Production of statins by fungal fermentation
Mishal Subhan A B , Rani Faryal B and Ian Macreadie B CA Department of Microbiology, Faculty of Biological Sciences, Quaid-I-Azam University, Islamabad 45320, Pakistan
B School of Science, RMIT University, Bundoora, Vic. 3083, Australia
C Tel: +61 9925 6627, Email: ian.macreadie@rmit.edu.au
Microbiology Australia 38(2) 70-72 https://doi.org/10.1071/MA17031
Published: 30 March 2017
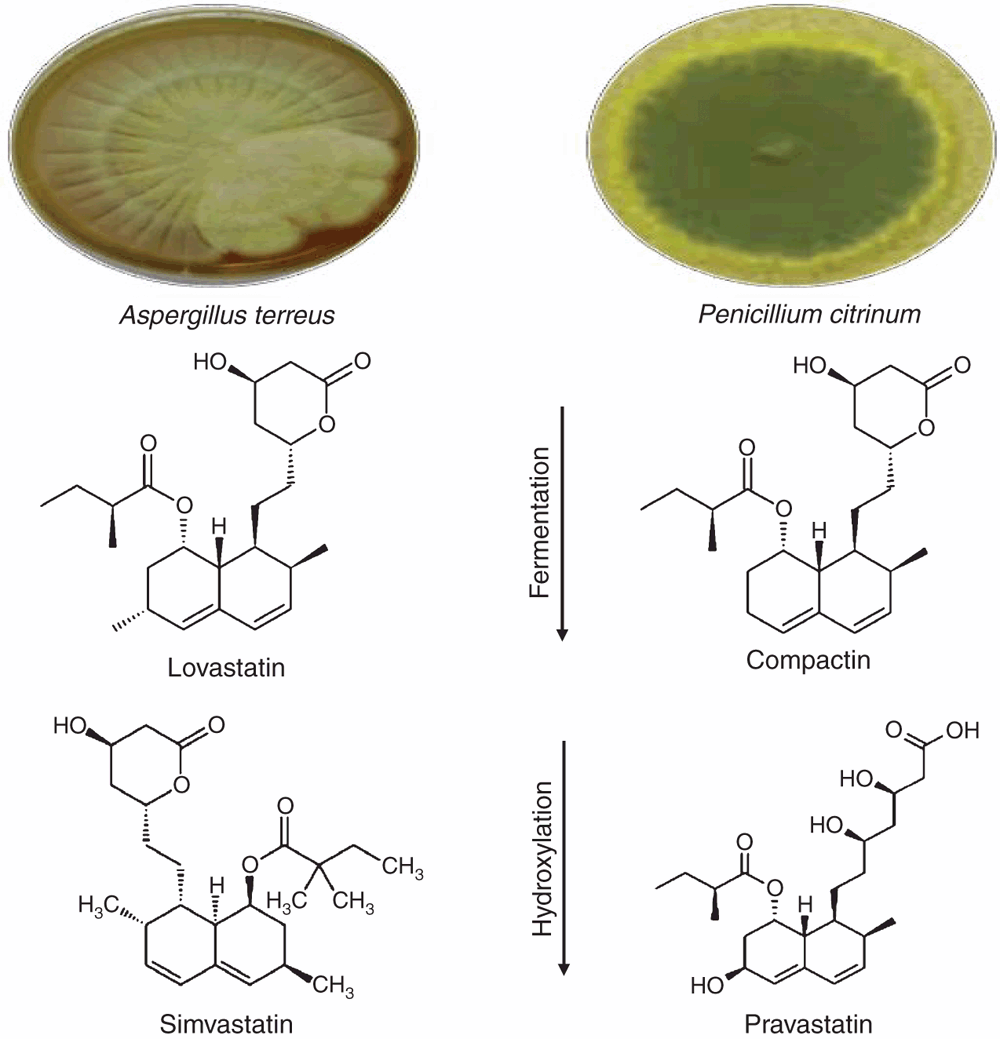
Fungi are used industrially to obtain a variety of products, from low value bulk chemicals to high value drugs like, immunosuppressants, antibiotics, alkaloids and statins. Lovastatin and compactin are natural statins produced as secondary metabolites by predominantly Aspergillus and Penicillium species, following a polyketide pathway. Lovastatin was one of the first cholesterol-lowering drugs. Many statins are now chemically synthesised but lovastatin is still required to produce simvastatin. Apart from reducing blood cholesterol levels simvastatin causes pleotropic effects and has potential to treat various kinds of disorders including neurodegenerative disease and cancer.
