071/MA14063
Published: 30 October 2014
Acid sulfate soils and their associated sulfidic sediments present a major hazard to sustainable farming, water security and urban infrastructure. Traditionally these soils are limed in order to neutralise the ‘leachate’ that is a public health hazard and toxic to aquatic organisms. It may be more sustainable to exploit the soil microorganisms capable of sequestering metals to remediate these soils. Until recently, little was known about the microbial ecology of these environments. The soils have a moderately acidic (pH 4) chemistry and a unique ecosystem where the microbial community composition is correlated to bioaccessible metal concentrations. These environments have the potential to provide novel insights into how environmental conditions shape the microbiome that can be exploited for biotechnologies.
The formation and management of acid sulfate soils
Worldwide, the pressure for land to meet food security and urban development needs can lead to the disturbance of waterlogged soils containing iron-sulfide minerals. These minerals are oxidised to form acid sulfate soils (Figure 1); a toxic legacy of rain-induced acidic and metalliferous groundwaters that lead to the corrosion of infrastructure and the severe deterioration of water quality. The associated formation of monosulfides in affected waterways is equally problematic, capable of de-oxygenating the aquatic ecosystem in seconds if disturbed.
Current management and treatment practices of acid sulfate soils include minimising the formation of acidity; neutralising acidity and/or turning land back to wetlands. The ongoing cost of these disturbed environments in Queensland alone is estimated at $180 million per year1.
Up until recently, little was known about the microbial community in coastal acid sulfate soils. Nonetheless; rates of mineral transformation2 and mineral transformation products3,4 cannot be fully explained by abiotic processes, indicating that soil bacteria play an essential, but unknown role in element cycling in this environment. Furthermore, under biotic, redox cycling conditions, the rapid (days to months) transformation of meta-stable minerals to stable minerals, which concomitantly sequesters trace contaminants could be possible3. Thus, understanding the microbial ecology, and particularly the identification of bacteria capable of iron-sulphur transformations, is an essential step towards exploiting the microbial community to remediate these environments.
Microbial ecology of acid sulfate soils and sulfidic drain sediments
The microbial ecology was determined using pyrosequencing from samples collected from the model coastal acid sulfate soil site at Blacks Drain, Tweed Valley, NSW, Australia. The sampling strategy is fully detailed elsewhere5. This site is considered to be a model setting as it is a pollution hotspot (contaminating adjacent waterways with acidity, Al, Mn and Fe) has undergone detailed geochemical characterisations of the soils and sediments over the past decade5.
The acid sulfate soils and sulfidic drain sediments contained an average 186 phylotypes per sample in comparison to non-pyritic soils, which contain 5-fold higher ecosystem complexity, with an average 1,017 phylotypes per sample6. Ecosystem complexity is controlled by selection pressures that reduce species diversity7. Acid sulfate soils contain a number of selection pressures associated with the seasonal oxidation of the unstable iron minerals; however, in comparison to acid mine drainage environments (pH 2–4) that contain an average of 61 phylotypes per sample8, these soils have a 3-fold higher ecosystem complexity.
A total of 23 phyla were identified, of which five phyla dominate (>90%) the community composition, with four common to all environments (Proteobacteria, Acidobacteria, Firmicutes and Chloroflexi), and Bacteriodetes as a major component of sulfidic sediments and Actinobacteria a major component of acid sulfate soil field horizons.
The subdivision of the phyla totalled 48 classes. Focusing on the dominant phyla, drain sediments had two-fold higher abundance of Proteobacteria; and were dominated by δ-proteobacteria. All soil horizons contained δ-proteobacteria but were dominated by β-proteobacteria and α-proteobacteria. Splitting the phylum Chloroflexi into classes revealed that drain sediments were dominated by Anaerolineae in comparison to the soils that were dominated by the Chloroflexi class. The phylum Acidobacteria, also showed that the drain sediments were dominated by the Acidobacteria class and soil horizons dominated by Holophagae. The phylum Firmicutes was dominated by the classes Bacilli and Clostridia across both settings.
Interestingly, there was a low abundance of the class Nitrospira, a group that contains acidophilic Fe(II) oxidising bacteria (Leptospirillum sp.). This is the most important phylotype in acid mine biofilms7; is moderately abundant (average 12%) in acid mine drainage sites8, but has a low abundance in acid sulfate soils at <3%. The abundance of this group may be determined by pH due to their limited metabolic capacity (derive energy solely from Fe(II) oxidation) thus are adapted to extremely acidic environments (pH <4)8 where competing abiotic Fe(II) oxidation kinetics are very slow.
