Rumen microbiome response to methane inhibition
Ibrahim Ahmad A * , Richard P. Rawnsley A , John P. Bowman A and Apeh A. Omede AA

Ibrahim Ahmad is a veterinarian and a PhD candidate at the Tasmanian Institute of Agriculture (TIA), University of Tasmania. He has several years of experience in general veterinary practice, meat inspection and research. His research interests include mitigation of enteric methane emissions from grass-fed dairy and beef production systems, rumen microbiology, animal nutrition and livestock systems modelling. |

Assoc. Prof. Richard Rawnsley is the academic lead for the Livestock Production Centre within the TIA. His research focuses on developing practical, science-based solutions to enhance both animal performance and the sustainability of pasture-based livestock systems. |
Prof. John P. Bowman is a microbiologist based in Launceston, Tas., where he is the professor of microbiology at the University of Tasmania. He is the leader of the Centre for Food Safety and Innovation within the Tasmanian Institute of Agriculture. His interests are broad and cover many aspects of food and agricultural systems, microbial ecology and systematics. He is a Fellow of the Australian Society of Microbiology. He completed his PhD (and DSc) at The University of Queensland and has been a researcher and lecturer at the University of Tasmania since the mid-1990s. |

Dr Apeh A. Omede is a lecturer and researcher in animal production at the TIA, University of Tasmania. He is interested in researching the interphase between nutritional strategies for sustainable animal production, the environment and gut microbiome in farm animals. He was a Marie Curie COFUND postdoc at Teagasc Ireland before joining the University of Tasmania. |
Abstract
Rumen microbiota ferment feed into various by-products, some of which are used by methanogens to produce enteric methane (CH4), a potent greenhouse gas (GHG) that significantly contributes to climate change. The use of CH4 inhibitors, such as Asparagopsis spp. and 3-nitrooxypropanol (3-NOP), in ruminant feed has been shown to be effective in reducing methanogenesis. However, inhibition of enteric CH4 leads to inconsistent productivity gains, partly due to the shift in significant fermentative microbes away from acetogenesis and the inability to convert increased ruminal dihydrogen (H2) spared from methanogenesis into absorbable nutrients beneficial to the host animals. Mitigation of CH4 synthesis paired with redirection of ruminal H2 to preserve normal rumen functions could be one of the most promising strategies to improve ruminant productivity while feeding CH4 inhibitors. This review highlights the growing knowledge of the rumen microbiome response to CH4 inhibition and explores the potential benefits of co-supplementing CH4 inhibitors with electron acceptors to optimise rumen function as a future direction to supporting the voluntary on-farm adoption of anti-methanogenic feed additives (AMFAs) across livestock systems.
Keywords: 3-NOP, Asparagopsis, feed additive, greenhouse gas, methane emission, methane inhibition, methanogenesis, rumen fermentation, rumen microbiome, ruminants.
