The impact of tree removal on standing grass biomass, seedling establishment and growth of woody species
P. Monegi A B * , N. R. Mkhize A B , T. J. Tjelele A , D. Ward C and Z. Tsvuura B
A B * , N. R. Mkhize A B , T. J. Tjelele A , D. Ward C and Z. Tsvuura B
A Agricultural Research Council, Animal Production, Range and Forage Sciences, Irene 0062, South Africa.
B School of Life Sciences, University of KwaZulu-Natal, Scottsville 3209, South Africa.
C Department of Biological Sciences, Kent State University, Kent, OH 44240, USA.
The Rangeland Journal 44(1) 25-32 https://doi.org/10.1071/RJ21003
Submitted: 19 January 2021 Accepted: 11 February 2022 Published: 6 April 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of the Australian Rangeland Society. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
The removal of trees in rangelands can create gaps and lead to increased grass production that could suppress subsequent tree seedling establishment and growth. However, gaps can also enhance the growth of remaining trees. We conducted a field experiment at two savanna sites with different soil texture and woody species. We used 24 plots at each site to determine the effect of tree-removal intensities (0%, 10%, 20%, 50%, 75% and 100%) on grass production, tree-seedling establishment and growth, and growth of the remaining large trees. Site 1 was on previously cultivated severely-eroded clay-dominated soils, encroached by a monospecific stand of Vachellia tortilis. Site 2 had never been cultivated, and was on sandy soils with several woody species. At Site 1, 75 and 100% tree removal significantly reduced standing grass biomass towards the end of the first growing season, with no differences towards the end of the second season. At Site 2, tree removal significantly increased standing grass biomass. There was no significant effect of tree removal on tree seedling establishment at Site 1, but at Site 2 tree removal had a significantly negative effect on overall tree seedling establishment. At both sites, there were no significant differences in tree seedling growth. Moderate (50%) to high (75%) removal of trees had a positive effect on the growth of remaining large trees at both study sites. We found that tree seedling establishment could be affected by the level of grass biomass following tree removal, but other factors including soil erosion are also important considerations. Reduced tree competition facilitates growth of remaining large trees. An implication of these findings is that, regardless of the substantial costs of woody plant control, the recovery of key ecosystem services such as an increased forage production may not be realised. However, we recognise that this may be system-specific.
Keywords: forage production, grass competition, rangeland management, restoration, soil erosion, tree clearing, tree competition, woody plant encroachment.
Introduction
Interactions among mature trees play a significant role in structuring savannas (Meyer et al. 2007; Schleicher et al. 2011a). Although these interactions can either reduce or facilitate woody plant encroachment (Meyer et al. 2008; Pillay and Ward 2012), in savanna rangelands (Jeltsch et al. 2000), these interactions may lead to woody plant encroachment. Given the negative effects of woody plant encroachment on pastoral productivity, ecologists and land users have often considered tree removal (also termed tree thinning) as a management option (Smit 2005; Ndhlovu et al. 2016). High tree densities in savannas may negatively affect tree growth because of competition among woody species (Kambatuku et al. 2011a; Pillay and Ward 2012). Competition among woody species is associated with a reduction in the size of one or more neighbours (Meyer et al. 2007). However, the removal of some trees may result in a substantial increase in the size of remaining individuals (Smit 2001; Schleicher et al. 2011b). Moreover, increased woody plant size can also benefit rangelands by increasing understorey grass and forb biomass because of increased water availability and/or nutrient content below canopies (Treydte et al. 2008; Schleicher et al. 2011b).
In savannas, large trees have been reported to limit tree seedling establishment by outcompeting seedlings for resources (Loth et al. 2005; Brudvig and Asbjornsen 2009), so tree removal can promote tree seedling establishment and growth (Kambatuku et al. 2011a; Smit 2014). Tree removal in rangelands can open the canopy while maintaining a pool of recruits to replace large, older trees when they die (Schnitzer et al. 2001; Smit 2014). However, gaps created by tree removal can increase grass production (Beale 1973; Sagar et al. 2012), which negatively affects tree seedling germination, survival and growth (Kambatuku et al. 2011b; Grellier et al. 2012). Increased grass biomass is expected to reduce tree seedling establishment similarly to tree establishment. Evidence suggests that Vachellia seeds do not germinate under Vachellia trees (Loth et al. 2005). The suppressive effect of grass competition has also been reported to affect larger trees (Riginos 2009). Regardless, there is considerable variance in this relationship; some studies have found that grasses facilitate tree seedling survival and growth (Duncan and Chapman 2003; Tomlinson et al. 2019), whereas others have found non-significant effects of grasses on tree seedling performance (Scariot et al. 2008).
