Phyllode fall and nutrient content in a mulga (Acacia aneura F.Muell. ex Benth.) community in central Australia in response to rainfall
J. C. Turner A , M. H. Friedel B E and M. Neumann
B E and M. Neumann  C D
C D
A Newcastle, NSW 2300, Australia.
B Research Institute for the Environment and Livelihoods, Charles Darwin University, Grevillea Drive, Alice Springs, NT 0870, Australia.
C Department of Chemistry and Biotechnology, Faculty of Science, Engineering and Technology, Swinburne University of Technology, Hawthorn, Vic. 3122, Australia.
D Institute of Silviculture, University of Natural Resources and Life Sciences, 1190 Vienna, Austria.
E Corresponding author. Email: mhfriedel@outlook.com
The Rangeland Journal 43(1) 1-9 https://doi.org/10.1071/RJ21007
Submitted: 5 February 2021 Accepted: 6 April 2021 Published: 22 May 2021
Abstract
The fall of phyllodes from Acacia aneura F.Muell. ex Benth. (mulga) in central Australia was studied over 22 months from mid-1958 at four locations within a livestock reserve north of Alice Springs in the Northern Territory, in order to identify rainfall or seasonal triggers. Phyllode fall increased by at least an order of magnitude for short periods following rain of 15–20 mm or more on a ‘mature’ mulga site and similar trends were apparent for ‘young’ and ‘desert form’ mulga on the same site, and on a second, independent, ‘mature’ site. When rates of phyllode fall were high after substantial rain, at both ‘mature’ locations, ~30% of nitrogen and ~50% of phosphorus were withdrawn before abscission, suggesting that mulga was markedly conservative of these nutrients. Conversely, under dry conditions, when phyllode fall was relatively low (<200 mg m−2 day−1), concentrations of nitrogen and phosphorus in fallen phyllodes were higher. Concentrations of potassium, calcium, magnesium and sodium did not vary consistently with increasing rate of phyllode fall, although overall levels of calcium and potassium were considerably higher in the second ‘mature’ location. This legacy study is of renewed interest given the potential of mulga communities to contribute to national carbon stocks, and the consequent need for robust field-based data on growth dynamics and carbon fluxes.
Keywords: litterfall, arid shrubland, nitrogen, phosphorus, nutrient withdrawal, carbon sequestration, rainfall trigger.
Introduction
From mid-1958 to April 1960 a study of the time patterns in the shedding of phyllodes by Acacia aneura F.Muell. ex Benth. in a central Australian mulga community in response to rainfall was conducted. The nutrient content of the phyllodes was also examined. At the time there were no published studies of leaf or phyllode fall in arid Australian plant communities. Studies on the rates of fall of leaves and other plant materials on to the soil surface most commonly considered forest vegetation in the middle latitudes of the northern hemisphere. An early review of published data worldwide was undertaken by Bray and Gorham (1964).
In 2004, Liu et al. (2004) conducted a meta-analysis of litterfall observations in Eurasian forests (min-max leaf litterfall 23–1075 g m−2 year−1), and Neumann et al. (2018) analysed litterfall collected by a European monitoring network (mean ± s.d. leaf litterfall 260 ± 119 g m−2 year−1). An equivalent global summary for drylands has not been undertaken, but Holland et al. (2015) collated globally available litterfall observations to 1997 that included 31 records for drylands. The mean leaf litterfall for these 31 records was 298 g m−2 year−1 (range of minimum to maximum of 13–862 g m−2 year−1). In a dry forest in Mexico, measured litterfall ranged from 277 to 344 g m−2 year−1, including non-foliar components (Búrquez et al. 1999). Thus, global data suggest that dryland vegetation shed a similar weight of leaves annually to more mesic vegetation. Leaf litterfall was positively correlated with the annual rainfall sum irrespective of species and forest types (Bray and Gorham 1964; Liu et al. 2004; Neumann et al. 2018). The rate of leaf shedding increased seasonally during summer months for evergreen species when rainfall was deficient (Bray and Gorham 1964). Deciduous species, however, had peak litterfall in autumn, when leaves senesce.
Published work in Australia is concentrated on Eucalyptus forest communities in south-eastern and south-western Australia (e.g. Hatch 1955; McColl 1966; Adams and Attiwill 1991). According to Pook et al. (1997) and Crockford and Richardson (1998), leaf fall in evergreen eucalypts had a clear seasonal pattern, and temperature (or daylength), rainfall, and soil moisture could trigger leaf fall. There is scant information on litterfall for arid and semiarid woodlands and shrublands in Australia (Neumann et al. in press) where aridity is delimited by a median annual rainfall of <350 mm (Bureau of Meteorology 2005). Hart (1995) studied litterfall of Callitris-Eucalyptus communities in central New South Wales and McIvor (2001) measured litterfall in eucalypt woodlands in Queensland. Limited information on litterfall in mulga shrublands suggests that season is not influential. Wilcox (1960, 1974) found that maximum ‘leaf and seed (pod)’ fall appeared to occur only after substantial rains following a period of very low rainfall in a mulga community in Western Australia. Winkworth (1973) also reported peak rates of phyllode fall after rain in a central Australian mulga community. Burrows (1976) concluded that litterfall from mulga in south-west Queensland appeared to be independent of both season and rainfall, but his 4-weekly sampling regime may have been too infrequent to detect short-term rainfall responses. However, south-west Queensland has a particularly favourable water regime for mulga and is bioclimatically different from the rest of the mulga zone (Nix and Austin 1973). Consequently, mulga growth and phyllode fall may be a more continuous process in that region than elsewhere.