Background
The rumen is a complex ecosystem that harbours a diverse anaerobic microbiota known as the rumen microbiome, which consists of bacteria, protozoa, fungi and methanogenic archaea, listed in order of relative abundance and diversity.1 Ruminal bacteria make up the largest component of the microbial biomass, protozoa can account for up to half of this biomass, whereas fungi were initially estimated to be ~8% but may reach 20% in sheep, and the methanogenic archaea comprise ~0.3–4% of the microbial biomass.2 These microbial communities (i.e. bacteria, fungi and protozoa) present in the rumen enable the hydrolysis and fermentation of various feed components, including polysaccharides, non-fibre carbohydrates, proteins and lipids consumed by ruminant animals, into substantial amounts of volatile fatty acids (VFAs), carbon dioxide (CO2), H2, as well as other end products such as methanol, methylamines and methylated sulfur compounds. However, most of the H2 produced during fermentation is utilised by methanogenic archaea to reduce CO2, resulting in methane (CH4) production. Several H2-consuming bacteria, including capnophilic bacteria like Prevotella, Fibrobacter, Ruminococcus, Lachnospira and Succinomonas, as well as nitrogen-compound or sulfur-respiring bacteria such as Denitrobacterium detoxificans and Wolinella succinogenes, can compete with methanogens for H2. Methanogens also form symbiotic relationships with H2-producing bacteria, protozoa and fungi, which prevent the redirection of H2 to alternative sinks. These associations facilitate the interspecies transfer of H2 between H2 producers and users for CH4 synthesis, which is fundamental in the normal functioning of an anaerobic ecosystem in an uninhibited rumen.3
Excess electrons in the rumen, particularly H2, are mainly removed by archaeal methanogens through CH4 production during the final stages of fermentation, with some contribution from hydrogenotrophic bacteria that produce VFA through nitrate and sulfate reduction.4 Therefore, the production of CH4 prevents the partial pressure of H2 from increasing to concentrations that could potentially inhibit the effective functioning of hydrogenases involved in electron transfer reactions (i.e. interspecies H2 transfer), particularly NADH dehydrogenase, which could lead to the accumulation of NADH within microbial cells (i.e. bacteria, protozoa and fungi) and, in turn, a reduction in rumen fermentation and degradation of plant fibre.3,5 Thus, this paper provides an understanding of the current knowledge on the varied responses of the hydrogenotrophic and fermentative rumen microbiome, with a focus on the potential implications for ruminant productivity from enteric CH4 inhibition.
Feed additives: methane inhibitors
Rumen microbiota ferment ingested feed into different end products, including VFA, CO2 and H2, which are utilised by methanogenic archaea in the rumen to produce CH4. Enteric CH4 emissions from ruminant livestock significantly contribute to global anthropogenic GHG emissions and represent a loss of gross energy intake.6 Hence, there has been heightened global interest in exploring practical measures to decrease CH4 emissions from ruminants, driven mainly by the potential benefits of improving production efficiency and reducing contribution to global GHG emissions.7 Several strategies have been proposed to mitigate enteric CH4 emissions, including selective animal breeding, management improvements and enhancing forage quality,8 with a particular emphasis on the use of anti-methanogenic feed additives (AMFAs) to manipulate the rumen environment.9 Rumen modifiers, such as ionophores, enhance microbial fermentation to improve feed efficiency by promoting the production of propionate and reducing the availability of H2 for methanogens, which indirectly lowers CH4 emissions. By contrast, CH4 inhibitors directly target methanogenesis by blocking key enzymes, resulting in a more immediate and significant reduction in CH4 production. The red seaweed Asparagopsis spp. (e.g. A. armata and A. taxiformis) and the synthetic compound 3-NOP are two well-known inhibitors that have consistently demonstrated high anti-methanogenic activity and effectiveness in reducing CH4 emissions.
