Filoviruses and bats
Amy J Schuh A , Brian R Amman A and Jonathan S Towner A BA Viral Special Pathogens Branch, Division of High-Consequence Pathogens and Pathology, Centers for Disease Control and Prevention, Atlanta, GA 30329, USA
B Tel: +1 404 639 4561, Fax: +1 404 639 1509, Email: jit8@cdc.gov
Microbiology Australia 38(1) 12-16 https://doi.org/10.1071/MA17005
Published: 17 February 2017
While Reston and Lloviu viruses have never been associated with human disease, the other filoviruses cause outbreaks of hemorrhagic fever characterised by person-to-person transmission and high case fatality ratios. Cumulative evidence suggests that bats are the most likely reservoir hosts of the filoviruses. Ecological investigations following Marburg virus disease outbreaks associated with entry into caves inhabited by Rousettus aegyptiacus bats led to the identification of this bat species as the natural reservoir host of the marburgviruses. Experimental infection of R. aegyptiacus with Marburg virus has provided insight into the natural history of filovirus infection in bats that may help guide the search for the reservoir hosts of the ebolaviruses.
Filovirus history and geographic range
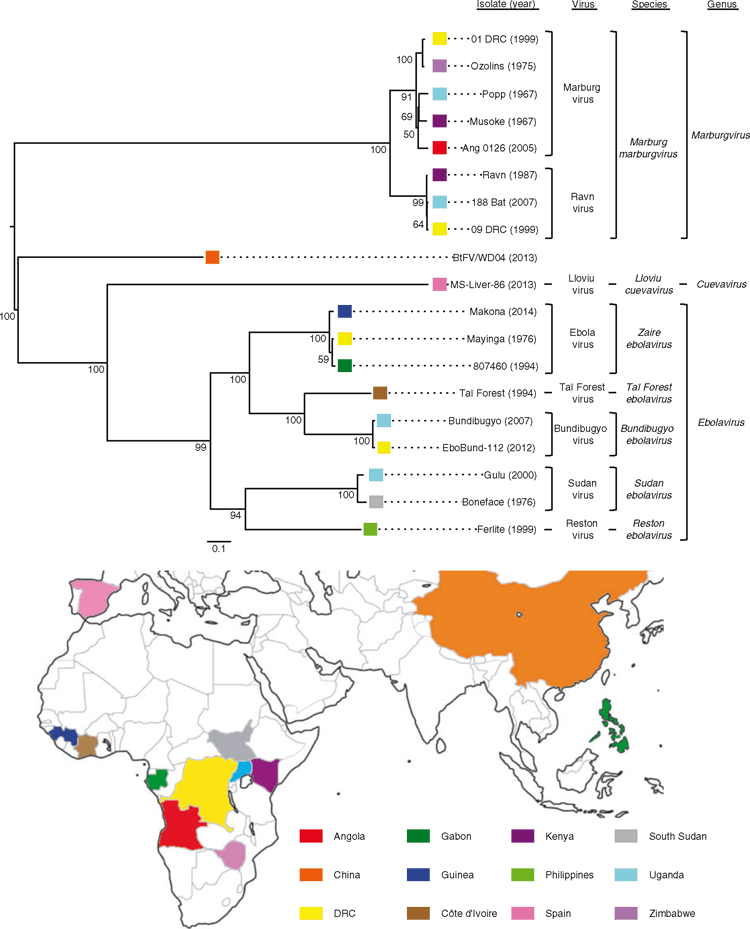
The phylogeny illustrates the genetic relationships between the filoviruses and the associated map shows the known range of filovirus circulation according to virus (Figure 1). Marburg virus (MARV) was first described in 1967 following two successive filovirus hemorrhagic fever (FHF) outbreaks among German and former-Yugoslavian laboratory workers that had handled primates imported from Uganda1. Ravn virus (RAVV), also a marburgvirus, was initially isolated from a 1987 fatal case in Kenya2. Nearly simultaneous FHF outbreaks in present-day South Sudan and the Democratic Republic of the Congo (DRC), led to the identification of Sudan virus (SUDV)3 and Ebola virus (EBOV)4, respectively. Reston virus was discovered in 1989 following an epizootic of FHF among macaques exported to the United States from the Philippines5. Taϊ Forest virus has been isolated once only from a nonfatal case that became ill following the necropsy of a chimpanzee that died from a hemorrhagic disease in Côte d’Ivoire in 19946. Bundibugyo virus was initially isolated during a FHF outbreak in Uganda in 20077. Lloviu virus was identified during the investigation of a die-off of Miniopterus schreibersii bats in Spain in 20028. A partial genomic sequence recovered from a Rousettus leschenaultii bat captured in China in 2013 likely represents a novel filovirus9. Ecological niche modelling has confirmed the known range of filovirus circulation and has predicted additional areas throughout sub-Saharan Africa and Southeast Asia that are suitable for zoonotic transmission of filoviruses10–13.