Nutrient cycling is important given Australia’s old and strongly leached soils, but there is usually sufficient nitrogen due to large atmospheric pools and nitrogen-fixing bacteria. In contrast, phosphorus can be limiting, but on a global scale, resorption of phosphorus in Australian ecosystems is relatively efficient (Du et al. 2020). Burrows (1976) assessed levels of nitrogen and phosphorus in litterfall in a mulga community, comparing them with pre-abscission levels, and concluded that nutrient conservation was less than might be expected for the infertile soils there. He proposed that mulga depended on a ‘tight extrinsic cycle’ of nutrients achieved through its extensive root system and that, coupled with nutrient withdrawal before abscission, efficient cycling could be achieved.
Modelling analysis and remote sensing suggest that growth anomalies in semiarid ecosystems of the southern hemisphere, particularly those in Australia, can have substantial impacts on global carbon dioxide concentration (Poulter et al. 2014). Within Australia, the eucalypt woodlands of Queensland are an important contributor to national carbon stocks (Burrows et al. 2002). Given the wide distribution of mulga communities across arid and semiarid Australia, these communities are also potentially substantial carbon sinks. Mulga communities in south-west Queensland and north-west New South Wales, in particular, are now being used for carbon sequestration under the Avoided Deforestation and Human Induced Regeneration mechanisms of the Emissions Reduction Fund (ERF 2020; R. B. Hacker, pers. comm.). The robustness of these assessments for mulga communities Australia-wide is constrained by limited information on growth dynamics and carbon fluxes, with the possible exception of south-west Queensland (Fensham et al. 2012).
We investigated the relationship between rainfall and phyllode fall, and observations on the initiation of growth, in a central Australian mulga community to identify rainfall or seasonal triggers and to test which patterns found elsewhere apply to this species in central Australia. In addition, we investigated the relationship between the phyllode fall data and the nutrient content of the phyllodes. Although the measurements were confined to phyllode fall under canopies, we estimated the role of mulga in carbon storage and carbon cycling by accounting for community-wide canopy cover, to indicate the potential of mulga communities to contribute to national carbon stocks.
Methods
Study area
The study sites were located in a mulga community within a livestock reserve some 24 km north of the township of Alice Springs in the Northern Territory of Australia, at an elevation of ~670 m. The reserve encompassed a watering point, Sixteen Mile Bore, at ~23°30′S, 133°50′E. The reserve was only used for brief periods by grazing cattle and thus the vegetation was in near pristine condition except within ~100 m of the bore (D. J. Nelson, pers. comm.). At Alice Springs (elevation 580 m), mean annual rainfall at the time of the study was ~267 mm (283 mm in 2020), with 190 mm falling in the summer months from October to March. The rainfall is highly variable on both annual and monthly time scales (Bureau of Meteorology 2020). Temperatures ranged from mean monthly minima and maxima of 4°C and 19°C in July, to 21°C and 35° in January. Rainfall and temperature means were based on data collected between 1941 and 2020 (Bureau of Meteorology 2020).
Mature mulga trees on the reserve typically reached a height of 5 to 6 m. They were the only tree species present on the study sites. Occurring on the gentle slope (~1 : 500) of a deeply weathered peneplain, they tended to be grouped together into linear groves, several trees wide and 100 m or more long. The groves were situated more or less along contour lines. The intergrove areas were usually several times wider than the groves, and in most places had a sparse ground cover of a perennial tussock grass, Eragrostis eriopoda Benth. Locally in the intergroves there were clumps of young mulga with individuals ranging in height from ~0.5 to 2.0 m. The phyllodes of the common variety of Acacia aneura present on the site were ~1–3 mm wide, 1 mm thick and 3–10 cm long.
Methods of collection
Phyllode traps were made by covering square wooden frames on one side with cotton mosquito netting. The wood was of cross-section ~2 × 2 cm and the interior dimensions of the resulting frames were 75 × 75 cm, giving a collection area of 0.5625 m2. The traps were made large enough, on the basis of a preliminary trial, for there usually to be measurable amounts of phyllode material (>0.01 g) fallen in 1 or 2 weeks and yet not so large that they were difficult to install flat on the usually heterogeneous ground surface near the bases of mulga trees. The frames were laid out flat so that the mosquito netting was in contact with the underlying soil surface. The latter had previously been cleared by carefully removing litter from the immediate area to be covered by the frames; otherwise minimal disturbance was caused in the vicinity of each trap.
Placement of traps
On 10 June 1958, 12 traps were placed under mulga in groves within a fenced 5 ha experimental area (hereafter termed the ‘mature-1’ site) 2.6 km north of the bore. Rainfall was measured daily at this site. Positions for traps were chosen subjectively to meet the following requirements: adequate representation within the groves present on the site; reasonably dense foliage vertically above each trap; minimum obvious interference with any instruments already installed (e.g. rain gauges; see Slatyer 1965). The canopy cover at the ‘mature-1’ site was ~20% overall and soils were red earth.
On 30 September 1958, an additional 15 traps were set out as follows.
-
Five traps under young mulga (≤2 m tall) in thickets located in intergroves within the ‘mature-1’ site, hereafter called the ‘young’ location.
-
Three traps under individuals of the ‘desert form’ of Acacia aneura (more recently identified as Acacia minyura Randell) within the ‘mature-1’ site, hereafter the ‘desert form’ location. There were several individuals of this variety located in the intergroves. The variety was characterised by phyllodes 3 to 4 mm wide, ~1 mm thick, 1 to 2 cm long, broader and lighter in colour than the common variety. It usually had a shrub growth habit.