The effect of CH4 reduction from Asparagopsis supplementation is due mainly to the bioactive compound bromoform (CHBr3), found at a concentration up to 100-fold higher than the next most abundant organobromine compound.10 When fed at minimal effective inclusion levels (0.45–1.58 mg CHBr3 kg–1 body weight, BW day–1), methanogens metabolise CHBr3 through reductive dehalogenation, which limits the transfer of the active ingredient to animal tissues and food products.11 Romero et al.6 reported a 90% degradation within the first 3 h, and that progressive dehalogenation continued to over 99% after 12 h of CHBr3 inclusion in the rumen. The synthetic compound, 3-NOP (marketed as Bovaer, DSM-Firmenich AG, Kaiseraugst, Switzerland), has been developed that also persistently reduces CH4 emissions in dairy cows.12 However, 3-NOP has differential effects on individual methanogenic lineages and thus, the efficacy to inhibit different methanogens is variable among ruminant species,13 suggesting structural differences in the subunits composition of the methyl coenzyme M reductase (MCR) enzyme contribute to the variation in tolerance to 3-NOP.14 Investigations into the metabolism of aliphatic nitro compounds in the rumen have shown that 3-NOP is reduced to 3-nitropropanol, which is then absorbed from the rumen and further metabolised in the liver and other tissues.15
Asparagopsis supplementation has been shown to significantly decreased enteric CH4 emission by up to 67 and 99% in dairy16 and beef17 cattle. Similarly, dietary inclusion of 3-NOP resulted in a reduction of CH4 emission by 20–80% in beef and dairy cattle.18,19 Overall, the dietary supplementation of said inhibitors has consistently shown significant potential for reducing enteric CH4 emissions to different extents based on the dosage of the active ingredient, the basal diet and the livestock system.20 However, mitigation of enteric CH4 emissions in ruminants using feed additive inhibitors leads to inconsistent productivity gains, partly due to the shift in significant fermentative microbes away from acetogenesis and the inability to convert increased ruminal H2 spared from methanogenesis into absorbable nutrients beneficial to the animals.16,21
Effects of inhibitors on the rumen microbiome
Supplementation of CH4 inhibitors in ruminant diets lowers the abundances of rumen methanogen populations, with shifts in bacterial groups and fermentation from acetate to propionate production, using Asparagopsis22 and 3-NOP.21 These supplements share a similar mode of action by inactivation of the MCR enzyme to inhibit the final step of methanogenesis.11 However, the inclusion of AMFAs resulted in both positive and negative effects on hydrogenogenic and hydrogenotrophic microbes in the rumen, a consequence of methanogenesis suppression and increased H2 production.21,23
Published studies by Krizsan et al.22 and Zhang et al.23 examined the effects of dietary supplementation of Asparagopsis on the rumen microbiome of dairy cows. Krizsan et al.22 observed that the inclusion of Asparagopsis in a diet composed of high forage resulted in a decrease in dry matter intake (DMI) by 13.8%, a 3% reduction in milk yield, and a five-fold increase in enteric H2 emissions from dairy cows. Further observations revealed a shift in rumen fermentation from acetate to propionate production, a reduced relative abundance of Prevotella in the bacterial population and a shift from Methanobrevibacter to Methanomethylophilaceae in the archaeal population. Methanomethylophilaceae are methanogens that utilise H2 as an electron donor and substrates such as methanol and methylamines to synthesise CH4.24 Zhang et al.23 evaluated the effects of Asparagopsis supplementation on the rumen microbial communities in lactating dairy cows. The reduction of enteric CH4 from the supplemented animals was coupled with a decrease in the population of rumen methanogens and a significant reduction in the transcription of methanogenesis pathways. Subsequent findings by Zhang et al.23 revealed increased H2 production associated with the upregulation of hydrogenases that mediate hydrogenotrophic metabolism, which may provide a competitive advantage in the conversion of excess H2 in the rumen. In addition, a trial conducted by Roque et al.16 that examined Asparagopsis supplementation in a high-forage diet reported a reduction in DMI and milk yield of respectively 38 and 11.6% in lactating dairy cows. The negative effects on production in studies by Krizsan et al.22 and Roque et al.16 coincided with increased emissions of enteric H2 from the experimental animals. The findings of Zhang et al.23 were based on continued analyses to the trial study by Roque et al.16
Similarly, Ni et al.21 analysed rumen microbiota responses to 3-NOP supplementation in dairy calves fed ration composed of low forage (7%) feed. The results showed an increased ruminal H2 levels, which caused a shift in microbial fermentation away from acetate production towards undesirable compounds such as ethanol, without adverse effects on feed intake or animal performance. Supplementation of 3-NOP in dairy calves by Gaofeng et al.21 resulted in a significant reduction in the methanogens population and stimulated reductive acetogens, especially novel uncultivated lineages such as ‘Candidatus Faecousia’. Gruninger et al.25 reported that supplementation of 3-NOP in a high-forage diet reduced the relative abundance of Methanobrevibacter methanogens. This change resulted in a 37-fold increase in H2 emissions and also increased the relative abundance of Bacteroidota.