Evidence suggests that bats are natural reservoir hosts of the filoviruses
Although contact with non-human primate or duiker tissue has been linked to FHF outbreaks1,14–16, the high mortality caused by filoviruses in these animals indicate that they are only incidental hosts. However, FHF outbreak investigations have revealed that many of the index cases had entered environments inhabited by bats prior to disease onset. In 1975, MARV disease occurred in a tourist that had stayed in two hotels populated with bats and visited Chinhoyi Caves in present-day Zimbabwe 8–9 days prior to disease onset17. The index case in the 1976 outbreak of SUDV disease worked at a cotton factory containing Mops trevori18 and the index case in the 1979 SUDV disease outbreak worked at the same factory19. Fifteen days before becoming ill, the index case in the 1980 MARV disease outbreak had entered Kenya’s bat-populated Elgon Caves2 and the 1987-isolated case of RAVV disease had visited Kenya’s Kitum Cave prior to becoming ill20. After the large 1995 epidemic of EBOV disease in present-day DRC, 24 plant and 19 vertebrate and invertebrate native species were experimentally inoculated with EBOV21. Three bat species (Mops condylurus, Chaerephon pumilus and Epomophorus wahlbergi) supported EBOV replication and seroconverted in the absence of overt clinical disease, while the remaining animal and plant species were refractory to virus infection. These findings supported the accumulating number of links between FHF index cases and prior exposure to environments inhabited by bats. This linkage became stronger when it was discovered that 52% of the 154 cases in a series of MARV disease outbreaks in the DRC between 1998 and 2000 worked in the underground Goroumbwa Mine known to house hundreds of thousands of bats22. In 2007, an EBOV disease outbreak followed a reported annual migration of Hypsignathus monstrosus and Epomops franqueti and the putative index case had purchased bats for consumption23. The index cases in a series of MARV and RAVV disease outbreaks in 2007 worked in Kitaka Mine, Uganda24 and two cases of MARV disease were found in tourists that had separately visited nearby-Python Cave in 200825,26.
Rousettus aegyptiacus identified as a natural reservoir host for the marburgviruses
Ecological investigations following the 2007–2008 MARV and RAVV disease outbreaks in Uganda revealed that Kitaka Mine and Python Cave were inhabited by large numbers of R. aegyptiacus24,27. Follow-up longitudinal studies of R. aegyptiacus populations at these sites revealed a consistent prevalence of both MARV and RAVV infection in 2–5% of the bats. Genetically diverse marburgviruses were isolated from bat tissues that were genetically similar to those sequences generated from outbreak cases. Further, the studies found a temporal association between marburgvirus spillover events, biannual pulses of active MARV infection in juvenile bats and the biannual birthing season. These studies provided the evidence needed to definitively identify R. aegyptiacus as a natural reservoir host of the marburgviruses and a source of spillover into the human population.
Natural history of MARV infection in R. aegyptiacus
Following the discovery of R. aegyptiacus as the natural reservoir host for the marburgviruses, experimental studies were initiated to investigate the natural history of virus infection in this bat species. The first published study by Paweska et al. found that bats inoculated by the intraperitoneal and subcutaneous routes with a Vero cell-adapted, human-derived MARV strain exhibited viral replication in multiple tissues in the absence of overt illness followed by seroconversion, while bats dually inoculated by the oral and nasal routes showed no evidence of infection within the 21-day study period28. A second study by Amman et al. found that bats subcutaneously inoculated with a low-passage, bat-derived MARV strain shed virus in their oral secretions up to 11 days following infection and led to the hypothesis that the virus may be horizontally transmitted between bats through direct and/or indirect contact with infectious oral secretions or biting29. To investigate the mechanisms of bat-to-bat MARV transmission, a third study by Paweska et al. housed groups of donor bats inoculated with a human MARV strain with naïve contact bats in direct, indirect or airborne contact and monitored for evidence of infection for 42 days30. No evidence of infection was detected in the contact bats; however, the inoculated bats shed little to no MARV in their bodily fluids and were serially sacrificed as the study progressed. The possibility that hematophagous ectoparasitic argasid ticks (Ornithodoros faini) found in large colonies of R. aegyptiacus might facilitate marburgvirus transmission was ruled-out when >3000 O. faini ticks collected from Python Cave tested negative for marburgvirus RNA31. Further studies are needed to determine how MARV is maintained in its natural reservoir host.
