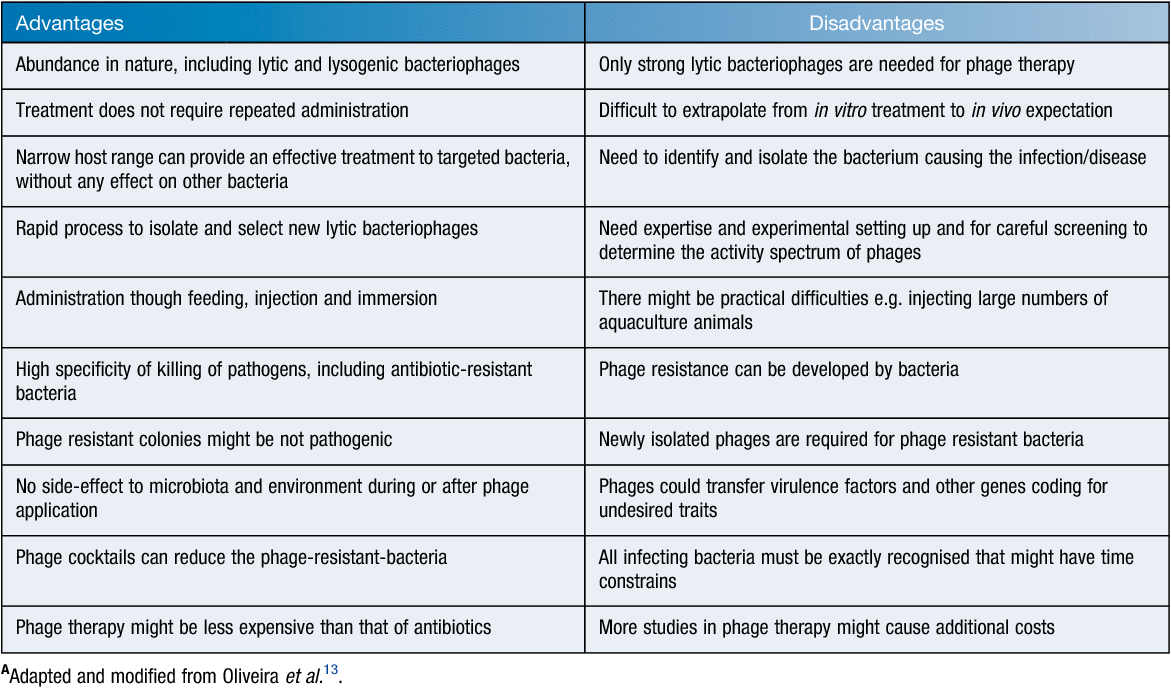
Bacteriophages as biocontrol agents in aquaculture
Son Tuan Le A B and İpek Kurtböke A CA University of the Sunshine Coast, School of Science and Engineering and the GeneCology Research Centre, Maroochydore DC, Qld 4558, Australia
B Research Institute for Marine Fisheries, 224 Le Lai, Ngo Quyen, Hai Phong 180000, Vietnam. Email: Tuan.Son.Le@research.usc.edu.au
C Email: IKurtbok@usc.edu.au
Microbiology Australia 40(1) 37-41 https://doi.org/10.1071/MA19003
Published: 27 February 2019
Aquaculture production (inland and marine) has been increasing globally reaching 80.1 million metric tons in 2016. Simultaneously the utilisation of fish food per capita has also been risen reaching 20.0 kg per year in 2016. However, the growing industry also experiences problems including diseases caused by viruses, bacteria, fungi, protozoans, helminths and parasitic crustaceans on valuable seafood products resulting in economic losses. Antimicrobial agents and chemical control strategies used to control such diseases are creating environmentally detrimental effects as well as encouraging development and dissemination of antibiotic resistant bacteria. Vaccine developments are costly and lengthy with application difficulties in farm settings. Accordingly, alternative therapies for controlling bacterial pathogens in aquaculture are gaining importance. One such measure is to use bacteriophages that are specific to disease causing bacteria.
