Seafood-borne parasitic diseases in Australia: how much do we know about them?
Shokoofeh ShamsiSchool of Animal and Veterinary Sciences
Charles Sturt University
Wagga Wagga, NSW 2650, Australia
Tel: +61 2 6933 4887
Email: sshamsi@csu.edu.au
Microbiology Australia 37(1) 27-29 https://doi.org/10.1071/MA16015
Published: 12 February 2016
Fish are host to many parasites, some of which can cause disease in humans. With the increase in cultural and culinary diversity and the increased popularity of eating raw or slightly cooked seafood dishes in Australia it is speculated that seafood-borne parasitic infections in Australian consumers may rise. Seafood-borne zoonotic parasites are recognised as a significant public health concern worldwide. In Australia there are few reports of infection in humans in the medical literature. Australian Government enforcement agencies rate the risk of seafood-borne zoonosis as low; however, the prevalence of seafood-borne zoonoses may be under-reported in Australia due to misdiagnosis. Although food safety regulations and import controls for seafood in Australia are strict, the focus is more on the control of food-borne bacterial, viral and chemical contaminant related illnesses rather than parasitic diseases.
Increasing demand for raw and exotic seafood has significantly expanded the geographical and demographic limits of fish-borne parasitic infections. Medical literature on the regular consumption of seafood is plentiful. It is heavily promoted for prolonging life, aiding in childhood development, increasing brain stimulation and even to help our pets. However, there is an increasing risk of contracting parasitic zoonoses from consuming raw and undercooked seafood, which should be of concern in Australia. Zoonotic parasites are common in Australian seafood, and can pose a major health risk to people who consume seafood or work in a fishing industry1. Among zoonotic parasites in seafood, Anisakid nematodes are of the greatest significance due to their high prevalence in wild caught fish2 and also due to the severity of the disease they cause, which is known as anisakidosis. Other nematodes such as Gnathostoma and Angiostrongylus as well as platyhelminths such as Diphylobothrium, Clonorchis and Paragonimus, can occasionally cause seafood-borne zoonotic infections3.
Infection in humans (zoonosis)
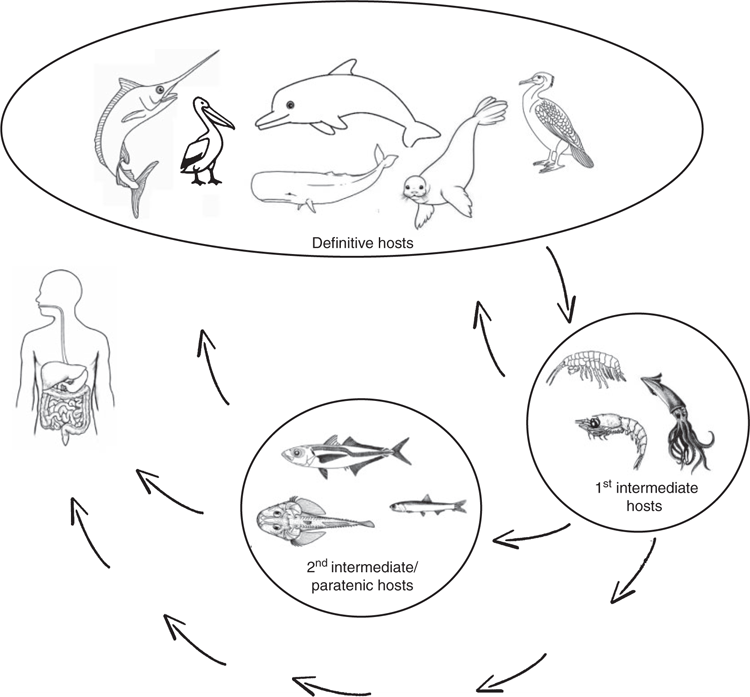
Anisakidosis results from accidental infection with one or more larvae of species of certain genera of anisakids, including Anisakis spp, Contracaecum spp and Pseudoterranova spp. Infection with larvae of Anisakis and Pseudoterranova is of common occurrence, whereas infection with other genera, such as Contracaecum has been reported less frequently. Humans usually become infected with these parasites after eating raw, undercooked or improperly processed fish or seafood (Figure 1). Clinically, several different types of human anisakidosis have been described based on the location of the parasite. This includes gastro-intestinal, visceral, oropharyngeal and transient luminal anisakidosis4. The first two types are associated with severe symptoms such as vomiting, severe pain in lower abdomen and fever.
In recent years, it has been recognised that an allergic response can occur in humans due to live anisakids or food in which worms have been killed by cooking or pasteurisation5. Other allergic disorders, such as chronic urticaria may also result from parasitism6. There is anecdotal evidence in other countries (e.g. Spain) that some allergic reactions to seafood are indeed allergic reaction to anisakids in seafood.