-
Seven traps under parts of two groves contained in a 0.8 ha fenced site (hereafter the ‘mature-2’ site) that had been heavily grazed by horses previously and was ~3 km from the ‘mature-1’ site. This site was in a shallow un-incised drainage line and soils were loamier that those at the ‘mature-1’ site. Canopy cover of mulga was <20% across the ‘mature-2’ site.
Collections were made regularly from all traps until 12 April 1960.
Treatment of collected phyllodes
For most of the collection period, fallen phyllodes were gathered from the traps every 2 weeks. After the first 9 months it became evident that the rate of fall of phyllodes increased greatly in the first week or two after a substantial fall of rain. In consequence, the measurements were intensified to ~weekly collections at such times to get more detailed insight into phyllode fall dynamics.
At collection times, the phyllodes were gathered into paper envelopes, one envelope per trap. The samples were dried overnight at 105°C and weighed. The oven-dried phyllodes were stored in air-tight jars until chemical analyses were carried out. No collection was made of any flowers, fruits or branches which fell into the traps.
Chemical analyses of nitrogen, phosphorus, potassium, calcium, magnesium and sodium content were conducted on finely ground phyllode material. Citations for analytical methods for specific nutrients are unknown but see Williams and Twine (1967) for representative methodologies. The chemical analyses were limited to phyllodes from the ‘mature-1’ and ‘mature-2’ locations, because there was insufficient material from the ‘young’ and ‘desert form’ locations.
Nitrogen was determined by the Kjeldahl method, using concentrated sulfuric acid and potassium sulfate for the digestion, together with selenium as a catalyst. Phosphorus was determined colourimetrically at 400 nm by means of the molybdate-vanadate yellow colour complex. The aliquot for colouring-up was taken from a triple acid digest containing perchloric, sulfuric and nitric acids. The other elements were determined from an HCl extract of the ash from ~2 g of the ground material. The ignition took place in a muffle furnace at ~500°C. Calcium was determined by titration of a precipitate of the oxalate with standard potassium permanganate solution. Magnesium was determined on the filtrate from the calcium oxalate by precipitation as magnesium ammonium phosphate, solution of the washed precipitate in dilute HCl and colourimeteric determination of the phosphate. Potassium and sodium were determined using an EEL flame photometer.
Data analyses
We calculated average phyllode fall by collection period (n = 63 for the ‘mature-1’ location), for the four locations (‘mature-1’, ‘young’, ‘desert form’, ‘mature-2’). Daily on-site rainfall measurements were plotted against the phyllode-fall time series. Tests for significant relationships between phyllode fall and rainfall were not used due to the inconsistent timeframes of phyllode fall sampling (see ‘Treatment of collected phyllodes’). For instance, comparing weekly with biweekly data would have violated the comparability of the observations. We linked phyllode fall and nutrient data for the ‘mature-1’ and ‘mature-2’ locations and used segmented regression to detect breakpoints in the data. We used paired t-tests to check for significant differences between locations and treatments, after excluding the ‘mature-1’ data collected before inclusion on 30 September 1958 of the ‘mature-2’ site. The data used in these analyses are presented in the Supplementary material Table S1, available at the journal’s website. All analyses and visualisations were computed using the R language and environment (R Development Core Team 2016).
Results
Phyllode fall
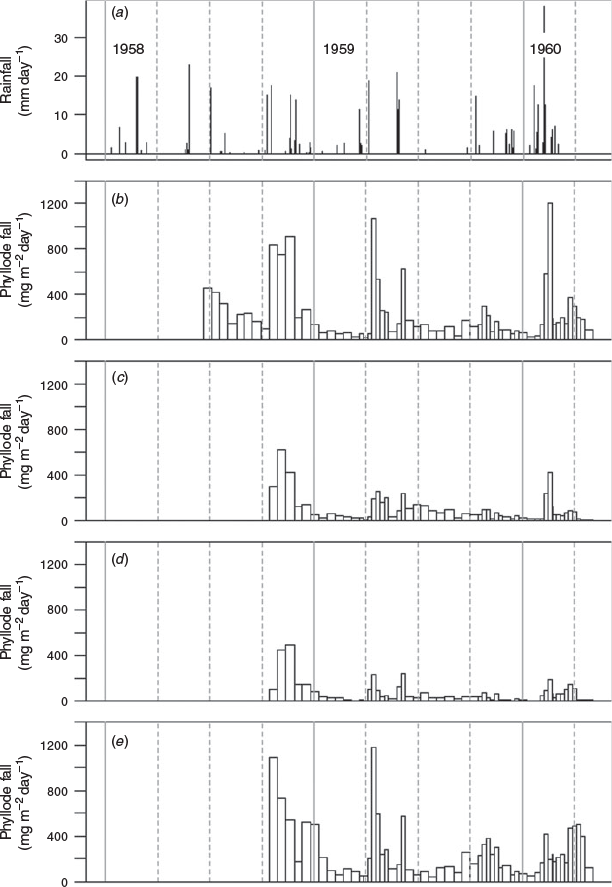
There was a close association between substantial falls of rain and onset of increased mean rates of phyllode fall of up to an order of magnitude (Fig. 1a, b). Rainfalls of ~5 mm or less in the interval between collections produced no discernible effect on the rate of phyllode fall in the ‘mature-1’ location. Six small falls of ~1–6 mm, totalling 26.9 mm and occurring between 23 November 1959 and 7 December 1959, were also ineffective. An isolated fall of 14.7 mm on 3 October 1959 was followed by an approximate doubling of the rate of phyllode fall at the ‘mature-1’ location in the next week. However, in March 1959 a total of 15.9 mm of rain over three successive days (11.4, 2.5 and 2.0 mm) produced no appreciable change in the pattern of phyllode fall. Fourteen days later on 30 March 1959 when another 18.8 mm fell, there was an order of magnitude increase in the rate of phyllode fall at the ‘mature-1’ location during the following week. It seems likely that the soil had retained sufficient moisture from the earlier event to supplement that from the 30 March fall.
|
The results suggest that ~15–20 mm is the minimum rainfall necessary for a major phyllode fall (defined as an order of magnitude or greater increase in rate) following several months of very little rain.
