Amoebic gill disease: a growing threat
Jessica Johnson-Mackinnon A , Tina Oldham A and Barbara Nowak A BA University of Tasmania, Institute of Marine and Antarctic Studies, Locked Bag 1370, Launceston, Tas. 7250, Australia
B Corresponding author. Tel: + 61 3 6324 3814, Email: B.Nowak@utas.edu.au
Microbiology Australia 37(3) 140-142 https://doi.org/10.1071/MA16048
Published: 30 August 2016
The risk of disease outbreaks is predicted to increase due to climate change. For farmed fish an example is amoebic gill disease (AGD). While initially reported only in farmed salmonids in Washington State, USA, and Tasmania, Australia, it has now become an issue for Atlantic salmon farming worldwide and affects a range of other farmed marine fish species. Local high temperature anomalies and a lack of rainfall have been associated with the outbreaks of AGD. This worldwide presence is at least partly due to the cosmopolitan nature of the parasite and its low host-specificity. The disease can be treated using freshwater or hydrogen peroxide baths, but the treatments increase the cost of salmon production. Management of AGD contributes 20% to production costs of Atlantic salmon in Tasmania.
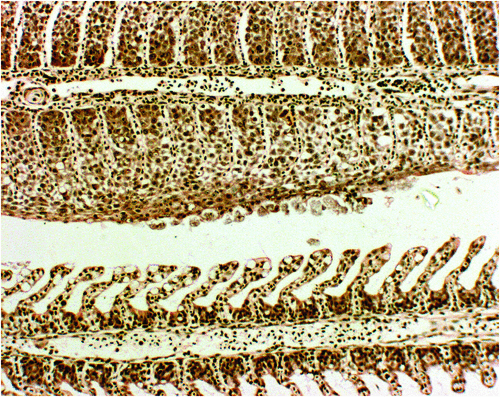
AGD, caused through infection of fish gills by the facultative parasite Neoparamoeba perurans, was first documented in sea-caged salmonids in 19851. Since its initial observation AGD has become a primary health concern globally for the marine salmonid industry, resulting in mortalities as great as 80% when left untreated2. Clinical signs of AGD include respiratory distress, lethargy and inappetence, which are associated with grossly visible gill lesions3. Histologically, gill lesions are characterised by epithelial hyperplasia, interlamellar vesicles with associated amoebae and lamellar fusion4 (Figure 1).
|
Because N. perurans was only recently identified5 and shown to cause AGD6, minimal information is available on its biology and ecology. Amoebae of the genus Neoparamoeba (Amoebozoa, Dactylopodida) are ubiquitous in the marine environment7, and N. perurans specifically have been detected throughout the water column on and near Atlantic salmon (Salmo salar L.) farms8,9. All species from the genus Neoparamoeba harbour at least one intracellular endosymbiont known as a Perkinsela amoebae-like organism (PLO)10. The details of the symbiotic relationship between the PLOs and Neoparamoeba are unknown; however, the strict phylogenetic congruence of PLOs and their Neoparamoeba hosts suggests that PLOs are vertically transmitted from parent to daughter cells during mitotic division10. Species of Neoparamoeba all share the same general ultrastructural characteristics and cannot be differentiated morphologically11.
Despite numerous studies investigating potential reservoirs of N. perurans, no significant reservoir outside farmed salmon has been identified9. Extensive surveys of the water column8,12 and wild fish13–15 have detected only minimal evidence of N. perurans. Studies of metazoan ectoparasites, for example copepods or isopods, on farmed salmon have detected low frequencies of N. perurans16,17, but no evidence of a reservoir population. Additional studies using genus specific identification methods detected Neoparamoeba spp. in sediment samples18 and net biofouling19, but as yet no species specific testing has been conducted to detect N. perurans in these potential reservoirs.
