Diversity, ecology and biotechnological potential of mangrove sediment microbiomes in Malaysia
Mohd Faidz Mohamad Shahimin A B *A
B

Dr Mohd Faidz Mohamad Shahimin is a Senior Lecturer and Research Fellow at Universiti Malaysia Perlis (UniMAP). He specialises in environmental microbiology and anaerobic biodegradation, with research focused on microbial solutions for pollution and other biotechnological applications. His work contributes to sustainable environmental management and the bioenergy sector. |
Abstract
Mangrove ecosystems are globally recognised for their ecological and climate-regulating functions, yet their sediment microbiomes remain underexplored, particularly in Southeast Asia. Recent metagenomic investigations in Northwest Peninsular Malaysia have revealed diverse and rich microbial taxa across various mangrove zones, including Firmicutes, Chloroflexota, Proteobacteria, Thermoproteota, and Asgardarchaeota; many members of which have been implicated to contributing to carbon sequestration, nutrient cycling, and pollutant degradation in other environments. Although the ecological roles of specific enriched taxa in the sediment samples were not ascertained, novel genes and metabolic pathways may be uncovered with advanced high-throughput sequencing and metagenomic profiling. Further studies in characterisation of dominant microbial groups in various metabolic activities will shed some insights into their various biotechnological applications, including targeted pollutant degradation, pilot-scale bioremediation in mangrove-mimic systems, and bioenergy generation through microbial fuel cells and methanogenesis. This paper intends to highlight the untapped potential of mangrove microbiomes in environmental biotechnology and underscore the importance of functional characterisation and translational research to support sustainable coastal management and climate resilience.
Keywords: anaerobic microbiology, blue carbon sink, environmental biotechnology, mangrove sediments, microbial diversity, microbiome.
Introduction
Mangrove forests are among the world’s most productive and ecologically important ecosystems. They thrive in tropical and subtropical coastal zones where land meets the sea, and they are regularly flooded by tides, exposing them to fluctuating salinity and water levels. Mangrove ecosystems also provide high carbon sequestration capacity (38.3 Tg C year−1), thereby playing an important role in global coastal biogeochemical reactions and climate change.1 The soil in these ecosystems is layered: the surface is oxygen-rich, while deeper layers are anoxic, creating a unique environment that supports diverse microbial communities. However, previous culture-based methods only managed to identify a small fraction of mangrove microbial diversity, while the bulk of unculturable microorganisms, under standard laboratory conditions, remain unknown. With the ever-increasing anthropogenic activities in recent decades, mangroves are exposed to various pollutants, including hydrocarbons, plastics, pesticides, persistent organic pollutants (POPs), and pharmaceutical waste, which further increases the complexity of the microbial communities’ structure in mangrove sediments. Despite their importance, the microbial communities in mangrove sediments, especially in Malaysia, remain vastly unknown. Mangrove sediments hold a hidden wealth of microbial diversity, uniquely adapted to a saline, anoxic environment rich in organic matter. These microbial communities are vital drivers of carbon sequestration,2,3 nutrient cycling4,5 and pollution resilience,6 yet they remain underexplored for their biotechnological potential, including in the degradation of pollutants and bioenergy production.
Microbial diversity of mangrove sediments and their ecosystem roles
Due to mangroves’ complex biogeochemical reactions, the microbial structure in mangrove soils is shaped by a complex interplay of biotic and abiotic factors, including vegetation type, soil depth, salinity, tidal cycles, and anthropogenic influences. Recent studies have revealed that mangrove soils harbour a wide array of microbial taxa, many of which are novel or poorly understood.5,7,8 Microbial communities in mangrove soils are also not uniform; they vary significantly across different ecological niches. Our initial microbial communities screening also showed non-uniformity of microbial community structures in mangrove sediments sampled from six mangrove locations in Northwest Peninsular Malaysia (detailed in Table 1) at three different depths (Fig. 1). Coastal and estuarine mangrove sediments from Kuala Perlis and Matang Mangrove Forest Reserve were chosen to compare the microbial community structures in two different states of Northern Peninsular Malaysia, whereas Pulau Langkawi estuarine mangrove sediments were also examined to determine the effects of anthropogenic activities on microbial community structures.