The Australian scenario
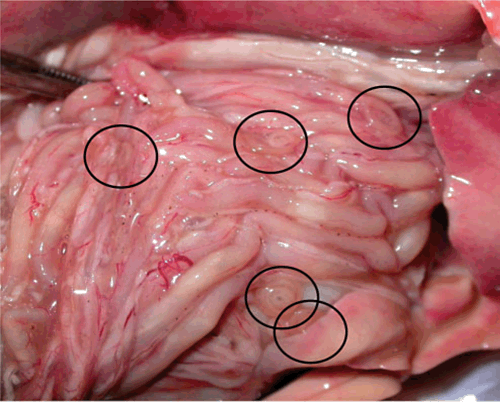
In Australia our knowledge about these important parasites is poor. Although anisakid nematodes are commonly found in Australian marine fish (Figure 2), such as mackerel, flathead, snapper and whiting2,7,8, all forms of seafood are available and popular in Australia and 40% of fish consumed raw in Australia have been considered as infected with Anisakis at the time of processing for trade1, there are only a handful of documented confirmed cases acquired in Australia.
|
Case 1: There has been only one documented confirmed case of infection with anisakid nematodes acquired in Australia9. This case involved a 41-year-old South Australian woman of Tongan descent who became ill after eating raw mackerel that had been caught locally. She was hospitalised with severe gastrointestinal pain, diarrhoea and vomiting that progressively worsened for 3 weeks. The diagnosis only occurred after a worm was passed in her faeces. The initial presumptive identification of the larva was an intestinal nematode, possibly a species of the Trichostrongylus or Ascaris genera. However, on further detailed microscopic examination, the larva was identified as a species of Contracaecum. Had the worm not been found, or properly examined by a taxonomist, misdiagnosis would have occurred. It is very rare for anisakid larvae to be found after passing through the gastro-intestinal tract as was the case in this Australian patient.
Since then the author has come across several suspected cases of anisakidosis, mostly among Australian travellers after returning home (unpublished cases). The cases could not be confirmed due to the lack of knowledge, clinical suspicion and a reliable diagnostic technique in Australia. In many countries, the disease is usually diagnosed by endoscopy, radiography, or surgery if the worm has embedded within the gastro-intestinal tract in patients with symptoms and a history of consuming raw seafood. Serological tests to detect parasite allergens have also been recently developed10. However, in Australia there is no standard test available for diagnosis of these important parasites. General health practitioners are not aware of the presence and high abundance of these parasites in Australian fish and diagnostic laboratory staff are not trained to identify these worms. As a result, the full extent of hidden cases of the disease in Australia remains unknown.
Case 2: Another confirmed case report of fish-borne parasitic disease in humans in Australia occurred in 201111. In this case, a couple became infected with Gnathostoma species after consumption of ‘Black Bream’ (possibly Acanthopagrus berda or Hephaestus jenkinsi), which they caught in the Calder River in Western Australia. They presented with recurring skin swellings. The fish had been cooked on a camp fire, but the cooking time was unclear. The diagnosis was based on eosinophilia and a positive serology. Humans become infected accidentally by consuming third-stage larvae. Gnathostoma larvae are unable to mature inside the human body, and instead migrate through visceral and cutaneous tissues, causing a variety of generalised, non-specific symptoms. Gnathostomiasis is generally endemic in parts of the world where seafood is consumed raw, such as in South-East Asia and Japan, but also more recently in Latin America, India and Africa, and in travellers returning from these areas12.
Another aspect of Australia’s seafood consumption that must be considered is the large volume of imported seafood that Australians consume. The most important sources of seafood products are Thailand, New Zealand, Vietnam and China (frdc.com.au/knowledge/Factsheets/Factsheet_Imported_Seafood_in_Australia.pdf). The literature describes several zoonotic parasites affecting fish endemic to these countries13. CSIRO conducted a comprehensive review of AQIS’s imported seafood testing protocols14 and concluded that imported seafood does not pose any greater health risk to the consumer than locally produced seafood. Nevertheless proper precautions when preparing seafood in order to reduce the risk of illness or disease has been recommended. However, recommendations to eliminate or reduce parasites have not been dealt with in details.
Prevention
Gastro-intestinal anisakidosis can be prevented by properly cooking fish to an internal temperature of approximately 63°C or freezing at or below −20°C for 7 days15. This will most likely prevent most other parasites as well.
Consuming raw seafood in reputable restaurants only, where chefs are trained to recognise infected seafood.
Conclusion
In the absence of standard diagnostic techniques it is difficult to have a realistic estimation of occurrence of seafood borne parasitic diseases in the country. As Chai et al.12 stated it appears that fish borne parasitic disease in Australia remains a ‘public health orphan’ with very little research having been carried out on what the real risks are. The economic value of the fishing industry and health of consumers must be protected. Therefore further research is required to fill in the current knowledge gaps of the biology and ecology of these parasites and the risk they pose to consumers and workers.