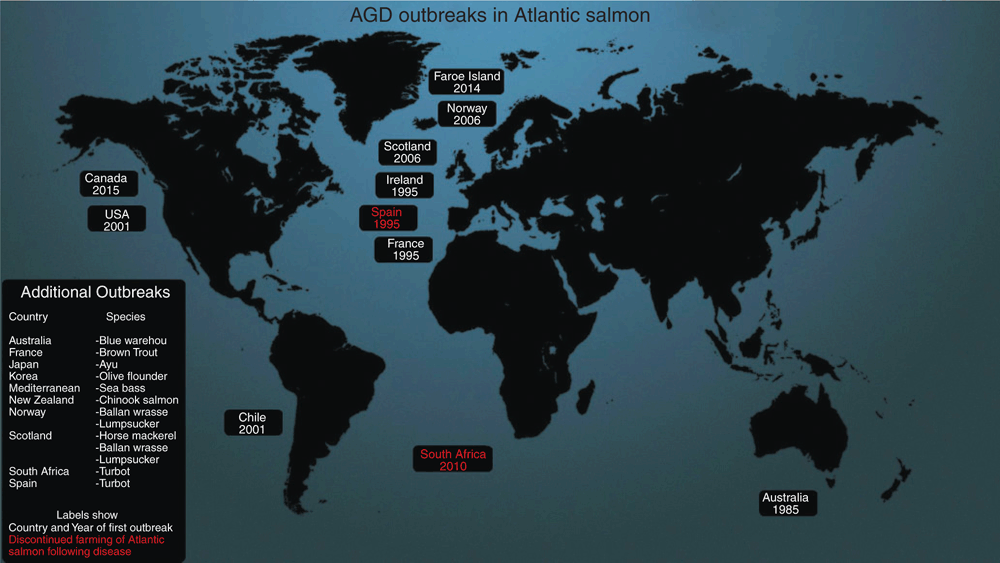
Along with seemingly no reservoir, N. perurans is also an opportunistic parasite with no apparent host specificity. The pathogen has been detected not only in the commercially important Atlantic salmon20, but also in a variety of farmed and non-farmed finfish species around the world9 (Figure 2). Presently AGD is a major issue for aquaculture in Tasmania, Ireland, Scotland, Norway and the United States with varying levels of impact from 10% to 82% mortality in some cases9. Additional outbreaks have been reported in Chile, France, Spain, South Africa, and most recently Canada and the Faroe Islands9,21.
|
Beyond salmon, N. perurans has been found on the gills of an additional 14 finfish species including ayu in Japan22, sea bass in the Mediterranean23, and olive flounder in Korea24. There is no traceable pattern from one of these outbreaks to another making it unlikely that it is a specific sub-population that causes the disease or that amoebae are transferred from one outbreak site to another. What is known of its lifecycle suggests an asexual clonal evolutionary pattern. It has been postulated by statistical analysis that the sheer number of individuals in any given microbial species is so large that dispersal would rarely be restricted by contrived geographical barriers25, especially in marine environments7.
The cosmopolitan nature of N. perurans and lack of host specificity make discerning trends and risk factors for AGD challenging. A recent meta-analysis which considered all reports of AGD to date suggests locally high temperature anomalies, rather than absolute temperature, are related to disease outbreak9. Salinity also plays an important role in AGD. N. perurans is a marine amoeba with minimal tolerance for low salinity. Freshwater bathing for 2–4 hours is the most commonly utilised commercial treatment for AGD26, and though many reports do not include information on rainfall, the few which have report lower than average rainfall preceding outbreaks3,27–29. Given the predicted increase in ocean temperatures and altered rainfall patterns associated with climate change, there is concern that AGD associated costs will continue to increase for the salmonid industry moving forward30.
Although research into AGD has come a long way in the past 30 years there are still many knowledge gaps in key areas from basic biology to industry research. For instance, little is known about the parasite N. perurans. The mechanisms behind the successful transfer of the PLO from mother to daughter cell, and benefits of the symbiosis, are not yet known. In addition there is little information on how the amoebae cause disease and whether there is potential for vaccines or drug targets. On a more practical side, extensive and thorough testing of sediment, biofouling and other potential reservoirs would also be beneficial for predicting outbreaks and controlling this globally increasing threat.
References
[1] Kent, M. et al. (1988) Paramoeba pemaquidensis (Sarcomastigophora: Paramoebidae) infestation of the gills of coho salmon Oncorhynchus kisutch reared in sea water. Dis. Aquat. Organ. 5, 163–169.| Paramoeba pemaquidensis (Sarcomastigophora: Paramoebidae) infestation of the gills of coho salmon Oncorhynchus kisutch reared in sea water.Crossref | GoogleScholarGoogle Scholar |
[2] Steinum, T. et al. (2008) First cases of amoebic gill disease (AGD) in Norwegian seawater farmed Atlantic salmon, Salmo salar L., and phylogeny of the causative amoeba using 18S cDNA sequences. J. Fish Dis. 31, 205–214.
| First cases of amoebic gill disease (AGD) in Norwegian seawater farmed Atlantic salmon, Salmo salar L., and phylogeny of the causative amoeba using 18S cDNA sequences.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXktVSgtr8%3D&md5=27431fc6eba06c79ddc8c163ca72677fCAS | 18261034PubMed |
[3] Munday, B. et al. (1990) Paramoebic gill infection and associated pathology of Atlantic salmon, Salmo salar and rainbow trout, Salmo gairdneri in Tasmania, in Pathology in marine science. Proceedings of the Third International Colloquium on Pathology in Marine Aquaculture, 2–6 October 1988, Gloucester Point, Virginia, USA. pp. 215–222.