Examples of a time lag between rain and an increase in the rate of phyllode fall are apparent (Fig. 1) in late March–early April 1959, late May 1959, early October 1959, and mid to late January 1960. Taken together, these cases suggest a lag of about a week or slightly less.
The length of time between successive substantial rainfalls may affect the pattern of phyllode fall. With two such rains less than 2 weeks apart, as in mid to late January 1960, the rate of phyllode fall, already increased by an order of magnitude after the first rain, increased still further after the second rain (Fig. 1b–e). Lesser second peaks were apparent in April to May 1959, with six weeks between successive falls (Fig. 1b, c, e). The ‘young’ and ‘desert form’ mulga had only about half of the ‘mature-1’ phyllode fall, while the amount or timing of phyllode fall at the ‘mature-2’ location (Fig. 1e) was similar to that at the ‘mature-1’ location. For the whole observation period, the phyllode falls at the ‘young’ and ‘desert form’ locations were slightly different from one another using paired t-tests (P = 0.003), whereas the ‘mature-1’ and ‘mature-2’ locations were not significantly different (P = 0.174).
Nutrient analyses
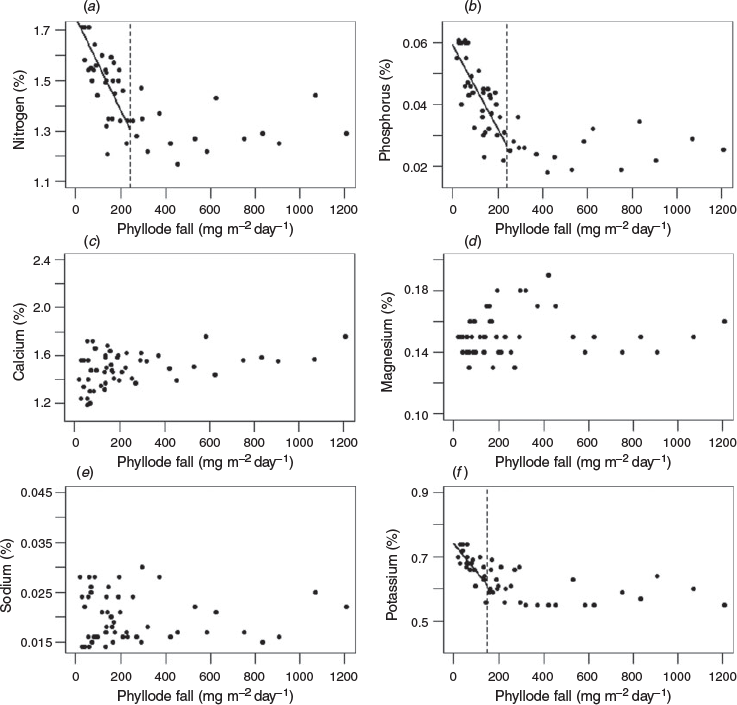
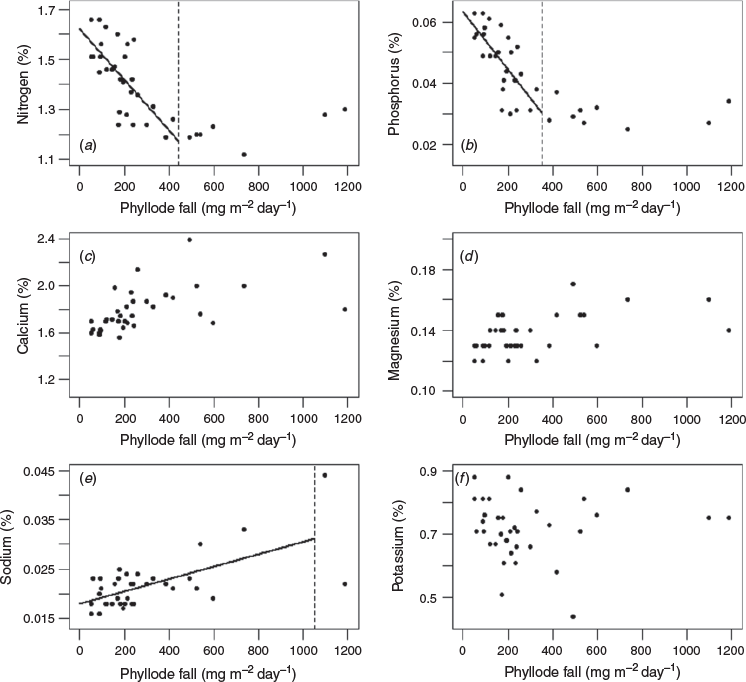
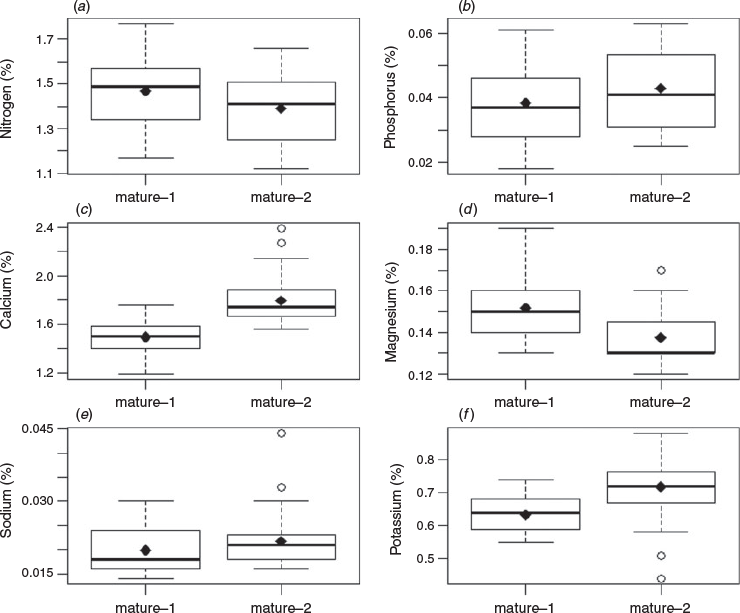
Significant inverse relationships between the rate of phyllode fall and concentrations of nitrogen and phosphorus in the phyllodes were identified for both ‘mature-1’ and ‘mature-2’ locations (Figs 2a, b, 3a, b) up to a value of ~200 and ~400 mg m−2 day−1 respectively. A significant inverse relationship between the rate of phyllode fall and concentration of potassium in the phyllodes was also found but only for the ‘mature-1’ location, whereas there was a positive relationship between the rate of phyllode fall and concentration of sodium in the phyllodes in the ‘mature-2’ location only. For some nutrient concentrations (nitrogen, calcium, magnesium and potassium) a paired t-test showed significant differences (P < 0.001) between the two locations (Fig. 4). The greatest differences in absolute terms were for potassium and calcium and both were larger at the ‘mature-2’ location (Fig. 4).