Vibrio species are the main pathogens responsible for disease outbreaks which can result in 98.5–100% of mortality of the host animal within 72–96 h causing huge economic losses to hatcheries1. Examples include V. tubiashii infections that caused mortalities of oyster larvae in North America from 2006 to 2008 resulting in a decline of 59% in production at that time2. Moreover, antibiotic resistant species of Vibrio have also been on the increase. Mass mortality in the larvae of black tiger shrimp (Penaeus monodon) was reported to be caused by multi-drug resistant V. harveyi strains with resistance to cotrimoxazole, chloramphenicol, erythromycin and streptomycin3. Resistance of 15 V. alginolyticus isolates from oysters farmed in Korea against 16 different antibiotics including ampicillin, vancomycin and erythromycin has also been reported4. Vibrio spp. isolated from fish pond facilities in Nigeria were also reported to be resistant to tetracyclineoxazole (100%), oxytetracycline (99.4%) and chloramphenicol (73.1%)5.
Aeromonas has been another pathogenic genus causing significant economic losses for aquaculture operations. Antibiotics again are extensively used to control diseases caused by the pathogenic species of this genus: examples include amoxicillin, ampicillin, cephamycin, cotrim and kanamycin6,7. However, according to the results from an antimicrobial susceptibility survey taken between 2013 and 2014, the sensitivity of these pathogens against the above-listed antibiotics decreased over this time thus devaluing the efficiency of antibiotic treatment. Highly virulent and antibiotic resistant strains to co-trimoxazole, tetracycline, florfenicol, ampicillin, trimethoprim/sulfamethoxazole, nalidixic acid, chloramphenicol, and nitrofurantoin were also reported7–9. Strains with complete resistance to methicillin, rifampicin, bacitracin and novobiocin were also reported for the same pathogen isolated from fish and prawns in South India9.
Fish nocardiosis caused by Nocardia species in particular by N. seriolae is also on the increase in the South East Asia Pacific region. Erythromycin, oxolinic acid and fosfomycin resistant strains of the pathogen have also been reported10.