Search for the natural reservoir hosts of the ebolaviruses
Although the index cases of ebolavirus disease outbreaks have been linked to bats, they have never been associated with a particular environment, such as caves, like the index cases of marburgvirus disease outbreaks. Therefore, the search for the reservoir hosts of the ebolaviruses has involved testing a wide-range of wild-caught, forest-dwelling bats for evidence of ebolavirus infection. Serological reactivity of bat sera with ebolavirus antigen has been detected in 307 bats representing at least 17 species throughout sub-Saharan Africa and Asia32–40. Evidence of active ebolavirus infection has been found in seven bat species – EBOV RNA has been detected in three solitary, forest-dwelling frugivorous species (E. franqueti, H. monstrosus and Myonycteris torquata) captured in Gabon and the Republic of Congo32 and RESTV RNA has been detected in four diverse species (Chaerephon plicatus, Cynopterus brachyotis, Miniopterus australis and M. schreibersii) captured in the Philippines39. However, infectious ebolavirus has never been isolated from any of these bat species. Consequently, it is unknown whether they are primary reservoir hosts of the virus, secondary reservoir hosts that play a minor role in virus maintenance or incidental dead-end hosts that are susceptible to infection, but do not shed infectious virus. It is interesting to note that MARV RNA in the absence of infectious virus has been detected in Miniopterus inflatus, Rhinolophus eloquens and Hipposideros sp. bats that roost with R. aegyptiacus24,41. Similarly, investigations examining the susceptibility of R. aegyptiacus bats to experimental infection with each of the five ebolaviruses demonstrated very limited replication and no viral shedding followed by seroconversion42,43. These findings suggest that sporadic detection of filovirus RNA or IgG antibodies from wild-caught bats may only represent virus spillover resulting from contact with a primary reservoir host.
Expectations of a filovirus natural reservoir host
Based on what we have learned about marburgvirus infection in R. aegyptiacus, we would expect the reservoir hosts of the ebolaviruses to have a consistent prevalence of both active and past infection, shed sufficiently high levels of infectious virus to maintain virus circulation in the population and exhibit host population dynamics conducive to virus transmission. Host population-level virus persistence is highly dependent on host population dynamics, particularly community size and annual fluctuations in age-structure from births and deaths. Mathematical modelling of marburgvirus transmission in a closed population of R. aegyptiacus revealed that the virus was only able to persist if the model included: (1) a biannual breeding component that provided a twice-yearly influx of susceptible juveniles; (2) a latent period of ≥21 days; and (3) a host population size ≥20 00044. This suggests that if the natural reservoirs of the ebolaviruses are a solitary bat species that only congregates during the breeding season(s), host population-level virus maintenance may depend on other mechanisms such as persistent infection with intermittent shedding, as has been observed with other bat-borne viruses45–49. The large number of bat species within the geographical range of ebolavirus circulation complicates the search for the natural reservoir host of these viruses. In an effort to guide field sampling efforts, Peterson et al. used a series of biological principles to develop a priority list of mammalian clades that coincided with past filovirus disease outbreaks50 and Han et al. used a machine learning algorithm to identify potential filovirus-positive bat species based on intrinsic trait similarity with known filovirus RNA-, isolation- and antibody- positive bat species51.
For more information on filoviruses and bats, we would like to direct readers to recent overviews published by Olival and Hayman52, Wood et al.53, Leendertz et al.54 and Amman et al55.
Acknowledgement
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
[1] Smith, M.W. (1982) Field aspects of the Marburg virus outbreak: 1967. Primate Supply 7, 11–15.[2] Smith, D.H. et al. (1982) Marburg-virus disease in Kenya. Lancet 319, 816–820.
| Marburg-virus disease in Kenya.Crossref | GoogleScholarGoogle Scholar |
[3] Report of a WHO/International Study Team (1978) Ebola haemorrhagic fever in Sudan, 1976. Bull. World Health Organ. 56, 247–270.