|
|
Discussion
Phyllode fall
At the time of this study, R. O. Slatyer was also working at the ‘mature-1’ location. He reported (Slatyer 1962) that 3–4 days after a rainfall of at least 13.7 mm, the mulga phyllodes reached maximum values of relative water content. Thus, the sharp increases in the rate of phyllode fall observed soon after substantial rainfall in this study (Fig. 1) probably occurred because Acacia aneura lost its dormancy induced by low soil moisture and began active growth. It is likely that older phyllodes were shed as soon as shoot elongation and new phyllode enlargement began. A brief delay between the stimulus of plentiful soil moisture and the resultant response of resumption of active metabolism is to be expected, given Slatyer’s (1962) results. Both Wilcox (1960, 1974) in Western Australia and Winkworth (1973) in central Australia also observed peak rates of mulga phyllode fall after substantial rain. Mulga litterfall in Queensland, on the other hand, appeared to be independent of season and rainfall (Burrows 1976), although the 4-weekly sampling regime used in Burrows’ study may have been insufficient to detect short-term rainfall responses. Annual average rainfall at Burrows’ (1976) mulga site was 75% higher than that at Alice Springs (see Nix and Austin 1973 for a bioclimatic analysis of the mulga zone) and rainfall during his study was above average, in contrast to this study, where rainfall over the phyllode-collection period was below the long-term average.
We noted differences in phyllode fall between the four locations, in terms of magnitude and timing (Fig. 1b–e). The initiation of a major fall of phyllodes will likely be dependent on variables such as age of trees, efficiency of the branches in promoting trunk flow of water, root distribution, season and local soil permeability (e.g. Slatyer 1962, 1965). Some of these factors will influence soil moisture availability and others will affect the tree’s ability to take up water.
Slatyer’s (1962) results also indicated that, if rain fell again a month or so later, the trees would still be growing slowly or perhaps just entering growth dormancy. A high proportion of their phyllodes would probably have been produced in response to the earlier rainfall and be relatively young. This could explain why a secondary peak in the rate of phyllode fall was less than the peak which followed the first rain after a dry period, in this study (e.g. April–May 1959; Fig. 1). Mulga can also shed phyllodes under severe drought conditions, which may lead to the death of the plants (D. J. Nelson, pers. comm.). These conditions were not experienced during this study but prevailed in subsequent years to 1966 (see Marsden-Smedley et al. 2012 for the definition of drought).
There were some anomalous results. One is the heavy fall of phyllodes in the ‘mature-1’ location after rains in early October 1958 (Fig. 1), which appeared to peak about a month after the rain whereas later episodes of heavy phyllode fall lasted for only a week or two. This result may be an artefact of the 2-weekly sampling regime used at that time. The second apparent departure from the commonly observed pattern occurred in March 1960, when the rate of phyllode fall rose to a minor peak at all four locations about a month after the last major rain. There was a general flowering of mulga at that time and some additional phyllode shedding may have been associated with reproductive processes. The peak phyllode fall in late October–early November 1958 may also have been associated with flowering but there were no phenological records being kept at the time.
Our results and the available literature suggest that major phyllode falls after heavy rains were a physiological response of mulga to increased soil water availability. When the plants were in growth dormancy it is likely that loss of phyllodes was accidental and physically caused (e.g. detachment by wind, birds, insects). In that case, there would have been no physiological trigger to initiate withdrawal of nutrients and thus during dormancy nitrogen and phosphorus reached their highest observed concentrations (see Figs 2, 3). We can hypothesise that during the declining phase of major phyllode fall episodes, as soil moisture stress gradually increased, some phyllodes were still being shed under physiological control, enabling withdrawal of some nitrogen and phosphorus, whereas others were being lost by physical agency without nutrient withdrawal.