The effects of tree removal can differ within similar environments (Archer and Predick 2014), with site-specific drivers such as plant species and soils perhaps responsible for these variations (Ding and Eldridge 2019). Few studies have compared the response of rangelands to removal of different tree species, which leaves open whether differences in tree species’ traits influence the ecological or management outcomes of removal (Ding and Eldridge 2019). For example, multi-specific stands usually have a higher productivity and tree density compared with monospecific stands because monospecific stands are often associated with more intense self-thinning, resulting in a lower tree density (Pretzsch 2014). Mixed tree species may also improve resource use compared with monospecific stands by improving resource supply and capture (Forrester 2015).
Soil texture can also affect plant growth. Because water extraction is more difficult from clayey than sandy soils, particularly at low soil moisture contents (Fensham et al. 2015), soil texture could alter tree and grass physiological responses to soil moisture variability, aggravating water stress, suggesting that differences in plant species and soil texture could be important determinants of ecological services after tree removal.
We evaluated the effects of different intensities of tree removal on standing grass biomass, seedling establishment and growth, and the growth of the remaining large trees at Roodeplaat farm, Gauteng Province, South Africa. We tested the following predictions:
standing grass biomass will increase with increasing tree removal because of reduced competition from woody plants;
increased grass biomass after tree removal will reduce subsequent tree seedling establishment; and
reduced tree competition through moderate (50%) and high (75%, 100%) tree removal will significantly increase tree seedling growth (stem diameter, height and canopy size) and the growth of remaining trees, whereas low removal intensities (i.e. 10% and 20%) will have no significant effect.
Materials and methods
Study area
The study was conducted at the Agricultural Research Council’s Roodeplaat experimental farm (25°56′S, 28°35′E) in Gauteng Province, South Africa. The natural vegetation component used for livestock and wild-herbivore production comprises approximately 2100 ha. The vegetation type is Marikana Thornveld (Mucina and Rutherford 2006), characterised by Vachellia karroo (Hayne) Banfi & Glasso (previously Acacia) and Senegalia (previously Acacia) caffra (Thunb.) P. J. H. Hurter & Mabb (Kyalangalilwa et al. 2013). The farm is also dominated by V. tortilis (Forssk.) Galasso & Banfi, Ziziphus mucronata (Willd.), and some Euclea species. Main grass species are Digitaria eriantha Steud, Panicum maximum Jacq, Setaria sphacelata Stapf & C. E. Hubb, Eragrostis curvula Schrad, Themeda triandra Forssk and Heteropogon contortus (L.) Roem. & Schult. Mean annual rainfall is 646 mm, largely falling between November and March. Minimum and maximum summer and winter temperature ranges are 20–29°C and 2–16°C respectively. The study area is situated on the Roodeplaat Igneous Complex (Panagos et al. 1998).
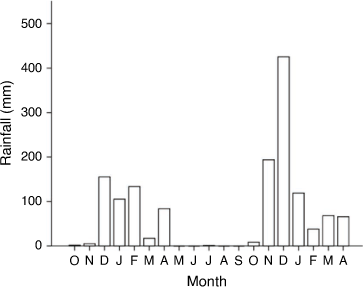
The study was conducted at two sites. The sites were selected on the basis of differences in tree species and soils. Site 1 was on clay-dominated soils (38% sand; 17% silt; 45% clay) characterised by severe soil degradation with surface erosion and crust formations. It occupied approximately 39.4 ha and had been under crop cultivation more than 20 years ago, but is now encroached by a monospecific stand of V. tortilis (mean density of 2961 plants ha−1). Site 2 was on sandy soils (67% sand; 16% silt; 17% clay) with several woody species (Dichrostachys cinerea (L.) Wight & Arn, S. caffra, V. karroo, V. nilotica (L.) P. J. H. Hurter & Mabb, V. robusta (Burch.) Kyalangalilwa & Boatwright, V. tortilis and Ziziphus mucronata). Site 2 (approximately 130.8 ha) was never cultivated, but was encroached at a mean density of 4065 plants ha−1. The sites were approximately 1.8 km apart and were fenced to exclude grazing animals during the study. Rainfall and temperature data were received from an accredited Agricultural Research Council’s Campus for Soil, Climate and Water (Fig. 1, Supplementary Fig. S1). Study duration was 18 months (two growing seasons), from October 2018 to April 2020.
|
Study design
Mechanical removal of trees in woody plant-encroached rangelands is expensive, and to obtain the most benefit at least cost, it may be worthwhile to use a gradient of levels of tree removal. Moreover, low tree-removal intensities could open the canopy for game viewing and continued use of the rangeland by browsing mammals. Accordingly, low removal intensities (10 and 20%) were included in the design.