While control of bacterial diseases has been attempted via different strategies during farming, after harvest unhygienic practices also constitute serious public health risk issues. Cross-contamination with pathogenic bacteria (e.g. Escherichia coli, Campylobacter and Salmonella spp) is one of the main causes of food poisoning after harvest. These pathogens can easily be spread to ready-to-eat foods, such as raw oysters and salads, through handling and contaminated equipment or surfaces. In particular, during shucking of oysters, a significant risk of cross contamination can occur due to poor hygiene leading to gastrointestinal infections. The costs of foodborne diseases to the industry can be significantly high: e.g. US$10–83 billion in USA11 and >AU$1.2 billion annually in Australia12.
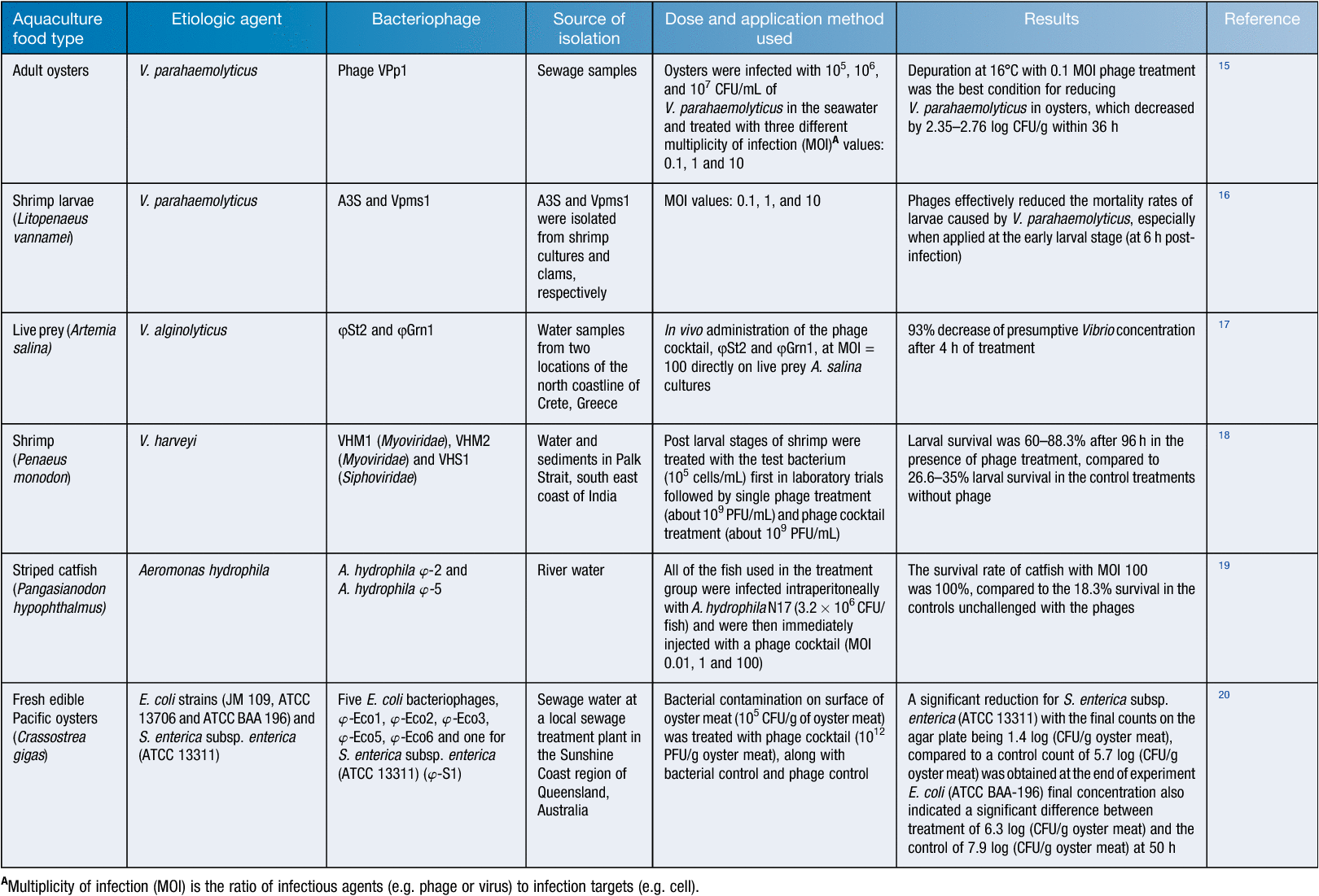
To reduce antibiotic use in the control of the above-mentioned pathogens in aquaculture farms alternative measures, in particular, those of biological origin, are being sought by the industry. One such measure is the use of bacteriophages that are specific to the disease-causing bacteria (Table 1). Phage therapy so far has displayed encouraging results in aquaculture settings via the use of diverse types of administrations: (1) direct application of phage suspensions in water; (2) oral administration of phages mixing with food; and (3) injections13,14 (Table 2).
|
|
At the University of the Sunshine Coast (USC) in Queensland, Australian research in this field has also been carried out over the past 10 years and specific examples are listed below:
-
Research study jointly conducted with the USC and the Research Institute for Marine Fisheries, Hai Phong and the Research Institute for Aquaculture No. 2, Ho Chi Minh, Vietnam, Le et al.19 was able to reduce the incidence of disease due to Aeromonas hydrophila that causes Motile Aeromonas Septicemia (MAS) in Striped Catfish (Pangasianodon hypophthalmus). It is one of the most important farmed fish species in the South East Asia Pacific region including Vietnam, Thailand, Cambodia, Laos and, more recently, the Philippines and Indonesia21. In 2015, Vietnam supplied 90% of catfish production with a value of US$1.1–1.7 billion; however, an increase in Motile Aeromonas Septicemia cases and the detection of antibiotic resistant species of the pathogen has been threatening the productivity of the industry. Thus, the development of world first bacteriophage treatment against Aeromonas hydrophila with successful field trials conducted in Vietnam19 now offers an alternative disease control strategy for the farmers.