Nutrient cycling
It is generally recognised that nitrogen and phosphorus are physiologically mobile when leaves are shed (Crockford and Richardson 1998; Drenovsky et al. 2019). Potassium can also be mobile (Ruck and Gregory 1955; Stenlid 1958; Guha and Mitchell 1966; Turner and Lambert 1983). However, in this study potassium withdrawal was only apparent at the ‘mature-1’ location (Figs 2a, 4). Burrows (1976) reported the withdrawal of 50–60% of phosphorus before abscission in mallee eucalypts and mulga and withdrawal of ~50% of nitrogen in mallee but only ~25% in mulga. Burrows (1976) proposed that the leguminous nitrogen-fixing mulga did not require tight intrinsic cycling of nitrogen and instead relied on an efficient extrinsic cycle. Since nutrient content of phyllodes before abscission was not measured in our study we cannot explore the point further.
Accumulation of calcium and magnesium in physiologically shed leaves is widely reported (Ruck and Gregory 1955; Stenlid 1958; Guha and Mitchell 1966; Thomas 1969; Turner and Lambert 1983) but there was no such evidence in our data (Figs 2, 3). We noted significantly more calcium and potassium and significantly less magnesium in phyllodes from the ‘mature-2’ location compared with those from the ‘mature-1’ location (Fig. 4). Sodium was the only nutrient that increased in concentration with increasing phyllode fall for the ‘mature-2’ location and thus showed a contrasting behaviour to the main nutrients, nitrogen, phosphorus and potassium (Fig. 3). This result had the lowest coefficient of determination and may simply be attributable to site differences and-or past grazing, in the absence of any replication.
Phyllode fall at rates exceeding ~200 mg m−2 day−1 accounted for ~71% by weight of the total fall over the entire measurement period at the ‘mature-1’ location. For rates of phyllode fall exceeding ~200 and ~400 mg m−2 day−1 at the ‘mature-2’ location, the proportion by weight of the total fall over the entire measurement period was ~74 and ~47% respectively. The substantial withdrawal of nitrogen (~30%) and phosphorus (~50%) at times of high rates of phyllode fall (see Figs 2, 3) suggests that the mulga tree is markedly conservative of the available nitrogen and phosphorus resources.
Carbon cycling
Low carbon flux of mulga of ~149 mg C m−2 day−1 (assuming 50% carbon content and observed average phyllode fall 297 mg m−2 day−1) results in ~54 g C m−2 transferred from canopies to the ground every year within the groves of the ‘mature-1’ site. We note that this is considerably less than mean leaf litterfall observed in other dryland vegetation of 149 g C m−2 year−1, assuming 50% carbon content (Holland et al. 2015). Intergroves have considerably less canopy cover and, based on our data for ‘desert form’ mulga (Fig. 1), the phyllode fall in intergroves is ~36% (106 mg m−2 day−1) compared with mature mulga in groves. The assumption of 20% overall canopy cover reduces the carbon flux due to phyllode fall in this central Australian mulga community to 11 g C m−2 year−1 under dry conditions (annual rainfall 194 mm in 1958 and 140 mm in 1959). Phyllode fall measured in this study represents ~17% of the 64 g C m−2 year−1 net primary production (i.e. carbon allocated into plant biomass) calculated with the BIOS2 modelling environment. BIOS2 combines meteorological, soil and vegetation to derive carbon and water fluxes across Australia and is used for calculating the Australian carbon budget (Raupach et al. 2001; Haverd et al. 2013). Linking data on phyllode fall observed in this study with measurements of grass and herbaceous production, above and below ground tree growth and mortality would capture the main carbon fluxes in mulga and enable validation of the BIOS2 results, that to date are largely unchecked for mulga ecosystems. Our results and the available literature suggest that mulga through phyllode shedding has considerable but variable effects on terrestrial carbon storage and cannot be ignored in national or global accounting, given that mulga systems cover ~150 million ha (Silcock et al. 2016). Expected reductions in the present lengthy fire interval of 20–100 years common for mulga communities (Murphy et al. 2013) will need to be incorporated in future estimates of carbon fluxes and nutrient cycling in mulga.
Despite being collected over six decades ago now, this legacy data gave us new insights into arid mulga ecosystems. This would not have been possible without the ‘off-site’ storage of data sheets and records by the lead author and his willingness to make this hard-won data accessible to the scientific community.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
J. C. Turner gratefully acknowledges the work of D. J. Nelson, D. N. McCowan, D. Hobby, F. Bichler, W. Edwards, G. Hogben, G. Malimonenko, and K. Bunn for the collection and weighing of phyllodes; Mrs A. Komarowski for the preparation of samples for analysis; Mrs I. M. Sergeant for the analyses of nitrogen and phosphorus; L. R. Murray, V. A. Lee and Mrs Ellis for the analyses for potassium, calcium, magnesium and sodium; R. A. Perry, R. E. Winkworth and Professor R. O. Slatyer for their criticism of an original manuscript and for provision of technical services. M. H. Friedel and M. Neumann greatly appreciate John Turner’s and his wife Ruby’s help in rediscovering the original data and supporting us to bring it to publication. We are also most grateful to Des Nelson and his diaries for clarifying locations and events for us, and for general assistance. We thank Ron Hacker, Mark Adams, and anonymous reviewers for constructive comments on earlier drafts of this paper. M. H. Friedel appreciates the support of Charles Darwin University and M. Neumann acknowledges support by the Austrian Science Fund (FWF), Grant No. J4211-N29.
References
Adams, M. A., and Attiwill, P. M. (1991). Nutrient balance in forests of northern Tasmania. 1. Atmospheric inputs and within-stand cycles. Forest Ecology and Management 44, 93–113.| Nutrient balance in forests of northern Tasmania. 1. Atmospheric inputs and within-stand cycles.Crossref | GoogleScholarGoogle Scholar |
Bray, J. R., and Gorham, E. (1964). Litter production in forests of the world. Advances in Ecological Research 2, 101–157.
| Litter production in forests of the world.Crossref | GoogleScholarGoogle Scholar |
Bureau of Meteorology (2005). Climate classification maps. Available at: http://www.bom.gov.au/jsp/ncc/climate_averages/climate-classifications/index.jsp?maptype=seasb (accessed 9 November 2020).
