Understanding the spatiotemporal dynamics of understorey biomass in semi-arid woodlands of south-eastern Australia
Linda Riquelme A * , Libby Rumpff A , David H. Duncan A and Peter A. Vesk A
A * , Libby Rumpff A , David H. Duncan A and Peter A. Vesk A
A School of Ecosystem and Forest Sciences, The University of Melbourne, Vic. 3010, Australia.
The Rangeland Journal 44(1) 47-59 https://doi.org/10.1071/RJ21060
Submitted: 9 December 2021 Accepted: 7 March 2022 Published: 19 April 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of the Australian Rangeland Society. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
When managing grazing pressure for conservation, understanding forage dynamics is essential. In south-eastern Australia, ongoing grazing is inhibiting regeneration in several semi-arid woodland communities. Western grey kangaroos (Macropus fuliginosus (Desmarest, 1817)) have been identified as a key component of total grazing pressure. They are thought to switch from grass to lower-quality browse, including tree seedlings, when grass biomass falls below 400 kg ha−1. One static threshold may not adequately capture the spatial and temporal hazard associated with kangaroo grazing, and this study aimed to explore how grassy biomass varies across a case-study landscape. Understorey biomass and species composition data were collected in the field on seven occasions between December 2016 and May 2019. We used Generalised Linear Mixed Models (GLMMs) to describe the influence of environmental and herbivory variables on total (live and dead) understorey, live understorey, and grass (live and dead) biomass. Canopy cover showed the strongest influence on understorey biomass, with more biomass found in open sites than in woodland. Understorey biomass levels were lowest in summer and autumn. Grass biomass, in particular, fell below the 400 kg ha−1 forage-switch threshold in wooded areas during this time. We anticipate that an increased understanding of understorey biomass dynamics will inform managers as to when and where to focus management efforts to promote regeneration and sustained recovery of these semi-arid woodlands. Results of this study suggest that conducting management efforts before the summer/autumn decline in understorey biomass, particularly in woodlands, is critical in reducing the browsing risk to seedlings.
Keywords: grazing management, grazing pressure, herbage mass, herbivory by kangaroos, regeneration, semiarid rangelands.
Introduction
Understanding and quantifying aboveground plant biomass is important for a range of applications, such as fire management (Franke et al. 2018), estimating carbon stocks and fluxes (Bradshaw and Warkentin 2015), and grazing and livestock production (Michez et al. 2019). In some ecosystems, anthropogenic modification of the landscape has resulted in populations of large, native vertebrate herbivores reaching levels where their impacts become an issue for conservation values, such as native species diversity and recruitment (Magioli et al. 2019; Prowse et al. 2019; Tucker et al. 2021). Ground layer biomass provides forage for herbivores, and so knowledge of available forage is critical when managing large, generalist herbivore populations (Noy-Meir 1975).
The distribution of understorey biomass is not uniform across the landscape; rather, it reflects complex feedbacks that can make estimation of standing understorey biomass a challenge (House et al. 2003; Blaser et al. 2013). Biomass accumulation is affected by the intensity and timing of rainfall events, soil texture, and landscape features (Ludwig et al. 1997; Nano and Pavey 2013). Dependencies on vegetation growth form can complicate this relationship, in various ways. For instance, larger growth forms (i.e. shrubs, trees) tend to have a higher biomass per unit of area (Flombaum and Sala 2007). Overstorey type and cover can influence species composition in the understorey due to the differences in soil moisture, the microclimate, nutrient concentration, and the seed bank (Warnock et al. 2007; Horner et al. 2012), but also directly influence biomass through increased competition for light, water, and nutrients (Jameson 1967; Beale 1973). Soil texture also shapes composition: fine-textured soils are more favourable for grasses; conversely, coarse-textured soils are better suited to shrubs (Sala et al. 1997).
Adding large vertebrate herbivores to the equation further complicates matters, as standing biomass will vary depending on their feeding and shelter requirements. Large herbivores preferentially reduce plant biomass through grazing and browsing (Mysterud 2006). The selection of particular species or growth forms may vary over time as relative availability changes (Norbury 1987). Moreover, green grass is generally favoured over dry vegetation, as it is more rapidly digested (Dawson and Munn 2007). Herbivores also tend to graze differentially throughout the landscape, depending on factors such as topography, forage quality, and distance to water and shelter (Bailey et al. 1996).
These complex interactions can make modelling understorey biomass through space and time challenging for managers seeking to control herbivore populations based on forage availability. Land managers are faced with this issue in several semi-arid woodlands in south-eastern Australia, including the endangered Buloke Woodlands of the Riverina and Murray-Darling Depression Bioregions (Department of Agriculture, Water and the Environment 2020). Since European colonisation, these woodlands have been extensively cleared for grazing and agriculture; some of the largest remaining stands now occur within national parks (Cheal et al. 2011). Livestock grazing was phased out in these national parks by 1996, but subsequent monitoring has found little or no tree regeneration, particularly buloke (Sandell 2011). One of the key reasons for this is the grazing pressure posed by introduced rabbits (Oryctolagus cuniculus (Linnaeus, 1758)) and goats (Capra hircus (Linnaeus, 1758)), and the native western grey kangaroos (Macropus fuliginosus (Desmarest, 1817)) (Bennett et al. 2020). Rabbits and goats are also identified threats to native vegetation, the former even at very low densities (Mutze et al. 2016; Bennett et al. 2020) and annual monitoring and control of these herbivores is undertaken in the region (Sandell 2011; Taylor and Pegler 2016). In recent years, goat incursions have been infrequent in our study area, and rabbit populations have been maintained at or below target densities (Taylor and Pegler 2016). Under these circumstances, western grey kangaroos have been presumed to be the herbivore with most potential to limit woodland regeneration. Kangaroos have become more abundant since European colonisation due to higher forage availability as a result of clearing, the provision of watering points across the landscape, and the removal of hunting pressure by Indigenous people and dingoes (Canis lupus dingo) (Cheal 1986; Prowse et al. 2019). The focus on kangaroos is further motivated by the need for a strong evidence base for justifying population control, as culling can be controversial (Cheal 1986). It has been estimated that when grass biomass falls below a threshold of 400 kg ha−1 because of drought or overabundant competitors, some western grey kangaroos will switch from grass to less palatable browse, such as shrubs and tree seedlings (Norbury 1987). Sustained periods below this threshold are expected to result in seedling herbivory and mortality, thus preventing recruitment of woody species (Sluiter et al. 1997). Managers currently undertake annual kangaroo culls, with targets based on annual population surveys (Morris et al. 2019). However, data on the availability of grassy biomass across the landscape could be combined with kangaroo density estimates to set more informed cull targets. The aim of this study, and the first step in incorporating predictions of biomass into existing kangaroo population models, was to explore how grassy biomass varies across the landscape of interest.
To explore landscape variation, we developed an explanatory model to examine how climatic, environmental, and herbivory variables influence ground layer biomass across the semi-arid Pine Plains area of Wyperfeld National Park, south-eastern Australia, over a period of 2.5 years. Using this model, we explored when and where grassy biomass was most likely to fall below the proposed 400 kg ha−1 forage-switch threshold, thus identifying periods of increased browsing risk to tree seedlings. We then cross-validated the model using biomass data collected in autumn 2019, which were sampled from new and existing sites.