References
[1] Anantanawat, S. et al. (2012) A semi-quantitative risk assessment of harmful parasites in Australian finfish. South Australian Research & Development Institute, 2012.[2] Shamsi, S. et al. (2011) Occurrence and abundance of anisakid nematode larvae in five species of fish from southern Australian waters. Parasitol. Res. 108, 927–934.
| Occurrence and abundance of anisakid nematode larvae in five species of fish from southern Australian waters.Crossref | GoogleScholarGoogle Scholar | 21057811PubMed |
[3] Butt, A.A. et al. (2004) Infections related to the ingestion of seafood. Part II: parasitic infections and food safety. Lancet Infect. Dis. 4, 294–300.
| Infections related to the ingestion of seafood. Part II: parasitic infections and food safety.Crossref | GoogleScholarGoogle Scholar | 15120346PubMed |
[4] Smith, J.W. (1999) Ascaridoid nematodes and pathology of the alimentary tract and its associated organs in vertebrates, including man: a literature review. Helminthological Abstracts 68, 49–96.
[5] Audicana, M.T. and Kennedy, M.W. (2008) Anisakis simplex: from obscure infectious worm to inducer of immune hypersensitivity. Clin. Microbiol. Rev. 21, 360–379.
| Anisakis simplex: from obscure infectious worm to inducer of immune hypersensitivity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXmtFKlsrY%3D&md5=9aa3524f91d041d032fb818f153f0bbdCAS | 18400801PubMed |
[6] Daschner, A. and Pascual, C.Y. (2005) Anisakis simplex: sensitization and clinical allergy. Curr. Opin. Allergy Clin. Immunol. 5, 281–285.
| Anisakis simplex: sensitization and clinical allergy.Crossref | GoogleScholarGoogle Scholar | 15864089PubMed |
[7] Doupe, R.G. et al. (2003) Larval anisakid infections of some tropical fish species from north-west Australia. J. Helminthol. 77, 363–365.
| 1:STN:280:DC%2BD3srkvVKnsA%3D%3D&md5=ba2a1e0eb75472834aceb2284cc505eaCAS | 14627454PubMed |
[8] Shamsi, S. et al. (2011) Mutation scanning-coupled sequencing of nuclear ribosomal DNA spacers (as a taxonomic tool) for the specific identification of different Contracaecum (Nematoda: Anisakidae) larval types. Mol. Cell. Probes 25, 13–18.
| 1:CAS:528:DC%2BC3MXhs1ansbY%3D&md5=a5e0b2f60e7d6a403dd2fbaf6dd5369dCAS | 20933594PubMed |
[9] Shamsi, S. and Butcher, A.R. (2011) First report of human anisakidosis in Australia. Med. J. Aust. 194, 199–200.
| 21401462PubMed |
[10] García, M. et al. (1997) The use of IgE immunoblotting as a diagnostic tool in Anisakis simplex allergy. J. Allergy Clin. Immunol. 99, 497–501.
| The use of IgE immunoblotting as a diagnostic tool in Anisakis simplex allergy.Crossref | GoogleScholarGoogle Scholar | 9111494PubMed |
[11] Jeremiah, C.J. et al. (2011) Gnathostomiasis in remote northern Western Australia: the first confirmed cases acquired in Australia. Med. J. Aust. 195, 42–44.
| 21728942PubMed |
[12] Herman, J.S. and Chiodini, P.L. (2009) Gnathostomiasis, another emerging imported disease. Clin. Microbiol. Rev. 22, 484–492.
| Gnathostomiasis, another emerging imported disease.Crossref | GoogleScholarGoogle Scholar | 19597010PubMed |
[13] Chai, J.-Y. et al. (2005) Fish-borne parasitic zoonoses: status and issues. Int. J. Parasitol. 35, 1233–1254.
| Fish-borne parasitic zoonoses: status and issues.Crossref | GoogleScholarGoogle Scholar | 16143336PubMed |
[14] Moir, C. (2009) Review of the current testing protocols for imported seafood products. CSIRO Report reference number R-661-03-11. 90 pp.
[15] Hochberg, N.S. and Hamer, D.H. (2010) Anisakidosis: perils of the deep. Clin. Infect. Dis. 51, 806–812.
| 20804423PubMed |
Biography
Dr Shokoofeh Shamsi is a senior lecturer in veterinary Parasitology. After completing a Masters Degree in Medical Parasitology (Tehran University of Medical Sciences) she gained her PhD in Veterinary Parasitology from The University of Melbourne. She is a taxonomist who couples conventional morphological techniques with modern molecular technologies to diagnose infections and identify parasite species, especially aquatic parasites. This has resulted in the discovery of several new pathogenic species and strains, and their recognition as disease causing agents in humans and animals in Australia and worldwide.