-
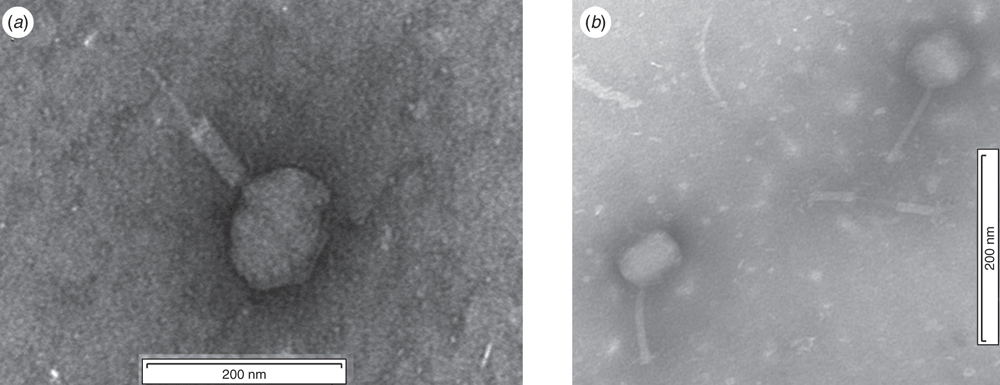
One of the main sources of Vibrio infections in aquaculture is the use of microalgae infested by the pathogen as feed in the aquaculture tanks. Bacteriophages were again successfully used to eliminate Vibrio infestations on microalgae used as a food source for oyster larvae in oyster hatcheries at the USC in a study jointly conducted with the Port Stephens Fisheries Institute in NSW, Australia. The morphology of one of these phages is illustrated in Figure 1a.
-
Two key vectors for potential Vibrio spp. contamination in the hatchery include broodstock and seawater22. Bacteriophages were again successfully used to treat Vibrio infections in Sydney rock oyster larvae (Saccostrea glomerata) and this improved oyster survival rate in the USC in a study again jointly conducted with the Port Stephens Fisheries Institute in NSW, Australia. The morphology of one of these phages is illustrated in Figure 1b.
-
Human pathogenic bacteria can contaminate sea-food because of unhygienic handling practices leading to foodborne diseases. This is a particular problem for oysters which are often eaten raw or only lightly cooked which might not remove human pathogens from the product23. Again, at the USC, Le et al.20 successfully isolated five different E. coli phages and a Salmonella phage and treatments of shucked oysters with these phages resulted in significant decrease in the numbers of E. coli and Salmonella. Reduction in the numbers of extended spectrum beta-lactamase resistant E. coli strain (ATCC BAA 196) was also achieved20.
-
Moreover, off-flavor compound producing bacteria present in the sediments of unlined aquaculture tanks can result in the diffusion of earthy-musty compounds into fish flesh lowering the sale value of the product. Recently, in a joint study between the USC and the SeaFood Team of the Department of Agriculture and Fisheries in QLD, Jonns et al.24 reported a decrease in odours caused by geosmin and 2-methyl-iso-borneol (2-imb) producing streptomycetes when they used streptophages in simulated aquaculture tank experiments in the laboratory. This method provides a safe alternative strategy to farmers whose business is detrimentally impacted by the odour producing bacteria e.g. the barramundi farmers.
|
Conclusions
The rising incidence of antibiotic resistance in bacteria and problems with antibiotic residues in aquatic environments and aquaculture products, highlight the need for, alternative therapies for control of pathogenic bacteria in aquaculture. Bacteriophage-mediated biocontrol can be one of these alternative methods15–26. The cases presented above demonstrate the potential of phage therapy in controlling diseases associated with aquaculture although further data is required for the acceptance and successful application of bacteriophages in aquaculture settings.
There are other factors to be considered before widespread application of bacteriophage therapy can occur such as existence of phage resistant bacteria. Examples include phage-resistant Streptococcus iniae causing beta-hemolytic streptococcicosis in Japanese flounder Paralichthys olivaceus27.