| No. | Location (coordinates) | Type of mangrove | Environmental setting | |
|---|---|---|---|---|
| 1 | Kuala Perlis (6°24′20.4″N, 100°07′31″E) | Coastal mangrove | Coastal zone | |
| 2 | Kuala Perlis (6°22′22.9″N, 100°08′33.1″E) | Estuarine mangrove | River estuary | |
| 3 | Pulau Langkawi (6°15′09.4″N, 99°49′27″E) | Estuarine mangrove | Anthropogenically impacted | |
| 4 | Pulau Langkawi (6°12′27.4″N, 99°48′42″E) | Estuarine mangrove | Pristine/natural environment | |
| 5 | Matang Mangrove Forest Reserve (4°47′07.7″N, 100°36′28.4″E) | Coastal mangrove | Coastal zone | |
| 6 | Matang Mangrove Forest Reserve (4°48′58″N, 100°37′23.9″E) | Estuarine mangrove | River estuary |
Relative abundance of prokaryotic phyla in mangrove sediment samples from Kuala Perlis coast, Kuala Perlis estuary, Pulau Langkawi anthropogenic estuary, Pulau Langkawi pristine estuary, Matang Mangrove Forest Reserve (MMFR) coast, and MMFR estuary. Sequence reads were taxonomically classified at the family level, and relative abundances were calculated per sample. Taxa contributing >5% in at least one sample are individually shown. Taxa contributing <5% across all samples were grouped as ‘Others <5%’. Shifts in microbial abundance across sites and depths reflect changes in biogeochemical processes such as carbon cycling, nutrient transformation, and pollutant degradation, highlighting the ecological sensitivity of mangrove sediments to environmental conditions.
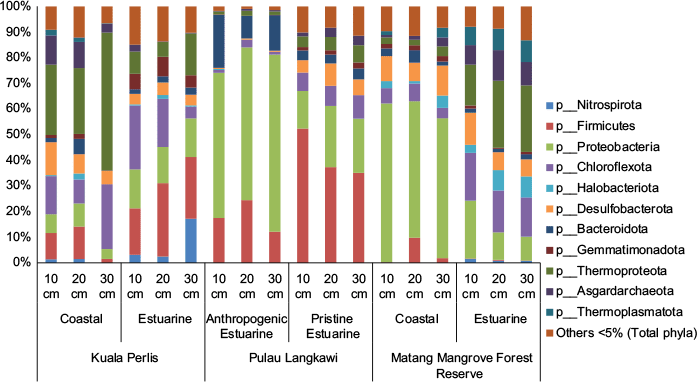
Dominant phyla characterised using 16S rRNA Illumina sequencing included Firmicutes, Chloroflexota and Proteobacteria among the bacterial community, and Thermoproteota and Asgardachaeota among the archaeal community, with notable shifts in abundance from site to site and with depth within each site (Fig. 1). These microbes were known to be involved in many important biogeochemical reactions in mangrove sediments including hydrocarbons degradation, carbon cycling, nutrients cycling and mercury methylation (Table 2). A recent study9 analysing the root-associated microbiomes of four mangrove species (Acanthus ilicifolius, Bruguiera gymnorrhiza, Clerodendrum inerme, and Lumnitzera racemosa), found distinct bacterial distributions in the root, rhizosphere, and non-rhizosphere soils.
| Phylum | Roles in mangrove sediments | References | |
|---|---|---|---|
| Firmicutes | Anaerobic fermentation of organic matter; hydrocarbon and polycyclic aromatic hydrocarbons (PAH) degradation under anaerobic conditions | 10,11 | |
| Chloroflexota | Anaerobic degradation of complex organic matter; syntrophic interactions aiding carbon cycling under anoxic conditions | 12,13 | |
| Proteobacteria | Sulfate reduction, nitrogen cycling (denitrification, nitrification), hydrocarbon degradation; sulfur and phosphorus cycling | 14,15 | |
| Thermoproteota | Ammonia oxidation (AOA) supporting nitrification and nitrogen cycling under low-oxygen conditions | 10,16 | |
| Asgardarchaeota | Anaerobic organic matter degradation, mercury methylation, nutrient cycling and carbon cycling in sediments | 17–19 |
Despite their importance, the microbial communities in these mangrove sediments are poorly characterised. However, understanding the microbial community structure in mangrove sediments is essential for conservation and restoration efforts. These microbes not only support mangrove health through nutrient cycling and pollutant degradation but also act as indicators of environmental change, reflecting shifts in salinity, pollution, and hydrology. Systematic monitoring of microbial diversity, combined with advanced analytical tools such as metagenomics and machine learning tools, can inform sustainable management and targeted restoration interventions for mangrove ecosystems.