Iron and sulphur cycling bacteria in acid sulfate soils
A patchy species distribution and a high proportion of unclassified bacteria characterised acid sulfate soils. Interestingly, these soils lacked an abundance of known acid tolerant Fe(II) oxidisers, of which there are 22 known acidophilic Fe(II) oxidising species across four phyla9. Only the Firmicutes, Alicyclobacillus tolerans was detected in these soils. Instead, the microbial community associated with Fe(II) oxidation was the Betaproteobacteria, Sideroxydans lithotrophicus and Sideroxydans paludicola, with a known ability for Fe(II) oxidation over neutrophilic pH range (4–7.5) under micro-aerobic conditions. Another interesting finding was that Chloroflexus was highly abundant in the acid sulfate soil horizons. Little is known about the role of Chloroflexus in iron cycling, but research from microbial mat zones indicate a positive relationship to zones of enriched Fe(II) oxidation10 and the fully sequenced Chloroflexus aurantiacus contains a candidate Fe(II) trafficking protein (ATCC strain 29364).
Iron reduction is a ubiquitous microbiological mechanism11; but only the neutrophile Anaeromyxobacter dehalogenans was abundant in the acid sulfate soil horizon. Substrate based studies offer the potential to discover the largely unknown Fe(III) reducing the community in these soils as carbon substrates differ between acidophiles and neutrophiles. For example, acetate is key to Geobacter sp.12; however, acetate inhibits Fe(III) reduction in acidic conditions and sugars are a key substrate that stimulates Fe(III) reduction by acidophiles13.
Few S-cycling bacteria were found in the acid sulfate soils; furthermore, considering there are 220 bacterial species known to be involved in sulfate reduction14, it was surprising that no sulfate-reducing species were identified at this taxonomic level. This suggests that there is a significant repository of unknown S-cycling bacteria present in these environments.
Iron and sulphur cycling bacteria in sulfidic sediments
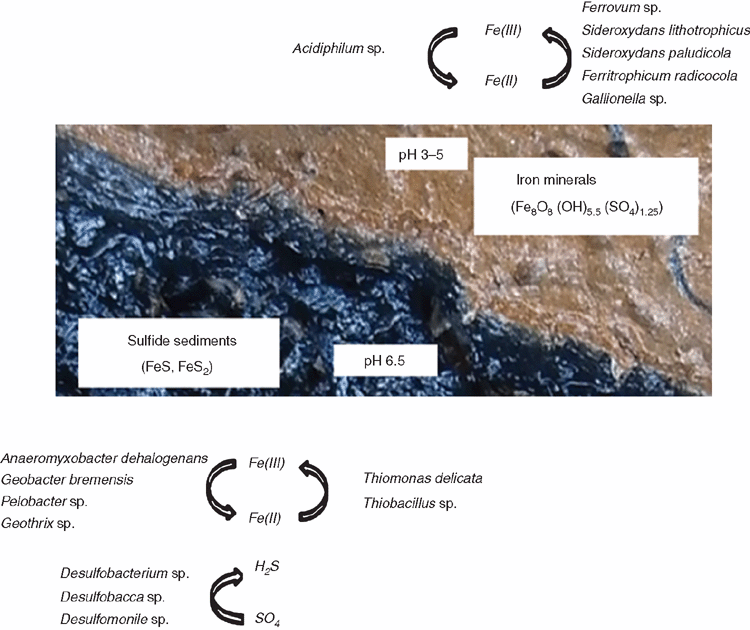
Unlike the acid sulfate soils, the sulfidic sediments were reasonably well resolved and a conceptual model of microbially mediated iron cycling and sulfate reduction in the sulfidic drain sediments was developed (Figure 2).
The sulfidic drain sediments contained an abundance of Fe(II) oxidising bacteria, for example, Ferrovum. sp; that oxidises Fe(II) aerobically using CO2. Further, there was an abundance of microbes with capabilities to reduce Fe(III) in the sulfidic sediments which included the acidophile Acidobacterium capsulatum that reduces Fe(III) between pH 2–5, and the neutrophiles Anaeromyxobacter dehalogenans and Geobacter sp. The sulfidic drain sediments contained an abundance of S-compound oxidising bacteria, dominated by Thiomonas delicata. However, similar to the soils, no sulfate reducing species were detected, suggesting that there are many unknown S-cycling bacteria in these environments.