Bacteriophages can also mediate toxicity such as the one encountered when Penaeus monodon gets infected with V. harveyi28. Accidental introduction of lysogenic phages was pointed out as an inherent risk for shrimp farmers29. V. harveyi Siphophage 1 (VHS1) was found to lose its ability to lyse cells but retained its ability to lysogenise after boiling for 10 min. Accordingly, cooking of crustaceans may not be sufficient for full inactivation of phages that might be present in the seafood thus resulting in lysogenic conversions29.
In-depth understanding on the fascinating interactions between the host and bacteriophages will facilitate development of effective management systems including the use of several techniques in rotation including the bacteriophage therapy13.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
Tuan Son Le gratefully acknowledges a MOET-VIED/USC PhD scholarship. This research did not receive any specific funding.
References
[1] Prado, S. et al. (2005) Pathogenic bacteria isolated from disease outbreaks in shellfish hatcheries. First description of Vibrio neptunius as an oyster pathogen. Dis. Aquat. Organ. 67, 209–215.| Pathogenic bacteria isolated from disease outbreaks in shellfish hatcheries. First description of Vibrio neptunius as an oyster pathogen.Crossref | GoogleScholarGoogle Scholar | 16408836PubMed |
[2] Elston, R.A. et al. (2008) Re-emergence of Vibrio tubiashii in bivalve shellfish aquaculture: severity, environmental drivers, geographic extent and management. Dis. Aquat. Organ. 82, 119–134.
| Re-emergence of Vibrio tubiashii in bivalve shellfish aquaculture: severity, environmental drivers, geographic extent and management.Crossref | GoogleScholarGoogle Scholar | 19149375PubMed |
[3] Karunasagar, I. et al. (1994) Mass mortality of Penaeus monodon larvae due to antibiotic-resistant Vibrio harveyi infection. Aquaculture 128, 203–209.
| Mass mortality of Penaeus monodon larvae due to antibiotic-resistant Vibrio harveyi infection.Crossref | GoogleScholarGoogle Scholar |
[4] Kang, C.-H. et al. (2016) Antimicrobial susceptibility of Vibrio alginolyticus isolated from oyster in Korea. Environ. Sci. Pollut. R. 23, 21106–21112.
[5] Igbinosa, E.O. (2016) Detection and antimicrobial resistance of Vibrio isolates in aquaculture environments: Implications for public health. Microb. Drug Resist. 22, 238–245.
| Detection and antimicrobial resistance of Vibrio isolates in aquaculture environments: Implications for public health.Crossref | GoogleScholarGoogle Scholar | 26540391PubMed |
[6] Nguyen, H.N.K. et al. (2016) Antibiotic resistance associated with aquaculture in Vietnam. Microbiol. Aust. 37, 108–111.
| Antibiotic resistance associated with aquaculture in Vietnam.Crossref | GoogleScholarGoogle Scholar |
[7] Thi, Q.V.C. et al. (2014) The current status antimicrobial resistance in Edwardsiella ictaluri and Aeromonas hydrophila cause disease on the striped catfish farmed in the Mekong Delta. ạp chí Khoa học trường đại học Cần Thơ. Số chuyên đề: Thủy sản 2, 7–14.
[8] Nguyen, H.N.K. et al. (2014) Molecular characterization of antibiotic resistance in Pseudomonas and Aeromonas isolates from catfish of the Mekong Delta, Vietnam. Vet. Microbiol. 171, 397–405.
| Molecular characterization of antibiotic resistance in Pseudomonas and Aeromonas isolates from catfish of the Mekong Delta, Vietnam.Crossref | GoogleScholarGoogle Scholar |
[9] Vivekanandhan, G. et al. (2002) Antibiotic resistance of Aeromonas hydrophila isolated from marketed fish and prawn of south India. Int. J. Food Microbiol. 76, 165–168.