Statins are drugs prescribed to reduce serum cholesterol levels. The first statin to be approved by the FDA, lovastatin, is produced as a result of fermentation of Aspergillus terreus1. Statins act by inhibiting HMG-CoA reductase (HMGCR) through competitive inhibition. This blocks the activity of HMGCR, the rate limiting enzyme in the synthesis of cholesterol2. Natural statins, including lovastatin and mevastatin (commonly known as compactin) are produced by direct fungal fermentation. Semi-synthetic statins, simvastatin and pravastatin (Figure 1), are synthesised by the stereoselective hydroxylation of natural statins. Chemically synthesised statins include atorvastatin, rosuvastatin, fluvastatin and pitavastatin3.
Discovery of statins
Compactin, discovered by Akira Endo in 1973 as a structural analogue for the HMG-CoA substrate, was produced by Penicillium citrinum. Lovastatin, previously known as mevinolin K and mevinolin, was produced from cultures of Monascus ruber and Aspergillus terreus respectively. It was the first statin to be approved by the FDA in 19874. Pravastatin, the semi-synthetic derivative of compactin was commercialised in 1989. Simvastatin remains a commonly prescribed statin1,5.
Fungal fermentation and statin production
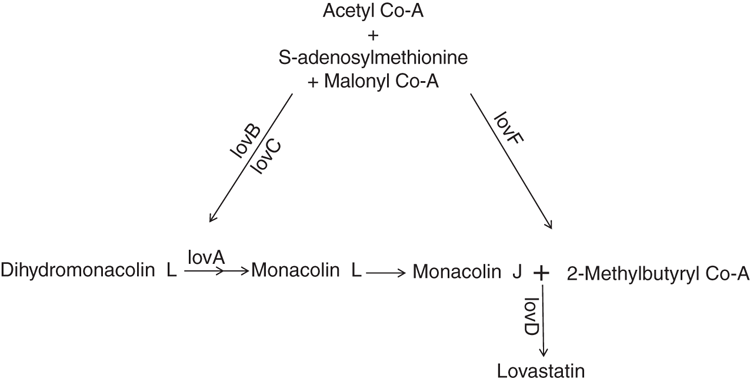
Statins are produced as a secondary metabolite from a polyketide pathway. This pathway is regulated by polyketide synthase genes such as Lov B, lovF and LovD, that are responsible for the transcription regulation and production of these secondary metabolites6,7. Statins are produced as a secondary metabolite during stress of the fungi. Acetyl Co-A acts as precursor molecule that plays an important role in bridging the primary metabolism with the secondary metabolism leading to production of various secondary metabolites such as terpenes and polyketides including statins8,9. All fungi producing lovastatin or compactin utilise the pathway shown in Figure 210.
Lovastatin is commercially produced by fermentation of A. terreus and simvastatin is produced by further chemical treatment of lovastatin usually involving direct alkylation11,12. Compactin is not as effective in inhibiting HMGCR as lovastatin, however, a semi-synthetic derivative of compactin, pravastatin, is highly effective in lowering blood cholesterol levels. Different strategies have been adopted for the efficient and economic scale up of these metabolites, such as media optimisation, using cheap raw substrates, mutagenesis and bioreactor optimisation3.
Unlike simvastatin where conversion of lovastatin to simvastatin is a chemical reaction, the reaction for pravastatin synthesis is biotransformation4. Lovastatin can be directly methylated or deacylated for the synthesis of simvastatin13. This involves a single step fermentation process and then direct chemical conversion. Pravastatin production involves the hydroxylation of compactin produced by P. citrinum by the biotransformation using the bacterium Streptomyces carbophilus. This organism produces cytochrome P450 enzyme that is responsible for the hydroxylation of compactin14,15. This dual step fermentation is economically not feasible. Recent studies have reported the production of pravastatin in a single fermentative step. Enzymes responsible for the hydroxylation are genetically incorporated in the penicillin-producing fungus Penicillium chrysogenum. This results in efficient production of pravastatin at industrial scale16.
Different fermentation techniques including solid state fermentation (SSF) and submerged fermentation (SmF) can be used for statin production. Large scale commercial production utilises submerged batch fermentation. There is a controlled aeration and agitation in a bioreactor during SmF, which increases the oxygen mass transfer and constant distribution of nutrients to fungal mycelia, resulting in increased production of statins12,17. Some studies have also reported fed-batch fermentation that were carried out in a bioreactor with a capacity of 1000 L. Repeated fed batch processes can also improve the productivity of desired metabolites18.
In our studies a new species of A. terreus, MS-7, was isolated from agricultural soils. Its identity was confirmed by ITS sequence analysis and it was found to be the potent producer of lovastatin as determined by analytical HPLC. Fermentation on modified soybean meal media resulted in more lovastatin produced using SSF (13.9 mg/g) in comparison to SmF (10.3 mg/g). As lovastatin produced by the fungus is inhibitory, production by SSF might result in minimal contact of mycelia with the lovastatin underneath, enhancing the productivity. Another factor that can result in greater production is that SSF promotes more mycelial growth as compared to the SmF.