Recent studies have provided foundational and new insights into the metagenomic and metatranscriptomic evaluation of the microbiome in rumen fluid from ruminants supplemented with CH4 inhibitors.21,23 However, the effects of CH4 inhibitors on the rumen microbiome, such as shifts in microbial composition, the regulation of methanogenic pathways, and the overall transcriptional dynamics of the rumen microbiome during methanogenesis inhibition, are still not well understood.23 The lack of information on alternative states of the rumen microbiome under methanogenesis inhibition by AMFAs emphasises a significant gap in the current understanding of rumen microbiology. Inhibition of CH4 production in the rumen could spare reducing equivalents, such as H2, which can then be used to produce beneficial nutrients for the host animal23 and ultimately improve both the feed efficiency and total productivity of ruminants.16
Inhibiting CH4 production in the rumen, while potentially beneficial for environmental reasons, may also have some negative effects on the animal. Although some research suggests improved animal productivity with CH4 inhibition, this is not consistently observed and, in some cases, CH4 inhibition can lead to changes in rumen fermentation, potentially affecting feed digestibility and other metabolic processes. The inhibition of enteric CH4 production is not an isolated intervention and may have indirect consequences on animal physiology, including inflammation of the rumen epithelium. This has been potentially linked to a dose-dependent negative effect of CHBr3-containing Asparagopsis supplementation, as reported in some studies.26,27 However, it remains unclear whether rumenitis arises directly from the additive itself or indirectly due to changes in diet composition following a reduction in DMI. A decline in DMI can impair rumen function, potentially leading to the development of rumenitis, whereas the likelihood of direct toxicity from CHBr3 at inclusion rates between 0.45 and 1.58 mg CHBr3 kg–1 BW appears low when compared with the no-observed-adverse-effect level (NOAEL) of 72 mg CHBr3 kg–1 BW reported in mice.28 Therefore, in cases where rumen health impacts such as rumenitis have been reported, these are most likely in response to the direct effects of CH4 inhibition on DMI and fibre digestion.
Exploring co-supplementation to improve rumen function
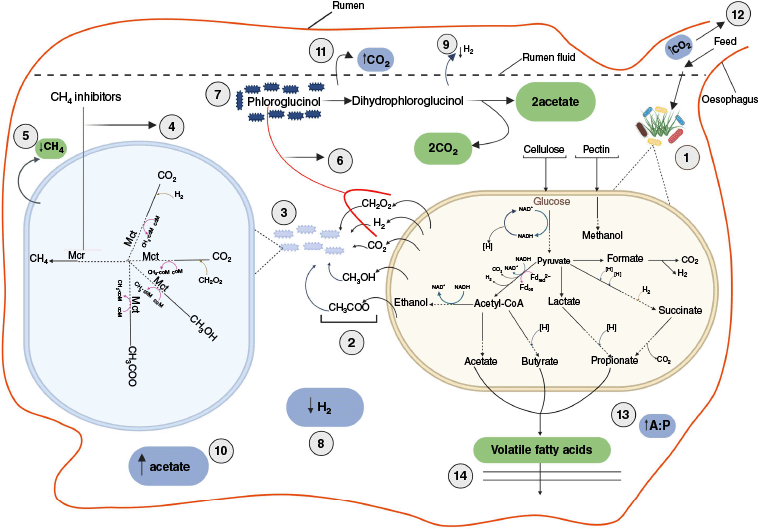
Under conditions of high CH4 inhibition there is currently a paucity of knowledge relating to the mechanisms that regulate the flow of electrons in the rumen, including the processes and microbes involved in H2 production and consumption.21 Mitigation of enteric CH4 emissions paired with redirection of ruminal H2 flow toward alternative hydrogenotrophic pathways for the production of absorbable nutrients, such as acetogenesis (as illustrated in Fig. 1), could be one of the most promising strategies to enhance ruminant productivity.29 For example, Romero et al.30 studied the effects of co-supplementation of a CH4 inhibitor, Asparagopsis, with an electron (H2) acceptor, phloroglucinol, on the rumen microbiome and fermentation in vitro. Combined treatment of Asparagopsis and phloroglucinol significantly inhibited CH4 production and lowered the abundance of methanogenic archaea, protozoa and fungi, as well as H2 accumulation in the supplemented batch compared to the control batch. This co-supplementation also promoted fermentation that resulted in increased acetate production, total VFA and a high acetate-to-propionate ratio (Fig. 1). This combination of supplements shows potential for reducing the negative environmental impact of rumen methanogenesis while maintaining rumen function and enhancing ruminant productivity.