Methods
Study area
This study was conducted in the Pine Plains area of Wyperfeld National Park, located in south-eastern Australia (Fig. 1), between summer 2016 and autumn 2019. Climate is semi-arid, with a mean annual rainfall of 332 mm (Bureau of Meteorology 2021a). However, over the sampling period the region experienced both higher (404 mm in 2016 and 348 mm in 2017) and lower than average rainfall (143 mm in 2019) (Bureau of Meteorology 2021a; see Supplementary Fig. S1). Rainfall is winter-dominant, with approximately 60% of mean annual precipitation falling between May and October (Bureau of Meteorology 2021a). Vegetation growth broadly follows the rainfall pattern, though it may be suppressed by low temperatures in mid-winter. Mean minimum daily temperatures range between 4.8 and 15.4°C (Bureau of Meteorology 2021b), while mean maximum temperatures range from 14.7 to 32.0°C (Bureau of Meteorology 2021c).

|
Pine Plains was extensively cleared and heavily grazed by sheep and cattle for almost 160 years until 1996 (Sandell 2011). The current threats associated with this endangered vegetation community now include grazing by native and non-native herbivores, further clearing, inappropriate fire management, and drought (Department of Agriculture, Water and the Environment 2020).
Pine Plains is a mosaic of vegetation types, largely classified into four communities (Miller et al. 1998): ‘Buloke Woodland’: buloke woodland often co-dominated by slender cypress pine (Callitris gracilis R. T. Baker), with a mixed grassy and herbaceous understorey; ‘Lakebed’: a mixed herbaceous grassland, often interspersed with chenopod shrubs, located on ephemeral lakebeds; ‘River Red Gum/Black Box Woodland’: dominated by river red gum (Eucalyptus camaldulensis Dehnh.) and black box (Eucalyptus largiflorens F. Muell.), found along the boundaries of lakebeds and drainage lines, and ‘Mallee/Scrub/Heath’: areas with a sparse herbaceous/shrubby understorey, that may have an overstorey dominated by several species of mallee eucalypts. Site stratification in our sampling design broadly followed the above classification. ‘Derived Grassland’ was added to specify areas of former Buloke Woodland that were subject to extensive historical clearing.
Data collection
Site selection
Candidate site locations were generated using ArcGIS 10.3.1 (Esri 2015) by placing random points in each of the following communities: Buloke Woodland, Black Box Woodland, Lakebed, and Derived Grassland. The Mallee vegetation community was omitted as low western grey kangaroo numbers have been recorded in this vegetation type, compared to grassland and non-eucalypt woodland (0.48 ± 0.36 km−2 in mallee vs 18.92 ± 10.12 km−2 in non-mallee vegetation in 2020) (MacKenzie 2020). Mallee vegetation is generally considered unsuitable habitat for kangaroos, due to a general lack of available grassy forage and permanent watering points (Short and Grigg 1982).
Random points were discarded upon ground truthing if they were located in areas of heterogeneous vegetation or ecotones, within fenced areas, or if they were <30 m from a road or fence. Ten sites were selected for each vegetation type, with a total of 40 sites. Sites were 90 m × 90 m and were at least 500 m apart. Sites were sampled on six occasions between December 2016 and May 2018.
Validation dataset
In May 2019, 24 additional sites, six in each vegetation type, were selected and sampled in addition to 24 of the existing 40 sites. We selected sites based on visual inspection of plotted site means across all seasons. Though sites were chosen from across the biomass range, we particularly wanted to refine our understorey biomass estimates around the 400 kg ha−1 threshold, as sites with lower biomass were more variable. Of the 24 existing sites selected, 54% fell within the lower half of the biomass distribution. Six sites were selected from each vegetation type. Data collected on this occasion served as a validation dataset to test model performance across seasons, as well as across the landscape.
Response variables: total understorey, live understorey, and grass biomass
We clipped total aboveground understorey biomass (live and dead, all growth forms), live aboveground understorey biomass (all growth forms), and aboveground grass biomass (live and dead, annual and perennial graminoids only). Pilot studies were conducted to determine clipping effort. Five quadrats per site were sampled, as bootstrapping showed that the standard error did not greatly reduce with increased sub sampling (Supplementary Fig. S2).
At each site, orthogonal transects were set up from the south-west to the north-east corner, and from the south-east corner to the north-west. Three quadrats were placed along the first transect at 42, 63 (intersection of transects), and 84 m. Two were placed along the second transect at 42 and 84 m. Quadrat locations were chosen to focus sampling on the centre of each site (30 m × 30 m area). Each quadrat was 50 cm × 50 cm in size. Within each quadrat, biomass was clipped to ground level, separated into live and dead material, and placed into paper bags. Woody material was not clipped when sampling shrubs. Samples were dried at 70°C for 72 h and weighed (a more detailed explanation of field methods can be found in the Supplementary materials).
Explanatory variables: soil moisture, vegetation, soil texture, and herbivory
Soil moisture data were obtained for each site from the Bureau of Meteorology Australian Landscape Water Balance website (Bureau of Meteorology 2020). These values were derived using the Australian Water Resources Assessment Landscape model (AWRA-L), which had a spatial resolution of 5 km × 5 km (Frost et al. 2018). These values represented the percentage of available water content in the top 1 m of the soil profile, also known as Root Zone Soil Moisture (Bureau of Meteorology 2020). Daily soil moisture was averaged over periods of 1, 2, and 3 months prior to clipping dates. We tested lag periods of 1, 2, 3, and 4 weeks between the various calculated average soil moisture periods and clipping dates.
Vegetation was divided into overstorey (trees) and understorey (graminoids, forbs, and shrubs). The overstorey was then further stratified into open and wooded canopy types (Table 1). Canopy type was assigned based on vegetation type: sites found in Lakebed or Derived Grassland were classed as having an ‘open’ canopy, and sites in Black Box or Buloke Woodland a ‘wooded’ canopy. The understorey was classified into the dominant growth forms present: annual graminoids, perennial graminoids, forbs, and shrubs (Table 1). To determine the dominant growth form at each site during a given season, species composition was estimated at each site according to the dry-weight-rank method (’t Mannetje and Haydock 1963). Before clipping, the top three species in each quadrat were visually ranked in terms of their dry weight. A set of multipliers was then used to convert rankings to percentage composition. Species were classified into growth forms, and a dry-weight estimate by growth form for each site calculated. Grass biomass was calculated by multiplying the proportion of total biomass classified as a grassy growth form, by the total understorey dry weight (’t Mannetje and Haydock 1963).

|
Soil texture data, from the Soil Landscape Grid of Australia, consisted of modelled percentage clay, silt, and sand at a 90 m × 90 m resolution (Grundy et al. 2015).
Western grey and red kangaroos (Osphranter rufus (Desmarest, 1822)) were present in the study area, although red kangaroos were also present in much lower densities during the study period (B. Rodgers pers. comm.). Western grey kangaroos tend to favour areas of heterogeneous habitat, foraging close to tree cover (Coulson and Norbury 1988). We therefore expected that increasing distance from tree cover would have a generally positive effect on understorey biomass. Existing kangaroo density estimates were available for Pine Plains, however these are single annual estimates for the area as a whole (MacKenzie 2020). We used the natural logarithm of the distance of each open site to cover as a proxy for kangaroo activity. Wooded sites were assigned a value of zero.
All data are available via an Open Science Framework repository (Riquelme et al. 2021).