The integration of high-throughput sequencing and omics technologies has significantly advanced our understanding of mangrove microbial communities. Long-read sequencing platforms like PacBio have enabled the recovery of high-quality metagenome-assembled genomes (MAGs), revealing novel metabolic pathways and microbial interactions that underpin key biogeochemical processes. However, functional insights remain limited without complementary approaches such as metatranscriptomics, which can link microbial presence to gene expression and activity under varying environmental conditions. The use of RNA stabilisation agents, coupled with AI-driven data analysis, is making such studies increasingly feasible.15,20
Towards biotechnological applications
Microbial isolation strategies
Several strategies can be employed to optimise the utilisation of functional microorganisms for biotechnological applications. One of such strategies is targeted isolation of microbial taxa from mangrove sediments, capable of removing specific pollutants, such as hydrocarbons, microplastics, heavy metals, and other xenobiotics, for environmental remediation. Targeted microbial isolation may present several disadvantages, including incomplete pollutant degradation, inactivity due to sensitivity to environmental conditions, and unintended consequences to the targeted environment, but using specific indigenous microorganisms isolated from pollutant-impacted sites tailored to degrade particular contaminants can potentially lead to faster and more efficient cleanup.21
Omics-based functional discovery
Besides targeted microbial isolation, functional gene discovery via metagenomic and metatranscriptomic profiling may uncover functional genes involved in pollutant degradation, stress tolerance, and nutrient cycling without the need for culturing.22 For instance, genes encoding key enzymes involved in the degradation of xenobiotics, such as monooxygenases, dioxygenases, and hydrolases, have been identified in mangrove sediment samples.22 These insights not only help identify novel biodegradation pathways but also guide the engineering of microbial consortia tailored for specific pollutants.
Future directions
Future work should prioritise functional validation of candidate taxa and pathways inferred from metagenomic and amplicon data. Approaches such as stable‑isotope probing (e.g. 13C, 15N) coupled to metatranscriptomics, metaproteomics, and metabolomics can directly link taxa to in situ carbon and nitrogen transformations and pollutant degradation. In parallel, generating high-quality MAGs with long‑read assemblies and applying single‑cell genomics will improve recovery of uncultured lineages and resolve novel enzymes relevant to xenobiotic breakdown.
To translate these findings into practical applications, mesocosm and field‑scale pilot studies that replicate mangrove conditions (i.e. salinity, anaerobic zones and root structures) should be set up to test bioaugmentation (introducing pollutant-degrading microbes) and biostimulation (enhancing native microbial functions) for hydrocarbons, plastics, and POPs, while quantifying trade‑offs (e.g. greenhouse gas fluxes, sulfate reduction vs methanogenesis). Bioaugmentation and biostimulation have been successfully tested in such setups, demonstrating accelerated pollutant breakdown and improved ecosystem resilience.15 Developing eDNA/ddPCR (environmental DNA surveillance coupled with droplet digital PCR) sentinel assays for key indicator taxa and genes could enable routine microbial monitoring to support restoration and compliance programs.
On the data side, the integration of machine learning with harmonised multi‑omics and environmental metadata will enhance the prediction of biodegradation potential. Algorithms trained on large omics datasets can predict biodegradation pathways, identify key microbial players, and estimate pollutant removal efficiencies. This computational approach not only enhances the accuracy of bioremediation models but also enables real-time monitoring and adaptive management of remediation strategies.15 Additionally, adoption of community metadata standards and open data deposition will increase reproducibility and reuse across sites.
Beyond remediation, mangrove microflora also shows potential in bioenergy applications. Certain anaerobic bacteria and archaea from mangrove sediments are capable of producing biohydrogen23 and methane through fermentation and methanogenesis.24 For example, sulfate-reducing bacteria and methanogens isolated from mangrove mud have been used in microbial fuel cells (MFCs)25 and anaerobic digesters to generate electricity and biogas from organic waste. These systems not only offer renewable energy solutions but also contribute to waste valorisation in coastal communities.
Together, these innovations underscore the untapped potential of mangrove microbiomes in biotechnology. By combining microbial ecology with cutting-edge genomics and computational tools, researchers can develop sustainable solutions to some of the most pressing environmental challenges, including pollution mitigation and ecosystem restoration. Importantly, these insights can inform policymakers by providing robust, science-based evidence to guide the development of effective conservation strategies, coastal management policies, and climate resilience planning, ensuring that mangrove ecosystems continue to deliver essential services to both nature and society.