At each site, 24 plots (30 m × 30 m) were established, separated by 5 m wide fire breaks. Tree removal treatments were replicated four times and randomly allocated to plots. Trees were cut with a chainsaw to the approximate removal equivalents of 0% (control, no removal), 10%, 20%, 50%, 75% and 100% (complete removal of trees) in October 2018 at the beginning of the wet season.
Standing grass biomass was assessed using five randomly -placed 50 cm × 50 cm quadrats in each plot and all biomass was harvested. Grass samples were collected towards the end of the wet season in March 2019 and 2020, ovendried at 70°C for 72 h and dry-matter yield was calculated.
All tree seedlings in each plot were counted before treatments were applied and at the end of the study period. To investigate effects of tree removal on the growth of tree seedlings and the large tree growth at Site 1, five seedlings and five large trees of V. tortilis from each plot were randomly marked and monitored over the two growing seasons. At Site 2, seven tree species (D. cinerea, S. caffra, V. karroo, V. nilotica, V. robusta, V. tortilis and Z. mucronata) were monitored (two seedlings and two large trees per species per plot). Seedlings were defined as pre-reproductive trees <1 m in height.
Tree and seedling growth was measured by recording height, canopy area (maximum and perpendicular lengths) and stem diameter at the beginning and end of the study. Seedling stem diameter was measured at the stem base; tree diameter was re-measured at a permanently marked point to minimise error. A flexible tape measure was used to measure large tree diameter and Vernier callipers for tree seedlings. Four healthy shoots on each tree from both the upper and lower canopy (Smit 2001) were randomly selected, permanently marked, and monitored for growth (length). Tree measurements (all trees were >2 m in height) were recorded at the beginning and end of the study. No trees marked for monitoring growth died during the study. Tree and seedling canopy sizes were calculated using an ellipse function (C = abπ/4.0), where ‘a’ = long axis and ‘b’ = perpendicular short axis of the canopy (Smith and Grant 1986). Plant growth rate was calculated using the equation:
Relative growth rate (RGR) = (ln W2 − ln W1)/(t2 − t1)
where W1 and W2, refer to log‐transformed plant measurements at times t1 and t2 (Hoffmann and Poorter 2002).
Data analysis
Data were log10 transformed to conform to ANOVA test assumptions. We used multivariate analysis of covariance (MANCOVA) to test the effects of tree removal on standing grass biomass, where grass biomass recorded after the first and the second growing seasons were considered the dependent variables. Standing grass biomass and tree density recorded before tree removal were used as covariates. We used MANCOVA to reduce Type 1 error caused by testing multiple dependent variables. Wilks’ lambda test statistic was used to investigate the effect of the removal treatments on the measured parameters. For significant MANCOVA results, we used univariate ANOVA to compare tree-removal densities. ANCOVA was used to test the effects of tree removal on seedling establishment, where the number of seedlings after tree removal was considered the dependent variable. The number of seedlings recorded before tree removal was used as a covariate. Tree and seedling stem diameter, height and canopy area (growth rates) were analysed using MANOVA. To determine the effects of tree removal on tree canopy shoot -growth, we used one-way ANOVA. A Bonferroni post hoc test was applied for pairwise comparisons among the removal treatments. Data from the two sites were analysed separately. IBM SPSS v. 26 (IBM 2019) was used for data analysis.
Results
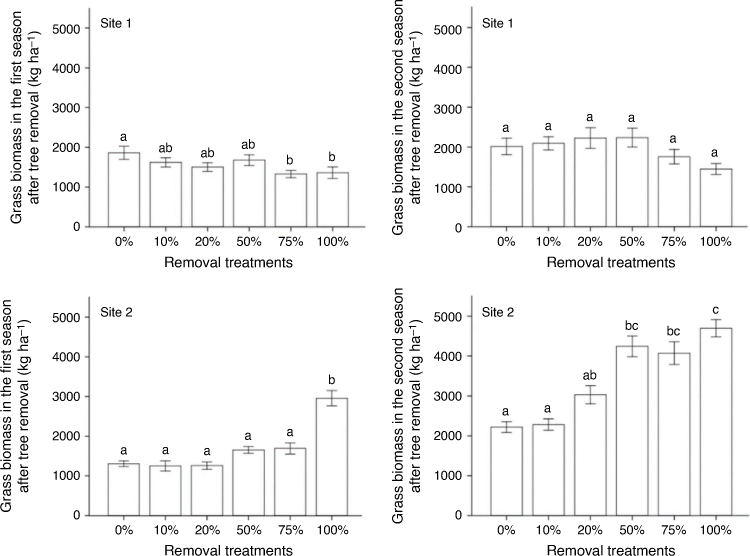
There were significant differences in standing grass biomass among tree-removal treatments at Site 1 (Wilks’ λ = 0.330; F = 2.223; P = 0.044; Fig. 2) only in the first growing season (F = 5.357; P = 0.004). A Bonferroni post hoc test showed that the control plots recorded greater grass biomass than did the 75% and 100% removal treatments. The grasses Digitaria eriantha (Steud.) and Sporobolus africanus (Poir.) Robyns & Tournay dominated Site 1.