Data analysis and modelling
Generalised Linear Mixed Models (GLMMs) were used to explore variation in understorey biomass. The response variables modelled were total understorey (live and dead, all growth forms), live understorey (all growth forms), and grass (live and dead, annual and perennial graminoid growth forms) biomass. In all models, the biomass data were cube-root-transformed to provide an approximately normal error distribution (Quinn and Keough 2002). Explanatory variables used in the models are outlined in Table 1. Preliminary analysis of understorey biomass per vegetation type showed a greater difference between the open (Lakebed and Derived Grassland) and wooded (Black Box and Buloke Woodlands) vegetation types than between individual vegetation types (Fig. 2). Therefore, vegetation was grouped into open and wooded canopy types for further analysis. To account for any effect of understorey composition on biomass, understorey vegetation was classified into growth forms (annual graminoids, perennial graminoids, forbs, and shrubs), and the dominant understorey growth form at each site included as a fixed effect in the models. Modelling was carried out with R software (v. 4.0.2, R Core Team 2020) using the lme4 package (v. 1.1-23, Bates et al. 2015).
|
The initial understorey biomass models included various mean soil moisture intervals, canopy type, and dominant understorey growth form as fixed effects. Site was included as a random effect to account for site differences in biomass, as well as temporal dependence (Zuur and Ieno 2016). A moving window of mean daily soil moisture values was favoured over daily values, as this would presumably average out any large rainfall events that might confound results. The relationships between understorey biomass and varying mean soil moisture windows were compared (see Supplementary Figs S4–S7).
Interactions between different variables were also tested. Three alternatives were proposed: no interactions (all variables considered independent), an interaction between soil moisture and canopy type (effect of soil moisture on biomass differs between open and woodland areas), or an interaction between soil moisture and dominant understorey growth form (effect of soil moisture on biomass differs depending on dominant understorey growth form).
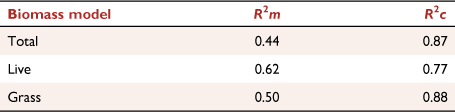
A model selection process was carried out for each response variable (i.e. total understorey, live understorey, and grass biomass). Models were ranked based on Akaike’s Information Criterion (AIC) (Akaike 1973) and Akaike weight (Burnham and Anderson 2002). We also present the marginal R2 (R2m: the proportion of variance explained by the fixed effects only), the conditional R2 (R2c: the proportion of variance explained by both the fixed and random effects) (Nakagawa and Schielzeth 2013), and the degrees of freedom for each model. The model with the lowest AIC and highest Akaike weight was selected as the best explanatory model.
We then explored the effects of kangaroo activity (using the logarithm of distance to cover as a proxy) and soil texture on biomass, using the top-performing biomass model structure. Another model selection process was conducted to select the optimum understorey biomass model.
Model validation
Data collected in May 2019 were used to test the performance of the final top-ranked models, which were trained using data collected between December 2016 and May 2018. Biomass was predicted to May 2019 and compared to observed data using Pearson’s correlation.
Results
Standing crop biomass
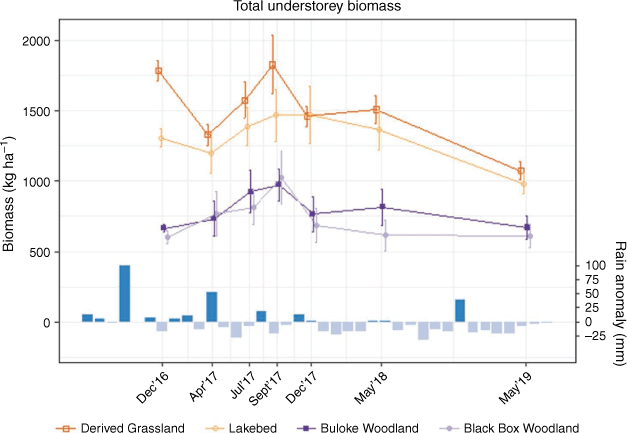
Open sites clearly differed from wooded sites, with more understorey biomass in open than in wooded sites (Figs 2, 3). Among open vegetation types, Derived Grassland sites had consistently higher mean biomass than Lakebed sites, although the 95% confidence intervals overlapped. There was no obvious difference in understorey biomass between the two wooded types throughout the study period.
|
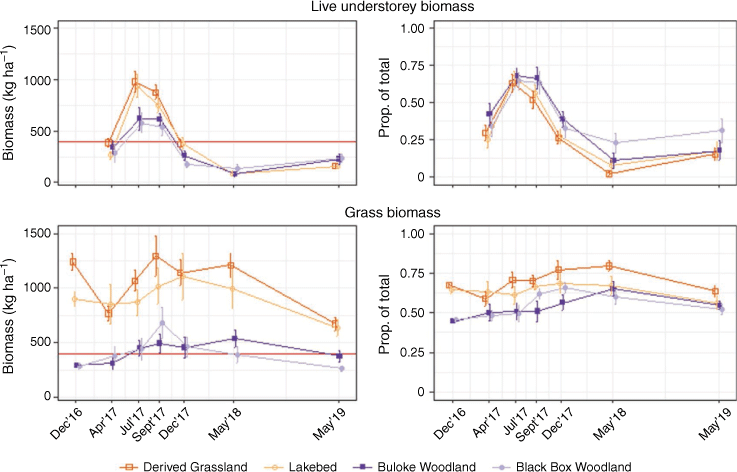
Hereafter, margins of error reported are 95% confidence intervals. Throughout the study period, there was a mean of 1383 ± 31 kg ha−1 total understorey biomass in open sites, and 707 ± 25 kg ha−1 in wooded sites (Fig. 2). It peaked in spring (September) 2017, with 1647 ± 141 kg ha−1 in open, and 1001 ± 110 kg ha−1 in wooded sites. Mean grass biomass in open sites was 952 ± 30 kg ha−1 and in wooded sites 365 ± 15 kg ha−1 (Fig. 3). Grass biomass was also at a maximum in spring (September) 2017, with 1156 ± 124 kg ha−1 in open (70% of total understorey biomass) and 588 ± 85 kg ha−1 in wooded sites (59% of total understorey biomass). There was little difference in the proportion of live understorey biomass between open and wooded sites, though open sites generally had a greater biomass than wooded ones (overall mean 374 ± 25 kg ha−1 and 313 ± 22 kg ha−1, respectively) (Fig. 3). Live understorey biomass peaked in winter (July) 2017, with 960 ± 74 kg ha−1 in open and 601 ± 73 kg ha−1 in wooded sites.
Lowest forage availability was generally recorded in autumn. For example, from its peak to the following autumn (May 2018) total understorey biomass declined by 13% in open areas, and by 29% in wooded (Fig. 2). However, live understorey showed the sharpest decline during this time, where it fell by 93% in open sites and by 81% in wooded sites (Fig. 3).
Biomass sampling was conducted in autumn in each of the three study years, with biomass declining over that period (Figs 2, 3). Between autumn (April) 2017 and autumn (May) 2019, total understorey biomass declined by 19% in open and by 15% in wooded sites (Fig. 2). Grass biomass fell by 19 and 7% in open and wooded, respectively (Fig. 3). Again, live understorey biomass showed the most dramatic decrease, falling by 51% in open and by 27% in wooded over the same period (Fig. 3).
In wooded areas, grass biomass fell close to or below the proposed 400 kg ha−1 forage-switch threshold (Norbury 1987), particularly during the drier summer and autumn periods (Fig. 3). Only in the spring of 2017 did average grass biomass remain above the threshold in woodland.