Bureau of Meteorology (2020). Climate data online. Available at: http://www.bom.gov.au/climate/data/ (accessed 11 November 2020).
Búrquez, A., Martínez-Yrízar, A., and Núñez, S. (1999). Sonoran Desert productivity and the effect of trap size on litterfall estimates in dryland vegetation. Journal of Arid Environments 43, 459–465.
| Sonoran Desert productivity and the effect of trap size on litterfall estimates in dryland vegetation.Crossref | GoogleScholarGoogle Scholar |
Burrows, W. H. (1976). Aspects of nutrient cycling in semi arid mallee and mulga communities. PhD thesis, Australian National University, Canberra, ACT, Australia.
Burrows, W. H., Henry, B. K., Back, P. V., Hoffmann, M. B., Tait, L. J., Anderson, E. R., Menke, N., Danaher, T., Carter, J. O., and McKeon, G. M. (2002). Growth and carbon stock change in eucalypt woodlands in northeast Australia: ecological and greenhouse sink implications. Global Change Biology 8, 769–784.
| Growth and carbon stock change in eucalypt woodlands in northeast Australia: ecological and greenhouse sink implications.Crossref | GoogleScholarGoogle Scholar |
Crockford, R. H., and Richardson, D. P. (1998). Litterfall, litter and associated chemistry in a dry sclerophyll eucalypt forest and a pine plantation in south-eastern Australia: 2. Nutrient recycling by litter, throughfall and stemflow. Hydrological Processes 12, 385–400.
| Litterfall, litter and associated chemistry in a dry sclerophyll eucalypt forest and a pine plantation in south-eastern Australia: 2. Nutrient recycling by litter, throughfall and stemflow.Crossref | GoogleScholarGoogle Scholar |
Drenovsky, R. E., Pietrasiak, N., and Short, T. H. (2019). Global temporal patterns in plant nutrient resorption plasticity. Global Ecology and Biogeography 28, 728–743.
Du, E., Terrer, C., Pellegrini, A. F. A., Ahlström, A., van Lissa, C. J., Zhao, X., Xia, N., Wu, X., and Jackson, R. B. (2020). Global patterns of terrestrial nitrogen and phosphorus limitation. Nature Geoscience 13, 221–226.
| Global patterns of terrestrial nitrogen and phosphorus limitation.Crossref | GoogleScholarGoogle Scholar |
ERF (2020). Emission Reduction Fund. Available at: http://www.cleanenergyregulator.gov.au/ERF (accessed 16 November 2020).
Fensham, R. J., Fairfax, R. J., and Dwyer, J. M. (2012). Potential aboveground biomass in drought-prone forest used for rangeland pastoralism. Ecological Applications 22, 894–908.
| Potential aboveground biomass in drought-prone forest used for rangeland pastoralism.Crossref | GoogleScholarGoogle Scholar | 22645819PubMed |
Guha, M. M., and Mitchell, R. L. (1966). The trace and major element composition of the leaves of some deciduous trees. II. Seasonal changes. Plant and Soil 24, 90–112.
| The trace and major element composition of the leaves of some deciduous trees. II. Seasonal changes.Crossref | GoogleScholarGoogle Scholar |
Hart, D. M. (1995). Litterfall and decomposition in the Pilliga State Forests, New South Wales, Australia. Australian Journal of Ecology 20, 266–272.
| Litterfall and decomposition in the Pilliga State Forests, New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Hatch, A. B. (1955). ‘The influence of plant litter on the jarrah forest soils of the Dwellingup region, Western Australia. Leaflet No. 70.’ (Forestry and Timber Bureau: Canberra, ACT, Australia.)
Haverd, V., Raupach, M. R., Briggs, P. R., Canadell, J. G., Isaac, P., Pickett-Heaps, C., Roxburgh, S. H., Van Gorsel, E., Viscarra Rossel, R. A., and Wang, Z. (2013). Multiple observation types reduce uncertainty in Australia’s terrestrial carbon and water cycles. Biogeosciences 10, 2011–2040.
| Multiple observation types reduce uncertainty in Australia’s terrestrial carbon and water cycles.Crossref | GoogleScholarGoogle Scholar |
Holland, E. A., Post, M. W., Matthews, E., Sulzman, J., Staufer, R., and Krankina, O. (2015). ‘A global database of litterfall mass and litter pool carbon and nutrients.’ (Oak Ridge National Laboratory, Distributed Active Archive Center: Oak Ridge, Tennessee, USA.)
Liu, C., Westman, C. J., Berg, B., Kutsch, W., Wang, G. Z., Man, R., and Ilvesniemi, H. (2004). Variation in litterfall-climate relationships between coniferous and broadleaf forests in Eurasia. Global Ecology and Biogeography 13, 105–114.
| Variation in litterfall-climate relationships between coniferous and broadleaf forests in Eurasia.Crossref | GoogleScholarGoogle Scholar |
Marsden-Smedley, J. B., Albrecht, D., Allan, G. E., Brock, C., Duguid, A., Friedel, M., Gill, A. M., King, K. J., Morse, J., Ostendorf, B., and Turner, D. (2012). Vegetation–fire interactions in central arid Australia: towards a conceptual framework. Ninti One Research Report NR001. Ninti One Limited, Alice Springs, NT, Australia.