| Antibiotic resistance of Aeromonas hydrophila isolated from marketed fish and prawn of south India.Crossref | GoogleScholarGoogle Scholar | 12038573PubMed |
[10] Itano, T. and Kawakami, H. (2002) Drug susceptibility of recent isolates of Nocardia seriolae from cultured fish. Fish Pathol. 37, 152–153.
| Drug susceptibility of recent isolates of Nocardia seriolae from cultured fish.Crossref | GoogleScholarGoogle Scholar |
[11] Nyachuba, D.G. (2010) Foodborne illness: is it on the rise? Nutr. Rev. 68, 257–269.
| Foodborne illness: is it on the rise?Crossref | GoogleScholarGoogle Scholar | 20500787PubMed |
[12] Angulo, F.J. et al. (2008) Foodborne disease in Australia: the OzFoodNet experience. Clin. Infect. Dis. 47, 392–400.
| Foodborne disease in Australia: the OzFoodNet experience.Crossref | GoogleScholarGoogle Scholar |
[13] Oliveira, J. et al. (2012) Bacteriophage therapy as a bacterial control strategy in aquaculture. Aquacult. Int. 20, 879–910.
| Bacteriophage therapy as a bacterial control strategy in aquaculture.Crossref | GoogleScholarGoogle Scholar |
[14] Rao, B.M. and Lalitha, K. (2015) Bacteriophages for aquaculture: are they beneficial or inimical. Aquaculture 437, 146–154.
| Bacteriophages for aquaculture: are they beneficial or inimical.Crossref | GoogleScholarGoogle Scholar |
[15] Rong, R. et al. (2014) Reductions of Vibrio parahaemolyticus in oysters after bacteriophage application during depuration. Aquaculture 418–419, 171–176.
| Reductions of Vibrio parahaemolyticus in oysters after bacteriophage application during depuration.Crossref | GoogleScholarGoogle Scholar |
[16] Lomelí-Ortega, C.O. and Martínez-Díaz, S.F. (2014) Phage therapy against Vibrio parahaemolyticus infection in the whiteleg shrimp (Litopenaeus vannamei) larvae. Aquaculture 434, 208–211.
| Phage therapy against Vibrio parahaemolyticus infection in the whiteleg shrimp (Litopenaeus vannamei) larvae.Crossref | GoogleScholarGoogle Scholar |
[17] Kalatzis, P.G. et al. (2016) Isolation and characterization of two lytic bacteriophages, ϕSt2 and ϕGrn1; phage therapy application for biological control of Vibrio alginolyticus in aquaculture live feeds. PLoS One 11, e0151101.
| Isolation and characterization of two lytic bacteriophages, ϕSt2 and ϕGrn1; phage therapy application for biological control of Vibrio alginolyticus in aquaculture live feeds.Crossref | GoogleScholarGoogle Scholar | 26950336PubMed |
[18] Stalin, N. and Srinivasan, P. (2017) Efficacy of potential phage cocktails against Vibrio harveyi and closely related Vibrio species isolated from shrimp aquaculture environment in the south east coast of India. Vet. Microbiol. 207, 83–96.
| Efficacy of potential phage cocktails against Vibrio harveyi and closely related Vibrio species isolated from shrimp aquaculture environment in the south east coast of India.Crossref | GoogleScholarGoogle Scholar | 28757045PubMed |
[19] Le, T.S. et al. (2018)a Protective effects of bacteriophages against Aeromonas hydrophila causing motile Aeromonas septicemia (MAS) in striped catfish. Antibiotics 7, 16.
| Protective effects of bacteriophages against Aeromonas hydrophila causing motile Aeromonas septicemia (MAS) in striped catfish.Crossref | GoogleScholarGoogle Scholar |
[20] Le, T.S. et al. (2018)b Bacteriophages as biological control agents of enteric bacteria contaminating edible oysters. Curr. Microbiol. 75, 611–619.
| Bacteriophages as biological control agents of enteric bacteria contaminating edible oysters.Crossref | GoogleScholarGoogle Scholar | 29282504PubMed |
[21] Nguyen, N. (2016) Improving sustainability of striped catfish (Pangasianodon hypophthalmus) farming in the Mekong Delta, Vietnam through recirculation technology. PhD thesis, Wageningen University, Wageningen, The Netherlands. https://library.wur.nl/WebQuery/wda/2196098
[22] Southgate, P.C. (2012) Foods and feeding. In Aquaculture: Farming Aquatic Animals and Plants, 2nd edition. pp. 188–213. Wiley-Blackwell Publishing Inc.