Metal bioaccessibility is correlated to genera abundances
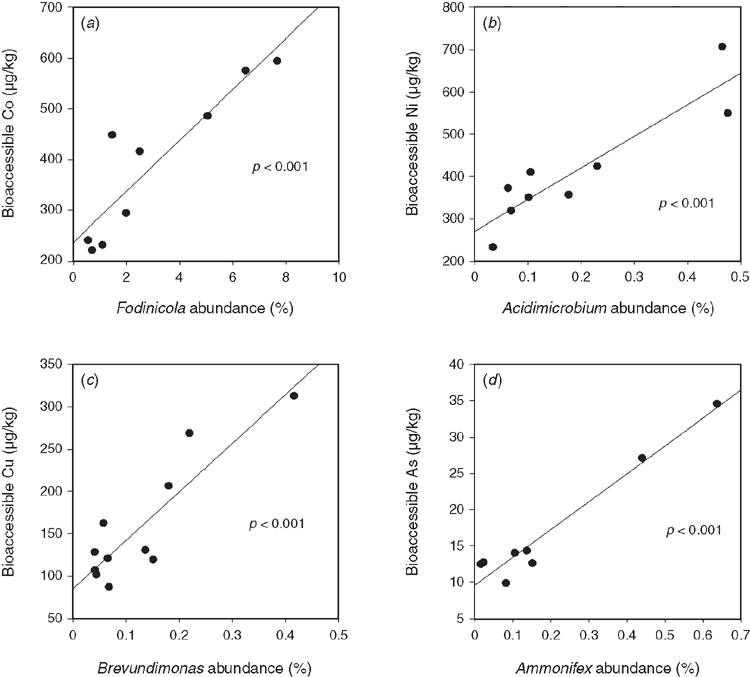
Acid sulfate soil and sulfidic sediments contain highly elevated bioavailable metal(loid) concentrations that are taken up by plants and can be used as biomonitors to isolate pollution hotspots15. A suite of metal(loid)s (Al, As, Cr, Co, Cu, Fe, Mn, Ni and Zn) were positively correlated to genera abundance (Figure 3), indicating that generally genera in acid sulfate environments have high tolerance capabilities to high metal(loid) bioaccessibility, which shape microbial community composition.
|
A key finding was the abundance characteristics of the Acidobacteria and Crenarcheota to bioaccessible Manganese concentrations. Recently, these phyla were found to be enriched in Mn-stimulated microcosms and linked to Mn-dependent organic compound oxidation16. Thus, this demonstrates the need for high phylogenetic resolution to investigate environmental factors; because only genus level resolution identified this relationship to soil chemistry.
Conclusion
Understanding the biogeochemistry of acid sulfate soils and sulfidic sediments would transform the management and remediation of these environmentally deleterious sites. The microbial ecology has few parallels to geochemically similar environments, with a microbial community composition including both acidophiles and neutrophiles associated with iron and sulphur cycling. Furthermore, acid sulfate soils and sulfidic sediments house a repository of uncharacterised microbes but abundance patterns correlated to soil chemistry. Taken together, acid sulfate soils may be a model environment that can be used to understand the role environmental conditions play on microbial community compositions, and this information could be used to develop novel biotechnologies.
References
[1] Fitzpatrick, R.W. et al. (2008) Atlas of Australian acid sulfate soils. In: Inland Acid Sulfate Soil Systems Across Australia (Fitzpatrick, R. W. and Shand, P., eds) vol. 249, pp. 75–89. Perth, CRC LEME.[2] Burton, E.D. et al. (2006) Acid-volatile sulfide oxidation in coastal flood plain drains: iron−sulfur cycling and effects on water quality. Environ. Sci. Technol. 40, 1217–1222.
| Acid-volatile sulfide oxidation in coastal flood plain drains: iron−sulfur cycling and effects on water quality.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xlt1OgtQ%3D%3D&md5=e3f6b89515bf59c65cf142ff8253a99fCAS | 16572778PubMed |
[3] Collins, R.N. et al. (2010) Schwertmannite stability in acidified coastal environments. Geochim. Cosmochim. Acta 74, 482–496.
| Schwertmannite stability in acidified coastal environments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFWmu73K&md5=bde750348504a6c67826f3cbb116dea3CAS |
[4] Burton, E.D. and Johnston, S.G. (2012) Impact of silica on the reductive transformation of schwertmannite and the mobilization of arsenic. Geochim. Cosmochim. Acta 96, 134–153.
| Impact of silica on the reductive transformation of schwertmannite and the mobilization of arsenic.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsV2jsrzL&md5=68153e3574371a7caf4989a621ae7824CAS |
[5] Stroud, J.L. et al. () Metal(loid) bioaccessibility dictates microbial community composition in acid sulfate soil horizons and sulfidic drain sediments. Environ. Sci. Technol., , .