Conclusion
Mangrove microbial communities in Northwest Peninsular Malaysia represent a rich resource for environmental biotechnology, from pollutant degradation to bioenergy production. Systematic characterisation, coupled with advanced sequencing and AI analytics, can unlock these microbes’ potential for sustainable remediation strategies and conservation efforts while ensuring the health of mangrove ecosystems against increasing anthropogenic pressures. Future research should focus on functional validation of key microbial taxa and scaling biotechnological applications to support regional conservation and climate adaptation strategies. Importantly, advances in biotechnology ultimately rely on a robust ecological understanding of mangrove microbial communities, as their natural roles in nutrient cycling, carbon sequestration, and pollutant degradation provide the foundation for applied innovations. By explicitly integrating ecological insights with biotechnological development, we can ensure that practical applications remain ecologically relevant and sustainable.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Declaration of funding
This project was financially supported by the Fundamental Research Grant Scheme (FRGS) under a grant number of FRGS/1/2020/WAB02/UNIMAP/02/4 granted to MFMS by the Ministry of Higher Education (MoHE) Malaysia.
References
1 Zhu JJ, Yan B. Blue carbon sink function and carbon neutrality potential of mangroves. Sci Total Environ 2022; 822: 153438.
| Crossref | Google Scholar | PubMed |
2 Ali S, Dey G, Nuong NHK, Rahman A, Wang L-C, Sukul U, Das K, Sharma RK, Wang S-L, Chen CY. Carbon sequestration in mangrove ecosystems: sources, transportation pathways, influencing factors, and its role in the carbon budget. Earth Sci Rev 2025; 269: 105184.
| Crossref | Google Scholar |
3 Duan F, Tan F, Zhao X, Feng H, Wang J, Peng H, Zhang N, Huang Y. Microbial life strategies-mediated differences in carbon metabolism explain the variation in SOC sequestration between Kandelia obovata and Sonneratia apetala. For Ecosyst 2025; 14: 100341.
| Crossref | Google Scholar |
4 Alongi DM. Macro-and micronutrient cycling and crucial linkages to geochemical processes in mangrove ecosystems. J Mar Sci Eng 2021; 9: 456.
| Crossref | Google Scholar |
5 Hu M, Sardans J, Sun D, Yan R, Wu H, Ni R, Peñuelas J. Microbial diversity and keystone species drive soil nutrient cycling and multifunctionality following mangrove restoration. Environ Res 2024; 251: 118715.
| Crossref | Google Scholar | PubMed |
6 Capdeville C, Pommier T, Gervaix J, Fromard F, Rols JL, Leflaive J. Mangrove facies drives resistance and resilience of sediment microbes exposed to anthropic disturbance. Front Microbiol 2018; 9: 3337.
| Crossref | Google Scholar | PubMed |
7 Zhuang W, Yu X, Hu R, Luo Z, Liu X, Zheng X, Xiao F, Peng Y, He Q, Tian Y, Yang T, Wang S, Shu L, Yan Q, Wang C, He Z. Diversity, function and assembly of mangrove root-associated microbial communities at a continuous fine-scale. NPJ Biofilms Microbiomes 2020; 6: 52.
| Crossref | Google Scholar | PubMed |
8 He M, Jiang S, Li X, Yao L, Shui B. Seasonal dynamics of bacterial communities in mangrove sediments of Shupaisha island, Zhejiang Province, China. Front Microbiol 2025; 16: 1526730.
| Crossref | Google Scholar |
9 Sui J, He X, Yi G, Zhou L, Liu S, Chen Q, Xiao X, Wu J. Diversity and structure of the root-associated bacterial microbiomes of four mangrove tree species, revealed by high-throughput sequencing. PeerJ 2023; 11: e16156.
| Crossref | Google Scholar | PubMed |
10 Zhang K, Hu Z, Zeng F, Yang X, Wang J, Jing R, Zhang H, Li Y, Zhang Z. Biodegradation of petroleum hydrocarbons and changes in microbial community structure in sediment under nitrate-, ferric-, sulfate-reducing and methanogenic conditions. J Environ Manage 2019; 249: 109425.
| Crossref | Google Scholar | PubMed |
11 Cotta SR, Cadete LL, van Elsas JD, Andreote FD, Dias A. Exploring bacterial functionality in mangrove sediments and its capability to overcome anthropogenic activity. Mar Pollut Bull 2019; 141: 586-594.
| Crossref | Google Scholar | PubMed |
12 Zhang C-J, Pan J, Duan C-H, Wang Y-M, Liu Y, Sun J, Zhou H-C, Song X, Li M. Prokaryotic diversity in mangrove sediments across Southeastern China fundamentally differs from that in other biomes. mSystems 2019; 4: 10.1128/msystems.00442-19.