[4] Report of an International Commission (1978) Ebola haemorrhagic fever in Zaire, 1976. Bull. World Health Organ. 56, 271–293.
[5] Miranda, M.E. et al. (1999) Epidemiology of Ebola (subtype Reston) virus in the Philippines, 1996. J. Infect. Dis. 179, S115–S119.
| Epidemiology of Ebola (subtype Reston) virus in the Philippines, 1996.Crossref | GoogleScholarGoogle Scholar |
[6] Formenty, P. et al. (1999) Human infection due to Ebola virus, subtype Cote d’Ivoire: clinical and biologic presentation. J. Infect. Dis. 179, S48–S53.
| Human infection due to Ebola virus, subtype Cote d’Ivoire: clinical and biologic presentation.Crossref | GoogleScholarGoogle Scholar |
[7] Towner, J.S. et al. (2008) Newly discovered Ebola virus associated with hemorrhagic fever outbreak in Uganda. PLoS Pathog. 4, e1000212.
| Newly discovered Ebola virus associated with hemorrhagic fever outbreak in Uganda.Crossref | GoogleScholarGoogle Scholar |
[8] Negredo, A. et al. (2011) Discovery of an ebolavirus-like filovirus in Europe. PLoS Pathog. 7, e1002304.
| Discovery of an ebolavirus-like filovirus in Europe.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsVGlu7fO&md5=b0ec413c00e4f27f41699c3c7b1a8150CAS |
[9] He, B. et al. (2015) Filovirus RNA in fruit bats, China. Emerg. Infect. Dis. 21, 1675–1677.
| Filovirus RNA in fruit bats, China.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XitVGltLvK&md5=98f15aa57eda7b86d0ef09059586d676CAS |
[10] Peterson, A.T. et al. (2004) Ecologic and geographic distribution of filovirus disease. Emerg. Infect. Dis. 10, 40–47.
| Ecologic and geographic distribution of filovirus disease.Crossref | GoogleScholarGoogle Scholar |
[11] Peterson, A.T. et al. (2006) Geographic potential for outbreaks of Marburg hemorrhagic fever. Am. J. Trop. Med. Hyg. 75, 9–15.
[12] Pigott, D.M. et al. (2014) Mapping the zoonotic niche of Ebola virus disease in Africa. eLife 3, e04395.
| Mapping the zoonotic niche of Ebola virus disease in Africa.Crossref | GoogleScholarGoogle Scholar |
[13] Pigott, D.M. et al. (2015) Mapping the zoonotic niche of Marburg virus disease in Africa. Trans. R. Soc. Trop. Med. Hyg. 109, 366–378.
| Mapping the zoonotic niche of Marburg virus disease in Africa.Crossref | GoogleScholarGoogle Scholar |
[14] Le Guenno, B. et al. (1995) Isolation and partial characterisation of a new strain of Ebola virus. Lancet 345, 1271–1274.
| Isolation and partial characterisation of a new strain of Ebola virus.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2M3msVyiug%3D%3D&md5=5fa0f06761c198a041e67868152ff183CAS |
[15] Leroy, E.M. et al. (2004) Multiple Ebola virus transmission events and rapid decline of central African wildlife. Science 303, 387–390.
| Multiple Ebola virus transmission events and rapid decline of central African wildlife.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjtFGmug%3D%3D&md5=9d55f73736416758f239978919fa2aa7CAS |
[16] Georges, A.J. et al. (1999) Ebola hemorrhagic fever outbreaks in Gabon, 1994–1997: epidemiologic and health control issues. J. Infect. Dis. 179, S65–S75.
| Ebola hemorrhagic fever outbreaks in Gabon, 1994–1997: epidemiologic and health control issues.Crossref | GoogleScholarGoogle Scholar |
[17] Conrad, J.L. et al. (1978) Epidemiologic investigation of Marburg virus disease, Southern Africa, 1975. Am. J. Trop. Med. Hyg. 27, 1210–1215.
| 1:STN:280:DyaE1M%2FotVGisA%3D%3D&md5=9caa849108825c028b8aa3b935a4c42cCAS |
[18] Arata, A.A. et al. (1978) Approaches towards studies on potential reservoirs of viral haemorrhagic fever in southern Sudan (1977). In Ebola virus haemorrhagic fever (Pattyn, S.R., ed), pp. 191–202, Elsevier/Netherland Biomedical.