Application of statins
The mevalonate pathway is not only responsible for the synthesis of cholesterol but also for the synthesis of other non-sterol isoprenoids that are involved in protein prenylation such as binding and regulation of target proteins. Statins may decrease protein prenylation, a key step during a cell growth and signalling pathway. Statins can be used in combination with cancer drugs to treat cancer19. Statins also reduce hepatic cholesterols leading to reduced gallstone formation and reduced platelet aggregation20,21. Recent studies have reported the role of statins in cognitive impairment after sepsis by reversing the microvascular dysfunction and reducing neuroinflammation22. Simvastatin has been found to reduce the incidence of neurodegenerative disorders such as Alzheimer’s disease and Parkinson’s disease23.
Acknowledgements
We thank the HEC commission of Pakistan for their funding and support.
References
[1] Endo, A. (2004) The origin of the statins. Int. Congr. Ser. 1262, 3–8.| The origin of the statins.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtFOqtr7L&md5=bab2903c25939f1c0e1ac29e8311ee2fCAS |
[2] Istvan, E. (2003) Statin inhibition of HMG-CoA reductase: a 3-dimensional view. Atheroscler. Suppl. 4, 3–8.
| Statin inhibition of HMG-CoA reductase: a 3-dimensional view.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXjtFWiu7Y%3D&md5=3403421c51ef6162a13e34ed03461cc0CAS |
[3] Subhan, M. et al. (2016) Exploitation of Aspergillus terreus for the production of natural statins. J Fungi 2, 1–13.
| Exploitation of Aspergillus terreus for the production of natural statins.Crossref | GoogleScholarGoogle Scholar |
[4] Tobert, J.A. (2003) Lovastatin and beyond: the history of the HMG-CoA reductase inhibitors. Nat. Rev. Drug Discov. 2, 517–526.
| Lovastatin and beyond: the history of the HMG-CoA reductase inhibitors.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXks1Oru7k%3D&md5=86348d92ea5655dc56943a6d73d6de9dCAS |
[5] Lorenz, R.T. and Parks, L. (1990) Effects of lovastatin (mevinolin) on sterol levels and on activity of azoles in Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 34, 1660–1665.
| Effects of lovastatin (mevinolin) on sterol levels and on activity of azoles in Saccharomyces cerevisiae.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXls1yrt7g%3D&md5=ac0fd2a932f8404184d613b62109b230CAS |
[6] Cacho, R.A. et al. (2015) Understanding programming of fungal iterative polyketide synthases: the biochemical basis for regioselectivity by the methyltransferase domain in the lovastatin megasynthase. ý J. Am. Chem. Soc. 137, 15688–15691.
| Understanding programming of fungal iterative polyketide synthases: the biochemical basis for regioselectivity by the methyltransferase domain in the lovastatin megasynthase. ýCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhvFGnsL%2FL&md5=c764d6741c6d70f12babe3163bba1421CAS |
[7] Xu, W. et al. (2013) LovG: The thioesterase required for dihydromonacolin L release and lovastatin nonaketide synthase turnover in lovastatin biosynthesis. Angew. Chem. Int. Ed. 52, 6472–6475.
| LovG: The thioesterase required for dihydromonacolin L release and lovastatin nonaketide synthase turnover in lovastatin biosynthesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXnt1ShsL0%3D&md5=59495dabbbf606e31b949e8813b93729CAS |
[8] Shi, L. and Tu, B.P. (2015) Acetyl-CoA and the regulation of metabolism: mechanisms and consequences. Curr. Opin. Cell Biol. 33, 125–131.
| Acetyl-CoA and the regulation of metabolism: mechanisms and consequences.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXis1aqsrc%3D&md5=7fc6d5e16401589db2a92cf643cb8080CAS |
[9] Chiang, Y.M. et al. (2010) Unraveling polyketide synthesis in members of the genus Aspergillus. Appl. Microbiol. Biotechnol. 86, 1719–1736.
| Unraveling polyketide synthesis in members of the genus Aspergillus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXltVylt7k%3D&md5=9729efde67ec6c0397e59a5cebcc6af1CAS |
[10] Campbell, C.D. and Vederas, J.C. (2010) Biosynthesis of lovastatin and related metabolites formed by fungal iterative PKS enzymes. Biopolymers 93, 755–763.
| Biosynthesis of lovastatin and related metabolites formed by fungal iterative PKS enzymes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXotVajtLc%3D&md5=706103e761a25bcecbaf02b5223c31e0CAS |
[11] Barrios-González, J. and Miranda, R.U. (2010) Biotechnological production and applications of statins. Appl. Microbiol. Biotechnol. 85, 869–883.