[6] Lauber, C.L. et al. (2009) Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl. Environ. Microbiol. 75, 5111–5120.
| Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtVSgtrjJ&md5=67ea04b9c9a01e748192981392af7cf2CAS | 19502440PubMed |
[7] Baker, B.J. and Banfield, J.F. (2003) Microbial communities in acid mine drainage. FEMS Microbiol. Ecol. 44, 139–152.
| Microbial communities in acid mine drainage.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXjtFWku7c%3D&md5=7c07d6d6d1fb8be38794ec4c39cabd45CAS | 19719632PubMed |
[8] Kuang, J.-L. et al. (2013) Contemporary environmental variation determines microbial diversity patterns in acid mine drainage. ISME J. 7, 1038–1050.
| 1:CAS:528:DC%2BC3sXms1Wit7c%3D&md5=708cbd7c4f36959c4d5ca2319d95ffdaCAS | 23178673PubMed |
[9] Bonnefoy, V. and Holmes, D.S. (2012) Genomic insights into microbial iron oxidation and iron uptake strategies in extremely acidic environments. Environ. Microbiol. 14, 1597–1611.
| Genomic insights into microbial iron oxidation and iron uptake strategies in extremely acidic environments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhtleht7%2FM&md5=2e7ce99659ed3a91152a3c346d7f6383CAS | 22050575PubMed |
[10] Trouwborst, R.E. et al. (2007) Biogeochemistry of Fe(II) oxidation in a photosynthetic microbial mat: implications for Precambrian Fe(II) oxidation. Geochim. Cosmochim. Acta 71, 4629–4643.
| Biogeochemistry of Fe(II) oxidation in a photosynthetic microbial mat: implications for Precambrian Fe(II) oxidation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtFyku7vJ&md5=8c6c51e9abb79eb549756e4f72e88e2dCAS |
[11] Weber, K.A. et al. (2006) Microorganisms pumping iron: anaerobic microbial iron oxidation and reduction. Nat. Rev. Microbiol. 4, 752–764.
| Microorganisms pumping iron: anaerobic microbial iron oxidation and reduction.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XpsFOktLg%3D&md5=c1e86d695d47d392fb0a0adcf4b04760CAS | 16980937PubMed |
[12] Lovley, D.R. and Phillips, E.J. (1987) Competitive mechanisms for inhibition of sulfate reduction and methane production in the zone of ferric iron reduction in sediments. Appl. Environ. Microbiol. 53, 2636–2641.
| 1:CAS:528:DyaL1cXhvV2rtg%3D%3D&md5=dd5bb4858669edb15ec118a23c9fbb80CAS | 16347483PubMed |
[13] Küsel, K. (2003) Microbial cycling of iron and sulfur in acidic coal mining lake sediments. Water Air Soil Pollut. Focus 3, 67–90.
| Microbial cycling of iron and sulfur in acidic coal mining lake sediments.Crossref | GoogleScholarGoogle Scholar |
[14] Barton, L.L. and Fauque, G.D. (2009) Biochemistry, physiology and biotechnology of sulfate-reducing bacteria. Adv. Appl. Microbiol. 68, 41–98.
| Biochemistry, physiology and biotechnology of sulfate-reducing bacteria.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXpsFeis7s%3D&md5=0897400abeee0a1d13f1dff1ca7f009fCAS | 19426853PubMed |
[15] Stroud, J.L. and Collins, R.N. (2014) Improved detection of coastal acid sulfate soil hotspots through biomonitoring of metal(loid) accumulation in water lilies (Nymphaea capensis). Sci. Total Environ. 487, 500–505.
| Improved detection of coastal acid sulfate soil hotspots through biomonitoring of metal(loid) accumulation in water lilies (Nymphaea capensis).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXosVWls7c%3D&md5=22804983fd5f4e5a65874742b6dff225CAS | 24805963PubMed |
[16] Beal, E.J. et al. (2009) Manganese- and iron-dependent marine methane oxidation. Science 325, 184–187.
| Manganese- and iron-dependent marine methane oxidation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXos1Srtr0%3D&md5=f9ffe8fd6f6d7efdbbda3c0922147751CAS | 19589998PubMed |
Biographies
Jackie Stroud was a post-doc at UNSW. Her research interests are environmental soil science and its applications to sustainable agriculture and bioremediation.
Mike Manefield is an Associate Professor at UNSW. His interests are in environmental microbiology and biotechnology development.