[19] Baron, R.C. et al. (1983) Ebola virus disease in southern Sudan: hospital dissemination and intrafamilial spread. Bull. World Health Organ. 61, 997–1003.
| 1:STN:280:DyaL2c7otV2jug%3D%3D&md5=ffa686acfc57739ee809a5154cbe9f0bCAS |
[20] Johnson, E.D. et al. (1996) Characterization of a new Marburg virus isolated from a 1987 fatal case in Kenya. Arch. Virol. Suppl. 11, 101–114.
| 1:STN:280:DyaK28znvFGnsw%3D%3D&md5=a54b046cf969daa1cec5c236c5126563CAS |
[21] Swanepoel, R. et al. (1996) Experimental inoculation of plants and animals with Ebola virus. Emerg. Infect. Dis. 2, 321–325.
| Experimental inoculation of plants and animals with Ebola virus.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2s7isF2gsw%3D%3D&md5=f085ad68a75176ea2ff2278ba6cde5a2CAS |
[22] Bausch, D.G. et al. (2006) Marburg hemorrhagic fever associated with multiple genetic lineages of virus. N. Engl. J. Med. 355, 909–919.
| Marburg hemorrhagic fever associated with multiple genetic lineages of virus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XovFCgtL0%3D&md5=0cb515b485fc6fcb99041de6d722806eCAS |
[23] Leroy, E.M. et al. (2009) Human Ebola outbreak resulting from direct exposure to fruit bats in Luebo, Democratic Republic of Congo, 2007. Vector Borne Zoonotic Dis. 9, 723–728.
| Human Ebola outbreak resulting from direct exposure to fruit bats in Luebo, Democratic Republic of Congo, 2007.Crossref | GoogleScholarGoogle Scholar |
[24] Towner, J.S. et al. (2009) Isolation of genetically diverse Marburg viruses from Egyptian fruit bats. PLoS Pathog. 5, e1000536.
| Isolation of genetically diverse Marburg viruses from Egyptian fruit bats.Crossref | GoogleScholarGoogle Scholar |
[25] Timen, A. et al. (2009) Response to imported case of Marburg hemorrhagic fever, the Netherlands. Emerg. Infect. Dis. 15, 1171–1175.
| Response to imported case of Marburg hemorrhagic fever, the Netherlands.Crossref | GoogleScholarGoogle Scholar |
[26] Centers for Disease Control and Prevention (2009) Imported case of Marburg hemorrhagic fever – Colorado, 2008. Morb. Mortal. Weekly Rep. 58, 1377–1381.
[27] Amman, B.R. et al. (2012) Seasonal pulses of Marburg virus circulation in juvenile Rousettus aegyptiacus bats coincide with periods of increased risk of human infection. PLoS Pathog. 8, e1002877.
| Seasonal pulses of Marburg virus circulation in juvenile Rousettus aegyptiacus bats coincide with periods of increased risk of human infection.Crossref | GoogleScholarGoogle Scholar |
[28] Paweska, J.T. et al. (2012) Virological and serological findings in Rousettus aegyptiacus experimentally inoculated with Vero cells-adapted Hogan strain of Marburg virus. PLoS One 7, e45479.
| Virological and serological findings in Rousettus aegyptiacus experimentally inoculated with Vero cells-adapted Hogan strain of Marburg virus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsVWjsLzM&md5=280f96d4e7b6a7be3ee172accf2d0cb9CAS |
[29] Amman, B.R. et al. (2015) Oral shedding of Marburg virus in experimentally infected Egyptian fruit bats (Rousettus aegyptiacus). J. Wildl. Dis. 51, 113–124.
| Oral shedding of Marburg virus in experimentally infected Egyptian fruit bats (Rousettus aegyptiacus).Crossref | GoogleScholarGoogle Scholar |
[30] Paweska, J.T. et al. (2015) Lack of Marburg virus transmission from experimentally infected to susceptible in-contact Egyptian fruit bats. J. Infect. Dis. 212, S109–S118.