[4] Nowak, B.F. (2012) Fish parasites, pathobiology and protection. (Woo, P.T.K. and Buchmann, K. eds), pp. 1–18, CAB International.
[5] Young, N.D. et al. (2007) Neoparamoeba perurans n. sp., an agent of amoebic gill disease of Atlantic salmon (Salmo salar). Int. J. Parasitol. 37, 1469–1481.
| Neoparamoeba perurans n. sp., an agent of amoebic gill disease of Atlantic salmon (Salmo salar).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtVOlsrfO&md5=3dd4bda8ce8e74aa56758e7ac1b64f70CAS | 17561022PubMed |
[6] Crosbie, P.B.B. et al. (2012) In vitro cultured Neoparamoeba perurans causes amoebic gill disease in Atlantic salmon and fulfils Koch’s postulates. Int. J. Parasitol. 42, 511–515.
| In vitro cultured Neoparamoeba perurans causes amoebic gill disease in Atlantic salmon and fulfils Koch’s postulates.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC38rpsFWisQ%3D%3D&md5=1ab6295e1ef321ecbf751e34575ec311CAS |
[7] Page, F.C. (1987) The classification of ‘naked’ amoebae (Phylum Rhizopoda). Arch. Protistenkd. 133, 199–217.
| The classification of ‘naked’ amoebae (Phylum Rhizopoda).Crossref | GoogleScholarGoogle Scholar |
[8] Wright, D. et al. (2015) Depth distribution of the amoebic gill disease agent, Neoparamoeba perurans, in salmon sea cages. Aquacult. Environ. Interact. 7, 67–74.
| Depth distribution of the amoebic gill disease agent, Neoparamoeba perurans, in salmon sea cages.Crossref | GoogleScholarGoogle Scholar |
[9] Oldham, T. et al. (2016) Incidence and distribution of amoebic gill disease (AGD)—an epidemiological review. Aquaculture 457, 35–42.
| Incidence and distribution of amoebic gill disease (AGD)—an epidemiological review.Crossref | GoogleScholarGoogle Scholar |
[10] Young, N.D. et al. (2014) Support for the coevolution of Neoparamoeba and their endosymbionts, Perkinsela amoebae-like organisms. Eur. J. Protistol. 50, 509–523.
| Support for the coevolution of Neoparamoeba and their endosymbionts, Perkinsela amoebae-like organisms.Crossref | GoogleScholarGoogle Scholar | 25243758PubMed |
[11] Dyková, I. et al. (2005) Neoparamoeba branchiphila n. sp., and related species of the genus Neoparamoeba Page, 1987: morphological and molecular characterization of selected strains. J. Fish Dis. 28, 49–64.
| Neoparamoeba branchiphila n. sp., and related species of the genus Neoparamoeba Page, 1987: morphological and molecular characterization of selected strains.Crossref | GoogleScholarGoogle Scholar | 15660793PubMed |
[12] Bridle, A. et al. (2010) Rapid detection and quantification of Neoparamoeba perurans in the marine environment. Aquaculture 309, 56–61.
| Rapid detection and quantification of Neoparamoeba perurans in the marine environment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtl2ntr7F&md5=b5eceebb5ade8feadc40d50b875c3f4fCAS |
[13] Stagg, H.E.B. et al. (2015) Detection of Paramoeba perurans in Scottish marine wild fish populations. Bull. Eur. Assoc. Fish Pathol. 35, 217–226.
[14] Kent, M. et al. (1998) Survey of salmonid pathogens in ocean-caught fishes in British Columbia, Canada. J. Aquat. Anim. Health 10, 211–219.
| Survey of salmonid pathogens in ocean-caught fishes in British Columbia, Canada.Crossref | GoogleScholarGoogle Scholar |
[15] Douglas-Helders, G. et al. (2002) Wild fish are not a significant reservoir for Neoparamoeba pemaquidensis (Page, 1987). J. Fish Dis. 25, 569–574.
| Wild fish are not a significant reservoir for Neoparamoeba pemaquidensis (Page, 1987).Crossref | GoogleScholarGoogle Scholar |
[16] Nowak, B.F. et al. (2010) Do salmon lice, Lepeophtheirus salmonis, have a role in the epidemiology of amoebic gill disease caused by Neoparamoeba perurans? J. Fish Dis. 33, 683–687.
| Do salmon lice, Lepeophtheirus salmonis, have a role in the epidemiology of amoebic gill disease caused by Neoparamoeba perurans? Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cnntVCmtg%3D%3D&md5=9422cfc978d2f5957b38a39f86d19e4cCAS | 20412358PubMed |
[17] Gonzalez-Poblete, L. (2015) Ectoparasites and associated pathogens affecting farmed salmon during marine grow-out in Chile and Tasmania. PhD Thesis, University of Tasmania.