Species and growth form composition
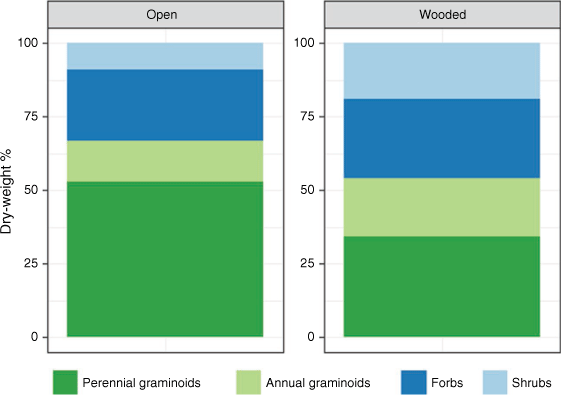
Open sites generally had a higher proportion of graminoids than wooded sites, particularly native perennials, which composed >50% dry weight in open sites (Fig. 4). Wooded sites had a more even mix of growth forms (Fig. 4). Proportions remained relatively stable over time across all sites (see Supplementary Figs S8, S9 for composition by site and season).
|
Biomass models
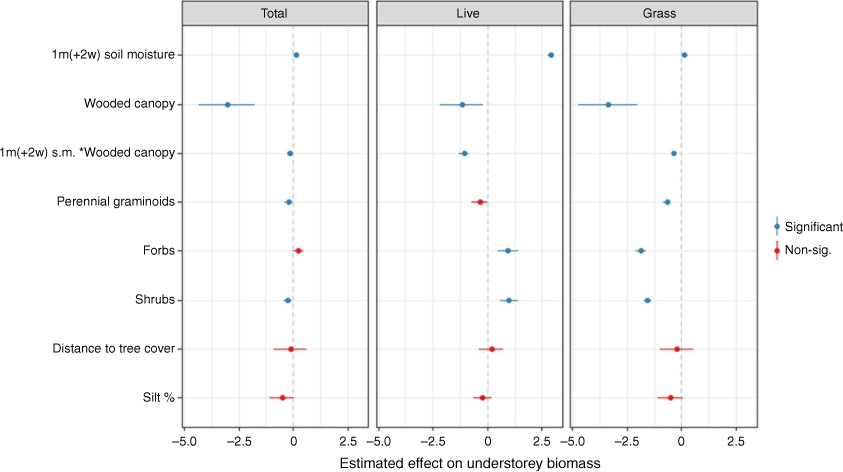
Initial modelling tested various averaged soil moisture windows and lags, as well as interactions with over- and understorey vegetation types. Though we were able to identify a series of ‘best fit’ models, multiple GLMMs had similar explanatory power within each biomass type (i.e. total understorey, live understorey, and grass biomass) (full results in Supplementary materials). Soil moisture had an overall positive effect on all understorey biomass types, though it had the most significant effect on live understorey biomass. A mean soil moisture window of 1 month with a 2-week lag explained the greatest amount of variation in live biomass, with the top-ranked model also having the highest explanatory power (when excluding site effect) of any understorey biomass model. Model outputs also suggested a significant interaction between soil moisture and canopy type, where the positive effects of soil moisture on understorey biomass were reduced under a wooded canopy (see Supplementary Tables S1–S3).
The effects of distance to tree cover – a proxy for kangaroo grazing activity – and soil texture on understorey biomass were then explored using the top-ranked live model structure. Including the distance to tree cover and soil texture broadly improved GLMMs, particularly total understorey and grass models. Distance alone had no significant effect on any understorey biomass type (Fig. 5). However, when included with a soil texture variable, there was an indication that soil refined the effect of distance: for example, when including silt content, there was an average improvement of 7% in marginal R2 (refer to Supplementary Tables S4–S6 for full results). As with the initial models, there was little difference in explanatory power between models within each understorey biomass type, especially for live understorey biomass.
The general structure of the top-performing GLMMs was:
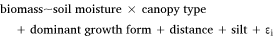
where ‘biomass’ refers to the understorey biomass at a given site, ‘soil moisture’ represents the average daily soil moisture over the previous month (plus a 2-week lag), ‘canopy type’ specifies whether a site is open (i.e. no trees) or wooded, ‘dominant growth form’ refers to the most prominent plant group in the understorey (i.e. perennial or annual grasses, forbs, or shrubs), ‘distance’ denotes the logarithm of the distance of the edge of a site to nearest tree cover (used as a proxy for kangaroo activity), and ‘silt’ refers to the percentage silt content in the top 5 cm of soil. The symbol ‘ε’ represents residual error, specified by a site random effect. This residual error also includes any correlated change in biomass through time. Season was not explicitly included in the models, as it would have competed with soil moisture: biomass varied over seasons due to changes in soil moisture, rather than due to season itself. In addition, the sample of seasons was too small to confidently include it as a random effect.
Models were better at explaining variation in understorey biomass when taking site effects into account, than when modelling with main effects only (R2c and R2m, respectively; Table 2). Depending on understorey biomass type, the difference between R2m and R2c ranged from 0.15 to 0.38 (Table 2). The top-ranked total understorey biomass model improved by 0.43 when including site effect, and the top-ranked grass model improved by 0.38, whereas there was a smaller effect of site on live biomass, with an improvement of 0.15. The grass biomass model had the highest explanatory power, when including site effects, of all understorey biomass models (R2c = 0.88).
|
Trees had the strongest influence on understorey biomass, with an overall negative effect (Fig. 5). Soil moisture had the greatest effect on live understorey biomass, with only a slight positive effect on total understorey and grass biomass. Dominant understorey growth forms had mixed effects. Sites where perennial graminoids predominated generally had less understorey biomass compared to sites dominated by annual graminoids. There tended to be less total understorey biomass in shrubby sites, and more in forb-dominated sites, although this effect was not significant. Live understorey biomass tended to be higher in forb and shrub-dominated sites. Distance to tree cover only had a positive effect on live understorey biomass, though the effect of distance on all biomass types was non-significant. Percentage of silt had a negative effect on all understorey biomass, though this was not significant.
Model validation
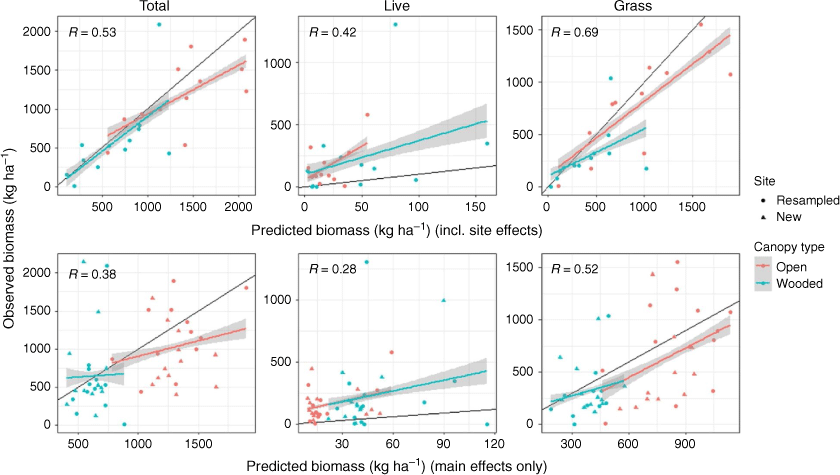
The predictive performance of the models was evaluated by comparing predicted to observed understorey biomass for May 2019 (Fig. 6). Predictions were made in two ways: by taking site effects into account (Fig. 6: top row) and by considering only the main effects (Fig. 6: bottom row). When including site random effect, predictions were more similar to observed values than predictions made considering only the main effects. This highlights the importance of site effects on biomass.
|
For total understorey biomass, the model tended to overpredict at the higher biomass end, but not at lower biomass levels. Live understorey biomass was consistently underpredicted by the model, both with and without site effect included. The grass biomass model performed best, particularly when including site effects, though grass was generally overpredicted at the medium- and high-end of the biomass range (Fig. 6).