At Site 2, tree removal significantly increased grass biomass at the end of both growing seasons (Wilks’ λ = 0.067; F = 8.624; P < 0.001). Standing grass biomass increased in the plots totally cleared of trees in the first growing season at Site 2 (F = 14.280; P = 0.001). Towards the end of the second growing season, grass biomass was higher than in the previous season across all treatments, with substantial increases at 50%, 75% and 100% removal (F = 7.713; P = 0.001). Grass biomass largely consisted of Panicum maximum and Setaria sphacelata var. sericea (Stapf) Clayton in the cleared plots.
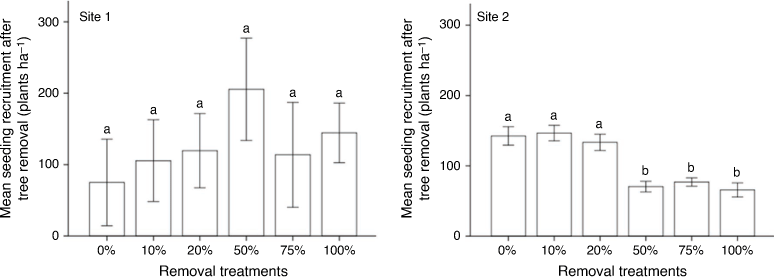
No significant (P > 0.05) differences in tree seedling establishment were recorded among treatment levels at either site before tree removal. The level of tree removal did not significantly (P > 0.05) influence tree seedling establishment at Site 1, but did at Site 2 (P < 0.05), with greatest reductions at 50%, 75% and 100% removal (Fig. 3). Seedling establishment after tree removal was significantly different between the 50–100% removed and the 0–20% removed at Site 2. However, there were no significant differences in seedling growth among treatment levels at either site (Wilks’ λ = 0.809; F = 1.406; P = 0.410 and Wilks’ λ = 0.878; F = 1.374; P = 0.156 for Sites 1 and 2 respectively).
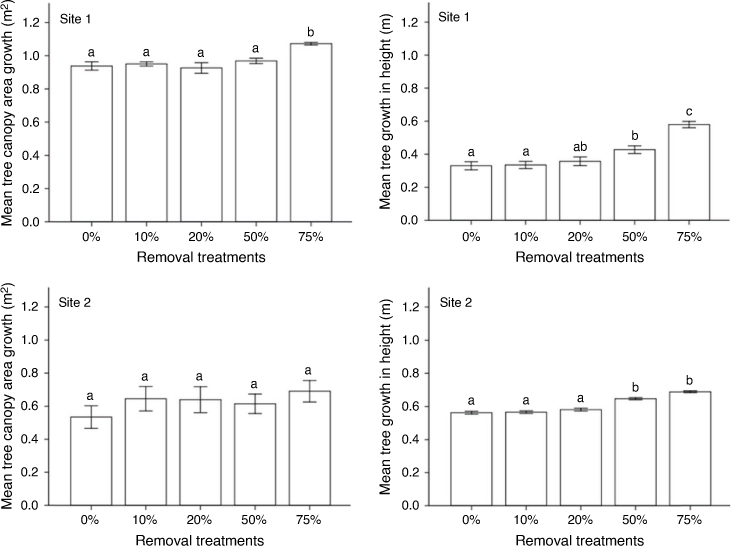
Significant differences in mean large tree growth were recorded among treatments at Site 1 (Wilks’ λ = 0.377; F = 8.956; P < 0.001; Fig. 4). A Bonferroni post hoc test indicated that only trees in the 75% removal treatment significantly increased in stem diameter, height and canopy area compared with other treatments. Large tree canopy-area results were supported by results for shoot growth, which showed that length of canopy shoots in the 75% removal treatment increased significantly more following tree removal than that in trees in other treatments (P = 0.001). Large trees in the 50% removal treatment showed a greater increase in canopy area and height than did those in the control (no removal). A significant increase in large-tree height was recorded at Site 2 in the 50% and 75% removal treatments (Wilks’ λ = 0.410; F = 14.594; P < 0.001), but not for canopy area (P = 0.639). At Site 2, growth in stem diameter was not significantly affected by tree-removal level (P = 0.147). The canopy-area results were supported by the canopy shoot-growth results that showed no significant differences among treatments (P = 0.856).