A schematic diagram of the effects of dietary co-supplementation of methane inhibitors with electron acceptor on the rumen microbiome and fermentation. (1) Microbial hydrolysis and fermentation of feed by anaerobic bacteria, protozoa and fungi. (2) Substrates for methanogenesis. (3) Methanogenesis by methanogenic archaea using fermentation metabolites. (4) Inhibition of methanogenesis pathways by Asparagopsis bromoform (CHBr3) or 3-nitrooxypropanol (3-NOP) acting on methyl coenzyme M reductase (Mcr). (5) Decreased methane (CH4) production. (6) Capture of dihydrogen (H2) or formate (CH2O2) by electron acceptor, phloroglucinol. (7) Degradation of phloroglucinol by ruminal bacteria, particularly strains of Streptococcus and Coprococcus.31 (8) Decreased dihydrogen (H2) concentrations in the rumen fluid. (9) Decreased ruminal dihydrogen (H2) gas production. (10) Increased ruminal acetate concentrations. (11) Increased carbon dioxide (CO2) production. (12) Increased carbon dioxide (CO2) emissions. (13) Increased acetate-to-propionate ratio (A:P). (14) Absorption of volatile fatty acids through the rumen wall, which the animal host utilises as a source of energy, carbon for diverse metabolites and glucose. Abbreviation: acetyl-CoA is acetyl-coenzyme A; Mct is methyl coenzyme M transferase. Created in BioRender (see https://BioRender.com/c505wob).
Conclusions and recommendations
The inhibition of methanogens through dietary AMFAs reduces enteric CH4 production and alters rumen function, potentially resulting in changes in feed conversion efficiency, DMI and animal productivity. Although there is a growing body of knowledge on the response of the rumen microbiome to CH4 inhibition, future research needs to explore the role and function of hydrogenotrophic microbes to minimise H2 accumulation in the rumen and reduce the risk of decreased DMI. Additionally, investigating the potential benefits of co-supplementation with electron acceptors could help optimise rumen function and animal performance. Such advancements are critical for the effective and voluntary adoption of CH4 inhibitors beyond controlled research and feeding environments, particularly in grazing systems, where day-to-day and animal-to-animal variability in intake and nutrient profile is a key consideration. Without a reliable approach to optimising animal performance under commercial conditions, the adoption of CH4 inhibitors on-farm may be limited to conservative feeding rates, constraining their potential contribution to CH4 mitigation.
Data availability
Data sharing is not applicable as no new data were generated or analysed during this study.
Declaration of funding
This project is supported by the Department of Natural Resources and Environment Tasmania Agricultural Development Fund. Ibrahim Ahmad also receives a Grant-In Top-Up Stipend from the Tim Healey Memorial Scholarship of The AW Howard Memorial Trust Inc. for promoting sustainable agriculture in Australia (University of Tasmania reference number 00030728).
References
1 Mizrahi I et al. (2021) The rumen microbiome: balancing food security and environmental impacts. Nat Rev Microbiol 19, 553-566.
| Crossref | Google Scholar | PubMed |
2 Tapio I et al. (2017) The ruminal microbiome associated with methane emissions from ruminant livestock. J Anim Sci Biotechnol 8, 7.
| Crossref | Google Scholar | PubMed |
3 Morgavi DP et al. (2010) Microbial ecosystem and methanogenesis in ruminants. Animal 4, 1024-1036.
| Crossref | Google Scholar | PubMed |
4 Ungerfeld EM (2020) Metabolic hydrogen flows in rumen fermentation: principles and possibilities of interventions. Front Microbiol 11, 589.