Discussion
Tree cover had the greatest influence on overall understorey biomass, with significantly lower understorey biomass in wooded areas compared to open sites. These results are supported by previous studies demonstrating that trees generally reduce the productivity of understorey biomass, leading to reduced forage in woodland (Jameson 1967; Beale 1973). We found that grass biomass was more likely to fall below a proposed forage-switch threshold (400 kg ha−1) in these areas during summer and autumn, which are known to be critical periods of forage stress for western grey kangaroos (Norbury 1987; Coulson et al. 1990). This suggests an increased grazing threat to tree seedlings in wooded areas during these times. This fall occurred even during years of average, or even relatively high, rainfall.
The grazing threat in wooded areas is likely to be compounded by the fact that kangaroos also spend time sheltering within tree cover (Coulson and Norbury 1988). The negative effects on vegetation by kangaroos at high densities have been well-documented (Cheal 1986; Coulson and Norbury 1988; Prowse et al. 2019), with research in semi-arid south-eastern Australia suggesting a density of about 3 kangaroos km−2 or less is required to maintain grass biomass above the 400 kg ha−1 threshold (Sluiter et al. 1997). However, distance to cover (used as a proxy for kangaroo activity) was not found to have a significant effect on understorey biomass. Studies in similar environments have yielded mixed results: for example, Sluiter et al. (1997) determined that kangaroo density, and therefore grazing, had a significant effect on grass biomass; however, Travers et al. (2020) found that although kangaroo grazing reduced understorey biomass, grazing did not explain much variance in biomass, nor did it improve overall model fit.
There are several possible explanations for why we found no apparent effect of distance from tree cover on understorey biomass. First, the no effect finding in our study may simply reflect lower kangaroo densities as a result of culling, compared to those in Coulson and Norbury’s (1988) or Sluiter’s et al. (1997) work. Unlike those studies, kangaroo survey data specific to our sites were unavailable; such data may have highlighted a more complex relationship between distance from cover and kangaroo foraging activity than assumed in our models. Home ranges of western grey kangaroos have been estimated to average around 6.9 km2 (Priddel et al. 1988), though they may be smaller in areas of high habitat diversity, where a home range of <5 km2 may be large enough to meet forage and shelter requirements (Coulson and Norbury 1988). However, in times of drought, kangaroos are likely to venture further out into open habitat to forage (Coulson and Norbury 1988). The effect of distance in the model was assumed to be the same across all seasons, despite the range in climatic conditions throughout the study period. It should also be recognised that the functional response of western grey kangaroos grazing on forage is not linear, regardless of distance from cover: there is a logarithmic relationship between forage biomass and forage intake, with intake decreasing as biomass increases (Short 1986). In addition, there are complex feedbacks between forage availability, composition, herbivore density, and forage selection and consumption (Snape et al. 2018), which are unlikely to be picked up with our proxy measure. Grazing effects on understorey biomass may also be masked by heterogeneous resource accumulation and depletion across the landscape (Tongway and Ludwig 1994). There is still more work to be done to ascertain the effects of kangaroo grazing on biomass, such as through kangaroo exclosure experiments (e.g. Sluiter et al. 1997).
In this study, mean soil moisture for the previous month plus a 2-week lag was associated with a general increase in understorey biomass, though this influence was greatest on live biomass. The modest effect of soil moisture on grass and total understorey biomass can be explained by the fact that they comprised both live and dead biomass, which were affected by soil moisture in different ways: as soil moisture increased, live biomass also increased. On the other hand, dead biomass would not increase with soil moisture, as it had already senesced, and may have increased as soil moisture declined. This resulted in a muted response to soil moisture. In addition, the soil moisture data had a resolution of approximately 5 km × 5 km (Frost et al. 2018), which will not reflect landscape variation that can occur at a finer spatial scale (Tongway and Ludwig 1994). Furthermore, weather patterns can be very localised in semi-arid areas, increasing the challenge for interpolation of rainfall records for input into hydrological models (Noy-Meir 1973; Robertson et al. 1987). Installing soil moisture loggers throughout the study area would refine those estimates, and could provide a clearer picture of the effects of soil moisture on understorey biomass.
Some studies have found that seasonal pulses of increased soil moisture have significant effects on biomass (Reynolds et al. 2004; Nano and Pavey 2013; Robinson et al. 2013), while others have found 6–12 month timeframes to be important (Robertson 1987; Sluiter et al. 1997). Biomass production rarely responds instantaneously to changes in soil moisture; response times tend to be between 1 week and 1 month (Svoray and Karnieli 2011; Cissé et al. 2016), though they may vary between species and growth forms (Ludwig et al. 1997). In our study, we established that shorter windows (1–2 months) of mean soil moisture, with longer lags (2 weeks to 1 month), correlated best with understorey biomass in this region. A longer window with a longer lag period was better suited to grass and total understorey biomass than the 1-month and 2-week lag that best explained live biomass. This is likely because live biomass tends to respond more rapidly to changes in soil and weather conditions than total understorey, which comprises live and dead matter (Fletcher 2006). However, the relationship between the shorter window and live biomass was the strongest of any biomass type, when not considering site effects.
On average, model predictive performance increased from 36 to 57% when taking site effects into account, compared to predicting with only the main effects. Site effects were stronger when modelling grass and total understorey biomass, though they were less pronounced for live understorey biomass. This may be partly explained by different site understories being dominated by different growth forms, which in turn can lead to varying rates of long-term dead standing biomass accumulation and decomposition (Facelli and Pickett 1991; McLaren and Turkington 2010). The models for total and grass biomass tended to overpredict at higher biomass levels (especially for open sites), though predicted values fitted more closely to observed values at the lower biomass end. Conversely, live predictions were consistently lower than observed values. As summer and autumn are seasons of low forage availability (Coulson et al. 1990), the ability to accurately estimate biomass in this period, especially grass, is critical. If grass is underpredicted in a given season, managers may decide to cull more kangaroos than necessary; on the other hand, if the amount of grass on the ground has been overestimated, the cull target may not be large enough to avoid a forage switch, threatening any tree seedlings present. Models were trained using data from high through to low rainfall years (2016–2018); even so, the study period was relatively short, given the temporal and spatial variability in rainfall in semi-arid areas (Noy-Meir 1973). Long-term observations would improve our knowledge of understorey biomass dynamics, especially the role of legacies of rainfall anomalies on biomass production during subsequent years (Sala et al. 2012).