Discussion
The impact of tree removal on grass production
At Site 2, the increased standing grass biomass in response to tree removal was consistent with our prediction, whereas the diminished grass biomass at Site 1 could perhaps be explained by encroachment of the V. tortilis, which has been reported to enhance water infiltration and soil nutrients below rather than outside canopies (Ludwig et al. 2003; Abdallah et al. 2008; Yadeta et al. 2018). Therefore, canopy gaps created through tree removal may not be beneficial in increasing overall herbaceous biomass production in a V. tortilis monospecific stand. Recovery of herbaceous biomass may depend not only on the reduction in tree competition but also on other factors such as traits of the target species (Ding et al. 2020). In addition, site-specific characteristics such as elevated soil erosion during rainy events at Site 1 could help explain why grass biomass responded negatively. One of us (PM) observed severe soil erosion at Site 1 after rainy events during the study period, which may have been exacerbated by removal of vegetation cover.
The effects of tree removal on tree seedling establishment and growth
Both grass presence and absence have been reported to affect seedling growth (Vadigi and Ward 2014; Morrison et al. 2018). At Site 1, low grass cover (possibly caused by the observed soil erosion) across all treatments may have enhanced tree seedling growth, resulting in similar seedling growth among treatments (Grellier et al. 2012; Vadigi and Ward 2014). However, at Site 2, we postulate that grass cover, regardless of the differences in biomass, could have been sufficient across all treatments to suppress the tree seedling growth.
The reduced tree seedling establishment in response to moderate and high intensities of tree removal at Site 2 supported our predictions. Although tree seedling establishment at Site 1 was not affected by thinning, the results suggest that causal factors are perhaps complex and site specific. Site 1 factors over-riding the ‘grass suppression of seedlings hypothesis’ could be related to undetermined factors such as the predation of V. tortilis seeds by insects and a possible reduction in the soil seed bank (Jiao et al. 2009; Ward et al. 2010). V. tortilis seeds are known to be highly infested by seed-predating insects, particularly bruchid beetles, which reduces the natural regeneration of these plants (Ward et al. 2010). Soil erosion at Site 1 may also have reduced the soil seed bank, resulting in diminished tree seedling establishment. The greater grass biomass following tree removal in 50%, 75% and 100% removal treatments may have reduced the establishment of tree seedlings at Site 2 (Grellier et al. 2012; Pillay and Ward 2021). Results suggest that changes in grass biomass after tree removal influence tree seedling establishment, seedling survival and growth, and, consequently, woody plant encroachment in savannas.
The impact of tree removal on large-tree growth
Data from both sites were consistent with the prediction that thinning at moderate (50%) to high (75%) removal intensities would significantly increase remaining tree growth because of reduced tree competition, a result consistent with the results elsewhere (Smit 2001; Brudvig et al. 2011). Reduced tree competition through moderate to high intensities of tree removal may facilitate growth of remaining trees. However, we caution against high intensities (75–100%) of tree removal because this may result in large gaps between the remaining trees and/or lack of woody vegetation, which may favour an increase in soil erosion, particularly when the grass biomass is low (Smit 2014).
Conclusions
Tree removal may increase standing grass biomass in multi-tree-species systems on healthy soils, but may not be effective in monospecific stands, especially on eroded clay soils. Research including grazing animals would be useful in identifying long-term management options for controlling woody plant encroachment while promoting the herbaceous layer, as would studies that test the effects of tree removal on species composition.
The implications of these results for woody plant encroachment and management are that in rangelands severely affected by woody plant encroachment, removal of some of the woody material may release the remaining individuals from competition-induced size limits. A further implication of these findings is that, regardless of investment in woody species control, the recovery of key ecosystem services such as an increased forage production may not be realised, or may be system-specific.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
The current study was funded by the National Research Foundation (grant number: 99405) and the Agricultural Research Council.
Supplementary material
Supplementary material is available online.
Acknowledgements
We thank Bongani Ndlalane, Kabelo Molopo and Lepuase Chiloane for helping with preparing the experiment. The authors appreciate the assistance of Nothando Ngcobo, Nchaupa Rasekgokga and Michelle Monegi for helping with data collection.
References
Abdallah, F, Noumi, Z, Touzard, B, Belgacem, AO, Neffati, M, and Chaieb, M (2008). The influence of Acacia tortilis (Forssk.) subsp. raddiana (Savi) and livestock grazing on grass species composition, yield and soil nutrients in arid environments of South Tunisia. Flora 203, 116–125.| The influence of Acacia tortilis (Forssk.) subsp. raddiana (Savi) and livestock grazing on grass species composition, yield and soil nutrients in arid environments of South Tunisia.Crossref | GoogleScholarGoogle Scholar |
Archer, SR, and Predick, KI (2014). An ecosystem services perspective on brush management: research priorities for competing land-use objectives. Journal of Ecology 102, 1394–1407.
| An ecosystem services perspective on brush management: research priorities for competing land-use objectives.Crossref | GoogleScholarGoogle Scholar |
Beale, IF (1973). Tree density effects on yields of herbage and tree components in south-west Queensland mulga (Acacia aneura F.Muell.) scrub. Tropical Grasslands 7, 135–142.