| Lack of Marburg virus transmission from experimentally infected to susceptible in-contact Egyptian fruit bats.Crossref | GoogleScholarGoogle Scholar |
[31] Schuh, A.J. et al. (2016) No evidence for the involvement of the argasid tick Ornithodoros faini in the enzootic maintenance of marburgvirus within Egyptian rousette bats Rousettus aegyptiacus. Parasit. Vectors 9, 128.
| No evidence for the involvement of the argasid tick Ornithodoros faini in the enzootic maintenance of marburgvirus within Egyptian rousette bats Rousettus aegyptiacus.Crossref | GoogleScholarGoogle Scholar |
[32] Leroy, E.M. et al. (2005) Fruit bats as reservoirs of Ebola virus. Nature 438, 575–576.
| Fruit bats as reservoirs of Ebola virus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXht1GqsrfL&md5=5cc4e0b830510fc55cb7b1e55b64dd21CAS |
[33] Pourrut, X. et al. (2007) Spatial and temporal patterns of Zaire ebolavirus antibody prevalence in the possible reservoir bat species. J. Infect. Dis. 196, S176–S183.
| Spatial and temporal patterns of Zaire ebolavirus antibody prevalence in the possible reservoir bat species.Crossref | GoogleScholarGoogle Scholar |
[34] Pourrut, X. et al. (2009) Large serological survey showing cocirculation of Ebola and Marburg viruses in Gabonese bat populations, and a high seroprevalence of both viruses in Rousettus aegyptiacus. BMC Infect. Dis. 9, 159.
| Large serological survey showing cocirculation of Ebola and Marburg viruses in Gabonese bat populations, and a high seroprevalence of both viruses in Rousettus aegyptiacus.Crossref | GoogleScholarGoogle Scholar |
[35] Hayman, D.T. et al. (2010) Long-term survival of an urban fruit bat seropositive for Ebola and Lagos bat viruses. PLoS One 5, e11978.
| Long-term survival of an urban fruit bat seropositive for Ebola and Lagos bat viruses.Crossref | GoogleScholarGoogle Scholar |
[36] Hayman, D.T. et al. (2012) Ebola virus antibodies in fruit bats, Ghana, West Africa. Emerg. Infect. Dis. 18, 1207–1209.
| Ebola virus antibodies in fruit bats, Ghana, West Africa.Crossref | GoogleScholarGoogle Scholar |
[37] Yuan, J. et al. (2012) Serological evidence of ebolavirus infection in bats, China. Virol. J. 9, 236.
| Serological evidence of ebolavirus infection in bats, China.Crossref | GoogleScholarGoogle Scholar |
[38] Olival, K.J. et al. (2013) Ebola virus antibodies in fruit bats, Bangladesh. Emerg. Infect. Dis. 19, 270–273.
| Ebola virus antibodies in fruit bats, Bangladesh.Crossref | GoogleScholarGoogle Scholar |
[39] Jayme, S.I. et al. (2015) Molecular evidence of Ebola Reston virus infection in Philippine bats. Virol. J. 12, 107.
| Molecular evidence of Ebola Reston virus infection in Philippine bats.Crossref | GoogleScholarGoogle Scholar |
[40] Ogawa, H. et al. (2015) Seroepidemiological prevalence of multiple species of filoviruses in fruit bats (Eidolon helvum) migrating in Africa. J. Infect. Dis. 212, S101–S108.
| Seroepidemiological prevalence of multiple species of filoviruses in fruit bats (Eidolon helvum) migrating in Africa.Crossref | GoogleScholarGoogle Scholar |
[41] Swanepoel, R. et al. (2007) Studies of reservoir hosts for Marburg virus. Emerg. Infect. Dis. 13, 1847–1851.
| Studies of reservoir hosts for Marburg virus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXjs1Wrsw%3D%3D&md5=20f11db9c61564796f807dc2f0303b53CAS |
[42] Jones, M.E. et al. (2015) Experimental inoculation of Egyptian rousette bats (Rousettus aegyptiacus) with viruses of the Ebolavirus and Marburgvirus genera. Viruses 7, 3420–3442.
| Experimental inoculation of Egyptian rousette bats (Rousettus aegyptiacus) with viruses of the Ebolavirus and Marburgvirus genera.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhslOqt7vO&md5=e4ecb174284ff96183a0a68a21ff1cc8CAS |
[43] Paweska, J.T. et al. (2016) Experimental inoculation of Egyptian fruit bats (Rousettus aegyptiacus) with Ebola virus. Viruses 8, 29.