Western grey kangaroos show a preference for grasses and forbs (Short 1986); indeed, grasses have been found to comprise at least 75% of their diet (Norbury 1987). Although the proposed 400 kg ha−1 threshold is based on live and dead grass biomass (Norbury 1987), we believe understanding understorey biomass as a whole is important for kangaroo management, as all understorey biomass could be considered potential forage (Short 1986; Barker 1987). As conditions become drier and grass less available, the proportion of grass and forbs in kangaroo diets decreases, and their intake of chenopod shrubs and other browse increases (Barker 1987). In addition, it remains unclear how the 400 kg ha−1 forage-switch threshold might be affected by different ratios of live to dead grass in the field. With decreasing soil moisture availability, the proportion of live and dead vegetation will change. Though western grey kangaroos are able to survive on a diet of dry, fibrous vegetation (Priddel et al. 1988), they show a preference for green vegetation (Dawson and Munn 2007). Therefore, the relative importance of live and dead biomass might change throughout the year: green grass biomass may be more important during a good season, but total grass (or even total understorey) biomass may become more important during a dry season. Further studies on preference relative to changes in soil moisture, forage quality and availability, and herbivore densities would help clarify this.
This research has provided insight into dynamics of grassy biomass in a semi-arid environment where the abundance of standing understorey biomass is cast as a threshold indicator of herbivore density management, itself a means objective toward the ultimate objective of woodland restoration. In extant wooded areas, grassy forage tends to fall below the threshold during the drier months, and western grey kangaroos tend to shelter in and forage close to woodland. Therefore, undertaking management actions before this critical dry period is recommended to avoid browsing of seedlings, particularly in woodland areas. Understanding when and where increased grazing and browsing may occur can also help managers identify areas where vegetation assets require additional protection (Bennett et al. 2020). In order for grassy biomass estimates to be better integrated into an adaptive management approach, further work could refine biomass predictions across space and time, combining data on both herbivore density and vegetation response (Morris et al. 2019). A more dynamic spatial and temporal model of kangaroo foraging behaviour with respect to forage availability may better enable managers to anticipate periods and areas of higher grazing threat. Such information would aid the decision to trigger and scale management actions, including lethal control for the management of total grazing pressure.
Conclusions
We have demonstrated a model able to predict understorey biomass to new sites and seasons, explaining 53% of the variation in grass biomass in external validation. This information is a valuable guide for managers seeking to understand when and where understorey biomass may fall to levels that result in significant grazing threat to conservation outcomes in the semi-arid woodlands of south-eastern Australia, such that they can manage accordingly.
Data availability
The data that support this study are available via an Open Science Framework repository at doi: 10.17605/OSF.IO/GHQTC (Riquelme et al. 2021).
Conflicts of interest
The authors declare no conflict of interest.
Declaration of funding
This study was funded by the Parks Victoria Research Partners Panel, the Holsworth Wildlife Research Endowment (Ecological Society of Australia), the David H Ashton Scholarship (University of Melbourne Botany Foundation), the Bill Borthwick Student Scholarship (Victorian Environmental Assessment Council), and the National Environmental Science Program-Threatened Species Research Hub.
Supplementary material
Supplementary material is available online.
References
Akaike H (1973) Information Theory and an Extension of the Maximum Likelihood Principle. In ‘Proceeding of the Second International Symposium on Information Theory’. pp. 267–281. (Akademiai Kiado: Budapest, Hungary)Bailey, DW, Gross, JE, Laca, EA, Rittenhouse, LR, Coughenour, MB, Swift, DM, and Sims, PL (1996). Mechanisms that result in large herbivore grazing distribution patterns. Journal of Range Management 49, 386–400.
| Mechanisms that result in large herbivore grazing distribution patterns.Crossref | GoogleScholarGoogle Scholar |
Barker RD (1987) The diet of herbivores in the sheep rangelands. In ‘Kangaroos: their ecology and management in the sheep rangelands of Australia’. (Eds G Caughley, N Shepherd, J Short) pp. 69–83. (Cambridge University Press: Cambridge, UK)
Bates, D, Mächler, M, Bolker, B, and Walker, S (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67, 1–48.
Beale, IF (1973). Tree density effects on yields of herbage and tree components in south west Queensland mulga (Acacia aneura F. Muell.) scrub. Tropical Grasslands 7, 135–142.
Bennett, A, Duncan, DH, Rumpff, L, and Vesk, PA (2020). Disentangling chronic regeneration failure in endangered woodland ecosystems. Ecosphere 11, e02998.
| Disentangling chronic regeneration failure in endangered woodland ecosystems.Crossref | GoogleScholarGoogle Scholar |
Blaser, WJ, Sitters, J, Hart, SP, Edwards, PJ, and Venterink, HO (2013). Facilitative or competitive effects of woody plants on understorey vegetation depend on N-fixation, canopy shape and rainfall. Journal of Ecology 101, 1598–1603.
| Facilitative or competitive effects of woody plants on understorey vegetation depend on N-fixation, canopy shape and rainfall.Crossref | GoogleScholarGoogle Scholar |
Bradshaw, CJA, and Warkentin, IG (2015). Global estimates of boreal forest carbon stocks and flux. Global and Planetary Change 128, 24–30.
| Global estimates of boreal forest carbon stocks and flux.Crossref | GoogleScholarGoogle Scholar |
Bureau of Meteorology (2020) Australian Landscape Water Balance. Bureau of Meteorology. Available at http://www.bom.gov.au/water/landscape/#/sm/Actual/day/ [Accessed 5 February 2020]
Bureau of Meteorology (2021a) Monthly Rainfall - 076065 - Bureau of Meteorology. Available at http://www.bom.gov.au/jsp/ncc/cdio/weatherData/av?p_nccObsCode=139&p_display_type=dataFile&p_startYear=&p_c=&p_stn_num=076065 [Accessed 23 November 2021]
Bureau of Meteorology (2021b) Mean Minimum Temperature - 076064 - Bureau of Meteorology. Available at http://www.bom.gov.au/jsp/ncc/cdio/weatherData/av?p_nccObsCode=38&p_display_type=dataFile&p_startYear=&p_c=&p_stn_num=076064 [Accessed 23 November 2021]
Bureau of Meteorology (2021c) Mean Maximum Temperature - 076064 - Bureau of Meteorology. Available at http://www.bom.gov.au/jsp/ncc/cdio/weatherData/av?p_nccObsCode=36&p_display_type=dataFile&p_startYear=&p_c=&p_stn_num=076064 [Accessed 23 November 2021]
Burnham KP, Anderson DR (2002) ‘Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach’, 2nd edn. (Springer-Verlag: New York, NY, USA)
Cheal, D (1986). A park with a kangaroo problem. Oryx — The International Journal of Conservation 20, 95–99.
| A park with a kangaroo problem.Crossref | GoogleScholarGoogle Scholar |
Cheal D, Lucas A, Macauley L (2011) Recovery Plan for Buloke Woodlands of the Riverina and Murray-Darling Depression Bioregions.
Cissé, S, Eymard, L, Ottlé, C, Ndione, JA, Gaye, AT, and Pinsard, F (2016). Rainfall intra-seasonal variability and vegetation growth in the Ferlo Basin (Senegal). Remote Sensing 8, 66.
| Rainfall intra-seasonal variability and vegetation growth in the Ferlo Basin (Senegal).Crossref | GoogleScholarGoogle Scholar |
Coulson G, Norbury G (1988) Ecology and management of Western Grey Kangaroos (Macropus fuliginosus) at Hattah-Kulkyne National Park. Arthur Rylah Institute for Environmental Research.