Brudvig, LA, and Asbjornsen, H (2009). Dynamics and determinants of Quercus alba seedling success following savanna encroachment and restoration. Forest Ecology and Management 257, 876–884.
| Dynamics and determinants of Quercus alba seedling success following savanna encroachment and restoration.Crossref | GoogleScholarGoogle Scholar |
Brudvig, LA, Blunck, HM, Asbjornsen, H, Mateos-Remigio, VS, Wagner, SA, and Randall, JA (2011). Influences of woody encroachment and restoration thinning on overstory savanna oak tree growth rates. Forest Ecology and Management 262, 1409–1416.
| Influences of woody encroachment and restoration thinning on overstory savanna oak tree growth rates.Crossref | GoogleScholarGoogle Scholar |
Ding, J, and Eldridge, DJ (2019). Contrasting global effects of woody plant removal on ecosystem structure, function and composition. Perspectives in Plant Ecology, Evolution and Systematics 39, 125460.
| Contrasting global effects of woody plant removal on ecosystem structure, function and composition.Crossref | GoogleScholarGoogle Scholar |
Ding, J, Travers, SK, Delgado-Baquerizo, M, and Eldridge, DJ (2020). Multiple tradeoffs regulate the effects of woody plant removal on biodiversity and ecosystem functions in global rangelands. Global Change Biology 26, 709–720.
| Multiple tradeoffs regulate the effects of woody plant removal on biodiversity and ecosystem functions in global rangelands.Crossref | GoogleScholarGoogle Scholar | 31518466PubMed |
Duncan, RS, and Chapman, CA (2003). Tree-shrub interactions during early secondary forest succession in Uganda. Restoration Ecology 11, 198–207.
| Tree-shrub interactions during early secondary forest succession in Uganda.Crossref | GoogleScholarGoogle Scholar |
Fensham, RJ, Butler, DW, and Foley, J (2015). How does clay constrain woody biomass in drylands? Global Ecology and Biogeography 24, 950–958.
| How does clay constrain woody biomass in drylands?Crossref | GoogleScholarGoogle Scholar |
Forrester, DI (2015). Transpiration and water-use efficiency in mixed-species forests versus monocultures: effects of tree size, stand density and season. Tree Physiology 35, 289–304.
| Transpiration and water-use efficiency in mixed-species forests versus monocultures: effects of tree size, stand density and season.Crossref | GoogleScholarGoogle Scholar | 25732385PubMed |
Grellier, S, Barot, S, Janeau, JL, and Ward, D (2012). Grass competition is more important than seed ingestion by livestock for Acacia recruitment in South Africa. Plant Ecology 213, 899–908.
| Grass competition is more important than seed ingestion by livestock for Acacia recruitment in South Africa.Crossref | GoogleScholarGoogle Scholar |
Hoffmann, WA, and Poorter, H (2002). Avoiding bias in calculations of relative growth rate. Annals of Botany 80, 37–42.
| Avoiding bias in calculations of relative growth rate.Crossref | GoogleScholarGoogle Scholar |
Jeltsch, F, Weber, G, and Grimm, V (2000). Ecological buffering mechanisms in savannas: a unifying theory of long-term tree-grass coexistence. Plant Ecology 150, 161–171.
| Ecological buffering mechanisms in savannas: a unifying theory of long-term tree-grass coexistence.Crossref | GoogleScholarGoogle Scholar |
Jiao, JY, Zou, HY, Jia, YF, and Wang, N (2009). Research progress on the effects of soil erosion on vegetation. Acta Ecologica Sinica 29, 85–91.
| Research progress on the effects of soil erosion on vegetation.Crossref | GoogleScholarGoogle Scholar |
Kambatuku, JK, Cramer, MD, and Ward, D (2011a). Intraspecific competition between shrubs in a semi-arid savanna. Plant Ecology 212, 701–713.
| Intraspecific competition between shrubs in a semi-arid savanna.Crossref | GoogleScholarGoogle Scholar |
Kambatuku, JK, Cramer, MD, and Ward, D (2011b). Savanna tree-grass competition is modified by substrate type and herbivory. Journal of Vegetation Science 22, 225–237.