[23] Carrasco, E. et al. (2012) Cross-contamination and recontamination by Salmonella in foods: a review. Food Res. Int. 45, 545–556.
| Cross-contamination and recontamination by Salmonella in foods: a review.Crossref | GoogleScholarGoogle Scholar |
[24] Jonns, J.A. et al. (2017) Streptophage-mediated control of off-flavour taint producing streptomycetes isolated from barramundi ponds. Synth. Syst. Biotechnol. 2, 105–112.
| Streptophage-mediated control of off-flavour taint producing streptomycetes isolated from barramundi ponds.Crossref | GoogleScholarGoogle Scholar | 29062967PubMed |
[25] Vinod, M.G. et al. (2006) Isolation of Vibrio harveyi bacteriophage with a potential for biocontrol of luminous vibriosis in hatchery environments. Aquaculture 255, 117–124.
| Isolation of Vibrio harveyi bacteriophage with a potential for biocontrol of luminous vibriosis in hatchery environments.Crossref | GoogleScholarGoogle Scholar |
[26] Wang, Y. et al. (2017) Bacteriophage therapy for the control of Vibrio harveyi in greenlip abalone (Haliotis laevigata). Aquaculture 473, 251–258.
| Bacteriophage therapy for the control of Vibrio harveyi in greenlip abalone (Haliotis laevigata).Crossref | GoogleScholarGoogle Scholar |
[27] Matsuoka, S. et al. (2007) Phage therapy against beta-hemolytic streptococcicosis of Japanese flounder Paralichthys olivaceus. Fish Pathol. 42, 181–189.
| Phage therapy against beta-hemolytic streptococcicosis of Japanese flounder Paralichthys olivaceus.Crossref | GoogleScholarGoogle Scholar |
[28] Ruangpan, L. et al. (1999) Lethal toxicity of Vibrio harveyi to cultivated Penaeus monodon induced by a bacteriophage. Dis. Aquat. Organ. 35, 195–201.
| Lethal toxicity of Vibrio harveyi to cultivated Penaeus monodon induced by a bacteriophage.Crossref | GoogleScholarGoogle Scholar |
[29] Flegel, T.W. et al. (2005) Evidence for phage-induced virulence in the shrimp pathogen Vibrio harveyi. In Diseases in Asian Aquaculture V (Walker, P., et al., eds). pp. 329–337. Fish Health Section, Asian Fisheries Society, Manila.
Biographies
Son Tuan Le currently is a PhD student at the University of the Sunshine Coast (USC) as well as an environmental researcher at Research Institute for Marine Fisheries (Vietnam). He obtained his Bachelor of Environmental Science degree in 2009 from the Vietnam National University in Hanoi. He subsequently obtained his Master of Fisheries Sciences degree in 2012 at the Pukyong National University in the Republic of Korea. His MSc research project involved the application of bacteria for biodegradation of fish waste water in Korea. He then moved to Australia for his PhD studies at the University of the Sunshine Coast under the principal supervision of Dr İpek Kurtböke where he investigates the use of bacteriophages to control bacterial diseases in aquaculture. He is currently the recipient of the MOET-VIED/USC PhD scholarship.
Dr İpek Kurtböke has been working in the field of biodiscovery and has been an active member of the international actinomycete research community since 1982. She currently conducts research and teaches in the field of applied microbiology and biotechnology and is senior lecturer at the University of the Sunshine Coast (USC), Queensland. She has also been an active member of the World Federation of Culture Collections (WFCC) including serving as the Vice-President of the Federation (2010–2013) and currently is the President of the Federation (2017–2020).