[18] Crosbie, P.B.B. et al. (2005) Distribution of Neoparamoeba sp. in sediments around marine finfish farming sites in Tasmania. Dis. Aquat. Organ. 67, 61–66.
| Distribution of Neoparamoeba sp. in sediments around marine finfish farming sites in Tasmania.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD28%2FgtVSnsQ%3D%3D&md5=4029c7c636b9ef05005271b722f4ac45CAS |
[19] Tan, C.K. et al. (2002) Biofouling as a reservoir of Neoparamoeba pemaquidensis (Page, 1970), the causative agent of amoebic gill disease in Atlantic salmon. Aquaculture 210, 49–58.
| Biofouling as a reservoir of Neoparamoeba pemaquidensis (Page, 1970), the causative agent of amoebic gill disease in Atlantic salmon.Crossref | GoogleScholarGoogle Scholar |
[20] Munday, B.L. (1986) Diseases of Salmonids, in Proceedings of the Workshop on Diseases of Australian Fish and Shellfish.
[21] Ruane, N. and Jones, S. (2013) Amoebic gill disease (AGD) of farmed Atlantic salmon (Salmo salar L.). ICES Identification leaflets for diseases and parasites of fish and shellfish.
[22] Crosbie, P.B.B. et al. (2010) Amoebic gill disease in hatchery-reared ayu, Plecoglossus altivelis (Temminck & Schlegel), in Japan is caused by Neoparamoeba perurans. J. Fish Dis. 33, 455–458.
| Amoebic gill disease in hatchery-reared ayu, Plecoglossus altivelis (Temminck & Schlegel), in Japan is caused by Neoparamoeba perurans.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmt1aqtLw%3D&md5=c0ff1d2ff7d26270ca1f95b787ba01ffCAS |
[23] Dyková, I. et al. (2001) Comments on diagnosis of amoebic gill disease (AGD) in turbot, Scophthalmus maximus. Bull. Eur. Assoc. Fish Pathol. 21, 40–44.
[24] Kim, H.J. et al. (2005) Neoparamoeba sp. infection on gills of olive flounder, Par-alichthys olivaceus in Korea. J. Fish Pathol. 18 125 131
[25] Finlay, B.J. (2002) Global dispersal of free-living microbial eukaryote species. Science 296, 1061–1063.
| Global dispersal of free-living microbial eukaryote species.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xjslagsb0%3D&md5=43dd318aace9c12314385be6fe51abd1CAS | 12004115PubMed |
[26] Powell, M.D. et al. (2015) Freshwater treatment of amoebic gill disease and sea-lice in seawater salmon production: Considerations of water chemistry and fish welfare in Norway. Aquaculture 448, 18–28.
| Freshwater treatment of amoebic gill disease and sea-lice in seawater salmon production: Considerations of water chemistry and fish welfare in Norway.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXpt1Kru7o%3D&md5=d42433cf2d572df7a0eedf2cfce2c740CAS |
[27] Bustos, P.A. et al. (2011) Amoebic gill disease (AGD) in Atlantic salmon (Salmo salar) farmed in Chile. Aquaculture 310, 281–288.
| Amoebic gill disease (AGD) in Atlantic salmon (Salmo salar) farmed in Chile.Crossref | GoogleScholarGoogle Scholar |
[28] Rozas, M. et al. (2012) Epidemiology of amoebic gill disease (AGD) in Chilean salmon industry between 2007 and 2010. Bull. Eur. Assoc. Fish Pathol. 32, 181–188.
[29] Rodger, H.D. and McArdle, J.F. (1996) An outbreak of amoebic gill disease in Ireland. Vet. Rec. 139, 348–349.
| An outbreak of amoebic gill disease in Ireland.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2s%2FlvVKqtQ%3D%3D&md5=bb5aea744d80415551b2cd4135a76da7CAS | 8903016PubMed |
[30] Battaglene, S.C. et al. (2008) Scoping study into adaptation of the Tasmanian Salmonid Aquaculture Industry to potential impacts of climate change. National Agriculture and Climate Change Action Plan: Implementation Programme report, p. 83. http://www.imas.utas.edu.au/right-column-content/publications-search?queries_publication_type_query=Research+Reports
Biographies
Jessica Johnson-Mackinnon is a Canadian PhD student in the Aquatic Animal Health group at the University of Tasmania. Her primary interest is investing pathogens associated with industry using molecular techniques.
Tina Oldham is a PhD student in the Aquatic Animal Health group at the University of Tasmania. Her primary interest is in development of sustainable, resilient aquaculture.
Barbara Nowak is a Professor at Institute of Marine and Antarctic Studies at University of Tasmania. Her research focuses on aquatic animal health. She has been working on amoebic gill disease for the past 25 years.