| Savanna tree-grass competition is modified by substrate type and herbivory.Crossref | GoogleScholarGoogle Scholar |
Kyalangalilwa, B, Boatwright, JS, Daru, BH, Maurin, O, and van der Bank, M (2013). Phylogenetic position and revised classification of Acacia s.l. (Fabaceae: Mimosoideae) in Africa, including new combinations in Vachellia and Senegalia. Botanical Journal of the Linnean Society 172, 500–523.
| Phylogenetic position and revised classification of Acacia s.l. (Fabaceae: Mimosoideae) in Africa, including new combinations in Vachellia and Senegalia.Crossref | GoogleScholarGoogle Scholar |
Loth, PE, De Boer, WF, Heitkönig, IMA, and Prins, HHT (2005). Germination strategy of the East African savanna tree Acacia tortilis. Journal of Tropical Ecology 21, 509–517.
| Germination strategy of the East African savanna tree Acacia tortilis.Crossref | GoogleScholarGoogle Scholar |
Ludwig, F, Dawson, TE, Kroon, H, Berendse, F, and Prins, HHT (2003). Hydraulic lift in Acacia tortilis trees on an East African savanna. Oecologia 134, 293–300.
| Hydraulic lift in Acacia tortilis trees on an East African savanna.Crossref | GoogleScholarGoogle Scholar | 12647135PubMed |
Meyer, KM, Wiegand, K, Ward, D, and Moustakas, A (2007). The rhythm of savanna patch dynamics. Journal of Ecology 95, 1306–1315.
| The rhythm of savanna patch dynamics.Crossref | GoogleScholarGoogle Scholar |
Meyer, KM, Ward, D, Wiegand, K, and Moustakas, A (2008). Multi-proxy evidence for competition between savanna woody species. Perspectives in Plant Ecology, Evolution and Systematics 10, 63–72.
| Multi-proxy evidence for competition between savanna woody species.Crossref | GoogleScholarGoogle Scholar |
Morrison, TA, Holdo, RM, Rugemalila, DM, Nzunda, M, and Anderson, TM (2018). Grass competition overwhelms effects of herbivores and precipitation on early tree establishment in Serengeti. Journal of Ecology 107, 216–228.
| Grass competition overwhelms effects of herbivores and precipitation on early tree establishment in Serengeti.Crossref | GoogleScholarGoogle Scholar |
Mucina I, Rutherford MC (2006) ‘The vegetation of South Africa, Lesotho and Swaziland. Strelitzia 19’, (South Africa National Biodiversity Institute: Pretoria)
Ndhlovu, T, Milton, SJ, and Esler, KJ (2016). Effect of Prosopis (mesquite) invasion and clearing on vegetation cover in semi-arid Nama Karoo rangeland, South Africa. African Journal of Range and Forage Science 33, 11–19.
| Effect of Prosopis (mesquite) invasion and clearing on vegetation cover in semi-arid Nama Karoo rangeland, South Africa.Crossref | GoogleScholarGoogle Scholar |
Panagos, MD, Westfall, RH, van Staden, JM, and Zacharias, PJK (1998). The plant communities of the Roodeplaat Experimental Farm, Gauteng, South Africa and the importance of classification verification. South African Journal of Botany 64, 44–61.
| The plant communities of the Roodeplaat Experimental Farm, Gauteng, South Africa and the importance of classification verification.Crossref | GoogleScholarGoogle Scholar |
Pillay, T, and Ward, D (2012). Spatial pattern analysis and competition between Acacia karroo trees in humid savannas. Plant Ecology 213, 1609–1619.
| Spatial pattern analysis and competition between Acacia karroo trees in humid savannas.Crossref | GoogleScholarGoogle Scholar |
Pillay, TP, and Ward, D (2021). Grass competition is more important than fire for suppressing encroachment of Acacia sieberiana seedlings. Plant Ecology 222, 149–158.
| Grass competition is more important than fire for suppressing encroachment of Acacia sieberiana seedlings.Crossref | GoogleScholarGoogle Scholar |
Pretzsch, H (2014). Canopy space filling and tree crown morphology in mixed-species stands compared with monocultures. Forest Ecology and Management 327, 251–264.
| Canopy space filling and tree crown morphology in mixed-species stands compared with monocultures.Crossref | GoogleScholarGoogle Scholar |
Riginos, C (2009). Grass competition suppresses savanna tree growth across multiple demographic stages. Ecology 90, 335–340.
| Grass competition suppresses savanna tree growth across multiple demographic stages.Crossref | GoogleScholarGoogle Scholar | 19323216PubMed |
Sagar, R, Pandey, A, and Singh, JS (2012). Composition, species diversity, and biomass of the herbaceous community in dry tropical forest of northern India in relation to soil moisture and light intensity. Environmentalist 32, 485–493.