Coulson, G, Norbury, G, and Walters, B (1990). Forage biomass and kangaroo populations (Marsupialia: Macropodidae) in summer and autumn at Hattah-Kulkyne National Park, Victoria. Australian Mammalogy 13, 219–221.
| Forage biomass and kangaroo populations (Marsupialia: Macropodidae) in summer and autumn at Hattah-Kulkyne National Park, Victoria.Crossref | GoogleScholarGoogle Scholar |
Dawson TJ, Munn AJ (2007) How much do kangaroos of differing age and size eat relative to domestic stock?: implications for the arid rangelands. In ‘Animals of Arid Australia: out on their own?’. (Eds C Dickman, D Lunney, S Burgin) (Royal Zoological Society of New South Wales)
Department of Agriculture, Water and the Environment (2020) Buloke Woodlands of the Riverina and Murray-Darling Depression Bioregions. Department of Agriculture, Water and the Environment. Available at https://www.awe.gov.au/environment/biodiversity/threatened/assessments/buloke-woodlands [Accessed 17 March 2022]
Facelli, JM, and Pickett, STA (1991). Plant litter: Its dynamics and effects on plant community structure. The Botanical Review 57, 1–32.
| Plant litter: Its dynamics and effects on plant community structure.Crossref | GoogleScholarGoogle Scholar |
Fletcher D (2006) Population Dynamics of Eastern Grey Kangaroos in Temperate Grasslands. PhD Thesis, University of Canberra, Canberra, ACT, Australia.
Flombaum, P, and Sala, OE (2007). A non-destructive and rapid method to estimate biomass and aboveground net primary production in arid environments. Journal of Arid Environments 69, 352–358.
| A non-destructive and rapid method to estimate biomass and aboveground net primary production in arid environments.Crossref | GoogleScholarGoogle Scholar |
Franke, J, Barradas, ACS, Borges, MA, Menezes Costa, M, Dias, PA, Hoffmann, AA, Orozco Filho, JC, Melchiori, AE, and Siegert, F (2018). Fuel load mapping in the Brazilian Cerrado in support of integrated fire management. Remote Sensing of Environment 217, 221–232.
| Fuel load mapping in the Brazilian Cerrado in support of integrated fire management.Crossref | GoogleScholarGoogle Scholar |
Frost AJ, Ramchum A, Smith A (2018) The Australian Landscape Water Balance model (AWRA-L v6). Technical Description of the Australian Water Resources Assessment Landscape model version 6. Bureau of Meteorology.
Grundy, MJ, Rossel, RAV, Searle, RD, Wilson, PL, Chen, C, and Gregory, LJ (2015). Soil and Landscape Grid of Australia. Soil Research 53, 835–844.
| Soil and Landscape Grid of Australia.Crossref | GoogleScholarGoogle Scholar |
Horner, GJ, Cunningham, SC, Thomson, JR, Baker, PJ, and Mac Nally, R (2012). Forest structure, flooding and grazing predict understorey composition of floodplain forests in southeastern Australia. Forest Ecology and Management 286, 148–158.
| Forest structure, flooding and grazing predict understorey composition of floodplain forests in southeastern Australia.Crossref | GoogleScholarGoogle Scholar |
House, JI, Archer, S, Breshears, DD, and Scholes, RJ (2003). Conundrums in mixed woody-herbaceous plant systems: Woody-herbaceous plant systems. Journal of Biogeography 30, 1763–1777.
| Conundrums in mixed woody-herbaceous plant systems: Woody-herbaceous plant systems.Crossref | GoogleScholarGoogle Scholar |
Jameson, DA (1967). The Relationship of Tree Overstory and Herbaceous Understory Vegetation. Journal of Range Management 20, 247–249.
| The Relationship of Tree Overstory and Herbaceous Understory Vegetation.Crossref | GoogleScholarGoogle Scholar |
Ludwig JA, Tongway DJ, Freudenberger DO, Noble JC, Hodgkinson KC (Eds) (1997) ‘Landscape Ecology, Function and Management.’ (CSIRO Publishing: Melbourne)
MacKenzie DI (2020) Distance Analysis of Mallee Parks Kangaroo Census Data 2020. Report for Parks Victoria, Proteus Client Report: 2020-06. Proteus, Outram, New Zealand.
Magioli, M, Moreira, MZ, Fonseca, RCB, Ribeiro, MC, Rodrigues, MG, and de Barros Ferraz, KMPM (2019). Human-modified landscapes alter mammal resource and habitat use and trophic structure. Proceedings of the National Academy of Sciences 116, 18466–18472.
| Human-modified landscapes alter mammal resource and habitat use and trophic structure.Crossref | GoogleScholarGoogle Scholar |
McLaren, JR, and Turkington, R (2010). Plant functional group identity differentially affects leaf and root decomposition. Global Change Biology 16, 3075–3084.
| Plant functional group identity differentially affects leaf and root decomposition.Crossref | GoogleScholarGoogle Scholar |
Michez, A, Lejeune, P, and Bauwens, S (2019). Mapping and monitoring of biomass and grazing in pasture with an unmanned aerial system. Remote Sensing 11, 473.
| Mapping and monitoring of biomass and grazing in pasture with an unmanned aerial system.Crossref | GoogleScholarGoogle Scholar |
Miller J, Gibson M, Westbrooke M, Wilcock P, Brown G (1998) Condition of vegetation in the Riverine Woodlands of Wyperfeld National Park. Centre for Environmental Management, University of Ballarat, Victoria, Australia.
Morris WK, Duncan DH, Vesk P (2019) Control and monitoring of kangaroo populations in the Mallee Parks of semi-arid Northwest Victoria (NESP Technical Report) - Version 2. The University of Melbourne, Parks Victoria, & Commonwealth Department of Environment and Energy, Melbourne, Australia. Available at https://osf.io/w8krz/
Mutze, G, Cooke, B, and Jennings, S (2016). Estimating density-dependent impacts of European rabbits on Australian tree and shrub populations. Australian Journal of Botany 64, 142–152.
| Estimating density-dependent impacts of European rabbits on Australian tree and shrub populations.Crossref | GoogleScholarGoogle Scholar |
Mysterud, A (2006). The concept of overgrazing and its role in management of large herbivores. Wildlife Biology 12, 129–141.
| The concept of overgrazing and its role in management of large herbivores.Crossref | GoogleScholarGoogle Scholar |
Nakagawa, S, and Schielzeth, H (2013). A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods in Ecology and Evolution 4, 133–142.
| A general and simple method for obtaining R2 from generalized linear mixed-effects models.Crossref | GoogleScholarGoogle Scholar |
Nano, CEM, and Pavey, CR (2013). Refining the ‘pulse-reserve’ model for arid central Australia: Seasonal rainfall, soil moisture and plant productivity in sand ridge and stony plain habitats of the Simpson Desert. Austral Ecology 38, 741–753.
| Refining the ‘pulse-reserve’ model for arid central Australia: Seasonal rainfall, soil moisture and plant productivity in sand ridge and stony plain habitats of the Simpson Desert.Crossref | GoogleScholarGoogle Scholar |
Norbury GL (1987) Diet Selection and Demography of the Western Grey Kangaroo: Macropus fuliginosus melanops Desmarest in Hattah-Kulkyne National Park, Victoria. PhD Thesis, Monash University, Clayton, Victoria, Australia.