| Experimental inoculation of Egyptian fruit bats (Rousettus aegyptiacus) with Ebola virus.Crossref | GoogleScholarGoogle Scholar |
[44] Hayman, D.T. (2015) Biannual birth pulses allow filoviruses to persist in bat populations. Proc. Biol. Sci. 282, 20142591.
| Biannual birth pulses allow filoviruses to persist in bat populations.Crossref | GoogleScholarGoogle Scholar |
[45] Constantine, D.G. et al. (1964) Latent infection of Rio Bravo virus in salivary glands of bats. Public Health Rep. 79, 1033–1039.
| Latent infection of Rio Bravo virus in salivary glands of bats.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaF2M%2FjvVOmug%3D%3D&md5=5beece3437fd3d3e013c6b30382e9296CAS |
[46] Baer, G.M. et al. (1966) Bat salivary gland virus carrier state in a naturally infected Mexican freetail bat. Am. J. Trop. Med. Hyg. 15, 769–771.
| 1:STN:280:DyaF2s%2FhsVaktQ%3D%3D&md5=f88d44af9b6028d3c1dac1328dcd0ddaCAS |
[47] Lumsden, W.H. et al. (1961) A virus from insectivorous bats in Uganda. Ann. Trop. Med. Parasitol. 55, 389–397.
| A virus from insectivorous bats in Uganda.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaF38%2FmvVOqsA%3D%3D&md5=f1da0a7502b8de86dce2fd66a957ccefCAS |
[48] Bell, J.F. et al. (1964) A new virus, ‘MML’, enzootic in bats (Myotis lucifugus) of Montana. Am. J. Trop. Med. Hyg. 13, 607–612.
| 1:STN:280:DyaF2M%2FgtlSnsQ%3D%3D&md5=3a6b14410db36dd07eb20ca1f9a0a9d7CAS |
[49] Sulkin, S.E. et al. (1959) Studies on the pathogenesis of rabies in insectivorous bats. I. Role of brown adipose tissue. J. Exp. Med. 110, 369–388.
| Studies on the pathogenesis of rabies in insectivorous bats. I. Role of brown adipose tissue.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaF3c3gs1elsA%3D%3D&md5=3c0c5af751e32823fcd9c8f6f6db5068CAS |
[50] Peterson, A.T. et al. (2004) Potential mammalian filovirus reservoirs. Emerg. Infect. Dis. 10, 2073–2081.
| Potential mammalian filovirus reservoirs.Crossref | GoogleScholarGoogle Scholar |
[51] Han, B.A. et al. (2016) Undiscovered bat hosts of filoviruses. PLoS Negl. Trop. Dis. 10, e0004815.
| Undiscovered bat hosts of filoviruses.Crossref | GoogleScholarGoogle Scholar |
[52] Olival, K.J. and Hayman, D.T.S. (2014) Filoviruses in bats: current knowledge and future directions. Viruses 6, 1759–1788.
| Filoviruses in bats: current knowledge and future directions.Crossref | GoogleScholarGoogle Scholar |
[53] Wood, J.L. et al. (2016) Ebola, bats and evidence-based policy: informing Ebola policy. EcoHealth 13, 9–11.
| Ebola, bats and evidence-based policy: informing Ebola policy.Crossref | GoogleScholarGoogle Scholar |
[54] Leendertz, S.A. et al. (2016) Assessing the evidence supporting fruit bats as the primary reservoirs for Ebola viruses. EcoHealth 13, 18–25.
| Assessing the evidence supporting fruit bats as the primary reservoirs for Ebola viruses.Crossref | GoogleScholarGoogle Scholar |
[55] Amman, B.R. et al. (In press) Ecology of filoviruses. In Marburg and Ebola viruses: from ecosystems to molecules (Muehlberger, E. et al., eds). Current Topics in Microbiology and Immunology, Springer.
Biographies
Amy Schuh, PhD is a microbiologist, Brian Amman, PhD is an ecologist and Jonathan Towner, PhD is the Team Lead of the Virus-Host Ecology Section at the Viral Special Pathogens Branch at the United States Centers for Disease Control and Prevention. They conduct ecological investigations aimed at identifying the reservoir hosts of the filoviruses and use captive-born R. aegyptiacus bats to study the mechanisms of filovirus maintenance and virus spillover to humans.