| Crossref | Google Scholar |
13 Lin X, Hetharua B, Lin L, Xu H, Zheng T, He Z, Tian Y. Mangrove sediment microbiome: adaptive microbial assemblages and their routed biogeochemical processes in Yunxiao Mangrove National Nature Reserve, China. Microb Ecol 2019; 78: 57-69.
| Crossref | Google Scholar | PubMed |
14 Andreote FD, Jiménez DJ, Chaves D, Dias AC, Luvizotto DM, Dini-Andreote F, Fasanella CC, Lopez MV, Baena S, Taketani RG, de Melo IS. The microbiome of Brazilian mangrove sediments as revealed by metagenomics. PLoS One 2012; 7: e38600.
| Crossref | Google Scholar | PubMed |
15 Allard SM, Costa MT, Bulseco AN, Helfer V, Wilkins L, Hassenrück C, Zengler K, Zimmer M, Erazo N, Mazza Rodrigues JL, Duke N, Melo V, Vanwonterghem I, Junca H, Makonde HM, Jiménez DJ, Tavares T, Fusi M, Daffonchio D, Duarte CM, Peixoto RS, Rosado AS, Gilbert JA, Bowman J. Introducing the Mangrove Microbiome Initiative: identifying microbial research priorities and approaches to better understand, protect, and rehabilitate mangrove ecosystems. mSystems 2020; 5: e00658-20.
| Crossref | Google Scholar | PubMed |
16 Dias ACF, Dini-Andreote F, Taketani RG, Tsai SM, Azevedo JL, de Melo IS, Andreote FD. Archaeal communities in the sediments of three contrasting mangroves. J Soils Sediments 2011; 11: 1466-1476.
| Crossref | Google Scholar |
17 Macleod F, Kindler GS, Wong HL, Chen R, Burns BP. Asgard archaea: Diversity, function, and evolutionary implications in a range of microbiomes. AIMS Microbiol 2019; 5: 48-61.
| Crossref | Google Scholar | PubMed |
18 Zhang CJ, Liu YR, Cha G, Liu Y, Zhou XQ, Lu Z, Pan J, Cai M, Li M. Potential for mercury methylation by Asgard archaea in mangrove sediments. ISME J 2023; 17: 478-485.
| Crossref | Google Scholar | PubMed |
19 Banciu HL, Gridan IM, Zety AV, Baricz A. Asgard archaea in saline environments. Extremophiles 2022; 26: 21.
| Crossref | Google Scholar | PubMed |
20 Mohamad Shahimin MF, Bakri M, Mustafa AR. Addressing knowledge gaps on the role of anaerobic microbial communities in mangrove ecosystems: implications for contaminant biodegradation and ecosystem conservation. IOP Conf Ser Earth Environ Sci 2025; 1467: 012028.
| Crossref | Google Scholar |
21 Mendoza-Burguete Y, de la Luz Pérez-Rea M, Ledesma-García J, Campos-Guillén J, Ramos-López MA, Guzmán C, Rodríguez-Morales JA. Global situation of bioremediation of leachate-contaminated soils by treatment with microorganisms: a systematic review. Microorganisms 2023; 11: 857.
| Crossref | Google Scholar | PubMed |
22 Zhang ZF, Liu LR, Pan YP, Pan J, Li M. Long-read assembled metagenomic approaches improve our understanding on metabolic potentials of microbial community in mangrove sediments. Microbiome 2023; 11: 188.
| Crossref | Google Scholar | PubMed |
23 Mullai P, Rene ER, Sridevi K. Biohydrogen production and kinetic modeling using sediment microorganisms of Pichavaram Mangroves, India. Biomed Res Int 2013; 2013: 265618.
| Crossref | Google Scholar | PubMed |
24 Zhang CJ, Chen YL, Sun YH, Pan J, Cai MW, Li M. Diversity, metabolism and cultivation of archaea in mangrove ecosystems. Mar Life Sci Technol 2021; 3: 252-262.
| Crossref | Google Scholar | PubMed |
25 Sabaruddin MF, Fahimi AF, Ibrahim NB. Potential bioelectricity generation from mangrove sediments. J Adv Res Mat Sci 2016; 25: 10-17.
| Google Scholar |
 Dr Mohd Faidz Mohamad Shahimin is a Senior Lecturer and Research Fellow at Universiti Malaysia Perlis (UniMAP). He specialises in environmental microbiology and anaerobic biodegradation, with research focused on microbial solutions for pollution and other biotechnological applications. His work contributes to sustainable environmental management and the bioenergy sector. |