McColl, J. G. (1966). Accession and decomposition of litter in spotted gum forests. Australian Forestry 30, 191–198.
| Accession and decomposition of litter in spotted gum forests.Crossref | GoogleScholarGoogle Scholar |
McIvor, J. G. (2001). Litterfall from trees in semiarid woodlands of north-east Queensland. Austral Ecology 26, 150–155.
| Litterfall from trees in semiarid woodlands of north-east Queensland.Crossref | GoogleScholarGoogle Scholar |
Murphy, B. P., Bradstock, R. A., Boer, M. M., Carter, J., Cary, G. J., Cochrane, M. A., Fensham, R. J., Russell-Smith, J., Williamson, G. J., and Bowman, D. M. J. S. (2013). Fire regimes of Australia: a pyrogeographic model system. Journal of Biogeography 40, 1048–1058.
| Fire regimes of Australia: a pyrogeographic model system.Crossref | GoogleScholarGoogle Scholar |
Neumann, M., Ukonmaanaho, L., Johnson, J., Benham, S., Vesterdal, L., Novotný, R., Verstraeten, A., Lundin, L., Thimonier, A., Michopoulos, P., and Hasenauer, H. (2018). Quantifying carbon and nutrient input from litterfall in European forests using field observations and modeling. Global Biogeochemical Cycles 32, 784–798.
| Quantifying carbon and nutrient input from litterfall in European forests using field observations and modeling.Crossref | GoogleScholarGoogle Scholar |
Neumann, M., Turner, J., Lewis, T., McCaw, L., Cook, G., and Adams, M. (in press). Dynamics of necromass in woody Australian ecosystems. Ecosphere , .
Nix, H. A., and Austin, M. P. (1973). Mulga: a bioclimatic analysis. Tropical Grasslands 7, 9–21.
Pook, E. W., Gill, A. M., and Moore, P. H. R. (1997). Long-term variation of litter fall, canopy leaf area and flowering in a Eucalyptus maculata forest on the south coast of New South Wales. Australian Journal of Botany 45, 737–755.
| Long-term variation of litter fall, canopy leaf area and flowering in a Eucalyptus maculata forest on the south coast of New South Wales.Crossref | GoogleScholarGoogle Scholar |
Poulter, B., Frank, D., Ciais, P., Myneni, R. B., Andela, N., Bi, J., Broquet, G., Canadell, J. G., Chevallier, F., Liu, Y. Y., Running, S. W., Sitch, S., and Van Der Werf, G. R. (2014). Contribution of semi-arid ecosystems to interannual variability of the global carbon cycle. Nature 509, 600–603.
| Contribution of semi-arid ecosystems to interannual variability of the global carbon cycle.Crossref | GoogleScholarGoogle Scholar | 24847888PubMed |
R Development Core Team (2016). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at: https://www.R-project.org/ (accessed 14 April 2021).
Raupach, M. R., Kirby, J. M., Barrett, D. J., and Briggs, P. R. (2001). Balances of water, carbon, nitrogen and phosphorus in Australian landscapes: (1) Project description and results. CSIRO Land and Water Technical Report 40/01. CSIRO Land and Water, Canberra, ACT, Australia.
Ruck, H. C., and Gregory, F. G. (1955). Mobility of manganese, magnesium and potassium in leaf tissues. Nature 175, 378–379.
Silcock, J. L., Witt, G. B., and Fensham, R. J. (2016). A 150-year fire history of mulga (Acacia aneura F. Muell. ex Benth.) dominated vegetation in semiarid Queensland, Australia. The Rangeland Journal 38, 391–415.
| A 150-year fire history of mulga (Acacia aneura F. Muell. ex Benth.) dominated vegetation in semiarid Queensland, Australia.Crossref | GoogleScholarGoogle Scholar |
Slatyer, R. O. (1962). Internal water balance of Acacia aneura F. Muell. in relation to environmental conditions. UNESCO Arid Zone Research 16, 137–146.
Slatyer, R. O. (1965). Measurement of precipitation interception by an arid zone plant community (Acacia aneura F.Muell.). UNESCO Arid Zone Research 25, 181–192.
Stenlid, G. (1958). Salt losses and redistribution of salts in higher plants. Encyclopedia of Plant Physiology 4, 615–637.
| Salt losses and redistribution of salts in higher plants.Crossref | GoogleScholarGoogle Scholar |
Thomas, W. A. (1969). Accumulation and cycling of calcium by dogwood trees. Ecological Monographs 39, 101–120.
| Accumulation and cycling of calcium by dogwood trees.Crossref | GoogleScholarGoogle Scholar |
Turner, J., and Lambert, M. J. (1983). Nutrient cycling within a 27-year-old Eucalyptus grandis plantation in New South Wales. Forest Ecology and Management 6, 155–168.
| Nutrient cycling within a 27-year-old Eucalyptus grandis plantation in New South Wales.Crossref | GoogleScholarGoogle Scholar |
Wilcox, D. G. (1960). Studies in the mulga pastoral zone. 2. Some aspects of the value of mulga scrub. Journal of the Department of Agriculture, Western Australia, Series 4 1, 581–586.
Wilcox, D. G. (1974). ‘Morphogenesis and Management of Woody Perennials in Australia.’ US Department of Agriculture Miscellaneous Publication 1271, Paper 6, pp. 60–71. (USDA: Washington, DC, USA.)
Williams, C. H., and Twine, J. R. (1967). Determinations of nitrogen, sulphur, phosphorus, potassium, sodium, calcium and magnesium in plant material by automatic analysis. Technical Paper No. 24. Division of Plant Industry, CSIRO, Canberra, ACT, Australia.
Winkworth, R. E. (1973). Eco-physiology of mulga (Acacia aneura). Tropical Grasslands 7, 43–48.