Noy-Meir, I (1973). Desert ecosystems: environment and producers. Annual Review of Ecology and Systematics 4, 25–51.
| Desert ecosystems: environment and producers.Crossref | GoogleScholarGoogle Scholar |
Noy-Meir, I (1975). Stability of grazing systems: an application of predator-prey graphs. Journal of Ecology 63, 459–481.
| Stability of grazing systems: an application of predator-prey graphs.Crossref | GoogleScholarGoogle Scholar |
Priddel, D, Shepherd, N, and Wellard, G (1988). Home Ranges of Sympatric Red Kangaroos Macropus-Rufus, and Western Grey Kangaroos Macropus-Fuliginosus, in Western New-South-Wales. Wildlife Research 15, 405–411.
| Home Ranges of Sympatric Red Kangaroos Macropus-Rufus, and Western Grey Kangaroos Macropus-Fuliginosus, in Western New-South-Wales.Crossref | GoogleScholarGoogle Scholar |
Prowse, TAA, O’Connor, PJ, Collard, SJ, and Rogers, DJ (2019). Eating away at protected areas: Total grazing pressure is undermining public land conservation. Global Ecology and Conservation 20, e00754.
| Eating away at protected areas: Total grazing pressure is undermining public land conservation.Crossref | GoogleScholarGoogle Scholar |
Quinn G, Keough M (2002) ‘Experimental Design & Data Analysis for Biologists.’ (Cambridge University Press: Cambridge, UK)
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at https://www.R-project.org/
Reynolds, JF, Kemp, PR, Ogle, K, and Fernández, RJ (2004). Modifying the ‘pulse–reserve’ paradigm for deserts of North America: precipitation pulses, soil water, and plant responses. Oecologia 141, 194–210.
| Modifying the ‘pulse–reserve’ paradigm for deserts of North America: precipitation pulses, soil water, and plant responses.Crossref | GoogleScholarGoogle Scholar | 15042457PubMed |
Riquelme L, Rumpff L, Duncan DH, Vesk PA (2021) OSF | biom_data.csv. Open Science Framework. Available at https://osf.io/4kg6d/ [Accessed 25 November 2021]
Robertson G (1987) Plant dynamics. In ‘Kangaroos: their ecology and management in the sheep rangelands of Australia’. (Eds G Caughley, N Shepherd, J Short) pp. 50–68. (Cambridge University Press: Cambridge, UK)
Robertson G, Short J, Wellard G (1987) The environment of the Australian sheep rangelands. In ‘Kangaroos: their ecology and management in the sheep rangelands of Australia’. (Eds G Caughley, N Shepherd, J Short) pp. 14–34. (Cambridge University Press: Cambridge, UK)
Robinson, TMP, Pierre, KJL, Vadeboncoeur, MA, Byrne, KM, Thomey, ML, and Colby, SE (2013). Seasonal, not annual precipitation drives community productivity across ecosystems. Oikos 122, 727–738.
| Seasonal, not annual precipitation drives community productivity across ecosystems.Crossref | GoogleScholarGoogle Scholar |
Sala OE, Lauenroth WK, Golluscio RA (1997) 11 Plant functional types in temperate semi-arid regions. In ‘Plant functional types: their relevance to ecosystem properties and global change’. (Eds TM Smith, HH Shugart, FI Woodward) pp. 217–233. (Cambridge University Press: Cambridge, UK)
Sala, OE, Gherardi, LA, Reichmann, L, Jobbágy, E, and Peters, D (2012). Legacies of precipitation fluctuations on primary production: theory and data synthesis. Philosophical Transactions of the Royal Society B: Biological Sciences 367, 3135–3144.
| Legacies of precipitation fluctuations on primary production: theory and data synthesis.Crossref | GoogleScholarGoogle Scholar |
Sandell P (2011) Victoria’s rangelands: in recovery or in transition: report from a Parks Victoria sabbatical project. Parks Victoria, Melbourne, Victoria, Australia.
Short, J (1986). The effect of pasture availability on food intake, species selection and grazing behaviour of kangaroos. Journal of Applied Ecology 23, 559–571.
| The effect of pasture availability on food intake, species selection and grazing behaviour of kangaroos.Crossref | GoogleScholarGoogle Scholar |
Short, J, and Grigg, G (1982). The abundance of Kangaroos in suboptimal habitats: wheat, intensive pastoral, and Mallee. Wildlife Research 9, 221–227.
| The abundance of Kangaroos in suboptimal habitats: wheat, intensive pastoral, and Mallee.Crossref | GoogleScholarGoogle Scholar |
Sluiter IRK, Allen GG, Morgan DG, Walker IS (1997) Vegetation responses to stratified kangaroo grazing pressure at Hattah-Kulkyne National Park, 1992–96. Department of Natural Resources and Environment.
Snape M, Caley P, Baines G, Fletcher D (2018) Kangaroos and conservation: Assessing the effects of kangaroo grazing in lowland grassy ecosystems. Environment, Planning and Sustainable Development Directorate, ACT Government.
Svoray, T, and Karnieli, A (2011). Rainfall, topography and primary production relationships in a semiarid ecosystem. Ecohydrology 4, 56–66.
| Rainfall, topography and primary production relationships in a semiarid ecosystem.Crossref | GoogleScholarGoogle Scholar |
’t Mannetje, L, and Haydock, KP (1963). The Dry-Weight-Rank Method for the Botanical Analysis of Pasture. Grass and Forage Science 18, 268–275.
| The Dry-Weight-Rank Method for the Botanical Analysis of Pasture.Crossref | GoogleScholarGoogle Scholar |
Taylor L, Pegler P (2016) Total grazing management plan for the restoration of semi-arid woodland and floodplain vegetation communities in north-western (Mallee) parks 2016–2021. Parks Victoria, Melbourne, Victoria, Australia.
Tongway, DJ, and Ludwig, JA (1994). Small-scale resource heterogeneity in semi-arid landscapes. Pacific Conservation Biology 1, 201–208.
| Small-scale resource heterogeneity in semi-arid landscapes.Crossref | GoogleScholarGoogle Scholar |
Travers, SK, Eldridge, DJ, Koen, TB, Val, J, and Oliver, I (2020). Livestock and kangaroo grazing have little effect on biomass and fuel hazard in semi-arid woodlands. Forest Ecology and Management 467, 118165.
| Livestock and kangaroo grazing have little effect on biomass and fuel hazard in semi-arid woodlands.Crossref | GoogleScholarGoogle Scholar |
Tucker, MA, Santini, L, Carbone, C, and Mueller, T (2021). Mammal population densities at a global scale are higher in human‐modified areas. Ecography 44, 1–13.
| Mammal population densities at a global scale are higher in human‐modified areas.Crossref | GoogleScholarGoogle Scholar |
Warnock, AD, Westbrooke, ME, Florentine, SK, and Hurst, CP (2007). Does Geijera parviflora Lindl. (Rutaceae) facilitate understorey species in semi-arid Australia? The Rangeland Journal 29, 207–216.
| Does Geijera parviflora Lindl. (Rutaceae) facilitate understorey species in semi-arid Australia?Crossref | GoogleScholarGoogle Scholar |
Zuur, AF, and Ieno, EN (2016). A protocol for conducting and presenting results of regression-type analyses. Methods in Ecology and Evolution 7, 636–645.
| A protocol for conducting and presenting results of regression-type analyses.Crossref | GoogleScholarGoogle Scholar |