| Crossref | Google Scholar | PubMed |
5 McAllister TA et al. (2025) International symposium on ruminant physiology: rumen fungi, archaea and their interactions. J Dairy Sci [Published online early 15 January 2025].
| Crossref | Google Scholar | PubMed |
6 Romero P et al. (2023) Rumen microbial degradation of bromoform from red seaweed (Asparagopsis taxiformis) and the impact on rumen fermentation and methanogenic archaea. J Anim Sci Biotechnol 14, 133.
| Crossref | Google Scholar | PubMed |
7 Belanche A et al. (2025) Feed additives for methane mitigation: a guideline to uncover the mode of action of antimethanogenic feed additives for ruminants. J Dairy Sci 108, 375-394.
| Crossref | Google Scholar | PubMed |
8 Hristov AN et al. (2013) Special topics — Mitigation of methane and nitrous oxide emissions from animal operations: I. A review of enteric methane mitigation options. J Anim Sci 91, 5045-5069.
| Crossref | Google Scholar | PubMed |
9 van Gastelen S et al. (2024) Long-term effects of 3-nitrooxypropanol on methane emission and milk production characteristics in Holstein–Friesian dairy cows. J Dairy Sci 107, 5556-5573.
| Crossref | Google Scholar | PubMed |
10 Machado L et al. (2016) Identification of bioactives from the red seaweed Asparagopsis taxiformis that promote antimethanogenic activity in vitro. J Appl Phycol 28, 3117-3126.
| Crossref | Google Scholar |
11 Glasson CRK et al. (2022) Benefits and risks of including the bromoform containing seaweed Asparagopsis in feed for the reduction of methane production from ruminants. Algal Res 64, 102673.
| Crossref | Google Scholar |
12 Dijkstra J et al. (2018) Short communication: Antimethanogenic effects of 3-nitrooxypropanol depend on supplementation dose, dietary fiber content, and cattle type. J Dairy Sci 101, 9041-9047.
| Crossref | Google Scholar | PubMed |
13 Duin EC et al. (2016) Mode of action uncovered for the specific reduction of methane emissions from ruminants by the small molecule 3-nitrooxypropanol. Proc Natl Acad Sci USA 113, 6172-6177.
| Crossref | Google Scholar | PubMed |
14 Pitta D et al. (2022) Symposium review: Understanding the role of the rumen microbiome in enteric methane mitigation and productivity in dairy cows. J Dairy Sci 105, 8569-8585.
| Crossref | Google Scholar | PubMed |
15 Majak W, Clark LJ (1980) Metabolism of aliphatic nitro compounds in bovine rumen fluid. Can J Anim Sci 60, 319-325.
| Crossref | Google Scholar |
16 Roque BM et al. (2019) Inclusion of Asparagopsis armata in lactating dairy cows’ diet reduces enteric methane emission by over 50 percent. J Clean Prod 234, 132-138.
| Crossref | Google Scholar |
17 Cowley FC et al. (2024) Bioactive metabolites of Asparagopsis stabilized in canola oil completely suppress methane emissions in beef cattle fed a feedlot diet. J Anim Sci 102, skae109.
| Crossref | Google Scholar | PubMed |
18 Hristov AN et al. (2015) An inhibitor persistently decreased enteric methane emission from dairy cows with no negative effect on milk production. Proc Natl Acad Sci USA 112, 10663-10668.
| Crossref | Google Scholar | PubMed |
19 Vyas D et al. (2016) Effects of sustained reduction of enteric methane emissions with dietary supplementation of 3-nitrooxypropanol on growth performance of growing and finishing beef cattle1. J Anim Sci 94, 2024-2034.
| Crossref | Google Scholar | PubMed |
20 Durmic Z et al. (2025) Feed additives for methane mitigation: recommendations for identification and selection of bioactive compounds to develop antimethanogenic feed additives. J Dairy Sci 108, 302-321.