| Biotechnological production and applications of statins.Crossref | GoogleScholarGoogle Scholar |
[12] Seenivasan, A. et al. (2008) Microbial production and biomedical applications of lovastatin. Indian J. Pharm. Sci. 70, 701–709.
| Microbial production and biomedical applications of lovastatin.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3M3jsVKltQ%3D%3D&md5=f6617d9105b2ccf2ff9c487569701334CAS |
[13] Thaper, R.K. et al. (1999) A cost-efficient synthesis of simvastatin via high-conversion methylation of an alkoxide ester enolate. Org. Process Res. Dev. 3, 476–479.
| A cost-efficient synthesis of simvastatin via high-conversion methylation of an alkoxide ester enolate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXls1aitrw%3D&md5=5cbab4555bd155e1f0b8f63ed1594a00CAS |
[14] Matsuoka, T. et al. (1989) Purification and characterization of cytochrome P-450sca from Streptomyces carbophilus. Eur. J. Biochem. 184, 707–713.
| Purification and characterization of cytochrome P-450sca from Streptomyces carbophilus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXltFSjtQ%3D%3D&md5=aed48001aa833eafd0f7132e7eb3f02eCAS |
[15] Park, J.W. et al. (2003) Bioconversion of compactin into pravastatin by Streptomyces sp. Biotechnol. Lett. 25, 1827–1831.
| Bioconversion of compactin into pravastatin by Streptomyces sp.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXotlajsL8%3D&md5=d181ec3209e91e60ea88189820f58631CAS |
[16] McLean, K.J. et al. (2015) Single-step fermentative production of the cholesterol-lowering drug pravastatin via reprogramming of Penicillium chrysogenum. Proc. Natl. Acad. Sci. USA 112, 2847–2852.
| Single-step fermentative production of the cholesterol-lowering drug pravastatin via reprogramming of Penicillium chrysogenum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXivFWqu78%3D&md5=539f8091e3ab286ba204f61f82bb4b23CAS |
[17] Singh, S.K. and Pandey, A. (2013) Emerging approaches in fermentative production of statins. Appl. Biochem. Biotechnol. 171, 927–938.
| Emerging approaches in fermentative production of statins.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXht1WhsbzJ&md5=b355e6ef3fd6c9d0773bbc348e1a1d06CAS |
[18] Kumar, M.S. et al. (2000) Repeated fed-batch process for improving lovastatin production. Process Biochem. 36, 363–368.
| Repeated fed-batch process for improving lovastatin production.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXnvF2nsLs%3D&md5=e8e122403ade7819fdd7c295caf349c1CAS |
[19] García-Ruiz, C. et al. (2012) Statins and protein prenylation in cancer cell biology and therapy. Anticancer. Agents Med. Chem. 12, 303–315.
| Statins and protein prenylation in cancer cell biology and therapy.Crossref | GoogleScholarGoogle Scholar |
[20] Oesterle, A. et al. (2017) Pleiotropic effects of statins on the cardiovascular system. Circ. Res. 120, 229–243.
| Pleiotropic effects of statins on the cardiovascular system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2sXjsFyltQ%3D%3D&md5=32ce28242278582b080d5adb345cf702CAS |
[21] Erichsen, R. et al. (2011) Long-term statin use and the risk of gallstone disease: a population-based case-control study. Am. J. Epidemiol. 173, 162–170.
| Long-term statin use and the risk of gallstone disease: a population-based case-control study.Crossref | GoogleScholarGoogle Scholar |
[22] Reis, P.A. et al. (2017) Statins prevent cognitive impairment after sepsis by reverting neuroinflammation, and microcirculatory/endothelial dysfunction. Brain Behav. Immun. 60, 293–303.
| Statins prevent cognitive impairment after sepsis by reverting neuroinflammation, and microcirculatory/endothelial dysfunction.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XhvVGqtbvJ&md5=93f5cc51282044f597291405d8b7b4cdCAS |
[23] Wolozin, B. et al. (2007) Simvastatin is associated with a reduced incidence of dementia and Parkinson’s disease. BMC Med. 5, 20.
| Simvastatin is associated with a reduced incidence of dementia and Parkinson’s disease.Crossref | GoogleScholarGoogle Scholar |
Biographies
Mishal Subhan is student of PhD from Quaid-I-Azam University Pakistan, working in collaboration with Ian Macreadie at RMIT University Australia. Her research interests include fermentation technology, bioprocessing and yeast biotechnology, related to production of statins and their therapeutic applications.
Rani Faryal is Associate Professor at Quaid-I-Azam University Pakistan. Her research interests include molecular genetics and immunology, specifically signalling pathways and lymphoma.
Ian Macreadie is a Professor at RMIT University Australia. His research interests include fermentation, molecular biology, yeast biotechnology and development of yeast bioassays for preventatives of neurodegenerative diseases and aging.