| Composition, species diversity, and biomass of the herbaceous community in dry tropical forest of northern India in relation to soil moisture and light intensity.Crossref | GoogleScholarGoogle Scholar |
Scariot A, Vieira DL, Sampaio AB, Guarino E, Sevilha A (2008) Recruitment of dry forest tree species in central Brazil pastures. In ‘Post-agricultural succession in the neotropics’. (Ed. RW Myster) pp. 231–244. (Springer: New York, NY, USA)
Schleicher, J, Wiegand, K, and Ward, D (2011a). Changes of woody plant interaction and spatial distribution between rocky and sandy soil areas in a semi-arid savanna, South Africa. Journal of Arid Environments 75, 270–278.
| Changes of woody plant interaction and spatial distribution between rocky and sandy soil areas in a semi-arid savanna, South Africa.Crossref | GoogleScholarGoogle Scholar |
Schleicher, J, Meyer, KM, Wiegand, K, Schurr, FM, and Ward, D (2011b). Disentangling facilitation and seed dispersal from environmental heterogeneity as mechanisms generating associations between savanna plants. Journal of Vegetation Science 22, 1038–1048.
| Disentangling facilitation and seed dispersal from environmental heterogeneity as mechanisms generating associations between savanna plants.Crossref | GoogleScholarGoogle Scholar |
Schnitzer, SA, Mascaro, J, and Carson, WP (2001). Treefall gaps and the maintenance of plant species diversity in tropical forests. Ecology 82, 913–919.
| Treefall gaps and the maintenance of plant species diversity in tropical forests.Crossref | GoogleScholarGoogle Scholar |
Smit, GN (2001). The influence of tree thinning on the vegetative growth and browse production of Colophospermum mopane. South African Journal of Wildlife Research 31, 99–114.
Smit, GN (2005). Tree thinning as an option to increase herbaceous yield of an encroached semi-arid savanna in South Africa. BMC Ecology 5, e4.
| Tree thinning as an option to increase herbaceous yield of an encroached semi-arid savanna in South Africa.Crossref | GoogleScholarGoogle Scholar |
Smit, N (2014). Response of Colophospermum mopane to different intensities of tree thinning in the Mopane Bushveld of southern Africa. African Journal of Range and Forage Science 31, 173–177.
| Response of Colophospermum mopane to different intensities of tree thinning in the Mopane Bushveld of southern Africa.Crossref | GoogleScholarGoogle Scholar |
Smith, TM, and Grant, K (1986). The role of competition in the spacing of trees in a Burkea africana–Terminalia sericea savanna. Biotropica 8, 219–223.
| The role of competition in the spacing of trees in a Burkea africana–Terminalia sericea savanna.Crossref | GoogleScholarGoogle Scholar |
Tomlinson, KW, Sterck, FJ, Barbosa, ERM, de Bie, S, Prins, HHT, and van Langevelde, F (2019). Seedling growth of savanna tree species from three continents under grass competition and nutrient limitation in a greenhouse experiment. Journal of Ecology 107, 1051–1066.
| Seedling growth of savanna tree species from three continents under grass competition and nutrient limitation in a greenhouse experiment.Crossref | GoogleScholarGoogle Scholar |
Treydte, AC, Looringh van Beeck, FA, Ludwig, F, and Heitkoenig, IMA (2008). Improved quality of beneath-canopy grass in South African savannas: local and seasonal variation. Journal of Vegetation Science 19, 663–670.
| Improved quality of beneath-canopy grass in South African savannas: local and seasonal variation.Crossref | GoogleScholarGoogle Scholar |
Vadigi, S, and Ward, D (2014). Herbivory effects on saplings are influenced by nutrients and grass competition in a humid South African savanna. Perspectives in Plant Ecology, Evolution and Systematics 16, 11–20.
| Herbivory effects on saplings are influenced by nutrients and grass competition in a humid South African savanna.Crossref | GoogleScholarGoogle Scholar |
Ward, D, Musli, I, Or, K, Gbenro, T, and Skutelsky, O (2010). Bruchid seed infestation and development time in three host species of Acacia (Coleoptera, Bruchidae). Zoology in the Middle East 51, 95–103.
| Bruchid seed infestation and development time in three host species of Acacia (Coleoptera, Bruchidae).Crossref | GoogleScholarGoogle Scholar |
Yadeta, T, Veenendaal, E, Sykora, K, Tessema, ZK, and Asefa, A (2018). Effect of Vachellia tortilis on understory vegetation, herbaceous biomass and soil nutrients along a grazing gradient in a semi-arid African savanna. Journal of Forestry Research 29, 1601–1609.
| Effect of Vachellia tortilis on understory vegetation, herbaceous biomass and soil nutrients along a grazing gradient in a semi-arid African savanna.Crossref | GoogleScholarGoogle Scholar |