| Crossref | Google Scholar | PubMed |
21 Ni G et al. (2024) Methanogenesis inhibition remodels microbial fermentation and stimulates acetogenesis in ruminants. bioRxiv 2024, 2024.08.15.608071 [Preprint, published 15 August 2024].
| Crossref | Google Scholar |
22 Krizsan SJ et al. (2023) Effects on rumen microbiome and milk quality of dairy cows fed a grass silage-based diet supplemented with the macroalga Asparagopsis taxiformis. Front Anim Sci 4, 1112969.
| Crossref | Google Scholar |
23 Zhang P et al. (2024) Red seaweed supplementation suppresses methanogenesis in the rumen, revealing potentially advantageous traits among hydrogenotrophic bacteria. bioRxiv 2024, 2024.06.07.597961 [Preprint, published 7 June 2024].
| Crossref | Google Scholar |
24 Khairunisa BH et al. (2023) Evolving understanding of rumen methanogen ecophysiology. Front Microbiol 14, 1296008.
| Crossref | Google Scholar | PubMed |
25 Gruninger RJ et al. (2022) Application of 3-nitrooxypropanol and canola oil to mitigate enteric methane emissions of beef cattle results in distinctly different effects on the rumen microbial community. Anim Microbiome 4, 35.
| Crossref | Google Scholar | PubMed |
26 Li X et al. (2018) Asparagopsis taxiformis decreases enteric methane production from sheep. Anim Prod Sci 58, 681.
| Crossref | Google Scholar |
27 Muizelaar W et al. (2021) Safety and transfer study: transfer of bromoform present in Asparagopsis taxiformis to milk and urine of lactating dairy cows. Foods 10, 584.
| Crossref | Google Scholar | PubMed |
28 Condie LW et al. (1983) Comparative renal and hepatotoxicity of halomethanes: bromodichloromethane, bromoform, chloroform, dibromochloromethane and methylene chloride. Drug Chem Toxicol 6, 563-578.
| Crossref | Google Scholar | PubMed |
29 Greening C et al. (2019) Diverse hydrogen production and consumption pathways influence methane production in ruminants. ISME J 13, 2617-2632.
| Crossref | Google Scholar | PubMed |
30 Romero P et al. (2023) Evaluating the effect of phenolic compounds as hydrogen acceptors when ruminal methanogenesis is inhibited in vitro – part 2. Dairy goats. Animal 17, 100789.
| Crossref | Google Scholar | PubMed |
31 Tsai CG, Jones GA (1975) Isolation and identification of rumen bacteria capable of anaerobic phloroglucinol degradation. Can J Microbiol 21, 794-801.
| Crossref | Google Scholar | PubMed |
 Ibrahim Ahmad is a veterinarian and a PhD candidate at the Tasmanian Institute of Agriculture (TIA), University of Tasmania. He has several years of experience in general veterinary practice, meat inspection and research. His research interests include mitigation of enteric methane emissions from grass-fed dairy and beef production systems, rumen microbiology, animal nutrition and livestock systems modelling. |
 Assoc. Prof. Richard Rawnsley is the academic lead for the Livestock Production Centre within the TIA. His research focuses on developing practical, science-based solutions to enhance both animal performance and the sustainability of pasture-based livestock systems. |
 Prof. John P. Bowman is a microbiologist based in Launceston, Tas., where he is the professor of microbiology at the University of Tasmania. He is the leader of the Centre for Food Safety and Innovation within the Tasmanian Institute of Agriculture. His interests are broad and cover many aspects of food and agricultural systems, microbial ecology and systematics. He is a Fellow of the Australian Society of Microbiology. He completed his PhD (and DSc) at The University of Queensland and has been a researcher and lecturer at the University of Tasmania since the mid-1990s. |
 Dr Apeh A. Omede is a lecturer and researcher in animal production at the TIA, University of Tasmania. He is interested in researching the interphase between nutritional strategies for sustainable animal production, the environment and gut microbiome in farm animals. He was a Marie Curie COFUND postdoc at Teagasc Ireland before joining the University of Tasmania. |


